Abstract
Objective
To demonstrate the safety and effectiveness of responsive stimulation at the seizure focus as an adjunctive therapy to reduce the frequency of seizures in adults with medically intractable partial onset seizures arising from one or two seizure foci.
Methods
Randomized multicenter double-blinded controlled trial of responsive focal cortical stimulation (RNS System). Subjects with medically intractable partial onset seizures from one or two foci were implanted, and 1 month postimplant were randomized 1:1 to active or sham stimulation. After the fifth postimplant month, all subjects received responsive stimulation in an open label period (OLP) to complete 2 years of postimplant follow-up.
Results
All 191 subjects were randomized. The percent change in seizures at the end of the blinded period was −37.9% in the active and −17.3% in the sham stimulation group (p = 0.012, Generalized Estimating Equations). The median percent reduction in seizures in the OLP was 44% at 1 year and 53% at 2 years, which represents a progressive and significant improvement with time (p < 0.0001). The serious adverse event rate was not different between subjects receiving active and sham stimulation. Adverse events were consistent with the known risks of an implanted medical device, seizures, and of other epilepsy treatments. There were no adverse effects on neuropsychological function or mood.
Significance
Responsive stimulation to the seizure focus reduced the frequency of partial-onset seizures acutely, showed improving seizure reduction over time, was well tolerated, and was acceptably safe. The RNS System provides an additional treatment option for patients with medically intractable partial-onset seizures.
Keywords: Cortical stimulation, Partial seizures, Focal seizures, Responsive stimulation, Neurostimulator

Dr. Christianne N. Heck is the Medical Director of the USC Comprehensive Epilepsy Program at the Keck School of Medicine at the University of Southern California in Los Angeles, California, and Principal Investigator of the RNS System clinical trials at this site.
Thirty percent to 40% of patients with partial-onset seizures have intractable epilepsy, defined by the International League Against Epilepsy (ILAE) as a failure to control seizures after two seizure medications that have been appropriately chosen and used.1 These patients may be candidates for surgical removal of the seizure focus or for vagus nerve stimulation (VNS). However, these treatments are not appropriate or helpful for all.2
Direct brain stimulation is one approach to treating medically intractable partial-onset seizures. The RNS System (NeuroPace, Inc., Mountain View, CA, U.S.A.) is a cranially implanted neurostimulator that provides responsive stimulation directly to the seizure focus when epileptiform activity is detected. The intent is to disrupt epileptiform activity before a seizure can develop.
A randomized, multicenter, double-blinded, sham-stimulation controlled pivotal study assessed efficacy and safety of responsive direct brain stimulation as an adjunctive therapy to reduce the frequency of seizures in adults with medically intractable partial onset seizures from one or two foci. Efficacy and safety results of the blinded controlled portion of the trial were previously reported.3 This manuscript provides study results during the open label period of this trial with up to 2 years of postimplant follow-up.
Methods
The RNS System (NeuroPace, Inc) is an investigational device that provides responsive cortical stimulation via the RNS Neurostimulator, a cranially implanted programmable neurostimulator, which is connected to one or two depth and/or subdural cortical strip leads that are surgically placed in the brain according to the seizure focus (Fig.1). Each of the four electrodes in a lead can sense and stimulate, and provides eight sensing and stimulating electrodes in total. The Neurostimulator continually senses electrocorticographic activity and is programmed by the physician to detect specific abnormalities on electrocorticography (ECoG) and then to provide brief pulses of stimulation in response to the detection. In most patients, the Neurostimulator is programmed to detect and provide stimulation to interictal epileptiform abnormalities. The physician adjusts detection and stimulation parameters for each patient as needed to optimize control of clinical seizures.
Figure 1.
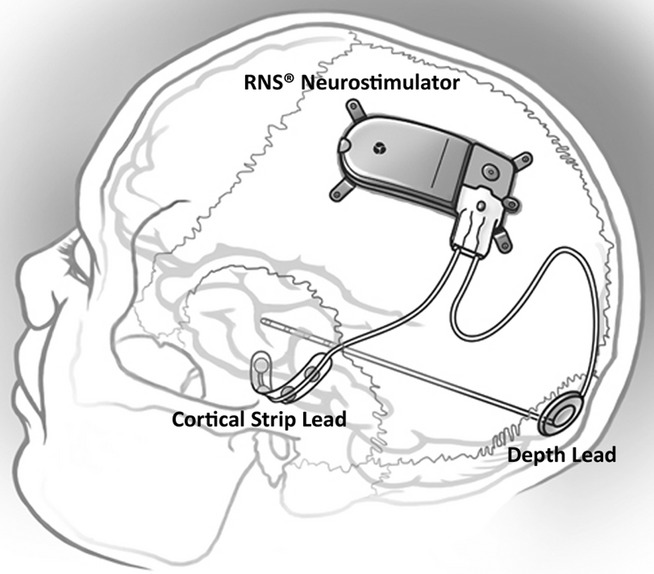
Implanted RNS Neurostimulator and NeuroPace Depth Lead and NeuroPace Cortical Strip Lead.
Subjects participating in the RNS System Pivotal trial were 18–70 years of age, had partial-onset seizures that had not been controlled with two or more trials of antiepileptic drugs (AEDs), had three or more disabling seizures per month on average, and had undergone standard diagnostic testing that localized seizures to one or two foci. Disabling seizures included simple partial motor, complex partial, and secondarily generalized tonic–clonic. Seizures were recorded in daily seizure diaries. Subjects who were implanted with a vagus nerve stimulator were required to have vagus nerve stimulation turned off during the baseline period and to have the vagus nerve stimulator pulse generator (but not leads) removed prior to implantation of the RNS System.
The study protocol was approved by the institutional review boards of all participating investigation sites. All patients gave written informed consent. The study was registered on www.clinicaltrials.gov (NCT00264810).
The Pivotal trial design is provided in Figure2. To be eligible for implant, subjects had to have three or more disabling seizures per month on average over a 12-week Baseline Period while on stable AED regimens. For the first 4 weeks after implantation, the Neurostimulator was programmed to sense and record the ECoG, but not to deliver stimulation (Postoperative Stabilization Period). Subjects were then randomized 1:1 to the Treatment group (active stimulation) or to the Sham group (no stimulation). Stimulation was adjusted for the Treatment group over the next 4 weeks (Stimulation Optimization Period) and continued over the 12-week Blinded Evaluation Period (BEP). Both Treatment and Sham group subjects received responsive stimulation treatment throughout the Open Label Period (OLP), which began after the fifth postimplant month and continued to 2 years postimplant. AEDs were to remain constant through the end of the BEP but could be adjusted in the OLP. Study design and subject flow are presented in Figure2.
Figure 2.
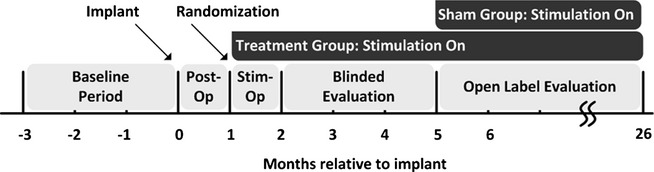
RNS System Pivotal Study Design.
Changes in seizures during postimplant periods were compared to the preimplant baseline. During the BEP, the primary effectiveness outcome was the change from baseline in seizure frequency in the Treatment group compared to the Sham stimulation group as estimated by a generalized estimating equation (GEE) model. Over the OLP, changes in seizures were expressed as median percent change for each 3-month period compared to the preimplant baseline, and as responder rate (the percentage of subjects with a 50% or greater reduction in seizures). Significance was assessed by the GEE.
Quality of life in individual subjects was assessed by the Quality of Life in Epilepsy Inventory (QOLIE-89)4 at baseline and for the OLP at 1 and 2 years after implantation. Clinically significant individual improvements in the overall QOLIE-89 score and primary scales were defined as an increase in the T-score of 5 points, which is equivalent to 0.5 standard deviations.5,6 Group-averaged changes in the QOLIE overall score and primary scale scores were compared to baseline using the paired one-sample t-test.
Safety was assessed by the rate and types of spontaneously reported adverse events (AEs), which were classified by the investigators as serious or mild. An adverse event was identified as serious if it resulted in a hospital admission or invasive procedure, posed significant risks or consequences to the subject's acute or long-term health, or caused serious injury or death. AEs were further classified as device-related (definitely or potentially related to the RNS System) or not device-related, and anticipated or unanticipated. An independent data monitoring committee reviewed all AEs and a second committee determined whether deaths met criteria for sudden unexplained death in epilepsy (SUDEP).
Other safety assessments were neuropsychological function and mood, which were evaluated at baseline and at 1 and 2 years after implantation. Standardized tests of neuropsychological functioning assessed a variety of domains that included visual and verbal memory, language, and cognitive flexibility. Mood was assessed by summary scores of validated surveys of affective status (the Beck Depression Inventory [BDI-II], Profile of Mood States [POMS], and the Center for Epidemiological Studies Depression Scale [CES-D]).
Results
One hundred ninety-one subjects were implanted with the RNS System at 31 investigational centers in the United States from May 2006 to May 2009. The last subject completed the 2-year Pivotal trial in May 2011. Demographics and baseline characteristics for the implanted subjects are presented in Table1.
Table 1.
Demographic and baseline characteristics of implanted subjects
| Characteristic | All implanted (N = 191) | Treatment (N = 97) | Sham (N = 94) |
|---|---|---|---|
| Mean ± SD (min–max) or % (n) | |||
| Age (years) | 34.9 ± 11.6 (18–66) | 34.0 ± 11.5 (18–60) | 35.9 ± 11.6 (18–66) |
| Female | 48 (91) | 48 (47) | 47 (44) |
| Duration of epilepsy (years) | 20.5 ± 11.6 (2–57) | 20.0 ± 11.2 (2–57) | 21.0 ± 12.2 (2–54) |
| Number of AEDs at enrollment | 2.8 ± 1.2 (0–8) | 2.8 ± 1.3 (1–8) | 2.9 ± 1.1 (0–6) |
| Mean seizure frequency during Preimplant Period (seizures/month) | 34.2 ± 61.9 (3–338) median = 9.7 | 33.5 ± 56.8 (3–295) median = 8.7 | 34.9 ± 67.1 (3–338) median = 11.6 |
| Seizure onset location – mesial temporal lobe only (vs. other)a | 50 (95) | 49 (48) | 50 (47) |
| Number of seizure foci -two (vs. one)a | 55 (106) | 49 (48) | 62 (58) |
| Prior therapeutic surgery for epilepsya | 32 (62) | 35 (34) | 30 (28) |
| Prior EEG monitoring with intracranial electrodes | 59 (113) | 65 (63) | 53 (50) |
| Prior VNS | 34 (64) | 31 (30) | 36 (34) |
Characteristics used as strata in randomization algorithm.
Of the 191 subjects who were implanted with the RNS Neurostimulator and Leads, 50% (95) had seizures arising from mesial temporal lobe structures. Mesial temporal onsets were bilateral in 73% (69), left in 18% (17), and right in 9% (9). Three of the 17 subjects with unilateral left and five of the nine subjects with unilateral right mesial temporal lobe onsets had already undergone resective surgery.
All 191 implanted subjects were randomized. One hundred eighty-seven subjects completed the BEP (through 5 months postimplant), 182 subjects completed at least 1 year postimplant, and 175 subjects completed the entire 2 years postimplant. Subject accountability and reasons for withdrawal are presented in Figure3. There were 379 years of implant experience and >328 patient years of experience with responsive stimulation enabled. One hundred seventy-three of these subjects enrolled in a subsequent study to be followed for an additional 7 years (Long-term Treatment study). This study is ongoing.
Figure 3.
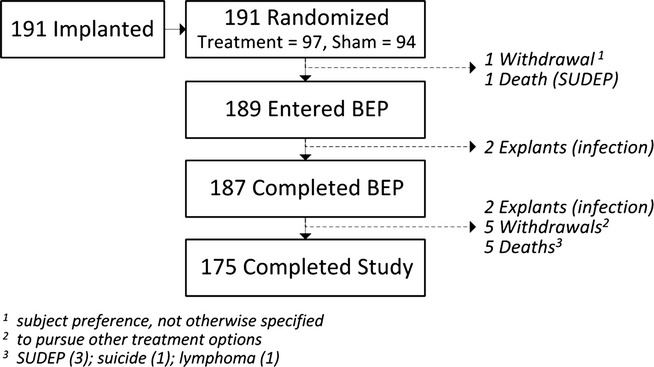
RNS System Pivotal Study Subject Disposition.
Default detection settings were programmed after implantation to detect changes in frequency and power of the ECoG signal (using a line length detector). Detection was adjusted depending on the electrocorticographic patterns that the physician wanted detected. For the majority of subjects, stimulation was initially programmed to a frequency of 200 Hz, pulse width of 160 μs, and burst duration of 100 msec; these settings were usually not changed. The current amplitude was typically started at 0.5 mA and then titrated upward as tolerated. At the completion of the OLP, stimulation current amplitude was <4.0 mA in 53.8% of subjects, 4.0–7.9 mA in 34.8% of subjects, and 8.0–11.9 mA in 8.7% of subjects. The maximum current amplitude of 12.0 mA was programmed in 2.7% of subjects. Stimulation of the hippocampus was usually delivered with adjacent electrodes serving as cathode and anode (bipolar), and stimulation in the neocortex was usually delivered with all electrodes serving as anodes and the Neurostimulator case serving as the cathode. The total duration of stimulation was 5.9 min/day on average (median 4.7 min/day). Seventy-five percent of subjects received <7.3 min of stimulation a day.
Seizure reduction
There was a significantly greater reduction in the frequency of total disabling seizures in the Treatment group (37.9%) compared to the Sham group (17.3%) during the BEP relative to the Preimplant Period of the investigation (p = 0.012).3 After an initial reduction in seizures related to the implant procedure, there was a progressive reduction in seizures in the Treatment group and a return toward baseline seizure frequency in the Sham group, so that by the final month of the BEP, the reduction in seizures in the Treatment group reached 41.5% compared to a 9.4% reduction in the Sham group. Treatment with responsive stimulation in the OLP reduced seizure frequency in the subjects that had been randomized to the Sham group. The reduction in seizure frequency in the second through fifth months of stimulation (months 6 through 9 post-implant) was significant compared to their preimplant baseline p = 0.04, paired t-test).
Both groups continued to have a reduction in seizures during the OLP, which improved over time. The median percent reduction in seizures and responder rates over each 3-month period of the OLP is presented in Figure4. Note that all subjects were receiving responsive stimulation during the OLP. The median percent reduction at 1 year was 44% and at 2 years was 53% compared to the preimplant baseline. The responder rate was also 44% at 1 year, and 55% at 2 years. The continued improvement over the OLP reached statistical significance for both the median percent reduction and the responder rate (p < 0.0001).
Figure 4.
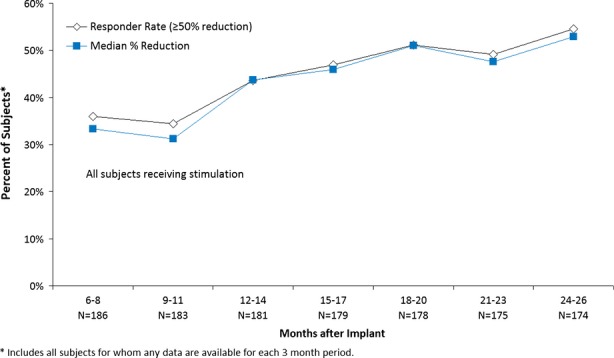
Responder rate and median percent reduction in seizure frequency.
The study was not powered to provide conclusions regarding subsets of subjects; however, descriptive analyses were performed to assess the response in subjects with seizure onsets in the mesial temporal lobe (MTLE, N = 90) compared to those who had onsets outside of the mesial temporal lobe (non-MTLE, N = 93). The responses were similar. At the end of the OLP, the median percent reduction in seizures was 55% for subjects with MTLE and 58% for subjects with non-MTLE.
AEDs could be changed in the OLP. The seizure response in subjects in whom AEDs remained stable was similar to those who had any type of change. Over the last 3 months for which data were available in the OLP, the reduction in seizures was 54% in subjects, with no change in any AED (N = 87), 61% in subjects who added or increased the dose of an AED (N = 40) and in subjects who discontinued or decreased any AED (N = 11), and 45% in subjects who both increased and decreased any AED (N = 45). Therefore, the favorable response to responsive stimulation was not due to changes in antiepileptic medication therapy.
The majority of subjects experienced a clinically meaningful reduction in seizure frequency and some had extended periods of seizure freedom. Figure5 shows the percent change in seizures during the most recent 3 months of open label data for all implanted subjects compared to their baseline seizure frequency. Eighty-two percent of subjects (150/183) had some improvement in seizure frequency. Fifty-four percent (99/183) had a 50% or greater reduction in seizures, compared to 7% (13/183) who had a 50% or greater increase. Nine percent of subjects (16/183) were seizure free over the last 3 months of their participation in the Pivotal study.
Figure 5.
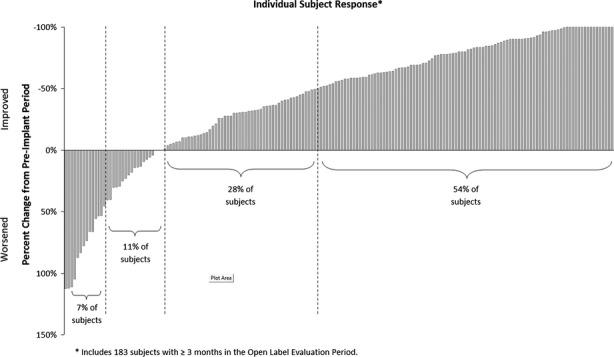
Seizure frequency percent change by subject: most recent 3 months.
The demographic characteristics presented in Table1 were examined in those subjects who were seizure free (N = 16) and those who had a 50% or greater increase in seizures (N = 13). All but two demographic characteristics were similar. The exceptions were that seizure-free subjects were more likely to have one seizure focus than two (75% of seizure free and 41% of those not seizure free; p = 0.01 chi-square test) and subjects with a 50% or greater increase in seizures were somewhat younger (29 years old, range 19–51) compared to the other subjects (35 years old, range 19–51; p = 0.03 per t-test), although there was no difference between these groups in the duration of epilepsy. The meaningfulness of these differences is not clear because the study was not powered to perform subset analyses for multiple demographic characteristics.
Quality of life
There were statistically significant improvements in quality of life at 1 and 2 years after implantation. Statistically significant group improvements occurred in quality of life overall (p < 0.001) and in 10 of the 17 primary scale scores at 1 year and 10 of the 17 at 2 years (year 2 shown in Fig.6). There were no statistically significant declines in any of the QOLIE scales.
Figure 6.
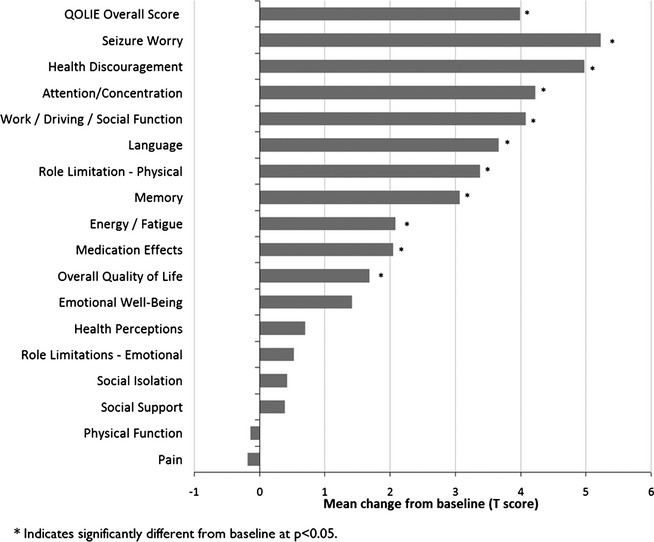
Changes in QOLIE-89 primary scale scores at 2 years after implantation of the RNS Neurostimulator and Leads.
Adverse events
SAEs that occurred in 2.5% or more of the 191 subjects over the entire 2 year postimplant period are provided in Table2.
Table 2.
Serious adverse events affecting ≥2.5% of implanted subjects
| % Subjects with events (# subjects) | % Subjects with device-relateda events (# subject) | |
|---|---|---|
| Related to the implanted device | ||
| Implant site infection | 3.7 (7) | 3.7 (7) |
| Device lead revision | 3.7 (7) | 2.1 (4) |
| Device lead damage | 2.6 (5) | 2.6 (5) |
| Related to seizures | ||
| Complex partial seizures increased | 5.2 (10) | 3.1 (6) |
| Tonic–clonic seizures exacerbated | 3.7 (7) | 0.5 (1) |
| Tonic–clonic seizures increased | 3.7 (7) | 2.6 (5) |
| Other serious adverse events | ||
| EEG monitoring | 7.3 (14) | 0.5 (1) |
| Death | 3.1 (6) | 0.5 (1) |
| Therapeutic agent toxicityb | 2.6 (5) | – |
Includes device-related and device-relation uncertain.
Four related to antiepileptic medication and one related to acetaminophen toxicity.
All SAEs that occurred at implant or within the first postoperative month were anticipated and resolved without neurologic sequelae. The most frequent SAE during the postoperative period was implant site infection, occurring in 5 subjects (2.6%): one of these subjects had the Neurostimulator and Leads explanted. Four subjects (2.1%) had an intracranial hemorrhage; two of these four subjects had postoperative epidural hematomas that were evacuated, the third had a subdural hematoma evacuated, and the fourth had a small computerized tomography (CT) diagnosed intraventricular hemorrhage and was observed for 1 day in the hospital. Other SAEs that occurred during the first postoperative month were transient apraxia and dysphemia (one subject) and a procedure to revise the location of a lead (one subject).
Over the blinded periods (months 2 through 5 post-implant) there was no difference between the Treatment and Sham group subjects in the rate or types of SAEs. The only statistically significant difference in mild adverse events during the blinded periods was related to side effects of AEDs, which occurred in six subjects in the Sham group but in no subjects in the Treatment group (p = 0.013).
Three subjects had a total of five SAEs during the blinded periods that were considered device related. One subject in the Treatment group had an SAE related to an increase in the frequency of complex partial seizures, and one subject in the Sham group had three SAEs (one SAE related to an increase in the frequency of complex partial seizures, one SAE due to an increase in the frequency of simple partial sensory seizures, and one SAE related to a new type of simple partial sensory seizure; this was an SAE because the patient was briefly hospitalized for observation). The third subject had inpatient video–electroencephalography (EEG) monitoring, which was considered an SAE because the procedure was performed in the hospital. Other SAEs in the blinded period that were not considered to be device related included two subjects who had implant site infections (both were attributed to seizure-related head trauma and not considered to be device related) and two subjects who underwent procedures to revise the location of the leads.
Over the OLP, the most common SAE was admission to an inpatient epilepsy monitoring unit in order to perform video-EEG monitoring (13 subjects; 7.0%). Four subjects in the OLP had a lead revision to improve the lead location. Five subjects had damaged leads. Four of these five subjects had a depth lead that was damaged where the lead was secured at the burr hole, and the fifth subject had a strip lead that was damaged between the skull and a titanium plate and a second lead that was inadvertently cut. These were considered SAEs because there was a procedure to replace the leads. Three subjects had implant site infections during the OLP and one of these had the device explanted. Two subjects had subdural hemorrhages that were attributed to seizure related head trauma during the OLP. None of these subjects had neurologic consequences.
SAEs related to a change in seizures during the OLP included an increased frequency of complex partial (nine subjects; 4.8%) or tonic–clonic seizures (seven subjects; 3.7%), and an exacerbation (increased severity or duration) in tonic–clonic (seven subjects; 3.7%) or complex partial seizures (two subjects; 1.1%). There were three additional subjects with SAEs related to simple partial motor seizures; one subject's seizures were more severe and two subject's seizures were more frequent. In each of these cases, these were considered SAEs because the subject was hospitalized to change or administer AEDs and/or to be monitored. No subject withdrew from the trial because of an adverse event related to a change in seizures.
Six subjects died during the Pivotal trial. Four of these deaths were attributed to possible or definite SUDEP; one of these subjects was randomized to the Sham group and did not have stimulation enabled. One subject died of lymphoma and one of suicide. The subject who died of suicide had a preexisting history of depression including scores on the mood inventories that indicated severe depression with high depressive symptoms but was clinically stable at the time of enrollment in the investigator's opinion.
Neuropsychological function and mood
There were no negative effects of treatment with the RNS System on neuropsychological functioning. Group comparison of the changes in neuropsychological assessment scores at the end of the BEP relative to baseline showed no negative effects on any of the cognitive variables and no difference between Treatment and Sham group. There was no deterioration in the neuropsychological measures at 1 and 2 years postimplant, indicating that there were no delayed or longer-term adverse effects of responsive stimulation on neuropsychological function.
Subset analyses of the neuropsychological data were also performed for the subjects with MTLE and subjects with non-MTLE. There was no deterioration on any measure in either group. Subjects with MTLE had statistically significant group improvements at 1 and 2 years (p < 0.05) in some measures of cognitive flexibility (DKEFS Design Fluency) and visual spatial abilities (WAIS-III Block Design Task). Subjects with non-MTLE had statistically significant group improvements at 1 and 2 years (p < 0.05) in some measures of language (Boston Naming Test), cognitive flexibility (DKEFS Design Fluency), and general verbal ability (WAIS-III Information).
Treatment with the RNS System had no negative effect on mood as assessed by the three validated mood inventories. There was no deterioration in any score at the end of the BEP or across the OLP.
Scores for the BDI-II were also assessed separately for subjects with MTLE (N = 93) and non-MTLE subjects (N = 94). At baseline, mean BDI-II scores were significantly higher in MTLE subjects (12.6) compared to non-MTLE subjects (9.4; p < 0.01, two-sample t-test), indicating more depressive symptoms. At 1 and 2 years, there was no worsening in BDI-II scores in the non-MTLE subjects. There were statistically significant improvements in the MTLE subjects at 1 year (reduction in BDI of 2.8, p = 0.012 paired t-test) and at 2 years (reduction in BDI of 2.3, p = 0.049).
Conclusion
A multicenter double-blinded, sham stimulation–controlled trial of the RNS System as an adjunctive treatment for medically intractable partial-onset seizures in adults whose seizures were localized to one or two seizure foci demonstrated acceptable safety and a statistically significant reduction in seizure frequency that was sustained long term. The average subject had a >20-year history of epilepsy and was having frequent seizures despite treatment with multiple AEDs. About one third of the subjects had already been treated with vagus nerve stimulation, and about one third had already had a therapeutic epilepsy surgery. These subjects were not considered to be current epilepsy surgery candidates.
There was a statistically significantly greater reduction in seizure frequency during the BEP relative to the Preimplant Period in the Treatment group compared to the Sham stimulation group.3 Ultimately, what is most important to patients is that a treatment works over the long term. The reduction in seizures with the RNS System increased over the first and second years after implant and was sustained at around 50%. These group improvements were not related to changes in antiepileptic medications. Because 92% of implanted subjects completed the entire study, this improvement cannot be attributed to patients with poorer clinical responses dropping out.
Increased efficacy over time has also been reported with other neurostimulation devices for treatment of partial-onset seizures such as the VNS,7–9 and with stimulators that deliver scheduled deep brain stimulation of the anterior nucleus of the thalamus,10 as well as with deep brain stimulation for movement and psychiatric disorders.11 The mechanism by which stimulation exerts its therapeutic effect is not known, but these consistent observations of acute and then continued improvement over the first 1–2 years of treatment suggest that there are multiple mechanisms of action. The acute effects of stimulation may be mediated by local cellular inhibition and/or excitation.12 Other acute effects could be related to changes in cerebral blood flow and to the release of neurotransmitters from axons and bordering astrocytes. Later and sustained therapeutic effects could be related to alterations in neuronal networks related to changes in synaptic plasticity, neurogenesis, and cortical reorganization.11,13–15
The clinical meaningfulness of the response to treatment is demonstrated by the significant group improvements in overall quality of life and in perception of cognitive function, relationships and social function, overall health, and vulnerability to seizures. Improvements in health discouragement and seizure worry are strongly associated with improved quality of life in persons with intractable epilepsy.16,17
The safety data from the Pivotal study of the RNS System demonstrate that the risks of implantation are low and that treatment is well tolerated and safe over time. There was no difference in the SAE rate in the subjects in the Treatment group (active stimulation) and subjects in the Sham group (no stimulation). Adverse events were consistent with the known risks of an implanted medical device, seizures, and other therapies for epilepsy. Treatment with the RNS System did not cause deterioration in any aspect of neuropsychological function or mood.
Specific adverse events of special relevance with any implanted device to treat seizures include hemorrhage and implant site infection, and changes in seizures. The rate of acute and chronic hemorrhages and the rate of infections of the implant site in subjects treated with the RNS System were not higher than the rates of hemorrhage or infection in patients implanted with intracranial electrode to localize the seizure focus18–21 or with epilepsy surgery18,22,23 or with deep brain stimulation for treatment of movement disorders.24,25 Adverse changes in seizures are expected in any trial of an epilepsy therapy and likely represent the natural fluctuation of seizures in patients who do not respond to the treatment. The numbers of adverse events related to seizures in the RNS System trial was not higher than what has been reported in randomized controlled trials of antiepileptic medications approved by the U.S. Food and Drug Administration (FDA) for adjunctive treatment of partial-onset seizures.26–30
At least 30% of adults with partial-onset seizures do not have their seizures controlled with antiepileptic medications,31,32 and a similar percentage experience medication-related side effects that impact quality of life, such as impaired cognition, fatigue, problems with coordination, nausea, or other gastrointestinal symptoms.31–34 Some of these patients will consider epilepsy surgery or the VNS. However, not all patients are candidates for these treatments and these treatments do not always work. Subjects in the RNS System Pivotal trial were not candidates for epilepsy surgery, and one third had already failed treatment with a VNS.
Despite the risks associated with all epilepsy treatments, the risks of doing nothing are often greater. Patients with more frequent seizures have poorer cognitive function; significant increases in anxiety, depression, and suicidality; poorer employment status; a lower quality of life; and worse overall health than patients with fewer seizures.35–37 Patients do not need to achieve complete seizure freedom in order to experience positive life changes.38 A reduction in seizure frequency, even without seizure freedom, can improve mood, employment, perceived health, and quality of life.39,40 These observations reinforce the need to find new therapies that can reduce the burden of seizures.
Responsive stimulation to the seizure focus reduced the frequency of partial-onset seizures acutely and over the long-term, was well tolerated, and was acceptably safe in a population of persons with frequent and disabling partial-onset seizures who had failed multiple epilepsy therapies. There were enduring improvements in quality of life. Adverse systemic effects common with AEDs did not occur with treatment with responsive stimulation; adverse events related to coordination, gastrointestinal side effects, and allergic reactions were not higher in treated subjects compared to untreated subjects during the blinded periods. There was no deterioration in overall group measures of cognition or mood. The results of this study indicate that the RNS System provides an additional treatment option for patients with medically intractable partial-onset seizures who are not good candidates for epilepsy surgery.
Acknowledgments
University of Southern California: Rami G. Apelian, MD, Vidya Hawkins, DO, Neda Heidari, MD, Laura A. Kalayjian, MD, Reed L. Levine, MD, Charles Y. Liu, MD, PhD, Andrew D. Ly, MD, Johnson L. Moon, MD, Jason S. Muir, MD, Ron A. Shatzmiller, MD, MS, Parastou Shilian, MD, and Steve N. Sykes, MD; California Pacific Medical Center: Kenneth D. Laxer, MD, and Peter B. Weber, MD; Via Christi Comprehensive Epilepsy Center: Kore Liow, MD, and Nazih Moufarrij, MD; The Cleveland Clinic Foundation: Andreas V. Alexopoulos, MD, MPH, William E. Bingaman, MD, Lara Jehi, MD, Prakash Kotagal, MD, and Imad Michael Naijm, MD; Dartmouth-Hitchcock Medical Center: Krzysztof A. Bujarski, MD, Ann-Christine Duhaime, MD, Gregory L. Holmes, MD, Erik Kobylarz, MD, PhD, Richard P. Morse, MD, David W. Roberts, MD, and Vijay M. Thadani, MD, PhD; Henry Ford Hospital: Konstantin V. Elisevich, MD, PhD, Shailaja Gaddam, MD, Madhuri L. Koganti, MD, Amit Ray, MD, Brien J. Smith, MD, Andrea F. Sneider, DO, Marianna Spanaki-Varelas, MD, PhD, and Vibhangini S. Wasade, MD; Indiana University: Andrew J. Kalnin, MD, Omkar N. Markand, MD, Dragos Sabau, MD, Thomas C. Witt, MD, and Robert M. Worth, MD, PhD; Massachusetts General Hospital: Sydney S. Cash, MD, PhD, Emad N. Eskandar, MD, and Daniel B. Hoch, MD, PhD; Rush University Medical Center/Epilepsy Center: Donna C. Bergen, MD; Richard W. Byrne, MD, and Marvin A. Rossi, MD, PhD; Swedish Medical Center: Lisa M. Caylor, MD, Michael J. Doherty, MD, John D. Morgan, MD, and David G. Vossler, MD; Thomas Jefferson University: James J. Evans, MD, Scott E. Mintzer, MD, Maromi Nei, MD, Ashwini D. Sharan, MD, Michael R. Sperling, MD, and Andre Zangaladze, MD, PhD; University of Texas Southwestern Medical Center: Mark A. Agostini, MD, Sachin Dave, MD, Ramon Diaz-Arrastia, MD, PhD, Puneet K. Gupta, MD, MSE, Christopher J. Madden, MD, Pradeep N. Modur, MD, MS, and Louis Anthony Whitworth, MD; Johns Hopkins University School of Medicine: George I. Jallo, MD, Eric H. W. Kossoff, MD, Frederick A. Lenz, MD, PhD, and Eva Katharina Ritzl, MD; Medical College of Georgia/Georgia Regents University: Cole A. Giller, MD, PhD, MBA, Ki-Hyeong Lee, MD, MS, Mark R. Lee, MD, PhD, Anthony M. Murro, MD, Jeffrey M. Politsky, MD, Joseph R. Smith, MD, Suzanne M. Strickland, MD, and Jeffrey A. Switzer, DO; Miami Children's Hospital: Sanjiv Bhatia, MD, Michael Duchowny, MD, Prasanna Jayakar, MD, PhD, Glen Morrison, MD, John Ragheb, MD, and Trevor J. Resnick, MD; Saint Barnabas Medical Center: Orrin Devinsky, MD, Werner Doyle, MD, Mangala A. Nadkarni, MD, and Peter P. Widdess-Walsh, BA, MB, BCh, BAO, MRCPI; University of Wisconsin Hospital and Clinics: Mustafa K. Baskaya, MD, Brad R. Beinlich, MD, Rahul Dewan, MD, Victor Diaz-Cotrina, MD, John C. Jones, MD, Lincoln F. Ramirez, MD, PhD, Edgar A. Samaniego, MD, MS, Raj D Sheth, MD, Karl A. Sillay, MD, and Evelyn C. Tunnell, MD; Mayo Clinic – Arizona: Joseph F. Drazkowski, MD, Katherine H. Noe, MD, PhD, and Joseph I. Sirven, MD; Oregon Health & Science University: James J. Cereghino, MS, MD, Felicia A. Ferguson, MD, Mary M. Ransom, MD, Martin C. Salinsky, MS, MD, and William Brewster Smith, MD; Baylor College of Medicine: Ian L. Goldsmith, MD, Eli M. Mizrahi, MD, and Daniel Yoshor, MD; Medical University of South Carolina: Jimmy E. Couch, DO, Steven S. Glazier, MD, Jonathan J. Halford, MD, Justin M. Nolte, MD, Holly J. Skinner, DO, and Mimi Sohn, MD; George Washington University: Anthony Caputy, MD, and Samuel J.Potolicchio Jr., MD; Mayo Clinic – Florida: David R. Chabolla, MD, Kent C. New, MD, PhD, Jerry J. Shih, MD, and William Tatum, DO; University of Rochester: Michel Berg, MD, Guiseppe Erba, MD, Robert A. Gross, MD, PhD, John Craig Henry, MD, Lynn C. Liu, MD, Webster H. Pilcher, MD, PhD, and Jason M. Schwalb, MD; University of Virginia: William J. Elias, MD, Ilona S. Humes, MD, Paul D. Lyons, MD, Gabriel U. Martz, MD, Rhunnelle C. Murray, MD, Mark Quigg, MD, MS, Utku Uysal, MD, and Christopher J. Wright, MD; Columbia University Medical Center: Hyunmi Choi, MD, MS, Daniel Friedman, MD, Robert R. Goodman, MD, PhD, Steven C. Karceski, MD, Derek J, Chong, MD, and Carl W. Bazil, MD, PhD; Mayo Clinic – Rochester: Jeffrey W. Britton, MD, Gregory D. Cascino, MD, and Richard Marsh, MD; Emory University: Charles M. Epstein, MD, Sandra L. Helmers, MD, Suzette M. LaRoche, MD, Kimford J. Meador, MD, Page B. Pennell, MD, and Denise Taylor, DO; University of Florida, Gainesville: Jeffrey M. Chung, MD, George A. Ghacibeh, MD, Kimford J. Meador, MD, and Steven N. Roper, MD; Yale University School of Medicine: Pue Farooque, DO, Evan J. Fertig, MD, Alexander M. Papaastassiou, MD, Susan S. Spencer, MD, Dennis D. Spencer, MD, and Kenneth P. Vives, MD; Wake Forest University Health Sciences: William L. Bell, MD, FACP, Mary L. Campagna-Gibson, MD, Joao Carlos De Toledo, MD, Thomas L. Ellis, MD, and Maria C. Sam, MD, MS, FAASM.
Disclosures or Conflicts of Interest
Author Felice T. Sun certifies that she has equity ownership/stock options with NeuroPace and is an employee of NeuroPace. Author Tracy A. Courtney certifies that she has equity ownership/stock options with NeuroPace and is an employee of NeuroPace. Author Cairn G. Seale certifies that she has equity ownership/stock options with NeuroPace and is an employee of NeuroPace. Author Martha J. Morrell certifies that she has equity ownership/stock options with NeuroPace and is an employee of NeuroPace. The remaining authors have no conflicts of interest which are relevant to this research activity. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51:1069–1077. doi: 10.1111/j.1528-1167.2009.02397.x. [DOI] [PubMed] [Google Scholar]
- 2.IOM (Institute of Medicine) Epilepsy Across the Spectrum: Promoting Health and Understanding. Washington, DC: The National Academies Press; 2012. [PubMed] [Google Scholar]
- 3.Morrell MJ. RNS® system in Epilepsy Study Group Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. 2011;77:1295–1304. doi: 10.1212/WNL.0b013e3182302056. [DOI] [PubMed] [Google Scholar]
- 4.Devinsky O, Vickrey BG, Cramer J, et al. Development of the quality of life in epilepsy inventory. Epilepsia. 1995;36:1089–1104. doi: 10.1111/j.1528-1157.1995.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 5.Borghs S, de la Loge C, Cramer JA. Defining minimally important change in QOLIE-31 scores: estimates from three placebo-controlled lacosamide trials in patients with partial-onset seizures. Epilepsy Behav. 2012;23:230–234. doi: 10.1016/j.yebeh.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 6.Wiebe S, Matijevic S, Eliasziw M, et al. Clinically important change in quality of life in epilepsy. J Neurol Neurosurg Psychiatry. 2002;73:116–120. doi: 10.1136/jnnp.73.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elliott RE, Morsi A, Tanweer O, et al. Efficacy of vagus nerve stimulation over time: review of 65 consecutive patients with treatment-resistant epilepsy treated with VNS >10 years. Epilepsy Behav. 2011;20:478–483. doi: 10.1016/j.yebeh.2010.12.042. [DOI] [PubMed] [Google Scholar]
- 8.DeGiorgio CM, Schachter SC, Handforth A, et al. Prospective long-term study of vagus nerve stimulation for the treatment of refractory seizures. Epilepsia. 2000;41:1195–1200. doi: 10.1111/j.1528-1157.2000.tb00325.x. [DOI] [PubMed] [Google Scholar]
- 9.Morris GL, III, Mueller WM. Long-term treatment with vagus nerve stimulation in patients with refractory epilepsy. The Vagus Nerve Stimulation Study Group E01-E05. Neurology. 1999;53:1731–1735. doi: 10.1212/wnl.53.8.1731. [DOI] [PubMed] [Google Scholar]
- 10.Fisher R, Salanova V, Witt T, et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51:899–908. doi: 10.1111/j.1528-1167.2010.02536.x. [DOI] [PubMed] [Google Scholar]
- 11.Lozano AM, Lipsman N. Probing and regulating dysfunctional circuits using deep brain stimulation. Neuron. 2013;77:406–424. doi: 10.1016/j.neuron.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 12.Hess CW, Vaillancourt DE, Okun MS. The temporal pattern of stimulation may be important to the mechanism of deep brain stimulation. Exp Neurol. 2013;247:296–302. doi: 10.1016/j.expneurol.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boon P, Raedt R, De Herdt V, et al. Electrical stimulation for the treatment of epilepsy. Neurotherapeutics. 2009;6:218–227. doi: 10.1016/j.nurt.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stone SS, Teixeira CM, Devito LM, et al. Stimulation of entorhinal cortex promotes adult neurogenesis and facilitates spatial memory. J Neurosci. 2011;31:13469–13484. doi: 10.1523/JNEUROSCI.3100-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stavrinou LC, Boviatsis EJ, Stathis P, et al. Sustained relief after discontinuation of DBS for dystonia: implications for the possible role of synaptic plasticity and cortical reorganization. J Neurol Surg A Cent Eur Neurosurg. 2012;73:175–178. doi: 10.1055/s-0032-1313590. [DOI] [PubMed] [Google Scholar]
- 16.Hessen E, Lossius MI, Gjerstad L. Health concerns predicts poor quality of life in well-controlled epilepsy. Seizure. 2009;18:487–491. doi: 10.1016/j.seizure.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 17.Loring DW, Meador KJ, Lee GP. Determinants of quality of life in epilepsy. Epilepsy Behav. 2004;5:976–980. doi: 10.1016/j.yebeh.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 18.Behrens E, Schramm J, Zentner J, et al. Surgical and neurological complications in a series of 708 epilepsy surgery procedures. Neurosurgery. 1997;41:1–9. doi: 10.1097/00006123-199707000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Wong CH, Birkett J, Byth K, et al. Risk factors for complications during intracranial electrode recording in presurgical evaluation of drug resistant partial epilepsy. Acta Neurochir (Wien) 2009;151:37–50. doi: 10.1007/s00701-008-0171-7. [DOI] [PubMed] [Google Scholar]
- 20.Silberbusch MA, Rothman MI, Bergey GK, et al. Subdural grid implantation for intracranial EEG recording: CT and MR appearance. AJNR Am J Neuroradiol. 1998;19:1089–1093. [PMC free article] [PubMed] [Google Scholar]
- 21.Spencer SS, Spencer DD, Williamson PD, et al. Combined depth and subdural electrode investigation in uncontrolled epilepsy. Neurology. 1990;40:74–79. doi: 10.1212/wnl.40.1.74. [DOI] [PubMed] [Google Scholar]
- 22.Engel J, Jr, Wiebe S, French J, et al. Practice parameter: temporal lobe and localized neocortical resections for epilepsy: report of the Quality Standards Subcommittee of the American Academy of Neurology, in association with the American Epilepsy Society and the American Association of Neurological Surgeons. Neurology. 2003;60:538–547. doi: 10.1212/01.wnl.0000055086.35806.2d. [DOI] [PubMed] [Google Scholar]
- 23.Wiebe S, Blume WT, Girvin JP, et al. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345:311–318. doi: 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]
- 24.Weaver FM, Follett K, Stern M, et al. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA. 2009;301:63–73. doi: 10.1001/jama.2008.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The Deep-Brain Stimulation for Parkinson's Disease Study Group. Deep-brain stimulation of the subthalamic nucleus or the pars interna of the globus pallidus in Parkinson's disease. N Engl J Med. 2001;345:956–963. doi: 10.1056/NEJMoa000827. [DOI] [PubMed] [Google Scholar]
- 26.Krauss GL, Perucca E, Ben-Menachem E, et al. Perampanel, a selective, noncompetitive alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor antagonist, as adjunctive therapy for refractory partial-onset seizures: interim results from phase III, extension study 307. Epilepsia. 2013;54:126–134. doi: 10.1111/j.1528-1167.2012.03648.x. [DOI] [PubMed] [Google Scholar]
- 27.Ben-Menachem E, Biton V, Jatuzis D, et al. Efficacy and safety of oral lacosamide as adjunctive therapy in adults with partial-onset seizures. Epilepsia. 2007;48:1308–1317. doi: 10.1111/j.1528-1167.2007.01188.x. [DOI] [PubMed] [Google Scholar]
- 28.Cereghino JJ, Biton V, Bou-Khalil B, et al. Levetiracetam for partial seizures: results of a double-blind, randomized clinical trial. Neurology. 2000;55:236–242. doi: 10.1212/wnl.55.2.236. [DOI] [PubMed] [Google Scholar]
- 29.U.S. Department of Health and Human Services. Center for Devices and Radiological Health, FDA. Keppra NDA 21-035. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/1999/21035lbl.pdf. Accessed June 14, 2013.
- 30.Anhut H, Ashman P, Feuerstein TJ, et al. Gabapentin (Neurontin) as add-on therapy in patients with partial seizures: a double-blind, placebo-controlled study. The International Gabapentin Study Group. Epilepsia. 1994;35:795–801. doi: 10.1111/j.1528-1157.1994.tb02513.x. [DOI] [PubMed] [Google Scholar]
- 31.Marson AG, Al-Kharusi A, Alwaidh M, et al. The SANAD study of effectiveness of carbamazepine, gabapentin, lamotrigine, oxcarbazepine, or topiramate for treatment of partial epilepsy: an unblinded randomised controlled trial. Lancet. 2007;369:1000–1015. doi: 10.1016/S0140-6736(07)60460-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.French JA, Kanner AM, Bautista J, et al. Efficacy and tolerability of the new antiepileptic drugs II: treatment of refractory epilepsy: report of the Therapeutics and Technology Assessment Subcommittee and Quality Standards Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2004;62:1261–1273. doi: 10.1212/01.wnl.0000123695.22623.32. [DOI] [PubMed] [Google Scholar]
- 33.Perucca P, Carter J, Vahle V, et al. Adverse antiepileptic drug effects: toward a clinically and neurobiologically relevant taxonomy. Neurology. 2009;72:1223–1229. doi: 10.1212/01.wnl.0000345667.45642.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zaccara G, Franciotta D, Perucca E. Idiosyncratic adverse reactions to antiepileptic drugs. Epilepsia. 2007;48:1223–1244. doi: 10.1111/j.1528-1167.2007.01041.x. [DOI] [PubMed] [Google Scholar]
- 35.Poochikian-Sarkissian S, Sidani S, Wennberg RA, et al. Psychological impact of illness intrusiveness in epilepsy – comparison of treatments. Psychol Health Med. 2008;13:129–145. doi: 10.1080/13548500701294515. [DOI] [PubMed] [Google Scholar]
- 36.Gilliam F. The impact of epilepsy on subjective health status. Curr Neurol Neurosci Rep. 2003;3:357–362. doi: 10.1007/s11910-003-0014-0. [DOI] [PubMed] [Google Scholar]
- 37.Jacoby A, Baker GA, Steen N, et al. The clinical course of epilepsy and its psychosocial correlates: findings from a U.K. Community study. Epilepsia. 1996;37:148–161. doi: 10.1111/j.1528-1157.1996.tb00006.x. [DOI] [PubMed] [Google Scholar]
- 38.Jones JE, Berven NL, Ramirez L, et al. Long-term psychosocial outcomes of anterior temporal lobectomy. Epilepsia. 2002;43:896–903. doi: 10.1046/j.1528-1157.2002.43201.x. [DOI] [PubMed] [Google Scholar]
- 39.Hermann BP, Seidenberg M, Dow C, et al. Cognitive prognosis in chronic temporal lobe epilepsy. Ann Neurol. 2006;60:80–87. doi: 10.1002/ana.20872. [DOI] [PubMed] [Google Scholar]
- 40.Leidy NK, Elixhauser A, Vickrey B, et al. Seizure frequency and the health-related quality of life of adults with epilepsy. Neurology. 1999;53:162–166. doi: 10.1212/wnl.53.1.162. [DOI] [PubMed] [Google Scholar]


