Abstract
This systematic review and meta-analysis of effectiveness trials comparing multicomponent behavioural weight management programmes with controls in overweight and obese adults set out to determine the effectiveness of these interventions implemented in routine practice. To be included, interventions must have been multicomponent, delivered by the therapists who would deliver the intervention in routine practice and in that same context, and must be widely available or feasible to implement with little additional infrastructure or staffing. Searches of electronic databases were conducted, and augmented by screening reference lists and contacting experts (November 2012). Data were extracted by two reviewers, with mean difference between intervention and control for 12-month change in weight, blood pressure, lipids and glucose calculated using baseline observation carried forward. Data were also extracted on adverse events, quality of life and mood measures. Although there were many published efficacy trials, only eight effectiveness trials met the inclusion criteria. Pooled results from five study arms providing access to commercial weight management programmes detected significant weight loss at 12 months (mean difference −2.22 kg, 95% confidence interval [CI] −2.90 to −1.54). Results from two arms of a study testing a commercial programme providing meal replacements also detected significant weight loss (mean difference −6.83 kg, 95% CI −8.39 to −5.26). In contrast, pooled results from five interventions delivered by primary care teams showed no evidence of an effect on weight (mean difference −0.45 kg, 95% CI −1.34 to 0.43). One study testing an interactive web-based intervention detected a significant effect in favour of the intervention at 12 months, but the study was judged to be at high risk of bias and the effect did not persist at 18 months. Few studies reported other outcomes, limiting comparisons between interventions. Few trials have examined the effectiveness of behavioural weight loss programmes delivered in everyday contexts. These trials suggest that commercial interventions delivered in the community are effective for achieving weight loss. There is no evidence that interventions delivered within primary care settings by generalist primary care teams trained in weight management achieve meaningful weight loss.
Keywords: Adult, behaviour therapy, obesity, weight loss
Introduction
The World Health Organization estimates that, every year, overweight and obesity cause at least 2.8 million deaths and the loss of 35.8 million disability-adjusted life years, imposing a penalty on individuals and a large financial burden on health care systems and the wider economy 1,2. Multicomponent behavioural weight management programmes, incorporating diet, exercise and behavioural counselling, can lead to significant weight loss, but their efficacy is highly variable 3,4.
Much of the evidence on these programmes comes from efficacy trials of interventions designed and delivered by specialist researchers and provides proof of principle that this kind of programme can work, typically in ideal circumstances. Such data can establish the efficacy of these programmes, but do not necessarily establish their effectiveness in everyday practice. The Dietary Approaches to Stop Hypertension (DASH) trials illustrate this distinction. In a proof of principle trial, consumption of the DASH diet reduced blood pressure by 6/3 mm of mercury compared with control 5 and an intensive behavioural intervention delivered by a dietician-produced similar reductions 6. However, a pragmatic trial of a DASH intervention delivered by generalists (primary care nurses) had negligible effects on blood pressure 7. These trials indicate the context and person who delivers the intervention are critical to its success. Several systematic reviews have examined the efficacy of behavioural weight loss interventions, but without an explicit focus on whether the intervention was delivered in the setting in which it would be delivered outside of a trial and by personnel who would be involved in programme delivery in routine practice 3,4,8–10. In addition, studies of weight management programmes often have high attrition levels, and researchers use different approaches to participants lost to follow-up, with some presenting complete case data, and others using a variety of imputation methods for missing data. Previous reviews have taken weight change outcomes as reported and synthesized this. Such synthesis risks introducing spurious differences between studies.
We aimed to determine the clinical effectiveness of multicomponent behavioural weight management programmes for overweight and obese adults compared with minimal intervention controls, as tested in randomized controlled trials. To inform implementation in practice, we included only those interventions that are already widely available or may be readily implemented with current or minimal additional infrastructure or staffing and tested in the same context as would be used to implement these programmes outside of a trial. In particular, to be included, the people delivering the intervention had to be available outside of a trial context and interventions had to be delivered in the same setting in the trial that would apply in a wider roll-out (e.g. commercial programmes in their standard settings, primary care-delivered interventions in primary care). To ensure that spurious differences between programmes were not introduced because of different approaches to accounting for participants lost to follow-up, we recalculated outcomes using a consistent approach across all included studies.
Methods
A review protocol was agreed prior to commencing work (see File S1). We searched BIOSIS, the Cochrane Database of Systematic Reviews, CENTRAL, the Conference Proceedings Citation Index, the Database of Abstracts of Reviews and Effects, EMBASE, the Health Technology Assessment database, MEDLINE, PsycINFO, and the Science Citation Index in November 2012 for randomized and quasi-randomized controlled trials, using terms for overweight and obesity, diet and exercise, and weight loss interventions. We used the comprehensive search in a review of behavioural weight management programmes to identify studies published prior to May 2009 and updated this 3. The electronic search strategy for MEDLINE is listed in Table S1. We also screened references from systematic reviews and primary studies identified through our search and sought further studies from experts in the field. Searches were not restricted by country or language.
Studies were included if they recruited adults (≥18 years) with a body mass index (BMI) of ≥25 kg m−2 (or a BMI of ≥23 kg m−2 in Asian populations). Our focus was on weight loss interventions for the general population so we excluded studies in pregnant women, people with eating disorders and trials of weight loss programmes provided as a treatment for a particular medical condition. For example, we would exclude trials where every single participant had hypertension and the weight loss programme was presented as a treatment for hypertension, but include trials that provided weight loss programmes for almost all who sought support to lose weight, which were open to participants with comorbid conditions, including hypertension and type 2 diabetes.
To be included, interventions had to be structured weight management programmes, as opposed to non-specific advice, and had to incorporate diet, physical activity and a behaviour change strategy. Each component had to be clearly described (using a pre-defined set of criteria, see Protocol S1 for detail) and the intervention had to involve multiple contacts. We included only programmes where the interventionists were available outside of a trial context and where the programme was delivered in the same setting in the trial that would apply in any wider roll-out. We were interested in behavioural weight management programmes specifically, and hence excluded programmes that involved surgery or medication and programmes that aimed to tackle several behavioural issues such as problem drinking and smoking.
Studies were required to report a measure of weight change at 12 months or greater from baseline and to include a control group ranging from no intervention at all to seeing someone without specific training in delivering a weight management programme.
Data collection
Titles and abstracts were assessed by a single reviewer with a sample checked by a second reviewer. Two reviewers independently conducted data extraction and quality assessment. Any discrepancies were resolved by discussion, or where needed, by referral to a third reviewer. As well as data on participants and outcomes, we extracted descriptive data on the characteristics of the components of the interventions, the type of person delivering the intervention, the delivery setting and mode, and the behavioural approaches used, including goals set. Where further detail was required, we contacted study authors and conducted web searches for treatment protocols and further publications.
Our primary outcome was difference in mean weight change between intervention and control at 12 and 24 months. Where outcome data were missing, we contacted authors for further information. Where available, we also extracted data on changes in systolic and diastolic blood pressure, high-density lipoprotein (HDL) and low-density lipoprotein (LDL) cholesterol, fasting blood glucose, quality of life, mood, and adverse events. We also extracted other weight change data as a secondary outcome at 12 months (change in BMI and percentage weight change from baseline).
Reviewers critically appraised each included study using criteria developed by the York Centre for Reviews and Dissemination 11. Risk of bias was assessed on the basis of generation of the randomization sequence, concealment of allocation, selective reporting and attrition. Studies were considered to be at low risk of bias for selective reporting where all predefined outcomes were reported. In the absence of formal criteria with which to judge attrition bias in weight loss studies, we considered studies to be at low risk of attrition bias if over 50% of participants were followed up at 12 months and if the percentage followed up was similar across all arms (<20% difference).
Statistical analysis
To ensure we did not introduce spurious differences in effectiveness, we presented results using baseline observation carried forward (BOCF), recalculating where necessary 12. BOCF is an intention-to-treat analysis that makes the assumption that the weight (or other biomedical measure) of those who do not attend an assessment is the same as at baseline. We conducted meta-analyses in Review Manager 5.2 (The Nordic Cochrane Centre, Copenhagen, Denmark) to examine the difference in weight change between intervention groups and control at 12 and 24 months, and to examine changes in biomedical outcomes at 12 months or longer, where reported 13. There were insufficient data to meta-analyse adverse events, quality of life or mood, hence we report these narratively.
We pooled four subgroups of studies to test an a priori hypothesis that these groups of programmes would have different effects: commercial programmes without meal replacements (all included studies in this category were group-based, and this subgroup is hence referred to as ‘group-based commercial programmes’ for simplicity); commercial programmes providing meal replacements; primary care interventions; and web programmes. We used a random effects model to account for differences in the nature of the populations and the interventions being tested. Pooled results are presented as mean differences in kilogrammes (kg) with 95% confidence intervals (CI), and the I2 statistic is used to present statistical heterogeneity 14. Where a study contributed more than one intervention arm to a given subgroup, we split the control group equally to avoid double counting in the pooled result.
Where data allowed, we tested for differences in the effectiveness of the interventions by subgroups by calculating the mean difference in weight loss and χ2 test for differences, using the largest subgroup (group-based commercial programmes) as a reference to improve precision.
Results
The search retrieved 2,210 references in total, 1,966 of which were identified through database searches, 91 of which were identified from Loveman et al., and 153 of which were identified from other sources. After screening titles and abstracts, we excluded 1,894 references that were not randomized controlled trials of behavioural weight management programmes, and retrieved full texts for 206 references. Of these, 196 were excluded after full-text screening, with the most common reason being study design. Thirty-two references met our criteria for intervention type, participants, outcomes and comparators, but 22 of these were excluded because the interventions were not delivered in the settings or by the people who might deliver them in a routine, non-trial context, or because the intervention being tested required substantial infrastructure or staffing that was not already widely available (Table S2). The 10 included references represent eight studies, 13 intervention arms and eight control arms in total. A flow chart detailing the search and screening process can be found in Fig. S1.
Characteristics of included studies
The eight included studies represented over 3,700 participants. The number of participants in each study ranged from 261 to 1,755, with a mean of 600 participants per study. Four studies were conducted in the United States, two were conducted in England, one was conducted in Switzerland, and one was a multi-centre study conducted in Australia, Germany and England. The mean age of study participants ranged from 40 to 52 years, and as is common in weight loss studies, the majority of participants were women (ranging from 71% to 100%). The mean BMI ranged from 31.4 to 39.0 kg m−2. Four of the eight included studies had an upper limit for baseline BMI: one study of a commercial programme had a BMI cut-off point of 35 kg m−2 15; two further studies of commercial programmes had a cut-off of 40 kg m−2 16,17; and one study of a primary care intervention had a cut-off of 50 kg m−2 18. All studies reported weight change at 12 months from baseline. Four studies also reported longer follow-ups: one reported weight change at 18 months and three followed up participants at two years. Reporting of secondary outcomes was sparse, with five of the eight studies reporting some biomedical outcomes and fewer than half reporting on quality of life, depression or adverse events.
Intervention characteristics are described by a subgroup below. Further details of the intervention and control arms for each included study, including diet and physical activity components, can be found in Tables 1 and 2.
Table 1.
Characteristics of included studies: commercial programmes
| Study ID (arm) | Country | Intervention | Diet | Physical activity | Control | % followed up at 12 months | Notes |
|---|---|---|---|---|---|---|---|
| Commercial programme providing meal replacements | |||||||
| Rock 2010 (in person) 17 | USA | Jenny Craig Individual; phone, web, in person Delivered by: trained lay people 104 sessions over 24 months Session length: not reported |
Low fat and reduced energy (5,020–8,370 kJ d−1, aiming for deficit of 2,010–4,180 kJ d−1). Includes free, pre-packaged meals | Recommended physical activity, intensity not specified, 5 or more days a week for 30 min a session. CDs and DVDs provided for support. | Consultation with research staff + written info at baseline and 6 months, monthly check-ins by email or phone. | Intervention: 95% Control: 91% |
|
| Rock 2010 (phone) 17 | USA | As above, but phone and web only | As above | As above | As above | Intervention: 95% Control: 91% |
|
| Group-based commercial programmes | |||||||
| Heshka 2006 16 | USA | Weight Watchers Group; in-person and web Delivered by: trained lay people 104 sessions over 24 months Session length: 60 min |
Energy-restricted balanced diet using a points system | Recommend minimum 30-min moderate-intensity aerobic activity 5+ d week−1 with 2+ resistance sessions per week | 20-min consultation with a dietician who provided basic information | 80%, no difference between arms | Raw data from authors |
| Jebb 2011 15 | England, Germany and Australia | Weight Watchers Group; phone, web, and in-person Delivered by: trained lay people 52 sessions over 12 months Session length: 60 min |
Energy-restricted balanced diet using a points system | Recommend minimum of 30 min of moderate-intensity aerobic activity on 5+ d week−1 with 2+ resistance sessions per week | Repeated contact with nurse practitioner untrained in weight management | Intervention: 61% Control: 54% |
|
| Jolly 2011 RC 19 | England | Rosemary Conley Group; in-person Delivered by: trained lay people 12 sessions over 12 weeks Session length: 1 h |
Reduced energy low-fat, low-GI diet, energy goals of weeks 1 and 2: 5,020 kJ; weeks 3 and 4: 5,860 kJ; weeks 5+: personal energy allowance | Recommended physical activity and one 45-min dance-based exercise session per week | One-off advice, offered 12 free entries to local sports centre | Intervention: 68% Control: 70% |
|
| Jolly 2011 SW 19 | England | Slimming World Group; in-person Delivered by: trained lay people 12 sessions over 12 weeks Session length: 1 h |
No set energy intake. Low-fat, low-energy density diet, includes free foods (no restriction) + allowances for other types of food. | Recommended physical activity, building to 10 × 15 min of moderate activity or 5 × 30 min weekly | As above | Intervention: 62% Control: 70% |
|
| Jolly 2011 WW 19 | England | Weight Watchers Group; in-person Delivered by: trained lay people 12 sessions over 12 weeks Session length: 1 h |
Energy-restricted balanced diet using a points system | Recommend minimum of 30 min of moderate-intensity aerobic activity on 5+ d week−1 with 2+ resistance sessions per week | As above | Intervention: 78% Control: 70% |
|
GI, glycaemic index; RC, Rosemary Conley; SW, Slimming World; WW, Weight Watchers.
Table 2.
Characteristics of included studies: Internet and primary care
| Study ID (arm) | Country | Intervention | Diet | Physical activity | Control | % followed up at 12 months | Notes |
|---|---|---|---|---|---|---|---|
| Automated Internet based intervention | |||||||
| Hersey 2012 22 | USA | Individual Web Feedback on diet and exercise, frequency dependent on participants providing diet and exercise records Access available for 18 months |
No set energy intake. Encouraged reduction in energy, saturated fats, and ‘junk foods’ and increase in fruits and vegetables, low-fat protein, low-fat dairy, and whole grains | Recommended increase in moderate and vigorous physical activity | Discussion in one-off session and access to static website (i.e. information only, no interactive components) | Intervention: 32% Control: 28% |
Third arm with counselling not included. |
| Primary care interventions delivered by generalists | |||||||
| Jolly 2011 GP 19 | England | Individual; in-person Delivered by: practice nurses (and some GP involvement) 12 sessions over 12 weeks Session length: 20 min |
Reduced energy low-fat diet based on healthy eating principles | Recommended physical activity incremental to 30 min of moderate activity 5 d week−1 | One-off advice, offered 12 free entries to local sports centre | Intervention: 66% Control: 70% |
Two arms (NHS Size Down and choice of intervention) not included – Size Down is UK only and varies by location. |
| Jolly 2011 pharmacist 19 | England | Exactly as per GP arm, but delivered by pharmacist | As per GP arm | As per GP arm | As above | Intervention: 57% Control: 70% |
|
| Munsch 2003 21 | Switzerland | Group; in-person Delivered by: GP trained by psychologist and dietician 16 sessions over 4 months Session length: 90 min |
Balanced diet with fat intake target of 20 g day−1 | Recommended 15 min of exercise daily with examples swimming, walking and incorporation into daily life. | Non-specific comments about general measures to lose weight from GP. | Intervention: 77% Control: 47% |
Third arm conducted in clinic not included in this analysis. |
| Nanchahal 2011 20 | England | Individual; in-person Delivered by: Health trainers (lay people with NHS training) 14 sessions over 8 months Session length: 30 min |
Calorie-reduced diet based on the Eatwell plate. | Recommended exercise focussing on walking with exercise diaries provided. | Usual care: received British Health Foundation booklet at baseline | Intervention: 61% Control: 60% |
Pragmatic trial. Some participants used drugs or weight loss surgery during study period (15 intervention, 12 control). |
| Wadden 2011 18 | USA | Individual Delivered by: medical assistant (practice staff) Phone and in-person Number of sessions: 25 Duration: 24 months Session length: not reported |
Energy restriction: If weight <113, 5,020–6,280 kcal per day; and if 113.4 kg or more, 6,280–7,530 per day | Recommended moderate-intensity physical activity for minimum 30 min, 6 d week−1 | Quarterly PCP visits. At each visit, PCP spent 5–7 min reviewing weight change and discussing info in handouts. | Intervention: 83% Control: 86% |
Third arm received weight loss medication, not included in this review. |
GP, general practitioner; NHS, National Health Service; PCP, primary care practitioner.
Intervention characteristics
Commercial programmes
Three studies, representing five intervention arms and 1,605 participants, evaluated community-based commercial programmes that did not provide meal replacements. All were group-based. All interventions were delivered in person, although some provided additional individual support via phone or Internet. Three studies provided access to Weight Watchers: two provided vouchers for weekly sessions for a year or longer 15,16 and one provided vouchers for 12 weekly sessions 19. One study also provided vouchers for 12 weekly sessions of Rosemary Conley or Slimming World (UK-based programmes) 19. In all programmes, physical activity was recommended (not supervised), although in one commercial intervention, participants were offered a 45-min dance class each week 19. Four of the five interventions involved energy restrictions, and the fifth involved a low-energy density diet, but did not specify a set energy intake. In two studies, the control group received multiple contacts from a generalist with no specific training in weight management and in one study the control group received one-off advice.
The one study of a commercial intervention providing meal replacements (Jenny Craig) included 442 participants in total 17. This study had two intervention arms: one received in-person counselling and the other received telephone counselling for two years. All counselling were given weekly and individually. Participants were set a low-fat and reduced-energy diet including free, pre-packaged meals. Physical activity was recommended. The control group had two face-to-face sessions with the research staff, and was provided with brief written materials.
Primary care-based interventions
Five study arms were classed as primary care-based interventions, representing almost 1,000 participants in total. All but one programme was delivered by staff already based in the practice where the intervention was delivered (see Tables1 and 2 for further detail) 20. An additional study that used trained lay people was also included as it was judged to require only minimal additional infrastructure. All interventions were delivered in person; one in a group setting 21 and the remainder via one-to-one counselling. There was a wide range in the frequency and duration of sessions offered, from 14 weekly sessions to monthly sessions for two years. Four of the interventions set energy restrictions, and the fifth restricted fat intake only 21. All programmes recommended physical activity of moderate intensity. Control groups ranged from multiple sessions with someone untrained in weight management (two studies), to a single session (one study) and written advice only (one study).
Web-based programmes
One study of an automated web-based intervention met our criteria; participants could submit diet and exercise logs and receive weekly feedback tailored by a computer algorithm 22. Access was available for 18 months. The intervention recommended an increase in moderate and vigorous physical activity. It did not set an energy prescription, but encouraged reduction in energy, especially high-energy foods. The study included over 800 participants. The control group received one face-to-face counselling session and access to a static, information-only website (i.e. no interactive components).
Risk of bias
Four of the eight included studies were judged to be at low risk of bias across all domains 15–18. In two studies (one primary care, one web-based), the randomization procedures were not described in enough detail to evaluate risk of bias 21,22. These two studies were also judged to be at high risk of attrition bias because of low 22 and differential 21 rates of follow-up at 12 months. A third study, which evaluated both commercial and primary care interventions was also judged to be at high risk of attrition bias because of differential rates of follow-up 19. Finally, one study was judged to be at high risk of selection bias as it did not report all of the outcomes that it had pre-specified 20. Further details can be found in Table 3.
Table 3.
Risk of bias judgements for included studies*
| Study ID | Random sequence generation | Allocation concealment | Attrition | Selective reporting | Notes |
|---|---|---|---|---|---|
| Hersey 2012 22 | Unclear | Unclear | High | Low | Randomization procedures not described. Follow-up <50% at 12 months |
| Heshka 2006 16 | Low | Low | Low | Low | |
| Jebb 2011 15 | Low | Low | Low | Low | |
| Jolly 2011 19 | Low | Low | High | Low | Between arm differences in rates of follow-up >20% |
| Munsch 2003 21 | Unclear | Unclear | High | Low | Randomization process not described, significant baseline imbalances. Between arm differences in rates of follow-up >20% |
| Nanchahal 2011 20 | Low | Low | Low | High | Psychological variables measured, but not reported |
| Rock 2010 17 | Low | Low | Low | Low | |
| Wadden 2011 18 | Low | Low | Low | Low |
Where ‘low’ indicates low risk of bias in that domain, ‘unclear’ indicates insufficient information with which to judge, and ‘high’ indicates high risk of bias in that domain.
Weight change
Commercial programmes
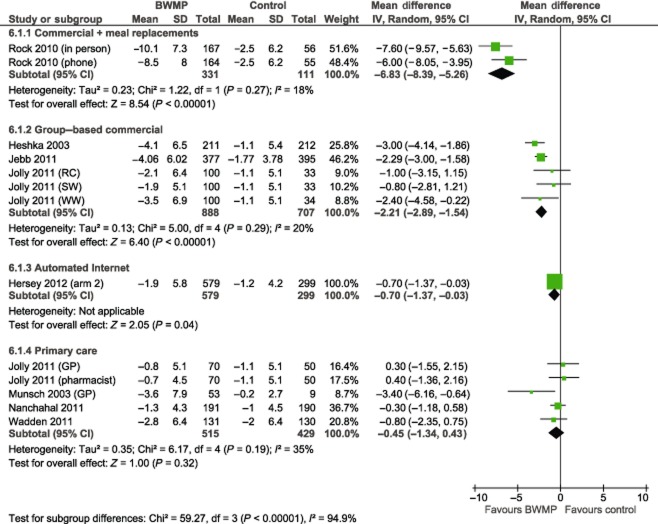
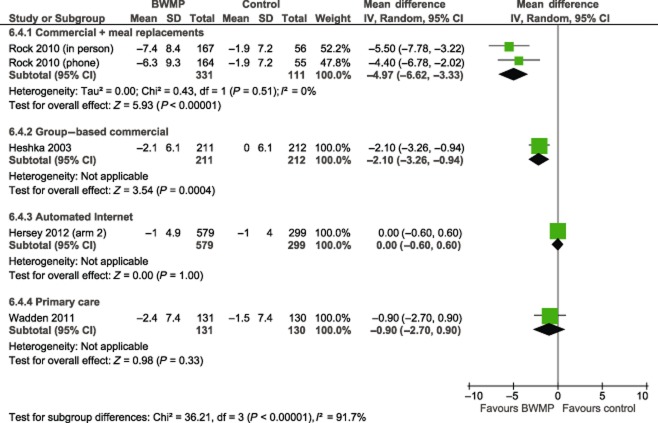
Pooled results from three studies comparing commercial group-based weight loss programmes (Weight Watchers, Slimming World and Rosemary Conley) with control yielded a mean difference of −2.22 kg in favour of the intervention group, with 95% CI of −2.89 to −1.54 at 12 months (Fig. 1, Hedge's adjusted g −0.42, 95% CI −0.53 to −0.30). This represents five intervention arms in total. Statistical heterogeneity was low (I2 = 20%). In the one study that reported follow-up at two years, a statistically significant difference between intervention and control arms remained (risk ratio −2.10, 95% CI −3.26 to −0.94, Fig. 2).
Figure 1.
Mean difference in weight change (kg) at 12 months. Note: BWMP, behavioural weight management programme; CI, confidence interval; GP, general practitioner; RC, Rosemary Conley; SW, Slimming World; SD, standard deviation; WW, Weight Watchers.
Figure 2.
Mean difference in weight change (kg) at 24 months. Note: BWMP, behavioural weight management programme; CI, confidence interval; SD, standard deviation.
The pooled result from two arms testing phone or in-person delivery of a commercial programme providing meal replacements (Jenny Craig) showed the programme to be more effective than minimal intervention at 12 months, with a mean difference of −6.83 kg (95% CI −8.39 to −5.26) (Hedge's adjusted g −0.93, 95% CI −1.21 to −0.65). At two years, the difference was smaller but still significant (mean difference −4.97 kg, 95% CI −6.62 to −3.33). At one and two years, the in-person arm had a slightly greater mean weight loss than the phone arm, but there was no statistically significant difference between them at either follow-up.
Automated Internet intervention
The one study that tested an automated Internet programme detected a statistically significant effect of the intervention at 12 months, but the effect was clinically small (mean difference −0.70 kg, 95% CI −1.37 to −0.03; Hedge's adjusted g −0.13, 95% CI −0.27 to 0.01). At 18 months, there was no difference between intervention and control arms (mean difference 0.00 kg, 95% CI −0.60 to 0.60).
Interventions delivered by generalists in primary care
Of the five interventions delivered by generalists in primary care, only one detected a statistically significant effect (mean difference −3.40, 95% CI −6.16 to −0.64; Hedge's adjusted g −0.06, 95% CI −0.19 to 0.07). However, this was a small study (n = 62) and judged to be at high risk of bias because of, among other things, significant differences among groups in terms of baseline variables and attrition 21. Pooled results from the five primary care interventions did not detect a statistically significant difference in weight change between intervention and control groups at 12 months (mean difference −0.45 kg, 95% CI −1.34 to 0.43). Statistical heterogeneity was moderate (I2 = 35%); a sensitivity analysis removing the one study at high risk of bias reduced this to 0%. Only one study reported follow-up at two years; this also did not detect a significant effect (mean difference −0.90 kg, 95% CI −2.70 to 0.90).
Between-group differences
Subgroups showed varying degrees of weight loss, with significant between group differences overall (χ2 = 59.3, P < 0.001, I2 = 94.9%). There were significant differences between the reference group, group-based commercial programmes and each of the other types of programmes. The χ2 difference with commercial programmes providing meal replacements was χ2 = 28.0, P < 0.001, with automated Internet programmes χ2 = 9.7, P = 0.002, and with primary care χ2 = 9.6, P = 0.002.
Changes in other weight outcomes
We also looked at other weight-related outcomes as secondary outcomes at 12 months. Five out of the eight included studies reported change in BMI and four out of the eight reported mean change in percentage of weight at baseline. In both cases, results mirrored those seen in weight change, with significant differences observed in commercial programmes with and without meal replacements, and no significant effect observed in interventions delivered in primary care. The study of an automated Internet programme did not report on these outcomes. Results are tabulated in Table 4, with forest plots in Figs S2 and S3.
Table 4.
Changes in secondary weight outcomes at 12 months, pooled results using baseline observation carried forward* and random effects meta-analysis
| Outcome | Subgroup | Number of studies | Total n | Mean difference and 95% CI |
|---|---|---|---|---|
| Change in BMI | Commercial + meal replacements | 1 | 442 | −2.5 kg m−2, 95% CI −3.0 to −2.0 |
| Group-based commercial | 1 | 1,020 | −1.0 kg m−2, 95% CI −1.5 to −0.5 | |
| Primary care | 3 | 712 | −0.5 kg m−2, 95% CI −1.0 to +0.1 | |
| Change from baseline weight by percentage | Commercial + meal replacements | 1 | 442 | −7.5%, 95% CI −9.2 to −5.8 |
| Group-based commercial | 1 | 400 | −1.5%, 95% CI −2.7 to −0.5 | |
| Primary care | 3 | 882 | −0.4%, 95% CI −1.2 to +0.5 |
Secondary outcomes for Jolly only available in multiple imputation as opposed to baseline observation carried forward form.
BMI, body mass index; CI, confidence interval.
Two studies reported percentage of participants who gained weight between baseline and 12 months: in a group-based commercial study, 20% of intervention versus 46% of control participants weighed more at 12 months than they did at baseline 16, and in a primary care study 30% intervention versus 41% control weighed more at 12 months than at baseline 18.
Biomedical outcomes
Because of limited data, meta-analyses of biomedical outcomes involved fewer participants than for weight outcomes. No significant effects were found, with the exception of a marginal increase in HDL (mean difference +0.03 mmol L−1, 95% CI 0.00–0.05, P = 0.02) and decrease in diastolic blood pressure (mean difference −0.92 mmHG, 95% CI −1.72 to −0.13) in participants in group-based commercial programmes. Results are tabulated in Table 5, with forest plots in Figs S4–S8. No significant subgroup differences were detected for any biomedical outcome.
Table 5.
Changes in biomedical outcomes at 12 months, pooled results using baseline observation carried forward and random effects meta-analysis
| Outcome | Subgroup | Number of studies | Total n | Mean difference and 95% CI |
|---|---|---|---|---|
| Blood pressure (systolic) | Group-based commercial | 2 | 1,195 | −0.8 mmHg, 95% CI −2.0 to 0.4 |
| Primary care | 2 | 642 | −0.2 mmHg, 95% CI −2.3 to 1.8 | |
| Blood pressure (diastolic) | Group-based commercial | 2 | 1,195 | −0.9 mmHg, 95% CI −1.7 to −0.1 |
| Primary care | 2* | 642 | −0.6 mmHg, 95% CI −1.9 to 0.8 | |
| Lipids (LDL) | Commercial with meal replacements | 1 | 442 | 0.15 mmol L−1, 95% CI −0.10 to 0.40 |
| Group-based commercial | 1 | 772 | −0.07 mmol L−1, 95% CI −0.14 to 0.00 | |
| Primary care | 1 | 261 | 0.07 mmol L−1, 95% CI −0.10 to 0.24 | |
| Lipids (HDL) | Commercial with meal replacements | 1 | 442 | 0.02 mmol L−1, 95% CI −0.01 to 0.05 |
| Group-based commercial | 3 | 1,195 | 0.03 mmol L−1, 95% CI 0.00–0.05, P = 0.02 | |
| Primary care | 1 | 261 | −0.02 mmol L−1, 95% CI −0.07 to 0.03 | |
| Fasting glucose | Group-based commercial | 2 | 1,195 | −0.03 mmol L−1, 95% CI −0.08 to 0.02 |
| Primary care | 1 | 261 | −0.25 mmol L−1, 95% CI −0.56 to 0.06 |
One additional study based in primary care did not present results by study arm and hence was not included in the comparison. A significant reduction in blood pressure was reported across all participants who had hypertension at baseline.
CI, confidence interval; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Adverse events
Only one of the eight included studies reported adverse events 18. This study of a primary care-based intervention detected no significant between-group differences in serious adverse events over the two-year trial period (P = 0.56). One study of a commercial programme with meal replacements administered the Eating Disorder Examination Questionnaire to participants at baseline and follow-up. Scores improved over time in all groups, and no significant between-group differences were detected 17.
Quality of life and depression scales
Five studies measured quality of life at baseline and follow-up. Of these, two did not report any results and two studies, one of a commercial group programme and one of a primary care intervention, did not report numeric results, but stated that no differences were found between groups in change in quality of life measures 16,20. The fifth study, of a commercial weight loss programme providing meal replacements, also did not detect significant differences in quality of life between baseline and 12 months in any groups 17.
Three studies measured depression indices at baseline and follow-up. One primary care study reported that depression decreased in the intervention group, but did not provide numeric figures or report on changes in depression in the control group 21. In the complete case analysis, the study of the commercial programme providing meal replacements detected a significant intervention effect on the Beck Depression Inventory, with improvements in both intervention arms at 12 months 17. A further primary care study found no differences between groups 20.
Discussion
Surprisingly few trials have tested weight loss interventions delivered in the settings and by the practitioners who would deliver such interventions in routine practice. Such studies provide crucial information for informing clinical practice. The data that were available show evidence that group-based commercial interventions are effective. Limited evidence suggests that commercial interventions that supply food are even more effective, although we would temper drawing conclusions from this as results were from one study. In addition, the cost of providing meal replacements is considerably more than purely behavioural support programmes and less likely to be deployed at scale in routine practice. There was some evidence, clouded by methodological concerns, that Internet interventions could be effective in the short term. The available evidence shows that interventions delivered by primary care teams are not effective. Scant evidence on outcomes other than weight change suggests that these interventions achieve only modest benefits on blood pressure, lipids, and fasting glucose, although the power of this review to detect changes was limited by the fact that few studies reported on these outcomes. There was insufficient evidence on quality of life but no evidence this improved or worsened. There was no evidence that such programmes led to mood problems or adverse events, but data were limited.
The difference in weight loss between control condition and commercial weight management support may appear modest, at 2.2 kg. However, as the control condition lost over 1 kg in every case, the total weight loss achieved by people on these programmes is over 3 kg. This is somewhat equivalent to the increase in mean weight in the United States over a decade in the latter part of the 20th century 23, when the prevalence of obesity went from 23% to 31% during one such decade 24. Thus the challenge of these weight control interventions is to achieve widespread uptake, which might ‘take a decade off’ the obesity epidemic. Doing so could have a modest, but important impact on population health. For the individuals concerned, a weight reduction of this magnitude, approximately one BMI unit, would reduce their risk of mortality by more than 6% 25, decrease the risk of developing diabetes by over 30% 26, and improve HbA1c and blood pressure in people already living with type 2 diabetes 27.
There are two major strengths to our review compared with other reviews in the area. Firstly, we recognize that delivery of complex behavioural interventions such as these is a skilled task. Interventions delivered by experts in ideal contexts may achieve much better results than those delivered in routine practice. For example, we excluded outcomes from a study of the Diabetes Prevention Program because the intervention was delivered by qualified dieticians and people with masters degrees in relevant areas. In this study, the intervention group lost 6.1 kg more than the control group at 12 months 28. By contrast, a study of an abbreviated version of the same programme adapted for use by generalists and included in our review achieved 0.8 kg greater weight loss than control 18. By rigorously focusing on the nature of the implementation, we have shown what is currently known about what can and cannot be achieved in weight management in the ‘real world’.
Secondly, weight loss studies vary greatly in how they present weight loss data, and previous reviews have taken weight loss as reported and summarized this. As attrition is often substantial in these trials, combining data presented in different ways can lead to spurious differences between studies. To ensure a consistent approach to weight change outcomes from all included studies, there are only two methods open to the reviewer: complete case analysis and imputation via BOCF. We used previously published methods to calculate BOCF and used these data as our primary outcome. BOCF is an appropriate means of imputation as it provides a conservative estimate suitable for assessing population benefit. Although complete case data were available for most included studies, we favoured BOCF as a more conservative approach. A sensitivity analysis using complete case data produced similar results (Fig. S9). Reporting BOCF as an outcome in future trials, whether as a sensitivity analysis or as an aid to future evidence synthesis, will enable these meta-analyses to be readily updated.
Our scope and approach to data synthesis differ from other reviews in this area, and thus offer a different perspective designed to be more suitable for informing current clinical practice. In terms of scope, our review is most similar to a 2011 review of primary care-relevant treatments, conducted on behalf of the U.S. Preventive Services Task Force, which included studies conducted in primary care and studies that tested programmes that could, in theory, operate in or be referred to from primary care 29. The authors’ approach differed from ours in several key respects: they do not exclude efficacy trials; they do not consider primary care-delivered interventions separately from those that could be referred to from primary care; they use weight change data as reported; and their inclusion criteria allow for non-randomized controlled trials and single component interventions. Their overall pooled effect is consistent with our results from commercial weight management programmes only; by separately evaluating those interventions delivered in primary care by generalists, our results present a more nuanced picture. Other reviews have chosen to focus either on commercial programmes or those delivered in primary care. Incorporating three recent trials, our meta-analysis produces more convincing findings that commercial weight management programmes can induce significant weight loss at one year and longer than a 2005 review by Tsai and colleagues 9, which concluded that, with the exception of one trial of Weight Watchers 16, evidence was suboptimal. Our results are consistent with those of a 2013 narrative review of obesity management in primary care, which found that current evidence does not support the use of low- to moderate-intensity primary care counselling for obesity and sounded a note of caution as to the transferability of the more intensive interventions that had been tested 10. By conducting a meta-analysis, we are able to support this conclusion with numeric estimates of the effects of such programmes.
There are several limitations of our review. Firstly, BOCF, as with all imputation methods, has limitations, although, as discussed earlier, use of BOCF removed a possible reason for spurious differences among studies, and our findings were not sensitive to the use of BOCF as opposed to complete case data 30. Secondly, our inclusion criteria were deliberately strict. There is evidence that diet and exercise lead to greater weight loss than either alone 31–33, and hence we focused on multicomponent interventions containing diet, exercise and behaviour. We also included only those interventions judged to be readily transferable into routine practice. This meant that we excluded studies delivered by specialists in primary care settings, as it was not clear from trial reports if staff were already employed by the practices or were hired in to deliver the intervention. However, a sensitivity analysis including these studies did not affect the overall results. Furthermore, despite attempts to contact authors, several studies with insufficient or inappropriate data had to be excluded. Finally, given the small number of studies, we were unable to formally test for publication bias, and given the lack of reporting of secondary outcomes, we were unable to draw conclusions as to the impact of such programmes on biomedical results. In addition, because of limited reporting of intervention characteristics, we are missing some detail on programme content that may have added further context, an issue that we have also noted in efficacy trials 34. Our results were further limited by the quality of included studies, with only half rated at low risk of bias.
Although we did not evaluate cost-effectiveness, cost-effectiveness analyses are available for two of our included studies (one commercial, one Internet). Both found the intervention to be cost-effective 22,35. Many primary care practices in the United Kingdom and the United States are currently referring patients to commercial weight loss services, and findings from our review support this course of action 10,36. This analysis is unable to establish why programmes delivered by primary care staff appear less likely to induce significant weight loss than commercial programmes. As with the commercial programmes, to be included, primary care programmes were required to be complex interventions with multiple contacts, and unpublished data suggest that there were few differences in the delivery, training and behavioural change techniques used by commercial and primary care programmes. Our findings are also consistent with evidence from direct comparisons 19. It would be helpful to understand the reasons for the lack of efficacy of primary care programmes and to rectify this, especially given that commercial programmes may not be available or suited to everyone, and in light of evidence that some people would prefer a primary care-delivered programme over a commercial one 19,37.
Our analyses add to the body of evidence by demonstrating that some behavioural weight management programmes can lead to significant weight loss at one year and sometimes longer without requiring major changes to current staffing or infrastructure. However, our results suggest only a limited range of current options with regard to such programmes, with promising evidence, albeit limited by a small number of studies, of the effectiveness of commercial programmes and disappointing results from programmes delivered by generalists within primary care settings. This review highlights the need for further research into the reasons for the lack of efficacy of primary care programmes being tested and for further studies of web-based interventions. Future studies of weight loss interventions should carefully consider their subsequent applicability (e.g. staffing, location and other resources) to routine practice.
Acknowledgments
The review protocol was designed and agreed upon by the National Institute for Health and Care Excellence (NICE) to support the development of NICE Guidance on managing overweight and obese adults – lifestyle weight management services and by members of the Behavioural Weight Management Review Group, which, in addition to the authors, consists of Jane Ogden, Igho Onakpoya and Dawn Phillips. IO also contributed to data extraction. The work on which this paper is based was funded by NICE to support the development of NICE Guidance on managing overweight and obese adults – lifestyle weight management services and the protocol was agreed with them. The opinions expressed in this paper are those of the authors and do not represent either NICE's position on these matters or constitute NICE guidance. The UK Medical Research Council (U105960389 Nutrition and Health) and the University of Oxford also provided funding. Paul Aveyard is funded by The UK Centre for Tobacco and Alcohol Studies, a UKCRC Public Health Research Centre of Excellence. Funding from the British Heart Foundation, Cancer Research UK, Economic and Social Research Council, Medical Research Council and the Department of Health, under the auspices of the UK Clinical Research Collaboration, is gratefully acknowledged.
Glossary
- BMI
body mass index
- kg
kilogramme
Conflict of interest statement
The National Institute for Health and Care Excellence (NICE) provided support for the original review upon which the submitted work was based. The MRC received grants from Weight Watchers International for work where Susan Jebb was principal investigator. She received no personal remuneration with regard to this work. Susan Jebb has received personal fees from Rosemary Conley, and hospitality from Weight Watchers International outside of the submitted work. Paul Aveyard has received hospitality from Weight Watchers and Slimming World outside of the submitted work. Paul Aveyard and Susan Jebb were each authors on one study included in the review.
Supporting Information
Additional Supporting Information may be found in the online version of this article, http://dx.doi.org/10.1111/obr.12220
Figure S1. PRISMA flow diagram of review process.
Figure S2. Mean difference in BMI (kg m−2) at 12 months.
Figure S3. Mean difference in percentage weight change from baseline at 12 months.
Figure S4. Mean difference in systolic blood pressure change (mmHg) at 12 months.
Figure S5. Mean difference in diastolic blood pressure change (mmHg) at 12 months.
Figure S6. Mean difference in LDL change (mmol L−1) at 12 months.
Figure S7. Mean difference in HDL change (mmol L−1) at 12 months.
Figure S8. Mean difference in glucose change (mmol L−1) at 12 months.
Figure S9. Mean difference in weight change (kg) at 12 months: sensitivity analysis using complete case data.
Table S1. MEDLINE search strategy.
Table S2. Studies excluded because interventions not delivered in routine context.
References
- World Health Organization. Global health risks: mortality and burden of disease attributable to selected major risks. Geneva: World Health Organization; 2009. URL http://www.who.int/healthinfo/global_burden_disease/GlobalHealthRisks_report_full.pdf (accessed June 2014) [Google Scholar]
- Butland B, Jebb SA, Kopelman P, et al. Foresight. Tackling Obesities: Future Choices – Project Report. UK: Government Office for Science; 2007. [DOI] [PubMed] [Google Scholar]
- Loveman E, Frampton GK, Shepherd J, et al. The clinical effectiveness and cost-effectiveness of long-term weight management schemes for adults: a systematic review. Health Technol Assess. 2011;15:1–182. doi: 10.3310/hta15020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk SF, Penney TL, McHugh TL, Sharma AM. Effective weight management practice: a review of the lifestyle intervention evidence. Int J Obes (Lond) 2012;36:178–185. doi: 10.1038/ijo.2011.80. [DOI] [PubMed] [Google Scholar]
- Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. NEJM. 1997;336:1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- Writing Group of the PREMIER Collaborative Research Group. Effects of comprehensive lifestyle modification on blood pressure control: main results of the premier clinical trial. JAMA. 2003;289:2083–2093. doi: 10.1001/jama.289.16.2083. [DOI] [PubMed] [Google Scholar]
- Little P, Kelly J, Barnett J, Dorward M, Margetts B, Warm D. Randomised controlled factorial trial of dietary advice for patients with a single high blood pressure reading in primary care. BMJ. 2004;328:1054. doi: 10.1136/bmj.38037.435972.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCombie L, Lean MEJ, Haslan D. Effective UK weight management services for adults. Clin Obes. 2012;23:96–102. doi: 10.1111/j.1758-8111.2012.00049.x. [DOI] [PubMed] [Google Scholar]
- Tsai AG, Wadden TA. Systematic review: an evaluation of major commercial weight loss programs in the United States. Ann Intern Med. 2005;142:56–66. doi: 10.7326/0003-4819-142-1-200501040-00012. [DOI] [PubMed] [Google Scholar]
- Carvajal R, Wadden TA, Tsai AG, Peck K, Moran CH. Managing obesity in primary care practice: a narrative review. Ann N Y Acad Sci. 2013;1281:191–206. doi: 10.1111/nyas.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NHS Centre for Reviews and Dissemination. Systematic Reviews: CRD's Guidance for Undertaking Reviews in Healthcare. Centre for Reviews and Dissemination: York, UK; 2008. [Google Scholar]
- Kaiser KA, Affuso O, Beasley TM, Allison DB. Getting carried away: a note showing baseline observation carried forward (BOCF) results can be calculated from published complete-cases results. Int J Obes (Lond) 2012;36:886–889. doi: 10.1038/ijo.2011.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Review Manager (RevMan) The Nordic Cochrane Centre. Copenhagen: The Cochrane Collaboration; 2012. [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jebb SA, Ahern AL, Olson AD, et al. Primary care referral to a commercial provider for weight loss treatment versus standard care: a randomised controlled trial. Lancet. 2011;378:1485–1492. doi: 10.1016/S0140-6736(11)61344-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heshka S, Anderson JW, Atkinson RL, et al. Weight loss with self-help compared with a structured commercial program: a randomised trial. JAMA. 2003;289:1792–1798. doi: 10.1001/jama.289.14.1792. [DOI] [PubMed] [Google Scholar]
- Rock CL, Flatt SW, Sherwood NE, et al. Effect of a free prepared meal and incentivized weight loss program on weight loss and weight loss maintenance in obese and overweight women: a randomized controlled trial. JAMA. 2010;304:1803–1810. doi: 10.1001/jama.2010.1503. [DOI] [PubMed] [Google Scholar]
- Wadden TA, Volger S, Sarwer DB, et al. A two-year randomised trial of obesity treatment in primary care practice. NEJM. 2011;365:1969–1979. doi: 10.1056/NEJMoa1109220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly K, Lewis A, Beach J, et al. Comparison of range of commercial or primary care led weight reduction programmes with minimal intervention control for weight loss in obesity: Lighten Up randomised controlled trial. BMJ. 2011;343:d6500. doi: 10.1136/bmj.d6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanchahal K, Power T, Holdsworth E, et al. A pragmatic randomised controlled trial in primary care of the Camden Weight Loss (CAMWEL) programme. BMJ Open. 2012;2 doi: 10.1136/bmjopen-2011-000793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munsch S, Biedert E, Keller U. Evaluation of a lifestyle change programme for the treatment of obesity in general practice. Swiss Med Wkly. 2003;133:148–154. doi: 10.4414/smw.2003.10109. [DOI] [PubMed] [Google Scholar]
- Hersey JC, Khavjou O, Strange LB, et al. The efficacy and cost-effectiveness of a community weight management intervention: a randomized controlled trial of the health weight management demonstration. Prev Med. 2012;54:42–49. doi: 10.1016/j.ypmed.2011.09.018. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Fryar CD, Carroll MD, Flegal KM. Mean Body Weight, Height, and Body Mass Index, United States 1960–2002. Advance Data from Vital and Health Statistics; No. 347. Hyattsville, MD: National Center for Health Statistics; 2004. [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- Prospective Studies Collaboration. Body-mass index and cause-specific mortality in 900,000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–1096. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick HE, Valsania P, Halter JB, Lin X. Relation of weight gain and weight loss on subsequent diabetes risk in overweight adults. J Epidemiol Community Health. 2000;54:596–602. doi: 10.1136/jech.54.8.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481–1486. doi: 10.2337/dc10-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. NEJM. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc ES, O'Connor E, Whitlock EP, Patnode CD, Kapka T. Effectiveness of primary care-relevant treatments for obesity in adults: a systematic evidence review for the US Preventive Services Task Force. Ann Intern Med. 2011;155:434–471. doi: 10.7326/0003-4819-155-7-201110040-00006. [DOI] [PubMed] [Google Scholar]
- Liu-Seifert H, Zhang S, D'Souza D, Skljarevski V. A closer look at the baseline-observation-carried-forward (BOCF) Patient Prefer Adherence. 2010;4:11–16. doi: 10.2147/ppa.s8135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster-Schubert KE, Alfano CM, Duggan CR, et al. Effect of diet and exercise, alone or combined, on weight and body composition in overweight-to-obese postmenopausal women. Obesity. 2012;20:1628–1638. doi: 10.1038/oby.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skender ML, Goodrick GK, Del Junco DJ, et al. Comparison of 2-year weight loss trends in behavioural treatments of obesity: diet, exercise and combination interventions. J Am Diet Assoc. 1996;96:342–346. doi: 10.1016/S0002-8223(96)00096-X. [DOI] [PubMed] [Google Scholar]
- Villareal DT, Chode S, Parimi N, et al. Weight loss, exercise, or both and physical function in obese older adults. NEJM. 2011;364:1218–1229. doi: 10.1056/NEJMoa1008234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann-Boyce J, Johns DJ, Jebb SA, Aveyard P Behavioural Weight Management Review Group. Effect of behavioural techniques and delivery mode on effectiveness of weight management: systematic review, meta-analysis and meta-regression. Obes Rev. 2014;15:598–609. doi: 10.1111/obr.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller NR, Colagiuri S, Schofield D, et al. A within-trial cost-effectiveness analysis of primary care referral to a commercial provider for weight loss treatment, relative to standard care-an international randomised controlled trial. Int J Obes (Lond) 2013;37:828–834. doi: 10.1038/ijo.2012.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahern AL, Olson AD, Aston LM, Jebb SA. Weight Watchers on prescription: an observational study of weight change among adults referred to Weight Watchers by the NHS. BMC Public Health. 2011;11:434. doi: 10.1186/1471-2458-11-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahern AL, Boyland EJ, Jebb SA, Cohn SR. Participants’ explanatory model of being overweight and their experiences of 2 weight loss interventions. Ann Fam Med. 2013;11:251–257. doi: 10.1370/afm.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. PRISMA flow diagram of review process.
Figure S2. Mean difference in BMI (kg m−2) at 12 months.
Figure S3. Mean difference in percentage weight change from baseline at 12 months.
Figure S4. Mean difference in systolic blood pressure change (mmHg) at 12 months.
Figure S5. Mean difference in diastolic blood pressure change (mmHg) at 12 months.
Figure S6. Mean difference in LDL change (mmol L−1) at 12 months.
Figure S7. Mean difference in HDL change (mmol L−1) at 12 months.
Figure S8. Mean difference in glucose change (mmol L−1) at 12 months.
Figure S9. Mean difference in weight change (kg) at 12 months: sensitivity analysis using complete case data.
Table S1. MEDLINE search strategy.
Table S2. Studies excluded because interventions not delivered in routine context.