Abstract
Background
Prospective cohort studies have indicated that serum vitamin D levels are inversely related to risk of type 2 diabetes. However, such studies cannot determine the source of vitamin D. Therefore, we examined the association of dietary vitamin D intake with incident type 2 diabetes within the European Prospective Investigation into Cancer and Nutrition (EPIC)-InterAct study in a heterogeneous European population including 8 countries with large geographical variation.
Methods
Using a case-cohort design, 11,245 incident cases of type 2 diabetes and a representative subcohort (N=15,798) were included in the analyses. Hazard ratios (HR) and 95% confidence intervals (CIs) for type 2 diabetes were calculated using a Prentice-weighted Cox regression adjusted for potential confounders. 24-h diet recall data from a subsample (N=2347) were used to calibrate habitual intake data derived from dietary questionnaires.
Results
Median follow-up time was 10.8 years. Dietary vitamin D intake was not significantly associated with the risk of type 2 diabetes. HR and 95 % CIs for the highest compared to the lowest quintile of uncalibrated vitamin D intake was 1.09 (0.97-1.22), (ptrend=0.17). No associations were observed in a sex-specific analysis. The overall pooled effect [HR (95% CI)] using the continuous calibrated variable was 1.00 (0.97-1.03) per increase of 1 μg/day dietary vitamin D.
Conclusion
This observational study does not support an association between higher dietary vitamin D intake and type-2 diabetes incidence. This result has to be interpreted in light of the limited contribution of dietary vitamin D on the overall vitamin D status of a person.
Keywords: vitamin D, type-2 diabetes, dietary intake, observational study, EPIC
Introduction
Besides the ‘classical’ function of vitamin D in calcium homeostasis, additional biological effects of vitamin D on diabetes-related outcomes have been identified. The results of experimental cell, animal and human studies support a role of vitamin D in the prevention of type 2 diabetes.1-4 However, the mechanisms by which vitamin D may affect type 2 diabetes risk are not fully understood. Effects of vitamin D on insulin secretion, insulin sensitivity as well as on inflammatory and autoimmune conditions associated with diabetic conditions are reported.4-9
Evidence from prospective observational studies for an inverse association between vitamin D status, as characterized by serum 25-hydroxyvitamin D [25(OH)D] measurements, and type 2 diabetes is strong.10-12 After combining the data from nine prospective studies that measured serum 25(OH)D, participants in the highest (versus lowest) quartile of 25(OH)D showed a 41% (95% CI, 33%, 48%) lower risk of type-2 diabetes though any causal inference of this association remains unconfirmed.13 Moreover, a recent metaanalysis combining results from 14 observational studies reported a significant inverse association for circulating 25(OH)D but not for dietary vitamin D.14 Serum 25(OH)D concentration, the accepted biomarker of vitamin D status, reflects both the endogenous synthesis in the skin (following UVB exposure) and the dietary intake of vitamin D. Though in general the contribution of dietary vitamin D to the overall vitamin D status is of minor importance as compared to the endogenous synthesis of vitamin D, intra- and inter-subject variation in UVB exposure can be high, and the importance of vitamin D from diet substantially increases in situations with limited UVB exposure. Thus, analyses on the impact of dietary vitamin D and diabetes risk are meaningful. Mitri and colleagues combined data from three cohort studies and found a borderline significant inverse association between vitamin D intake >500 U/d (12.5 μg/d) and type 2 diabetes incidence (versus subjects with a vitamin D intake < 200 U/d (5 μg/d)); the relative risk was 0.87 (95% CI, 0.76-0.99).11 However, only one out of three cohort studies reported a significant inverse association.15-17
Due to its large sample size, the InterAct project within the European Prospective Investigation into Cancer and Nutrition (EPIC) provides the opportunity to investigate the association between the incidence of type 2 diabetes and dietary vitamin D prospectively in both men and women. Furthermore, the study allows a relatively wide range of dietary intake levels since data from different European countries are included.
Material and Methods
Study design and population
EPIC is a multi-centre prospective cohort study conducted since 1992 in 10 European countries.18 In the present EPIC-InterAct study, a nested case-cohort design within 8 of the 10 countries contributing to the EPIC cohort was used.19 The current analysis is based on data from EPIC participants in Denmark, France, Germany, Italy, the Netherlands, Spain, Sweden, and the United Kingdom (UK).
Following the case-cohort design, the study population consisted of a random baseline sample of 16.154 participants (subcohort) and 12.403 incident cases of type 2 diabetes (including 778 from the subcohort), i.e. all participants of the entire cohort with a first diagnosis of type 2 diabetes between recruitment and December 31, 2007.19
The final study sample after excluding participants without dietary information (N=736) consisted of 27,043 participants, including 15,798 members of the subcohort and 11,994 incident diabetes cases (including 749 cases from the subcohort).
Dietary assessment
Diet over the previous twelve months was assessed using dietary assessment instruments that were specifically developed for each participating country.18 The questions were structured by common food groups except for the questionnaires used in Italy and Spain, where questions were structured by country-specific meals.
All participants were asked to report their average consumption of each food by structured categories ranging from never or less than once per month to six or more times per day. In Germany, Italy, The Netherlands and Spain individual average portions were estimated, whereas standard portions were assigned to the participants in Denmark, the United Kingdom and Umeå, Sweden. A combination of methods for estimating portion size was used in Malmo, Sweden. All dietary measurement instruments have been validated previously in a series of studies within the various source populations participating in EPIC.20
Daily intake of vitamin D, calcium, and magnesium was calculated by means of a standardized food composition data base across EPIC countries 21.
Demographic and lifestyle factors
To collect information on education, medical history (surgeries and previous illnesses), tobacco and alcohol consumption, and physical activity and other lifestyle factors, additional questionnaires were used. Height and weight were measured at baseline, except for the France and Oxford-UK study centre, where height and weight was self-reported.18
Ascertainment of type 2 diabetes
Ascertainment and verification of incident diabetes has been described in detail elsewhere.19 In brief, incident cases were identified by self-reports (history of diabetes, physician-diagnosed diabetes, antidiabetic drug use), linkage to primary and secondary care registers, linkage to drug registers, hospital admissions and mortality data. Further verification of the diabetic cases came from individual medical record reviews.
Statistical analysis
Cox proportional hazard models adapted for case-cohort design according to the Prentice method were used to calculate hazard ratios (HR) and 95% confidence intervals (CI).22 A Cox model was used with stratification by age (in 1-year categories), centre and sex; Incident type 2 diabetes was the outcome variable and age was used as the underlying timescale.
Hazard ratios for dietary vitamin D intake are presented comparing quintiles taking the lowest quintile as reference. Quintile cutpoints were defined among subcohort members only. Different models were run to disentangle the effects of dietary vitamin D on type 2 diabetes: model 1: adjusted for total energy (kcal/day, cont.); model 2: adjusted for non-fat-energy (kcal/day, cont.), fat (g/day, cont., monounsaturated, polyunsaturated and saturated fat), physical activity (occupational, recreational and household activity; inactive, moderately inactive, moderately active, active, missing)23, and model 3: as model 2 with further adjustment for a-priori defined potential confounders and risk factors for diabetes: BMI (kg/m2, cont.), education level (none or primary school, technical/professional school, secondary school, longer education incl. university degree, unknown), smoking status (never, former, current, unknown), alcohol intake (g/day, cont.). Sensitivity analyses were performed excluding incident cases that occurred within the first two years of follow-up as well as additional adjustment for waist-hip-ratio.
In order to account for different levels of sunlight exposure, the EPIC centres were divided into 7 latitude group: below 42°N (Granada, Murcia, Ragusa, and Naples); 42°N - 44°N (Asturias, Navarra, San Sebastian, Florence, and South coast of France – centred in Marseille); 45°N - 46°N (Varese, Turin, and South of France – centred in Lyon); 47°N - 49°N (North-East and West of France – centred in Nantes and Paris, respectively – and Heidelberg); 50°N - 51°N (Potsdam, Utrecht, Bilthoven, Cambridge, and Oxford); 52°N - 56°N (Malmö, Aarhus, and Copenhagen); and above 57°N (Norway, and Umeå). A further sensitivity analysis was thus performed with additional adjustment for latitude.
Statistical interaction (heterogeneity) was evaluated with the Wald test by including cross-product terms of potential interaction variables (i.e., sex, physical activity, BMI) in the respective models. Tests for trend over quintiles of intake (coded 1 to 5) were performed using the Wald statistics.
To correct for measurement error, dietary intake data calculated from questionnaire data were calibrated against 24 hour dietary recall data for a subsample (N = 2347). A fixed-effects linear model was used in which centre and sex-specific recall data were regressed on the dietary intakes.24-26 The calibrated dietary data were used to model dietary intake of vitamin D continuously in the whole sample and in the country-specific analysis. We used a meta-analytic approach to investigate heterogeneity across centres (metan procedure in STATA) by pooling the adjusted HR’s per centre using the random effects model.
All tests were two-sided and considered to be statistically significant with a p-value of ≤0.05. All analyses were conducted using SAS version 9.1 (SAS Institute, Cary, North Carolina) and STATA version 10 for meta-analysis (College Station, Texas).
Results
Median vitamin D intake in the subcohorts of the respective countries by sex is shown in Table 1. Dietary vitamin D intake was highest in Sweden and lowest in Italy. Vitamin D intake was higher in men across all countries.
TABLE 1. Dietary vitamin D intake by sex and country in the subcohort1: the EPIC-InterAct study.
| Country | Men | Women | ||
|---|---|---|---|---|
| Median | IQR | Median | IQR | |
| All | 3.6 | 1.6-7.7 | 2.4 | 1.1-4.6 |
|
| ||||
| France | -- | -- | 1.3 | 0.7-2.5 |
| Italy | 1.3 | 0.8-2.7 | 1.0 | 0.5-2.1 |
| Spain | 2.7 | 1.3-8.7 | 1.7 | 0.9-4.4 |
| UK | 3.5 | 1.7-5.2 | 2.4 | 1.7-3.8 |
| Netherlands | 3.4 | 2.3-7.4 | 2.5 | 1.8-3.9 |
| Germany | 2.0 | 1.1-3.6 | 1.5 | 0.8-3.3 |
| Sweden | 7.1 | 4.5-9.6 | 4.7 | 3.3-6.7 |
| Denmark | 2.9 | 1.4-7.7 | 1.7 | 1.0-3.9 |
Vitamin D intake in μg/day from 24-hour recall data (N =2347); IQR, inter-quartile range
Median age at baseline was 52.7 years (IQR 46.5-59.2). The median follow-up time for the study population was 10.8 years. Descriptive characteristics of the subcohort by quintiles of vitamin D intake are shown in Table 2. The percentage of current smokers was lower in men but higher in women with higher vitamin D intake. The increasing numbers of participants with unknown physical activity with increasing vitamin D intake is due to physical activity data not being collected in Umeå, Sweden, where vitamin D intake is high. No differences in BMI were observed by quintiles of vitamin D intake. Total fish, meat, calcium and energy intake was higher in both men and women with increasing vitamin D intake (table 2).
TABLE 2. Baseline characteristics in the subcohort by sex and quintiles of dietary vitamin D intake: the EPIC-InterAct study.
| Vitamin D (μg/day) | Quintile 1 < 2.19 (Median: 1.6) | Quintile 2 2.19-<3.13 (Median: 2.6) | Quintile 3 3.13-<4.27 (Median: 3.7) | Quintile 4 4.27-<6.07 (Median: 5.0) | Quintile 5 ≥6.07 (Median: 7.9) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Men | N | % | N | % | N | % | N | % | N | % |
| N = 744 | N = 903 | N = 1109 | N = 1418 | N = 1794 | ||||||
| Smoking status | ||||||||||
| Never | 201 | 27 | 275 | 30 | 353 | 32 | 461 | 33 | 591 | 33 |
| Former | 278 | 37 | 349 | 39 | 402 | 36 | 515 | 36 | 627 | 35 |
| Current | 259 | 35 | 269 | 30 | 333 | 30 | 430 | 30 | 567 | 32 |
| Unknown | 6 | 1 | 10 | 1 | 21 | 2 | 12 | 1 | 9 | 1 |
| Education | ||||||||||
| Primary school or less | 348 | 47 | 335 | 37 | 413 | 37 | 502 | 35 | 731 | 42 |
| Technical/professional school | 140 | 19 | 200 | 22 | 260 | 23 | 351 | 25 | 388 | 22 |
| Other secondary education | 92 | 12 | 133 | 15 | 132 | 12 | 160 | 11 | 260 | 14 |
| University | 154 | 21 | 220 | 24 | 281 | 25 | 385 | 27 | 398 | 22 |
| Unknown | 10 | 1 | 15 | 2 | 23 | 2 | 20 | 1 | 17 | 1 |
| Physical activity | ||||||||||
| Inactive | 201 | 27 | 258 | 29 | 284 | 26 | 374 | 26 | 348 | 19 |
| Moderately inactive | 243 | 33 | 274 | 30 | 339 | 31 | 397 | 28 | 603 | 34 |
| Moderately active | 224 | 30 | 261 | 29 | 314 | 28 | 359 | 25 | 474 | 26 |
| Active | 60 | 8 | 79 | 9 | 91 | 8 | 114 | 8 | 108 | 6 |
| Unknown | 16 | 2 | 31 | 3 | 81 | 7 | 174 | 12 | 261 | 15 |
|
| ||||||||||
| Median | IQR | Median | IQR | Median | IQR | Median | IQR | Median | IQR | |
|
| ||||||||||
| Age at recruitment | 52.7 | 46.3-58.4 | 52.8 | 45.9-58.5 | 52.9 | 46.5- 58.6 | 53.0 | 47.3- 59.6 | 53.8 | 48.6- 60.3 |
|
| ||||||||||
| BMI (kg/m2) | 26.7 | 24.5-28.9 | 26.4 | 24.1-28.8 | 26.2 | 24.2-28.6 | 26.3 | 24.5-28.7 | 26.2 | 24.0-28.7 |
| Total energy (Kcal/d) | 2048 | 1685-2457 | 2256 | 1860-2713 | 2330 | 1963-2736 | 2443 | 2052-2869 | 2678 | 2273-3150 |
| Saturated fat (g/d) | 24.5 | 18.6-31.0 | 30.1 | 23.4-37.4 | 32.2 | 24.9-40.8 | 34.8 | 27.0-43.5 | 41.2 | 31.8-52.4 |
| Monounsaturated fat (g/d) | 28.3 | 21.0-38.9 | 31.5 | 23.0-41.9 | 31.5 | 24.3-42.0 | 33.6 | 26.5-42.7 | 39.2 | 31.6-48.7 |
| Polyunsaturated fat (g/d) | 9.9 | 7.6-13.6 | 11.6 | 9.2-15.3 | 13.2 | 10.4-16.8 | 14.0 | 10.9-18.4 | 16.7 | 13.2-21.7 |
| Alcohol (g/d) | 13.5 | 3.1-34.0 | 16.3 | 5.4-38.4 | 16.4 | 5.2-37.3 | 14.8 | 4.9-37.8 | 13.2 | 4.1-31.0 |
| Calcium (mg/d) | 804 | 595-1047 | 885 | 669-1173 | 931 | 720-1233 | 968 | 735-1261 | 1109 | 836-1400 |
| Magnesium (g/d) | 335 | 267-410 | 351 | 295-421 | 369 | 312-438 | 391 | 323-458 | 412 | 347-483 |
| Coffee (g/d) | 120 | 56-413 | 175 | 65-500 | 287 | 90-600 | 385 | 131-665 | 400 | 145-675 |
| Soft drinks (g/d) | 0.0 | 0.0-39.4 | 6.7 | 0.0-66.7 | 8.1 | 0.0-85.7 | 16.4 | 0.0-97.5 | 21.3 | 0.0-114 |
| Total meat (g/d) | 101 | 66-146 | 119 | 86-159 | 130 | 91-170 | 134 | 98-178 | 142 | 102-193 |
| Total fish (g/d) | 10.0 | 3.1-22.4 | 16.4 | 8.2-28.4 | 22.7 | 12.7-36.3 | 29.4 | 15.3-47.6 | 37.7 | 15.4-67.7 |
| Vitamin D (μg/day) | Quintile 1 < 2.19 (Median: 1.6) | Quintile 2 2.19-<3.13 (Median: 2.6) | Quintile 3 3.13-<4.27 (Median: 3.7) | Quintile 4 4.27-<6.07 (Median: 5.0) | Quintile 5 ≥6.07 (Median: 7.9) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Women | N | % | N | % | N | % | N | % | N | % |
| N = 2415 | N = 2257 | N = 2050 | N = 1742 | N = 1366 | ||||||
| Smoking status | ||||||||||
| Never | 1416 | 59 | 1244 | 55 | 1143 | 56 | 963 | 55 | 694 | 51 |
| Former | 482 | 20 | 523 | 23 | 440 | 21 | 350 | 20 | 296 | 22 |
| Current | 494 | 20 | 476 | 21 | 447 | 22 | 408 | 23 | 367 | 27 |
| Unknown | 23 | 1 | 14 | 1 | 20 | 1 | 21 | 1 | 9 | 1 |
| Education | ||||||||||
| Primary school or less | 1077 | 45 | 916 | 41 | 780 | 38 | 672 | 39 | 571 | 42 |
| Technical/professional school | 443 | 18 | 499 | 22 | 508 | 25 | 447 | 26 | 366 | 27 |
| Other secondary education | 438 | 18 | 393 | 17 | 363 | 18 | 249 | 14 | 157 | 11 |
| University | 420 | 17 | 408 | 18 | 356 | 17 | 339 | 19 | 255 | 19 |
| Unknown | 37 | 2 | 41 | 2 | 43 | 2 | 35 | 2 | 17 | 1 |
| Physical activity | ||||||||||
| Inactive | 274 | 11 | 263 | 12 | 248 | 12 | 197 | 11 | 151 | 11 |
| Moderately inactive | 611 | 25 | 551 | 24 | 466 | 23 | 435 | 25 | 418 | 31 |
| Moderately active | 1237 | 51 | 1111 | 49 | 951 | 46 | 769 | 44 | 588 | 43 |
| Active | 244 | 10 | 238 | 11 | 214 | 10 | 156 | 9 | 102 | 7 |
| unknown | 49 | 2 | 94 | 4 | 171 | 8 | 185 | 11 | 107 | 8 |
|
| ||||||||||
| Median | IQR | Median | IQR | Median | IQR | Median | IQR | Median | IQR | |
|
| ||||||||||
| Age at recruitment | 52.3 | 45.3-58.8 | 52.4 | 46.3-58.7 | 51.9 | 46.0-58.5 | 52.1 | 46.1-59.3 | 53.3 | 47.1-61.0 |
|
| ||||||||||
| BMI (kg/m2) | 24.9 | 22.4-28.3 | 24.9 | 22.5-27.9 | 25.0 | 22.6-28.1 | 24.7 | 22.5-28.0 | 25.0 | 22.6-28.2 |
| Total energy (Kcal/d) | 1625 | 1353-1926 | 1836 | 1529-2185 | 1916 | 1604-2282 | 2001 | 1695-2404 | 2185 | 1861-2563 |
| Saturated fat (g/d) | 22.1 | 16.5-27.8 | 27.0 | 21.1-34.0 | 28.5 | 21.8-36.3 | 30.7 | 24.1-39.0 | 35.2 | 26.7-44.0 |
| Monounsaturated fat (g/d) | 22.3 | 16.3-30.5 | 25.4 | 19.1-33.8 | 26.4 | 20.5-35.0 | 27.8 | 22.4-35.8 | 32.3 | 26.3-40.2 |
| Polyunsaturated fat (g/d) | 8.8 | 6.8-11.4 | 10.7 | 8.4-14.0 | 11.5 | 8.9-14.7 | 12.3 | 9.4-16.4 | 14.2 | 11.3-18.2 |
| Alcohol (g/d) | 1.9 | 0.0-10.1 | 3.0 | 0.3-11.6 | 3.7 | 0.4-11.6 | 4.0 | 0.6-12.1 | 5.0 | 0.7-12.8 |
| Calcium (mg/d) | 806 | 614-1033 | 904 | 711-1147 | 957 | 727-1212 | 995 | 770-1244 | 1062 | 861-1326 |
| Magnesium (g/d) | 283 | 233-343 | 309 | 257-369 | 327 | 275-386 | 337 | 283-404 | 348 | 300-408 |
| Coffee (g/d) | 150 | 58-437 | 250 | 84-500 | 290 | 100-523 | 330 | 129-582 | 375 | 150-600 |
| Soft drinks (g/d) | 0.0 | 0.0-36.2 | 0.0 | 0.0-46.2 | 3.3 | 0.0-55.7 | 6.5 | 0.0-85.7 | 6.0 | 0.0-85.7 |
| Total meat (g/d) | 75 | 47-106 | 91 | 62-120 | 94 | 65-129 | 95 | 68-126 | 99 | 71-131 |
| Total fish (g/d) | 9.5 | 3.2-21.5 | 16.1 | 7.0-29.7 | 22.7 | 9.8-36.0 | 27.2 | 11.2-46.3 | 37.8 | 17.4-66.2 |
N = 15798; IQR: inter-quartile range
No significant association was observed between vitamin D intake and type 2 diabetes risk (Table 3). Hazard ratios (HR) and 95 % confidence intervals (CI) in the most adjusted model for the higher quintiles compared to the lowest were 1.04 (0.95-1.14), 1.02 (0.93-1.12), 1.06 (0.96-1.17) and 1.09 (0.97-1.22) (p-trend=0.17) (Table 3). We further performed a separate analysis by sex and did not find any significant association between diabetes incidence and vitamin D intake in either men or women (pinteraction = 0.40). No statistical interaction was observed between BMI or physical activity groups and dietary vitamin D (pinteraction> 0.05). Moreover, there was no statistical interaction between vitamin D and calcium intake (p = 0.93).
TABLE 3. Dietary vitamin D intake and risk of type 2 diabetes: the EPIC-InterAct study.
| Model 11 | Model 21 | Model 31 | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Quintiles of vitamin D intake (μg/day) | HR | 95% CI | HR | 95% CI | HR | 95% CI |
| < 2.19 | Ref. | Ref. | Ref. | |||
| 2.19-<3.13 | 1.04 | (0.96 - 1.13) | 1.02 | (0.94-1.11) | 1.04 | (0.95-1.14) |
| 3.13-<4.27 | 1.02 | (0.94 - 1.11) | 1.00 | (0.91-1.08) | 1.02 | (0.93-1.12) |
| 4.27-<6.07 | 1.09 | (1.00 - 1.19) | 1.05 | (0.96-1.15) | 1.06 | (0.96-1.17) |
| ≥6.07 | 1.18 | (1.06 - 1.30) | 1.10 | (0.99-1.22) | 1.09 | (0.97-1.22) |
| p-trend | 0.002 | 0.078 | 0.170 | |||
All models stratified by sex, age and study centre; model 1: adjustment for total energy; model 2: adjustment for non-fat energy, polyunsaturated fatty acids, monounsaturated fatty acids, saturated fatty acids, physical activity; model 3: as model 2 with further adjustment for education, BMI, smoking, alcohol intake; HR = hazard ratio, CI = confidence interval.
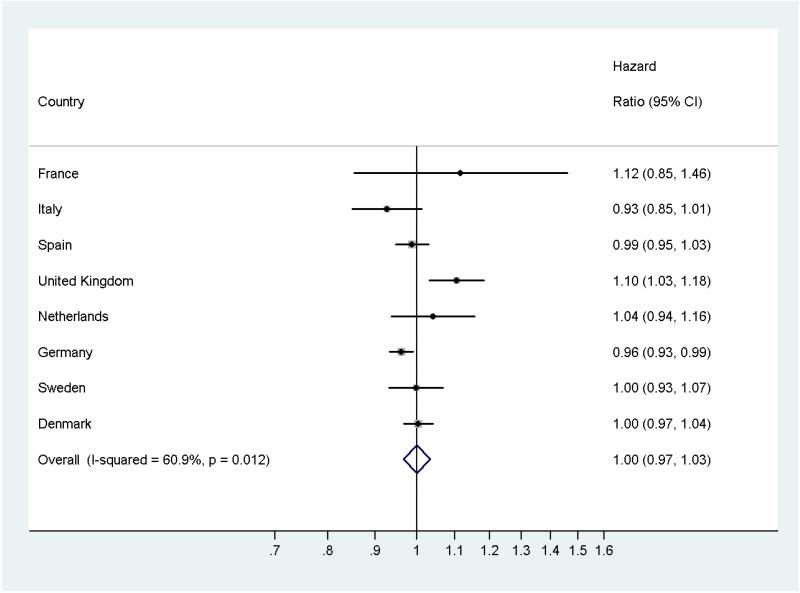
To account for potential measurement error when calculating dietary intake from questionnaire data, we further performed an analysis using the continuous intake data calibrated with 24-hr dietary recall data from a subsample of the subcohort. The overall estimate showed no association for vitamin D and diabetes risk [HR (95% CI) =1.00 (0.97-1.03), per 1 μg/day increment] (Figure 1). The corresponding effect estimate using the observed (continuous) data was HR (95% CI) =1.03 (0.98-1.08). Country specific hazard ratios for the calibrated vitamin D intake (per 1 μg/day increment) in comparison with the overall effect estimate are shown in the forest plot (Figure 1). There was statistically significant heterogeneity across countries (I2=60.9%, p = 0.012). Heterogeneity could in part be explained by the significant positive association in the UK data as exclusion of the UK data resulted in a non-significant and substantially decreased heterogeneity (I2=18.6%, p = 0.228). In a sensitivity analysis, we excluded incident cases that occurred within the first two years of follow-up. No considerable changes in the hazard ratios were observed. We furthermore additionally adjusted for waist-hip-ratio and did not observe any changes in the effect estimates (waist-hip-ratio data are missing for the Umeå centre, n = 1796). As a proxy for sun exposure and thus endogenous vitamin D production we divided the centres in 7 categories according to their latitude. Adjustment for latitude did not notably change the observed risk estimates for vitamin D and typ-2 diabetes risk. Furthermore, additional adjustment for calcium intake did not notably change the risk estimates.
FIGURE 1. Dietary vitamin D intake (per 1 μg/day) and risk of type 2 diabetes, by country and overall: the EPIC-InterAct study; CI = confidence interval.
(Models stratified by sex and age; adjusted for non-fat energy, polyunsaturated fatty acids, monounsaturated fatty acids, saturated fatty acids, physical activity, education, BMI, smoking, alcohol intake.)
Discussion
In this large prospective case-cohort analysis within the European Prospective Investigation into Cancer and Nutrition dietary vitamin D was not significantly associated with the incidence of type 2 diabetes. This was true for men and women. So far, only few cohort studies prospectively assessed the association between dietary vitamin D with the incidence of type 2 diabetes or diabetes related outcomes.15-17 In line with our results, the Nurses’ Health Study did not find a significant association between dietary or total intake of vitamin D and type 2 diabetes.16 Furthermore, our results corroborate the findings from a large Japanese cohort that reported no association in men or in women.17 However, the Women’s Health Study reported an inverse association between dietary vitamin D intake and the prevalence of metabolic syndrome15, and also a meta-analysis of these three studies found a borderline inverse association between vitamin D intake >500 U/d (>12.5 μg/d) and type 2 diabetes incidence.11
Systematic reviews focusing on eight randomized trials did also not provide clear evidence for an inverse association of vitamin D supplementation with hyperglycemia or incident type 2 diabetes.11 The so far largest trial, the Women’s Health Initiative (WHI) study, did not observe any risk reduction of type 2 diabetes over 7 years of follow-up in the intervention arm with supplementation of vitamin D (400 IU/d) and calcium (1000 mg/d).27 However, the authors noted, that higher doses of vitamin D may have been necessary to affect diabetes risk. Several explanations are possible as to why we did not observe any association between dietary vitamin D and type 2 diabetes risk in our study: 1) there is indeed no relationship; 2) the amount and variation of dietary vitamin D intake is too low in the population to detect potential effects; especially, dietary vitamin D intake is distinctly lower in most European countries as compared to the US where staple food is frequently fortified with vitamin D, and thus dietary vitamin D intake more effectively impacts on the overall vitamin D status of a person.
The present study is thus far the largest study prospectively assessing the association between dietary vitamin D intake and the risk of type 2 diabetes. Thus, power was high to detect even weak associations. Further strengths of the study were the high quality of physician-verified diagnoses of type 2 diabetes; the calibration of nutrient intake data from questionnaires with that from 24 h recall data; and the inclusion of detailed information on potential confounders in the analysis. However, due to the observational study design, residual confounding cannot completely be ruled out. A further strength of the study is the wide range of intake from different European countries with different eating habits. The intake data in our study was comparable to that in representative studies in Europe.28-30 Particularly, the here presented higher intake in the northern European countries, e.g. Sweden, where intake of vitamin D-rich foods and the contribution from fortified foods is high, is in line with previous reports.28,31 There are, however, several limitations that should be noted when interpreting the results. Firstly, we did not account for vitamin D from supplements. Additional adjustment on unspecified supplement intake in the study population did not change the risk estimates, but calculated amounts of vitamin D from supplements were not available. Secondly, the contribution of endogenous production of vitamin D (following UVB exposure) could not be addressed here. In a sensitivity analysis, we adjusted for centers’ latitude as a proxy for sun exposure, which did not affect the risk estimates. However, since the contribution of diet to the overall vitamin D status of a person is low, studies with information on the entire vitamin D status may lead to a different conclusion. Indeed, prospective observational studies with serum 25(OH)D measurements showed a strong inverse association between vitamin D status and type 2 diabetes.10-13 However, these studies can also be subject to substantial residual confounding, e.g. by imprecise assessment of outdoor physical activities which are correlated with both UVB exposure and, eventually, with vitamin D status as well as with diabetes risk. In summary, in the present study we did not observe an association between dietary vitamin D intake and the risk of type 2 diabetes in a large European study population. However, it has to be acknowledged that endogenous production of vitamin D in the skin following UVB exposure is the dominant predictor of vitamin D status in humans.
Acknowledgements
We thank all EPIC participants and staff for their contribution to the study. We thank Nicola Kerrison (MRC Epidemiology Unit, Cambridge) for managing the data for the InterAct project. We further thank Valentina Gallo (Imperial College London) for providing information on dividing the EPIC centres into latitude groups. Funding for the InterAct project was provided by the EU FP6 programme (grant number LSHM_CT_2006_037197). In addition, InterAct investigators acknowledge funding from the following agencies: E Ardanaz: Health Research Fund (FIS) of the Spanish Ministry of Health and Navarre Regional Government; JWJ Beulens: Verification of diabetes cases in EPIC-NL was additionally funded by NL Agency grant IGE05012 and an Incentive Grant from the Board of the UMC Utrecht; PW Franks: Swedish Research Council, Novo Nordisk, Swedish Diabetes Association, Swedish Heart-Lung Foundation; R Kaaks: German Cancer Aid, German Ministry of Research (BMBF); TJ Key: Cancer Research UK; KT Khaw: Medical Research Council UK, Cancer Research UK; T Kühn: German Cancer Aid; P Nilsson: Swedish Research Council; K Overvad: Danish Cancer Society; JR Quirós: Asturias Regional Government; O Rolandsson: The Västerboten County Council; I Sluijs: Verification of diabetes cases was additionally funded by NL Agency grant IGE05012 and an Incentive Grant from the Board of the UMC Utrecht; AMW Spijkerman: Dutch Ministry of Public Health, Welfare and Sports (VWS), Netherlands Cancer Registry (NKR), LK Research Funds, Dutch Prevention Funds, Dutch ZON (Zorg Onderzoek Nederland), World Cancer Research Fund (WCRF), Statistics Netherlands; A Tjonneland: Danish Cancer Society; R Tumino: AIRE-ONLUS Ragusa, AVIS-Ragusa, Sicilian Regional Government; DL van der A: Dutch Ministry of Public Health, Welfare and Sports (VWS), Netherlands Cancer Registry (NKR), LK Research Funds, Dutch Prevention Funds, Dutch ZON (Zorg Onderzoek Nederland), World Cancer Research Fund (WCRF), Statistics Netherlands; R Zamora-Ros: the Health Research Fund (FIS) of the Spanish Ministry of Health (RTICC DR06/0020/0091); E Riboli: Imperial College Biomedical Research Centre.
Footnotes
Conflict of interest: None declared.
References
- 1.Maestro B, Campion J, Davila N, Calle C. Stimulation by 1,25-dihydroxyvitamin D3 of insulin receptor expression and insulin responsiveness for glucose transport in U-937 human promonocytic cells. Endocr J. 2000;47:383–391. doi: 10.1507/endocrj.47.383. [DOI] [PubMed] [Google Scholar]
- 2.Danescu LG, Levy S, Levy J. Vitamin D and diabetes mellitus. Endocrine. 2009;35:11–17. doi: 10.1007/s12020-008-9115-5. [DOI] [PubMed] [Google Scholar]
- 3.Kadowaki S, Norman AW. Dietary vitamin D is essential for normal insulin secretion from the perfused rat pancreas. J Clin Invest. 1984;73:759–766. doi: 10.1172/JCI111269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palomer X, Gonzalez-Clemente JM, Blanco-Vaca F, Mauricio D. Role of vitamin D in the pathogenesis of type 2 diabetes mellitus. Diabetes Obes Metab. 2008;10:185–197. doi: 10.1111/j.1463-1326.2007.00710.x. [DOI] [PubMed] [Google Scholar]
- 5.Norman AW, Frankel JB, Heldt AM, Grodsky GM. Vitamin D deficiency inhibits pancreatic secretion of insulin. Science. 1980;209:823–825. doi: 10.1126/science.6250216. [DOI] [PubMed] [Google Scholar]
- 6.Cade C, Norman AW. Vitamin D3 improves impaired glucose tolerance and insulin secretion in the vitamin D-deficient rat in vivo. Endocrinology. 1986;119:84–90. doi: 10.1210/endo-119-1-84. [DOI] [PubMed] [Google Scholar]
- 7.Chiu KC, Chu A, Go VL, Saad MF. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr. 2004;79:820–825. doi: 10.1093/ajcn/79.5.820. [DOI] [PubMed] [Google Scholar]
- 8.Borissova AM, Tankova T, Kirilov G, Dakovska L, Kovacheva R. The effect of vitamin D3 on insulin secretion and peripheral insulin sensitivity in type 2 diabetic patients. Int J Clin Pract. 2003;57:258–261. [PubMed] [Google Scholar]
- 9.Calle C, Maestro B, Garcia-Arencibia M. Genomic actions of 1,25-dihydroxyvitamin D3 on insulin receptor gene expression, insulin receptor number and insulin activity in the kidney, liver and adipose tissue of streptozotocin-induced diabetic rats. BMC Mol Biol. 2008;9:65. doi: 10.1186/1471-2199-9-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007;92:2017–2029. doi: 10.1210/jc.2007-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitri J, Muraru MD, Pittas AG. Vitamin D and type 2 diabetes: a systematic review. Eur J Clin Nutr. 2011;65:1005–1015. doi: 10.1038/ejcn.2011.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pittas AG, Sun Q, Manson JE, Dawson-Hughes B, Hu FB. Plasma 25-hydroxyvitamin D concentration and risk of incident type 2 diabetes in women. Diabetes Care. 2010;33:2021–2023. doi: 10.2337/dc10-0790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forouhi NG, Ye Z, Rickard AP, Khaw KT, Luben R, Langenberg C, et al. Circulating 25-hydroxyvitamin D concentration and the risk of type 2 diabetes: results from the European Prospective Investigation into Cancer (EPIC)-Norfolk cohort and updated meta-analysis of prospective studies. Diabetologia. 2012;55:2173–2182. doi: 10.1007/s00125-012-2544-y. [DOI] [PubMed] [Google Scholar]
- 14.Khan H, Kunutsor S, Franco OH, Chowdhury R. Vitamin D, type 2 diabetes and other metabolic outcomes: a systematic review and meta-analysis of prospective studies. Proc Nutr Soc. 2013;72:89–97. doi: 10.1017/S0029665112002765. [DOI] [PubMed] [Google Scholar]
- 15.Liu S, Song Y, Ford ES, Manson JE, Buring JE, Ridker PM. Dietary calcium, vitamin D, and the prevalence of metabolic syndrome in middle-aged and older U.S. women. Diabetes Care. 2005;28:2926–2932. doi: 10.2337/diacare.28.12.2926. [DOI] [PubMed] [Google Scholar]
- 16.Pittas AG, Dawson-Hughes B, Li T, Van Dam RM, Willett WC, Manson JE, et al. Vitamin D and calcium intake in relation to type 2 diabetes in women. Diabetes Care. 2006;29:650–656. doi: 10.2337/diacare.29.03.06.dc05-1961. [DOI] [PubMed] [Google Scholar]
- 17.Kirii K, Mizoue T, Iso H, Takahashi Y, Kato M, Inoue M, et al. Calcium, vitamin D and dairy intake in relation to type 2 diabetes risk in a Japanese cohort. Diabetologia. 2009;52:2542–2550. doi: 10.1007/s00125-009-1554-x. [DOI] [PubMed] [Google Scholar]
- 18.Riboli E, Hunt KJ, Slimani N, Ferrari P, Norat T, Fahey M, et al. European Prospective Investigation into Cancer and Nutrition (EPIC): study populations and data collection. Public Health Nutr. 2002;5:1113–1124. doi: 10.1079/PHN2002394. [DOI] [PubMed] [Google Scholar]
- 19.Langenberg C, Sharp S, Forouhi NG, Franks PW, Schulze MB, Kerrison N, et al. Design and cohort description of the InterAct Project: an examination of the interaction of genetic and lifestyle factors on the incidence of type 2 diabetes in the EPIC Study. Diabetologia. 2011;54:2272–2282. doi: 10.1007/s00125-011-2182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Margetts BM, Pietinen P. European Prospective Investigation into Cancer and Nutrition: validity studies on dietary assessment methods. Int J Epidemiol. 1997;26(Suppl 1):S1–S5. doi: 10.1093/ije/26.suppl_1.s1. [DOI] [PubMed] [Google Scholar]
- 21.Slimani N, Deharveng G, Unwin I, Southgate DA, Vignat J, Skeie G, et al. The EPIC nutrient database project (ENDB): a first attempt to standardize nutrient databases across the 10 European countries participating in the EPIC study. Eur J Clin Nutr. 2007;61:1037–1056. doi: 10.1038/sj.ejcn.1602679. [DOI] [PubMed] [Google Scholar]
- 22.Barlow WE, Ichikawa L, Rosner D, Izumi S. Analysis of case-cohort designs. J Clin Epidemiol. 1999;52:1165–1172. doi: 10.1016/s0895-4356(99)00102-x. [DOI] [PubMed] [Google Scholar]
- 23.Wareham NJ, Jakes RW, Rennie KL, Schuit J, Mitchell J, Hennings S, et al. Validity and repeatability of a simple index derived from the short physical activity questionnaire used in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Public Health Nutr. 2003;6:407–413. doi: 10.1079/PHN2002439. [DOI] [PubMed] [Google Scholar]
- 24.Slimani N, Kaaks R, Ferrari P, Casagrande C, Clavel-Chapelon F, Lotze G, et al. European Prospective Investigation into Cancer and Nutrition (EPIC) calibration study: rationale, design and population characteristics. Public Health Nutr. 2002;5:1125–1145. doi: 10.1079/PHN2002395. [DOI] [PubMed] [Google Scholar]
- 25.Slimani N, Ferrari P, Ocke M, Welch A, Boeing H, Liere M, et al. Standardization of the 24-hour diet recall calibration method used in the European prospective investigation into cancer and nutrition (EPIC): general concepts and preliminary results. Eur J Clin Nutr. 2000;54:900–917. doi: 10.1038/sj.ejcn.1601107. [DOI] [PubMed] [Google Scholar]
- 26.Ferrari P, Day NE, Boshuizen HC, Roddam A, Hoffmann K, Thiebaut A, et al. The evaluation of the diet/disease relation in the EPIC study: considerations for the calibration and the disease models. Int J Epidemiol. 2008;37:368–378. doi: 10.1093/ije/dym242. [DOI] [PubMed] [Google Scholar]
- 27.de Boer I, Tinker LF, Connelly S, Curb JD, Howard BV, Kestenbaum B, et al. Calcium plus vitamin D supplementation and the risk of incident diabetes in the Women’s Health Initiative. Diabetes Care. 2008;31:701–707. doi: 10.2337/dc07-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ovesen L, Andersen R, Jakobsen J. Geographical differences in vitamin D status, with particular reference to European countries. Proc Nutr Soc. 2003;62:813–821. doi: 10.1079/PNS2003297. [DOI] [PubMed] [Google Scholar]
- 29.Elmadfa I, Weichselbaum E, Konig J, de Winter A-MR, Trolle E, Haapala I, et al. European nutrition and health report 2004. Forum Nutr. 2005:1–220. [PubMed] [Google Scholar]
- 30.Fabian E, Elmadfa I. Nutritional situation of the elderly in the European Union: data of the European Nutrition and Health Report (2004) Ann Nutr Metab. 2008;52(Suppl 1):57–61. doi: 10.1159/000115352. [DOI] [PubMed] [Google Scholar]
- 31.Lips P. Vitamin D status and nutrition in Europe and Asia. J Steroid Biochem Mol Biol. 2007;103:620–625. doi: 10.1016/j.jsbmb.2006.12.076. [DOI] [PubMed] [Google Scholar]