Abstract
While statin intake has been proven to reduce the risk of colorectal cancer (CRC), the mechanism of antitumor effects and clinical significance in survival benefits remain unclear. Statin-induced antiproliferative effects and its underlying mechanism were examined using six CRC cell lines. Statins except pravastatin showed antiproliferative effects (simvastatin ≥ fluvastatin > atorvastatin) even though both of simvastatin and pravastatin could activate mevalonate pathways, suggesting the statin-mediated antiproliferative effects depended on non-mevalonate pathway. Indeed, statin induced p27KIP1 expression by downregulation of histone methyltransferase enhancer of zeste homolog 2 (EZH2), which acts as an epigenetic gene silencer. Additionally, the use of simvastatin plus classII histone deacetylase (HDAC) inhibitor (MC1568) induced further overexpression of p27KIP1 by inhibiting HDAC5 induction originated from downregulated EZH2 in CRC cells and synergistically led to considerable antiproliferative effects. In the clinical setting, Statin intake (except pravastatin) displayed the downregulated EZH2 expression and inversely upregulated p27KIP1 expression in the resected CRC by immunohistochemical staining and resulted in the significantly better prognoses both in overall survival (p = 0.02) and disease free survival (p < 0.01) compared to patients without statin intake. Statins may inhibit tumor progression via an EZH2-mediated epigenetic alteration, which results in survival benefits after resected CRC. Furthermore, statin plus classII HDAC inhibitor could be a novel anticancer therapy by their synergistic effects in CRC.
Keywords: colorectal cancer, statins, EZH2, HDAC, p27
Colorectal cancer (CRC) is the third most prevalent cancer in the world as an estimated 1.2 million individuals are diagnosed with CRC annually and is the fourth most common cause of death from cancer, accounting for 8.1% of all cancer-related deaths in 2008.1 Apart from genetic cause of CRC development, obesity is one of the major risks of developing CRC. Indeed, a population-based large cohort study surprisingly revealed that the long-term use of statins, which block the pathway for synthesizing cholesterol by inhibiting 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, resulted in a 47% relative reduction in the risk of CRC after adjustment for other known risk factors.2 Thereafter, several clinical studies (phase I or II) have explored the possibility of statins as an anticancer reagent.3–7 Presently, their possibility as an anticancer reagent is still controversial. Statin plus 5-FU based neoadjuvant chemoradiation have improved the pathologic complete response rate against rectal cancer,7 whereas statin use in adjuvant setting failed to improve survival outcomes (disease-free survival [DFS], recurrence-free survival, overall survival [OS]) of stage III colon cancer patients.8
The main target of statin is the mevalonate pathway, which is an important metabolic pathway. Mevalobate pathway generates not only cholesterol but also produces many isoprenoids that are critical for multiple cellular processes such as cell growth and differentiation. Statins inhibit the step of HMG-CoA conversion to mevalonate and results in the decreased levels of various downstream products such as cholesterol, dolichol, ubiquinone, isopentenyladenine, farnesyl pyrophosphate, geranylgeranylated proteins.9 On the other hand, the anticancer effect of statins has been reported to differ among their kinds of statins (e.g., proapoptosis in lovastatin, cell cycle arrest in mevastatin and invasion in fluvastatin and lovastatin).10–12 In their findings, while the addition of geranylgeranylated proteins eliminated the lovastatin-induced apoptosis and the fluvastatin- or lovastatin-induced negative effect on invasion, the upstream molecules such as mevalonate or farnesyl pyrophosphate failed to prevent their effects intriguingly,10 suggesting the possibility of mevalonate pathway-independent mechanism in statin-induced anticancer effects.
What’s new? —
Although statin use is associated with reduced colorectal cancer risk, the mechanism by which the drugs exert antitumor effects and their benefits for survival remain unclear. Here, experimental and clinical statin-associated anticancer effects were found to differ for different kinds of statins. For example, while both simvastatin and pravastatin activated the mevalonate pathway in colon cancer cells, of the two drugs, only simvastatin displayed non-mevalonate-pathway antiproliferative effects via induction of p27KIP1 and epigenetic silencing by enhancer of zeste homolog 2 (EZH2). Statin-induced downregulation of EZH2 and upregulation of p27KIP1 was associated with improved overall and disease-free survival.
Oncogenesis is a complex process associated with accumulation of genetic and epigenetic defects that alter the transcriptional program. Proteins of the polycomb repressive complex 2 (PRC2) has been known to function as a transcriptional repressor through a methylation of histone H3 at lysine 27, and its activity is essential for cellular proliferation, differentiation and cell fate decisions. The histone methyltransferase enhancer of zeste homolog 2 (EZH2), a key member of PRC2 function, is associated with transcriptional repression, and thereby EZH2 acts mainly as a gene silencer.13 In several cancers such as prostate,14 breast15 and colon,16 the overexpression of EZH2 has been reported to be associated with aggressive and metastatic disease. Recently, in cancer epigenetics, collaboration and the functional links of epigenetic silencing enzymes such as EZH2, histone methytransferase, DNA methyltransferases and histone deacetylase (HDAC) are now increasingly recognized.17
Here, we showed the statin-induced antiproliferative effects on colon cancer cells (but not in pravastatin) via the EZH2-mediated epigenetic mechanism and its survival benefits (OS and DFS) in CRC patients with curative surgery. Additionally, combination of statin and classII HDAC inhibitor accelerated the antiproliferative effects on colon cancer cells by further induction of p27 compared to statin only, suggesting the novel epigenetic anticancer approach for CRC.
Material and Methods
Cell culture
The human CRC cell lines were obtained since July 2012. DLD1 was obtained from Institute of Development, Aging and Cancer, Cell Resource Center for Biomedical Research, Tohoku University which was checked by short tandem repeat PCR (STR-PCR). SW620 was purchased from European Collection of Cell Cultures. HCT116 were purchased from American Tissue Culture Collection. LoVo and colo320 were purchased from Riken BioResource Center CELL BANK. HT29 was kindly provided from Division of Gene Regulation, Institute of Advanced Medical Research, School of medicine, Keio University and checked by STR-PCR. The cells were cultured in the recommended medium supplemented with 10% fetal bovine serum (Gibco-BRL, CA, USA) at 37°C in a humidified atmosphere of 5% CO2 to 95% air. The gene profile of the cell lines is summarized in Supporting Information Table 1.
Growth assay
One thousand cells were cultured in a 96-well cell culture plate. Various doses of statin (pravastatin, simvastatin, fluvastatin, atorvastatin [Sigma, St. Louis, USA]), 5-fluorouracil (5-FU) (Kyowa, Tokyo, Japan), classII HDAC inhibitor MC1568 (AdooQ BioScience, CA, USA) were added to the cells 24 hr after seeding the cells. The medium was changed daily. After 5 days, living cells were counted by cell counting kit 8 (Dojindo, Kumamoto, Japan) according to the manufacturer.
Reverse Transcription Polymerase Chain Reaction (RT-PCR)
Total RNA of colon cancer cell line DLD1 was extracted using TRIZOL (Invitrogen, CA, USA) and treated with DNaseI (Roche, Basel, Switzerland) to remove genomic DNA. Five microgram of total RNA was reverse transcribed using Superscript III (Invitrogen) according to the instruction manual. The cDNA was amplified by PCR as follows: initial denature at 94°C for 3 min, followed by 25 cycles of amplification (94°C 30 sec, 55°C 30 sec, 72°C 30 sec), terminal extension at 72°C for 5 min. The primers used for screening HDACs and EZH2 are shown in Supporting Information Table 2. Amplification of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was done in the same condition but the cycle was decreased to 19 cycles.***
Protein extraction and Western blotting
The whole cell lysate was extracted by RIPA buffer. The cells were washed by phosphate buffered saline (PBS) and lysed by RIPA buffer containing protease phosphatase inhibitor cocktail (Thermo Scientific, MA, USA) on ice for 15 min. The lysate was centrifuged at 5,000 rpm for 5 min, and the supernatant was collected. Nuclear protein fraction was collected using NE-PER Nuclear and Cytoplasmic Extraction kit (Thermo Scientific) as described in the instruction manual. Samples were loaded on a SDS-PAGE gel and transferred to a polyvinylidene difluoride (PVDF) membrane (BioRad, CA, USA). The membranes were blocked with 5% non-fat dry milk (NFDM) in TBS/Tween 20 (0.1%) at room temperature for 1 hr and then were incubated with the first antibody for 1 hr at room temperature. After washing the membrane with TBS/Tween 20, the membranes were incubated with the second antibody for 1 hr at room temperature. The membranes were washed with tris buffered salin (TBS)/Tween 20 and with TBS, incubated with ECL-Plus (GE Healthcare, Little Chalfont, UK) and visualized using Image reader LAS 4000 (Fujifilm, Tokyo, Japan). The antibodies used were: HDAC3 (Cell Signaling, MA, USA), HDAC5 (Santa-Cruz, Texas, USA), HDAC7 (Santa Cruz), EZH2 (BD Biosciences, CA, USA), sterol regulatory element binding protein 2 (SREBP2; Cayman, Michigan, USA), p27 (BD Biosciences), β-actin (Cell Signaling), Histone1 (Sigma).
EZH2 suppression by small interfering RNA (siRNA) duplex oligo ribonucleotide
EZH2 suppression was performed using siRNA and Stealth RNAi siRNA duplex oligo nucleotides (Invitrogen). Cells were plated in a 12-well culture plate. The duplex siRNA was transfected to the cells using Lipofectamine RNAiMAX (Invitrogen) according to the instruction manual. The whole cell lysate was extracted 48 hr after transfection. Two kinds of siRNA were used. The sequences are, No. 1: 5′-UAU GAA AGG AGU GUA AGC UUU GCU C, No. 2: 5′-UUU CCU UGG AGG AGU AUC CAC AUC C and complement sequence of each oligo.
Immunohistochemical staining
Formalin fixed, paraffin-embedded blocks of CRC resected specimens were sliced to 3-µm sections. Sections were autoclave-pretreated in histofine antigen retrieval solution (pH9) (Nichirei, Tokyo, Japan). Endogenous peroxidase activity was blocked using 3% hydrogen peroxide, and the sections were incubated with 50 times diluted primary antibodies (EZH2 and p27) over night at 4°C. A subsequent reaction was performed with a biotin-free horseradish peroxidase enzyme-labeled polymer of the Envision Plus detection system (Dako, Glostrup, Denmark). The reaction was visualized with a diaminobenzidine solution, followed by counterstaining with Mayer’s hematoxylin.
Prognostic analyses
The records of 742 R0 resected CRC patients at Kumamoto Regional Medical Center, Kumamoto University, Minamata Regional Medical Center from January 2000 to December 2006 were analyzed. Of them, 61 patients had taken statins preoperatively and/or postoperatively and then categorized as statin (+) group. Sixty of the 61 patients had statin prior to operation and continued to take a statin at least 1 year after operation. One patient began to take statin after operation within 1 year. The remaining 681 patients were without statin intake and categorized as statin (−) group. Clinicopathological characteristics of statin (+) group (n = 61) and statin (−) group (n = 681) are shown in Supporting Information Table 3. The kind of statin was pravastatin (51%), simvastatin (20%), fluvastatin (15%), atorvastatin (11%) and others (3%). The dose of statin that the patients had was pravastatin 10 mg/day, simvastatin 5 mg/day, fluvastatin 20 mg/day, atorvastatin 10 mg/day. OS and DFS were calculated using the Kaplan-Meier method and compared using the log-rank test between in patients with and without statin intakes. Statistical analysis was done by using the SPSS 11.5 software program. p values less than 0.05 were considered as significant.
Results
Statins-induced antitumor effects against CRC cell lines
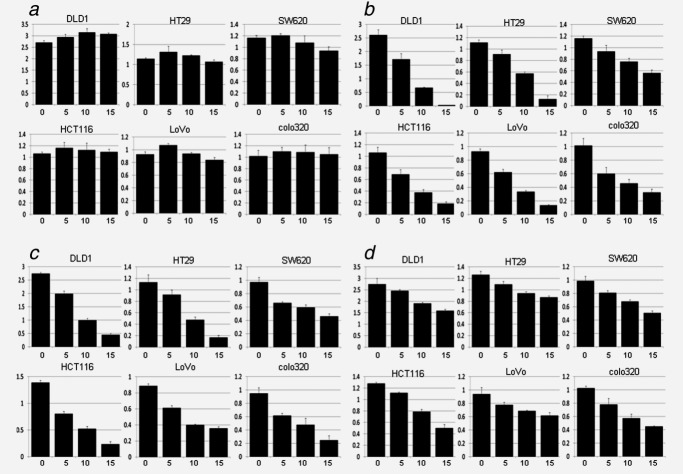
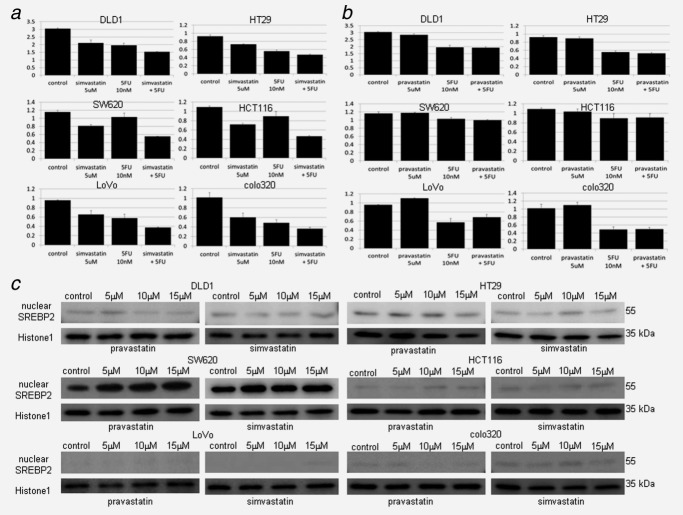
To assess the biological mechanism of statin-mediated antitumor effects in CRC, we investigated the growth inhibition effects against six CRC cell lines using four different statins (pravastatin, simvastatin, fluvastatin and atorvastatin) (Figs. 1a–1d). Simvastatin and fluvastatin showed strong growth suppressive effects. Atorvastatin revealed a relatively weak growth suppressive effect, whereas no growth suppressive effect was observed in pravastatin (simvastatin ≥ fluvastatin > atorvastatin > pravastatin). The rate of living cells compared to the control is shown in Supporting Information Table 4. Furthermore, the growth suppressive effects derived from statins were examined under the combined use of statin and 5-FU, which is the standard anticancer reagent for CRC. Then, the combined therapy (simvastatin and 5-FU)-induced antiproliferative effect was simply the sum of each effect, and there was no synergistic effects in all six CRC cell lines (Fig. 2a). In contrast, pravastatin 5 μM plus 5-FU 10 nM showed limited the only effects derived from 5-FU without any pravastatin-derived antiproliferative effects (Fig. 2b). The rate of living cells compared to the control is shown in Supporting Information Table 5. Thus, the growth inhibition effects derived from statins differ among their types such that pravastatin failed to inhibit the cell growth in vitro. To investigate if these growth inhibition effects work through the mevalonate pathway, the cleavage of SREBP2 protein, which is required to induce the mevalonate pathway, was examined. SREBP2 protein is cleaved by SREBP-cleavage-activating protein in the Golgi and turns into site 1 protease (SP1) and site 2 protease (SP2). SP2 transports into the nuclear and bind to sterol regulatory element, which leads to the downstream gene expressions such as low-density lipoprotein receptor, HMG-CoA reductase and SREBP2 itself.18 We examined statin-mediated nuclear transportation of SREBP2 (SP2) by Western blotting in CRC cells. Then, the nuclear fraction of 55 kDa SP2 in SW620 was induced by both pravastatin and simvastatin, whereas such the statin-mediated nuclear transportation were not found in DLD1, HT29, HCT116, LoVo and colo320 by both of pravastatin and simvastatin (Fig. 2c). Although pravastatin did not show growth inhibitory effect in SW620, the activation of mevalonate pathway derived from pravastatin worked as equally as simvastatin. These findings suggested that the majority of CRC cell lines except SW620 did not require activating mevalonate pathway, which generates not only cholesterol but also play a critical role in multiple cellular processes such as cell growth and differentiation, in their cell metabolism. Collectively, our results raised the possibility that the statin-induced growth suppressive effects depended on non-mevalonate pathway.
Figure 1.
Statins-induced antiproliferative effects against CRC cell lines in growth assay. (a) Pravastatin: 0, 5, 10, 15 μM of pravastatin was added to the cells for 5 days. Growth suppression was not observed in all six CRC cell line examined. (b) Simvastatin: 0, 5, 10, 15 μM of simvastatin was added to the cells for 5 days. Growth suppression was observed in all six CRC cell line examined. (c) Fluvastatin: 0, 5, 10, 15 μM of fluvastatin was added to the cells for 5 days. Growth suppression was observed in all six CRC cell line examined similar to simvastatin. (d) Atorvastatin: 0, 5, 10, 15 μM of atorastatin was added to the cells for 5 days. Growth suppression was observed in all six CRC cell line examined but weak compared to simvastatin and fluvastatin. The vertical scale is the measured OD detected using cell counting kit.
Figure 2.
Statin-mediated activation of mevalonate pathway, and antiproliferative effects by statin plus 5-FU in CRC cell line. (a) Growth assay of simvastatin 5 μM, 5-FU 10 nM and combination (simvastatin 5 μM plus 5-FU 10 nM). The drugs were added to the cells for 5 days, and the living cells were counted. The antieffect of both simvastatin and 5-FU was observed by combination in all six CRC cell lines examined but no synergistic effect was observed. The vertical scale is the measured OD detected using cell counting kit. (b) Growth assay of pravastatin 5 μM, 5-FU 10 nM and combination (pravastatin 5 μM plus 5-FU 10 nM). The drugs were added to the cells for 5 days, and the living cells were counted. Only the antitumor effect of 5-FU was observed in all six CRC cell lines examined. The vertical scale is the measured OD detected using cell counting kit. (c) Western blotting of nuclear fraction to determine nuclear SREBP2 (SP2) expression by statins. Nuclear protein was extracted 48 hr after statin was added at various doses (0, 5, 10, 15 μM) to the cells. SP2 (55kDa) was increased by both pravastatin (left lane) and simvastatin (right lane) in SW620 but not in other CRC cell lines. Histone1 was used for loading control of nuclear protein.
Statin-mediated epigenetic gene alteration in CRC
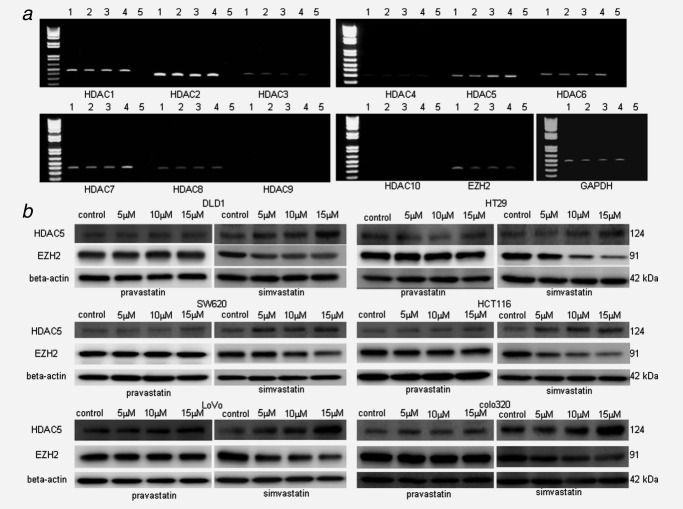
As a candidate of mevalonate pathway-independent mechanism with antiproliferative effects for CRC cells, we focused on the epigenetic gene silencing mechanism, which is now increasingly recognized as a novel therapeutic target in cancers. To investigate if statin induced epigenetic gene alteration in CRC cells, RT-PCR screening of epigenetic molecules (HDAC1 to HDAC10 and EZH2) was performed with statin treatments. DLD1 expressed various levels of HDAC1 to HDAC8 (weak expression of HDAC4) and EZH2, whereas the expressions of HDAC9 and HDAC10 were not found. The treatment with simvastatin in DLD1 attenuated the expressions of HDAC3 and EZH2 in contrast to the induction of HDAC5 and HDAC7 expressions by semiquantitative PCR (Fig. 3a). In Western blotting in six CRC cell lines, simvastatin induced HDAC5 expression and attenuated EZH2 expression in all CRC cell line, whereas pravastatin did not show any changes (Fig. 3b). Although HDAC3 protein was expressed in all CRC cell lines, no difference in protein expression level was observed by statin (simvastatin or pravastatin) treatment (data not shown). HDAC7 protein was expressed in DLD1 and HT29, but not in other cell lines, and then no difference in protein expression level was found by statin (simvastatin or pravastatin) treatment (data not shown). Eventually, EZH2 and HDAC5 were candidate for antiproliferative effectors in statin (except pravastatin)-induced epigenetic gene alteration.
Figure 3.
Screening of epigenetic molecules regulated by statins. (a) RT-PCR of HDAC1-10, EZH2. Total RNA of colon cancer cell line DLD1 was extracted 24 hr after various dose of simvastatin was added to the cells (0, 5, 10, 15 μM). Suppression of HDAC3, EZH2 and induction of HDAC5, HDAC7 was observed. Lane 1: 0 μM, lane 2: 5 μM, lane 3: 10 μM, lane 4: 15 μM, lane 5: negative control. GAPDH was used for control. (b) Western blotting of epigenetic molecules. The whole cell lysate was extracted 48 hr after various dose of pravastatin or simvastatin was added to the cells (0, 5, 10, 15 μM). HDAC5 (124 kDa) induction and EZH2 (91kDa) suppression was observed in all six CRC cell lines by simvastatin but not by pravastatin. β-Actin (42 kDa) was used for loading control.
Statin induced p27KIP1 protein via EZH2 suppression, and a combined use of statin plus classII HDAC inhibitor results in synergistically considerable induction of p27KIP1 in CRC
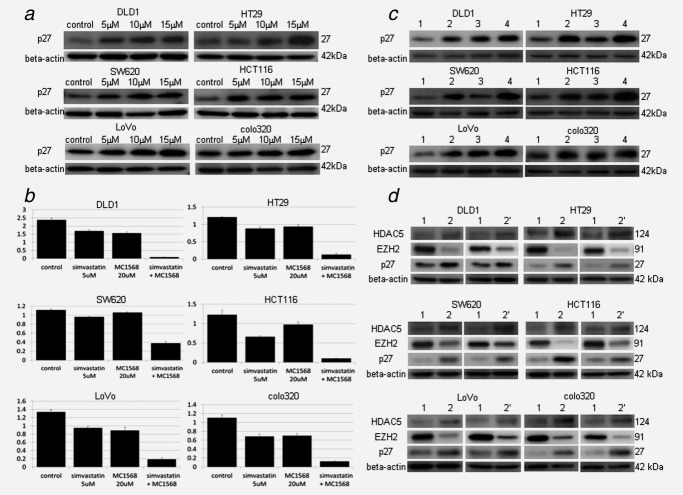
The histone methyltransferase EZH2 is associated with transcriptional repression, and thereby EZH2 has been reported to act as a gene silencer of cell-cycle-related suppressor proteins such as p27KIP1 in cancer progression.13 To further investigate the mechanism of statin-induced antiproliferative effects, we examined the protein expression levels of p27KIP1 with simvastatin treatment in six CRC cell lines. In all CRC cell lines, simvastatin induced p27KIP1 protein expression in contrast to the downregulation of EZH2 protein expression dose dependently (Fig. 4a), suggesting that statin-mediated EZH2 downregulation played the role in statin-induced antitumor effects via p27KIP1 induction. Next, to clear the role of the other candidate HDAC5 in statin-mediated antiproliferative effects in CRC cells, we examined if inhibition of HDAC5 affected to the cell growth and p27KIP1 protein expression level using specific class II HDAC inhibitor (MC1568) with simvastatin. While MC1568 revealed a weak growth suppression effect at 20 μM in CRC cells, a considerable antiproliferative effect was synergistically induced by combined use with simvastatin (Fig. 4b). The rate of living cells compared to the control is shown in Supporting Information Table 6. Western blotting confirmed the induction of p27KIP1 protein by combined use of MC1568 and simvastatin (Fig 4c). Thus, statin-induced HDAC5 expression played a negative role in statin-induced antiproliferative effects in CRC cells. Therefore, we considered the possibility that induction of HDAC5 was from the feedback of statin-induced EZH2 downregulation. Then, we found that EZH2 suppression by siRNA induced HDAC5 expression in all CRC cell lines (Fig. 4d), suggesting statin induced HDAC5 expression as a negative feedback of EZH2 downregulation. Indeed, additional treatment with MC1568 to simvastatin led to the further p27 expression and turned in the synergistically considerable cell growth inhibition in our study.
Figure 4.
Statin-induced p27 KIP1 protein expression and synergistically effects by epigenetic drug of class II HDAC inhibitor (MC1568). (a) Western blotting of p27 by various dose of simvastatin. The whole cell lysate was extracted 48 hr after various dose of simvastatin was added to the cells (0, 5, 10, 15 μM). p27 was induced by simvastatin in all six CRC cell lines examined. (b) Synergistic effect by combination of simvastatin and MC1568. Simvastatin 5 μM and/or MC1568 20 μM was added to the cells 24 hr after seeding the cells. The drugs were changed daily, and the living cells were counted after 5 days. Combination of simvastatin and MC1568 showed synergistic antitumor effect in all six CRC cell lines examined. The vertical scale is the measured OD detected using cell counting kit. (c) Western blotting of p27 by simvastatin 5 μM, MC1568 20 μM and combination (simvastatin 5 μM plus MC1568 20 μM). The whole cell lysate was extracted 24 hr after drugs were added. Combination of simvastatin and MC1568 induced high level of p27 compared to simvastatin or MC1568 alone in all six CRC cell lines examined. Lane 1 is control. Lane 2 is simvastatin 5 μM. Lane 3 is MC1568 20 μM. Lane 4 is combination of simvastatin 5 μM plus MC1568 20 μM. β-Actin (42 kDa) was used for loading control. (d) Suppression of EZH2 by two kinds of siRNA. Left line is siRNA-1, right line in siRNA-2. Lane 1 is control siRNA, Lane 2 is EZH2 siRNA. EZH2 was suppressed by both siRNA and in all CRC cell line. Induction of HDAC5 and p27 was observed in all CRC cell line. β-Actin (42 kDa) was used for loading control.
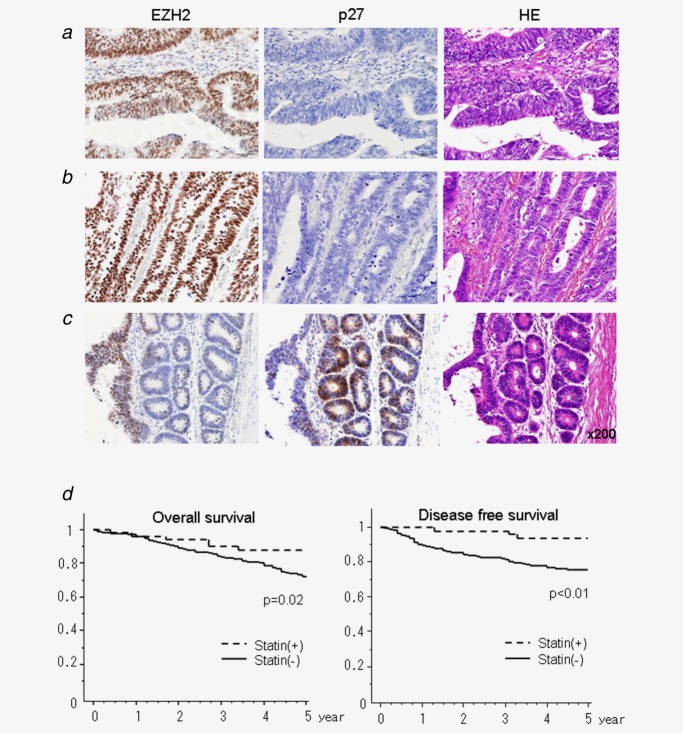
Statin intake displayed the downregulated EZH2 expression and inversely upregulated p27KIP1 expression in the resected CRC by immunohistochemical staining and improved the prognosis in the clinical setting
To assess the statin-mediated EZH2 downregulation in CRC patients, the protein expressions of EZH2 and p27KIP1 were examined by immunohistochemical staining (Figs. 5a–5c). The resected CRC specimens derived from patients without statin intake showed the upregulated EZH2 expression and downregulated p27 expression (Fig. 5a). The similar results were observed in patients with pravastatin intake (Fig. 5b). In contrast, the resected CRC specimens in patients with the other kinds of statins displayed the heterogeneous expressions of EZH2 and p27KIP1 including the upregulated p27KIP1 corresponding to the downregulated EZH2 (Fig. 5c). The wide view of Figure 5c is shown in Supporting Information Figure 1. Furthermore, to evaluate the clinical impact of statin intake for prognostic outcomes in CRC patients, OS and DFS were examined in comparison between statin (+) and statin (−) group. Statin (+) group showed significantly better survival rate in both OS and DFS (Fig. 5d) compared to stain (−) group. The recurrence rate was significantly lower in statin (+) group compared to statin (−) group (4.9% and 17%, respectively). Intriguingly, the three patients with recurrence in statin (+) group had pravastatin in all (recurrence rate who had pravastatin was 9.7%), whereas there were no recurrent patients who had other statins.
Figure 5.
Immunohistochemical staining of EZH2 and p27KIP1 and prognostic analyses in CRC patients with or without statin intakes. (a, b) The specimens derived from patients without statin intake (a) and pravastatin intake (b) showed upregulated EZH2 expression and downregulated p27KIP1 expression. (c) The specimens derived from patients derived from simvastatin intake displayed heterogeneous expressions including upregulated p27 corresponding to downregulated EZH2. The representative findings were shown. (d) Statin-derived survival benefits in R0 resected CRC patients. Left: Kaplan-Meier plot of OS of all patients with statin (+) group (n = 61) and statin (−) group (n = 681). Right: Kaplan-Meier plot of DFS of all patients with statin (+) group (n = 61) and statin (−) group (n = 681).
Discussion
Clinically statin intake has been proven to reduce the risk of CRC,2 however the mechanism of anticancer effects remains fully unclear. Intriguingly, although statin intake produced significantly better survival in patients with CRC, the statin-associated antiproliferative effects in CRC cell lines depended on kinds of statins (simvastatin ≥ fluvastatin > atorvastatin > pravastatin) in our study. Indeed, although statin intakes produced significantly better survival in patients with CRC, pravastatin intake revealed high recurrence rate of 9.7% as similar as that in statin (−) group, and there were no recurrent patients who had other kinds of statins. While pravastatin successfully activated of mevalonate pathway as equally as simvastatin, pravastatin did not show any antiproliferative effects in CRC cell lines, suggesting the statin-induced growth inhibitory effects depended on non-mevalonate pathway. Our results showed that statins except pravastatin revealed the anticancer effects by inhibiting EZH2-mediated gene silencing of p27KIP1 in CRC. On the other hand, it is reported that statin use did not improve survival of stage III colon cancer patients.8 Although the authors claim that the study is a prospective study, the use of statin depends on patient self-report in response to questionnaire. Furthermore, the kind of used statins (e.g., pravastatin, simvastatin, fluvastatin, atorvastatin and lovastatin) and its dose were not investigated in their study, and thereby clinical impact of different kind and used dose of statins in the survival benefit was unclear. In our study, there was no recurrent patient in stage III statin (+) group. The patients with resected stage III CRC usually take 5-FU-based adjuvant chemotherapy. The additional use of statins (except pravastatin) to 5-FU enhanced growth inhibitory effects in CRC cells in our results. The statin-mediated additional effects were sum of statin- and 5-FU-derived antiproliferative effects, and there was no synergistic effect. If a clinical trial to prove statin-induced survival benefits of stage III colon cancer patients will be set in the future, a use of simvastatin at least except pravastatin would be recommended from our results.
Here, we identified epigenetic alteration via EZH2 downregulation as a statin-mediated antiproliferative mechanism with p27KIP1 induction which was confirmed by both CRC cell line and CRC resected specimens. In our study, statins especially in simvastatin, but not in pravastatin, suppressed EZH2 expression and inversely induced HDAC5 expression in all CRC cell lines. Indeed, EZH2 depletion has been reported to accelerate the G1/S transition in CRC cells.16 On the other hand, the biological meaning of HDAC5 induction by statin was unclear at first. In colon cancer, the expression level of HDAC5 was lower compared to normal epithelium,19 and HDAC5 overexpression in CRC cell induced tumor cell growth inhibition and apoptosis,20 suggesting the antiproliferative role of HDAC5 in CRC. Thereby, we first made a hypothesis that inhibition of HDAC5 would rescue CRC cells from statin-induced antiproliferative effects. Surprisingly, an addition of the class II HDAC inhibitor MC1568 to simvastatin displayed a considerable synergistic antiproliferative effect in all CRC cell lines. Statin-induced HDAC5 expression may be to survive CRC cells from the feedback of EZH2 suppression, as downregulation of EZH2 by siRNA leads to HDAC5 induction. Indeed, the inhibition of the HDAC5 using additional MC1568 to statins lead to further p27KIP1 induction and turned in strong antiproliferative effect in CRC cells. To prevent side effect due from chemotherapy, it is important for anticancer therapy to gain high antitumor effect even in small amount of drug. In our study, the remarkable growth inhibitory effect derived from MC1568 was obtained at 20 μM by combined use with statins. Naldi et al. reported that single use of MC1568 failed to influence the distribution of cells in the cell cycle and to induce cell death even at 50 μM.21 By this meaning, statin plus low dose classII HDAC inhibitor could be a novel anticancer therapy by their synergistic effects in CRC.
In conclusion, as a novel mechanism of statin-induced antiproliferative effects in CRC cells, epigenetic alteration such as the downregulated EZH2 which resulted in p27KIP1 overexpression in CRC cells was identified. Furthermore, the use of simvastatin plus low dose classII HDAC inhibitor induced further overexpression of p27KIP1 in CRC cells by inhibiting HDAC5 induction originated from downregulated EZH2 and synergistically led to considerable antiproliferative effects. Statin plus classII HDAC inhibitor could be a novel anticancer therapy for CRC. Although statin intake lead to survival benefits in patients with CRC, the statin-associated antiproliferative effects in CRC differ from the kinds of statins (simvastatin ≥ fluvastatin > atorvastatin > pravastatin), indicating that the kind of statins should be considered in the setting of clinical trials.
Acknowledgments
The authors thank Mr. Keisuke Miyake, Ms. Naomi Yokoyama, Ms. Yuko Taniguchi and Ms. Kazumi Isechi for their kind supports preparing experiment reagents and analyzing clinical data.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
References
- International Agency for Research on Cancer. Globocan 2008 Facts Stats. 2010. [Google Scholar]
- Poynter JN, Gruber SB, Higgins PD, et al. Statins and the risk of colorectal cancer. N Engl J Med. 2005;352:2184–92. doi: 10.1056/NEJMoa043792. [DOI] [PubMed] [Google Scholar]
- Thibault A, Samid D, Tompkins AC, et al. Phase I study of lovastatin, an inhibitor of mevalonate pathway, in patients with cancer. Clin Cancer Res. 1996;2:483–91. [PubMed] [Google Scholar]
- Larner J, Jane J, Laws E, et al. A Phase I-II trial of lovastatin for anaplastic astrocytoma and glioblastoma multiforme. Am J Clin Oncol. 1998;21:579–83. doi: 10.1097/00000421-199812000-00010. [DOI] [PubMed] [Google Scholar]
- Kawata S, Yamasaki E, Nagase T, et al. Effect of pravastatin on survival in patients with advanced hepatocellular carcinoma. A randomize controlled trial. Br J Cancer. 2001;84:886–91. doi: 10.1054/bjoc.2000.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Jung KH, Park YS, et al. Simvastatin plus irinotecan, 5-fluorouracil, and leucovorin (FOLFIRI) as first-line chemotherapy in metastatic colorectal patients: a multicenter phase II study. Cancer Chemother Pharmacol. 2009;64:657–63. doi: 10.1007/s00280-008-0913-5. [DOI] [PubMed] [Google Scholar]
- Katz MS, Minsky BD, Saltz LB, et al. Association of statin use with a pathologic complete response to neoadjuvant chemoradiation for rectal cancer. Int J Radiat Oncol Biol Phys. 2005;62:1363–70. doi: 10.1016/j.ijrobp.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Ng K, Ogino S, Meyerhardt JA, et al. Relationship between statin use and colon cancer recurrence and survival: results from CALGB 89803. J Natl Cancer Inst. 2011;103:1540–51. doi: 10.1093/jnci/djr307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343:425–30. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- Agarwal B, Bhendwal S, Halmos B, et al. Lovastatin augments apoptosis induced by chemotherapeutic agents in colon cancer cells. Clin Cancer Res. 1999;5:2223–9. [PubMed] [Google Scholar]
- Wachtershauser A, Akoglu B, Stein J. HMG-CoA reductase inhibitor mevastatin enhances the growth inhibitory effect of butyrate in the colorectal carcinoma cell line Caco-2. Carcinogenesis. 2001;22:1061–7. doi: 10.1093/carcin/22.7.1061. [DOI] [PubMed] [Google Scholar]
- Kusama T, Mukai M, Iwasaki T, et al. Inhibition of epidermal growth factor induced RhoA translocation and invasion of human pancreatic cancer cells by 3-hydroxy-3-methylglutaryl-coenzym A reductase inhibitors. Cancer Res. 2001;61:4884–91. [PubMed] [Google Scholar]
- Cao R, Wang L, Wang H, et al. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science. 2002;298:1039–43. doi: 10.1126/science.1076997. [DOI] [PubMed] [Google Scholar]
- Varambally S, Dhanasekaran SM, Zhou M, et al. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature. 2002;419:624–9. doi: 10.1038/nature01075. [DOI] [PubMed] [Google Scholar]
- Kleer CG, Cao Q, Varambally S, et al. EZH2 is a marker of aggressive breast cancer and promotes neoplastic transformation of breast epithelial cells. Proc Natl Acad Sci USA. 2003;100:11606–11. doi: 10.1073/pnas.1933744100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluge Ø, Gravdal K, Carlsen E, et al. Expression of EZH2 and Ki-67 in colorectal cancer and associations with treatment response and prognosis. Br J Cancer. 2009;101:1282–9. doi: 10.1038/sj.bjc.6605333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon JA, Lange CA. Roles of the EZH2 histone methyltransferase in cancer epigenetics. Mutation Res. 2008;647:21–9. doi: 10.1016/j.mrfmmm.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Sato R, Inoue J, Kawabe Y, et al. Sterol-dependent transcriptional regulation of sterol regulatory element-binding protein-2. J Biol Chem. 1996;271:26461–64. doi: 10.1074/jbc.271.43.26461. [DOI] [PubMed] [Google Scholar]
- Scanlan MJ, Welt S, Gordon CM, et al. Cancer-related serological recognition of human colon cancer: identification of potential diagnostic and immunotherapeutic targets. Cancer Res. 2002;62:4041–47. [PubMed] [Google Scholar]
- Huang Y, Tan M, Gosink M, et al. Histone deacetylase 5 is not a p53 target gene, but its overexpression inhibits tumor cell growth and induces apoptosis. Cancer Res. 2002;62:2913–22. [PubMed] [Google Scholar]
- Naldi M, Calonghi N, Masotti L, et al. Histone post-translational modifications by HPLC-ESIMS after HT29 cell treatment with histone deacetylase inhibitors. Proteomics. 2009;9:5437–45. doi: 10.1002/pmic.200800866. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.