Abstract
Objectives
To evaluate our clinical experience with percutaneous image-guided radiofrequency ablation (RFA) of 200 renal tumours in a large tertiary referral university institution.
Patients and Methods
Image-guided RFA (ultrasonography or computed tomography [CT]) of 200 renal tumours in 165 patients from June 2004 to 2012 was prospectively evaluated. Institutional Review Board approval was granted.
The treatment response and technical success were defined by absence of contrast enhancement within the tumour on contrast enhanced CT or magnetic resonance imaging.
Both major and minor complications, glomerular filtration rate (GFR) before and after RFA, the management and outcomes of the complications, as well as oncological outcome were prospectively documented.
Multivariate analysis was used to determine variables associated with major complications and also the percentage GFR change after RFA.
The overall (OS), 5-year cancer-specific (CSS), local recurrence-free (LRFS) and metastasis-free survival (MFS) rates are presented using the Kaplan–Meier curves.
Results
In all, 200 tumours were RF ablated with a mean (range) tumour size of 2.9 (1–5.6) cm and the mean (range) patient age was 67.7 (21–88.6) years with a mean follow-up period of 46.1 months.
The primary technical and overall technical success rate was 95.5% and 98.5%, respectively. Two independent predictors of successful RFA in a single sitting were tumour size (<3 cm) and exophytic location in multivariate logistic regression analysis.
Major complications included ureteric injury (six patients), calyceal-cutaneous fistula (one), acute tubular necrosis (one) and abscess (two). Two independent predictors of ureteric injury were central location and lower pole position.
Within this cohort of patients, only four patients developed significant renal function deterioration i.e. >25% decreased in GFR. In all, 161 (98%) patients of the 165 patients have preservation of renal function. Any change in renal function after RFA was not influenced by tumour factors or solitary kidney status.
In our clinical series, this yielded a 5-year OS, CSS, LRFS and MFS rates of 75.8%, 97.9%, 93.5% and 87.7% respectively.
Conclusions
Image-guided RFA is a safe, nephron sparing and effective treatment for small renal cell carcinoma (RCC) tumours with a low rate of recurrence and has good 5-year CSS and MFS rates.
Keywords: radiofrequency ablation, renal cell carcinoma, complication, survival rates
Introduction
RCC is the commonest cancer of the kidney. It accounts for 3% of all cancers in adults 1. The detection of RCC has increased over the past decade and each year there are ≈270 000 cases worldwide 2,3. This has been partly due to the increase of incidentally detected RCC from wider use of radiological imaging 4 and it is also related to increased incidence of renal cancer in the general population secondary to cigarette smoking and obesity 5–8.
Incidentally detected renal masses are smaller and at an earlier stage than those tumours that present clinically with symptoms, e.g. pain, haematuria and palpable flank mass 9,10. About 80% of these incidental detected solid masses are RCC on histological diagnosis 11. Historical data has suggested that 60% of these small masses will grow gradually over time 12. Therefore, there remains a clinical risk with adopting a ‘watchful waiting’ approach for younger patients, as these tumours may become symptomatic or metastasise 12.
Currently, there is general consensus that smaller renal tumours (< 4 cm), should be treated with minimally invasive techniques to preserve renal function and avoid unnecessary surgical removal of the entire kidney 13. Nephron-sparing surgery (NSS) with either laparoscopic or open partial nephrectomy has replaced the ‘gold standard’ of radical nephrectomy whenever it is deemed technically possible to remove the small renal tumour and to preserve the rest of the kidney. It has been shown that radical nephrectomy leads to a higher incidence of chronic kidney disease, especially those with co-morbidity, e.g. diabetes, and this leads to increased mortality and morbidity 14. NSS has shown similar recurrence-free survival (RFS) and long-term survival outcomes as those of radical nephrectomy 15,16 and has good preservation of renal function 14,17. However, partial nephrectomy is technically challenging and associated with significant morbidity 15,16,18,19.
Given the surgical constraints, the initial exploratory work with the image-guided ablative treatment of small renal tumours with radiofrequency ablation (RFA), cryotherapy and microwave has developed at a rapid pace over the last decade. Percutaneous tumour ablation has proven to be a safe and effective treatment option for small renal tumours, and good oncological outcome data are emerging for RFA 20,21 and cryoablation 22.
Percutaneous RFA is now a well-established technique for treating small renal tumours 23–25. It uses a high-frequency, alternating current within the targeted tissue to cause ionic agitation generating frictional heat, which results in cancer cell destruction when the temperatures exceed 60°C. The present study evaluates our clinical experience in a single university institution in the treatment of 200 renal tumours with image-guided RFA.
Patients and Methods
Our ‘Percutaneous Renal RFA’ programme was established in 2004. All patients were referred through our local urology multi-disciplinary team (MDT) meeting. The MDT panel consisted of at least one consultant urologist, a consultant oncologist, a consultant radiologist and other supporting staff. The patient imaging was reviewed and the management options were discussed and a consensus achieved within the panel. In our institution, the diagnosis of renal tumour before nephrectomy has historically been based on imaging criteria alone, as established on CT when the tumour has a mean density of >20 HU and shows >20 HU enhancement after contrast 4,26 or on MRI when there is appropriate enhancement (15% from threshold) after gadolinium 27. The inclusion criteria for consideration of RFA treatment were: non-surgical candidates (in our early experience this was defined by an American Society of Anesthesiologists (ASA) score of >3, but subsequently all patients with an ASA score >3 unsuitable for general anaesthesia were also a relative contraindication for image-guided RFA) with incidental stage T1 renal tumours, renal tumours in a solitary kidney, synchronous primary renal tumour, patients with Von-Hippel-Lindau disease or patients with impaired renal function. In addition, depending on the clinical situation, the patient’s personal choice for a NS procedure, when partial nephrectomy was deemed technically impossible by the urologist and occasionally, patients with metastatic RCC undergoing immunotherapy were also considered.
After the MDT meeting, the patients were seen in the out-patient consultation clinic by both the consultant urologist and radiologist. A comprehensive consultation was provided by the urologist covering the various treatment options available for the patient. Patients who wished to consider percutaneous renal ablation would then be seen by the interventional radiologist (IR) in the clinic. The IR would provide in-depth discussion of the pros and cons of the treatment considering the size and location of the renal tumours and explanation of the implications of the percutaneous renal ablation with the requirement for long-term imaging follow-up. In the beginning of the development of this programme (2004–2009), image-guided renal RFA was the only treatment option and since 2009, both image-guided renal RFA and cryoablation were offered as treatment options. Depending on the tumour size and location, heat- or cold-based energy might be offered as a preferential technique. Informed written consent for the treatment with RFA of their renal tumours was obtained in all patients. In our programme, we also have a clinical specialist nurse who was available to support the patient’s journey. All patients were referred to the hospital pre-assessment clinic to assess their fitness for general anaesthesia and screened for day case admission. Routine baseline laboratory investigations were performed, including a clotting screen (international normalised ratio [INR] <1.5 was required at the time of treatment), renal function tests (creatinine measurement) and full blood counts.
From June 2004 to 2012, we have performed image-guided RFA of 200 renal tumours in 165 patients. The patients’ prospectively collected clinical database was evaluated for technical success, renal function, clinical complications and oncological treatment outcome. The review study was granted approval by our institution’s ethics committee chairman and informed consent was waived for the database review by the Institutional Ethical Board.
In all, 165 patients [109 men, 56 women; mean (range) age 67.7 (21–88.6) years] underwent percutaneous RFA of the 200 renal tumours in 210 treatment sessions. In all, 102 renal tumours were in the right kidney and 98 in the left kidney. The mean (range) tumours size was 2.9 (1–5.6) cm. Amongst the renal tumours RF ablated, tumours were >3 and <3 cm in 134 and 66 tumours, respectively. The polar position of the renal tumours was: upper (63), middle (86) and lower (51). The tumour treatment classification according to the criteria of Gervais et al. 28 was: exophytic (43), mixed (100), parenchymal (41) and central (16). All the patients had a baseline renal function test immediately before and at 24 h after RFA treatment.
Biopsy Procedure
In our institution, all patients undergo biopsies at the time of ablation of the renal tumour as part of their standard clinical care. The co-access sheath used is part of the RFA LeVeen needle electrode system (Boston Scientific, MA, USA) and allows biopsy without the need to reposition the sheath for RFA treatment. The co-access sheath was inserted into the renal tumour under CT guidance and all biopsies were taken with an 18-G core biopsy needle gun through a 16-G outer sheath (Boston Scientific, MA, USA). At least two core biopsies of each renal tumour were taken.
RFA
All the RFAs were performed under general anaesthesia as the preferred option of the anaesthetist, apart from three patients who were treated with i.v. conscious sedation at the patient’s choice and the discretion of the anaesthetist at the time of treatment. All patients received broad spectrum i.v. antibiotics amoxicillin trihydrate/potassium clavulanate (Co-amoxiclav 1.2 g) during the procedure and at 12 h after treatment, followed by a 10-day course of oral ciprofloxacin (500 mg twice daily). This routine prophylaxis had been adopted as policy after consultation with Breen et al. 29 but we acknowledged this remains a controversial practice amongst operators performing image-guided renal ablation. RFA was performed as an elective procedure in all patients with a routine admission the day before and observation overnight after RFA in our institution.
All RFAs were performed by one or two of the two consultant radiologists (T.M.W., H.C.I). In all, 200 renal tumours were RF ablated in 210 treatment sessions with an impedance-controlled pulsed current from a 200-W RF 3000 generator (Boston Scientific, MA, USA). RFA was performed with a varying size (3, 3.5 or 4 cm) umbrella-shaped multi-tines needle electrode (LeVeen CoAccess RFA needle electrode, Boston Scientific, MA, USA), selected to match the size of the tumour. During RFA, the number of overlapping ablations was dependent on the size and geometry of the lesion. For this system, the timing of individual ablations was impedance-controlled, depending upon the tissue vascularity and resistance. RCC target tissue cell death is achieved via tissue desiccation and consequently loses its ability to conduct current, hence the rise in the impedance. ‘Roll off’ equates to clinical endpoint where complete tissue coagulation is reached when the impedance reaches a clinically relevant level and there is concurrent power shutdown of the generator. A more vascular tumour will cause more heat-sink effect and this will lead to a longer treatment time.
From June 2004 to 2006, due to the initial arrangement of the programme, most of the renal RFAs were performed in an operating theatre under ultrasonographic (US) guidance (31 RFAs). Since June 2006, the treatment sessions were moved into our CT interventional suite, and all renal tumours were subsequently ablated under CT guidance (179) with US available to compliment guidance if necessary. The availability of CT imaging during treatment allows better assessment of the safety margin of the treatment, particularly to assess the proximity of the surrounding organs, e.g. bowel and ureter, in relation to the multi-tine needle electrode. In our institution, CT-guided RFA involves a contrast-enhanced study with 100 mL iodinated contrast medium given at 3 mL/s at the beginning to assist targeting of the renal tumour and a subsequent intra-procedural CT to guide RF electrode positioning, which was performed at 3-mm-collimation spiral acquisition. We do not perform immediate post procedural contrast-enhanced CT to assess treatment effect as this is performed at 1 month after treatment to allow the early post-RFA changes to resolve.
During treatment, the number of overlapping ablations was dependent on the size and geometry of the lesion and multi-planar reformatting of the multi-tines electrode positioning was important to determine the overall tumour coverage (Fig. 1A, B, C). The mean (range) ablated renal tumour size was 2.9 (1–5.6) cm. In each patient, the mean (range) total overlapping ablations was 2.5 (1–5) and the total ablation time was 26.3 (6–63.6) min.
Figure 1.
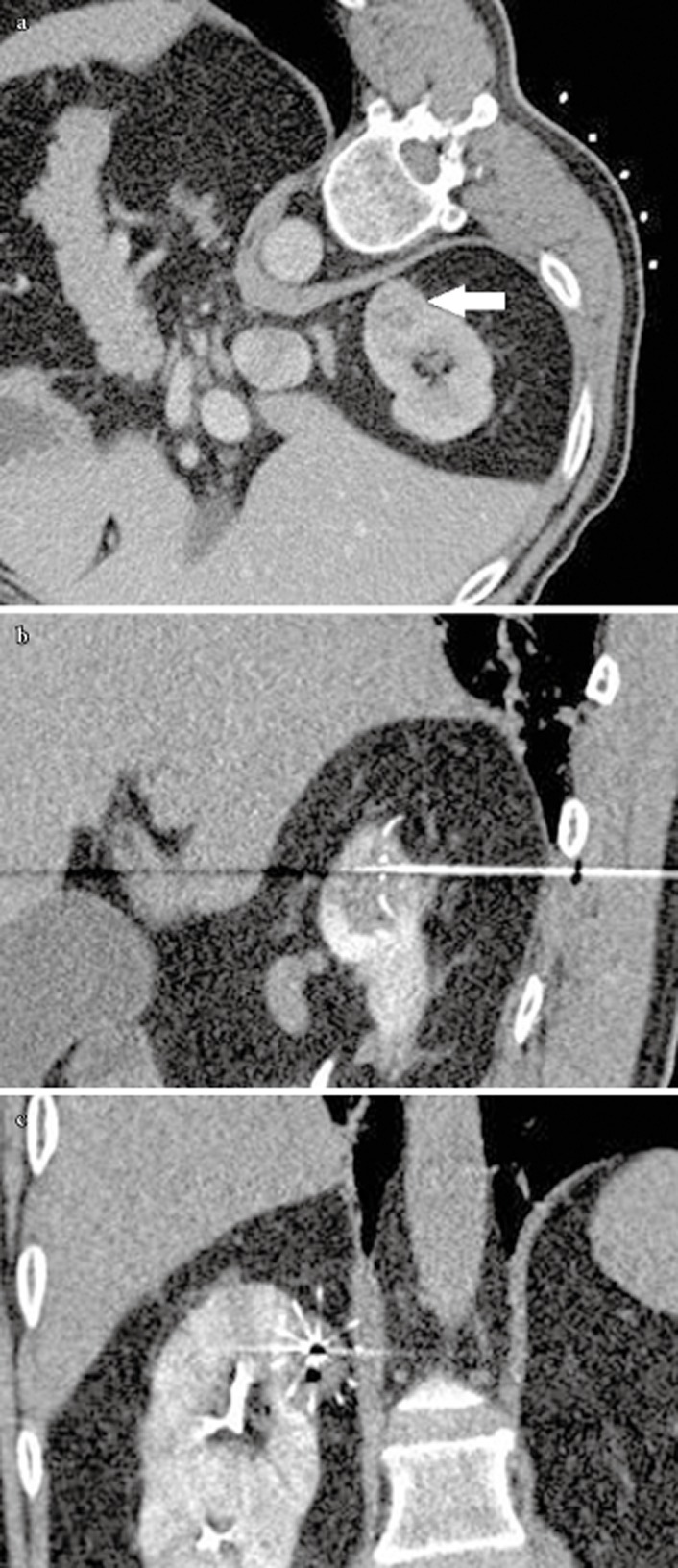
(A) Pre RFA axial contrast enhanced CT showed a 2.5 cm enhancing renal tumour at the upper pole of the right kidney (white arrow) (B) Sagittal reformatting showed the forward RF burn and (C) Coronal reformatting showed the overall coverage of the tumour by the multi-tines RF electrode.
After RFA, all patients were monitored in the theatre recovery area and then transferred to the ward for overnight observation and discharged home if clinically stable the following day, as the standard care. All the patients were monitored clinically and followed-up with radiology imaging by our institution during this period. Complications of the procedure were prospectively collated. Complications were classified as major or minor based on the classification of the Society of Interventional Radiology, with major complications requiring treatment or hospitalisation and minor complications needing only conservative monitoring. The average hospital stay was 2.8 days.
Cold Pyeloperfusion Technique
This technique was used when the renal tumour was centrally located or the treated tumour margin was close to the ureter and there was concern for renal pelvis or proximal ureteric injury (Figure 2) 30,31. This was initially described by us for patients having treatment in theatre 31 and since 2006, we have modified the technique to accommodate for the fact that all treatments are now performed in CT interventional suite 30. This in essence involved the patient lying supine on our CT table and retrograde cannulation of the ureter in the CT suite by the urologist. A guidewire was passed under direct vision using a flexible cystoscope into the renal pelvis. A 6 F open end flushing catheter (70 cm, COOK, Bloomington, IN, USA) was then passed over the guidewire and positioned into the renal pelvis. The position was then confirmed by performing a CT scout view with a small amount of contrast instilled retrogradely, to confirm the location. A 14 F Foley catheter was placed into the bladder, to which the ureteric catheter was taped to prevent its displacement. Cold 5% dextrose at 6°C was perfused via the ureteric catheter by gravity (80 cmH2O) and drained via the ureter into the bladder. At the end of the procedure, depending on the clinical requirement, we sometimes exchanged the ureteric catheter for a 7.5-F Optipur (Ettlingen, Germany) ureteric stent in the fluoroscopic suite under fluoroscopic guidance. The Foley catheter was removed 24 h after the procedure.
Figure 2.
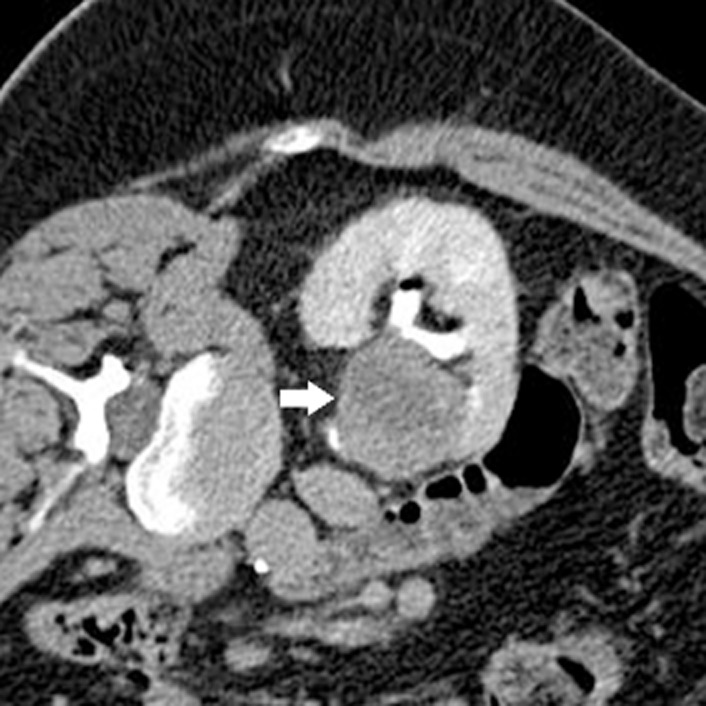
Pre RFA axial CT showed a centrally located renal tumour (white arrow) where it was abutting the ureter and PUJ.
In all, 13 RFA sessions in 10 patients with centrally located renal tumours were RF ablated using the cold pyeloperfusion technique. Two patients were at very high risk for ureteric injury as their tumours were obliterating the PUJ and ureter, and despite the risks were keen to go ahead with the treatment with the cold pyeloperfusion technique.
Hydrodissection Technique
Some patients had renal tumours that were in close proximity to bowel loops during treatment, either because of the location of the tumour (anterior and mid-polar location) or due to a lack of intra-abdominal fat. The hydrodissection technique was used in these instances, when there was <1 cm between the treatment margin and the bowel loop, to avoid thermal injury to the small or large bowel or any other surrounding vital organ 29,32. We routinely performed hydrodissection with a 16-G sheathed needle (Boston Scientific, MA, USA) and 150–500 mL 5% dextrose solution at room temperature was instilled to displace the bowel. In this series, this technique was performed in 25 patients who had 26 RFA sessions (mean volume 287.5 mL 5% dextrose).
Clinical and Radiological Follow-Up
All patients had dynamic contrast-enhanced (DCE) cross-sectional CT or MRI before and after RFA. We routinely performed DCE-MRI at baseline, 1, 3 and 6 months after RFA and annual CT for full staging (chest and kidneys) for a period of 10 years, as part of our Yorkshire Cancer Network (YCN) protocol. In patients with a serum creatinine of >200 μmol/L, DCE-MRI was used to assess the kidneys and unenhanced CT for chest staging annually. Triple-phase DCE-CT of the kidneys (which includes unenhanced, arterial and portal-venous phases) was performed to assess the zone of ablation if MRI was contraindicated (e.g. cardiac pacemaker) or the patient was claustrophobic. Occasionally, additional imaging was performed as a result of patients’ clinical symptoms.
All MRI examinations were acquired on a 1.5 T system (Symphony; Siemens Medical Systems, Erlangen, Germany). A dedicated four-element body array coil and integrated spine coil were used for signal reception. The MRI examination included T1, T2, true-FISP (Fast Imaging with Steady-state Precession) sequences in the axial, sagittal and coronal planes and pre and post gadolinium enhancement TI VIBE (volumetric interpolated breath-hold examination) sequences in both coronal and axial planes. Technical success was defined by absence of contrast enhancement within the tumour on CT or MRI (Fig. 3A, B and C). Residual disease was defined as persistent enhancement in an area of tumour after RFA seen on imaging at least 1 month after treatment. This usually presents as nodular and crescent enhancement around the periphery of the RFA zone 28,33. Recurrent disease was defined as new area of enhancement in the zone of ablation after at least one imaging (> 3 months) had shown complete lack of enhancement in the treated area (i.e. complete ablation). The imaging was reviewed by one of the two consultant radiologists (T.M.W., H.C.I.) and consensus was achieved if there was any uncertainty about the imaging findings.
Figure 3.
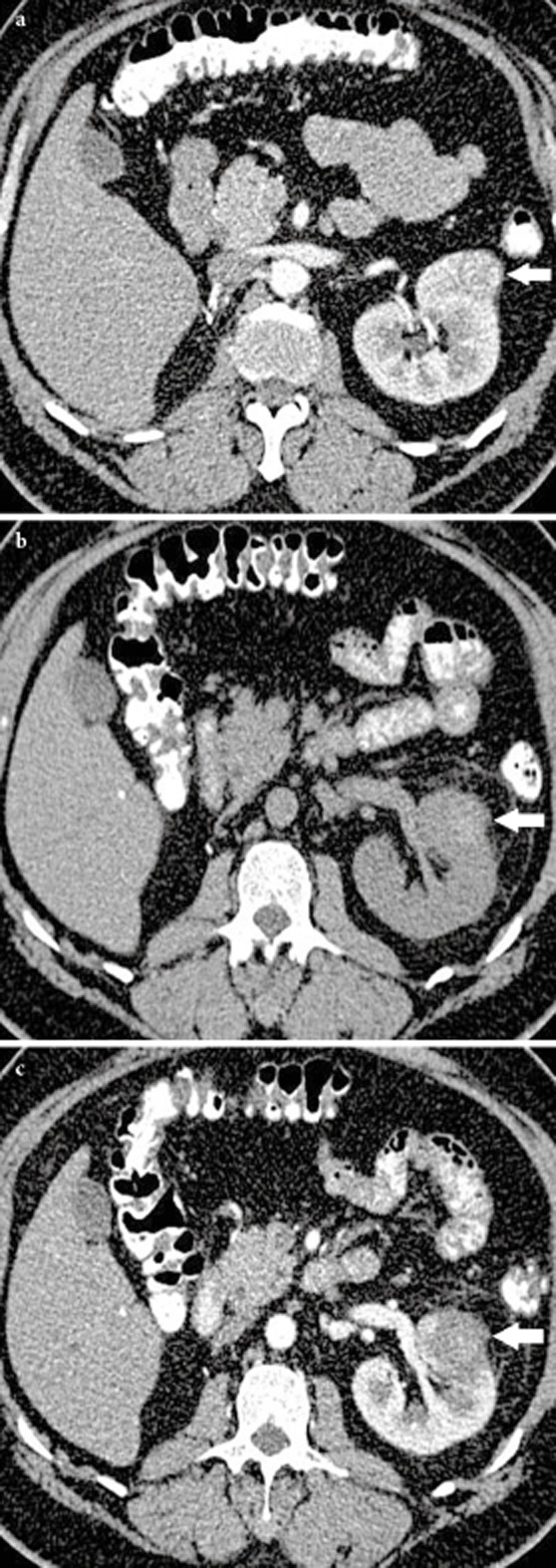
Axial contrast enhanced CT showed a small enhancing left renal tumour (white arrow) at the anterior cortex of the kidney pre-RFA (A) and the zone of ablation had high attenuation HU post RFA consistent with coagulation necrosis (white arrow) on the unenhanced CT (B) and displayed no enhancement (white arrow) post contrast administration (C).
Both major and minor complications, GFR before and after RFA, the management and outcomes of the complications, as well as oncological outcome, were prospectively documented. The overall (OS), cancer-specific (CSS), local RFS (LRFS) and metastasis-free survival (MFS) rates were also documented.
Data Analysis and Statistics
Descriptive statistics (e.g. mean, sd and variance) were reported and differences with a P < 0.05 were considered to indicate statistical significance.
Univariate analysis was performed using the Fisher’s exact test to assess the tumour size and location as predictors of technical success. The t-test was used to evaluate differences between the group means.
Multivariate logistic regression analysis was used to determine any association between the change in GFR before and after RFA (% GFR change) with the tumour size, polar position (upper, middle and lower pole of the kidney), tumour treatment location (exophytic, mixed, parenchymal and central), the total size of the tumour treated per RFA session, number of tumours treated and the solitary kidney status.
Multivariate logistic regression analysis was used to determine any association between the importance of tumour position in the kidney (upper, mid or lower), tumour treatment location (central, mixed, parenchymal and expohytic) and tumour size in influencing the technical success of the treatment or complication. Kaplan–Meier curves were used to determine the OS, CSS, LRFS and MFS and these were also documented.
Results
Renal Tumour Morphology and Histology
In all, 196 renal tumours were solid and four had cystic components within the renal tumour. The mean (range) ablated renal tumour size was 2.9 (1–5.6) cm and all the tumours were clinical stage T1.
In all, 188 core biopsies yielded an adequate sample for histological diagnosis with inadequate samples in 12 (6%) renal tumours. Amongst them, the histological subtype was: clear cell carcinoma (160), papillary (eight), distal nephron tumour (chromophobe or oesinophilic variant; 14), oncocytoma (one), fibrosis (three), metastasis from gastrointestinal tumour (one) and angiomyolipoma (one). Therefore, in our cohort of patients, 183 (91.5%) renal tumours had histological confirmation of malignancy and all were confirmed RCCs with various histological subtypes apart from the one metastasis from an oesophageal cancer. The five (2.5%) patients with a benign histological diagnosis did not need long-term follow-up. The 12 (6%) renal tumours with inconclusive biopsies, mainly due to the small sample size, were placed in an indeterminate group.
Technical Success (Primary and Overall)
Of the 200 treated tumours, 197 (98.5%) were completely ablated (191 in one RFA session, three after a second and three after a third session). Three patients declined re-treatment. Therefore the primary and overall technical success rates were 95.5 % and 98.5%, respectively. The local repeat RFA rate for the individual renal tumour was 3%.
Technical Success vs Tumour Location, Position and Size
The overall technical success of the RFA of all renal tumours is summarised in the Table 1. The results were categorised into tumour location, as well as tumour size.
Table 1.
The overall technical success rate vs size and location
| Tumour location | Technical success*, n/N (N = 200) | Total tumours >3 cm, n/N (N = 67) | Total tumours ≤3 cm, n/N (N = 133) |
|---|---|---|---|
| Exophytic | *43/43 | *15/15 | *28/28 |
| Parenchymal | *41/41 | *11/11 | *30/30 |
| Mixed | *98/100 | *32/34 | *66/66 |
| Central | *15/16 | *6/7 | *9/9 |
Technical success* = tumours with complete ablation/total number of tumours treated.
All exophytic and parenchymal renal tumours, regardless of location and size, were completely ablated in one RFA session. All six patients who required more than one treatment to achieve complete ablation had centrally located renal tumours (Table 2). In our series, there was a strong statistical association between central vs non-central locations (which includes exophytic and parenchymal location) and the primary technical success rate, i.e. successful ablation in one sitting (P < 0.001, Fisher’s exact test). Similar results were also seen when we compared the renal tumour location for central (16 tumours) vs exophytic (43) (P < 0.001, Fisher’s exact test). Three patients with residual disease refused further treatment, one had surgery and two were deemed unfit for surgery from the outset, the tumours location were central (one) and mixed (two). In addition, the size of the tumour was also a strong independent predictor in achieving complete RFA in one treatment session (Table 3), between renal tumours <3 vs >3 cm (P < 0.001, Fisher’s exact test). Size and location were shown to be independent predictors in multivariate logistic regression analysis. However, the primary technical success rate was shown to be not influenced by the tumour polar position (upper, middle or lower; P > 0.7, Fisher’s exact test).
Table 2.
Patients with residual disease or requiring repeated RFA
| Patients | Tumour location | Size, cm | Complete ablation achieved | Residual disease because patients refused further treatment | Number of RFA treatments |
|---|---|---|---|---|---|
| 1 | Central | 2.7 | Yes | No | 2 |
| 2 | Central | 3.4 | Yes | No | 3 |
| 3 | Central | 3.4 | Yes | No | 2 |
| 4 | Central | 3.5 | Yes | No | 3 |
| 5 | Central | 4 | Yes | No | 2 |
| 6 | Central | 4.8 | Yes | No | 3 |
| 7 | Central | 5.6 | No | Yes | 2 |
| 8 | Mixed | 4 | No | Yes | 1 |
| 9 | Mixed | 5.4 | No | Yes | 2 |
Table 3.
Achievement of complete ablation and number of ablation sessions based on tumour size
| Tumour size, cm | Number of tumours with complete ablation/ total number of tumours treated (%) | Number of tumours treated with one session | Number of tumours treated with two sessions | Number of tumours treated with three sessions |
|---|---|---|---|---|
| ≤ 3 | 133/133 (100) | 132 | 1 | NA |
| 3–5 | 62/63 (98.4)* | 57* | 3 | 3 |
| >5 | 2/4† | 2 | NA | NA |
In this group of renal tumours treated (3–5 cm), one renal tumour was treated with single session but the patient refused to have further treatment. †In this group of renal tumours (>5 cm), two renal tumours had residual disease after a single treatment session and patients did not undertake further treatment due to patients’ choice.
Technical Success vs Consultant IR Experience
During the 8-year period, 128 renal tumours were RF ablated from June 2004 to 2008 (<4-year experience) and a further 72 renal tumours were RF ablated from June 2008 to 2012 (>4-year experience). All the patients with residual disease (nine) were treated in the first 4 years. In the subsequent 4 years, the primary technical success rate was 100%. Therefore, in our series, the IR experience does influence the primary technical success rate when we compared the IR’s experience <4 vs >4-year period (P = 0.03, Fisher’s exact test). When experience of the operator was included in the multivariate logistic regression analysis, along with size and location, IR experience was no longer significant, although still with a similar trend (P = 0.18, Fisher’s exact test). It is likely that this merely reflects that the experience of the operator results in a better selection of tumours for treatment, with the larger and central tumours all occurring in the first 4 years.
Patients with Residual (Incompletely Treated) Disease
At the time of reporting, three patients within the present cohort were incompletely treated, one refused further treatment and two had repeated treatment but had residual disease and it was deemed inappropriate to treat further. The patient who refused further treatment has a MFS of 80.2 months and is still alive. One patient had ankylosing spondylitis and was treated initially under US guidance. He then had a repeat treatment under CT guidance but could not be positioned optimally in the CT scanner due to his ankylosing spondylitis, so that only part of the residual disease was treated. He subsequently opted for open radical nephrectomy. Another elderly patient was deemed inappropriate for a third ablation as the MDT decided that surveillance should be advocated instead and the patient had a MFS of 94.6 months after the second RFA before dying of ischaemic heart disease. All three patients were treated during our early experience (<3 years) and in our first 50 cohort of patients.
RFA Procedural Complications
Complications were classified as major or minor based on the classification of the Society of Interventional Radiology, with major complications requiring treatment or hospitalisation and minor complications needing only conservative monitoring. There have been a total of 11 major complications directly relating to our treatment technique, including ureteric stricture (seven), acute tubular necrosis resulting in permanent renal failure in a patient with solitary kidney (one), calyceal cutaneous fistula (one) and renal abscesses (two). There were 12 minor complications that include lateral cutaneous nerve paraesthesia (five), skin burn (two), self-limiting subcapsular haematoma (four) and self-limiting pneumothorax (one). In the present cohort, one patient developed myocardial infarction after general anaesthesia but recovered after appropriate medical treatment.
Haemorrhage
There was no major haemorrhage in the present cohort of patients requiring intervention, e.g. blood transfusion or vascular embolization. There were four patients who developed self-limiting subcapsular haematoma after renal RFA, which were present at the time of treatment completion.
Ureteric Stricture
There were seven (3.5%) ureteric strictures in 210 RFA treatment sessions. All the ureteric injuries occurred in the upper third of the ureter and PUJ. The management for the ureteric stricture was: retrograde ureteric stenting (four), conservative management (two) and radical nephrectomy (one). One patient had opted for radical nephrectomy as recurrent UTIs from the subsequent ureteric stent insertion resulted in persistent psoas abscess formation.
For the 16 central tumours the cold pyeloperfusion technique was used during 13 treatment sessions in 10 centrally located renal tumours and eight of these patients were treated successfully without the development of ureteric stricture. As we had predicted, the two high-risk patients who were deemed likely to develop ureteric strictures (tumour abutting the ureter with zero distance to the treatment margin; Fig. 2) had developed strictures despite using the protective mechanism. The remaining five patients also developed ureteric strictures without cold pyeloperfusion technique were in retrospect, all <1 cm margin from the ureter and the locations of the tumours were: central (one) and lower pole (four). Therefore, in this cohort (excluding the two high-risk patients), the use of the cold pyeloperfusion technique when treating renal tumours in close proximity of the ureter (<1 cm) would protect the ureter from injury (P < 0.003, Fisher’s exact test) but the protection is impossible if the margin between tumour and ureter is completely obliterated. In addition, two independent predictors of the likelihood of developing ureteric stricture are central vs non-central (exophytic and parenchymal) location (P = 0.02, Fisher’s exact test) and lower pole vs non-lower pole location (P = 0.01, Fisher’s exact test).
Renal Abscess
There were two patients who developed renal abscesses: one patient had a pre-existing ileal-conduit 34 and the other patient appeared to be immune-compromised. Both were treated with parenteral antibiotics and local drainage.
Calyceal-Cutaneous Fistula and Acute Tubular Necrosis
One patient who developed a calyceal cutaneous fistula had a pre-existing ileal conduit formed for previous cystectomy and had had recurrent UTIs. Another patient developed irreversible acute tubular necrosis after renal RFA. We have reported these cases previously 34,35.
Renal Function Measurement: GFR before and after RFA
The mean (sd) GFR before and after renal RFA was 54.7 (18.2) vs 52.7 (18.5) mL/min/1.73 m2. There was a significant difference between the GFR measurements before and after RFA with a mean difference of 2.03, where the GFR was higher before treatment (P < 0.001, Wilcoxon signed-rank test). Within this cohort of patients, only four patients developed significant renal function deterioration (>25% decreased in GFR). In all, 161 (98%) of the 165 patients had preservation of renal function.
The mean (sd) percentage change in GFR from before to after RFA was – 3.1 (15.2)%, i.e. worsened by 3.1% after RFA treatment. However, using multivariate logistic regression analysis there was no association between the percentage of GFR change with tumour size, polar position (upper, middle and lower pole of the kidney), tumour treatment location (exophytic, mixed, parenchymal and central), the size of the tumour treated per RFA session, number of tumours treated and the solitary kidney status.
RFA Oncological Outcome
All the patients were followed up both radiologically and clinically for a mean (range) of 47.6 (2.6–96) months. Most of our elderly patients (20) in the early cohort had succumbed to ischaemic heart disease or respiratory infection. In the overall cohort, nine patients presented with existing renal metastasis or pre-existing gastrointestinal cancer but were in remission at the time of initial RFA and they were excluded from the Kaplan–Meier curve analysis. The reason for treating the primary renal tumour in the setting of renal metastasis was with the intention to debulk the primary tumour before anti-angiogenic therapy or immunotherapy. In our clinical series, this yielded 5-year OS and CSS rates of 75.8% and 97.9%, respectively (Figs 5).
Figure 5.
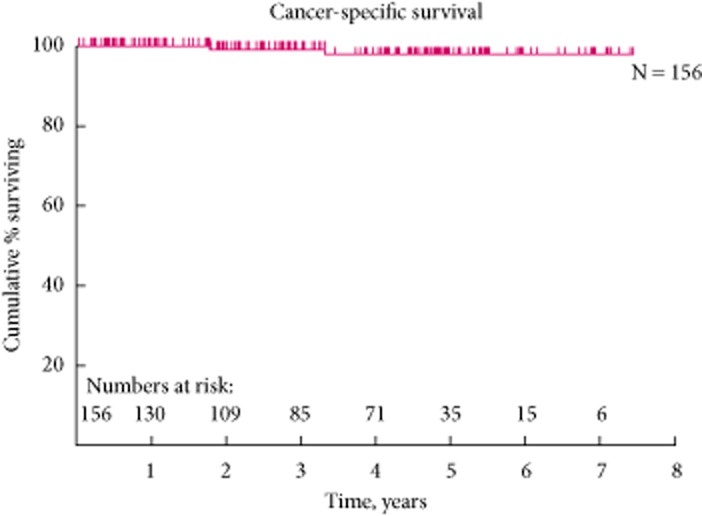
The 5-year cancer specific survival curve.
Figure 4.
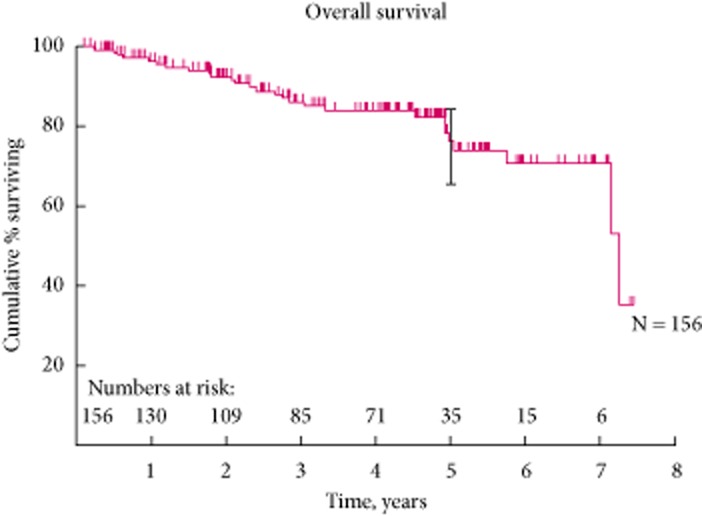
The overall survival curve.
Local Recurrence and Distant Metastasis
In the present cohort, there were five (2.5%) local recurrences and all were late recurrences (>4 years follow-up), with mean detection at 58.3 months (Table 4). All the local recurrences recurred at the inner margin of the zone of the ablation.
Table 4.
Local recurrence patients who had RFA and developed local recurrent disease after an interval with initial radiology confirmed complete ablation
| Months to local recurrence | Biopsy before RFA | Treatment of recurrence | Pathology after treatment | Follow-up |
|---|---|---|---|---|
| 78.3 | Grade 3 RCC | Nodular local disease: Surveillance as patient is diagnosed with dementia | N/A | Alive at 88.9 months |
| 53.2 | Grade 2 RCC | Local renal tumour thrombus: surveillance as per patient choice | N/A | Alive at 85.4 months |
| 52 | Grade 1 RCC | Nodular local disease: surveillance as per patient choice | N/A | Alive at 64.9 months |
| 53.8 | Grade 2 RCC | Nodular local disease: radical nephrectomy | Grade 2 RCC | Alive at 63.1 months |
| 54.5 | Grade 2 RCC | Nodular/crescent local disease: had repeated percutaneous cryoablation | Grade 2 RCC | Alive at 55.4 months |
In all, four (2%) patients developed distant metastasis with mean detection at 37.8 months (Table 5). Two patients have died from metastatic RCC and the other two patients are still alive.
Table 5.
Patients who had RFA and developed distant recurrent disease after an interval with initial radiology confirmed complete ablation and metastasis free
| Months to distant metastasis | Biopsy before RFA | Site and treatment of recurrence | Pathology after treatment | Follow-up |
|---|---|---|---|---|
| 53.8 | Grade 2RCC | Lung: active surveillance | N/A | Alive at 63.1 months |
| 31.7 | Grade 3 RCC | Lung and brain metastasis: palliative | N/A | Died at 43.3.months |
| 21.5 | Grade 4 RCC with sarcomatoid changes | Lung and liver metastasis: palliative | N/A | Died at 23 months |
| 44.2 | Grade 2 RCC | Lung: active surveillance | N/A | Alive at 50.9 months |
One of these patients died 23 months after treatment having developed both pulmonary and liver metastases, which were detected at 21.5 months during an acute CT examination after she had presented with abdominal pain. She had RFA of her 3.5-cm renal tumour, which had a histological diagnosis of Grade 4 sarcomatoid changes conventional RCC. Another patient known to have chronic renal impairment presented with pulmonary metastasis at 31.7 months after RFA of two renal tumours in the left kidney (4 and 2.5 cm), with histological diagnosis of grade 3 conventional RCC. He died 43 months later when he developed brain metastases.
A further two patients with distant metastasis are still alive. One patient developed local recurrence in the zone of ablation of the treated right grade 2 conventional RCC (3.3 cm) and a pulmonary metastasis (<10 mm) at 53.8 months. He underwent radical right nephrectomy at 55.3 months and his pulmonary metastasis was kept under surveillance. He developed bony metastases (ribs, iliac wing and sacrum) at 63.5 months and he is currently undergoing anti-angiogenic therapy with Sutent. One patient developed a presumed pulmonary metastasis at 44.2 months after RFA of a right grade 2 conventional RCC (4.2 cm) but no conclusive CT-guided pulmonary biopsies had been taken at 55.2 months. He had three negative histological biopsies to date (last one in October 2012).
Our 5-year LRFS and MFS rates of 93.5% and 87.7%, respectively (Figs 7).
Figure 7.
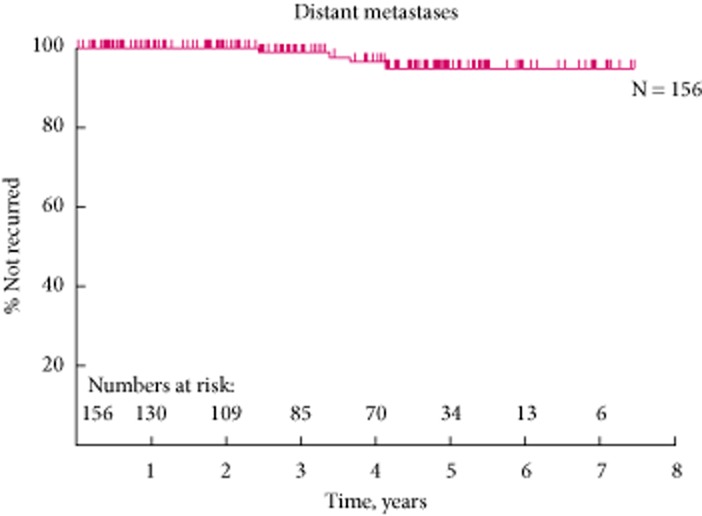
The 5-year distant metastasis free survival curve.
Figure 6.
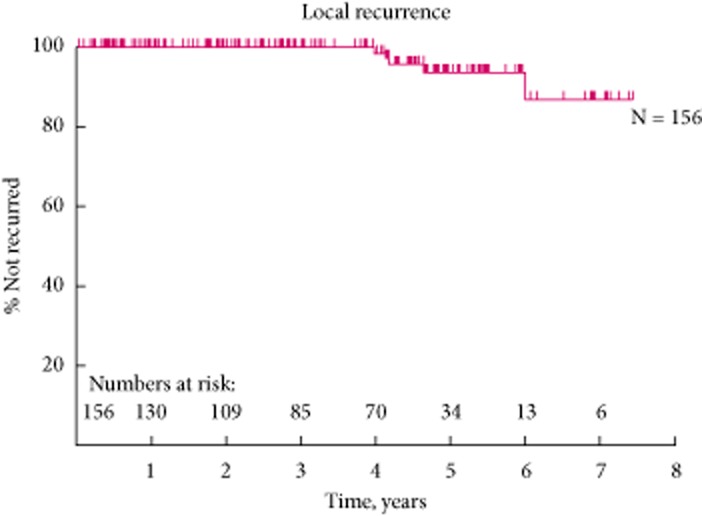
The 5-year local recurrence free survival curve.
Discussion
The new accepted standard treatment for small renal tumours (<4 cm) is a NS technique with either a surgical approach or image-guided ablative therapy 13.
We have reviewed our experience in a large tertiary university institution over an 8-year period from June 2004 to 2012 with the treatment of 200 renal tumours using percutaneous RFA and compared to a range of 41 to 185 patients reported in previous series with long-term follow-up 20,21,36–38. Our image-guided renal ablation programme is a supra-regional centre for the whole of the YCN, as well as outside YCN regions, e.g. South Yorkshire, Lincolnshire and Tyneside. Whilst our urologists received referral from a smaller referral base, e.g. Leeds and Harrogate, they had treated 129 patients with T1 renal tumours and these patients underwent either radical or partial nephrectomy during the same period.
We have evaluated our technical success, treatment complications, treatment effect on renal function and the 5-year OS, CSS, LRFS as well as MFS rates in the present cohort of patients. In addition, operator experience as well as tumour factors, e.g. size and location, were assessed to determine its influence on the technical success rate of the primary RFA treatment (i.e. achieve complete RFA after one setting). For renal tumours in close proximity to the ureter (<1 cm), we also reviewed our experience in the use of protective techniques with the cold pyeloperfusion technique and assessed whether this step provided protection from ureteric injury.
In the present series, the primary and overall technical success rate was 95.5 % (191/200) vs 98.5% (197/200) respectively for the 200 renal tumours that were RF ablated. The local repeat RFA rate was 3%. This overall technical success rate is similar to other published series with reported rates that ranged from 90 to 100% 21,29,39,40. In addition, it is interesting to note that our primary technical success rate (100%) has improved considerably after the initial years of the learning curve (> 4 years) and as stated earlier this is likely to be related to better case selection by the team. Similar observations were also reported by Poon et al. 41, with a definite learning curve in acquiring this specialist skill by a dedicated team in order to achieve good technical outcomes and a minimal complication rate.
We have also shown that tumour factors, e.g. size (<3 cm) and exophytic location, are two important independent predictors in achieving complete ablation with a single treatment 29,39,42. This is because a smaller vascular pedicle in the exophytic renal tumour as well as surrounding peri-renal fat allows more effective treatment than in central tumours.
In the present cohort, our major and minor complication rates are also comparable to other published series 29,39,42. Interestingly, we did not experience significant haemorrhage after RFA in the present cohort, as this is one of the commonest major complications reported previously 43.
The ureteric stricture rate in the present cohort was 3.5% and is similar to the reported range of 2–3% 42,43. This was in part related to our early success in treating central tumours with the cold pyeloperfusion technique 30,31, after which we have performed more RFAs of renal tumours in closer proximity to the ureters. Our overall experience has indicated that cold pyeloperfusion is effective in protecting most of the ureters (80%) but does not always confer protection to the ureter and PUJ during RFA, especially when there is zero distance between the tumour and the ureter. It is crucial during case selection and especially when providing consultation to patients, to highlight the potential higher risks of ureteric injury when the renal tumour has a central or lower pole location, so the patients are consented accordingly and aware of the potential risks that the procedure entails.
We have been meticulous in protecting vital organs, e.g. the colon, from the RFA treatment margin with the hydrodissection technique with 5% dextrose, and to date, we have not encountered any bowel injury during RFA in the present cohort of 200 renal tumours. This is similarly reported by other institutions and the use of protective techniques, e.g. hydro-dissection, is becoming increasingly routine when the distance between the treatment margin to bowel is <1 cm 32,43.
Development of chronic kidney disease, when the GFR is <60 mL/min/1.73 m2, is associated with higher incidence of death, cardiovascular events and hospital admissions 44. Therefore it is important that the NS procedures can be performed in patients with RCC that have compromised renal function, e.g. solitary kidney status or with pre-existing chronic kidney disease, with the aim to preserve renal function and maintain well-being. The published data have confirmed that renal preservation can be achieved with image-guided renal ablation 17,45–47. However, this is the first clinical series to examine the relationship between the change in the GFR (% GFR change) before and after RFA treatment with the tumour characteristics (tumour size, polar position and location), the total bulk of the tumour treated per ablation session, number of tumours treated and the solitary kidney status, and we have confirmed that there is no association with renal function change with any of these factors 48. The present result has also confirmed that there is a 3.1% worsening of the GFR after RFA treatment when compared with the GFR before treatment (P < 0.001). However, there were only four patients that developed significant deterioration of renal function (>25% decreased in GFR after treatment). Most (98%) of the present cohort had preservation of their renal function. It is important to note that the measurement of renal function at 1 day after the procedure is a routine clinical test in our institution and we are aware that in most patients renal function does recover and stabilise for a period of 3–6 months after RFA. The immediate renal function test in our practice was used as a clinical guide to decide which patients, especially the high-risks patients (e.g. with single kidney or impaired renal function), were safe to discharge immediately or if further renal function monitoring was required.
There are only limited series reporting the longer term oncological efficacy of RFA of renal tumours with a total of 417 patients reported 20,21,36–38. The present clinical series has shown 5-year OS, CSS, LRFS and MFS rates of: 75.8%, 97.9%, 93.5% and 87.7%, respectively. This is comparable to both the Tracy et al. 20 and Zagoria et al. 21 cohorts. Tracy et al. reported 5-year OS, CSS and RFS rates of 85%, 99% and 93%, respectively. In addition, Zagoria et al. reported similar findings; where 5-year OS, LRFS and disease-free rates were 66%, 88% and 83%, respectively. The OS rate in the present cohort is a reflection of elderly and unfit patients in our early experience. This is also comparable to the long-term outcome of laparoscopic renal cryoablation for small renal tumour reported by Aron et al. 49, where 5-year OS, CSS and RFS rates were 83%, 95% and 78%, respectively.
In the present cohort there was a local repeat RFA rate of 3%, local tumour progression rate of 2.5% and metastatic progression rate of 2%. These results also compare favourably with a meta-analysis of renal cryoablation vs RFA, where the cryoablation has local re-ablation, local tumour progression and metastatic progression rates of: 1%, 5% and 2%, respectively 50. Similar results have also been reported in other RFA series: 3%, 7% and 5% respectively by Tracy et al. 20 and Zagoria et al. 21 have also shown 12% local progression and 7% metastatic progression in their longer term cohort.
Overall it is reassuring to confirm that the present longer term survival outcome is within the reported range of the ‘gold standard’ partial nephrectomy, where the 5-year MFS after partial nephrectomy for T1 renal tumours is 86–97% 16 and the local recurrence rate after partial nephrectomy is 1–3% 51,52. However, it is prudent that we continue to monitor our technical success, and complication rates, as well as local disease and metastatic disease progression potential after local ablative therapy, and also adopt the nephrometry score to assess the complexity of case selection increasingly used in NSS (either the R.E.N.A.L. or Preoperative Aspects and Dimensions Used for an Anatomical [PADUA] score) 53,54, so that we may collectively provide long-term 10-year oncological data in the near future.
In conclusion, whilst there have been multiple previous publications on RFA in renal cancer, the present study is one of the largest series by far and increases the total number of cases reported from ≈400 to 600. This, together with our ability to analyse risk factors in the numbers available to us, means that it is now possible to begin to draw firm conclusions about the safety and efficacy of RFA and to reassure patients that, in the hands of experienced operators, they can be confident that the results are comparable to any alternative approach to NS surgical or non-surgical treatment for renal cancer.
Conflict of Interest
None declared.
Glossary
- ASA
American Society of Anesthesiologists
- DCE
dynamic contrast-enhanced
- IR
interventional radiologist
- (L)RFS
(local) recurrence-free survival
- MDT
multi-disciplinary team
- NS(S)
nephron-sparing (surgery)
- OS
overall survival
- RFA
radiofrequency ablation
- US
ultrasonograpy/ultrasound
- YCN
Yorkshire Cancer Network
References
- 1.Jemal A, Tiwari RC, Murray T. Cancer statistics, 2004. CA Cancer J Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Office for National Statistics. Cancer Statistics Registrations: Registrations of Cancer Diagnosed in 2008, England. London: Office for National Statistics, National Statistics; 2010. [Google Scholar]
- 3.2008. Globocan.
- 4.Zagoria RJ. Imaging of small renal masses: a medical success story. AJR Am J Roentgenol. 2000;175:945–955. doi: 10.2214/ajr.175.4.1750945. [DOI] [PubMed] [Google Scholar]
- 5.Jayson M, Sanders H. Increased incidence of serendipitously discovered renal cell carcinoma. Urology. 1998;51:203–205. doi: 10.1016/s0090-4295(97)00506-2. [DOI] [PubMed] [Google Scholar]
- 6.Hollingsworth JM, Miller DC, Daignault S, Hollenbeck BK. Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst. 2006;98:1331–1334. doi: 10.1093/jnci/djj362. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen MM, Gill IS, Ellison LM. The evolving presentation of renal carcinoma in the United States: trends from the Surveillance, Epidemiology, and End Results program. J Urol. 2006;176:2397–2400. doi: 10.1016/j.juro.2006.07.144. [DOI] [PubMed] [Google Scholar]
- 8.Pantuck AJ, Zisman A, Belldegrun AS. The changing natural history of renal cell carcinoma. J Urol. 2001;166:1611–1623. [PubMed] [Google Scholar]
- 9.Pantuck AJ, Zisman A, Rauch MK, Belldegrun A. Incidental renal tumors. Urology. 2000;56:190–196. doi: 10.1016/s0090-4295(00)00655-5. [DOI] [PubMed] [Google Scholar]
- 10.Luciani LG, Cestari R, Tallarigo C. Incidental renal cell carcinoma – age and stage characterization and clinical implications: study of 1092 patients (1982–1997) Urology. 2000;56:58–62. doi: 10.1016/s0090-4295(00)00534-3. [DOI] [PubMed] [Google Scholar]
- 11.Remzi M, Marberger M. Renal tumor biopsies for evaluation of small renal tumors: why, in whom, and how? Eur Urol. 2009;55:359–367. doi: 10.1016/j.eururo.2008.09.053. [DOI] [PubMed] [Google Scholar]
- 12.Abouassaly R, Lane BR, Novick AC. Active surveillance of renal masses in elderly patients. J Urol. 2008;180:505–509. doi: 10.1016/j.juro.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 13.Ljungberg B, Cowan NC, Hanbury DC. EAU guidelines on renal cell carcinoma: the 2010 update. Eur Urol. 2010;58:398–406. doi: 10.1016/j.eururo.2010.06.032. [DOI] [PubMed] [Google Scholar]
- 14.Huang WC, Levey AS, Serio AM. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol. 2006;7:735–740. doi: 10.1016/S1470-2045(06)70803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Poppel H, Becker F, Cadeddu JA. Treatment of localised renal cell carcinoma. Eur Urol. 2011;60:662–672. doi: 10.1016/j.eururo.2011.06.040. [DOI] [PubMed] [Google Scholar]
- 16.Uzzo RG, Novick AC. Nephron sparing surgery for renal tumors: indications, techniques and outcomes. J Urol. 2001;166:6–18. [PubMed] [Google Scholar]
- 17.Lucas SM, Stern JM, Adibi M, Zeltser IS, Cadeddu JA, Raj GV. Renal function outcomes in patients treated for renal masses smaller than 4 cm by ablative and extirpative techniques. J Urol. 2008;179:75–80. doi: 10.1016/j.juro.2007.08.156. [DOI] [PubMed] [Google Scholar]
- 18.Hafez KS, Novick AC, Butler BP. Management of small solitary unilateral renal cell carcinomas: impact of central versus peripheral tumor location. J Urol. 1998;159:1156–1160. [PubMed] [Google Scholar]
- 19.Thompson RH. Radical nephrectomy: too radical for small renal masses? Lancet. 2006;368:823–824. doi: 10.1016/S0140-6736(06)69302-1. [DOI] [PubMed] [Google Scholar]
- 20.Tracy CR, Raman JD, Donnally C, Trimmer CK, Cadeddu JA. Durable oncologic outcomes after radiofrequency ablation: experience from treating 243 small renal masses over 7.5 years. Cancer. 2010;116:3135–3142. doi: 10.1002/cncr.25002. [DOI] [PubMed] [Google Scholar]
- 21.Zagoria RJ, Pettus JA, Rogers M, Werle DM, Childs D, Leyendecker JR. Long-term outcomes after percutaneous radiofrequency ablation for renal cell carcinoma. Urology. 2011;77:1393–1397. doi: 10.1016/j.urology.2010.12.077. [DOI] [PubMed] [Google Scholar]
- 22.Duffey B, Nguyen V, Lund E, Koopmeiners JS, Hulbert J, Anderson JK. Intermediate-term outcomes after renal cryoablation: results of a multi-institutional study. J Endourol. 2012;26:15–20. doi: 10.1089/end.2011.0179. [DOI] [PubMed] [Google Scholar]
- 23.Zagoria RJ. Percutaneous image-guided radiofrequency ablation of renal malignancies. Radiol Clin North Am. 2003;41:1067–1075. doi: 10.1016/s0033-8389(03)00074-5. [DOI] [PubMed] [Google Scholar]
- 24.Gervais DA, McGovern FJ, Arellano RS, McDougal WS, Mueller PR. Renal cell carcinoma: clinical experience and technical success with radio-frequency ablation of 42 tumors. Radiology. 2003;226:417–424. doi: 10.1148/radiol.2262012062. [DOI] [PubMed] [Google Scholar]
- 25.Mayo-Smith WW, Dupuy DE, Parikh PM, Pezzullo JA, Cronan JJ. Imaging-guided percutaneous radiofrequency ablation of solid renal masses: techniques and outcomes of 38 treatment sessions in 32 consecutive patients. AJR Am J Roentgenol. 2003;180:1503–1508. doi: 10.2214/ajr.180.6.1801503. [DOI] [PubMed] [Google Scholar]
- 26.Silverman SG, Lee BY, Seltzer SE, Bloom DA, Corless CL, Adams DF. Small (< or = 3 cm) renal masses: correlation of spiral CT features and pathologic findings. AJR Am J Roentgenol. 1994;163:597–605. doi: 10.2214/ajr.163.3.8079852. [DOI] [PubMed] [Google Scholar]
- 27.Ho VB, Allen SF, Hood MN, Choyke PL. Renal masses: quantitative assessment of enhancement with dynamic MR imaging. Radiology. 2002;224:695–700. doi: 10.1148/radiol.2243011048. [DOI] [PubMed] [Google Scholar]
- 28.Gervais DA, McGovern FJ, Wood BJ, Goldberg SN, McDougal WS, Mueller PR. Radio-frequency ablation of renal cell carcinoma: early clinical experience. Radiology. 2000;217:665–672. doi: 10.1148/radiology.217.3.r00dc39665. [DOI] [PubMed] [Google Scholar]
- 29.Breen DJ, Rutherford EE, Stedman B. Management of renal tumors by image-guided radiofrequency ablation: experience in 105 tumors. Cardiovasc Intervent Radiol. 2007;30:936–942. doi: 10.1007/s00270-007-9090-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cantwell CP, Wah TM, Gervais DA. Protecting the ureter during radiofrequency ablation of renal cell cancer: a pilot study of retrograde pyeloperfusion with cooled dextrose 5% in water. J Vasc Interv Radiol. 2008;19:1034–1040. doi: 10.1016/j.jvir.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 31.Wah TM, Koenig P, Irving HC. Radiofrequency ablation of a central renal tumor: protection of the collecting system with a retrograde cold dextrose pyeloperfusion technique. J Vasc Interv Radiol. 2005;16:1551–1555. doi: 10.1097/01.RVI.0000175322.05225.0a. [DOI] [PubMed] [Google Scholar]
- 32.Farrell MA, Charboneau JW, Callstrom MR, Reading CC, Engen DE, Blute ML. Paranephric water instillation: a technique to prevent bowel injury during percutaneous renal radiofrequency ablation. AJR Am J Roentgenol. 2003;181:1315–1317. doi: 10.2214/ajr.181.5.1811315. [DOI] [PubMed] [Google Scholar]
- 33.Wile GE, Leyendecker JR, Krehbiel KA, Dyer RB, Zagoria RJ. CT and MR imaging after imaging-guided thermal ablation of renal neoplasms. Radiographics. 2007;27:325–340. doi: 10.1148/rg.272065083. [DOI] [PubMed] [Google Scholar]
- 34.Wah TM, Irving HC. Infectious complications after percutaneous radiofrequency ablation of renal cell carcinoma in patients with ileal conduit. J Vasc Interv Radiol. 2008;19:1382–1385. doi: 10.1016/j.jvir.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 35.Wah TM, Irving HC. Acute tubular necrosis following radiofrequency ablation of a renal cell carcinoma. Cardiovasc Intervent Radiol. 2009;32:591–592. doi: 10.1007/s00270-007-9191-6. [DOI] [PubMed] [Google Scholar]
- 36.McDougal WS, Gervais DA, McGovern FJ, Mueller PR. Long-term followup of patients with renal cell carcinoma treated with radio frequency ablation with curative intent. J Urol. 2005;174:61–63. doi: 10.1097/01.ju.0000162046.45024.2b. [DOI] [PubMed] [Google Scholar]
- 37.Levinson AW, Su LM, Agarwal D. Long-term oncological and overall outcomes of percutaneous radio frequency ablation in high risk surgical patients with a solitary small renal mass. J Urol. 2008;180:499–504. doi: 10.1016/j.juro.2008.04.031. [DOI] [PubMed] [Google Scholar]
- 38.Psutka SP, Feldman AS, McDougal WS, McGovern FJ, Mueller P, Gervais DA. Long-term oncologic outcomes after radiofrequency ablation for T1 renal cell carcinoma. Eur Urol. 2013;63:486–492. doi: 10.1016/j.eururo.2012.08.062. [DOI] [PubMed] [Google Scholar]
- 39.Gervais DA, Arellano RS, McGovern FJ, McDougal WS, Mueller PR. Radiofrequency ablation of renal cell carcinoma: part 1, Indications, results, and role in patient management over a 6-year period and ablation of 100 tumors. AJR Am J Roentgenol. 2005;185:64–71. doi: 10.2214/ajr.185.1.01850064. [DOI] [PubMed] [Google Scholar]
- 40.Matsumoto ED, Johnson DB, Ogan K. Short-term efficacy of temperature-based radiofrequency ablation of small renal tumors. Urology. 2005;65:877–881. doi: 10.1016/j.urology.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 41.Poon RT, Ng KK, Lam CM. Learning curve for radiofrequency ablation of liver tumors: prospective analysis of initial 100 patients in a tertiary institution. Ann Surg. 2004;239:441–449. doi: 10.1097/01.sla.0000118565.21298.0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zagoria RJ, Traver MA, Werle DM, Perini M, Hayasaka S, Clark PE. Oncologic efficacy of CT-guided percutaneous radiofrequency ablation of renal cell carcinomas. AJR Am J Roentgenol. 2007;189:429–436. doi: 10.2214/AJR.07.2258. [DOI] [PubMed] [Google Scholar]
- 43.Gervais DA, Arellano RS, McGovern FJ, McDougal WS, Mueller PR. Radiofrequency ablation of renal cell carcinoma: part 2, Lessons learned with ablation of 100 tumors. AJR Am J Roentgenol. 2005;185:72–80. doi: 10.2214/ajr.185.1.01850072. [DOI] [PubMed] [Google Scholar]
- 44.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 45.Wehrenberg-Klee E, Clark TW, Malkowicz SB. Impact on renal function of percutaneous thermal ablation of renal masses in patients with preexisting chronic kidney disease. J Vasc Interv Radiol. 2012;23:41–45. doi: 10.1016/j.jvir.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 46.Prevoo W, van den Munckhof MP, Meinhardt W, Horenblas S, van den Bosch MA. Radiofrequency ablation of kidney tumours in patients with a solitary kidney. Clin Radiol. 2010;65:230–236. doi: 10.1016/j.crad.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 47.Raman JD, Raj GV, Lucas SM. Renal functional outcomes for tumours in a solitary kidney managed by ablative or extirpative techniques. BJU Int. 2009;105:496–500. doi: 10.1111/j.1464-410X.2009.08776.x. [DOI] [PubMed] [Google Scholar]
- 48.Gervais DA. Preexisting chronic kidney disease and renal tumor ablation: preliminary answers and persistent questions. J Vasc Interv Radiol. 2012;23:46–47. doi: 10.1016/j.jvir.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 49.Aron M, Kamoi K, Haber GP. Laparoscopic renal cryoablation: long-term oncologic outcomes with minimum 5 year follow up. J Urol. 2008;179(Suppl):209–210. [Google Scholar]
- 50.Kunkle DA, Uzzo RG. Cryoablation or radiofrequency ablation of the small renal mass : a meta-analysis. Cancer. 2008;113:2671–2680. doi: 10.1002/cncr.23896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lane BR, Gill IS. 7-year oncological outcomes after laparoscopic and open partial nephrectomy. J Urol. 2010;183:473–479. doi: 10.1016/j.juro.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 52.Yossepowitch O, Bjartell A, Eastham JA. Positive surgical margins at partial nephrectomy: predictors and oncological outcomes. J Urol. 2008;179:2158–2163. doi: 10.1016/j.juro.2008.01.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kutikov A, Uzzo RG. The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol. 2009;182:844–853. doi: 10.1016/j.juro.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 54.Ficarra V, Novara G, Secco S. Preoperative aspects and dimensions used for an anatomical (PADUA) classification of renal tumours in patients who are candidates for nephron-sparing surgery. Eur Urol. 2009;56:786–793. doi: 10.1016/j.eururo.2009.07.040. [DOI] [PubMed] [Google Scholar]


