Summary
Background
Allergen-specific immunotherapy favours immune deviation from a Th2 to a Th1 response and increases the number of regulatory T cells (Tregs). Epicutaneous immunotherapy (EPIT) of sensitized mice decreases the clinical and the allergen-specific Th2 responses and increases local and peripheral Foxp3+ Tregs.
Objective
To investigate the role of Tregs in EPIT and characterize their phenotype and maintenance following EPIT.
Methods
Tregs were investigated using in vivo depletion or adoptive transfer into BALB/c mice. Tregs were depleted using anti-CD25 antibody injection during EPIT, and allergen-specific responses were compared with Sham, EPIT alone and naïve mice. To demonstrate that Tregs can mediate protection by their own, and to study their maintenance following the end of EPIT, CD25+CD4+ Tregs isolated from mice just after or 8 weeks after EPIT were transferred into peanut-sensitized mice. Foxp3-IRES-mRFP mice were transferred with EPIT-induced Tregs to analyse the induction of host Tregs.
Results
The anti-CD25 antibody injection to EPIT mice abrogated the induction of Tregs in spleen and the expression of Foxp3 in oesophagus. This resulted in levels of peanut-induced eosinophilic infiltration in oesophagus similar to Sham and significantly higher than EPIT. Whereas the transfer of Tregs from Sham-treated mice demonstrated no effect, the transfer of Tregs isolated just after EPIT prevented peanut-induced eosinophil infiltration and eotaxin expression and induced Foxp3 in oesophagus. The transfer of Tregs isolated 8 weeks after EPIT suppressed allergen-specific responses as efficiently as did Tregs isolated just after EPIT and increased spleen Foxp3+ CD25+ CD4+ cells similarly. The use of reporter mice demonstrated an increase in host Tregs.
Conclusions
These results confirm the Tregs-mediated mechanism of EPIT and demonstrate the persistence of efficient Tregs during a long period of time after treatment cessation. This suggests that EPIT induces long-term tolerance in peanut-sensitized mice.
Keywords: epicutaneous immunotherapy, peanut allergy, regulatory T cells
Introduction
The aim of allergen-specific immunotherapy (SIT) is to induce desensitization (i.e. reduced immune reactivity) or even definitive absence of reactivity (tolerance) to the concerned allergen. This is mediated by the generation of allergen non-responsive or allergen-tolerant T cells 1, as manifested by decreased IgE and increased IgG4 levels. SIT was shown to induce T cell tolerance (i.e. reduced allergen-specific response of PBMCs), to favour immune deviation from Th2 to Th1 responses 2,3 and to increase the number of regulatory T cells (Tregs) 4-6. The latter could be pivotal in the physiological immune response to allergens 7, and the increase in Tregs was recently suggested for the monitoring or as a prognosis biomarker for the clinical response to sublingual immunotherapy (SLIT) 8.
Encouraging results on epicutaneous immunotherapy (EPIT) have been reported in the treatment for various allergies. Using a specific epicutaneous delivery system (EDS) (Viaskin®, DBV-Technologies, Paris, France), Dupont et al. delivered allergen to treat children with severe allergy to cow's milk 9. Application of this EDS on intact skin enhances the skin hydration and results in allergen diffusion through the superficial layers of the skin. This is followed by an uptake of the allergen by skin dendritic cells, without free passage through the skin/dermis 10. The EDS has been proposed for the treatment of food allergy.
Preclinical data in peanut-sensitized mice in a model of allergic gut inflammation demonstrated that Viaskin® allows desensitization and protection during subsequent peanut oral exposure 11–13. This study also showed that protection against oesophagus injuries was associated with increased Foxp3 mRNA expression in the oesophageal mucosa. In ovalbumin-sensitized mice, EPIT induced a significant increase in peripheral CD4+CD25+Foxp3+ T cell levels 10.
Clinical trials and experimental models both showed that specific immunotherapy induces IL-10+ regulatory T cells 4,14,15. EPIT increases local and systemic Foxp3+ Tregs 10,11 and actually proved beneficial on the different routes of allergen exposure: bronchial hyperresponsiveness 12, eosinophils recruitment in skin 10 or peanut-induced gut inflammation 11. Therefore, the induction of Tregs by EPIT may establish a global tolerance. Previous studies showed an association between SIT and the induction of Tregs (for review see 16), but the precise role of Tregs in SIT is imprecise.
CD4+ Tregs consist of either naturally occurring Tregs (nTregs) or inducible Tregs (iTregs) (for review see 17 and 18). Among iTregs, different subsets have been described and differ in the way they can suppress the immune response. These include IL-10-producing Tr1 cells (Tr1), TGF-β-inducing Th3 cells and CD4+CD25+Foxp3+ Tregs (Foxp3+), which have been implicated in the regulation of allergies 19. Subcutaneous immunotherapy (SCIT), as well as SLIT, induces a transient increase in Tr-1 followed by an immune deviation towards the Th1 response 4,14,15. Foxp3 is critical for the stability of Tregs 20 and allows CD4+CD25+Foxp3+ Tregs to persist potentially for a longer period of time. EPIT-induced IL-10+ Tr1 and Foxp3+ Tregs both could play a major role in the long-term maintenance of these cells and therefore in the induction of tolerance.
Here, we show that depleting Tregs using an anti-CD25 antibody erased the beneficial effect of EPIT and that the protection offered by EPIT-induced Tregs could be transferred to untreated sensitized mice. This clearly suggests a pivotal role for these cells in the acquisition of tolerance. Moreover, EPIT induced both effector and naïve Foxp3+ Tregs, but not IL-10+ Tr1 cells. The suppressive activity of EPIT-induced Tregs is thus IL-10 independent and partially acts through CTLA-4. Finally, Tregs maintained a suppressive activity long after the end of EPIT, which confirms that EPIT can be a powerful treatment for food allergies.
Methods
Animals
Three-week-old female BALB/c mice, C57BL/6 (Charles Rivers, Lyon, France), and Foxp3-IRES-mRFP mice (C57BL/6J-Foxp3tm1flv, Genoway, Lyon, France) were purchased and housed under standard animal husbandry conditions. All experiments were performed according to the European Community rules on animal care, with permission 92–305 from the French Veterinary Services.
Study design
The first study (Fig. 1a) was conducted in BALB/c mice and comprised three phases: sensitization, EPIT and sustained oral ingestion of peanut. A naïve group (n = 8) remained unsensitized and untreated. Sensitization was performed as previously described 11 using six intragastric gavages once a week during 6 weeks with 200 μL with 1mg of peanut proteins extract (PPE) (Greer laboratories, Lenoir, NE, USA) mixed with 10 μg cholera toxin (CT – Servibio, USA). Following sensitization, and before oral exposure to the allergen, mice were allocated to five different treatment groups: (1) the EPIT group (n = 8) received active EPIT during 8 weeks; (2) the EPIT + αCD25 group (n = 8) received active EPIT and intraperitoneal injection with 100 μg anti-mouse CD25 antibody (Clone: PC61.5, eBioscience, France) 24 h before each application of the EDS; (3) the EPIT + isotype group (n = 8) received active EPIT and intraperitoneal injection with 100 μg isotype control antibody (eBioscience) 24 h before each application of the EDS; (4) the Sham group (n = 8) received placebo treatment; and (5) the Sham + αCD25 group (n = 8) received placebo treatment and intraperitoneal injection of 100 μg anti-mouse CD25 antibody at the same time as the EPIT + αCD25 group. Following treatment, animals were orally exposed to a 10-day sustained oral exposure to peanuts, leading to eosinophilic infiltration in the oesophagus as previously described 11. Eight (8) naïve (control) mice were also challenged upon the same procedure. The day after the last challenge, mice were killed for organ recovery.
Figure 1.
Study design of experiments. (a) BALB/c mice were sensitized to peanut proteins. Then, mice were treated by active EPIT, EPIT + αCD25, EPIT + isotype control antibody, αCD25 alone (Sham + αCD25) or placebo (Sham) during 8 weeks. Naïve mice remained unsensitized and untreated. Following treatment, animals were submitted to a 10-day sustained oral exposure to peanuts. The day after the last challenge, mice were killed for organ recovery. (b) BALB/c mice were sensitized and treated as described above (donor mice). After 8 weeks of treatment, donor mice were killed, and the CD4+CD25+ or CD4+CD25− T cells were sorted from spleen cells of each group and transferred into peanut-sensitized non-treated mice. Three days after the transfer, mice were submitted to a 10-day sustained oral exposure to peanuts using the same protocol. (c) BALB/c mice were sensitized and treated as described above (EPIT, Sham, naïve). After treatment, the PPE-specific cytokine response of splenocytes in the presence of blocking antibodies and the Tregs phenotype in spleen were analysed. (d) BALB/c mice were sensitized and treated as described above (donor mice: EPIT n = 40). After treatment (n = 20) or 8 weeks after the end of treatment (n = 20), donor mice were killed, and the CD4+CD25+ T cells were sorted from spleen cells and transferred into peanut-sensitized non-treated mice. Three days after the transfer, mice were submitted to a 10-day sustained oral exposure to peanuts using the same protocol. (e) C57BL/6 mice were sensitized and treated as described above (donor mice: EPIT n = 20). After 8 weeks of treatment, donor mice were killed, and the CD4+CD25+ T cells were sorted from spleen cells and transferred into peanut-sensitized non-treated Foxp3-IRES-mRFP mice (n = 8). Two weeks after the transfer, the PPE-specific cytokine response of splenocytes and expression of mRFP were compared to that of non-transferred mice (n = 8). EPIT or Sham-treated C57BL/6 mice, as well as naïve mice, were used as a control of EPIT efficacy in WT mice.
In a second study (Fig. 2b), BALB/c donor mice were sensitized and treated as above described (EPIT n = 20, Sham n = 20). Donor mice were killed after 8 weeks of treatment. The CD4+CD25+ or CD4+CD25− T cells were sorted from spleen cells of each group and transferred into peanut-sensitized non-treated mice. Four groups (n = 8) were adoptively transferred: two groups received CD4+CD25+ T cells, respectively, from EPIT and Sham, and two groups received CD4+CD25− T cells, respectively, from EPIT and Sham. Three days after the transfer, mice were submitted to a 10-day sustained oral exposure to peanuts using the same protocol as described in Study 1.
Figure 2.
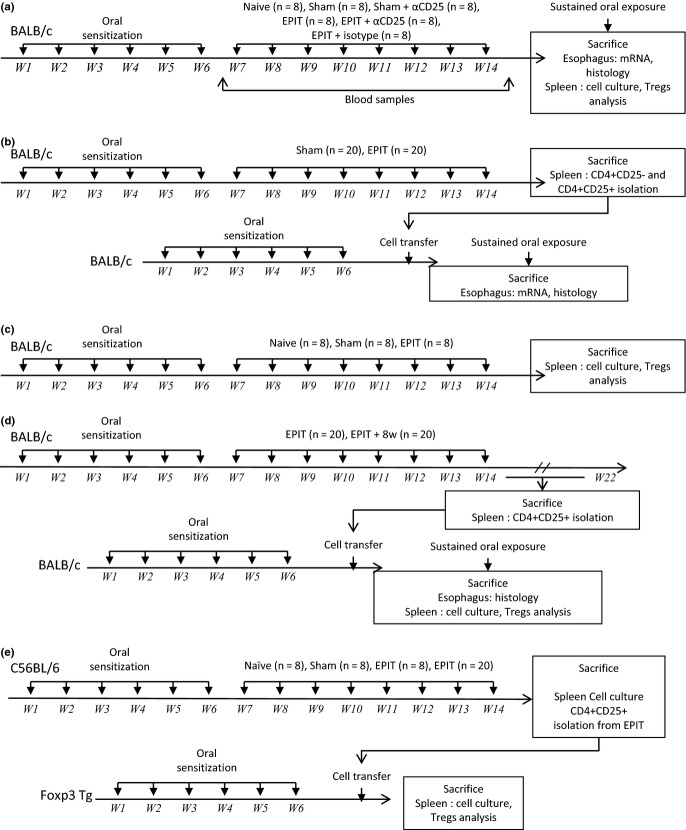
EPIT-induced desensitization was blocked by anti-CD25 antibodies. (a) Quantity of peanut-specific IgE (left panel) and IgG2a for each group after sensitization, before EPIT (d42) and after the treatment period (d104). (b) Measurement of IL-5 and IFN-γ secretion by splenocytes collected from each group of mice immediately after killing. Splenocytes were stimulated with peanut for 72 h. Cytokines were measured using Bioplex. (c) Proportion of Tregs in the spleen of mice from each group. Sham: peanut-sensitized untreated mice; EPIT: peanut-sensitized mice treated by EPIT; EPIT + αCD25: peanut-sensitized mice treated by EPIT and anti-CD25 antibody. Data are shown as means ± SEM of three independent experiments for each group of mice (n = 8 in each group). *P < 0.05, **P < 0.01, ***P < 0.001.
To characterize the phenotype and mechanism of action of EPIT-induced Tregs, 24 BALB/c mice were sensitized and treated as above described (EPIT n = 8, Sham n = 8, naïve n = 8). After the treatment, the PPE-specific cytokine response of splenocytes in the presence of blocking antibodies and the Tregs phenotype in spleen were analysed (Fig. 1c).
To investigate the maintenance of protective Tregs, BALB/c mice were sensitized and treated as above described (donor mice: EPIT n = 40). Donor mice were killed after the treatment (n = 20) or 8 weeks after the end of the treatment (n = 20). CD4+CD25+ T cells were sorted from spleen cells and transferred into peanut-sensitized non-treated mice. Three days after the transfer, mice underwent a 10-day sustained oral exposure to peanuts using the same protocol as in Study 1 (Fig. 1d).
To analyse the possible induction of host Tregs by the transfer of EPIT-induced Tregs, C57BL/6 mice were sensitized and treated as above described (donor mice: EPIT n = 20). Donor mice were killed after 8 weeks of treatment. The CD4+CD25+ T cells were sorted from spleen cells and transferred into peanut-sensitized non-treated Foxp3-IRES-mRFP mice (n = 8). Two weeks after the transfer, the PPE-specific cytokine response of splenocytes and the expression of mRFP were compared with non-transferred mice (n = 8). EPIT- or Sham-treated C57BL/6 mice, as well as naïve mice, were used as control of EPIT efficacy in wild-type (WT) mice (Fig. 1e).
All experiments were reproduced two or three times with similar results.
Epicutaneous immunotherapy and antibody treatment
Epicutaneous immunotherapy was performed using a patented epicutaneous delivery system (EDS), Viaskin® (DBV Technologies, Paris, France) 12. The EDS loaded with 100 μg of peanut protein was applied for 48 h once a week onto the back of mice, which hair had been previously removed. Skin preparation and EDS application were all performed under general anaesthesia using ketamine (Imalgen1000, Merial) (100 mg/kg body weight) and xylazine (Rompun®, Bayer) (10 mg/kg body weight).
Cell sorting and adoptive transfer
Spleens were teased into a single-cell suspension and washed in 10% heat-inactivated FCS in RPMI. After red blood cell lysis, splenocytes were washed three times in RPMI-1640 (Gibco, France). CD4+ T cells were enriched by depletion, and CD4+CD25+ and CD4+CD25− T cells were then sorted using CD4+CD25+ Regulatory T Cell Isolation Kit (Miltenyi Biotec), according to the manufacturer's instructions. Cells were counted and adjusted to inject 5.105 cells in 100 μL of PBS intravenously in tail vein of mice. Sorting purity was checked by flow cytometry and was over 90%.
Histological analysis of the oesophagus
Oesophagi were fixed in 4% neutral buffered formalin, embedded in paraffin wax, transversally cut into 5-μm-thick sections, fixed to positive charge slides and stained using a routine haematoxylin–eosin–safranin staining method (HES).
Three sections of oesophagus were analysed by blind reading. Image analysis was performed on oesophagus sections using a digital camera (Leïca DFC 420C, Nanterre, France) combined with image analysis software (Leïca LAS Software). Six high-powered fields were randomly selected around the oesophageal lumen, and eosinophil counts expressed in number of eosinophils/mm2.
Real-time PCR
Total RNA from oesophagus segments was extracted using the RNeasy Mini Kit (Qiagen, Courtaboeuf, France) according to the manufacturer's instructions. The concentration of RNA was determined, and complementary DNA (cDNA) was synthesized using 500 ng RNA through a reverse transcription reaction (SuperScript II RNase H reverse transcription reagents, Invitrogen, Cergy-Pontoise, France). Real-time PCR quantitative mRNA analyses were performed as previously described 11. The results were demonstrated as mRNA expression. Calculations to determine the relative level of gene expression were made by reference to the β-actin and SDHA in each sample, using the ΔCq method, and the results were depicted as arbitrary units. Negative controls without RNA and without retrotranscriptase were also performed.
Blood-specific IgE and IgG2a
Blood was collected from the retro-orbital venous plexus after sensitization (day 42) and after 8 weeks of EPIT (day 104) under isoflurane (Isoflurane Belamont, Nicholas Piramal, India). Specific antibodies were quantified using a quantitative ELISA developed in-house according to the 2001 FDA guidelines, as already published 12. Briefly, plasma samples were incubated into microtitre plates coated with peanut protein extract. The presence of specific IgE (sIgE) and specific IgG2a (sIgG2a) was detected by the addition of an anti-mouse IgE or IgG2a antibody labelled with phosphatase alkaline (Serotec, Oxford, England). Reagent (pNPP – Sigma, France) was used as an enzyme substrate, and optical density was measured at 405 nm.
Cytokine production
After treatment, spleens were teased into a single-cell suspension and washed three times in RPMI-1640 (Gibco, France). Cells were counted, and 2 × 106 cells were incubated in a 24-well microtitre plate (Nunc) in 1 mL of medium alone or medium with peanut protein (100 μg/mL). In some experiments, mouse-specific anti-IL-10 or anti-CTLA-4 blocking antibodies (4 μg/mL) were added to the culture to assess the respective contribution of these pathways on the suppression of allergen-specific responses. Supernatants were harvested after 72 h and analysed for the presence of cytokines (IL-5, IL-13, IL-10 and IFN-γ) using the Bio-Plex® system (Bio-Rad, Marnes-la-Coquette, France) according to the manufacturer's instructions. In some experiments, cells were harvested after culture, and expression of CD86 on dendritic cells was analysed by flow cytometry.
Regulatory T cell measurement
For Tregs analysis, spleen cells were stained with different combination of the following antibodies: anti-mouse CD4-PerCP-Cy5.5, CD25-FITC, IL10-PE, CD62L-APC, CD44-APC-Cy7 (all from BD Biosciences, Le Pont de Claix, France), CTLA-4-PE, PD-1-efluo450, Foxp3-PE and Foxp3-APC (from e-Bioscience) or control isotype. Intracellular staining with anti-IL-10 and anti-Foxp3 antibodies was performed after fixation and permeabilization, using the cytofix/cytoperm (BD Bioscience, Le Pont de Claix, France) and Foxp3 Fixation/Permeabilization kits (eBioscience), respectively. Flow cytometry was performed on Canto II and analysed using FlowJo software (TreeStar, Inc. Ashland, OR, USA). For analysis of Tregs, cells were gated on lymphocytes using FCS/SSC, and the percentages of CD4+CD25+Foxp3+ or CD4+CD25+IL-10+ cells were measured. Proportion of CD44hi/CD62L−, CD44lo/CD62L+, CTLA-4+ and PD1+ cells was analysed in CD4+CD25+Foxp3+ cells.
Statistical analysis
The GraphPad Prism Software 5.0 (San Diego, CA, USA) was used for statistical analysis. Results are expressed as mean ± standard deviation (SD) of three independent experiments. For histological analyses, antibodies levels, mRNA expression and cytokine responses, statistical significance comparing different sets of mice was determined using the Mann–Whitney U-test.
Results
Inhibition of EPIT by anti-CD25 antibody at systemic level
As already described in this model 11, a slight decrease in sIgE was observed in Sham-treated mice over the 8-week period of time. However, following EPIT, this decrease was significantly higher (P < 0.001 vs. Sham) (Fig. 2a) and was accompanied by increased peanut-sIgG2a (P < 0.001 vs. Sham). After EPIT, peanut-specific IL-5 production by splenocytes decreased significantly as compared with Sham (1.07 ± 0.23 vs. 2.95 ± 0.65 μg/mL, respectively, P < 0.05). IFN-γ production did not vary (Fig. 2b). As compared with Sham, EPIT significantly increased the proportion of CD4+CD25+Foxp3+ T cells in the spleen of peanut-sensitized mice (9.72 ± 0.63 vs. 6.78 ± 1.06%, respectively, P < 0.05) (Fig. 1c). In mice receiving intraperitoneal anti-CD25 antibody injections during EPIT (i.e. 24 h prior to any application of the EDS), the level of sIgE and sIgG2a (Fig. 2a) and the level of the Th2 cytokines (Fig. 2b) were not influenced by the treatment, as compared with EPIT alone. In addition, the proportion of CD4+CD25+Foxp3+ spleen cells was lowered (5.21 ± 0.41 vs. 9.72 ± 0.63%, respectively, P < 0.05) (Fig. 2c). Sham mice treated with anti-CD25 antibodies and EPIT mice treated with isotype control did not differ from Sham or EPIT alone, respectively (Fig. 2).
Inhibition of EPIT in the oesophageal mucosa by anti-CD25 antibodies
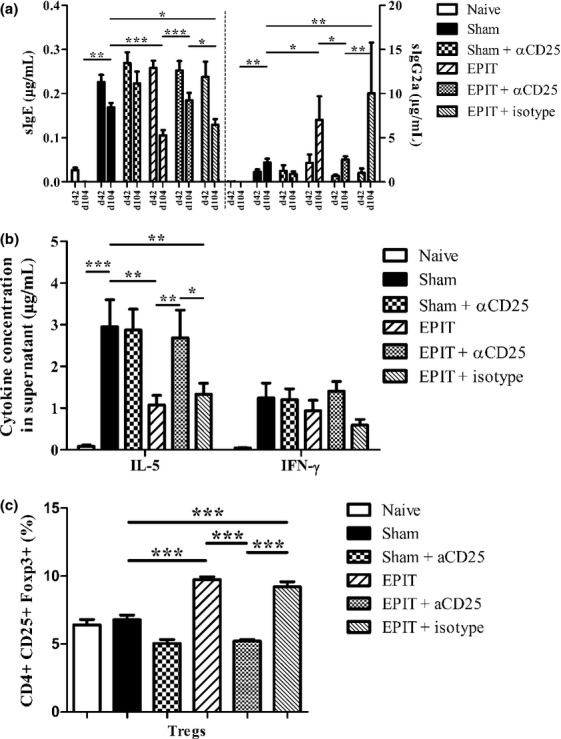
The inflammation of the oesophageal mucosa induced by oral peanut diet was lower in EPIT-treated mice, as compared with Sham. This was evidenced by lower eosinophilic infiltration (11.3 ± 4.2 vs. 36.4 ± 5.6 eosinophils/mm2, respectively, P < 0.05) (Fig. 3a and b), and lower expression of eotaxin (P < 0.05) and IL-5 (P = 0.061) mRNAs. Both reached levels similar to those of naïve mice (Fig. 3c and d). Expression of Foxp3 mRNA in the oesophageal mucosa was higher in EPIT than in Sham mice (P < 0.05) (Fig. 3E), suggesting a role for Tregs. In mice treated with anti-CD25 antibodies, the oesophageal inflammation induced by peanut diet was not prevented by EPIT, leading to oesophageal eosinophil counts significantly higher than with EPIT alone (38.9 ± 7.1 vs. 11.3 ± 4.2 eosinophils/mm2, respectively, P < 0.05) (Fig. 3a and b). The expression of eotaxin in the oesophagus was increased (P < 0.05) and, albeit not statistically significant, IL-5 expression reached a level similar to that of Sham mice (Fig. 3c and d). The blockage of CD25 also inhibited the induction of Foxp3 in the oesophagus of EPIT-treated mice (P < 0.05). The level of Foxp3 in EPIT + anti-CD25 mice was similar to that of Sham mice (Fig. 3e). Anti-CD25 did not modify the oesophageal eosinophil infiltration in Sham mice, and isotype control injection did not show any modification of EPIT efficacy (Fig. 3).
Figure 3.
EPIT modulated oesophagus immunity in mice orally exposed to peanut. (a) Mice were exposed to oral peanut for 10 days, and oesophagi were harvested. Formalin-fixed, paraffin-embedded tissue sections were stained with haematoxylin–eosin–safranine. Representative histology picture of oesophagus for naïve, Sham, EPIT and EPIT + αCD25 mice are shown. (b) Measurements of eosinophils infiltration in oesophagus were realized by double-blind reading at a 40-high-powered field. Results are expressed as mean number of eosinophils per mm2 ± SEM for each group of mice. (c–e) mRNAs were quantified by real-time PCR in oesophagus segments collected 24 h after the end of oral challenge. Results are presented as mean mRNA expression ± SEM of three independent experiments for each group of mice. The relative levels of IL-5 (c), eotaxin (d) and Foxp3 (e) expression were calculated by reference to the SDHA and the β-actin in each sample. Sham: peanut-sensitized untreated mice; EPIT: peanut-sensitized mice treated by EPIT; EPIT + αCD25: peanut-sensitized mice treated by EPIT and anti-CD25 antibody. *P < 0.05, **P < 0.01.
The effect of EPIT on oesophagus inflammation resides in CD4+CD25+ T cells
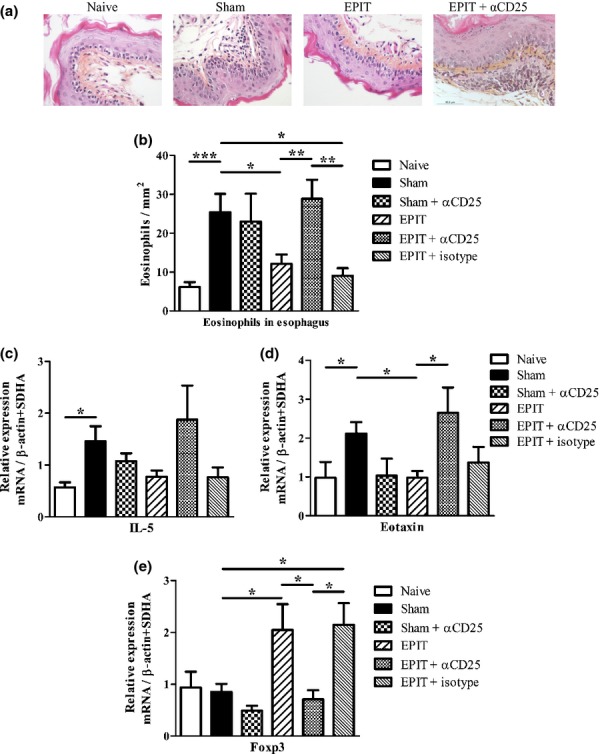
In mice sensitized to peanut, the transfer of either CD4+CD25+ or CD4+CD25− T cells from the Sham group did not influence the oesophageal eosinophil infiltration after peanut diet (72.1 ± 22 and 94.9 ± 28.6 eosinophils/mm2, respectively), as compared with non-transferred sensitized mice (69.4 ± 19.0). By contrast, the transfer of CD4+CD25+ T cells from the EPIT group to peanut-sensitized mice strongly influenced the oesophagus inflammation consecutive to oral peanut exposure and prevented eosinophilic infiltration in the oesophagus (12.2 ± 4.1 eosinophils/mm2), as compared with non-transferred sensitized mice (P < 0.01) (Fig. 4a). CD4+CD25− T cells transferred from the EPIT group did not influence the response to the peanut diet.
Figure 4.
Modulation of the oesophagus immunity by cell transfer into mice orally exposed to peanut. (a) Mice were sensitized and transferred with either CD4+CD25+ or CD4+CD25− T cells from either EPIT- or Sham-treated mice and were compared with non-transferred sensitized mice. Three days after transfer, mice were exposed to oral peanut for 10 days and oesophagi were harvested. Formalin-fixed, paraffin-embedded tissue sections were stained with haematoxylin–eosin–safranine, and measurements of eosinophils infiltration in oesophagus were realized by double-blind reading at a 40-high-powered field. Results are expressed as mean number of eosinophils per mm2 ± SEM for each group of mice (n = 8 in each group). (b–d) mRNAs were quantified by real-time PCR in oesophagus segments collected 24 h after the end of oral challenge. Results are demonstrated as mean mRNA expression ± SEM of two independent experiments for each group of mice. The relative levels of IL-5 (b), eotaxin (c) and Foxp3 (d) expression were calculated by reference to the SDHA and the β-actin in each sample. *P < 0.05, **P < 0.01.
As compared with non-transferred sensitized mice, IL-5 and eotaxin mRNA expressions in the oesophagus were significantly lower in sensitized mice transferred with CD4+CD25+ T cells from EPIT mice (P < 0.05), but not in mice transferred with CD4+CD25+ T cells or CD4+CD25− T cells from Sham mice, or with CD4+CD25− T cells from EPIT mice (Fig. 4b and c).
The transfer of CD4+CD25+ T cells from EPIT mice resulted in a significant induction of Foxp3 in the oesophagus after oral peanut exposure, as compared with non-transferred mice (P < 0.05). Likewise, oral peanut exposure did not induce any Foxp3 expression in mice that received CD4+CD25− T cells from EPIT or whatever cells from Sham (Fig. 4d).
Phenotypic and functional characterization of EPIT-induced Tregs
Epicutaneous immunotherapy increased CD4+CD25+Foxp3+ cells but not CD4+CD25+IL-10+ cells, as compared with Sham or naïve mice (P < 0.01) (Fig. 5a). Following EPIT, both naïve (CD44lo/CD62L+) and effector (CD44hi/CD62−) Foxp3 Tregs increased significantly, as compared with Sham and naïve mice (P < 0.001) (Fig. 5b). EPIT was also associated with a significant increase in both induced (CD304−) and natural (CD304+) Tregs, as compared with Sham and naïve mice (P < 0.01) (Fig. 5c). As compared with Sham and naïve mice, EPIT did not modify the proportion of Foxp3+ Tregs cells that expressed PD-1, but induced a higher proportion of CTLA-4+ Tregs (P < 0.01 vs. Sham and P < 0.05 vs. naïve mice) (Fig. 5d).
Figure 5.
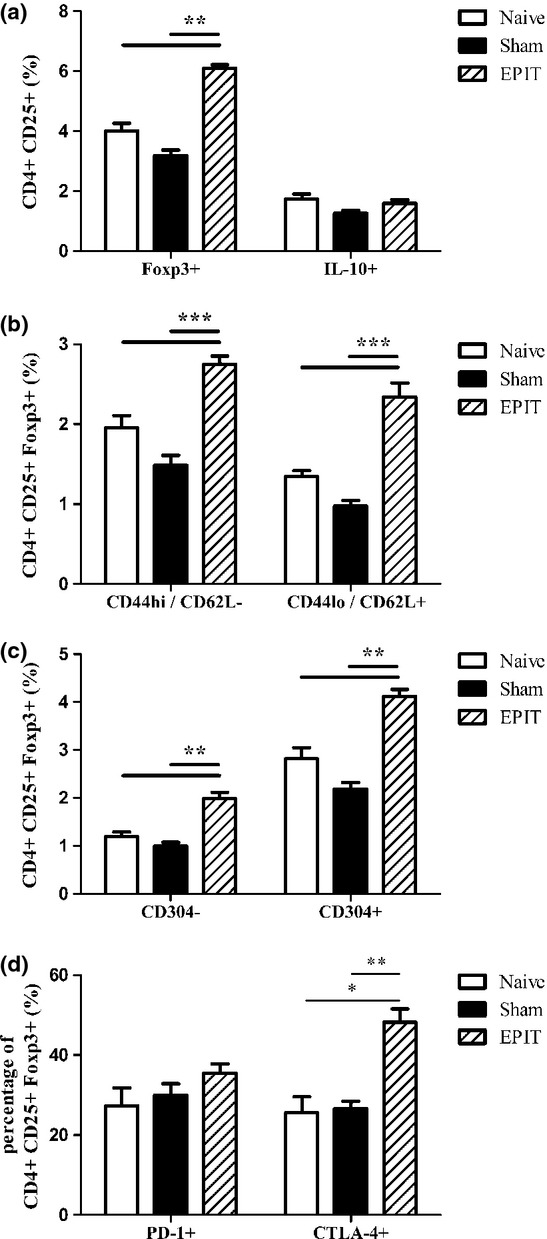
Phenotypic characterization of Tregs. Sensitized mice were EPIT- or Sham-treated for 8 weeks. At the end of treatment, spleen cells were harvested for flow cytometry analysis. (a and b) Cells were gated on CD4+ among the lymphocyte identified by FSC/SSC, the percentages of CD25+Foxp3+ or CD25+IL10+ (a), and percentages of CD25+Foxp3+CD44hiCD62L− or CD25+Foxp3+CD44loCD62L+ (b), and of CD25+Foxp3+CD304+ or CD25+Foxp3+CD304− (c) were analysed. (d) Among the lymphocyte identified by FSC/SSC, cells were gated on CD4+CD25+Foxp3+, and the percentage of cells expressing CTLA-4 and PD-1 was analysed. Data are shown as means ± SEM of three independent experiments for each group of mice (n = 8 in each group). *P < 0.05, **P < 0.01, ***P < 0.001
After EPIT, peanut-specific IL-5 and IL-13 production by splenocytes decreased significantly (P < 0.05 and P < 0.001 vs. Sham, respectively) (Fig. 6a and b), whereas IL-10 and IFN-γ production did not vary significantly (Fig. 6c and d). No production of TGF-β was observed (data not shown). Anti-IL-10 antibodies did not inhibit the suppressive effect observed in EPIT (Fig. 6). This lack of effect of anti-IL-10 antibodies demonstrated that EPIT inhibition was not IL-10 dependent as the concentration used was able to block the inhibitory effect of Tr1 cells (data not shown). By contrast, the injection of anti-CTLA-4 antibodies during the restimulation of splenocytes completely restored the production of IL-5 and partially restored the production of IL-13 (Fig. 6). Both anti-IL-10 and anti-CTLA-4 antibodies slightly increased the cytokine production by unstimulated splenocytes, without difference between groups (data not shown). Consistent with a CTLA-4-dependent mechanism, the allergen-specific activation of cultured dendritic cells (i.e. CD86 expression) decreased with EPIT (P < 0.05 vs. Sham) (Fig. 6e).
Figure 6.
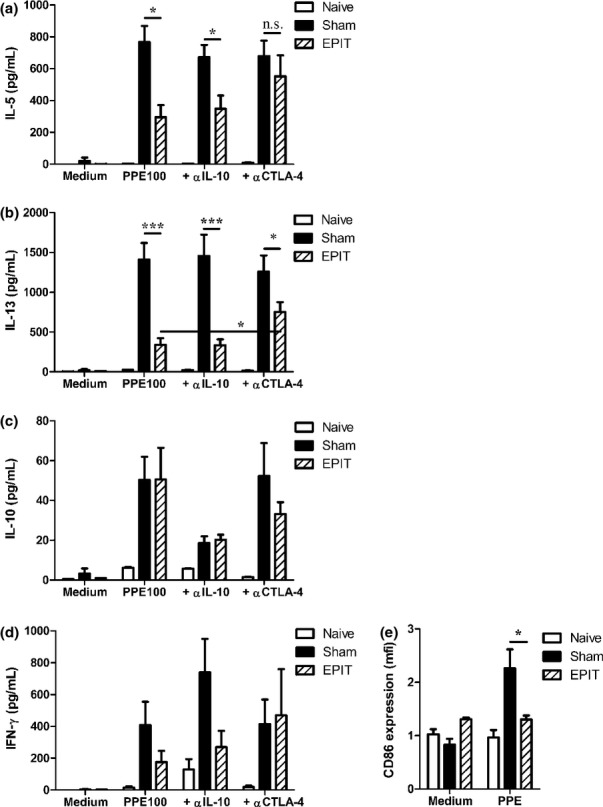
Mechanism of allergen-specific suppression by EPIT. Sensitized mice were EPIT- or Sham-treated for 8 weeks. At the end of the treatment, spleen cells were harvested and stimulated with peanut in the presence or absence of anti-IL-10 or anti-CTLA-4 blocking antibodies for 72 h. IL-5 (a), IL-13 (b), IL-10 (c) and IFN-γ (d) in supernatant were measured using Bioplex. (e) Cells were collected after culture and stained with anti-CD11c and CD86 antibody for flow cytometry analysis. mfi: mean intensity of fluorescence. Data are shown as means ± SEM of two independent experiments for each group of mice (n = 8 in each group). *P < 0.05, ***P < 0.001
Long-term maintenance of EPIT-induced Tregs after the end of treatment
To investigate the maintenance after the end of treatment of the suppressive capacity of Tregs induced by EPIT, CD4+CD25+ T cells were sorted from spleen cells of EPIT mice 8 weeks after the end of EPIT and then adoptively transferred intravenously to untreated peanut-sensitized mice. As previous experiments showed no effect of the transfer of CD4+CD25+ cells from Sham mice, and for ethical reasons, we focused on CD4+CD25+ cells of EPIT mice. The oesophageal inflammation after oral exposure to peanut and the allergen-specific response in receivers was compared with the response of non-transferred mice and of mice transferred with CD4+CD25+ T cells isolated just after the end of EPIT. As previously observed, the transfer of CD4+CD25+ T cells from EPIT-treated mice prevented the recruitment of eosinophils in the oesophagus after peanut oral exposure (P < 0.001) (Fig. 7a). The transfer of CD4+CD25+ T cells from EPIT-treated mice also induced a low but significant increase in the proportion of spleen Tregs in recipient mice P < 0.01 (Fig. 7b). This was accompanied by a significantly decreased peanut-specific production of Th2 cytokines (i.e. IL-5 and IL-13, P < 0.01 and P < 0.05, respectively) (Fig. 7c and d), a trend towards decreased production of IL-10 (P = 0.0813) (Fig. 7e) and no modification of IFN-γ production (Fig. 7f). Peanut-specific antibodies were not modified at the time of killing in transferred mice. This was probably due to the timing between cell transfer and killing of mice, too short to allow detectable changes in antibodies.
Figure 7.
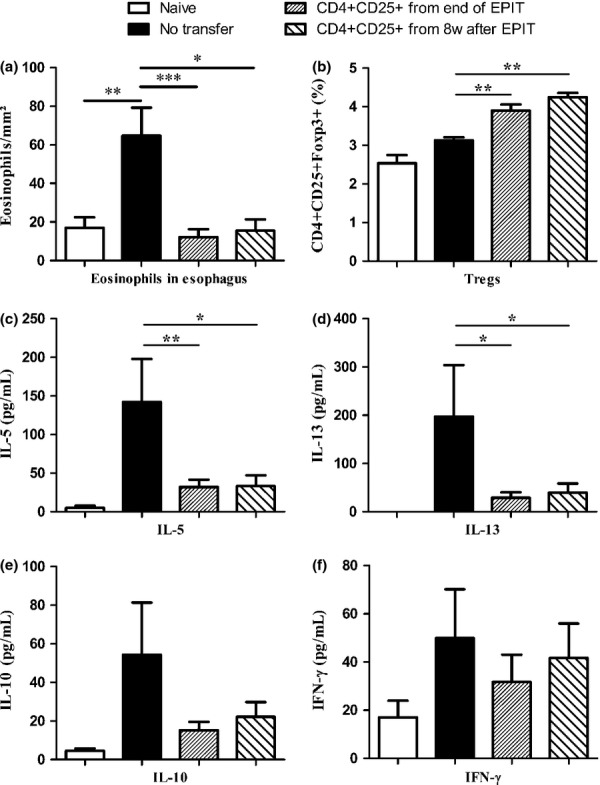
Long-term maintenance of Tregs after discontinuation of EPIT. Mice were sensitized and transferred with CD4+CD25+ T cells isolated just after or 8 weeks after the end of EPIT and were compared with non-transferred sensitized mice. Mice were exposed to oral peanut for 10 days, and oesophagi were harvested. (a) Formalin-fixed, paraffin-embedded tissue sections were stained with haematoxylin–eosin–safranine, and measurement of eosinophils infiltration in oesophagus was realized by double-blind reading at a 40-high-powered field. Results are expressed as mean number of eosinophils per mm2 ± SEM for each group of mice. (b) Proportions of Tregs were analysed in the spleen of naïve mice (white bar), peanut-sensitized non-transferred mice, peanut-sensitized mice transferred with CD4+CD25+ T cells isolated just after the end of EPIT and peanut-sensitized mice transferred with CD4+CD25+ T cells isolated 8 weeks after the end of EPIT. (c–f) Splenocytes were stimulated with peanut for 72 h. Concentrations of IL-5 (c), IL-13 (d), IL-10 (e) and IFN-γ (f) were measured in supernatants using Bioplex. Data are shown as means ± SEM of three independent experiments for each group of mice (n = 8 in each group). *P < 0.05, **P < 0.01, ***P < 0.001.
The transfer of CD4+CD25+ T cells isolated 8 weeks after the end of EPIT prevented the infiltration of eosinophil in the oesophagus, as efficiently as cells isolated just after the end of treatment did (P < 0.05 vs. non-transferred recipients) (Fig. 7a). It also increased the proportion of Tregs (P < 0.01 vs. non-transferred recipients) (Fig. 7b). These CD4+CD25+ T cells significantly inhibited the peanut-specific production of the IL-5 and IL-13 Th2 cytokines (P < 0.05) (Fig. 7c and d), showed a tendency towards inhibited production of IL-10 (Fig. 7e) and did not modify the production of IFN-γ (Fig. 7f). This demonstrates that EPIT-induced Tregs persisted after the end of treatment and kept their suppressive capacity.
CD4+CD25+ from EPIT mice increased host Tregs
The transfer of CD4+CD25+ T cells from EPIT-treated mice induced a low but significant increase in the proportion of Tregs in the spleen of recipient mice. To evaluate the origin of these increases in Tregs and to determine whether EPIT-induced Tregs could induce the increase in host Tregs, CD4+CD25+ cells were transferred into Foxp3-IRES-mRFP mice, in which host Tregs could be followed by the expression of mRFP.
Because reporter mice were on a C57BL/6 background, the efficacy of EPIT in C57BL/6 mice was confirmed by the decreased Th2 cytokine production of restimulated splenocytes of EPIT mice (Fig. 8a). Furthermore, the transfer of CD4+CD25+ T cells from EPIT-treated C57BL/6 mice induced a significant decrease in the peanut-induced production of Th2 cytokines (i.e. IL-5 and IL-13, P < 0.05), which was as efficient as the EPIT treatment of WT mice (Fig. 8a). The proportion of CD4+CD25+mRFP+ cells in the spleen of mice that received CD4+CD25+ from EPIT WT mice increased significantly (P < 0.01 vs. non-transferred mice) (Fig. 8b). As only host Tregs express mRFP, increased CD4+CD25+mRFP+ suggests that EPIT-induced Tregs could promote a de novo induction of Tregs.
Figure 8.
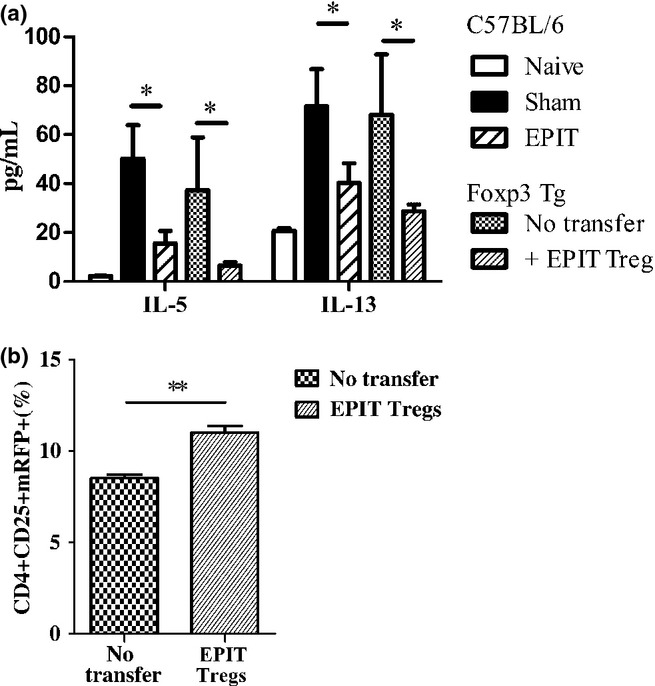
Transfer of EPIT-induced Tregs increase host Tregs. C57BL/6 mice were sensitized and treated by EPIT (n = 20). After 8 weeks of treatment, donor mice were killed, and the CD4+CD25+ T cells were sorted from spleen cells and transferred into peanut-sensitized non-treated Foxp3-IRES-mRFP mice (n = 8). Two weeks after transfer, the PPE-specific cytokine response of splenocytes and expression of mRFP were compared to that of non-transferred mice (n = 8). EPIT- or Sham-treated C57BL/6 mice as well as naïve mice were used as control of EPIT efficacy in C57BL/6 WT mice. (a) Splenocytes were stimulated with peanut for 72 h. IL-5 and IL-13 concentrations were measured in supernatants using Bioplex. (b) The proportions of CD4+CD25+mRFP+ Tregs were analysed in spleen of peanut-sensitized non-transferred Foxp3-IRES-mRFP mice and peanut-sensitized mice transferred with CD4+CD25+ T cells isolated from EPIT-treated C57BL/6 mice. Data are shown as means ± SEM for each group of mice (n = 8 in each group). *P < 0.05, **P < 0.01.
Discussion
Even if the role of Tregs in the allergic process remains unclear, clinical and experimental studies showed that they are generated during sublingual and subcutaneous specific immunotherapy (for review see 16). More recently, it was shown that Tregs are also induced by EPIT 9, in the spleen and mucosa of mice exposed to allergens 10,11. The present study clarifies the role of Tregs during EPIT and confirms their direct mediation in the desensitization of peanut-sensitized mice, and their protection from the digestive injuries induced by subsequent oral exposure to peanut. Moreover, EPIT induced a particular subset of Tregs that maintain a suppressive capacity for a long period of time following the end of treatment.
The role of Tregs in food allergy is still under investigation. Several studies suggest that the number or function of Tregs is impaired in allergic patients. Smith et al. showed a lower inhibitory capacity of Tregs in neonates who further developed an allergic disease 21. Accordingly, children who outgrew their allergy and became tolerant showed a higher proportion of Tregs and a decreased allergen-specific proliferation than children who did not 22,23. Higher frequencies of allergen-specific Tregs have been associated with a phenotype of mild clinical disease and favourable prognosis 24, and children with food allergy had a lower expression of Foxp3 and IL-10 than healthy children 22. Although Sicherer et al. 25 did not see any difference of Foxp3 or IL-10 expression between allergic and non-allergic children, Savilahti et al. 26 observed a higher expression of Foxp3 in children with persisting milk allergy. In animal models, the depletion of CD4+CD25+ T cells during allergic sensitization to peanut increases peanut-induced cytokine production, peanut-sIgE levels and response to challenge, even though it did apparently not affect mast cell responsiveness 27. In a model of cow's milk allergy in mice, Kanjaraw et al. also observed that the depletion of CD25+ cells before immunization enhanced BLG-specific CD4+ T cells and blood sIgE levels, which caused more severe symptoms upon BLG challenge 28. In our model, no difference was observed between naïve and sensitized mice in the frequency of Tregs. Moreover, the injection of anti-CD25 antibodies in Sham mice did not modify the different allergen-specific responses, and the transfer of cells isolated from Sham mice showed no effect on allergic responses. Tregs could thus have a role in controlling the allergic immune response during its initiation, whereas depletion of Tregs would be useless once sensitization is achieved. One limitation could be the putative depletion of activated T cells by anti-CD25 antibodies. However, the allergen-specific response in the Sham + anti-CD25 mice was not different from that observed in the Sham group, which indicates that the depletion of activated T cells is quite low in our model.
The role of Tregs in allergic diseases has been mostly suggested in immunotherapy studies. Clinical trials and experimental models showed that the frequency of IL-10+ regulatory T cells increases following specific immunotherapy 4,14,15. The proportion of Tregs, either Foxp3+ or CD25high cells, has also been investigated during venom immunotherapy 29,30. In peanut allergy, oral immunotherapy increased peanut-specific Foxp3 T cells during the 12 months following treatment, but the cells decreased to the pre-treatment level thereafter 31. On the contrary, Mori et al. did not see any modification of CD4+CD25+Foxp3+ cells during milk oral immunotherapy, questioning their role in this treatment 32. In our model, it has been demonstrated that EPIT increases both spleen and mucosal Foxp3+ cells 10,11. This difference could be due to the treatment route because some SCIT and SLIT studies did not evidence any increase in CD4+CD25+ Tregs 15,33,34. However, these studies differed in the methodology and markers used to define Tregs, underscoring difficulties to distinguish allergen-specific Tregs. The important role played by the EPIT-induced Tregs in our model is underlined by the ability of anti-CD25 antibody injection during EPIT to completely inhibit the induction of Tregs, as well as the inhibitory action of EPIT on sIgE, Th2-responses and peanut-induced oesophagus injuries. But this did not allow to directly conclude that the effect of EPIT solely resides in CD4+CD25+ T cells. As a consequence, CD4+CD25+ T cells from EPIT mice were adoptively transferred to peanut-sensitized mice before peanut oral exposure. This experiment showed that the suppressive activity of CD4+CD25+ T cells suffices to prevent the reaction to a subsequent oral allergen exposure. CD4+CD25+ T cells transferred from Sham mice had no effect on peanut-induced oesophagus injuries. This is consistent with the absence of any baseline regulatory capacity of CD4+CD25+ T cells, which need a prior induction by peanut EPIT. These results clearly demonstrate that the desensitization induced by EPIT resides in Tregs.
Whereas the above-mentioned nTregs develop during the normal process of T cell maturation in the thymus, iTregs develop in periphery, from mature T cells, under particular stimulatory conditions. Neuropilin 1 (CD304) has recently been proposed as a surface marker expressed by nTregs but not iTregs. In our study, we showed that EPIT induced both CD304+ nTregs and CD304− iTregs. Different subsets of iTregs have been described; the three most relevant classes being the IL-10-producing Tr1 cells, the TGF-β-producing Th3 cells and the CD4+CD25+ Tregs. Depending on the tissue, origin and stimulatory condition, the different subsets differ in their cytokine production and surface markers expression, and on how they can suppress immune responses. The suppressive capacity of Tr1 cells is IL-10 dependent, whereas CD4+CD25+ Tregs mediate suppression by cell–cell contact 35. Specific immunotherapy induces IL-10 and TGF-β production and increases the frequency of IL-10+ regulatory cells 4,14,15. Our model did not exhibit any increase in CD4+CD25+IL10+ cells or allergen-specific production of IL-10. Moreover, anti-IL-10 blocking antibodies did not reverse the inhibition of Th2 cytokine production, which clearly suggests that the suppressive activity of EPIT-induced Tregs does not depend on IL-10. Despite a decrease in allergen-specific responses and protection from inflammation induced by allergen exposure, EPIT did not induce allergen-specific IL-10 or TGF-β production by restimulated splenocytes and did not increase IL-10 levels in broncho-alveolar lavage of aerosol-challenged mice or in the oesophagus of sensitized mice orally exposed to peanut 11,12. Studies on patients allergic to birch pollen also demonstrated that allergen-specific Tregs do not require IL-10 to suppress the in vitro proliferation of effector T cells 33,36. By contrast, other teams observed that the suppressive activity of SIT-induced Tregs (by SLIT or peptide immunotherapy) was blocked by anti-IL-10 antibodies 15,34. As above suggested, different immunotherapy routes may induce different Tregs. EPIT increased peripheral Foxp3+CD4+ T cells and mucosal expression of Foxp3, associated with a decrease in allergen-specific cytokine production 10,11. This Foxp3+ Tregs-dominant process in EPIT contrasts with the Tr-1 cell-dependent mechanism of SLIT 4,37. Indeed, EPIT induced Tregs, which suppressive activity is partly mediated by CTLA-4, probably by cell–cell contact. CTLA-4 has been shown to act in the regulation of hypersensitivity responses to food allergens, especially peanut proteins 38. The induction of CTLA-4+ Tregs suggests that EPIT is a promising treatment for food allergies.
The protection mediated by EPIT-induced Tregs following their transfer to peanut-sensitized mice was associated with an increase in peripheral levels of Foxp3, which suggests that transferred cells proliferated by themselves and/or induced host Tregs. The transfer of EPIT-induced Tregs in Foxp3-IRES-mRFP mice induced an increase in mRFP-expressing cells, implying an induction of host Tregs. Activated Tregs can facilitate Tregs differentiation and block Th2 activation, independently of antigen specificity 39. We also observed a significant increase of Foxp3 in the oesophagus of transferred mice. This suggests that Tregs (transferred or host-induced) are able to migrate to the site of allergen exposure, to induce protection from eosinophil recruitment and Th2-induced inflammation and to induce local Tregs in response to allergen stimulation. EPIT actually proved to be beneficial on the different routes of allergen administration: bronchial hyperresponsiveness 12, eosinophils recruitment in skin 10 and on peanut-induced gut inflammation (in this study and 11). Foxp3+ Tregs constitute a large part of the CD4+ T cells population in the normal skin in humans and mice 40,41. Effector/memory Foxp3+ Tregs were shown to migrate from the skin towards draining lymph nodes in steady state as well as during inflammation and can return to skin upon antigen exposure 42. Therefore, we can postulate that EPIT induces Tregs, in skin or in draining lymph nodes after Langerhans cell migration, that these Tregs are able to recirculate, as shown by increased splenic Treg levels and that they are able to migrate to different tissues in response to allergen exposure. This suggests induction of a global tolerance rather than a local desensitization.
The aim of allergen immunotherapy is the induction of an immune tolerance that persists for years after treatment discontinuation. In this context, our study clearly showed that EPIT-induced Tregs retained their suppressive capacity for a long period of time following the end of treatment, thereby suggesting that EPIT can induce long-term tolerance, at least in our model. This differs from SCIT as well as SLIT, which induced a transient increase in Tr-1 followed by immune deviation towards Th1 response 4,14,15. During EPIT, sensitized mice presented a general decrease in allergen-specific responses associated with increased Foxp3+ Tregs. These Foxp3+ Tregs were of both the naïve and effector phenotypes. Whereas the naïve Tregs subset has been reported to preferentially proliferate in vitro, effector Tregs display higher suppressive activity in vivo, but they are prone to die and expand poorly in vitro 43,44. The induction of naïve Tregs by EPIT could participate in the long-term maintenance. The ability of EPIT-induced Tregs to promote de novo Tregs when transferred into host suggests that they could maintain the pool of Tregs in EPIT-treated mice even after the end of treatment. Moreover, EPIT induced both CD304− iTregs and CD304+ nTregs, suggesting that the mucosal induction of iTregs could also induce production or proliferation of nTregs, which then can participate to long-term maintenance of tolerance. The difference in Tregs induced by EPIT or SLIT implies different mechanisms of desensitization, which could both lead to long-term tolerance either by immune deviation for SLIT or by long-term maintenance of Tregs for EPIT. However, further studies, especially in humans, are needed to clearly conclude to a long-term effect of EPIT.
In summary, the induction of Tregs during allergen-specific immunotherapy seems to be central in the induction of long-term tolerance. The protection induced by EPIT was clearly Tregs dependent and transferable to other animals. EPIT modulates the allergen-specific T cell response via a mechanism that seems to differ from other specific immunotherapy routes, inducing strong Tregs, thus supporting a promising treatment for food allergies.
Acknowledgments
The authors would like to thank the staff of the animal facility of Paris XI University, School of pharmacy, for the animal care. The authors acknowledge Muriel Andrieu and Karine Labroquere of the Cochin Immunobiology Facility, and Maryline Favier and Corinne Lesaffre of the Cochin Histology Facility.
Conflict of interest
This study was supported by DBV Technologies, the developer and owner of Viaskins. Vincent Dioszeghy, Lucie Mondoulet, Veronique Dhelft, Melanie Ligouis, Emilie Puteaux are DBV Technologies employees. Pierre-Henri Benhamou is the Chief Executive Officer of DBV-technologies. Christophe Dupont received honoraria and/or compensation with regard to the study, as investigators, co-ordinators or experts, in relation with the time spent on the study.
References
- 1.Shamji MH, Durham SR. Mechanisms of immunotherapy to aeroallergens. Clin Exp Allergy. 2011;41:1235–46. doi: 10.1111/j.1365-2222.2011.03804.x. [DOI] [PubMed] [Google Scholar]
- 2.Ebner C, Siemann U, Bohle B, et al. Immunological changes during specific immunotherapy of grass pollen allergy: reduced lymphoproliferative responses to allergen and shift from TH2 to TH1 in T-cell clones specific for Phl p 1, a major grass pollen allergen. Clin Exp Allergy. 1997;27:1007–15. doi: 10.1111/j.1365-2222.1997.tb01252.x. [DOI] [PubMed] [Google Scholar]
- 3.O'Brien RM, Byron KA, Varigos GA, Thomas WR. House dust mite immunotherapy results in a decrease in Der p 2-specific IFN-gamma and IL-4 expression by circulating T lymphocytes. Clin Exp Allergy. 1997;27:46–51. [PubMed] [Google Scholar]
- 4.Bohle B, Kinaciyan T, Gerstmayr M, Radakovics A, Jahn-Schmid B, Ebner C. Sublingual immunotherapy induces IL-10-producing T regulatory cells, allergen-specific T-cell tolerance, and immune deviation. J Allergy Clin Immunol. 2007;120:707–13. doi: 10.1016/j.jaci.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 5.Jutel M, Akdis M, Budak F, et al. IL-10 and TGF-beta cooperate in the regulatory T cell response to mucosal allergens in normal immunity and specific immunotherapy. Eur J Immunol. 2003;33:1205–14. doi: 10.1002/eji.200322919. [DOI] [PubMed] [Google Scholar]
- 6.Scadding GW, Shamji MH, Jacobson MR, et al. Sublingual grass pollen immunotherapy is associated with increases in sublingual Foxp3-expressing cells and elevated allergen-specific immunoglobulin G4, immunoglobulin A and serum inhibitory activity for immunoglobulin E-facilitated allergen binding to B cells. Clin Exp Allergy. 2010;40:598–606. doi: 10.1111/j.1365-2222.2010.03462.x. [DOI] [PubMed] [Google Scholar]
- 7.Akdis M, Verhagen J, Taylor A, et al. Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen-specific T regulatory 1 and T helper 2 cells. J Exp Med. 2004;199:1567–75. doi: 10.1084/jem.20032058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujimura T, Yonekura S, Horiguchi S, et al. Increase of regulatory T cells and the ratio of specific IgE to total IgE are candidates for response monitoring or prognostic biomarkers in 2-year sublingual immunotherapy (SLIT) for Japanese cedar pollinosis. Clin Immunol. 2011;139:65–74. doi: 10.1016/j.clim.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 9.Dupont C, Kalach N, Soulaines P, Legoue-Morillon S, Piloquet H, Benhamou PH. Cow’s milk epicutaneous immunotherapy in children: a pilot trial of safety, acceptability, and impact on allergic reactivity. J Allergy Clin Immunol. 2010;125:1165–7. doi: 10.1016/j.jaci.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 10.Dioszeghy V, Mondoulet L, Dhelft V, et al. Epicutaneous immunotherapy results in rapid allergen uptake by dendritic cells through intact skin and downregulates the allergen-specific response in sensitized mice. J Immunol. 2011;186:5629–37. doi: 10.4049/jimmunol.1003134. [DOI] [PubMed] [Google Scholar]
- 11.Mondoulet L, Dioszeghy V, Larcher T, et al. Epicutaneous immunotherapy (EPIT) blocks the allergic esophago-gastro-enteropathy induced by sustained oral exposure to peanuts in sensitized mice. PLoS ONE. 2012;7:e31967. doi: 10.1371/journal.pone.0031967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mondoulet L, Dioszeghy V, Ligouis M, Dhelft V, Dupont C, Benhamou C. Epicutaneous immunotherapy on intact skin using a new delivery system in a murine model of allergy. Clin Exp Allergy. 2010;40:659–67. doi: 10.1111/j.1365-2222.2009.03430.x. [DOI] [PubMed] [Google Scholar]
- 13.Mondoulet L, Dioszeghy V, Vanoirbeek JA, Nemery B, Dupont C, Dupont C. Epicutaneous immunotherapy using a new epicutaneous delivery system in mice sensitized to peanuts. Int Arch Allergy Immunol. 2011;154:299–309. doi: 10.1159/000321822. [DOI] [PubMed] [Google Scholar]
- 14.Francis JN, James LK, Paraskevopoulos G, et al. Grass pollen immunotherapy: IL-10 induction and suppression of late responses precedes IgG4 inhibitory antibody activity. J Allergy Clin Immunol. 2008;121:1120–5. doi: 10.1016/j.jaci.2008.01.072. [DOI] [PubMed] [Google Scholar]
- 15.Mobs C, Slotosch C, Loffler H, Jakob T, Hertl M, Pfutzner W. Birch pollen immunotherapy leads to differential induction of regulatory T cells and delayed helper T cell immune deviation. J Immunol. 2010;184:2194–203. doi: 10.4049/jimmunol.0901379. [DOI] [PubMed] [Google Scholar]
- 16.Jutel M, Akdis CA. Immunological mechanisms of allergen-specific immunotherapy. Allergy. 2011;66:725–32. doi: 10.1111/j.1398-9995.2011.02589.x. [DOI] [PubMed] [Google Scholar]
- 17.Toda A, Piccirillo CA. Development and function of naturally occurring CD4+CD25+ regulatory T cells. J Leukoc Biol. 2006;80:458–70. doi: 10.1189/jlb.0206095. [DOI] [PubMed] [Google Scholar]
- 18.Wu K, Bi Y, Sun K, Wang C. IL-10-producing type 1 regulatory T cells and allergy. Cell Mol Immunol. 2007;4:269–75. [PubMed] [Google Scholar]
- 19.Akdis CA, Akdis M. Mechanisms and treatment of allergic disease in the big picture of regulatory T cells. J Allergy Clin Immunol. 2009;123:735–46. doi: 10.1016/j.jaci.2009.02.030. quiz 47–8. [DOI] [PubMed] [Google Scholar]
- 20.Williams LM, Rudensky AY. Main-tenance of the Foxp3-dependent developmental program in mature regulatory T cells requires continued expression of Foxp3. Nat Immunol. 2007;8:277–84. doi: 10.1038/ni1437. [DOI] [PubMed] [Google Scholar]
- 21.Smith M, Tourigny MR, Noakes P, Thornton CA, Tulic MK, Prescott SL. Children with egg allergy have evidence of reduced neonatal CD4(+)CD25(+)CD127(lo/-) regulatory T cell function. J Allergy Clin Immunol. 2008;121:1460–6. doi: 10.1016/j.jaci.2008.03.025. 1466.e1–7. [DOI] [PubMed] [Google Scholar]
- 22.Krogulska A, Borowiec M, Polakowska E, Dynowski J, Mlynarski W, Wasowska-Krolikowska K. FOXP3, IL-10, and TGF-beta genes expression in children with IgE-dependent food allergy. J Clin Immunol. 2011;31:205–15. doi: 10.1007/s10875-010-9487-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karlsson MR, Rugtveit J, Brandtzaeg P. Allergen-responsive CD4+CD25+ regulatory T cells in children who have outgrown cow's milk allergy. J Exp Med. 2004;199:1679–88. doi: 10.1084/jem.20032121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shreffler WG, Wanich N, Moloney M, Nowak-Wegrzyn A, Sampson HA. Association of allergen-specific regulatory T cells with the onset of clinical tolerance to milk protein. J Allergy Clin Immunol. 2009;123:43–52. doi: 10.1016/j.jaci.2008.09.051. [DOI] [PubMed] [Google Scholar]
- 25.Sicherer SH, Wood RA, Stablein D, et al. Immunologic features of infants with milk or egg allergy enrolled in an observational study (Consortium of Food Allergy Research) of food allergy. J Allergy Clin Immunol. 2010;125:1077–83. doi: 10.1016/j.jaci.2010.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Savilahti EM, Karinen S, Salo HM, et al. Combined T regulatory cell and Th2 expression profile identifies children with cow's milk allergy. Clin Immunol. 2010;136:16–20. doi: 10.1016/j.clim.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 27.van Wijk F, Wehrens EJ, Nierkens S, et al. CD4+CD25+ T cells regulate the intensity of hypersensitivity responses to peanut, but are not decisive in the induction of oral sensitization. Clin Exp Allergy. 2007;37:572–81. doi: 10.1111/j.1365-2222.2007.02681.x. [DOI] [PubMed] [Google Scholar]
- 28.Kanjarawi R, Dercamp C, Etchart N, et al. Regulatory T cells control type I food allergy to Beta-lactoglobulin in mice. Int Arch Allergy Immunol. 2011;156:387–96. doi: 10.1159/000323940. [DOI] [PubMed] [Google Scholar]
- 29.Pereira-Santos MC, Baptista AP, Melo A, et al. Expansion of circulating Foxp3+ D25bright CD4+ T cells during specific venom immunotherapy. Clin Exp Allergy. 2008;38:291–7. doi: 10.1111/j.1365-2222.2007.02887.x. [DOI] [PubMed] [Google Scholar]
- 30.Mamessier E, Birnbaum J, Dupuy P, Vervloet D, Magnan A. Ultra-rush venom immunotherapy induces differential T cell activation and regulatory patterns according to the severity of allergy. Clin Exp Allergy. 2006;36:704–13. doi: 10.1111/j.1365-2222.2006.02487.x. [DOI] [PubMed] [Google Scholar]
- 31.Jones SM, Pons L, Roberts JL, et al. Clinical efficacy and immune regulation with peanut oral immunotherapy. J Allergy Clin Immunol. 2009;124:292–300. doi: 10.1016/j.jaci.2009.05.022. 300.e1–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mori F, Bianchi L, Pucci N, Azzari C, De Martino M, Novembre E. CD4+CD25+Foxp3+ T regulatory cells are not involved in oral desensitization. Int J Immunopathol Pharmacol. 2010;23:359–61. doi: 10.1177/039463201002300136. [DOI] [PubMed] [Google Scholar]
- 33.Grindebacke H, Larsson P, Wing K, Rak S, Rudin A. Specific immunotherapy to birch allergen does not enhance suppression of Th2 cells by CD4(+)CD25(+) regulatory T cells during pollen season. J Clin Immunol. 2009;29:752–60. doi: 10.1007/s10875-009-9312-x. [DOI] [PubMed] [Google Scholar]
- 34.Yamanaka K, Yuta A, Kakeda M, et al. Induction of IL-10-producing regulatory T cells with TCR diversity by epitope-specific immunotherapy in pollinosis. J Allergy Clin Immunol. 2009;124:842–5. doi: 10.1016/j.jaci.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 35.Levings MK, Roncarolo MG. Phenotypic and functional differences between human CD4+CD25+ and type 1 regulatory T cells. Curr Top Microbiol Immunol. 2005;293:303–26. doi: 10.1007/3-540-27702-1_14. [DOI] [PubMed] [Google Scholar]
- 36.Nagato T, Kobayashi H, Yanai M, et al. Functional analysis of birch pollen allergen Bet v 1-specific regulatory T cells. J Immunol. 2007;178:1189–98. doi: 10.4049/jimmunol.178.2.1189. [DOI] [PubMed] [Google Scholar]
- 37.Mascarell L, Lombardi V, Louise A, et al. Oral dendritic cells mediate antigen-specific tolerance by stimulating TH1 and regulatory CD4+ T cells. J Allergy Clin Immunol. 2008;122:603–9. doi: 10.1016/j.jaci.2008.06.034. [DOI] [PubMed] [Google Scholar]
- 38.van Wijk F, Hoeks S, Nierkens S, et al. CTLA-4 signaling regulates the intensity of hypersensitivity responses to food antigens, but is not decisive in the induction of sensitization. J Immunol. 2005;174:174–9. doi: 10.4049/jimmunol.174.1.174. [DOI] [PubMed] [Google Scholar]
- 39.Hu G, Liu Z, Zheng C, Zheng SG. Antigen-non-specific regulation centered on CD25+Foxp3+ Treg cells. Cell Mol Immunol. 2010;7:414–8. doi: 10.1038/cmi.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tietz W, Allemand Y, Borges E, et al. CD4+ T cells migrate into inflamed skin only if they express ligands for E- and P-selectin. J Immunol. 1998;161:963–70. [PubMed] [Google Scholar]
- 41.Sather BD, Treuting P, Perdue N, et al. Altering the distribution of Foxp3(+) regulatory T cells results in tissue-specific inflammatory disease. J Exp Med. 2007;204:1335–47. doi: 10.1084/jem.20070081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tomura M, Honda T, Tanizaki H, et al. Activated regulatory T cells are the major T cell type emigrating from the skin during a cutaneous immune response in mice. J Clin Invest. 2010;120:883–93. doi: 10.1172/JCI40926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoffmann P, Eder R, Boeld TJ, et al. Only the CD45RA+ subpopulation of CD4+CD25high T cells gives rise to homogeneous regulatory T-cell lines upon in vitro expansion. Blood. 2006;108:4260–7. doi: 10.1182/blood-2006-06-027409. [DOI] [PubMed] [Google Scholar]
- 44.Huehn J, Siegmund K, Lehmann JC, et al. Developmental stage, phenotype, and migration distinguish naive- and effector/memory-like CD4+ regulatory T cells. J Exp Med. 2004;199:303–13. doi: 10.1084/jem.20031562. [DOI] [PMC free article] [PubMed] [Google Scholar]