Abstract
Objective
The goal of this study was to evaluate the effects of perforation edge approximation and direct application of basic fibroblast growth factor (bFGF) each alone on the healing of large traumatic tympanic membrane perforations with inverted edges in humans.
Study Design
Prospective, sequential allocation, three-armed, controlled clinical study.
Setting
University-affiliated teaching hospital.
Participants
Fifty-eight patients with large traumatic tympanic membrane perforations (i.e. affecting >50% of the surface area) with inverted edges were recruited. They were sequentially allocated to three groups: no intervention (n & 18), edge approximation alone (n & 20) and direct application of bFGF (n & 20). Otoscopy were performed before the treatment and at follow-up visits.
Main outcome measures
The closure rate, closure time and rate of otorrhoea.
Results
Application of bFGF yielded a significantly higher average rate of perforation closure (100%) than edge approximation (60%) and no intervention (56%) (P < 0.05). It also significantly shortened the average closure time (12.4 ± 3.6 days) as compared to edge approximation (46.3 ± 8.7 days) and no intervention control (48.2 ± 5.3 days) (P < 0.05). Purulent otorrhoea was observed in none of the three groups.
Conclusion
Edge approximation of inverted edges has little benefit in improving the healing outcome of large traumatic tympanic membrane perforations and thus is not an ideal treatment option for large traumatic tympanic membrane perforations. Application of bFGF materially improves the closure rate of large traumatic tympanic membrane perforations and significantly shortens the closure time.
In clinical practice, most traumatic tympanic membrane perforations (TMPs) tend to heal spontaneously. However, large perforations often fail to do so.1–5 Hence, controversy continues regarding the treatment of large traumatic TMPs. A majority of otolaryngologists suggest that early myringoplasty should be performed to improve the healing rate.6,7 Nevertheless, this procedure requires general anaesthesia and expensive equipment, resulting in a patients' high cost. Although some authors have suggested that patch treatment could shorten the healing time of large traumatic TMPs, it has little benefits in improving the overall healing rate.3,8–10 In addition, most large traumatic TMPs have curled edges, and the necessity to manipulate curled edges is also controversial. Some authors believe that curled edges may result in abnormal epithelium migration and failure of closure; in particular, inverted edges could migrate into the tympanum and develop middle ear cholesteatoma; ideal management should be to restore the original position.11–14
In one of our recent publications,3 we compared edge approximation plus gelfoam patch, gelfoam patch and spontaneous healing on the outcome of traumatic TMPs with inverted or everted edges. The results showed that gelfoam patch plus edge approximation only shortened the healing time, but did not improve the healing rate. This was in agreement with other reports.8 In addition, in another recent study of ours,5 we compared direct application of basic fibroblast growth factor (bFGF) alone and topical bFGF application plus gelfoam patching on the healing outcome of large traumatic TMPs. We observed that bFGF significantly improved the healing rate and shortened the closure time as compared to spontaneous healing. Some clinical and experimental studies have also demonstrated that topical application of exogenous fibroblast growth factor (FGF) facilitates the healing of experimental eardrum perforations and human chronic eardrum perforations and has no toxic side effects on the inner or middle ear.15–18 The present study was conducted to compare the effects of bFGF application and perforation edge approximation each alone on the healing of large traumatic TMPs with inverted edges.
Materials and methods
Ethical considerations
The study was reviewed and approved by the institutional ethical review board of Wenzhou Medical College-Affiliated Yiwu Hospital, China, and conducted in compliance with the Helsinki Declaration. An informed consent was obtained from each of the participants.
Subjects
A total of 135 patients were diagnosed with a traumatic TMP in the Department of Otorhinolaryngology at the Affiliated Yiwu Hospital of Wenzhou Medical College between February 2012 and May 2012. Of these patients, six refused to participate, 71 failed to fulfil the inclusion criteria and 58 were recruited. The inclusion criteria were as follows: (i) traumatic TMP involving >50% of the surface area with inverted edges or brown crusts derived from inverted edges and (ii) presentation within 7 days of the injury. All perforations were caused by blows to the ear. Patients were excluded if physical examinations revelled any of the following conditions: granulation tissue or purulent otorrhoea within the ear, severe vertigo, profound hearing loss or ossicular disruption to minimise the possible confounding effects of these condition on the treatment outcome.
Before treatment, the tympanic membrane (TM) was examined with an endoscope after cerumen or blood clots were carefully cleaned from the external auditory canal (EAC) with a cotton bud and the EAC was simultaneously photographed with a digital video camera, and the size of the perforation was analysed using Imagej software (NIH, Bethesda, MD, USA) and expressed as a percentage of the whole TM surface area. A large perforation was defined as a perforation estimated to comprise 50% or more of the entire TM. Patient age and sex, date and cause of traumatic injury, TMP size, presence or absence of otorrhoea, presence of ossicular disruption and associated clinical findings such as vertigo, tinnitus and perilymph leakage were all recorded. As it has been well established that healing of the perforation was always associated with successful closure of the air–bone gap,1,3,5,8 audiometric examination was not performed in this study.
Treatment allocation
The 56 subjects were allocated into three groups: no intervention control (n & 18); edge approximation (n & 20); and bFGF application (n & 20). This was performed by the principal investigator with the help of a registered nurse using a sequential allocation method. Specifically, consecutive subjects who both met the inclusion criteria and signed the consent form were alternatively allocated to the three groups based on the order of their initial hospital visit and date of returning the signed consent form.
Treatments
No intervention group
Patients in this group received no intervention, but underwent regular follow-up.
Perforation edge-approximation group
Under an otomicroscope, lidocaine (2%) and epinephrine (1 : 100 000 diluted) were injected into the bony cartilaginous junction of the EAC. The inverted edges, if not crusted and still visible, were moistened with a cotton ball soaked in Ringer's solution and then aligned back to the original position as much as possible using a Rosen needle or a small vacuum suction tip, but no scaffolding material was used.
Basic fibroblast growth factor (bFGF) group
The EAC was cleaned with a cotton bud soaked in povidone–iodine solution. Approximately 0.25 mL (4–5 drops) of a recombinant bovine bFGF (21 000 IU/5 mL) solution (Yi Sheng, Zhuhai City, China) was applied directly onto the TM, and no scaffolding material was used. The first application of bFGF drops was carried out by the doctor at the initial hospital visit. Afterwards, bFGF drops were applied daily by patients themselves at home as instructed until complete perforation closure was confirmed by the physician. The first follow-up examination was performed 2–3 days after the initial hospital visit, and the doctor determined whether the self-application of bFGF by patients at home was correct and whether purulent otorrhoea developed. Incorrect application of bFGF was corrected.
Follow-up
Oral amoxicillin was given to all subjects for 1 week. After the first follow-up visit, patients were followed up at least once per week until complete closure of the perforation or up to 6 months. The TM was examined repeatedly by endoscopy at all follow-up visits. To reduce clinician bias, clinical events such as TM closure or the presence of otorrhoea were photo-documented using colour slides. If the patient had severe vertigo, signs of perilymph leakage were evaluated and patients were excluded from the study. Perforation closure was confirmed by endoscopic examination. Demographic data and outcome measures were expressed as mean ± sd and analysed using a paired chi-squared test or t-test with the spss software (ver.11.0 for Windows; SPSS Inc., Chicago, IL, USA). Differences were considered statistically significant when P < 0.05.
Results
Outcomes
In the 58 patients, only two patients in the control group were lost to follow-up. The final analysis included 56 patients (22 males, 34 females) with a diagnosis of large traumatic TMP with inverted edges. Of these patients, a kidney-shaped perforation was observed in 27 patients, anterior perforation in 26 patients and posterior perforation in three patients.
Summarised in Table 1 are the demographic data of the 56 subject. No significant differences in patient age, sex, duration of injury or cause of injury were observed among the three groups. None of these patients developed purulent otorrhoea.
Table 1.
Demographic data of patients in the observation, edge-approximation and basic fibroblast growth factor application groups
| Group | Observation | Edge approximation | FGF | P |
|---|---|---|---|---|
| No. | 16 | 20 | 20 | |
| Sex, male:female | 5 : 11 | 8 : 12 | 9 : 11 | 0.84 |
| Age, years | 36.2 ± 11.8 | 34.6 ± 15.2 | 36.8 ± 13.7 | 0.64 |
| Duration, days | 4.7 ± 1.2 | 4.1 ± 1.4 | 5.1 ± 1.1 | 0.37 |
| Cause of injury, BI : EB | 15 : 1 | 18 : 2 | 18 : 1 | 0.52 |
| Position of perforation, kidney shape: anterior or posterior | 6 : 10 | 10 : 10 | 11 : 9 | 0.68 |
| The mean size of the perforation | 60.9% ± 4.9% | 59.0% ± 4.0% | 59.5% ± 4.8% | 0.41 |
Cause of injury: BI, blunt injury was primarily caused by a slap against the ear; EB, explosive blast injury was primarily caused by fireworks or firecrackers.
P < 0.05 was considered to indicate statistical significance.
χ2 test.
One-way analysis of variance.
At 6 months, the perforation closure rate in the control, edge approximation and bFGF application groups was 56% (9/16), 60% (12/20) and 100% (20/20), respectively. The mean closure time was 46.3 ± 8.7, 48.2 ± 5.3 and 12.4 ± 3.6 days, respectively. Fibroblast growth factor significantly improved the closure rate and shortened the closure time as compared to no intervention and edge approximation (P < 0.05). However, there was no significant difference either in the closure rate or in the closure time between no intervention and edge-approximation groups (P > 0.05).
Endoscopic findings
Of the 16 patients in the no intervention group, inverted edges gradually became necrotic within a week after injury. Centripetal migratory epithelium did not develop; however, a small amount of centripetal migratory epithelium was noticed in the region without inverted edges. About 2 weeks after injury, inverted edges formed a crust and slightly migrated away from the side of the annulus, and centripetal migratory epithelium developed on both sides with and without inverted edges. Over time, the amount of centripetal migratory epithelium gradually increased, and the perforation closed in nine patients. In other seven patients of this group, centripetal migratory epithelium was not seen along the edge of the malleus. Instead, yellow epithelialisation of the leading edge of centripetal migratory epithelium was seen at the annulus edge within 8–12 weeks, and the perforation failed to close completely (Fig. 1).
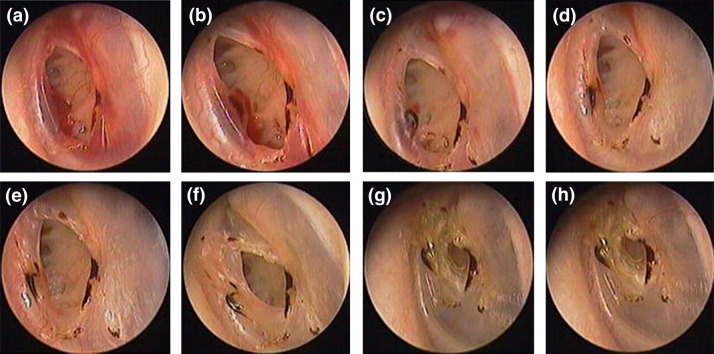
Figure 1.
The spontaneous healing process at various time points: 3 days (a), 6 days (b), 8 days (c), 12 days (d), 16 days (e), 4 weeks (f), 12 weeks (g) and 16 weeks (h).
Of the 20 patients in the edge-approximation group, the perforation obviously became smaller after edge approximation. However, about 1 week after the repair, the TM continually retracted to the original position and formed a crust in eight patients with inverted edges on the side of the malleus (Fig. 2); only mild retraction was seen in 12 patients with inverted edges on the side of the annulus. In addition, centripetal migratory epithelium did not emerge on the side of the inverted edge; nevertheless, centripetal migratory epithelium was seen on the side lacking the inverted edge. Within 6–12 weeks, 12 patients achieved a complete closure and nine patients showed failure in the closure. In eight unhealed patients, centripetal migratory epithelium was not noticed on the side of the malleus; in four of these eight patients, pinpoint perforation was seen between the repairing eardrum and healed eardrum; yellow epithelialisation of the pinpoint perforation edge was also visible (Fig. 3).
Figure 2.
The healing process at various time points after edge approximation: 2 days after perforation (a), approximation of the inverted edge (b), 2 days after treatment (c), 3 days after treatment (d), 1 week after treatment (e), 12 weeks after treatment (f).
Figure 3.
The healing process at various time points after edge approximation: 2 days after perforation (a), approximation of the inverted edge (b), 1 day after treatment (c), 2 days after treatment (d), 6 days after treatment (e), 2 weeks after treatment (f), 8 weeks after treatment (g), 12 weeks after treatment (h).
Of the 20 patients who underwent direct bFGF application, centripetal migratory regenerative epithelium was seen on the sides of both annulus and malleus. The regenerative eardrum was much thicker; however, granulation tissue formation was not evident (Fig. 4). All perforations closed completely with 7–16 days, and complications of auditory canal stenosis and vertigo were not observed during the follow-up period.
Figure 4.

The healing process at various time points after direct application of FGF: 6 days after perforation (a), 3 days after treatment (b), 7 days after treatment (c), 10 days after treatment (d), 2 weeks after treatment (e).
Discussion
Synopsis of findings
Perforation edge approximation as a sole management approach for large TMPs did not result in any significant outcome benefits in terms of the healing rate and mean closure time as compared to spontaneous healing. In contrast, direct application of FGF significantly facilitated the healing of large traumatic TMPs and improved the healing rate, without the curled edge approximation. In addition, no abnormal migratory epithelium was observed during the healing process of large traumatic TMPs with inverted edges.
Comparisons with other studies
It is believed that curled TMP edges may result in abnormal epithelial migration and closure failure.8,15 Benefits of approximation of curled perforation edges in reducing the perforation size and facilitating healing have been reported previously.8,11–14 In this study, curled edge approximation alone neither speeded up the closure nor improved the overall healing rate in cases of large perforations as compared to spontaneous healing. Otoscopic examinations showed that the approximated curled edges on the side of the malleus handle returned to their original positions. The underlying mechanisms were not clear, but contraction of the tympanic muscles and sustained vibration of the malleus handle might be involved. Otoscopic examinations also showed that the approximated perforation edges did not show any signs of healing and instead the perforation closure started on the non-curled edge side where centripetal migration of epithelial cells was evident. Nevertheless, in four cases of edge approximation, a needlepoint hole remained unclosed between the newly regenerated membrane and the approximated membrane by the end of the 6-month follow-up. This phenomenon was not seen in our previous studies where gelfoam patching alone or in combination with edge approximation was applied.3
In this study, direct application of bFGF alone improved the overall healing rate and shortened the average healing time in large TMPs as compared to spontaneous healing. These findings are consistent with results from both clinical studies of our own and experimental studies of other investigators.16–19 No adverse effects were observed either in this study or in our previous studies5,17 following the application of bFGF.
Strengths of the study
Only large traumatic TMPs with inverted edges were included in this study. No scaffold was used in edge-approximation group. In this way, we could accurately assess the pathologic changes of the approximated edges compared with the prior study with a scaffold.3,8–10,12–14 In addition, a relatively long follow-up (6-months) was used, allowing assessment of the large TMP healing process in an extended time course.
Limitations of study
In this study, the sample size was relatively smaller than that in our previous studies. Moreover, no histological assessment was performed. These limitations may somewhat comprise the significance of our findings. Histological assess-ment of the healing process in large traumatic TMPs following perforation edge approximation or direct FGF appli-cation in a large sample size is warranted in further studies.
Clinical implications
Approximation of curled edges seems not to be an ideal treatment option for large TMPs; it neither improves the closure rate nor reduces the closure time, and it requires local or general anaesthesia. In a sharp contrast, topical application of bFGF, which can be simply carried out by patients themselves at home, is able to improve the closure rate and shorten the closure time without significant adverse effects. As a result, bFGF drops may be considered as an ideal management option for large traumatic TMPs.
Conflict of interest
The authors have no funding, financial relationships or conflict of interest to disclose.
References
- 1.Orji FT. Agu CC. Determinants of spontaneous healing in traumatic perforations of the tympanic membrane. Clin. Otolaryngol. 2008;33:420–426. doi: 10.1111/j.1749-4486.2008.01764.x. [DOI] [PubMed] [Google Scholar]
- 2.Hempel JM, Becker A, Muller J, et al. Traumatic tympanic membrane perforations: clinical and audiometric findings in 198 patients. Otol Neurotol. 2012;33:1357–1362. doi: 10.1097/MAO.0b013e31826939b5. [DOI] [PubMed] [Google Scholar]
- 3.Lou ZC. He JG. A randomised controlled trial comparing spontaneous healing, gelfoam patching and edge-approximation plus gelfoam patching in traumatic tympanic membrane perforation with inverted or everted edges. Clin. Otolaryngol. 2011;36:221–226. doi: 10.1111/j.1749-4486.2011.02319.x. [DOI] [PubMed] [Google Scholar]
- 4.Ritenour AE, Wickley A, Ritenour JS, et al. Tympanic membrane perforation and hearing loss from blast overpressure in Operation Enduring Freedom and Operation Iraqi Freedom Wounded. J. Trauma. 2008;64:S174–S178. doi: 10.1097/TA.0b013e318160773e. [DOI] [PubMed] [Google Scholar]
- 5.Zheng cai L. Healing large traumatic eardrum perforations in humans using fibroblast growth factor applied directly or via gelfoam. Otol Neurotol. 2012;33:1553–1557. doi: 10.1097/JES.0b013e31826f5640. [DOI] [PubMed] [Google Scholar]
- 6.Sprem N, Branica S. Dawidowsky K. Tympanoplasty after war blast lesions of the eardrum: retrospective study. Croat. Med. J. 2001;42:642–645. [PubMed] [Google Scholar]
- 7.Conoyer M, Kaylie DM. Jackson CG. Otologic surgery following ear trauma. Otolaryngol. Head Neck Surg. 2007;137:757–761. doi: 10.1016/j.otohns.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 8.Park MK, Kim KH, Lee JD, et al. Repair of large traumatic tympanic membrane perforation with a Steri-Strips patch. Otolaryngol. Head Neck Surg. 2011;145:581–585. doi: 10.1177/0194599811409836. [DOI] [PubMed] [Google Scholar]
- 9.Camnitz PS. Bost WS. Traumatic perforations of the tympanic membrane: early closure with paper tape patching. Otolaryngol. Head Neck Surg. 1985;93:220–223. doi: 10.1177/019459988509300217. [DOI] [PubMed] [Google Scholar]
- 10.Han MA, Park SN, Park KH, et al. Therapeutic effect of multiple paper patching for traumatic tympanic membrane perforation-trial of quantitative analysis using image analyzer. Korean J. Otorhinolaryngol.-Head Neck Surg. 2008;51:518–523. [Google Scholar]
- 11.Bellucci RJ. Traumatic injuries of the middle ear. Otolaryngol. Clin. North Am. 1983;16:633–650. [PubMed] [Google Scholar]
- 12.Saito H, Kazama Y. Yazawa Y. Simple maneuver for closing traumatic eardrum perforation by micropore strip tape patching. Am. J. Otol. 1990;11:427–430. [PubMed] [Google Scholar]
- 13.Winerman I, Man A. Segal S. Early repair of traumatic perforations of the tympanic membrane in children. Int. J. Pediatr. Otorhinolaryngol. 1982;4:23–27. doi: 10.1016/0165-5876(82)90074-x. [DOI] [PubMed] [Google Scholar]
- 14.Armstrong B. Traumatic perforations of the tympanic membrane: observe or repair? Laryngoscope. 1972;82:1822–1830. doi: 10.1288/00005537-197210000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Somers T, Houben V, Goovaerts G, et al. Histology of the perforated tympanic membrane and its muco-epithelia junction. Clin. Otolaryngol. Allied Sci. 1997;22:162–166. doi: 10.1046/j.1365-2273.1997.00006.x. [DOI] [PubMed] [Google Scholar]
- 16.Fina M, Baird A. Ryan A. Direct application of basic fibroblast growth factor improves tympanic membrane perforation healing. Laryngoscope. 1993;103:804–809. doi: 10.1288/00005537-199307000-00015. [DOI] [PubMed] [Google Scholar]
- 17.Hakuba N, Iwanaga M, Tanaka S, et al. Basic fibroblast growth factor combined with atelocollagen for closing chronic tympanic membrane perforations in 87 patients. Otol Neurotol. 2010;31:118–121. doi: 10.1097/MAO.0b013e3181c34f01. [DOI] [PubMed] [Google Scholar]
- 18.Kase K, Iwanaga T, Terakado M, et al. Influence of topical application of basic fibroblast growth factor upon inner ear. Otolaryngol. Head Neck Surg. 2008;138:523–527. doi: 10.1016/j.otohns.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 19.Kanemaru S, Umeda H, Kitani Y, et al. Regenerative treatment for tympanic membrane perforation. Otol Neurotol. 2011;32:1218–1223. doi: 10.1097/MAO.0b013e31822e0e53. [DOI] [PubMed] [Google Scholar]