Abstract
Patients with severe haemophilia A experience frequent and spontaneous bleeding, causing debilitating damage to joints and decreasing quality of life. Prophylaxis with factor VIII (FVIII) reduces joint damage if initiated early. Circulating FVIII levels may be influenced by endogenous von Willebrand factor (VWF), a chaperone protein that binds and stabilizes FVIII. The aim of this study was to determine whether endogenous VWF antigen (VWF:Ag) levels are correlated with FVIII pharmacokinetic (PK) parameters and clinical outcomes in patients with severe haemophilia A. Previously treated, non-inhibitor patients in a multinational, randomized, double-blind, Ph II study received prophylaxis with once-weekly BAY 79-4980 (35 IU kg−1) or thrice-weekly recombinant sucrose-formulated FVIII (rFVIII-FS; 25 IU kg−1). PK parameters were evaluated at weeks 1 and 26. The number of bleeds per patient during the study was captured as part of the core efficacy endpoint. Spearman rank correlations assessed relationships of VWF:Ag levels with patient age, PK and annualized bleeding rate. Of 131 study patients (aged 13−64 years; BAY 79-4980, n = 63; rFVIII-FS, n = 68), 27 (21%; n = 15 and 12 respectively) were evaluable for PK assessment. Baseline VWF:Ag levels correlated with patient age (P < 0.0001). There was no significant difference in PK results between treatments; thus, PK parameters and VWF levels of all patients were analysed together. AUCnorm and T1/2 significantly increased with increased VWF:Ag (P < 0.001); clearance significantly decreased with increased VWF:Ag (P = 0.002). Annualized bleeding rate in patients treated with 3× per week rFVIII-FS significantly correlated with VWF:Ag and age (P = 0.038 and 0.021 respectively). PK parameters as well as the clinical outcome significantly correlated with endogenous VWF:Ag. The improved clinical outcome in subjects with high VWF:Ag levels may be explained by VWF:Ag influence on FVIII PK.
Keywords: age, factor VIII, pharmacokinetics, von Willebrand factor
Introduction
Patients with severe haemophilia A (<1% FVIII:C) experience frequent spontaneous haemorrhaging (bleeding) into soft tissue and joints, leading to debilitating joint damage, culminating in decreased quality of life 1–3. Many studies have demonstrated the means to prevent arthropathy through prophylaxis treatment with exogenous FVIII, which aims to preserve a level of FVIII:C in a haemophilia patient that would decrease the number of spontaneous bleeding episodes 4,5.
Prophylactic regimens that prevent the occurrence of spontaneous haemorrhage by maintaining adequate levels of FVIII:C require infusions approximately every 2–3 days, due to the short half-life of FVIII in circulation 6–10. The need for a long-acting FVIII product led to the development of BAY 79-4980, a recombinant sucrose-formulated FVIII (rFVIII-FS) reconstituted with a pegylated liposome solvent.
The core results of the Ph II study have been published and focused on the efficacy and safety data from the study 11. Briefly, there were no safety concerns with BAY 79-4980. However, the planned interim analysis by the data safety and monitoring board indicated that the primary and secondary efficacy endpoints would not be met. Prophylaxis with once a week BAY 79-4980 did not achieve non-inferiority compared to rFVIII-FS administered three times per week. Patients randomized to the study drug arm showed a statistically significant higher number of spontaneous bleeding and joint bleeding episodes compared to patients in the control arm. On the basis of the results of the interim analysis, the sponsor, Bayer Healthcare AG, halted the study. Despite the study not reaching completion, the high enrolment and patient number of the study as well as the comprehensive data gathered can potentially provide further insight into the factors that influence FVIII half-life and bleeding phenotype in patients with severe haemophilia A.
It is of great interest to the community whether circulating levels of von Willebrand factor (VWF) affect a haemophilia patient's response to FVIII treatment. It is known that FVIII relies on binding to VWF to remain stable and prevent degradation and clearance 12–14. However, there is a wide variation in each individual patient's measured FVIII half-life (from 6 to 29 h) and little is known mechanistically to explain why some patients present with relatively low or high FVIII half-lives 9,15–17. It has been shown that FVIII half-life correlates with VWF antigen preinfusion levels 18,19. If endogenous VWF levels can predict FVIII half-life, patients with lower VWF levels would theoretically have shorter bleed-free periods between FVIII infusions 20,21. Identification of patients with short FVIII half-lives would aid physicians in prescribing the optimum prophylaxis regimen that would prevent their patient from experiencing breakthrough bleeding. The objective of the current analysis was to determine if a correlation between VWF antigen (VWF:Ag) and FVIII pharmacokinetic (PK) parameters existed and whether this relationship translated to clinical outcomes with respect to bleeding episodes.
Materials and methods
Study design
The study design has been described previously 11. Briefly, this was a multicentre, multiregional, randomized, active-controlled, double-blind, prospective non-inferiority trial with two parallel treatment arms conducted in subjects with severe haemophilia A. The investigational treatment regimen was once-weekly 35 IU kg−1 body weight of BAY 79-4980 (consisting of 35 IU kg−1 rFVIII-FS reconstituted with 13 mg kg−1 pegylated liposome; investigational drug) plus two placebo injections and the control arm was standard prophylaxis (thrice-weekly treatment) with 25 IU kg−1 body weight of rFVIII-FS (Bayer HealthCare Pharmaceuticals Inc., Berkeley, CA, USA); active control; Fig.1). The study lasted from 30 June 2008 to 5 October 2010 and for each enrolled subject consisted of a screening period (3–8 weeks) and a treatment period (52 weeks). There was a planned enrolment of 250 subjects; however, the study was prematurely halted due to the failure to meet the primary endpoint at the planned interim analysis.
Fig 1.

Study design schematic. Subjects were randomly allocated to receive either BAY 79–4980 once a week at a dose of 35 IU kg−1 body weight plus two dummy infusions, or rFVIII-FS three times a week at a dose of 25 IU kg−1 body weight for a total evaluation period of 52 weeks. All subjects then underwent a run-in period of 3 weeks, during which time they received their first three liposomal infusions in the hospital under medical supervision before the home treatment was initiated. The N represents the planned enrolment number.
The study was conducted in compliance with the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, good clinical practice guidelines and the Declaration of Helsinki. The study protocol was approved by independent institutional review boards of all participating hospitals. All subjects provided signed informed consent prior to receiving any study-related tests or treatment.
Eligibility criteria
The study enrolled previously treated male patients (≥150 ED) aged between 12 and 70 years with severe haemophilia A (<1% FVIII). In addition to the inclusion and exclusion criteria set forth in the trial for selecting study participants for the efficacy and safety portion of the trial, the PK substudy excluded subjects who were receiving any pegylated medication (e.g. PEG interferon) in the month prior to the PK session. The inclusion in the PK sub-study was by patient's willingness in three prespecified study sites. Overall, 15 subjects were recruited at one site and all of them participated in the PK study.
PK assessment
The PK analysis was based on all subjects of the PK subgroup with evaluable PK data at week 1 as well as at week 26. Blood samples were drawn at the following time points: preinjection, 10 and 30 min, 1, 3, 6, 9, 24, 28, 32, 48 (72 optional) h post injection of study drug or control. The following PK parameters for FVIII were determined: AUC, AUC/D, AUCnorm, AUC(0-tn), Cmax, Cmax/D, Cmax,norm, tmax, in vivo half-life (T1/2), clearance, MRT, Vss and incremental recovery.
VWF:Ag assessment
Sandwich ELISA assay was used to determine the level of VWF:Ag in each sample and has been previously described 22. For this study, the following antibodies were used: AB polyclonal rabbit antihuman VWF antibody A 82 Dako (DAKO A/S, Glostrup, Denmark) and AB polyclonal rabbit antihuman VWF antibody peroxidase-labelled A 228 Dako (DAKO A/S). For determination of ADAMTS-13 antigen and activity a commercial reagent in a modified FRETS- test was used (Technozym ADAMTS13; Fa. Technoclon, Wien, Austria).
Bleeding rate assessment
Electronic patient diary (ePRO-LOG™; Arrowhead Electronic Healthcare, LLC., Austin, TX, USA) was used for documentation of home injection treatment and any bleeding episodes. Annualized bleeding rates were obtained for each subject by dividing his number of bleeds during the study by the number of days he was in the study, then multiplying by 365.25.
Statistical analysis
Demographic and baseline data of the investigated populations are summarized by descriptive statistics and t-test for statistical comparisons. Quantitative data (except PK-parameter) are described by summary statistics like arithmetic mean, arithmetic standard deviation, median and range. PK-parameters are summarized using geometric mean and geometric standard deviation. Qualitative data are condensed in frequency tables.
Patients were subdivided into two groups according to their VWF:Ag-values at baseline, a group with low values below a cut-off level and a group with high values above the cut-off level. The choice of the cut-off of 140% is somewhat arbitrary. However, looking at the maximum value of the VWF antigen at baseline, there were apparently two groups of patients in our data, that is one group with a maximum baseline value ≤131% and one group with values ≥161%. In between there was a gap. Thus, the choice of 140% used this gap to split the population. Nevertheless, two cut-off levels were used, 120% and 140%, to examine whether any findings depend on the choice of the cut-off level. For the comparison of PK-parameters between the patients with a baseline VWF:Ag below the cut-off level and those with a baseline VWF:Ag above the cut-off level, a t-test on the log-transformed PK-parameter was used.
To investigate a potential correlation between two variables, a scatter plot is provided including a linear regression line and Spearman and Pearson correlation statistics.
To investigate the relationship between PK-parameter (as dependent variable) and age and VWF:Ag, a regression analysis was performed including both independent variables.
Results
Subject characteristics at baseline
A total of 139 subjects (n = 67 BAY 79-4980; n = 72 rFVIII-FS) comprised the study population evaluable for safety (intent-to-treat group) while 131 subjects were evaluable for efficacy (n = 63 BAY 79-4980; n = 68 rFVIII-FS; per protocol group). Of these patients, 34 from the treatment arm and 41 from the control arm completed the study. The mean age, previous treatment regimen (prophylaxis vs. on-demand), bleeds during the previous 6 months and presence of target joints were comparable between the treatment and the control arms. Furthermore, patient baseline measurements of VWF:Ag, ADAMTS-13-Ag and ADAMTS-13-Act were not significantly different between the two treatment arms (Table 1; t-test; P > 0.1).
Table 1.
Patient baseline characteristics.
| BAY 79-4980 | rFVIII-FS | Total | |
|---|---|---|---|
| Intent-to-treat population | 67 | 72 | 139 |
| Per protocol population | 63 | 68 | 131 |
| Mean age years (median, range) | 32.8 (30, 14–61) | 34.4 (30.5, 13–64) | 33.6 (30, 13–64) |
| Previous treatment | |||
| N (%) prophylaxis | 29 (46) | 24 (35) | 54 (40) |
| N (%) on-demand | 34 (54) | 44 (65) | 78 (60) |
| Target joints | |||
| 1 Target joint N (%) | 52 (83) | 54 (79) | 106 (81) |
| >1 Target joint N (%) | 36 (57) | 39 (57) | 75 (57) |
| VWF:Ag % mean (median)* | 106.6 (104) | 110.9 (106.5) | 108.8 (106) |
| ADAMTS-13-Ag % mean (median)† | 78.8 (76) | 80.9 (78) | 79.9 (76) |
| ADAMTS-13-Act % mean (median)† | 97.5 (96) | 102.5 (99.5) | 100.1 (99) |
All patient characteristics correspond to per protocol population unless otherwise specified.
rFVIII-FS, recombinant sucrose-formulated FVIII; VWF, von Willebrand factor.
ITT population: BAY 79-4980 N = 67; rFVIII-FS N = 72; total N = 139.
BAY 79-4980 N = 66; rFVIII-FS N = 72; total N = 138.
To determine the VWF:Ag frequency at baseline, patients were categorized at cut-off levels of either 120% or 140% VWF:Ag levels. Among 135 subjects, 93 (68.9%) had VWF:Ag levels <120% and 42 (31.1%) had levels ≥120%. Using the higher cut-off value of 140%, 112 (82.9%) patients measured <140% and 23 (17.1%) had levels ≥140%. Table 2 demonstrates the mean VWF:Ag levels by age group. The results confirmed that VWF:Ag levels increased with age. VWF:Ag levels in subjects >50 years of age were twice as high as compared to subjects <20 years. Interestingly, while VWF:Ag showed a relationship with age (Spearman Rank correlation r = 0.46; P < 0.0001; ITT population,), ADAMTS-13-Ag levels and activity did not correlate with age (r = −0.04, P = 0.61; r = 0.156, P = 0.067 respectively). Body weight was taken into account and no relationship was detected between VWF:Ag and body weight (data not shown). Patients with blood group O (n = 54) had significantly (P < 0.0001) lower levels of VWF:Ag compared to non-O blood group (n = 63) patients (mean = 89.1 ± 33.7 blood group O; 128.3 ± 28.3 Non-O).Patients were checked for concomitant infective diseases and it was observed that those with a viral hepatitis disease (n = 94; mean VWF:Ag = 119.3 ± 19.3), either HBV or HCV, tended to have higher VWF levels than those without this concomitant disease (n = 45; mean VWF:Ag =90.9 ± 30.9).
Table 2.
Relationship between von Willebrand factor:Ag levels and age.
| Age group in years | N | Mean ± SD | Range |
|---|---|---|---|
| <20 | 9 | 75.4 ± 29 | 27–117 |
| 20 to <25 | 27 | 86.6 ± 26.6 | 41–137 |
| 25 to <30 | 30 | 103 ± 34.3 | 43–163 |
| 30 to <40 | 34 | 106.3 ± 38.6 | 29–210 |
| 40 to <50 | 20 | 129 ± 34.2 | 57–189 |
| 50 to <60 | 14 | 151.7 ± 64.1 | 78–272 |
| >60 | 5 | 177 ± 110.7 | 65–358 |
Spearman rank correlation r = 0.46, P < 0.0001.
During the study, high VWF values of >200% were seen in patients who developed an acute infection (e.g. acute bronchitis, angina, infection of prosthesis, reactivation of HCV; data on file).
A subset of patients from the study participated in the PK analysis. A total of 27 (21% of total population) patients were evaluated for PK. Interestingly, the subjects' VWF:Ag levels created two distinct groups: those with a maximum VWF:Ag value of ≤131% or ≥161%, creating a gap that is split by the 140% VWF:Ag cut-off, 20 subjects showing baseline VWF:Ag levels <140% and seven ≥140% and 11 subjects (42.3%) showed a VWF level of ≥120%. The demographics of the PK population are summarized in Table 3. All subsequent analyses were conducted using the PK subgroup population, unless otherwise noted. PK data at week 1 and 26 were available from 15 subjects who received BAY 79-4980 and 12 who received rFVIII-FS. In this study, the hemostatic properties and PK characteristics were not different between BAY 79-4980 and rFVIII-FS. Therefore, patients from both treatment groups were evaluated together for factors that influenced PK parameters.
Table 3.
PK population demographics.
| VWF:Ag < 140% | VWF:Ag ≥ 140% | Total | |
|---|---|---|---|
| N | 20 | 7 | 27 |
| Mean age in years | 29.2 | 42.2 | 32.1 |
| Median age (range) | 29 (20–45) | 39.5 (32–58) | 31 (20–58) |
| BAY 79-4980 arm | 11 | 4 | 15 |
| rFVIII-FS arm | 9 | 3 | 12 |
PK, pharmacokinetic; VWF, von Willebrand factor; rFVIII-FS, recombinant sucrose-formulated FVIII.
Relationship between VWF:Ag and PK parameters
The potential effect of VWF:Ag on patient FVIII PK parameters was examined. Although both week 1 and week 26 data were analysed for correlations between the variables, there were no relevant differences between the two time points, thus week 26 was representative of both. All data presented are from week 26 sampling. In addition, both VWF:Ag cut-offs of 120% and 140% were calculated and no relevant differences were found unless otherwise noted. Only the 140% VWF:Ag cut-off data are presented.
Decreased clearance was correlated with a high VWF:Ag level (Table 4). At the 26 week visit, a geometric mean clearance rate was greater in subjects with <140% VWF:Ag (n = 20) compared with those with ≥140% (n = 7) (P = 0.003; Fig.2). PK parameters of AUCnorm and T1/2 also were found to correlate with VWF:Ag with increased AUCnorm and T1/2 significantly associated with high VWF:Ag levels (P = 0.0005 for both; Table 4). The T1/2 ratio of ≥140%/<140% was 1.45, demonstrating that T1/2 was 1.45 longer in patients with ≥140% VWF:Ag levels compared with subjects with lower VWF:Ag. Spearman rank correlation statistics of week 26 PK parameters and VWF:Ag levels confirmed correlations in the clearance, AUCnorm and T1/2 parameters (P < 0.0001 for AUCnorm and T1/2, P = 0.0018 for clearance; Fig.3; Table 4). There was no influence on Cmax by VWF:Ag levels (P = 0.467).
Table 4.
Overview of correlation between VWF:Ag and PK parameters, week 26, geometric means.
| VWF:Ag < 40% | VWF:Ag ≥ 140% | P-value | Spearman rank coefficient (r) | P-value | |
|---|---|---|---|---|---|
| Clearance (dL h−1) | 2.1 | 1.3 | 0.0032 | −0.57 | 0.0018 |
| AUCnorm (kg × h dL−1) | 33.7 | 51.8 | 0.0005 | 0.69 | <0.0001 |
| T1/2 (h) | 11.4 | 16.5 | 0.0005 | 0.82 | <0.0001 |
PK, pharmacokinetic; VWF, von Willebrand factor.
Fig 2.

Correlation between von Willebrand factor (VWF):Ag with FVIII clearance. A decreased rate of FVIII clearance was correlated with increased levels of patient VWF:Ag at baseline. The figure demonstrates the data at week 26 during the study, however, week 1 results are not significantly different from the data shown.
Fig 3.
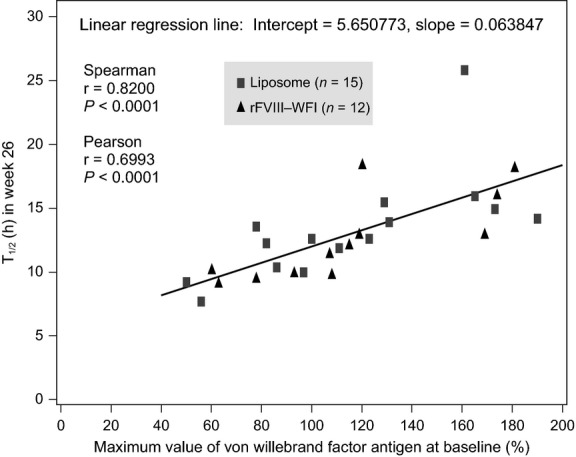
Correlation between von Willebrand factor (VWF):Ag with T1/2. Increased FVIII half-life was found to correlate with increased levels of patient VWF:Ag at baseline. The figure demonstrates the data at week 26 during the study, however, week 1 results are not significantly different from the data shown.
It was earlier shown that VWF:Ag levels increased with age and it was of interest to determine if the correlations between PK parameters and VWF:Ag were in actuality a result of age. Spearman rank correlation and regression analyses demonstrated that VWF:Ag levels had a significant influence on AUCnorm and T1/2 PK parameters. However, age was not correlated with the PK parameters, especially when excluding the two oldest patients aged 49 and 58 years, leaving a population of 25 patients aged between 20 and 45 years (Table 5).
Table 5.
Impact of VWF and age on PK parameters.
| Spearman rank correlation using only age or VWF:Ag |
Regression analysis using both age and VWF:Ag |
|||
|---|---|---|---|---|
| All ages | <49 years | All ages | <49 years | |
| r (P-value) | r (P-value) | P-value | P-value | |
| Age on AUCnorm | 0.37 (0.06) | 0.22 (0.29) | 0.26 | 0.58 |
| Age on T1/2 | 0.33 (0.09) | 0.17 (0.41) | 0.03 | 0.99 |
| VWF:Ag on AUCnorm | 0.69 (<0.0001) | N/A | 0.0006 | 0.0008 |
| VWF on T1/2 | 0.82 (<0.0001) | N/A | 0.0007 | <0.0001 |
N/A, not available; PK, pharmacokinetic; VWF, von Willebrand factor.
Relationship between VWF:Ag and bleeding rate
To determine if there was a clinical consequence of high or low VWF:Ag levels, the presence of a correlation between VWF:Ag level and annualized bleeding rate was explored in patients in the control arm of the study (n = 72). The subjects treated with rFVIII-FS three-times per week and who had VWF:Ag levels <140% demonstrated a greater number of bleeds (mean = 6.5 ± 7.1) compared to those in the same arm having ≥140% VWF:Ag levels (mean = 2.5 ± 4.3; P = 0.0354). The median number of bleeds per year in the two subgroups were 4.2 (0–22.8) and 1.1 (0–15.9) respectively. Therefore, patients with high VWF:Ag have lower bleeding rates during prophylaxis treatment of 25 IU kg−1 3× per week compared to patients with low VWF:Ag levels. Spearman rank analysis found a significant correlation between VWF:Ag and annualized bleeding rate (r = −0.25; P = 0.038) but a significant correlation was also observed between age and bleeding rate (r = −0.28; P = 0.021).
Discussion and conclusion
This multiregional, prospective, randomized, active-controlled trial in subjects with severe haemophilia A provided an opportunity to investigate the relationship between VWF and FVIII PK and bleeding. PK parameters were significantly correlated with VWF:Ag levels, specifically that high VWF:Ag increased AUC and half-life and decreased clearance. The correlations were confirmed using regression analysis. Interestingly, the influence of VWF:Ag on PK was not due to age in that although mean VWF:Ag levels increased with age, the PK parameters for all patients were not directly correlated with age.
The clinical significance of these findings were demonstrated by showing that under prophylaxis therapy, the annualized bleeding rate in patients with high VWF:Ag (≥140% in this study) was lower than that in patients with low VWF:Ag. Significant correlations between VWF:Ag and bleed rate and also between age and bleed rate were observed. The clinical observation of a reduced bleeding rate in patients with high VWF:Ag may be explained by longer FVIII half-life due to increased VWF:Ag. Patients with high VWF:Ag have measured FVIII half-lives of 1.49 times that of patients with low VWF:Ag. This observation can be applied to the clinical management of haemophilia A patients when determining their prophylactic treatment regimen 23. A patient with low VWF:Ag levels should theoretically be prescribed a shorter duration between FVIII infusions. Furthermore, although lifestyle is often cited as a reason that adults with haemophilia A have fewer bleeding episodes compared to paediatric patients, the increase in VWF:Ag levels in adults 24 could be a contributing factor as well. It is important to note that the bleeding analysis of this study was limited and did not control for other factors that could influence the bleed rate (e.g. age, number of target joints, etc.).
A controversial topic among the haemophilia community is the role of exogenous VWF and whether it impacts the efficacy of a FVIII product 25,26. In particular, low-purity plasma-derived FVIII (pdFVIII) products contain VWF whereas high-purity pdFVIII or rFVIII products do not. This study demonstrated a significant impact of endogenous VWF:Ag on PK parameters with clinical implications as regards to bleeding events. However, the question of whether infusing FVIII combined with exogenous VWF would also result in improved FVIII PK results compared to FVIII products not containing VWF is not answered in the scope of this analysis. PK analyses of FVIII products have not revealed a significant difference in half-life and clearance between VWF-containing or high-purity FVIII products. In other studies testing PKs of FVIII products, it would be of interest to note whether the patient groups were controlled for endogenous VWF:Ag levels.
In conclusion, this PK study was a subanalysis of 21% of the patients with PK data in the largest and double-blind controlled, prospective trial in haemophilia history. Factor VIII PK parameters were significantly influenced by patient endogenous VWF:Ag levels which manifested clinically by the corresponding effect on bleeding frequency. The results of the PK study provide further insight and evidence of the significance of endogenous VWF levels in the efficacy of FVIII products for the treatment of haemophilia A.
Acknowledgments
Jocelyn Hybiske, PhD for medical writing services, which was fully funded by Bayer Healthcare. LipLong study investigators: Austria: Ingrid Pabinger-Fasching, Wien; Belgium: Cedric Hermans, Brussels; Canada: Man-Chiu Poon, Calgary; Bruce Ritchie, Edmonton; Alan Tinmouth, Ottawa; Jerome Teitel, Toronto; Croatia: Silva Zupancic-Salek, Zagreb; Denmark: Jørgen Ingerslev, Åarhus N; France: Hervé Chambost, Marseille; Jean-Francois Schved, Montpellier; Benoit Guillet, Rennes; Germany: Johannes Oldenburg, Bonn; Robert Klamroth, Berlin; Israel: Uri Martinowitz, Tel Hashomer; Italy: Matteo Nicola Dario Di Minno, Napoli; Elena Santagostino, Milano; Piercarla Schinco, Torino; Roberto Musso, Catania; Massimo Morfini, Firenze; Angiola Rocino, Napoli; Netherlands: Karina Meijer, Groningen; Britta Laros-van Gorkom, Nijmegen; Norway: Pål Holme, Oslo; Poland: Jerzy Windyga, Warszawa; Aleksander Skotnicki, Krakow; Andrzej Hellmann, Gdansk; Krystyna Zawilska; Poznan, Tadeusz Robak, Lodz; Spain: Immaculada Soto, Oviedo; Saturnin Haya, Valencia; Turkey: Kaan Kavakli, Izmir; Bulent Antmen, Adana; Mehmet Akif Yesilipek, Antalya; United Kingdom: Charles Hay, Manchester; Jonathan Wilde, Birmingham; Savita Rangarajan, Greater London; United States: Jonathan Bernstein, Las Vegas; Lloyd Damon, San Francisco; Joan Gill, Wauwatosa; Ralph Gruppo, Cincinnati; Adam Cuker, Philadelphia; Philip Kuriakose, Detroit; Judith Lin, Boston; Marilyn Manco-Johnson, Aurora; Prasad Mathew, Albuquerque; Ellis Neufeld, Boston; Amit Soni, Orange County; Jerry Powell, Sacramento; Roshni Kulkarni, Lansing; Brian Wicklund, Kansas City.
Author contributions
HD and LS performed the statistical analyses; SL, UM, JZ provided the PK data; IS was the study investigator. All authors contributed to conceptualizing, editing of manuscript content and approval of final manuscript.
Disclosures
MME and HD are employees of Bayer Pharma AG. LS is an employee of Bayer Healthcare Pharmaceuticals Inc. IS has acted as a paid consultant to Bayer HealthCare. JW has acted as a paid consultant to Bayer, Baxter Pfizer, Octapharma, Novo Nordisk, and CSL Behring. UM has received fees for speaking, reimbursement of travel expenses and honorarium for advisory board to Bayer Healthcare. SL has received fees for speaking at Bayer sponsored meetings. SL and IS also have received reimbursement of travel expenses.
References
- 1.Aledort LM, Haschmeyer RH, Pettersson H. A longitudinal study of orthopaedic outcomes for severe factor-VIII-deficient haemophiliacs. The Orthopaedic Outcome Study Group. J Intern Med. 1994;236:391–9. doi: 10.1111/j.1365-2796.1994.tb00815.x. [DOI] [PubMed] [Google Scholar]
- 2.Fischer K, van der Bom JG, van den Berg HM. Health-related quality of life as outcome parameter in haemophilia treatment. Haemophilia. 2003;9(Suppl. 1):75–81. doi: 10.1046/j.1365-2516.9.s1.13.x. discussion 2. [DOI] [PubMed] [Google Scholar]
- 3.Royal S, Schramm W, Berntorp E, et al. Quality-of-life differences between prophylactic and on-demand factor replacement therapy in European haemophilia patients. Haemophilia. 2002;8:44–50. doi: 10.1046/j.1365-2516.2002.00581.x. [DOI] [PubMed] [Google Scholar]
- 4.Blanchette VS, McCready M, Achonu C, Abdolell M, Rivard G, Manco-Johnson MJ. A survey of factor prophylaxis in boys with haemophilia followed in North American haemophilia treatment centres. Haemophilia. 2003;9(Suppl. 1):19–26. doi: 10.1046/j.1365-2516.9.s1.12.x. discussion. [DOI] [PubMed] [Google Scholar]
- 5.Fischer K, Van Den Berg M. Prophylaxis for severe haemophilia: clinical and economical issues. Haemophilia. 2003;9:376–81. doi: 10.1046/j.1365-2516.2003.00764.x. [DOI] [PubMed] [Google Scholar]
- 6.Lofqvist T, Nilsson IM, Berntorp E, Pettersson H. Haemophilia prophylaxis in young patients–a long-term follow-up. J Intern Med. 1997;241:395–400. doi: 10.1046/j.1365-2796.1997.130135000.x. [DOI] [PubMed] [Google Scholar]
- 7.Nilsson IM, Berntorp E, Lofqvist T, Pettersson H. Twenty-five years' experience of prophylactic treatment in severe haemophilia A and B. J Intern Med. 1992;232:25–32. doi: 10.1111/j.1365-2796.1992.tb00546.x. [DOI] [PubMed] [Google Scholar]
- 8.Liesner RJ, Khair K, Hann IM. The impact of prophyactic treatment on children with severe haemophilia. Br J Haematol. 1996;92:973–8. doi: 10.1046/j.1365-2141.1996.420960.x. [DOI] [PubMed] [Google Scholar]
- 9.van Dijk K, van der Bom JG, Lenting PJ, et al. Factor VIII half-life and clinical phenotype of severe hemophilia A. Haematologica. 2005;90:494–8. [PubMed] [Google Scholar]
- 10.Collins PW, Blanchette VS, Fischer K, et al. Break-through bleeding in relation to predicted factor VIII levels in patients receiving prophylactic treatment for severe hemophilia A. J Thromb Haemost. 2009;7:413–20. doi: 10.1111/j.1538-7836.2008.03270.x. [DOI] [PubMed] [Google Scholar]
- 11.Powell J, Martinowitz U, Windyga J, et al. Efficacy and safety of prophylaxis with once-weekly BAY 79-4980 compared with thrice-weekly rFVIII-FS in haemophilia A patients. A randomised, active-controlled, double-blind study. Thromb Haemost. 2012;108:913–22. doi: 10.1160/TH12-03-0188. [DOI] [PubMed] [Google Scholar]
- 12.Lenting PJ, van Schooten CJ, Denis CV. Clearance mechanisms of von Willebrand factor and factor VIII. J Thromb Haemost. 2007;5:1353–60. doi: 10.1111/j.1538-7836.2007.02572.x. [DOI] [PubMed] [Google Scholar]
- 13.van Schooten CJ, Shahbazi S, Groot E, et al. Macrophages contribute to the cellular uptake of von Willebrand factor and factor VIII in vivo. Blood. 2008;112:1704–12. doi: 10.1182/blood-2008-01-133181. [DOI] [PubMed] [Google Scholar]
- 14.Schambeck CM, Grossmann R, Zonnur S, et al. High factor VIII (FVIII) levels in venous thromboembolism: role of unbound FVIII. Thromb Haemost. 2004;92:42–6. doi: 10.1160/TH04-02-0063. [DOI] [PubMed] [Google Scholar]
- 15.Fijnvandraat K, Peters M, ten Cate JW. Inter-individual variation in half-life of infused recombinant factor VIII is related to pre-infusion von Willebrand factor antigen levels. Br J Haematol. 1995;91:474–6. doi: 10.1111/j.1365-2141.1995.tb05325.x. [DOI] [PubMed] [Google Scholar]
- 16.Fischer K, Pendu R, van Schooten CJ, et al. Models for prediction of factor VIII half-life in severe haemophiliacs: distinct approaches for blood group O and non-O patients. PLoS ONE. 2009;4:e6745. doi: 10.1371/journal.pone.0006745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vlot AJ, Mauser-Bunschoten EP, Zarkova AG, et al. The half-life of infused factor VIII is shorter in hemophiliac patients with blood group O than in those with blood group A. Thromb Haemost. 2000;83:65–9. [PubMed] [Google Scholar]
- 18.Deitcher SR, Tuller J, Johnson JA. Intranasal DDAVP induced increases in plasma von Willebrand factor alter the pharmacokinetics of high-purity factor VIII concentrates in severe haemophilia A patients. Haemophilia. 1999;5:88–95. [PubMed] [Google Scholar]
- 19.Denis CV, Kwack K, Saffaripour S, et al. Interleukin 11 significantly increases plasma von Willebrand factor and factor VIII in wild type and von Willebrand disease mouse models. Blood. 2001;97:465–72. doi: 10.1182/blood.v97.2.465. [DOI] [PubMed] [Google Scholar]
- 20.Jenkins PV, O'Donnell JS. ABO blood group determines plasma von Willebrand factor levels: a biologic function after all? Transfusion. 2006;46:1836–44. doi: 10.1111/j.1537-2995.2006.00975.x. [DOI] [PubMed] [Google Scholar]
- 21.Schooten CJ, Tjernberg P, Westein E, et al. Cysteine-mutations in von Willebrand factor associated with increased clearance. J Thromb Haemost. 2005;3:2228–37. doi: 10.1111/j.1538-7836.2005.01571.x. [DOI] [PubMed] [Google Scholar]
- 22.Cejka J. Enzyme immunoassay for factor VIII-related antigen. Clin Chem. 1982;28:1356–8. [PubMed] [Google Scholar]
- 23.Gallinaro L, Cattini MG, Sztukowska M, et al. A shorter von Willebrand factor survival in O blood group subjects explains how ABO determinants influence plasma von Willebrand factor. Blood. 2008;111:3540–5. doi: 10.1182/blood-2007-11-122945. [DOI] [PubMed] [Google Scholar]
- 24.Coppola R, Mari D, Lattuada A, Franceschi C. von Willebrand factor in Italian centenarians. Haematologica. 2003;88:39–43. [PubMed] [Google Scholar]
- 25.Goudemand J. Inhibitor development in haemophilia A: the role of von Willebrand factor/factor VIII concentrates. Haemophilia. 2007;13(Suppl. 5):47–51. doi: 10.1111/j.1365-2516.2007.01571.x. [DOI] [PubMed] [Google Scholar]
- 26.Gouw SC, van der Bom JG, Auerswald G, Ettinghausen CE, Tedgard U, van den Berg HM. Recombinant versus plasma-derived factor VIII products and the development of inhibitors in previously untreated patients with severe hemophilia A: the CANAL cohort study. Blood. 2007;109:4693–7. doi: 10.1182/blood-2006-11-056317. [DOI] [PubMed] [Google Scholar]


