Abstract
Background
Concerns regarding the safety of transfused blood have led to the development of a range of interventions to minimise blood loss during major surgery. Anti‐fibrinolytic drugs are widely used, particularly in cardiac surgery, and previous reviews have found them to be effective in reducing blood loss, the need for transfusion, and the need for re‐operation due to continued or recurrent bleeding. In the last few years questions have been raised regarding the comparative performance of the drugs. The safety of the most popular agent, aprotinin, has been challenged, and it was withdrawn from world markets in May 2008 because of concerns that it increased the risk of cardiovascular complications and death.
Objectives
To assess the comparative effects of the anti‐fibrinolytic drugs aprotinin, tranexamic acid (TXA), and epsilon aminocaproic acid (EACA) on blood loss during surgery, the need for red blood cell (RBC) transfusion, and adverse events, particularly vascular occlusion, renal dysfunction, and death.
Search methods
We searched: the Cochrane Injuries Group's Specialised Register (July 2010), Cochrane Central Register of Controlled Trials (The Cochrane Library 2010, Issue 3), MEDLINE (Ovid SP) 1950 to July 2010, EMBASE (Ovid SP) 1980 to July 2010. References in identified trials and review articles were checked and trial authors were contacted to identify any additional studies. The searches were last updated in July 2010.
Selection criteria
Randomised controlled trials (RCTs) of anti‐fibrinolytic drugs in adults scheduled for non‐urgent surgery. Eligible trials compared anti‐fibrinolytic drugs with placebo (or no treatment), or with each other.
Data collection and analysis
Two authors independently assessed trial quality and extracted data. This version of the review includes a sensitivity analysis excluding trials authored by Prof. Joachim Boldt.
Main results
This review summarises data from 252 RCTs that recruited over 25,000 participants. Data from the head‐to‐head trials suggest an advantage of aprotinin over the lysine analogues TXA and EACA in terms of reducing perioperative blood loss, but the differences were small. Compared to control, aprotinin reduced the probability of requiring RBC transfusion by a relative 34% (relative risk [RR] 0.66, 95% confidence interval [CI] 0.60 to 0.72). The RR for RBC transfusion with TXA was 0.61 (95% CI 0.53 to 0.70) and was 0.81 (95% CI 0.67 to 0.99) with EACA. When the pooled estimates from the head‐to‐head trials of the two lysine analogues were combined and compared to aprotinin alone, aprotinin appeared more effective in reducing the need for RBC transfusion (RR 0.90; 95% CI 0.81 to 0.99).
Aprotinin reduced the need for re‐operation due to bleeding by a relative 54% (RR 0.46, 95% CI 0.34 to 0.62). This translates into an absolute risk reduction of 2% and a number needed‐to‐treat (NNT) of 50 (95% CI 33 to 100). A similar trend was seen with EACA (RR 0.32, 95% CI 0.11 to 0.99) but not TXA (RR 0.80, 95% CI 0.55 to 1.17). The blood transfusion data were heterogeneous and funnel plots indicate that trials of aprotinin and the lysine analogues may be subject to publication bias.
When compared with no treatment aprotinin did not increase the risk of myocardial infarction (RR 0.87, 95% CI 0.69 to 1.11), stroke (RR 0.82, 95% CI 0.44 to 1.52), renal dysfunction (RR 1.10, 95% CI 0.79 to 1.54) or overall mortality (RR 0.81, 95% CI 0.63 to 1.06). Similar trends were seen with the lysine analogues, but data were sparse. These data conflict with the results of recently published non‐randomised studies, which found increased risk of cardiovascular complications and death with aprotinin. There are concerns about the adequacy of reporting of uncommon events in the small clinical trials included in this review.
When aprotinin was compared directly with either, or both, of the two lysine analogues it resulted in a significant increase in the risk of death (RR 1.39, 95% CI 1.02, 1.89), and a non‐significant increase in the risk of myocardial infarction (RR 1.11 95% CI 0.82, 1.50). Most of the data contributing to this added risk came from a single study – the BART trial (2008).
Authors' conclusions
Anti‐fibrinolytic drugs provide worthwhile reductions in blood loss and the receipt of allogeneic red cell transfusion. Aprotinin appears to be slightly more effective than the lysine analogues in reducing blood loss and the receipt of blood transfusion. However, head to head comparisons show a lower risk of death with lysine analogues when compared with aprotinin. The lysine analogues are effective in reducing blood loss during and after surgery, and appear to be free of serious adverse effects.
Plain language summary
Anti‐fibrinolytic drugs for reducing blood loss and the need for red blood cell transfusions during and after surgery.
Aprotinin, although effective in reducing bleeding, had a higher rate of death than tranexamic acid and aminocaproic acid, which appeared free of serious side‐effects. Aprotinin has been withdrawn from world markets because of safety concerns. This review of over 250 clinical trials found that anti‐fibrinolytic drugs used at the time of major surgery reduce bleeding, the need for transfusions of red blood cells and the need for repeat surgery because of bleeding. With the exception of aprotinin the drugs appear safe.
Background
Public concern regarding the safety of transfused blood has prompted a reconsideration of the role of allogeneic blood transfusion (whole blood or packed red cells from an unrelated donor). The risks associated with receiving transfusion of allogeneic blood that has been screened by a competent blood transfusion program are considered minimal, with very low risks of transmission of HIV, and hepatitis C (Whyte 1997). However, this only applies where there is a safe, plentiful, well‐regulated supply. The majority of the world's population does not have access to such a system, and the risks of transfusion in developing countries may be much higher (McFarland 1997). Concerns of patients and clinicians regarding blood safety have generated enthusiasm for the use of technologies intended to reduce the use of allogeneic blood (Bryson 1998; Forgie 1998; Huet 1999; Laupacis 1997). Although allogeneic blood transfusion has had a unique place in medical practice, we are obliged to examine the evidence on the benefits, harms and costs of a range of techniques designed to minimise the use of this resource. Some of the alternatives to allogeneic blood have their own risks, and are expensive (Coyle 1999; Fergusson 1999).
Perioperative bleeding is one of the major indications for allogeneic blood transfusions worldwide (Levy 2006). However, massive surgical blood loss is a serious problem that affects many cardiac surgery patients in particular and has been shown to have a strong, independent association with in‐hospital mortality (Karkouti 2004). There is also considerable evidence that blood loss that leads to the transfusion of blood products is harmful, and that the degree of harm is directly related to the amount of blood loss (Karkouti 2006). To reduce perioperative blood loss a number of pharmacological agents have been used, these include the anti‐fibrinolytic drugs aprotinin, tranexamic acid (TXA), and epsilon aminocaproic acid (EACA).
Aprotinin is a non‐specific, serine protease inhibitor, derived from bovine lung, with anti‐fibrinolytic properties. It acts as an inhibitor of several serine proteases, including trypsin, plasmin, plasma‐kallikrein and tissue kallikrein. Aprotinin also inhibits the contact phase activation of coagulation that both initiates coagulation and promotes fibrinolysis (Fritz 1983; Royston 1998). During cardiopulmonary bypass (CPB) the negatively charged surface of the CPB circuit activates factor XII, converting prekallikrein to kallikrein which further activates factor XII. This positive feedback loop acts to intensify the intrinsic coagulation cascade. By inhibiting plasma kallikrein, aprotinin minimises derangements in coagulation and fibrinolysis (Smith 1998). There is also evidence that aprotinin exerts an indirect preservative effect on platelet function during extracorporeal circulation (ECC) (Mohr 1992). In many countries aprotinin is specifically indicated for the reduction of blood loss during cardiopulmonary bypass.
TXA and EACA are synthetic lysine analogues (synthetic derivatives of the amino acid lysine) that act as effective inhibitors of fibrinolysis. TXA and EACA act principally by blocking the lysine binding sites on plasminogen molecules, inhibiting the formation of plasmin and therefore inhibiting fibrinolysis (Faught 1998). Tranexamic acid is about ten times more potent than aminocaproic acid and binds much more strongly to both the strong and weak sites of the plasminogen molecule than EACA (Faught 1998; Mannucci 1998).
Why it is important to do this review
The efficacy of these three anti‐fibrinolytic drugs to reduce perioperative blood loss and allogeneic blood transfusion has been studied extensively. Systematic reviews of these drugs (Henry 1999; Laupacis 1997; Levi 1999; Munoz 1999; Sedrakyan 2004) have shown that the use of aprotinin is associated with statistically significant reductions in allogeneic blood transfusion requirements and re‐operation due to bleeding. Systematic reviews have also shown TXA to be effective in reducing exposure to allogeneic blood transfusion without significant increases in adverse effects (Henry 1999; Laupacis 1997). In the case of EACA, the evidence of effect is equivocal with most systematic reviews severely hampered by the small number of trials of this agent.
Based on the evidence of efficacy anti‐fibrinolytic drugs have become widely used, particularly in cardiac surgery. Because of their mode of actions there have been longstanding concerns about the possibility of adverse effects, with most attention directed at the risk of thrombosis and renal failure. However meta‐analyses of randomised trials, including previous versions of this Cochrane review (Henry 1999;Henry 2007), have been reassuring in providing no convincing evidence of an increased risk of these events in treated subjects. However, in the case of aprotinin, this view of an attractive benefit to harm ratio was thrown into doubt by the publication of several large non‐randomised studies (Mangano 2006; Mangano 2007;Schneeweiss 2008). The serious safety concerns raised by these and other studies prompted the United States Food and Drug Administration (FDA) to re‐evaluate its position regarding the use of aprotinin in cardiac surgery, some thirteen years after it was initially approved for prophylactic treatment to reduce perioperative blood loss and blood transfusion (Ferguson 2007). Aprotinin was finally removed from world markets in May 2008. The other drugs reviewed here are still in use.
In the light of these developments and in order to inform decisions about the use of the two lysine analogues as an alternative to aprotinin in cardiac surgery we have updated the Cochrane systematic review of the three anti‐fibrinolytic drugs used as blood‐sparing agents in surgery. This review updates previous estimates of the efficacy of aprotinin, tranexamic acid, and epsilon aminocaproic acid in reducing perioperative allogeneic blood transfusion in elective surgery. In light of the adverse findings from pharmaco‐epidemiological studies we also provide updated estimates of the effects of these drugs on clinical outcomes such as all‐cause mortality, thrombosis and renal failure.
Objectives
To examine the evidence for the efficacy of aprotinin, tranexamic acid, and epsilon aminocaproic acid in reducing allogeneic blood transfusion, and the evidence for any effect on clinical outcomes such as mortality and re‐operation rates and complications such as thrombosis and renal failure.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) with a concurrent control group, or randomised head‐to‐head comparative trials.
Types of participants
The study participants were adults (over 18 years). Trials were included if participants aged less than 18 years were enrolled, but the type of surgery was predominantly carried out in adult patients. The surgery performed was primarily elective but trials were included if urgent cases were proportionately similar across trial arms.
Types of interventions
The interventions considered are the anti‐fibrinolytic agents: aprotinin, tranexamic acid (TXA), and epsilon‐aminocaproic acid (EACA).
Types of outcome measures
Primary outcomes
the proportion of patients who were transfused with allogeneic blood, autologous blood, or with both;
the amounts of allogeneic and autologous blood transfused.
Secondary outcomes
perioperative blood loss,
re‐operation due to bleeding,
mortality,
post‐operative complications (myocardial infarction, stroke, deep vein thrombosis, pulmonary embolism, any thrombosis, renal failure),
length of hospital stay.
Search methods for identification of studies
We did not limit the searches by date language or publication status
Electronic searches
The original review drew on the literature search that was constructed as part of the International Study on Perioperative Transfusion (ISPOT) (Laupacis 1997). The original search is listed in full in Appendix 1.
July 2006 update
To maximise the sensitivity for the retrieval of all potentially relevant studies, the electronic searches of MEDLINE were initially unrestricted and updated to July 2006. In MEDLINE, two search filters were then used to restrict the electronic searches and improve the specificity of the updated searches. Firstly, the ISPOT filter, which identifies blood transfusion trials, and secondly, a modified version of the Cochrane Collaboration filter, which primarily identifies randomised controlled trials. These search filters were coupled with the specified MeSH headings and the relevant text‐word terms. These restricted searches were updated in MEDLINE to July 2006. Electronic database searches of Excerpta Medica (EMBASE) were updated to July 2006 using similar search strategies to those used in MEDLINE.
July 2010 update
Searches were carried out in July 2010 as part of a larger project to identify trials in the use of antifibrinolytics.
We searched the following databases;
the Cochrane Injuries Group's Specialised Register (searched July 2010),
Cochrane Central Register of Controlled Trials (The Cochrane Library 2010, Issue 3),
MEDLINE (Ovid SP) 1950 to July 2010,
EMBASE (Ovid SP) 1980 to July 2010.
Full details of the search strategies are presented in Appendix 2.
Searching other resources
The web sites of International Health Technology Assessment Agencies were also searched through the International Network of Agencies of Health Technology Assessment (INAHTA), and the International Society of Technology Assessment in Health Care (ISTAHC). The Internet was widely searched using Google™ and Google™ Scholar. Contact was also made with experts in the field to identify reports or projects in progress relevant to the review.
The reference lists of related reviews and identified articles were checked for relevant trials. In addition references in the identified trials were checked and authors contacted, where possible, to identify any additional published or unpublished data.
Data collection and analysis
Electronic database searches were carried out by the Cochrane Injuries Group Trials Search Co‐ordinator, who then collated the results and passed them on to the author (KK).
Selection of studies
The titles and abstracts identified in the electronic searches were independently screened by two authors to identify trials in which adult patients, scheduled for elective or urgent surgery, were randomised to either/or aprotinin, TXA, EACA or to a control group, who did not receive the intervention. From the results of the screened electronic searches, bibliographic searches, and contacts with experts, two of the authors independently selected trials that met previously defined inclusion criteria.
Data extraction and management
At least two authors independently extracted study characteristics and outcomes using an article extraction form. The extraction form was used to record information regarding randomisation criteria, methodology descriptions, the presence of a transfusion protocol, the type of surgery involved, treatment outcomes, and general comments.
Data on the following outcomes were recorded on the data extraction form: the number of patients exposed to allogeneic blood, the amount of allogeneic blood transfused, the number of patients receiving any transfusion (allogeneic blood, autologous blood, or both), the number of patients experiencing post‐operative complications (thrombosis, myocardial infarction, renal failure), and mortality. Data were also recorded on blood loss, and the proportion of patients requiring re‐operation for bleeding. Information regarding demographics (age, sex), type of surgery, and the presence or absence of a transfusion protocol was also recorded. Data were extracted for allogeneic blood transfusion if they were expressed as whole blood or packed red cells. Data were extracted regarding dose size for each drug regimen.
Assessment of risk of bias in included studies
Articles that met the inclusion criteria were independently assessed for methodological quality by two authors using criteria proposed by Schulz 1995. Disagreements were resolved by consensus. Methodological quality scores obtained for each trial using the criteria proposed by Schulz 1995 were then entered into Review Manager using the Cochrane Collaboration's tool for assessing risk of bias presented in Higgins 2009.
The following domains were assessed for each study:
sequence generation,
allocation concealment,
blinding.
We completed a risk of bias table for each study, incorporating a description of the study's performance against each of the above domains and our overall judgement of the risk of bias for each entry is as follows; 'Yes' indicates low risk of bias, 'Unclear' indicates unknown risk of bias (not enough information was reported to assess methodological quality); and 'No' indicates a high risk of bias.
Assessment of reporting biases
Funnel plots were inspected for evidence of publication bias.
Data synthesis
Extraction of trial data was performed by one author and checked by the review team's statistician if necessary. Data were checked and entered into Review Manager by one author. Articles identified as duplicate publications were combined to obtain one set of data. The study report with the greatest number of patients was then represented in the analysis. Studies that did not report data for the number or proportion of patients transfused with allogeneic blood, or the amounts of allogeneic blood transfused, were not included for review. However, trials not reporting blood transfusion data that could be used in the meta‐analysis were still included if they reported adverse event data. For dichotomous outcome data to be included in the analysis, trial reports had to provide either numeric data, that is the numbers of events that occurred in the treatment and control groups, or where there were no events recorded numerically, the trial report had to provide a clear statement qualifying and/or quantifying specific events had or had not occurred.
All analyses were performed using Review Manager software. Data on the numbers of patients exposed to allogeneic blood, and the numbers of patients in each treatment arm, were entered into Review Manager. The relative risks (RR) for allogeneic blood transfusion in the intervention group as compared with the control group, and the corresponding 95% confidence intervals (CI), were calculated for each trial using the random effects model. The presence of heterogeneity of treatment effect was assessed using the Q statistic, which has an approximate chi‐square distribution with degrees of freedom equal to the number of studies minus one (Der Simonian 1986). A P‐value less than or equal to 0.1 was used to define statistically significant heterogeneity. Statistical heterogeneity was also assessed using the I² test. The I² test describes the percentage of total variation across studies due to heterogeneity rather than chance. A value of 0% indicates no observed heterogeneity and larger values show increasing heterogeneity. Substantial heterogeneity is considered to exist when I² > 50% (Higgins 2002).
The mean number of units of allogeneic blood transfused to each group, and the corresponding standard deviations, were also entered. As the majority of trials reported the means and standard deviations for the amount of blood transfused in all patients in each comparison group, the data included a number of zero values for those patients not receiving transfusion. The data are therefore likely to be highly skewed. Wherever possible, the mean and standard deviation for the numbers of units of blood transfused in those receiving transfusion were recalculated. The new mean was calculated by dividing the total number of units transfused in the group by the number of patients transfused. The reported standard deviation and mean were used to calculate the sum of squares of the numbers of units transfused for the group. As this is equal to the sum of squares of the numbers of units transfused in those receiving transfusion, the new standard deviation was calculated using this, the new mean and the number of patients transfused. Thus the new values estimate the average amount of blood received by those transfused in each group. The new values were then entered into Review Manager to obtain the mean difference (MD) and 95% CIs to express the average reduction in the number of units of allogeneic blood given to those patients transfused. Data in millilitres (mls) were converted to units by dividing by 300.
Subgroup analysis and investigation of heterogeneity
Analysis of a‐priori subgroups was performed to determine whether effect sizes varied according to factors such as;
the type of surgery,
the use of transfusion protocols,
dose regimen, and
trial methodological quality.
The editorial group is aware that a clinical trial by Prof. Joachim Boldt has been found to have been fabricated (Boldt 2009). As the editors who revealed this fabrication point out (Reinhart 2011; Shafer 2011), this casts some doubt on the veracity of other studies by the same author. All Cochrane Injuries Group reviews which include studies by this author have therefore been edited to show the results with this author's trials included and excluded. Readers can now judge the potential impact of trials by this author on the conclusions of the review.
Results
Description of studies
Two hundred and fifty‐two trials met the inclusion criteria. Four trials were excluded (refer to 'Characteristics of excluded studies' section of this review). Of the 252 included trials, 131 evaluated aprotinin, 60 evaluated tranexamic acid (TXA), and 12 evaluated epsilon aminocaproic acid (EACA) versus control. Forty‐nine trials studied head‐to‐head comparisons of aprotinin, TXA, and EACA with or without an untreated control. Of these 49 trials, 25 compared aprotinin with TXA, 12 compared aprotinin with EACA, seven compared TXA with EACA, and five compared aprotinin with TXA and EACA. Trials were conducted in many countries including:
United States (n = 45), Germany (n = 24), UK (n = 17), Canada (n = 17), Italy (n = 16), Spain (n = 14), Belgium (n = 12), France (n = 10), Turkey (n = 9), Australia (n = 8), Sweden (n = 8),The Netherlands (n = 8), Japan (n = 7), China (n = 6), Austria (n = 5), Israel (n = 5), Switzerland (n = 5), Finland (n = 5), Czech Republic (n = 3), Denmark (n = 3), Taiwan (n = 2), Ireland (n = 2), Greece (n = 2), Poland (n = 2), Brazil (n = 1), Chile (n = 1), Dubai (n=1), Egypt (n = 1), India (n = 1), Norway (n = 1), Oman (n=1), Saudi Arabia (n=1) and South Africa (n = 1). Three studies were multicentre trials, one conducted across sites in the UK and the United States, one in sites in Australia, New Zealand, Asia and Europe and one in sites in the United States and Canada. The majority of included trials were published in English. Thirteen trials required translation (Carrera 1994; Corbeau 1995; Cvachovec 2001; Deleuze 1991; Gherli 1992; Hei 2005; Kahveci 1996; Katzel 1998; Kratzer 1997; Locatelli 1990; Maccario 1994; Trinh‐Duc 1992; Utada 1997). The data from these trials were included in the analysis.
Of the 252 included trials, 173 were conducted in cardiac surgery, 53 trials were in orthopaedic surgery, 14 involved liver surgery, five were conducted in vascular surgery, four involved thoracic surgery, one involved gynaecological surgery, one involved neurosurgery, and one trial was in orthognathic surgery.
The trial conducted by Lemmer 1994 stratified patients according to the type of procedure being performed, that is, either primary CABG or redo CABG surgery. Patients from each group were then randomised to either aprotinin or placebo. The data obtained from each of these two groups (primary CABG and redo CABG) have been analysed separately by the authors. Therefore from this single trial (Lemmer 1994), two comparisons of aprotinin versus control have been obtained. This review presents the data from this trial as follows: (1) Lemmer_1 1994: represents those patients who underwent primary CABG and were randomised to either aprotinin or placebo. (2) Lemmer_2 1994: represents those patients who underwent redo CABG and were randomised to either aprotinin or placebo.
Description of Dose Regimens
Aprotinin dose range
Three dose stratifications were used: (1) high‐dose aprotinin, (2) low‐dose aprotinin, and, (3) cardiopulmonary bypass (CPB) pump prime aprotinin. For the purposes of this review, any aprotinin regimen that did not follow the 'full Hammersmith' regimen, including those studies that described their regimens as 'half Hammersmith', were classified as low‐dose aprotinin. For those trials that did not involve cardiac surgery, classification of the dose‐regimen was based on the total quantity of aprotinin administered. Trials were classified as 'high‐dose' where participants received a total dose equal to or exceeding five million kallikrein inactivator units (KIU) or 700mg of aprotinin.
High‐dose aprotinin, described as the 'full Hammersmith' regimen, entails an initial loading dose of two million kallikrein inactivator units (KIU) of aprotinin given intravenously (IV) (280mg) over a 20 to 30 minute period commencing at the induction of anaesthesia, followed by a continuous infusion of 500,000 KIU per hour (70mg/hr) until the end of the operation. In addition, two million KIU of aprotinin (280mg) is added to the oxygenator prime or pump prime of the CPB. A 'half Hammersmith' regimen is described as follows: a loading dose of one million KIU (140mg) of aprotinin infused over a 20 to 30 minute period followed by a continuous IV infusion of 250,000 KIU of aprotinin per hour, until the end of the operation. An additional dose of one million KIU is added to the pump prime.
'Prime' dose aprotinin, for the purposes of this review, included those regimens that added aprotinin to the pump prime solution of the CPB exclusively. The dose of aprotinin used in the 'prime' regimen varied between trials. Sixteen trials studied the efficacy of 'prime' dose aprotinin and reported data on the proportion of participants exposed to allogeneic blood transfusion. Of these trials 12 studied a 'prime' dose of two million KIU of aprotinin, two studied a 'prime' dose of one million KIU of aprotinin, one studied a 'prime' dose of 500,000 KIU of aprotinin, and one trial studied a 'prime' dose of 25,000 KIU/kg (range 1.375 to 2.3 million KIU in total) of aprotinin.
Tranexamic acid (TXA) dose range
Of the 65 trials that studied the efficacy of TXA versus placebo or control (current standard practice) and were included in the meta‐analysis of allogeneic blood transfusion exposure; 34 involved cardiac surgery, 27 involved orthopaedic surgery, two involved liver surgery, one trial involved gynaecological surgery and one trial involved vascular surgery. Dose regimens for TXA varied significantly between trials with varying dose sizes and time frames for drug delivery. Of the 34 trials involving cardiac surgery, the TXA loading or bolus dose ranged from 2.5mg/kg to 100mg/kg. The maintenance dose of TXA for the cardiac trials, ranged from 0.25mg/kg/hr to 4.0mg/kg/hr delivered over 1 to 12 hours. Similar variation was observed in trials not involving cardiac surgery. More detailed information regarding dose regimens is provided in the 'Characteristics of included studies' section of this review.
Epsilon aminocaproic acid (EACA) dose range
Of the 16 trials that studied the efficacy of EACA versus placebo or control (current standard practice) and were included in the meta‐analysis of allogeneic blood transfusion exposure; 11 involved cardiac surgery, four involved orthopaedic surgery, and one involved liver surgery. Dose regimens for EACA also varied significantly between trials. Generally trials used different dose sizes and time frames for drug delivery. The EACA loading or bolus dose ranged from 80mg to 15g or 75 to 150mg/kg. The maintenance dose of EACA ranged from 1g/hr to 2g/hr or 12.5mg/kg/hr to 30mg/kg/hr infused over varying time periods. More detailed information regarding dose regimens is provided in the 'Characteristics of included studies' section of this review.
Transfusion 'triggers' / thresholds
Of the 189 trials of aprotinin, TXA, and EACA versus control included in the analysis of allogeneic blood transfusion exposure, 158 trials (84%) reported the use of a transfusion protocol, the remainder did not report the use of a transfusion protocol. Of those trials that reported the use of a transfusion protocol, all included a transfusion "trigger" value, that being the haemoglobin or haematocrit value, at which point a transfusion of allogeneic and/or autologous blood, was considered necessary. There was significant variation between trials in the type and value of transfusion threshold used. The lowest transfusion threshold level for haemoglobin was 5.0g/dL with blood being transfused if the haemoglobin level during CPB fell below 5.0g/dL (Green 1995). The transfusion protocol used by Brown 1997 advocated a haemoglobin threshold level of 6.0g/dL during CPB, whereas other trials involving CPB advocated a haemoglobin threshold level of 7.0g/dL, or haematocrit levels (Hct) between 18% to 20% during CPB. In general, post‐operative transfusion threshold levels ranged from Hb 7.0g/dL to 10.0g/dL, or Hct 20% to 30%.
Risk of bias in included studies
For further details regarding the performance of the studies against each domain, please see the 'Risk of bias' tables (Figure 1; Figure 2). A summary of the information in the tables is given below. Additionally, a visual summary of judgements about each methodological quality item for each included trial is shown in Figure 1.
1.
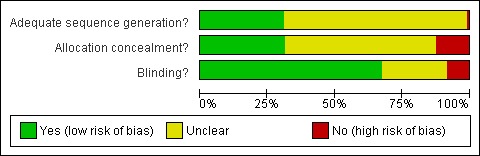
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Generation of allocation sequences
The method used to generate allocation sequences was judged to be adequate in only 78 trials. For all but two of the remaining trials the method used to generate allocation sequences was judged to be unclear. For two trials the method of randomisation that was judged to be inadequate. Refer to results presented in the 'Risk of bias' tables.
Allocation concealment
Only 79 trials were judged to have adequately concealed treatment allocation. For 31 trials the method used to conceal treatment allocation was judged to be inadequate. For the remaining trials allocation concealment was judged to be unclear. Refer to results presented in the 'Risk of bias' tables.
Blinding
For 170 trials blinding was judged to be adequate (double blinded), and unclear for 61 trials. Refer to results presented in the 'Risk of bias' tables.
Inclusion of all randomised participants
Of those trials able to be assessed for methodological quality, 124 trials either reported there were no exclusions, or used intention‐to‐treat analysis. In 80 trials, where exclusions were reported, these exclusions were judged unlikely to cause bias. For 37 trials exclusions were either judged to be excessive and likely to cause bias, or were not reported.
Effects of interventions
Aprotinin
There were 108 trials of aprotinin versus control that reported data on the proportion of patients exposed to allogeneic blood transfusion. These trials included a total of 11,172 patients, of whom 6259 were randomised to receive aprotinin and 4913 patients were randomised to a control group who did not receive aprotinin. The apparent imbalance between the aprotinin and control groups resulted from pooling data across different aprotinin dose groups within trials. Overall, the use of aprotinin significantly reduced the rate of allogeneic blood transfusion by a relative 34% (RR 0.66, 95% CI 0.60 to 0.72) compared to control. Heterogeneity between these trials was statistically significant (Chi² = 961.52, df = 107, P <0.00001; I² = 89%). The absolute risk reduction (ARR) was 20% (RD ‐0.20, 95% CI ‐0.24 to ‐0.17).
Type of surgery
There were 84 trials of aprotinin versus control that involved cardiac surgery and provided data on the number of patients exposed to allogeneic blood transfusion. These trials included a total of 9497 patients, of whom 5329 were randomised to receive aprotinin and 4168 patients were randomised to a control group who did not receive aprotinin. Overall, the use of aprotinin in cardiac surgery significantly reduced the need for allogeneic blood transfusion by a relative 32% (RR 0.68, 95% CI 0.63 to 0.73) compared to control. (The effect with the Boldt 1991trial excluded was unchanged (RR 0.68 (95% CI 0.63 to 0.73).) Heterogeneity between these trials was statistically significant (Chi² = 329.48, df = 83, P <0.00001; I² = 75%). The ARR was 21% (RD ‐0.21, 95% CI ‐0.24 to ‐0.18).
There were 15 trials of aprotinin versus control that involved orthopaedic surgery and provided data on the number of patients exposed to allogeneic blood transfusion. These trials included a total of 1146 patients, of whom 655 were randomised to receive aprotinin and 491 patients were randomised to a control group who did not receive aprotinin. Overall, the use of aprotinin in orthopaedic surgery significantly reduced the need for allogeneic blood transfusion by a relative 32% (RR 0.68, 95% CI 0.52 to 0.89) compared to control. Heterogeneity between these trials was statistically significant (Chi² = 45.47, df = 14, P <0.0001; I² = 69%). The ARR was 13% (RD ‐0.13, 95% CI ‐0.20 to ‐0.05).
There were three trials of aprotinin versus control that involved thoracic surgery and provided data on the number of patients exposed to allogeneic blood transfusion. These trials included a total of 78 patients, of whom 38 were randomised to receive aprotinin and 40 patients were randomised to a control group who did not receive aprotinin. The use of aprotinin in thoracic surgery significantly reduced the need for allogeneic blood transfusion by a relative 71% (RR 0.29, 95% CI 0.14 to 0.59). Heterogeneity between these trials was not statistically significant (Chi² = 0.37, df = 2, P = 0.83; I² = 0%).
There were two trials of aprotinin versus control that involved vascular surgery and provided data on the number of patients exposed to allogeneic blood transfusion. These trials included a total of 188 patients, of whom 105 were randomised to aprotinin and 83 patients were randomised to a control group who did not receive aprotinin. The use of aprotinin in vascular surgery had no effect on the need for allogeneic blood transfusion (RR 1.00, 95% CI 0.97 to 1.03). Heterogeneity between these trials was not statistically significant (Chi² = 0.01, df = 1, P = 0.84; I² = 0%).
There were two trials of aprotinin versus control that involved liver surgery and provided data on the number of patients exposed to allogeneic blood transfusion. These trials included a total of 177 patients, of whom 87 were randomised to aprotinin and 90 patients were randomised to a control group who did not receive aprotinin. The use of aprotinin in liver surgery reduced the need for allogeneic blood transfusion by a relative 42% (RR 0.58, 95% CI 0.37 to 0.90). Heterogeneity between these trials was not statistically significant (Chi² = 1.03, df = 1, P = 0.31; I² = 3%).
Data from the trials involving neurosurgery, and orthognathic surgery could not be analysed due to the small number of trials in each of these surgical subgroups.
Effect of transfusion protocols
There were 87 trials that compared aprotinin with control and reported the use of transfusion protocols. These trials included a total of 9974 patients, of whom 5599 were randomised to aprotinin and 4375 were randomised to a control group who did not receive aprotinin. In those trials where a transfusion protocol was used, aprotinin significantly reduced the need for allogeneic blood transfusion by a relative 35% (RR 0.65, 95% CI 0.59 to 0.71). Heterogeneity between these trials was statistical significant (Chi² = 924.12, df = 86, P < 0.00001; I² = 91%). (The effect was unchanged with the Boldt 1991 trial excluded (RR 0.65 (95% CI 0.59 to 0.71).)
There were 21 trials of aprotinin versus control that reported data on the number of patients exposed to allogeneic blood transfusion but did not report the use of a transfusion protocol. These trials included a total of 1198 patients, of whom 660 were randomised to aprotinin and 538 were randomised to a control group who did not receive aprotinin. The use of aprotinin statistically significantly reduced the need for allogeneic blood transfusion by a relative 29% (RR 0.71, 95% CI 0.61 to 0.84) compared to control. Heterogeneity between these trials was statistically significant (Chi² = 49.74, df = 20, P = 0.0002; I² = 60%).
Effect of dose
In those trials that used a low‐dose aprotinin regimen the RR of requiring an allogeneic blood transfusion was 0.65 (95% CI 0.55 to 0.77) compared to control. Whereas in those trials that used a high‐dose aprotinin regimen the RR of receiving an allogeneic blood transfusion was 0.66 (95% CI 0.61 to 0.71) compared to control. Therefore there was little difference in effect between high‐dose and low‐dose aprotinin. In cardiac surgery when aprotinin was given as a prime‐dose only, the RR of requiring allogeneic blood transfusion was 0.83 (95% CI 0.71 to 0.96). There was statistically significant heterogeneity present in all three subgroups (P >0.0001; I² >74%).
The study conducted by Green 1995 was not included in this analysis as it only provided aggregate data for the number of patients exposed to allogeneic blood transfusion, without stratifying allogeneic blood transfusion exposure by dose.
When the high‐dose analysis excludes the Boldt 1991 trial, the effect remains 0.66 (95% CI 0.61 to 0.71).
Volume of blood transfused
Seventy‐four trials of aprotinin versus control provided data on the volume of allogeneic blood transfused in all patients. These trials included a total of 7820 patients, of whom 4198 were randomised to aprotinin and 3622 were randomised to a control group who did not receive aprotinin. The use of aprotinin resulted in a significant saving of 1.02 units of allogeneic blood (MD ‐1.02 units, 95% CI ‐1.26 to ‐0.79 units). Heterogeneity between these trials was statistically significant (Chi² = 1627.35, df = 69, P <0.00001; I² = 96%).
Forty trials of aprotinin versus control provided data on the volume of allogeneic blood transfused in those patients transfused. These trials provided data for a total of 3563 patients, of whom 1680 were treated with aprotinin and 1883 did not receive aprotinin treatment. In those patients transfused the use of aprotinin resulted in a significant saving of 0.98 units of allogeneic blood per patient (MD ‐0.98 units, 95% CI ‐1.29 to ‐0.66 units). Heterogeneity between these trials was statistically significant (Chi² = 197.82, df = 36, P < 0.00001; I² = 82%).
Blood loss ‐ all surgery combined
A total of 16 trials of aprotinin versus control reported intra‐operative blood loss data. These trials included a total of 883 patients, of whom 449 were randomised to aprotinin and 434 were randomised to a control group. These trials involved cardiac surgery (n = 7), orthopaedic surgery (n = 5), thoracic surgery (n = 2), liver surgery (n = 2) and vascular surgery (n = 1). In aggregate, aprotinin treatment reduced intra‐operative blood loss on average by around 192 mls per patient (MD ‐191.87 mls, 95% CI ‐280.45 to ‐103.28 mls). Heterogeneity between these trials was statistically significant (Chi² = 40.04, df = 16, P = 0.0008; I² = 60%).
A total of 87 trials of aprotinin versus control reported post‐operative blood loss data. These trials included a total of 7896 patients, of whom 4394 were randomised to aprotinin and 3502 were randomised to a control group. These trials involved cardiac surgery (n = 75), orthopaedic surgery (n = 7), thoracic surgery (n = 2), orthognathic surgery (n = 1), liver surgery (n = 1), and vascular surgery (n = 1). In aggregate, aprotinin treatment significantly reduced post‐operative blood loss on average by around 346 mls per patient (MD ‐345.88 mls, 95% CI ‐383.47 to ‐308.29 mls). Heterogeneity between these trials was statistically significant (Chi² = 620.49, df = 86, P <0.00001; I² = 86%).
A total of 17 trials of aprotinin versus control reported total blood loss data (intra‐operative and post‐operative blood loss combined). These trials included a total of 1789 patients, of whom 932 patients were randomised to aprotinin and 857 were randomised to a control group. These trials involved cardiac surgery (n = 7) and orthopaedic surgery (n = 10). In aggregate, the use of aprotinin significantly reduced perioperative blood loss by around 416 mls per patient (MD ‐415.95 mls, 95% CI ‐520.38 to ‐311.51 mls). Heterogeneity between these trials was statistically significant (Chi² = 66.96, df = 16, P <0.0001; I² = 76%).
Blood loss ‐ cardiac surgery
Seven trials of aprotinin versus control involving cardiac surgery reported intra‐operative blood loss data. These trials included a total of 470 patients, of whom 242 were randomised to aprotinin and 228 were randomised to a control group. Aprotinin treatment in cardiac surgery appeared to be only marginally effective in reducing intra‐operative blood loss (MD ‐148.18 mls, 95% CI ‐240.21 to ‐56.14 mls). Heterogeneity between these trials was statistically significant (Chi² = 13.63, df = 6, P = 0.03; I² = 56%).
Seventy‐five trials of aprotinin versus control involving cardiac surgery reported post‐operative blood loss data. These trials included a total of 7371 patients, of whom 4132 were randomised to aprotinin and 3239 were randomised to a control group. The use aprotinin in cardiac surgery reduced post‐operative blood loss on average by 370 mls per patient (MD ‐369.62 mls, 95% CI ‐408.95 to ‐330.29 mls). Heterogeneity between these trials was statistically significant (Chi² = 513.91, df = 74, P <0.00001; I² = 86%). The effect excluding the trials Boldt 1991 and Boldt 1994 is MD ‐378.45 (95% CI ‐417.99 to ‐338.92).
Seven trials of aprotinin versus control involving cardiac surgery reported total blood loss data (intra‐operative and post‐operative blood loss combined). These trials included a total of 1359 patients, of whom 716 were randomised to aprotinin and 643 were randomised to a control group. The use of aprotinin in cardiac surgery significantly reduced the total volume of blood lost during the perioperative period (MD ‐448.86 mls, 95% CI ‐612.82 to ‐284.91 mls). Heterogeneity between these trials was statistically significant (Chi² = 42.60, df = 6, P<0.00001; I² = 86%).
Blood loss ‐ orthopaedic surgery
Five trials of aprotinin versus control involving orthopaedic surgery reported intra‐operative blood loss data. These trials included a total of 201 patients, of whom 103 were randomised to aprotinin and 98 were randomised to a control group. The use of aprotinin in orthopaedic surgery did not reduce the volume of blood lost during the intra‐operative period (MD ‐151.05 mls, 95% CI ‐317.63 to 15.52 mls). Heterogeneity between these trials was not statistically significant (Chi² = 6.62, df = 4, P = 0.16; I² = 40%).
Seven trials of aprotinin versus control involving orthopaedic surgery reported post‐operative blood loss data. These trials included a total of 318 patients, of whom 160 were randomised to aprotinin and 158 were randomised to a control group. The use of aprotinin in orthopaedic surgery was only marginally effective in reducing post‐operative blood loss (MD ‐113.58 mls, 95% CI ‐223.69 to ‐3.46 mls). Heterogeneity between these trials was statistically significant (Chi² = 18.56, df = 6, P = 0.005; I² = 68%).
Ten trials of aprotinin versus control involving orthopaedic surgery reported total blood loss data (intra‐operative and post‐operative blood loss combined). These trials included a total of 430 patients, of whom 216 were randomised to aprotinin and 214 were randomised to a control group. Aprotinin reduced the total volume of blood lost during the perioperative period on average by around 399 mls per patient (MD ‐399.09 mls, 95% CI ‐562.81 to ‐235.37 mls). Heterogeneity between these trials was statistically significant (Chi² = 22.67, df = 9, P = 0.007; I² = 60%).
Re‐operation for bleeding
Sixty‐one trials of aprotinin versus control reported data on re‐operation for bleeding. These trials included a total of 6117 patients, of whom 3392 were randomised to aprotinin and 2725 were randomised to a control group who did not receive aprotinin. The use of aprotinin significantly reduced the need for re‐operation for bleeding by a relative 54% (RR 0.46, 95% CI 0.34 to 0.62). Heterogeneity between these trials was not significant (Chi² = 35.44, df = 42, P = 0.75; I² = 0%). The Boldt 1994 trial had no events, and therefore provided no data to this analysis. When aprotinin was used in cardiac surgery, the RR of requiring re‐operation due to bleeding was 0.46 (95% CI 0.34 to 0.63). Again heterogeneity between these trials was not significant (Chi² = 34.56, df = 39, P = 0.67; I² = 0%).
Mortality
Sixty‐three trials of aprotinin versus control reported data on mortality. These trials included a total of 8876 patients, of whom 4889 were randomised to aprotinin and 3987 were randomised to a control group who did not receive aprotinin. The use of aprotinin was not associated with an increased risk of death (RR 0.81, 95% CI 0.63 to 1.06). Heterogeneity between these trials was not significant (Chi² = 29.54, df = 43, P = 0.94; I² = 0%). In the case of cardiac surgery, the use of aprotinin was not associated with an increased risk of death (RR 0.84, 95% CI 0.64 to 1.10).
Myocardial infarction
Forty‐nine trials of aprotinin versus control reported data for myocardial infarction. These trials included a total of 7137 patients, of whom 4032 were randomised to aprotinin and 3105 were randomised to a control group who did not receive aprotinin. The use aprotinin did not increase the risk of myocardial infarction (RR 0.87, 95% CI 0.69 to 1.11). Heterogeneity between these trials was not statistically significant (Chi² = 27.71, df = 38, P = 0.89; I² = 0%). When aprotinin was used in cardiac surgery, the relative risk of myocardial infarction was not statistically significant (RR 0.90, 95% CI 0.71 to 1.14).
Stroke
Twenty‐three trials of aprotinin versus control reported data for stroke. These trials included a total of 3122 patients, of whom 1862 were randomised to aprotinin and 1260 were randomised to a control group who did not receive aprotinin. The use aprotinin did not increase the risk of stroke (RR 0.82, 95% CI 0.44 to 1.52). Heterogeneity between these trials was not statistically significant (Chi² = 11.97, df = 19, P = 0.89; I² = 0%). The use of aprotinin in cardiac surgery was not associated with an increased risk of stroke (RR 0.81, 95% CI 0.40 to 1.67).
Deep vein thrombosis
Sixteen trials of aprotinin versus control reported data for deep vein thrombosis (DVT). These trials included a total of 1456 patients, of whom 854 were randomised to aprotinin and 602 were randomised to a control group who did not receive aprotinin. The use aprotinin did not increase the risk of deep vein thrombosis (RR 0.78, 95% CI 0.47 to 1.29). Heterogeneity between these trials was not statistically significant (Chi² = 6.22, df = 11, P = 0.86; I² = 0%). Three cardiac trials reported data for DVT. The use of aprotinin was not associated with a statistically significant increased risk of DVT (RR 1.29, 95% CI 0.36 to 4.58).
Pulmonary embolus
Four trials of aprotinin versus control reported data for pulmonary embolus (PE). These trials included a total of 585 patients, of whom 304 were randomised to aprotinin and 281 were randomised to a control group who did not receive aprotinin. The use of aprotinin did not statistically significantly increase the risk of PE (RR 1.49, 95% CI 0.42 to 5.29).
Renal failure / dysfunction
Twenty‐seven trials of aprotinin versus control reported data for renal failure / dysfunction. These trials included a total of 5185 patients, of whom 2904 were randomised to aprotinin and 2281 were randomised to a control group who did not receive aprotinin. The use aprotinin did not statistically significantly increase the risk of renal failure / dysfunction (RR 1.10, 95% CI 0.79 to 1.54). Heterogeneity between these trials was not statistically significant (Chi² = 7.64, df = 16, P = 0.96; I² = 0%). Although there appeared to be a trend toward an increased risk of renal failure/dysfunction when aprotinin was used in cardiac surgery, the result was not statistically significant (RR 1.07, 95% CI 0.76 to 1.51).
Length of hospital stay
Twenty‐three trials of aprotinin versus control reported data for hospital length of stay. These trials included a total of 2017 patients, of whom 1011 were randomised to aprotinin and 1006 were randomised to a control group who did not receive aprotinin. Aprotinin treatment did not reduce the length of hospital stay (MD ‐0.25 days, 95% CI ‐0.71 to 0.20 days). Heterogeneity between these trials was statistically significant (Chi² = 50.13, df = 22, P = 0.0006; I² = 56%).
Tranexamic acid
Sixty‐five trials compared TXA with control, and reported data on the number of patients exposed to allogeneic blood transfusion. These trials included a total of 4842 patients, of whom 2528 were randomised to TXA and 2314 were randomised to a control group who did not receive TXA. The use of TXA significantly reduced the need for allogeneic blood transfusion by a relative 39% (RR 0.61, 95% CI 0.53 to 0.70). Heterogeneity between these trials was statistically significant (Chi² = 249.33, df = 63, P <0.0001; I² = 75%). This represents an absolute risk reduction of 18% (RD ‐0.18, 95% CI ‐0.22 to ‐0.14).
Type of surgery
Thirty‐four trials of TXA versus control involved cardiac surgery. These trials included a total of 3006 patients, of whom 1578 were randomised to TXA, and 1428 were randomised to a control group who did not receive TXA. There was a significant 32% relative reduction in the rate of exposure to allogeneic blood transfusion in those patients treated with TXA (RR 0.68, 95% CI 0.57 to 0.81). Heterogeneity between these trials was statistically significant (Chi² = 137.35, df = 33, P <0.00001; I² = 76%).
Twenty‐seven trials of TXA versus control involved orthopaedic surgery. These trials included a total of 1381 patients of whom of whom 722 were randomised to TXA and 659 were randomised to a control group who did not receive TXA. Again there was a significant RR reduction of 51% in those participants treated with TXA (RR 0.49, 95% CI 0.39 to 0.62). Heterogeneity between these trials was statistically significant (Chi² = 53.86, df = 25, P = 0.0007; I² = 54%).
Two trials of TXA versus control involved liver surgery. These trials included a total of 296 patients of whom 148 were randomised to TXA and 148 were randomised to a control group who did not receive TXA. In liver surgery treatment with TXA did not reduce the risk of receiving an allogeneic blood transfusion (RR 0.16, 95% CI 0.00 to 32.47). Heterogeneity between these trials was statistically significant (Chi² = 14.23, df = 1, P = 0.0002; I² = 93%).
One trial of TXA versus control involved vascular surgery. This trial included 59 patients of whom 30 were randomised to TXA and 29 were randomised to a control group who did not receive TXA. In vascular surgery treatment with TXA reduced the risk of receiving an allogeneic blood transfusion (RR 0.56, 95% CI 0.33 to 0.96).
One trial of TXA versus control involved gynaecological surgery. This trial included 100 patients of whom 50 were randomised to TXA and 50 were randomised to a control group who did not receive TXA. In gynaecological surgery treatment with TXA did not reduce the risk of receiving an allogeneic blood transfusion (RR 1.50, 95% CI 0.75 to 3.01).
Effect of transfusion protocols
Fifty‐six trials of TXA versus control reported the use of transfusion protocols and provided data on the number of patients exposed to allogeneic blood transfusion. These trials included a total of 4125 patients, of whom 2156 were randomised to TXA and 1969 were randomised to a control group who did not receive TXA. The use of TXA reduced the need for allogeneic blood transfusion by a relative 43% (RR 0.57, 95% CI 0.48 to 0.67). Heterogeneity between these trials was statistically significant (Chi² = 248.97, df = 55, P <0.00001; I² = 78%). There were nine trials that did not report the use of transfusion protocols. These trials included a total of 717 patients of whom 372 were randomised to TXA and 345 were randomised to a control group. The use of TXA reduced the need for allogeneic blood transfusion compared to control (RR 0.76, 95% CI 0.61 to 0.96). Heterogeneity between these trials was statistically significant (Chi² = 15.48, df = 7, P = 0.03; I² = 55%).
Although the baseline rate of transfusion remained relatively constant across both subgroups (transfusion protocol 44% versus no transfusion protocol 45%) transfusion rates in the intervention arms was collectively greater in those trials that did not report the use of a transfusion protocol compared to those trials that did use a transfusion protocol to guide transfusion practice (37% versus 26%, respectively).
Volume of blood transfused
Twenty‐three trials of TXA versus control reported data on the volume of blood transfused in all patients. These trials included a total of 1814 patients, of whom 943 were randomised to TXA and 871 were randomised to a control group. The use of TXA resulted in a saving of 0.87 units of allogeneic blood per patient (MD ‐0.87 units, 95% CI ‐1.20 to ‐0.53 units). Heterogeneity between these trials was statistically significant (Chi² = 154.24, df = 20, P <0.00001; I² = 87%).
Thirteen trials of TXA versus control provided data on the volume of blood transfused in those patients transfused. All 481 patients received allogeneic blood transfusion. The use of TXA did not statistically significantly reduce the volume of blood transfused compared to control (MD ‐0.34 units, 95% CI ‐0.80 to 0.11 units). Heterogeneity between these trials was statistically significant (Chi² = 45.89, df = 12, P < 0.0001; I² = 74%).
Blood loss ‐ all surgery combined
A total of 17 trials of TXA versus control reported intra‐operative blood loss data. These trials included a total of 1173 patients, of whom 599 were randomised to TXA and 574 were randomised to a control group. These trials involved cardiac surgery (n = 4), orthopaedic surgery (n = 12) and gynaecological surgery (n = 1). In aggregate, TXA treatment reduced intra‐operative blood loss (MD ‐121.41 mls, 95% CI ‐180.19 to ‐62.63 mls). Heterogeneity between these trials was statistically significant (Chi² = 49.05, df = 16, P <0.0001; I² = 67%).
A total of 35 trials of TXA versus control reported post‐operative blood loss data. These trials included a total of 2501 patients, of whom 1285 were randomised to TXA and 1216 were randomised to a control group. These trials involved cardiac surgery (n = 22) orthopaedic surgery (n = 12) and gynaecological surgery (n = 1). In aggregate, TXA treatment significantly reduced post‐operative blood loss on average by around 247 mls per patient (MD ‐247.17 mls, 95% CI ‐294.76 to ‐199.58 mls). Heterogeneity between these trials was statistically significant (Chi² = 248.36, df = 34, P <0.00001; I² = 86%).
A total of 28 trials of TXA versus control reported total blood loss data (intra‐operative and post‐operative blood loss combined). These trials included a total of 1712 patients, of whom 875 patients were randomised to TXA and 837 were randomised to a control group. These trials involved cardiac surgery (n = 6), orthopaedic surgery (n = 20), gynaecological surgery (n = 1) and liver surgery (n = 1). In aggregate, the use of TXA significantly reduced perioperative blood loss by around 414 mls per patient (MD ‐414.06 mls, 95% CI ‐525.19 to ‐302.92 mls). Heterogeneity between these trials was statistically significant (Chi² = 249.58, df = 27, P <0.00001; I² = 89%).
Blood loss ‐ cardiac surgery
Four trials of TXA versus control involving cardiac surgery reported intra‐operative blood loss data. These trials included a total of 244 patients, of whom 138 were randomised to TXA and 106 randomised to a control group. The use of TXA in cardiac surgery reduced intra‐operative blood loss on average by around 167 mls per patient (MD ‐166.76 mls, 95% CI ‐331.24 to ‐2.27 mls). There is some evidence of statistical heterogeneity between these trials (Chi² = 5.36, df = 3, P = 0.15; I² = 44%).
Twenty‐two trials of TXA versus control involving cardiac surgery reported post‐operative blood loss data. These trials included a total of 1597 patients, of whom 827 were randomised to TXA and 770 were randomised to a control group. On average, TXA treatment reduced post‐operative blood loss by around 273 mls per patient compared to control (MD ‐272.87 mls, 95% CI ‐328.85 to ‐216.89 mls). Heterogeneity between these trials was statistically significant (Chi² = 83.41, df = 21, P <0.00001; I² = 75%).
Six trials of TXA versus control involving cardiac surgery reported total blood loss data (intra‐operative and post‐operative blood loss combined). These trials included a total of 391 patients, of whom 210 were randomised to TXA and 181 were randomised to a control group. TXA treatment reduced the total amount of blood lost during the perioperative period by around 300 mls per patient (MD ‐300.47 mls, 95% CI ‐470.74 to ‐130.21 mls). Heterogeneity between these trials was statistically significant (Chi² = 12.19, df = 5, P = 0.03; I² = 59%).
Blood loss ‐ orthopaedic surgery
Twelve trials of TXA versus control involving orthopaedic surgery reported intra‐operative blood loss data. These trials included a total of 829 patients, of whom 411 were randomised to TXA and 418 were randomised to a control group. The use of TXA in orthopaedic surgery reduced intra‐operative blood loss by around 116 mls per patient (MD ‐115.52 mls, 95% CI ‐187.88 to ‐43.16 mls). Heterogeneity between these trials was statistically significant (Chi² = 42.52, df = 11, P <0.0001; I² = 74%).
Twelve trials of TXA versus control involving orthopaedic surgery reported post‐operative blood loss data. These trials included a total of 804 patients, of whom 408 were randomised to TXA and 396 were randomised to a control group. On average, TXA treatment in orthopaedic surgery reduced post‐operative blood loss by around 229 mls per patient (MD ‐228.52 mls, 95% CI ‐321.76 to ‐135.27 mls). Heterogeneity between these trials was statistically significant (Chi² = 125.01, df = 11, P <0.00001; I² = 91%).
Twenty trials of TXA versus control involving orthopaedic surgery reported total blood loss data (intra‐operative and post‐operative blood loss combined). These trials included a total of 1201 patients, of whom 605 were randomised to TXA and 596 were randomised to a control group. The use of TXA in orthopaedic surgery significantly reduced the total amount of blood lost during the perioperative period (MD ‐446.19 mls, 95% CI ‐554.61 to ‐337.78 mls). Heterogeneity between these trials was statistically significant (Chi² = 85.30, df = 19, P <0.00001; I² = 78%).
Re‐operation for bleeding
Twenty‐seven trials of TXA versus control reported data on re‐operation for bleeding. These trials included a total of 2386 patients, of whom 1224 were randomised to TXA and 1162 were randomised to a control group. The use of TXA did not statistically significantly decrease the risk of re‐operation for bleeding (RR 0.80, 95% CI 0.55 to 1.17). Heterogeneity between these trials was not statistically significant (Chi² = 12.66, df = 23, P = 0.96; I² = 0%). Of the 27 trials of TXA that reported data for this outcome 26 involved cardiac surgery. Therefore in the context of cardiac surgery the use of TXA did not statistically significantly reduce the risk of re‐operation for bleeding (RR 0.79, 95% CI 0.54 to 1.17).
Mortality
Thirty trials of TXA versus control reported mortality data. These trials included a total of 2917 patients, of whom 1478 were randomised to TXA and 1439 were randomised to a control group. The use of TXA was not associated with an increased risk of death (RR 0.60, 95% CI 0.33 to 1.10). Heterogeneity between these trials was not statistically significant (Chi² = 10.00, df = 17, P = 0.90; I² = 0%). Of the 30 trials of TXA that reported data for mortality 23 involved cardiac surgery. The use of TXA in cardiac surgery was not associated with an increased risk of death (RR 0.58, 95% CI 0.26 to 1.28).
Myocardial infarction
Twenty‐one trials of TXA versus control reported data for myocardial infarction. These trials included a total of 2186 patients, of whom 1117 were randomised to TXA and 1069 were randomised to a control group who did not receive TXA. The use of TXA was not associated with an increased risk of myocardial infarction (RR 0.79, 95% CI 0.41 to 1.52). Heterogeneity between these trials was not statistically significant (Chi² = 7.84, df = 12, P = 0.80; I² = 0%). Of the 21 trials of TXA that reported data for myocardial infarction 19 involved cardiac surgery. The use of TXA in cardiac surgery did not increase the risk of myocardial infarction (RR 0.74, 95% CI 0.37 to 1.47).
Stroke
Eighteen trials of TXA versus control reported data for stroke. These trials included a total of 2027 patients, of whom 1050 were randomised to TXA and 977 were randomised to a control group. The use of TXA was not associated with a statistically significant increase in the risk of stroke (RR 1.23, 95% CI 0.49 to 3.07). Heterogeneity between these trials was not statistically significant (Chi² = 3.18, df = 7, P = 0.87; I² = 0%). Of the 18 trials of TXA that reported data for this outcome 17 involved cardiac surgery. In this surgical setting the risk of stroke was not statistically significantly increased with the use of TXA (RR 1.44, 95% CI 0.53 to 3.91).
Deep vein thrombosis
Twenty‐three trials of TXA versus control reported data for deep vein thrombosis. These trials included a total of 1472, of whom 746 were randomised to TXA and 726 were randomised to a control group. TXA treatment did not appear to be associated with an increase in the risk of developing a DVT (RR 0.71, 95% CI 0.35 to 1.43). Heterogeneity between these trials was not statistically significant (Chi² = 5.71, df = 11, P = 0.89; I² = 0%). Of the 23 trials of TXA that reported data for DVT four involved cardiac surgery. Of the 422 patients that underwent cardiac surgical procedures two patients developed a DVT. These were single events occurring in the control arms of two separate trials.
Pulmonary embolism
Fourteen trials of TXA versus control reported data for pulmonary embolism. These trials included a total of 1006 patients, of whom 527 were randomised to TXA and 479 were randomised to a control group who did not receive TXA. The use of TXA did not increase the risk of developing a pulmonary embolus (RR 0.67, 95% CI 0.23 to 1.99). Heterogeneity between these trials was not statistically significant (Chi² = 2.81, df = 7, P = 0.90; I² = 0%). Of the 16 trials that reported data for pulmonary embolism six involved cardiac surgery. Of the 569 patients that underwent cardiac surgical procedures only two patients developed a pulmonary embolus. As was the case with deep vein thrombosis these were single events occurring in the control arms of two separate trials.
Renal failure / dysfunction
Nine trials of TXA versus control provided data for renal failure / dysfunction. These nine cardiac surgery trials included a total of 912 patients, of whom 454 were randomised to TXA and 458 were randomised to a control group. Treatment with TXA did not appear to increase the risk of developing renal failure or renal dysfunction (RR 0.89, 95% CI 0.33 to 2.37). Heterogeneity between these trials was not statistically significant (Chi² = 2.52, df = 6, P = 0.87; I² = 0%).
Hospital length of stay
Ten trials of TXA versus control provided data for hospital length of stay. These trials included a total of 772 patients, of whom 379 were randomised to TXA and 393 were randomised to a control group. The use of TXA did not significantly impact on the length of hospital stay (MD ‐0.34 days, 95% CI ‐0.82 to 0.13 days). Heterogeneity between these trials was statistically significant (Chi² = 18.42, df = 9, P = 0.03; I² = 51%). For the five trials that involved cardiac surgery the use of TXA did not significantly reduce the length of hospital stay (MD ‐0.08 days, 95% CI ‐0.34 to 0.18 days).
Epsilon aminocaproic acid
Sixteen trials of EACA versus control provided data on the number of patients exposed to allogeneic blood transfusion. These trials included a total of 1035 patients, of whom 530 were randomised to EACA and 505 were randomised to a control who did not receive EACA. The use of EACA significantly reduced the need for allogeneic blood transfusion by a relative 19% (RR 0.81, 95% CI 0.67 to 0.99). Heterogeneity between these trials was statistically significant (Chi² = 41.12, df = 15, P = 0.0003; I² = 64%). This represents an absolute risk reduction of 10% (RD ‐0.10, 95% CI ‐0.18 to ‐0.03).
Type of surgery
Eleven trials of EACA versus control involved cardiac surgery. These trials included a total of 649 patients, of whom 338 were randomised to EACA and 311 were randomised to a control group. When used in cardiac surgery EACA reduced the need for allogeneic blood transfusion by a relative 30% (RR 0.70, 95% CI 0.52 to 0.93). There is some evidence of statistical heterogeneity between these trials (Chi² = 16.38, df = 10, P = 0.09; I² = 39%). Four trials of EACA versus control involved orthopaedic surgery. These trials included a total of 304 patients, of whom 150 were randomised to EACA and 154 patients were randomised to a control group. The use of EACA in orthopaedic surgery did not reduce the need for allogeneic blood transfusion compared to control (RR 1.00, 95% CI 0.93 to 1.08). Heterogeneity between these trials was not statistically significant (Chi² = 1.01, df = 3, P = 0.80; I² = 0%). One trial of EACA involved liver surgery. For this single trial the relative risk of requiring an allogeneic blood transfusion was 0.93 (95% CI 0.80 to 1.08).
Effect of transfusion protocols
Of the 16 trials of EACA versus control that provided data for the number of patients exposed to allogeneic blood transfusion, 15 reported the use of a transfusion protocol to guide transfusion practice. Therefore stratification of the data by the presence or absence of a transfusion protocol was uninformative.
Volume of blood transfused
Six trials of EACA versus control provided data for the volume of blood transfused in all patients. These trials included a total of 432 patients, of whom 215 were randomised to EACA and 217 were randomised to a control group who did not receive EACA. On average, the use of EACA reduced the volume of allogeneic blood transfused by 1.3 units per patient (MD ‐1.30 units, 95% CI ‐2.14 to ‐0.45 units). Heterogeneity between these trials was statistically significant (Chi² = 23.45, df = 5, P = 0.0003; I² = 79%). Three trials of EACA versus control provided data for the volume of blood transfused in those patients transfused. When the volume of allogeneic blood transfused was assessed in only those patients that actually received a blood transfusion the use of EACA did not reduce the amount of blood transfused (MD 0.22 units, 95% CI ‐0.34 to 0.79 units). Heterogeneity between these trials was not statistically significant (Chi² = 0.56, df = 2, P = 0.76; I² = 0%).
Blood loss ‐ all surgery combined
Five trials of EACA versus control reported intra‐operative blood loss data. These trials included a total of 353 patients, of whom 175 were randomised to EACA and 178 were randomised to a control group. These trials involved cardiac surgery (n = 2) and orthopaedic surgery (n = 3). In aggregate, EACA treatment reduced the amount of blood lost during the intra‐operative period by around 157 mls per patient (MD ‐156.63 mls, 95% CI ‐276.92 to ‐36.33 mls). Heterogeneity between these trials was not statistically significant (Chi² = 5.01, df = 4, P = 0.29; I² = 20%).
Fourteen trials of EACA versus control reported post‐operative blood loss data. These trials included a total of 1174 patients, of whom 580 were randomised to EACA and 594 were randomised to a control group. These trials involved cardiac surgery (n = 12) and orthopaedic surgery (n = 2). In aggregate, EACA treatment reduced post‐operative blood loss on average by 207 mls per patient (MD ‐207.49 mls, 95% CI ‐276.43 to ‐138.54 mls). Heterogeneity between these trials was statistically significant (Chi² = 97.46, df = 13, P < 0.00001; I² = 87%).
Two trials of EACA versus control reported total blood loss data (intra‐operative and post‐operative blood loss combined). These orthopaedic trials included a total of 92 patients, of whom 44 were randomised to EACA and 48 were randomised to a control group. The use of EACA in orthopaedic surgery was only marginally effective in reducing blood loss during the perioperative period (MD ‐299.69 mls, 95% CI ‐522.54 to ‐76.84 mls). Heterogeneity between these trials was not statistically significant (Chi² = 0.73, df = 1, P = 0.39; I² = 0%).
Blood loss ‐ cardiac surgery
Two trials of EACA versus control involving cardiac surgery reported intra‐operative blood loss data. These trials included a total of 79 patients, of whom 40 patients were randomised to EACA and 39 were randomised to a control group. On average, the use of EACA in cardiac surgery reduced the amount of blood lost during the intra‐operative period by around 214 mls per patient (MD ‐213.58, 95% CI ‐310.03 to ‐117.13 mls). Heterogeneity between these trials was not statistically significant (Chi² = 0.12, df = 1, P = 0.73; I² = 0%).
Twelve trials of EACA versus control involving cardiac surgery reported post‐operative blood loss data. These trials included a total of 946 patients, of whom 467 were randomised to EACA and 479 were randomised to control group who did not receive EACA treatment. The use of EACA in cardiac surgery reduced the amount of blood lost during the post‐operative period on average by around 200 mls per patient (MD ‐200.27 mls, 95% CI ‐273.44 to ‐127.09 mls). Heterogeneity between these trials was statistically significant (Chi² = 97.18, df = 11, P <0.00001, I² = 89%).
Blood loss ‐ orthopaedic surgery
Three trials of EACA versus control involving orthopaedic surgery provided intra‐operative blood loss data. These trials included a total of 274 patients, of whom 135 were randomised to EACA and 139 were randomised to a control group. EACA treatment in orthopaedic surgery did not reduce the amount of blood lost during the intra‐operative period (MD ‐40.66 mls, 95% CI ‐236.71 to 155.38 mls). Heterogeneity between these trials was not statistically significant (Chi² = 2.10, df = 2, P = 0.35; I² = 5%).
Two trials of EACA versus control involving orthopaedic surgery reported post‐operative blood loss. These trials included a total of 228 patients, of whom 113 were randomised to EACA and 115 were randomised to a control group. The use of EACA in orthopaedic surgery reduced blood loss during the post‐operative period by around 285 mls per patient (MD ‐285.06 mls, 95% CI ‐452.73 to ‐117.39 mls). Heterogeneity between these trials was not statistically significant (Chi² = 0.18, df = 1, P = 0.67; I² = 0%).
Two trials of EACA versus control involving orthopaedic surgery reported total blood loss data (intra‐operative and post‐operative blood loss combined). These trials included a total of 92 patients, of whom 44 were randomised to EACA and 48 were randomised to a control group. The use of EACA in orthopaedic surgery reduced blood loss during the perioperative period by around 300 mls per patient (MD ‐299.69 mls, 95% CI ‐522.54 to ‐76.84 mls). Heterogeneity between these trials was not statistically significant (Chi² = 0.73, df = 1, P = 0.39; I² = 0%).
Re‐operation for bleeding
Eight trials of EACA versus control reported data on the number of patients requiring re‐operation for bleeding. These trials included a total of 922 patients, of whom 470 were randomised to EACA and 452 were randomised to a control group. The use of EACA was not associated with an increased risk of re‐operation compared to control (RR 0.32, 95% CI 0.11 to 0.99). Heterogeneity between these trials was not statistically significant (Chi² = 1.84, df = 5, P = 0.87, I² = 0%). Of the eight trials of EACA that reported data on re‐operations, seven involved cardiac surgery. In this surgical setting the use of EACA did not increase the risk of re‐operation (RR 0.35, 95% CI 0.11 to 1.17).
Mortality
Eight trials of EACA versus control reported data on mortality. These trials included a total of 988 patients, of whom 504 were randomised to EACA and 484 were randomised to a control group. The use of EACA was not associated with a statistically significant increased risk of death compared to control (RR 1.07, 95% CI 0.44 to 2.57). Heterogeneity between these trials was not statistically significant (Chi² = 2.30, df = 5, P = 0.81; I² = 0%). Of the eight trials of EACA that reported data on mortality six involved cardiac surgery. In this surgical setting the use of EACA did not statistically significantly increase the risk of death (RR 1.65, 95% CI 0.50 to 5.43).
Myocardial infarction
Seven trials of EACA versus control reported data for myocardial infarction. These trials included a total of 896 patients, of whom 456 were randomised to EACA and 440 were randomised to a control group. The use of EACA was not associated with an increased risk of myocardial infarction compared to control (RR 0.88, 95% CI 0.48 to 1.63). Heterogeneity between these trials was not statistically significant (Chi² = 3.44, df = 4, P = 0.49; I² = 0%). Of the seven trials of EACA that reported data on myocardial infarction six involved cardiac surgery. In this surgical setting the use of EACA did not increase the risk of myocardial infarction (RR 0.88, 95% CI 0.48 to 1.63).
Stroke
Eight trials of EACA versus control reported data for stroke. These trials included a total of 936 patients, of whom 477 were randomised to EACA and 459 were randomised to a control group. The use of EACA was not associated with an increased risk of stroke compared to control (RR 0.62 95% CI 0.16 to 2.36). Heterogeneity between these trials was not statistically significant (Chi² = 1.84, df = 4, P = 0.77; I² = 0%). Of the eight trials of EACA that reported data on stroke, seven involved cardiac surgery. In this surgical setting the use of EACA did not increase the risk of stroke (RR 0.70, 95% CI 0.16 to 3.10).
Deep vein thrombosis
Four trials of EACA versus control reported data for DVT. These trials included a total of 304 patients, of whom 150 were randomised to EACA and 154 were randomised to a control group. The use of EACA was not associated with an increased risk of DVT compared to control (RR 0.78, 95% CI 0.20 to 3.03). Heterogeneity between these trials was not statistically significant (Chi² = 1.02, df = 1, P = 0.31; I² = 2%).
Pulmonary embolism
Three trials of EACA versus control provided data for pulmonary embolism. These trials included a total of 274 patients, of whom 135 were randomised to EACA and 139 were randomised to a control group. The use of EACA was not associated with an increased risk of pulmonary embolism compared to control (RR 0.34, 95% CI 0.06 to 2.13). Heterogeneity between these trials was not statistically significant (Chi² = 0.00, df = 1, P = 0.97; I² = 0%).
Renal failure / dysfunction
Two trials of EACA versus control reported data for renal failure / dysfunction. These trials included a total of 235 patients, of whom 117 were randomised to EACA and 118 were randomised to a control group. The use of EACA was not associated with an increased risk of renal failure / dysfunction (RR 0.41, 95% CI 0.14 to 1.22). Heterogeneity between these trials was not statistically significant (Chi² = 0.48, df = 1, P = 0.49; I² = 0%).
Hospital length of stay
Two trials of EACA versus control reported data for hospital length of stay. These trial included a total of 228 patients, of whom 113 were randomised to EACA and 115 were randomised to a control group. The use of EACA did not impact of the length of hospital stay (MD 0.58 days, 95% CI ‐3.17 to 4.33 days). Heterogeneity between these trials was statistically significant (Chi² = 3.13, df = 1, P = 0.08; I² = 68%).
Aprotinin versus tranexamic acid
Twenty‐one trials of aprotinin versus TXA reported data on the number of patients exposed to allogeneic blood transfusion. These trials included a total of 4185 patients, of whom 2124 were randomised to aprotinin and 2061 were randomised to TXA. There was no statistically significant difference in the rates of allogeneic blood transfusion between those patients treated with aprotinin compared to those treated with TXA (RR 0.90, 95% CI 0.81 to 1.01). Heterogeneity between these trials was statistically significant (Chi² = 60.78, df = 20, P <0.0001; I² = 67%). The effect with Mengistu 2008 excluded was RR 0.92 (95% CI 0.82 to 1.02).
Type of surgery
Eighteen of the 21 trials of aprotinin versus TXA that reported data on the number patients exposed to allogeneic blood transfusion involved cardiac surgery. These trials included a total of 3983 patients, of whom 2025 were randomised to aprotinin and 1958 were randomised to TXA. Compared to TXA, aprotinin reduced the rate of allogeneic blood transfusion (RR 0.87, 95% CI 0.76 to 0.99). Heterogeneity between these trials was statistically significant (Chi² = 45.01, df = 17, P = 0.0002; I² = 62%). When data from Mengistu 2008 were excluded, RR was 0.88 (95% CI 0.78 to 1.01).
Effect of transfusion protocols
Of the 21 trials of aprotinin versus TXA that reported data on the number patients exposed to allogeneic blood transfusion, all but one reported the use of a transfusion protocol to guide transfusion practice. Therefore stratification of the data by the presence or absence of a transfusion protocol proved uninformative.
Volume of blood transfused
Ten trials of aprotinin versus TXA provided data on the volume of allogeneic blood transfused in all patients. These trials included a total of 992 patients, of whom 496 were randomised to aprotinin and 496 were randomised to TXA. There was a small but statistically significant difference between aprotinin and TXA in the volume of allogeneic blood transfused (MD ‐0.24 units, 95% CI ‐0.45 to ‐0.04 units). Heterogeneity between these trials was not statistically significant (Chi² = 10.87, df = 9; P = 0.28; I² = 17%). (When data from Mengistu 2008 were removed, MD was ‐0.21 units, 95% CI ‐0.39 to ‐0.02 units). Six trials of aprotinin versus TXA provided data on the volume of allogeneic blood transfused in those patients transfused. These trials provided data for 207 transfused patients, of whom 97 were treated with aprotinin and 110 were treated with TXA. There was no statistically significant difference between aprotinin and TXA in the volume of allogeneic blood transfused in those patients transfused (MD ‐0.07 units, 95% CI ‐0.44 to 0.30 units). Heterogeneity between these trials was not statistically significant (Chi² = 0.97, df = 5, P = 0.97; I² = 0%).
Blood loss
Thirteen trials of aprotinin versus TXA involving cardiac surgery provided data for post‐operative blood loss. These trials included a total of 831 patients, of whom 412 were randomised to aprotinin and 419 were randomised to TXA. On average, aprotinin appeared to be more effective in reducing post‐operative blood loss than TXA (MD ‐145.81 mls, 95% CI ‐209.99 to ‐81.62 mls). Heterogeneity between these trials was statistically significant (Chi² = 33.86, df = 12, P = 0.0007; I² = 65%). (When data from Mengistu 2008 were removed, MD was ‐131.54 mls, 95% CI ‐192.15 to ‐70.94 mls.)
Re‐operation for bleeding
Seventeen trials of aprotinin versus TXA provided data on the number of patients requiring re‐operation for bleeding. These trials included a total of 4010 patients, of whom 2005 were randomised to aprotinin and 2005 were randomised to TXA. Aprotinin appeared to reduce the need for re‐operation compared to TXA (RR 0.69, 95% CI 0.51 to 0.93). Heterogeneity between these trials was not statistically significant (Chi² = 8.90, df = 13, P = 0.78; I² = 0%). The BART study (Fergusson 2008) provided 61.4% (weight) of the information for this outcome.
Mortality
Seventeen trials of aprotinin versus TXA reported mortality data. These trials included a total of 4130 patients, of whom 2060 were randomised to aprotinin and 2070 were randomised to TXA. There was no statistically significant difference between aprotinin and TXA (RR 1.35, 95% CI 0.94 to 1.93). Heterogeneity between these trials was not statistically significant (Chi² = 6.78, df = 9, P = 0.66, I² = 0%). BART study data (Fergusson 2008) dominated the analysis of this outcome (65.5% weight).
Myocardial infarction
Thirteen trials of aprotinin versus TXA reported data for myocardial infarction. These trials included a total of 3574 patients, of whom 1778 were randomised to aprotinin and 1796 were randomised to TXA. There was statistically significant difference between aprotinin and TXA (RR 1.00, 95% CI 0.71 to 1.42). Heterogeneity between these trials was not statistically significant (Chi² = 6.18, df = 10, P = 0.80; I² = 0%). The BART study (Fergusson 2008) provided 49.6% (weight) of the information for this outcome.
Stroke
Six trials of aprotinin versus TXA reported data for stroke. These trials include a total of 2030 patients of whom 1017 were randomised to aprotinin and 1013 were randomised to TXA. There was no statistically significant difference between aprotinin and TXA (RR 0.88, 95% CI 0.52 to 1.47). Heterogeneity between these trials was not statistically significant (Chi² = 1.91, df = 4, P = 0.75; I² = 0%). BART study data (Fergusson 2008) dominated the analysis of this outcome (88.5% weight).
Renal failure / dysfunction
Six trials of aprotinin versus TXA reported data for renal failure / dysfunction. These trials included a total of 2238 patients, of whom 1119 were randomised to aprotinin and 1119 were randomised to TXA. There was no statistically significant difference between aprotinin and TXA (RR 1.02, 95% CI 0.79 to 1.31). Heterogeneity between these trials was not statistically significant (Chi² = 1.20, df = 3, P = 0.75; I² = 0%). BART study data (Fergusson 2008) dominated the analysis of this outcome (94.5% weight).
Hospital length of stay
Six trials of aprotinin versus TXA reported data for hospital length of stay. These trials include a total of 2174 patients, of whom 1090 were randomised to aprotinin and 1084 were randomised to TXA. There was no statistically significant difference between aprotinin and TXA (MD ‐0.05, 95% CI ‐0.92 to 0.83 days). There was some evidence of statistical heterogeneity between these trials (Chi² = 9.14, df = 5, P = 0.10; I² = 45%).
Aprotinin versus epsilon aminocaproic acid
Twelve trials of aprotinin versus EACA reported data on the number of patients exposed to allogeneic blood transfusion. These trials included a total of 2200 patients, of whom 1102 were randomised to aprotinin and 1098 were randomised to EACA. The use of aprotinin significantly reduced the rate of allogeneic blood transfusion compared to EACA (RR 0.82, 95% CI 0.76 to 0.89). Heterogeneity between these trials was not statistically significant (Chi² = 9.33, df = 11, P = 0.59; I² = 0%).
Type of surgery
Of the 12 trials of aprotinin versus EACA that reported data on the number of patients exposed to allogeneic blood transfusion, 10 involved cardiac surgery and two involved orthopaedic surgery. Compared to EACA, aprotinin reduced the rate of allogenic blood transfusion in cardiac surgery (RR 0.82, 95% CI 0.76 to 0.89) but not in orthopaedic surgery (RR 0.82, 95% CI 0.48 to 1.40).
Effect of transfusion protocols
Of the 12 trials of aprotinin versus EACA that reported data on the number of patients exposed to allogeneic blood transfusion, nine reported the use of a transfusion protocol to guide transfusion practice and three did not. For the nine trials that reported the use of a transfusion protocol, aprotinin reduced the rate of allogeneic blood transfusions compared to EACA by a relative 18% (RR 0.82, 95% CI 0.76 to 0.89). Heterogeneity between these trials was not statistically significant (Chi² = 6.45, df = 8, P = 0.60; I² = 0%). For those trials that did not report the use of a transfusion protocol there was no statistically significant difference aprotinin and EACA (RR 0.78, 95% CI 0.47 to 1.31). Heterogeneity between these trials was not statistically significant (Chi² = 2.86, df = 2, P = 0.24; I² = 30%).
Volume of blood transfused
Five trials of aprotinin versus EACA reported data for the volume of allogeneic blood transfused in all patients. These trials included a total of 329 patients, of whom 166 were randomised to aprotinin and 163 were randomised to EACA. There was no statistically significant difference between aprotinin and EACA (MD ‐0.21 units, 95% CI ‐0.55 to 0.14 units). Heterogeneity between these trials was not statistically significant (Chi² = 5.14, df = 4, P = 0.27; I² = 22%). Two trials of aprotinin versus EACA provided data for the volume of allogeneic blood transfused in those patients transfused. These trials included a total of 63 transfused patients, of whom 28 were treated with aprotinin and 35 were treated with EACA. There was no statistically significant difference between aprotinin and EACA treatment (MD ‐0.18 units, 95% CI ‐0.63 to 0.28 units). Heterogeneity between these trials was not statistically significant (Chi² = 0.66, df = 1, P = 0.41; I² = 0%).
Blood loss
There were seven trials of aprotinin versus EACA involving cardiac surgery that reported post‐operative blood loss data. These trials included a total of 454 patients, of whom 230 were randomised to aprotinin and 224 were randomised to EACA. Aprotinin appeared to be marginally more effective in reducing post‐operative blood loss than EACA (MD ‐111.43 mls, 95% CI ‐220.64 to ‐2.21 mls). Heterogeneity between these trials was statistically significant (Chi² = 25.74, df = 6, P = 0.0002; I² = 77%).
Re‐operation for bleeding
Six trials of aprotinin versus EACA reported data on the number of patients requiring re‐operation for bleeding. These trials included a total of 2075 patients, of whom 1034 were randomised to aprotinin and 1041 were randomised to EACA. Although aprotinin appeared to be more effective than EACA in reducing the number patients requiring re‐operation due to bleeding the difference did not reach statistical significance (RR 0.70, 95% CI 0.49 to 1.00). Heterogeneity between these trials was not statistically significant (Chi² = 0.93, df = 2, P = 0.63; I² = 0%). However, the data from the BART study (Fergusson 2008) provided 90.1% of the information (weight) for this outcome. The results of this one trial showed that aprotinin was statistically significantly more effective than EACA in reducing the risk of re‐operation for bleeding (RR 0.67, 95% CI 0.46 to 0.98).
Mortality
There were five trials of aprotinin versus EACA that reported mortality data. These trials included a total of 1891 patients, of whom 949 were randomised to aprotinin and 942 were randomised to EACA. Although the result failed to reach statistical significance, there appeared to be a trend toward an increased risk of death in the aprotinin group compared to EACA (RR 1.51, 95% CI 0.99 to 2.30). Again, the results of the BART study (Fergusson 2008) provided most of the information for this outcome (89.9% weight). Heterogeneity between these trials was not statistically significant (Chi² = 0.26, df = 3, P = 0.97; I² = 0%).
Myocardial infarction
Four trials of aprotinin versus EACA reported data for myocardial infarction. These trials included a total of 1676 patients, of whom 830 were randomised to aprotinin and 846 were randomised to EACA. There was no statistically significant difference in the risk of myocardial infarction between aprotinin and EACA (RR 1.42, 95% CI 0.90 to 2.22). Heterogeneity between these trials was not statistically significant (Chi² = 1.27, df = 3, P = 0.74; I² = 0%). Data from the BART study (Fergusson 2008) dominated this outcome (68.2% weight).
Stroke
Two trials of aprotinin versus EACA reported data for stroke (cerebrovascular accident). These trials included a total of 1578 patients, of whom 785 were randomised to aprotinin and 793 were randomised to EACA. There was no difference in the risk of stroke between aprotinin and EACA (RR 1.05, 95% CI 0.60 to 1.85). Heterogeneity between these trials was not statistically significant (Chi² = 0.27, df = 1, P = 0.60; I² = 0%). The BART study (Fergusson 2008) results provided 94.2% of the information for this outcome.
Deep vein thrombosis
Four trials of aprotinin versus EACA reported data for deep vein thrombosis. These trials included a total of 300 patients, of whom 153 were randomised to aprotinin and 147 were randomised to EACA. One trial reported three cases of DVT all of which occurred in EACA treated patients (RR 0.14, 95% CI 0.01 to 2.51). There were no reported cases of DVT in the three remaining trials.
Pulmonary embolism
Three trials of aprotinin versus EACA reported data for pulmonary embolism. These trials included a total of 270 patients, of whom 138 were randomised to aprotinin and 132 were randomised to EACA. Three events of pulmonary embolism were reported; two in aprotinin treated patients and one in EACA treated patients. There was no statistically significant difference between aprotinin and EACA treatment (RR 1.33, 95% CI 0.10 to 18.42). Heterogeneity between these trials was not statistically significant (Chi² = 1.45, df = 1, P = 0.23; I² = 31%).
Renal failure / dysfunction
Two trials of aprotinin versus EACA reported data for renal failure / dysfunction. These trials included a total of 1595 patients, of whom796 were randomised to aprotinin and 799 were randomised to EACA. Although the analysis was dominated by the data from the BART study (71.6% weight) there was no statistically significant difference between aprotinin and EACA in the number patients experiencing renal failure / dysfunction (RR 1.33, 95% CI 0.59 to 2.99). Heterogeneity between these trials was moderate (Chi² = 2.12, df = 1, P = 0.15; I² = 53%).
Hospital length of stay
Two trials of aprotinin versus EACA reported data for hospital length of stay. These trials included a total of 1605 patients, of whom 803 were randomised to aprotinin and 802 patients were randomised to EACA. There was no statistically significant difference between aprotinin and EACA (MD ‐0.49 days, 95% CI ‐1.74 to 0.77 days).
Tranexamic acid versus epsilon aminocaproic acid
Eight trials provided direct 'head‐to‐head' comparisons of TXA and EACA and reported data on the number of patients exposed to allogeneic blood transfusion. These trials included a total of 2003 patients, of whom 1000 were randomised to TXA and 1003 were randomised to EACA. There was no statistically significant difference between TXA and EACA in the rates of allogeneic blood transfusion (RR 0.97, 95% CI 0.77 to 1.21). Heterogeneity between these trials was statistically significant (Chi² = 14.01, df = 7, P = 0.05; I² = 50%). All eight trials included in this analysis reported the use of a transfusion protocol to guide transfusion practice. Six of the eight trials included in this analysis involved cardiac surgery. A subgroup analysis of the data from these cardiac trials showed that the relative risk of receiving an allogeneic blood transfusion in patients treated with TXA compared to patients treated with EACA was 1.07 (95% CI 0.79 to 1.46).
Volume of blood transfused
Three trials of TXA versus EACA provided data for the volume of allogeneic blood transfused in all patients. These trials included a total of 268 patients, of whom 136 were randomised to TXA and 132 were randomised to EACA. There was no statistically significant difference between TXA and EACA (MD ‐0.28 units, 95% CI ‐0.59 to 0.03 units). Heterogeneity between these trials was not statistically significant (Chi² = 0.96, df = 2, P = 0.62; I² = 0%). Four trials of TXA versus EACA provided data for the volume of allogeneic blood transfused to those patients transfused. These trials included a total of 133 patients, of whom 59 were randomised to TXA and 74 were randomised to EACA. Again there was no statistically significant difference between TXA and EACA treatment (MD ‐0.34 units, 95% CI ‐0.74 to 0.07 units). Heterogeneity between these trials was not statistically significant (Chi² = 0.12, df = 2, P = 0.94; I² = 0%).
Blood loss
Six trials of TXA versus EACA involving cardiac surgery reported post‐operative blood loss data. These trials included a total of 402 patients, of whom 209 were randomised to TXA and 193 were randomised to EACA. There was no difference between TXA and EACA in the volume of blood lost during the post‐operative period (MD ‐4.36 mls, 95% CI ‐163.35 to 154.63 mls). Heterogeneity between these trials was statistically significant (Chi² = 33.81, df = 5, P <0.00001; I² = 85%).
Re‐operation for bleeding
Five trials of TXA versus EACA provided data on re‐operation for bleeding. These trials included a total of 1853 patients, of whom 922 were randomised to TXA and 931 were randomised to EACA. There was no statistically significant difference between TXA and EACA (RR 1.00, 95% CI 0.73 to 1.39). Heterogeneity between these trials was not statistically significant (Chi² = 1.81, df = 3, P = 0.61; I² = 0%). The data of the BART study (Fergusson 2008) dominated the results of this analysis (93.4% weight).
Mortality
Five trials of TXA versus EACA provided mortality data. These trials included a total of 1958 patients, of whom 980 were randomised to TXA and 978 were randomised to EACA. There was no statistically significant difference between TXA and EACA (RR 0.93, 95% CI 0.59 to 1.47). Heterogeneity between these trials was not statistically significant (Chi² = 1.43, df = 3, P = 0.70; I² = 0%).The data of the BART study (Fergusson 2008) dominated the results of this analysis (86.8% weight).
Myocardial infarction
Three trials of TXA versus EACA reported data for myocardial infarction. These trials included a total of 1687 patients, of whom 840 were randomised to TXA and 847 were randomised to EACA. There was no statistically significant difference between TXA and EACA (RR 1.33, 95% CI 0.80 to 2.23). Heterogeneity between these trials was not statistically significant (Chi² = 0.62, df = 2, P = 0.73; I² = 0%). The data of the BART study (Fergusson 2008) dominated the results of this analysis (82.9% weight).
Stroke
Three trials of TXA versus EACA reported data for stroke (cerebrovascular accident). These trials included a total of 1658 patients, of whom 820 were randomised to TXA and 838 were randomised to EACA. There was no statistically significant difference between TXA and EACA (RR 1.33, 95% CI 0.78 to 2.29). Heterogeneity between these trials was not statistically significant (Chi² = 0.30, df = 1, P = 0.58; I² = 0%). The data of the BART study (Fergusson 2008) provided 97.1% (weight) of the information for this analysis.
Pulmonary embolism
Three trials of TXA versus EACA reported data for pulmonary embolism. These trials included a total of 284 patients, of whom 150 were randomised to TXA and 134 were randomised to EACA. There was only one reported case of pulmonary embolism, this occurred in EACA treated patients.
Renal failure / dysfunction
Only the BART study (Fergusson 2008) provided data on renal failure / dysfunction in patients treated with either TXA or EACA. The results of the BART study showed that there was no statistically significant difference between TXA and EACA in the rates of patients experiencing renal failure / dysfunction (RR 0.98, 95% CI 0.76 to 1.27).
Hospital length of stay
Only the BART study (Fergusson 2008) hospital length of stay data in patients treated with either TXA or EACA. The results of the BART study showed that there was no statistically significant difference between TXA and EACA in the length of hospital stay (MD ‐0.64 days, 95% CI ‐1.82 to 0.54 days).
Aprotinin versus either lysine analogue
Thirty trials of aprotinin versus either TXA or EACA provided data on the number of patients exposed to allogeneic blood transfusion. These trials included a total of 5566 patients, of whom 2407 were randomised to aprotinin and 3159 were randomised to a lysine analogue. The use of aprotinin reduced the need for allogeneic blood transfusion by a relative 10% (RR 0.90, 95% CI 0.81 to 0.99). Heterogeneity between these trials was statistically significant (Chi² = 70.06, df = 29 (P < 0.0001; I² = 59%). (When data from Mengistu 2008 were removed, RR was 0.91 (95% CI 0.82 to 1.00).)
In view of the importance of the data on death and myocardial infarction we compared aprotinin with either tranexamic acid or aminocaproic acid. There were nineteen trials that reported on mortality. Of 2115 subjects randomised to aprotinin 71 died, compared with 85 of 3012 randomised to either lysine analogue. The increase in mortality with aprotinin was statistically significant (RR 1.39, 95% CI 1.02 to 1.89). Seventy percent of the statistical weight came from the Bart trial (Fergusson 2008). In contrast, there was no significant increase in the risk of myocardial infarction with aprotinin compared with either lysine analogue (RR 1.11, 95% CI 0.82 to 1.50).
Impact of trial quality
Aprotinin
Of the 108 trials of aprotinin that provided data on the number of patients exposed to allogeneic blood transfusion, 33 trials were assessed as having adequate allocation concealment of treatment schedule. For these 33 trials the use of aprotinin reduced the rate of allogeneic blood transfusion by a relative 36% (RR 0.64, 95% CI 0.53 to 0.79). Heterogeneity between these trials was statistically significant (Chi² = 665.70, df = 32, P <0.00001; I² = 95%). In the 63 trials where there was uncertainty regarding the method of allocation concealment (Unclear), the use of aprotinin reduced the rate of allogeneic blood transfusion by a relative 31% (RR 0.69, 95% CI 0.64 to 0.75). Heterogeneity between these trials was statistically significant (Chi² = 179.31, df = 62, P <0.00001; I² = 65%). In the remaining 12 trials where the method of allocation concealment was assessed as being inadequate (No), the use of aprotinin reduced the rate of allogeneic blood transfusion by a relative 37% (RR 0.63, 95% CI 0.54 to 0.75). Heterogeneity between these trials was not statistically significant (Chi² = 15.50, df = 11, P = 0.16; I² = 29%). These data indicate the effects of aprotinin were not significantly greater in those studies that reported inferior techniques for concealing the randomisation sequence.
Tranexamic acid
Of the 65 trials of TXA that provided data on the number of patients exposed to allogeneic blood transfusion, 28 were assessed as having adequate allocation concealment of treatment schedule. For these 28 trials the use of TXA reduced the rate of allogeneic blood transfusion by a relative 41% (RR 0.59, 95% CI 0.51 to 0.69). Heterogeneity between these trials was statistically significant (Chi² = 41.35, df = 27, P = 0.04; I² = 35%). In the 24 trials where there was uncertainty regarding the method of allocation concealment (Unclear), the use of TXA reduced the rate of allogeneic blood transfusion by a relative 47% (RR 0.53, 95% CI 0.37 to 0.76). Heterogeneity between these trials was statistically significant (Chi² = 209.62, df = 23, P <0.00001; I² = 89%). In the remaining 13 trials where the method of allocation concealment was assessed as being inadequate (No), the use of TXA reduced the rate of allogeneic blood transfusion by a relative 27% (RR 0.73, 95% CI 0.62 to 0.86). Heterogeneity between these trials was not statistically significant (Chi² = 16.38, df = 11 (P = 0.13), I² = 33%).
Epsilon aminocaproic acid
Of the 16 trials that provided data on the number of patients exposed to allogeneic blood transfusion, five were assessed as having adequate allocation concealment of treatment schedule. For these trials the use of EACA did not statistically significantly reduce the rate of allogeneic blood transfusion (RR 0.82, 95% CI 0.58 to 1.16). Heterogeneity between trials was statistically significant (Chi² = 14.35, df = 4, P = 0.006; I² = 72%). In the nine trials where there was uncertainty regarding the method of allocation concealment (Unclear), the use of EACA did not statistically significantly reduce the rate of allogeneic blood transfusion (RR 0.68, 95% CI 0.46 to 1.03). Heterogeneity between trials was not statistically significant (Chi² = 12.54, df = 8, P = 0.13; I² = 36%). In the remaining two trials where the method of allocation concealment was assessed as being inadequate (No), the use of EACA did not statistically significantly reduce the rate of allogeneic blood transfusion (RR 0.93, 95% CI 0.81 to 1.08). Heterogeneity between these trials was not statistically significant (Chi² = 0.13, df = 1, P = 0.72; I² = 0%).
Aprotinin versus tranexamic acid
Of the 21 trials that compared aprotinin to TXA, four were assessed as having adequate allocation concealment of treatment schedule. For these trials the RR of receiving an allogeneic blood transfusion in those patients treated with aprotinin compared to those patients treated with TXA was 0.80 (95% CI 0.69 to 0.92). Heterogeneity between these trials was not statistically significant (Chi² = 3.60, df = 3, P = 0.31; I² = 17%). In the 13 trials where there was uncertainty regarding the method of allocation concealment (Unclear), the RR of receiving an allogeneic blood transfusion was statistically significantly different between aprotinin and TXA (RR 0.97, 95% CI 0.88 to 1.07). Heterogeneity between these trials was not statistically significant (Chi² = 19.25, df = 12, P = 0.08; I² = 38%). (When Mengistu 2008 was removed from the analysis RR was 0.99 (95% CI 0.91 to 1.08). In the remaining four trials where the method of allocation concealment was assessed as being inadequate (No), the RR of receiving an allogeneic blood transfusion was not statistically significantly different between aprotinin treated patients and TXA treated patients (RR 0.93, 95% CI 0.62 to 1.39). Heterogeneity between these trials was statistically significant (Chi² = 10.29, df = 3, P = 0.02; I² = 71%).
Aprotinin versus epsilon aminocaproic acid
Of the 12 trials of aprotinin versus EACA that were assessed for methodological quality, three were assessed as having adequate allocation concealment. For these trials the RR of receiving an allogeneic blood transfusion in those patients treated with aprotinin compared to those patients treated with EACA was 0.86 (95%CI 0.71 to 1.05). Heterogeneity between these trials was not statistically significant (Chi² = 2.75, df = 2, P = 0.25; I² = 27%). For eight trials there was uncertainty regarding the method of allocation concealment (Unclear), the RR of receiving an allogeneic blood transfusion was not statistically significantly different between aprotinin and EACA (RR 0.76, 95% CI 0.58 to 0.99). Heterogeneity between these trials was not statistically significant (Chi² = 6.19, df = 7, P = 0.52; I² = 0%). For one trial the method of allocation concealment was assessed as being inadequate (No).
Tranexamic acid versus epsilon aminocaproic acid
Of the eight trials of TXA versus EACA that were assessed for methodological quality, one trial was assessed as having adequate allocation concealment (Yes). For five trials there was uncertainty regarding the method of allocation concealment (Unclear), and for two trials the method of allocation concealment was assessed as being inadequate (No). There were too few trials to formally assess the impact that methodological quality had on treatment effect.
Aprotinin versus lysine analogues (TXA and EACA combined)
Of the 29 trials that compared aprotinin to the lysine analogues, six were assessed as having adequate allocation concealment of treatment schedule. For these trials the RR of receiving an allogeneic blood transfusion in those patients treated with aprotinin compared to those patients treated with a lysine analogue was 0.82 (95% CI 0.71 to 0.95). Heterogeneity between these trials was not statistically significant (Chi² = 6.44, df = 3, P = 0.27; I² = 22%). In the 18 trials where there was uncertainty regarding the method of allocation concealment (Unclear), the RR of receiving an allogeneic blood transfusion was not statistically significantly different between aprotinin and the lysine analogues (RR 0.95, 95% CI 0.86 to 1.04). Heterogeneity between these trials was not statistically significant (Chi² = 26.77, df = 18, P = 0.08; I² = 33%). (When data from Mengistu 2008 were removed, RR was 0.97 (95% CI 0.89 to 1.06).) In the remaining five trials where the method of allocation concealment was assessed as being inadequate (No), the RR of receiving an allogeneic blood transfusion was not statistically significantly different between aprotinin treated patients and lysine analogue treated patients (RR 0.92, 95% CI 0.67 to 1.28). Heterogeneity between these trials was statistically significant (Chi² = 10.34, df = 4, P = 0.04; I² = 61%).
Discussion
This systematic review of the three anti‐fibrinolytic drugs, aprotinin, tranexamic acid (TXA), and epsilon aminocaproic acid (EACA), includes a total of 252 randomised controlled trials (RCTs), which recruited over 25,000 participants. The previous versions of this Cochrane review (Henry 1999; Henry 2007), included a total of 89 trials with 9876 participants and 211 trials with 20,781 participants, respectively. Although the three drugs differ somewhat in their modes of action, the results of this review confirm and strengthen previous findings that they reduce surgical blood loss and exposure to allogeneic red blood cell transfusion to a degree that is both statistically and clinically significant. Importantly, the risk of re‐operation necessitated by recurrent or continued bleeding after cardiac surgery was lowered by treatment with aprotinin and a clear trend was also seen with TXA for that outcome. These findings are not new, but this updated review provides additional information regarding two significant questions: how do the drugs compare with each other and to what extent are the clinical benefits offset by adverse effects, in particular vascular occlusion? In addressing these questions the updated review includes data from 49 active comparisons between aprotinin and the lysine analogues, compared with 29 in the previous review (Henry 2007). This updated review also adds to the information about vascular events ‐ capturing 54 more episodes of myocardial infarction than the earlier version. The analyses of active comparator trials (direct head‐to‐head comparisons) indicate that aprotinin was slightly more effective than TXA in reducing the need for red cell transfusion in patients undergoing cardiac surgery (RR 0.87, 95% CI 0.76 to 0.99). However, the results of the head‐to‐head comparison showed that aprotinin was marginally more effective than TXA in reducing post‐operative blood loss. In the context of cardiac surgery, aprotinin appeared to be more effective than EACA in reducing the need for red cell transfusion and post‐operative blood loss. Our confidence in ascribing an advantage to aprotinin needs to be moderated by evidence of possible publication bias and uncertainty over the comparative dose response relationships.
Mortality appeared to be unaffected by treatment with any of the drugs and there was no evidence that aprotinin, or the lysine analogues, increased the risks of myocardial infarction or other serious thrombosis. These latter results conflict with the findings of recently published observational studies by Mangano et al (Mangano 2006; Mangano 2007) and Karkouti et al (Karkouti 2006), which showed that the use of aprotinin in cardiac surgery was associated with an increase in the incidence of renal failure, myocardial infarction, and all‐cause mortality (over five years).
Measures of efficacy: blood loss and need for transfusion
Aprotinin appeared to be the most efficacious of the three drugs in reducing perioperative blood loss, the confidence interval (CI) for the average reduction in blood loss with aprotinin seen in placebo/inactive controlled trials does not overlap with those of either TXA or EACA. This conclusion was supported by the sparser literature from active comparator trials, which found that aprotinin reduced post‐operative blood loss to a greater extent than TXA; a similar result was seen in the comparison of aprotinin and EACA. It was notable that the apparent differences between the drugs were only seen in the context of cardiac surgery. There was no advantage of aprotinin over TXA when the drugs were used as an adjunct to orthopaedic procedures.
The three drugs were effective in reducing the proportions of patients who required transfusion with red blood cells. The pooled relative risk (RR) values from placebo/inactive controlled trials were similar. When considering these results it may be relevant that the baseline rates of transfusion differed considerably between the trials of aprotinin and the trials of TXA and EACA. The control‐arms of the aprotinin trials had an average transfusion rate of 62%, compared with 44% for the control‐arm of the TXA trials and 54% for the control‐arms of the EACA trials. A possible explanation for this difference is that aprotinin has been studied more extensively and for a longer period of time than TXA and EACA. It is generally accepted that improvements in surgical technique, advancements in cardiopulmonary bypass technology, the introduction of auto‐transfusion procedures and acceptance of lower transfusion thresholds have been responsible for a reduction in the rates of perioperative blood transfusion over time. This time dependant trend was observed in the trials of aprotinin in cardiac surgery. It is also possible that trials of aprotinin included more high‐risk patients than trials of the lysine analogues. Such high‐risk patients tend to have a greater propensity for blood loss and hence transfusion. Thus, comparisons between drugs based on the placebo/inactive controlled trials of anti‐fibrinolytic drugs may be confounded at trial level by differences in patient populations. Publication bias is a further consideration when considering the placebo/inactive controlled studies of these drugs. As in the previous versions of this review, an examination of the generated funnel plots suggested a degree of publication bias (favouring active treatment) in the aprotinin trials (Figure 3), and a similar pattern was also seen with the trials of TXA (Figure 4) and EACA (Figure 5).
3.
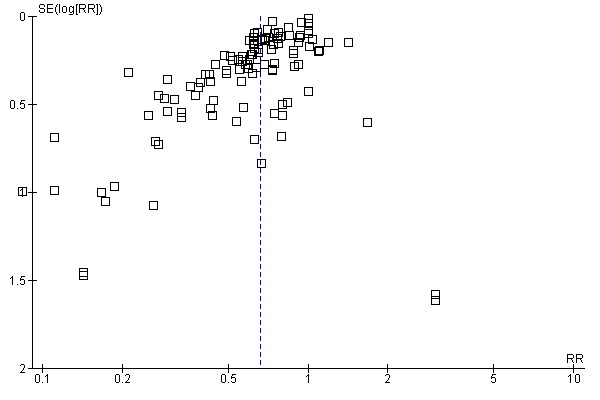
Funnel plot of comparison: 1 Aprotinin versus Control (Blood Transfusion & Blood Loss), outcome: 1.1 No. Exposed to Allogeneic Blood.
4.
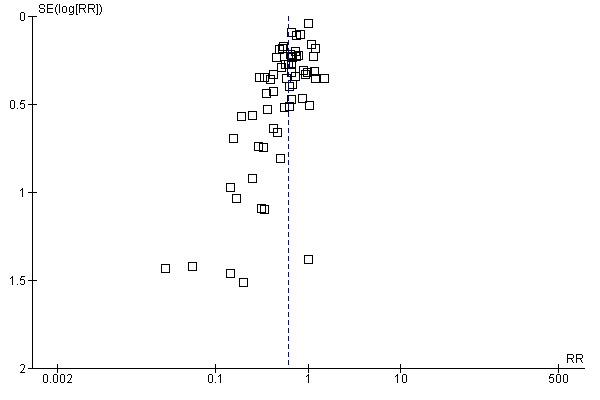
Funnel plot of comparison: 2 Tranexamic Acid versus Control (Blood Transfusion & Blood Loss), outcome: 2.1 No. Exposed to Allogeneic Blood.
5.
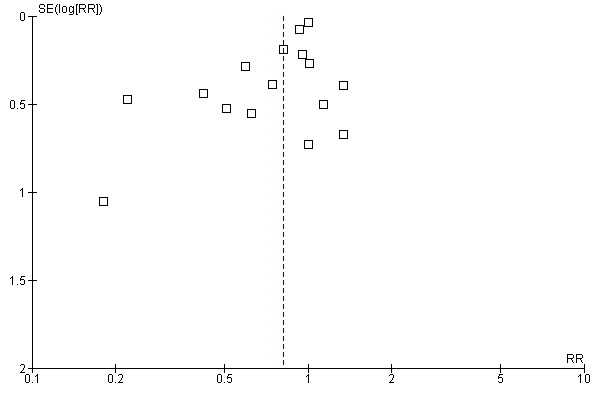
Funnel plot of comparison: 3 Epsilon Aminocaproic Acid versus Control (Blood Transfusion & Blood Loss), outcome: 3.1 No. Exposed to Allogeneic Blood.
In the case of cardiac surgery, when aprotinin was included in pairwise comparisons of blood transfusion requirements with TXA and EACA a small trend in favour of aprotinin was seen in each comparison. When we pooled data on blood transfusions for head‐to‐head comparisons of aprotinin with either of the lysine analogues the advantage of aprotinin was borderline significant ‐ pooled RR 0.90 (95% CI 0.81 to 0.99). We have previously published a meta‐analysis of the comparative trials of aprotinin and lysine analogues in cardiac surgery (Carless 2005). In that study we used a Bayesian meta‐analytic approach to determine if TXA and EACA could be considered equivalent (non‐inferior) to aprotinin in reducing the rate of allogeneic blood transfusion. Although hampered by the small number and size of the trials, our results showed that for blood transfusion, using a non‐inferiority boundary of 20%, the posterior probability that TXA is equivalent to aprotinin was 0.82.
In other words, the updated analyses make us less sure about the equivalence of the lysine analogues and aprotinin when used to reduce the need for red cell transfusion in cardiac surgery. But these conclusions do not take account of two additional factors, dose effects and the possibility of publication bias. As the funnel plots generated from the head‐to‐head trials of aprotinin and the lysine analogues show there appears to be a gap that should be occupied by small trials favouring the latter drugs (Figure 6; Figure 7; Figure 8; Figure 9). The data are sparse but if this represents non‐publication of such trials it could explain some of the apparent advantages of aprotinin seen in the overall analyses. This suggestion was made originally by Beattie 2006 and our updated analysis supports their conclusions. To find evidence of publication bias in the placebo‐controlled trials of these drugs is perhaps not surprising, but we thought it less likely to affect the active comparison studies. The commercial interests in the role of aprotinin (an expensive and popular drug) as an adjunct to cardiac surgery may lie behind this. However, it should be noted that none of the reports of the comparative trials mentions commercial sponsorship.
6.
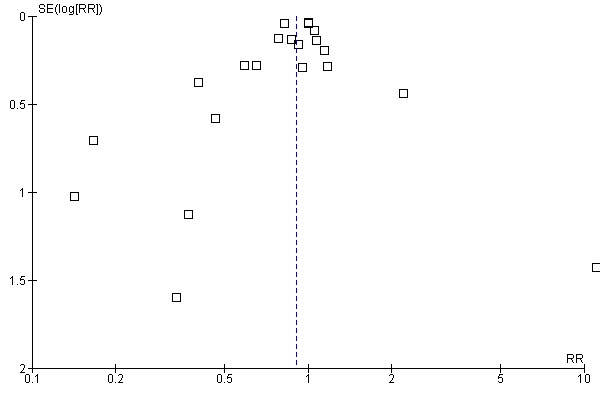
Funnel plot of comparison: 4 Aprotinin versus Tranexamic Acid (Blood Transfusion & Blood Loss), outcome: 4.1 No. Exposed to Allogeneic Blood.
7.
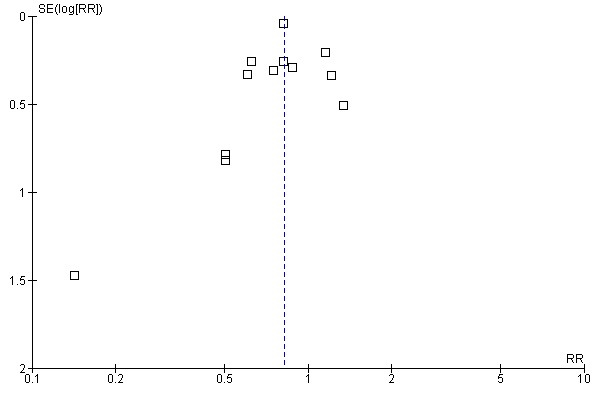
Funnel plot of comparison: 5 Aprotinin versus Epsilon Aminocaproic Acid (Blood Transfusion & Blood Loss), outcome: 5.1 No. Exposed to Allogeneic Blood.
8.
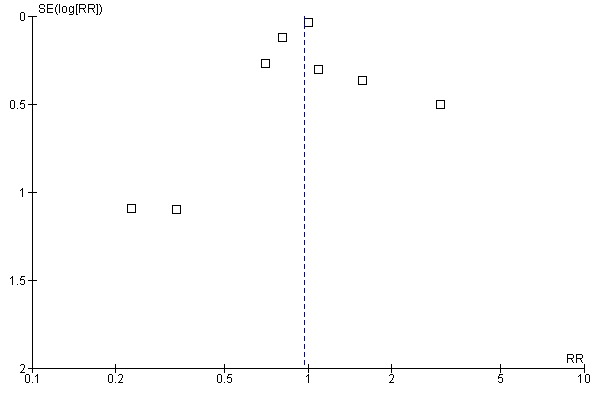
Funnel plot of comparison: 6 Tranexamic Acid versus Epsilon Aminocaproic Acid (Blood Transfusion & Blood Loss), outcome: 6.1 No. Exposed to Allogeneic Blood.
9.
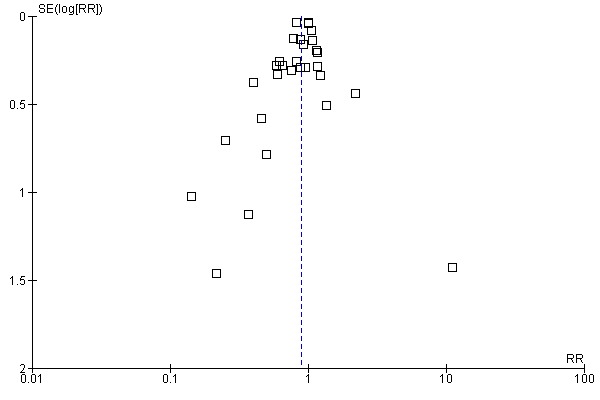
Funnel plot of comparison: 7 Aprotinin versus Lysine Analogues (Blood Transfusion), outcome: 7.1 No. Exposed to Allogeneic Blood.
In making comparisons between the average efficacy of the drugs it is important to consider the possible role of dose as a treatment effect modifier. When the pooled RR values for aprotinin were stratified, both low and high doses reduced the incidence of allogeneic red cell transfusion by around 35%. This was greater than the effect of aprotinin when given only as a priming dose ‐ a RR reduction of 17%. So extending duration of treatment beyond the priming dose may be important. TXA in doses of 2 to 10g and in doses below 2g had a similar effect, reducing allogeneic red cell transfusion by around 30%. There were insufficient data to explore dose effects in the head‐to‐head trials of aprotinin and TXA.
Analyses of the comparative trials of aprotinin and the lysine analogues in orthopaedic surgery were hampered by sparse data. When the results of placebo/inactive controlled trials were combined TXA appeared to be as effective as aprotinin in reducing the number of patients exposed to allogeneic blood transfusion. Conclusions about the relative efficacy of EACA and aprotinin in orthopaedic surgery were hampered by the small number of trials. Of the fourteen trials of aprotinin eight (57.1%) were published between 2000 and 2006. In comparison, of the 21 trials that compared TXA to placebo‐control 16 (76.2%) were published in this time period. As with cardiac surgery, conclusions about the relative efficacy of the drugs may be confounded by changes in surgical technique and transfusion practices that have occurred over time. However, as with the data on blood loss, the apparent advantage of aprotinin over the lysine analogues on the need for blood transfusion observed in cardiac surgery was not seen in orthopaedic surgery.
The analyses of the volumes of red cells transfused were difficult to interpret because of incomplete data in many trials. When all randomised subjects were included in the analyses (which included some who did not receive a transfusion) the average volumes of blood transfused were reduced modestly by all three drugs. When the analysis was confined to individuals who received red cell transfusions the reductions in volume were less marked and a statistically significant treatment effect was observed only for aprotinin. There were insufficient data from head‐to‐head trials to assess the comparative effectiveness of the three drugs in reducing the volumes of blood transfused.
Clinical significance of avoiding red cell transfusion
The true value of avoiding allogeneic red cell transfusion remains unclear (Vamvakas 2001). Patients who are concerned about the risks of contracting illness as a result of blood transfusion (or object to transfusion on religious grounds) will be more interested in avoiding it completely, rather than just reducing the volume of transfused blood. The importance of avoiding the need for transfusion depends on the probability of avoiding disease transmission or other adverse effects, in particular immunomodulation thought to be due to transfused white blood cells (Blumberg 1997; Vamvakas 2001). The significance of the latter remains although a number of countries now perform leukocyte depletion, either selectively, or universally, before administering red cell transfusions, despite a lack of convincing evidence that this provides clinical benefits (Vamvakas 2004). The rate of transmission of HIV or viral hepatitis in most developed countries is very low, because of the quality of screening of donated blood (Coyle 1999; Whyte 1997). These broad assumptions do not apply equally in developing countries. Allogeneic red cell transfusion is administered frequently and blood products may be inadequately screened; the prevalence of viral pathogens amongst donors is high (Kimball 1995; McFarland 1997). In these settings there may be much greater clinical value in a range of interventions that diminish or avoid the need for allogeneic blood. However, the costs of the drugs reviewed here are likely to be prohibitive in developing countries.
Most of the red cell transfusion data reviewed here have been collected in the context of major cardiac surgery, where blood loss may be substantial. The applicability of the efficacy data to clinical settings where blood loss is minor is questionable. Anti‐fibrinolytic drugs may be used alongside other interventions designed to minimise the need for allogeneic red cell transfusion. A variety of techniques have been employed; most involve the re‐infusion of autologous blood either from pre‐operative deposit, acute normovolemic haemodilution, or cell salvage. The latter, in most instances involves the re‐infusion of red blood cells that have been shed into the operative field. The evidence on the efficacy and safety of these techniques was reviewed extensively by the International Study on Perioperative Transfusion (ISPOT) (Bryson 1998; Forgie 1998; Huet 1999). The literature on re‐infusion techniques is generally viewed as being of indifferent quality, because of inadequate randomisation and lack of blinding of outcomes assessment. However, these techniques probably have a modest blood sparing effect. Significantly, the efficacy of autologous re‐infusion techniques appears lower when they are used in the context of a rigorous transfusion protocol. This and the growing evidence on the efficacy of transfusion triggers indicates that a more conservative approach to blood transfusion decisions is desirable (Carson 1998; Hebert 1999). This conservative approach, combined with the selective use of anti‐fibrinolytic drugs, may offer the best approach for managing the transfusion requirements of participants in high‐risk settings such as cardiac surgery.
Other measures of efficacy: need for re‐operation due to bleeding
If the significance of avoiding red cell transfusion is unclear the importance of avoiding re‐operation is not. Going back to theatre because of continued or recurrent bleeding is a serious development after cardiac surgery and any reduction in the incidence of this event is clinically significant (O'Brien 2002). The updated meta‐analysis confirmed that aprotinin reduces the rate of re‐operation due to bleeding by about half. This translates into an absolute risk reduction of 2% and a number needed‐to‐treat of 50 (95% CI 33 to 100). Similar trends were seen with TXA and EACA, but the data were sparse and the differences failed to reach statistical significance. We did not see evidence of publication bias in the data relating to re‐operation rates (Figure 10). When aprotinin was compared directly with TXA in head‐to‐head comparative trials the analysis suggested that aprotinin reduced re‐operations by 31%.
10.
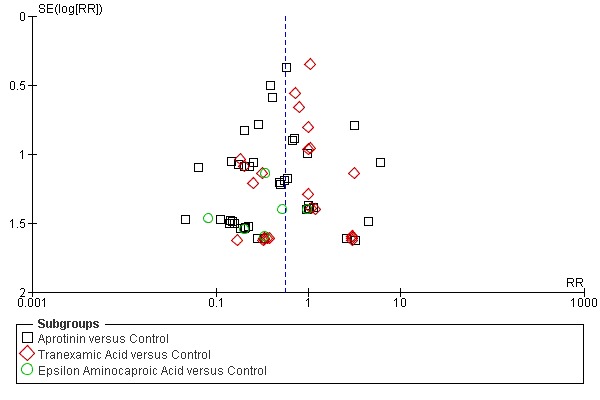
Funnel plot of comparison: 7 Adverse Events and Other Outcomes (Active versus Control), outcome: 7.1 Re‐operation for bleeding.
Effects of treatment on all cause mortality
Regardless of the type of surgery, when aprotinin was compared with no treatment there was no apparent effect on all‐cause mortality (RR 0.81, 95% CI 0.63 to 1.06). In the subset of cardiac surgery trials the result was similar: RR 0.84 (95% CI 0.64 to 1.10). Likewise, when TXA was compared to no treatment the effect on mortality rate in cardiac surgery was not statistically significant and the CI was fairly wide (RR 0.60, 95% CI 0.33 to 1.10). In head to head comparisons there was a trend to higher mortality with aprotinin than either tranexamic acid or aminocaproic acid but the analyses were constrained by the relatively small numbers of outcomes. As there were no qualitative differences between tranexamic acid and aminocaproic acid, and any quantitative differences between these drugs were small, we compared aprotinin with either lysine analogue for the outcomes of mortality and myocardial infarction. The risk of death was higher with aprotinin than with either lysine analogue, although this result was very dependent on the results of the BART trial (Fergusson 2008). There was no significant increase in the risk of myocardial infarction that could explain the higher mortality and indeed there were no other outcome analyses from the head to head trials that could provide an explanation. It is also possible that the difference was due to a benefit of the lysine analogues rather than an adverse effect of aprotinin. In any event this distinction is academic as aprotinin has been withdrawn from world markets and the lysine analogues appear almost equally effective in reducing the need for transfusion with allogeneic blood.
Adverse events and other outcomes
Neither aprotinin nor the lysine analogues appeared to increase the risk of myocardial infarction. In each case the pooled relative risk was close to one. Most data have been collected for aprotinin, which is more often used in cardiac surgery that the lysine analogues. This probably explains the higher rates of myocardial infarction in the placebo‐treated subjects in the aprotinin trials (4.5%) than the TXA trials (2.3%). Similarly, the risk of stroke was not increased by any of these drugs; nor was there any apparent increase in the risk of developing other thrombotic events (deep vein thrombosis, pulmonary embolism, 'other thrombosis').
Data aggregated from 28 randomised trials of aprotinin and nine trials of TXA showed that neither drug increased the risk of renal dysfunction compared to control. Although the event rate was slightly higher in aprotinin‐treated patients compared to the control group (2.4% versus 1.5%) the difference was not statistically significant.
Potential sources of bias in this review
In our review we found a large number of small trials. These continue to be published in the literature, even though individually they contribute very little additional information. In the case of aprotinin, redundancy in terms of new information has long since been reached and there is no justification for continuing to perform placebo‐controlled trials. Future investigation should involve large trials of the relative efficacy and safety of the different drugs (Hebert 2005). The small size of most of the existing trials raises concerns about the effects of publication bias. The funnel plot of the aprotinin trials reveals possible evidence of this ‐ in the form of a 'missing' population of small negative trials (Figure 3).
The main study outcome used in these trials was a practice variable ‐ the decision to transfuse a patient with allogeneic red cells. Although this requires a degree of subjectivity on the part of clinicians it is probably not a major source of bias in this meta‐analysis as around 70% of the trials were assessed as being double‐blind, involving the use of an identical placebo.
Sources of heterogeneity
Substantial heterogeneity in trial outcomes was seen. This was seen in the case of aprotinin for blood loss and blood transfusion outcomes. However, it was not apparent in the analyses of more significant clinical outcomes, such as re‐operation, myocardial infarction and death. It is therefore possible that the subjective nature of the intermediate outcomes, which require judgement about the degree of blood loss, and the need for transfusion, contributed to the between study heterogeneity. Despite this heterogeneity we have little doubt about the existence of a treatment benefit with these drugs. The variation for blood transfusion variables was in terms of the size, not the direction, of effect.
We considered a number of other factors that might explain variation in the size of the treatment effect for blood loss and rates of transfusion. In the case of transfusion, we stratified the data by the clinical setting, operation type, the concomitant use of clinical transfusion thresholds (transfusion triggers), and trial methodological quality. In the case of blood loss, we stratified the data by the type surgery performed and the period in which blood loss was assessed (that is, intra‐operative and/or post‐operative blood loss). Basically, none of these provided an adequate explanation for the degree of heterogeneity seen in these studies. Although effect size varied somewhat with dose, considerable heterogeneity was seen within dose strata. Likewise, there was substantial heterogeneity within the trials of aprotinin in cardiac surgery (that is, for intra‐and‐post‐operative blood loss, and the rates of transfusion). For the rates of exposure to allogeneic blood transfusion the adequacy of concealment of treatment allocation was associated with a small variation in treatment effect size, but once again there was heterogeneity within the different strata of methodological quality.
How do the results compare with the observational studies?
The most controversial aspect of this review is the lack of evidence of an increase in the risks of myocardial infarction, stroke, renal dysfunction and death with aprotinin when compared with no treatment. This is in keeping with previous published meta‐analyses of the randomised controlled trials of anti‐fibrinolytic drugs. In the case of aprotinin this review includes 77% more myocardial infarctions, but only 7% more deaths, than the previous version of this review. The updated data‐sets comparing aprotinin with no treatment conflict with those from four recent observational studies (Karkouti 2006; Mangano 2006; Mangano 2007; Schneeweiss 2008). Mangano and colleagues (2007) showed that during five years of follow‐up aprotinin‐treated patients had a death rate around 1.6 times higher than that of the untreated control group. The adjusted hazard ratio (HR) for all‐cause mortality was 1.48 (95% confidence interval 1.19 to 1.85). This study generated considerable scientific debate with calls for the use of aprotinin in cardiac surgery to be abandoned. In 2008 a large pharmaco‐epidemiological study by Schneeweiss 2008 confirmed the increased risk of death with aprotinin. These investigators studied the use of aprotinin (33,517 patients) or aminocaproic acid (44,682 patients) on the day coronary bypass surgery was performed. In this non‐randomised study they found that 1512 of the 33,517 aprotinin recipients (4.5%) and 1101 of the 44,682 aminocaproic acid recipients (2.5%) died. After adjustment, the estimated risk of death was 64% higher in the aprotinin group than in the aminocaproic acid group (relative risk, 1.64; 95% confidence interval [CI], 1.50 to 1.78). This difference remained statistically significant after a range of analytical procedures including a propensity score matched analysis and an instrumental variable analysis.
The first large observational study to find and adverse effect of aprotinin (Mangano 2006, Mangano 2007) was criticized on several grounds, including the fact that it was based on a multi‐centre patient registry, not a true population based cohort, that there were important differences between centres and that a range of selection biases may have influenced the between‐drug comparisons. These arguments will not be repeated here as full details are available in the relevant publications (Bidstrup 2006; Body 2006; Ferguson 2007; Levy 2006). Our view is not that the studies of Karkouti 2006; Mangano 2006 and Mangano 2007 were badly done, but that they have inherent limitations, mainly due to their observational nature and selection biases that probably cannot be completely overcome through statistical adjustments by propensity scores and co‐variates. These weaknesses were addressed in the larger study performed by Schneeweiss and colleagues (2008), described above. The agreement between these studies adds weight to the claim that aprotinin does indeed increase the chances of death.
In considering the apparently conflicting results of the different study types it is also important to acknowledge weaknesses in the database of randomised trials, in particular under‐recording of infrequent events that were not the primary outcomes of the trials. It is important to note that for dichotomous data to be included in our analyses, trial reports had to provide either numeric data, that is the numbers of events that occurred in the treatment and control groups, or where there were no events recorded, the trial report had to clearly state this. So, we have some confidence in the data included in the meta‐analyses. However, we acknowledge that under‐reporting of uncommon events that were not the primary outcomes of these generally small trials is a potential problem with this literature. In the case of aprotinin our analyses of myocardial infarction were based on data from 37 (49%) out of a total of 76 trials included in the analyses of blood transfusions. These trials were larger than average and included 64% of all participants. Nevertheless, the incomplete data are a potential source of bias in this and the analyses of other vascular outcomes. We are more confident of our analyses of mortality in cardiac surgery where, in the case of aprotinin, data were reported for 60% of all trials and 80% of participants. The most likely effect of under‐reporting is to make estimates imprecise, meaning that fairly small changes in mortality or occurrence of thrombotic events might have been missed.
There was a disappointing lack of information in the randomised trials regarding this putative adverse effect of the drug. Only 18 out of 76 trials of aprotinin documented this outcome, so there is a potential for bias due to under‐reporting. Based on analysis of 107 events in 4174 individuals the point estimate of the pooled RR with aprotinin (compared with placebo or no treatment) was 1.16 (95% CI 0.79 to 1.70), so we are not confident that we have ruled out a modest increase in risk. On the other hand the suggestion of an increase in risk from Mangano 2006 was based on a total of only 18 events, of which eight occurred in patients treated with aprotinin. Karkouti 2006 carried out a closely matched analysis of 898 individuals who received either aprotinin or TXA. Using a very sensitive measure of renal dysfunction they documented 182 instances, with a higher incidence in aprotinin treated subjects (RR 1.43, 95% CI 1.10 to 1.86).
There was greater agreement when we consider the results of the summary analyses of the head to head trials of aprotinin and lysine analogues and the observational studies described above. The comparison of aprotinin with the combined results of the lysine analogues found a significantly increased risk of death; similar in magnitude to what was found in the observational studies, but no apparent increase in the risk of major thrombotic events. The absence of a no treatment control group from these analyses means that we are unable to say whether the differences in mortality were due to an adverse effect of aprotinin or a protective effect of the lysine analogues. In addition, the meta‐analyses for death and myocardial infarction were heavily weighted by the results of the BART trial (Fergusson 2008). These factors limit our ability to draw firm conclusions about the true effects of the drugs. But the summary data now available, and the regulatory action taken against aprotinin, enable us to make some pragmatic recommendations. Despite the possibility that they are inferior to aprotinin in minimising perioperative blood loss and the need for allogeneic red cell transfusion both tranexamic acid and aminocaproic acid appear effective and safe. The experience is greatest with tranexamic acid and confidence in the use of this drug has been strengthened by the recent publication of the CRASH‐2 trial (CRASH‐2 2010), which found that two doses of tranexamic acid reduced overall mortality when administered soon after major trauma.
Conclusions
Antifibrinolytic drugs are effective in reducing blood loss, the need for allogeneic red cell transfusion, and the need for re‐operation due to continued post‐operative bleeding (in cardiac surgery). Aprotinin appears more effective than the lysine analogues in minimising peri and post operative blood loss when used as adjunctive therapy in cardiac surgery. Strictly speaking, based on their average effects on the need for red cell transfusion, the lysine analogues do not meet the criteria for being considered equivalent to aprotinin. However, comparisons between the drugs need to take account of the clinical significance of any small advantage of aprotinin, the dose response relationships for each of the drugs, and the possible effects of publication bias, which appears to favour aprotinin. Taking these factors into consideration it may reasonably be concluded that tranexamic acid is as effective as aprotinin, particularly when it is used as an adjunct to non‐cardiac surgical procedures. The data for epsilon aminocaproic acid are sparser and as a consequence not so convincing.
The updated meta‐analyses of the randomised trials comparing aprotinin with no treatment do not confirm the evidence from observational studies that aprotinin increases the risks of vascular occlusive events and mortality. However, there has been a degree of under‐reporting of these adverse events in trials of anti‐fibrinolytic drugs. The head to head comparisons of aprotinin and the lysine analogues have yielded results that are closer to those seen in the observational studies and indicate that aprotinin carries an increased risk of death. Consequently, the balance of benefit and harm favours the use of the lysine analogues over aprotinin, and justifies the regulatory action that resulted in the withdrawal of aprotinin from international markets in 2008.
Authors' conclusions
Implications for practice.
Tranexamic acid and epsilon aminocaproic acid provide worthwhile reductions in blood loss and the need for allogeneic red cell transfusion. Based on the results of randomised trials their efficacy does not appear to be offset by serious adverse effects. The evidence is stronger for tranexamic acid than for epsilon aminocaproic acid.
Implications for research.
There is no need for further placebo‐controlled trials of anti‐fibrinolytic drugs in cardiac surgery. The principal need is for large comparative trials to assess the relative efficacy, safety and cost‐effectiveness of the lysine analogues in different surgical procedures.
What's new
| Date | Event | Description |
|---|---|---|
| 10 February 2011 | New citation required but conclusions have not changed | The editorial group is aware that a clinical trial by Prof. Joachim Boldt has been found to have been fabricated (Boldt 2009). As the editors who revealed this fabrication point out (Reinhart 2011; Shafer 2011), this casts some doubt on the veracity of other studies by the same author. All Cochrane Injuries Group reviews which include studies by this author have therefore been edited to show the results with this author's trials included and excluded. Readers can now judge the potential impact of trials by this author ( Boldt 1991, Boldt 1994, Mengistu 2008) on the conclusions of the review. |
History
Protocol first published: Issue 1, 1999 Review first published: Issue 1, 1999
| Date | Event | Description |
|---|---|---|
| 31 May 2010 | New citation required and conclusions have changed | The searches were updated to February 2010. An additional 40 trials have been included. The updated data show a lower rate of death with the lysine analogues than aprotinin, which has been withdrawn from world markets. |
| 10 September 2008 | Amended | The text of 'Type of surgery' under 'Aprotinin' in the 'Effects of interventions' section was amended. |
| 8 September 2008 | Amended | Converted to new review format. |
Notes
The editorial group is aware that a clinical trial by Prof. Joachim Boldt has been found to have been fabricated (Boldt 2009). As the editors who revealed this fabrication point out (Reinhart 2011; Shafer 2011), this casts some doubt on the veracity of other studies by the same author. All Cochrane Injuries Group reviews which include studies by this author have therefore been edited to show the results with this author's trials included and excluded. Readers can now judge the potential impact of trials by this author ( Boldt 1991, Boldt 1994, Mengistu 2008) on the conclusions of the review.
Acknowledgements
The authors wish to thank Sonya Murray, Kim Henderson, Ann Wilkinson, Ketrina Sly, June McLeod, Jayne Fryer, Rosemary Aldrich, and Patricia McGettigan for their assistance at various stages during the development of this review.
Appendices
Appendix 1. Search strategy
The original search strategy at the outset of the review included the following terms;
Exploded MeSH terms: 'aprotinin' 'tranexamic acid' 'Aminocaproic acids' 'Blood transfusion' 'Hemorrhage' 'Anesthesia'.
Text‐word terms:aprotinin, antilysin, contrical, kallikrein‐trypsin, bovine pancreatic trypsin, tranexamic, cyklokapron, pharmacia, t‐amcha, amcha, ugurol, transamin, kabi, epsilon‐aminocaproic acid, aminocaproic, lederle, amicar, transfusion$, bleed$, blood loss$, hemorrhag$.
Appendix 2. Search strategy: 2010 update
Cochrane Injuries Group Specialised Register (searched July 2010)
(Aprotinin* or kallikrein‐trypsin inactivator* or bovine kunitz pancreatic trypsin inhibitor* or bovine pancreatic trypsin inhibitor* or basic pancreatic trypsin inhibitor* or BPTI or contrykal or kontrykal or kontrikal or contrical or dilmintal or iniprol or zymofren or traskolan or antilysin or pulmin or amicar or caprocid or epsamon or epsikapron or antilysin or iniprol or kontrikal or kontrykal or pulmin* or Trasylol or Antilysin Spofa or rp?9921 or antagosan or antilysin or antilysine or apronitin* or apronitrine or bayer a?128 or bovine pancreatic secretory trypsin inhibitor* or contrycal or frey inhibitor* or gordox or kallikrein trypsin inhibitor* or kazal type trypsin inhibitor* or (Kunitz adj3 inhibitor*) or midran or (pancrea* adj2 antitrypsin) or (pancrea* adj2 trypsin inhibitor*) or riker?52g or rp?9921or tracylol or trascolan or trasilol or traskolan or trazylol or zymofren or zymophren) or (tranexamic or Cyclohexanecarboxylic Acid* or Methylamine* or amcha or trans‐4‐aminomethyl‐cyclohexanecarboxylic acid* or t‐amcha or amca or kabi 2161 or transamin* or exacyl or amchafibrin or anvitoff or spotof or cyklokapron or ugurol oramino methylcyclohexane carboxylate or aminomethylcyclohexanecarbonic acid or aminomethylcyclohexanecarboxylic acid or AMCHA or amchafibrin or amikapron or aminomethyl cyclohexane carboxylic acid or aminomethyl cyclohexanecarboxylic acid or aminomethylcyclohexane carbonic acid or aminomethylcyclohexane carboxylic acid or aminomethylcyclohexanecarbonic acid or aminomethylcyclohexanecarboxylic acid or aminomethylcyclohexanocarboxylic acid or aminomethylcyclohexanoic acid or amstat or anvitoff or cl?65336 or cl65336 or cyclocapron or cyclokapron or cyklocapron or exacyl or frenolyse or hexacapron or hexakapron or tranex or TXA) or (aminocaproic or amino?caproic or aminohexanoic or amino?hexanoic or epsilon‐aminocaproic or E‐aminocaproic) adj2 acid*) or epsikapron or cy‐116 or cy116 or epsamon or amicar or caprocid or lederle or Aminocaproic or aminohexanoic or amino caproic or amino n hexanoic or acikaprin or afibrin or capracid or capramol or caprogel or caprolest or caprolisine or caprolysin or capromol or cl 10304 or EACA or eaca roche or ecapron or ekaprol or epsamon or epsicapron or epsilcapramin or epsilon amino caproate or epsilon aminocaproate or epsilonaminocaproic or etha?aminocaproic or ethaaminocaproich or emocaprol or hepin or ipsilon or jd?177or neocaprol or nsc?26154 or tachostyptan)
MEDLINE(Ovid) 1950 to July Week 2 2010 1. exp Antifibrinolytic Agents/ 2. (anti‐fibrinolytic* or antifibrinolytic* or antifibrinolysin* or anti‐fibrinolysin* or antiplasmin* or anti‐plasmin* or ((plasmin or fibrinolysis) adj3 inhibitor*)).ab,ti. 3. exp Aprotinin/ 4. (Aprotinin* or kallikrein‐trypsin inactivator* or bovine kunitz pancreatic trypsin inhibitor* or bovine pancreatic trypsin inhibitor* or basic pancreatic trypsin inhibitor* or BPTI or contrykal or kontrykal or kontrikal or contrical or dilmintal or iniprol or zymofren or traskolan or antilysin or pulmin or amicar or caprocid or epsamon or epsikapron or antilysin or iniprol or kontrikal or kontrykal or pulmin* or Trasylol or Antilysin Spofa or rp?9921 or antagosan or antilysin or antilysine or apronitin* or apronitrine or bayer a?128 or bovine pancreatic secretory trypsin inhibitor* or contrycal or frey inhibitor* or gordox or kallikrein trypsin inhibitor* or kazal type trypsin inhibitor* or (Kunitz adj3 inhibitor*) or midran or (pancrea* adj2 antitrypsin) or (pancrea* adj2 trypsin inhibitor*) or riker?52g or rp?9921or tracylol or trascolan or trasilol or traskolan or trazylol or zymofren or zymophren).ab,ti. 5. exp Tranexamic Acid/ 6. (tranexamic or Cyclohexanecarboxylic Acid* or Methylamine* or amcha or trans‐4‐aminomethyl‐cyclohexanecarboxylic acid* or t‐amcha or amca or kabi 2161 or transamin* or exacyl or amchafibrin or anvitoff or spotof or cyklokapron or ugurol oramino methylcyclohexane carboxylate or aminomethylcyclohexanecarbonic acid or aminomethylcyclohexanecarboxylic acid or AMCHA or amchafibrin or amikapron or aminomethyl cyclohexane carboxylic acid or aminomethyl cyclohexanecarboxylic acid or aminomethylcyclohexane carbonic acid or aminomethylcyclohexane carboxylic acid or aminomethylcyclohexanecarbonic acid or aminomethylcyclohexanecarboxylic acid or aminomethylcyclohexanocarboxylic acid or aminomethylcyclohexanoic acid or amstat or anvitoff or cl?65336 or cl65336 or cyclocapron or cyclokapron or cyklocapron or exacyl or frenolyse or hexacapron or hexakapron or tranex or TXA).ab,ti. 7. exp Aminocaproic Acids/ or exp 6‐Aminocaproic Acid/ 8. (((aminocaproic or amino?caproic or aminohexanoic or amino?hexanoic or epsilon‐aminocaproic or E‐aminocaproic) adj2 acid*) or epsikapron or cy‐116 or cy116 or epsamon or amicar or caprocid or lederle or Aminocaproic or aminohexanoic or amino caproic or amino n hexanoic or acikaprin or afibrin or capracid or capramol or caprogel or caprolest or caprolisine or caprolysin or capromol or cl 10304 or EACA or eaca roche or ecapron or ekaprol or epsamon or epsicapron or epsilcapramin or epsilon amino caproate or epsilon aminocaproate or epsilonaminocaproic or etha?aminocaproic or ethaaminocaproich or emocaprol or hepin or ipsilon or jd?177or neocaprol or nsc?26154 or tachostyptan).ab,ti. 9. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 10. randomi?ed.ab,ti. 11. randomized controlled trial.pt. 12. controlled clinical trial.pt. 13. placebo.ab. 14. clinical trials as topic.sh. 15. randomly.ab. 16. trial.ti. 17. 10 or 11 or 12 or 13 or 14 or 15 or 16 18. (animals not (humans and animals)).sh. 19.17 not 18 20. 9 and 19 EMBASE (Ovid) 1980 to 2010 Week 28 1. exp Antifibrinolytic Agent/ 2. (anti‐fibrinolytic* or antifibrinolytic* or antifibrinolysin* or anti‐fibrinolysin* or antiplasmin* or anti‐plasmin* or ((plasmin or fibrinolysis) adj3 inhibitor*)).ab,ti. 3. exp Aprotinin/ 4. (Aprotinin* or kallikrein‐trypsin inactivator* or bovine kunitz pancreatic trypsin inhibitor* or bovine pancreatic trypsin inhibitor* or basic pancreatic trypsin inhibitor* or BPTI or contrykal or kontrykal or kontrikal or contrical or dilmintal or iniprol or zymofren or traskolan or antilysin or pulmin or amicar or caprocid or epsamon or epsikapron or antilysin or iniprol or kontrikal or kontrykal or pulmin* or Trasylol or Antilysin Spofa or rp?9921 or antagosan or antilysin or antilysine or apronitin* or apronitrine or bayer a?128 or bovine pancreatic secretory trypsin inhibitor* or contrycal or frey inhibitor* or gordox or kallikrein trypsin inhibitor* or kazal type trypsin inhibitor* or (Kunitz adj3 inhibitor*) or midran or (pancrea* adj2 antitrypsin) or (pancrea* adj2 trypsin inhibitor*) or riker?52g or rp?9921or tracylol or trascolan or trasilol or traskolan or trazylol or zymofren or zymophren).ab,ti. 5. exp Tranexamic Acid/ 6. (tranexamic or Cyclohexanecarboxylic Acid* or Methylamine* or amcha or trans‐4‐aminomethyl‐cyclohexanecarboxylic acid* or t‐amcha or amca or kabi 2161 or transamin* or exacyl or amchafibrin or anvitoff or spotof or cyklokapron or ugurol oramino methylcyclohexane carboxylate or aminomethylcyclohexanecarbonic acid or aminomethylcyclohexanecarboxylic acid or AMCHA or amchafibrin or amikapron or aminomethyl cyclohexane carboxylic acid or aminomethyl cyclohexanecarboxylic acid or aminomethylcyclohexane carbonic acid or aminomethylcyclohexane carboxylic acid or aminomethylcyclohexanecarbonic acid or aminomethylcyclohexanecarboxylic acid or aminomethylcyclohexanocarboxylic acid or aminomethylcyclohexanoic acid or amstat or anvitoff or cl?65336 or cl65336 or cyclocapron or cyclokapron or cyklocapron or exacyl or frenolyse or hexacapron or hexakapron or tranex or TXA).ab,ti. 7. exp Aminocaproic Acid/ 8. (((aminocaproic or amino?caproic or aminohexanoic or amino?hexanoic or epsilon‐aminocaproic or E‐aminocaproic) adj2 acid*) or epsikapron or cy‐116 or cy116 or epsamon or amicar or caprocid or lederle or Aminocaproic or aminohexanoic or amino caproic or amino n hexanoic or acikaprin or afibrin or capracid or capramol or caprogel or caprolest or caprolisine or caprolysin or capromol or cl 10304 or EACA or eaca roche or ecapron or ekaprol or epsamon or epsicapron or epsilcapramin or epsilon amino caproate or epsilon aminocaproate or epsilonaminocaproic or etha?aminocaproic or ethaaminocaproich or emocaprol or hepin or ipsilon or jd?177or neocaprol or nsc?26154 or tachostyptan).ab,ti. 9. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 10. exp Randomized Controlled Trial/ 11. exp controlled clinical trial/ 12. randomi?ed.ab,ti. 13. placebo.ab. 14. *Clinical Trial/ 15. randomly.ab. 16. trial.ti. 17. 10 or 11 or 12 or 13 or 14 or 15 or 16 18. exp animal/ not (exp human/ and exp animal/) 19. 17 not 18 20. 9 and 19 Cochrane Central Register of Controlled Trials (The Cochrane Library 2010, Issue 3) #1 MeSH descriptor Antifibrinolytic Agents explode all trees #2 (anti‐fibrinolytic* or antifibrinolytic* or antifibrinolysin* or anti‐fibrinolysin* or antiplasmin* or anti‐plasmin* ):ab,ti or ((plasmin or fibrinolysis) near3 inhibitor*):ab,ti #3 MeSH descriptor Aprotinin explode all trees #4 (Aprotinin* or kallikrein‐trypsin inactivator* or bovine kunitz pancreatic trypsin inhibitor* or bovine pancreatic trypsin inhibitor* or basic pancreatic trypsin inhibitor* or BPTI or contrykal or kontrykal or kontrikal or contrical or dilmintal or iniprol or zymofren or traskolan or antilysin or pulmin or amicar or caprocid or epsamon or epsikapron or antilysin or iniprol or kontrikal or kontrykal or pulmin* or Trasylol or Antilysin Spofa or rp?9921 or antagosan or antilysin or antilysine or apronitin* or apronitrine or bayer a?128 or bovine pancreatic secretory trypsin inhibitor* or contrycal or frey inhibitor* or gordox or kallikrein trypsin inhibitor* or kazal type trypsin inhibitor or riker?52g or rp?9921or tracylol or trascolan or trasilol or traskolan or trazylol or zymofren or zymophren or midran):ab,ti or ((Kunitz near3 inhibitor*) or (pancrea* near3 antitrypsin) or (pancrea* near3 trypsin next inhibitor*)):ab,ti #5 MeSH descriptor Tranexamic Acid explode all trees #6 (tranexamic or Cyclohexanecarboxylic Acid* or Methylamine* or amcha or trans‐4‐aminomethyl‐cyclohexanecarboxylic acid* or t‐amcha or amca or kabi 2161 or transamin* or exacyl or amchafibrin or anvitoff or spotof or cyklokapron or ugurol oramino methylcyclohexane carboxylate or aminomethylcyclohexanecarbonic acid or aminomethylcyclohexanecarboxylic acid or AMCHA or amchafibrin or amikapron or aminomethyl cyclohexane carboxylic acid or aminomethyl cyclohexanecarboxylic acid or aminomethylcyclohexane carbonic acid or aminomethylcyclohexane carboxylic acid or aminomethylcyclohexanecarbonic acid or aminomethylcyclohexanecarboxylic acid or aminomethylcyclohexanocarboxylic acid or aminomethylcyclohexanoic acid or amstat or anvitoff or cl?65336 or cl65336 or cyclocapron or cyclokapron or cyklocapron or exacyl or frenolyse or hexacapron or hexakapron or tranex or TXA):ab,ti #7 MeSH descriptor Aminocaproic Acids explode all trees #8 MeSH descriptor 6‐Aminocaproic Acid explode all trees #9 (epsikapron or cy‐116 or cy116 or epsamon or amicar or caprocid or lederle or Aminocaproic or aminohexanoic or amino caproic or amino n hexanoic or acikaprin or afibrin or capracid or capramol or caprogel or caprolest or caprolisine or caprolysin or capromol or cl 10304 or EACA or eaca roche or ecapron or ekaprol or epsamon or epsicapron or epsilcapramin or epsilon amino caproate or epsilon aminocaproate or epsilonaminocaproic or etha?aminocaproic or ethaaminocaproich or emocaprol or hepin or ipsilon or jd?177or neocaprol or nsc?26154 or tachostyptan):ab,ti #10 (aminocaproic or amino?caproic or aminohexanoic or amino?hexanoic or epsilon‐aminocaproic or E‐aminocaproic):ab,ti #11 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10)
Data and analyses
Comparison 1. Aprotinin versus Control (Blood Transfusion & Blood Loss).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 No. Exposed to Allogeneic Blood | 108 | 11172 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.60, 0.72] |
| 2 No. Exposed to Allogeneic Blood ‐ Type of Surgery | 108 | 11172 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.60, 0.72] |
| 2.1 Cardiac surgery | 84 | 9497 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.63, 0.73] |
| 2.2 Orthopaedic surgery | 15 | 1146 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.52, 0.89] |
| 2.3 Thoracic surgery | 3 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.14, 0.59] |
| 2.4 Vascular surgery | 2 | 188 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.97, 1.03] |
| 2.5 Liver surgery | 2 | 177 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.37, 0.90] |
| 2.6 Neuro surgery | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.40, 1.35] |
| 2.7 Orthognathic surgery | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.02, 0.77] |
| 3 No. Exposed to Allogeneic Blood ‐ Transfusion Protocol | 108 | 11172 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.60, 0.72] |
| 3.1 Transfusion Protocol | 87 | 9974 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.59, 0.71] |
| 3.2 No Transfusion Protocol | 21 | 1198 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.61, 0.84] |
| 4 No. Exposed to Allogeneic Blood ‐ Dose | 107 | 12116 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.62, 0.73] |
| 4.1 Prime Dose | 16 | 1251 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.71, 0.96] |
| 4.2 Low Dose | 50 | 3601 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.55, 0.77] |
| 4.3 High Dose | 61 | 7264 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.61, 0.71] |
| 5 No. Exposed to Allogeneic Blood ‐ Dose (Cardiac Surgery) | 83 | 10423 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.65, 0.74] |
| 5.1 Prime Dose | 15 | 1191 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.69, 0.96] |
| 5.2 Low Dose | 29 | 2372 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.60, 0.80] |
| 5.3 High Dose | 58 | 6860 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.62, 0.72] |
| 6 Trial Methodological Quality ‐ Allocation Concealment | 108 | 11172 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.60, 0.72] |
| 6.1 Allocation concealment ‐ Yes | 33 | 2755 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.53, 0.79] |
| 6.2 Allocation concealment ‐ Unclear | 63 | 7489 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.64, 0.75] |
| 6.3 Allocation concealment ‐ No | 12 | 928 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.54, 0.75] |
| 7 Units of Allogeneic Blood Transfused ‐ Transfused Patients | 40 | 3563 | Mean Difference (IV, Random, 95% CI) | ‐0.98 [‐1.29, ‐0.66] |
| 8 Units of Allogeneic Blood Transfused ‐ All Patients | 74 | 7820 | Mean Difference (IV, Random, 95% CI) | ‐1.02 [‐1.26, ‐0.79] |
| 9 Blood loss ‐ Intra‐operative | 16 | 883 | Mean Difference (IV, Random, 95% CI) | ‐191.87 [‐280.45, ‐103.28] |
| 9.1 Cardiac surgery | 7 | 470 | Mean Difference (IV, Random, 95% CI) | ‐148.18 [‐240.21, ‐56.14] |
| 9.2 Orthopaedic surgery | 5 | 201 | Mean Difference (IV, Random, 95% CI) | ‐151.05 [‐317.63, 15.52] |
| 9.3 Thoracic surgery | 2 | 40 | Mean Difference (IV, Random, 95% CI) | ‐577.06 [‐893.71, ‐260.41] |
| 9.4 Liver surgery | 2 | 137 | Mean Difference (IV, Random, 95% CI) | ‐1200.40 [‐2943.39, 542.59] |
| 9.5 Vascular surgery | 1 | 35 | Mean Difference (IV, Random, 95% CI) | ‐102.00 [‐1004.32, 796.32] |
| 10 Blood loss ‐ Post‐operative | 87 | 7896 | Mean Difference (IV, Random, 95% CI) | ‐345.88 [‐383.47, ‐308.29] |
| 10.1 Cardiac surgery | 75 | 7371 | Mean Difference (IV, Random, 95% CI) | ‐369.62 [‐408.95, ‐330.29] |
| 10.2 Orthopaedic surgery | 7 | 318 | Mean Difference (IV, Random, 95% CI) | ‐113.58 [‐223.69, ‐3.46] |
| 10.3 Thoracic surgery | 2 | 83 | Mean Difference (IV, Random, 95% CI) | ‐359.31 [‐460.15, ‐258.48] |
| 10.4 Orthognathic surgery | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐513.0 [‐717.21, ‐308.79] |
| 10.5 Liver surgery | 1 | 44 | Mean Difference (IV, Random, 95% CI) | ‐105.0 [‐194.36, ‐15.64] |
| 10.6 Vascular surgery | 1 | 50 | Mean Difference (IV, Random, 95% CI) | ‐203.00 [‐404.93, ‐1.07] |
| 11 Blood loss ‐ Post‐operative ‐ Dose (Cardiac Surgery) | 75 | 8181 | Mean Difference (IV, Random, 95% CI) | ‐367.69 [‐403.50, ‐331.87] |
| 11.1 Prime Dose | 15 | 1158 | Mean Difference (IV, Random, 95% CI) | ‐343.08 [‐458.13, ‐228.04] |
| 11.2 Low Dose | 24 | 2038 | Mean Difference (IV, Random, 95% CI) | ‐274.58 [‐316.48, ‐232.67] |
| 11.3 High Dose | 52 | 4985 | Mean Difference (IV, Random, 95% CI) | ‐418.59 [‐470.96, ‐366.22] |
| 12 Blood loss ‐ Total | 17 | 1789 | Mean Difference (IV, Random, 95% CI) | ‐415.95 [‐520.38, ‐311.51] |
| 12.1 Cardiac surgery | 7 | 1359 | Mean Difference (IV, Random, 95% CI) | ‐448.86 [‐612.82, ‐284.91] |
| 12.2 Orthopaedic surgery | 10 | 430 | Mean Difference (IV, Random, 95% CI) | ‐399.09 [‐562.81, ‐235.37] |
1.1. Analysis.
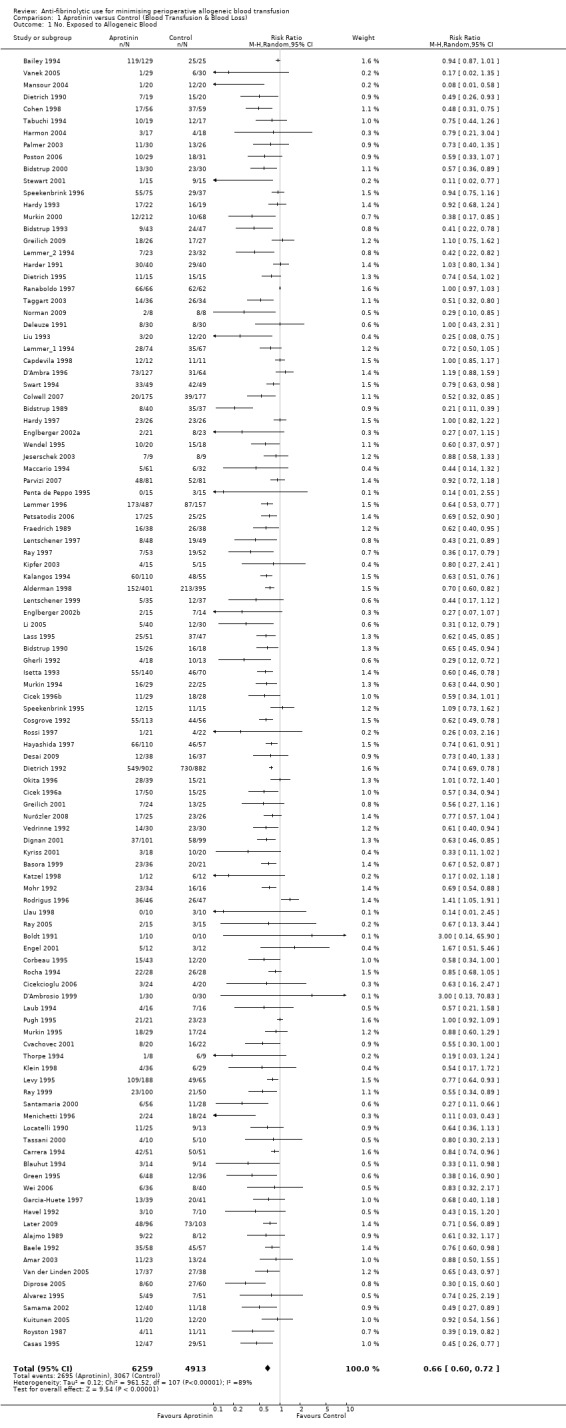
Comparison 1 Aprotinin versus Control (Blood Transfusion & Blood Loss), Outcome 1 No. Exposed to Allogeneic Blood.
1.2. Analysis.
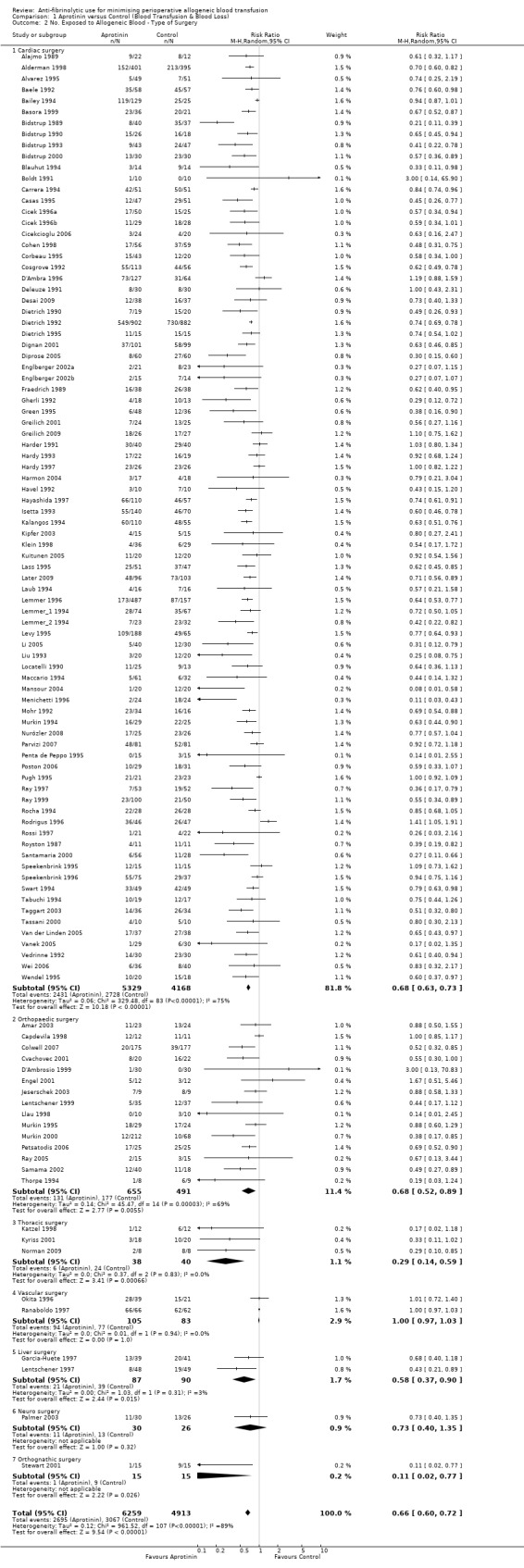
Comparison 1 Aprotinin versus Control (Blood Transfusion & Blood Loss), Outcome 2 No. Exposed to Allogeneic Blood ‐ Type of Surgery.
1.3. Analysis.
Comparison 1 Aprotinin versus Control (Blood Transfusion & Blood Loss), Outcome 3 No. Exposed to Allogeneic Blood ‐ Transfusion Protocol.
1.4. Analysis.
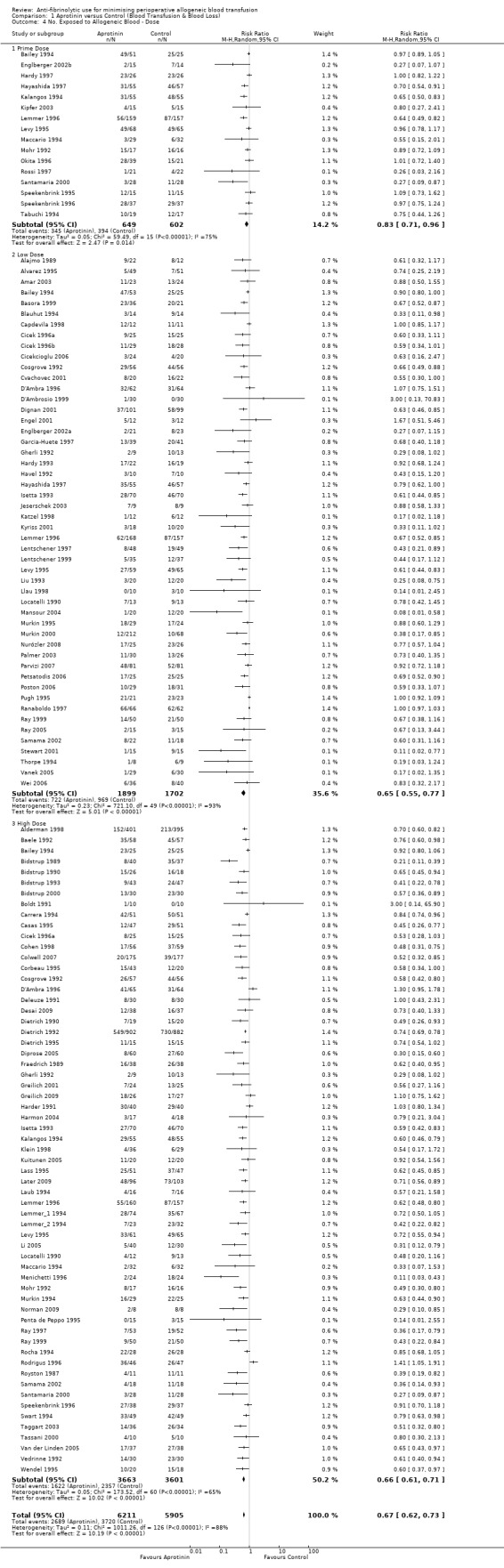
Comparison 1 Aprotinin versus Control (Blood Transfusion & Blood Loss), Outcome 4 No. Exposed to Allogeneic Blood ‐ Dose.
1.5. Analysis.
Comparison 1 Aprotinin versus Control (Blood Transfusion & Blood Loss), Outcome 5 No. Exposed to Allogeneic Blood ‐ Dose (Cardiac Surgery).
1.6. Analysis.
Comparison 1 Aprotinin versus Control (Blood Transfusion & Blood Loss), Outcome 6 Trial Methodological Quality ‐ Allocation Concealment.
1.7. Analysis.
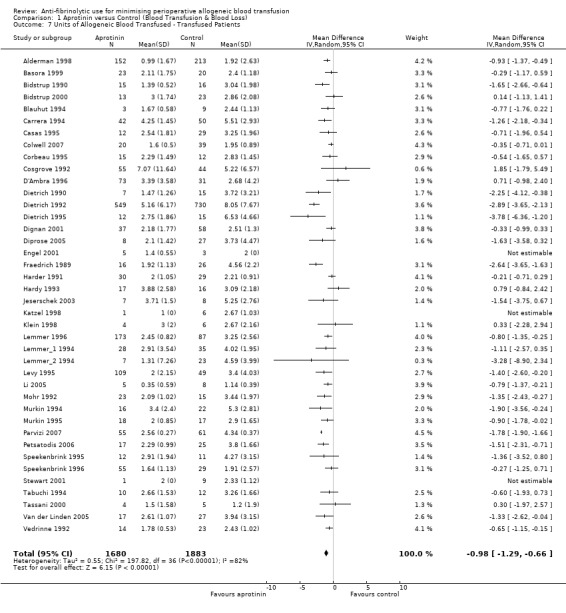
Comparison 1 Aprotinin versus Control (Blood Transfusion & Blood Loss), Outcome 7 Units of Allogeneic Blood Transfused ‐ Transfused Patients.
1.8. Analysis.
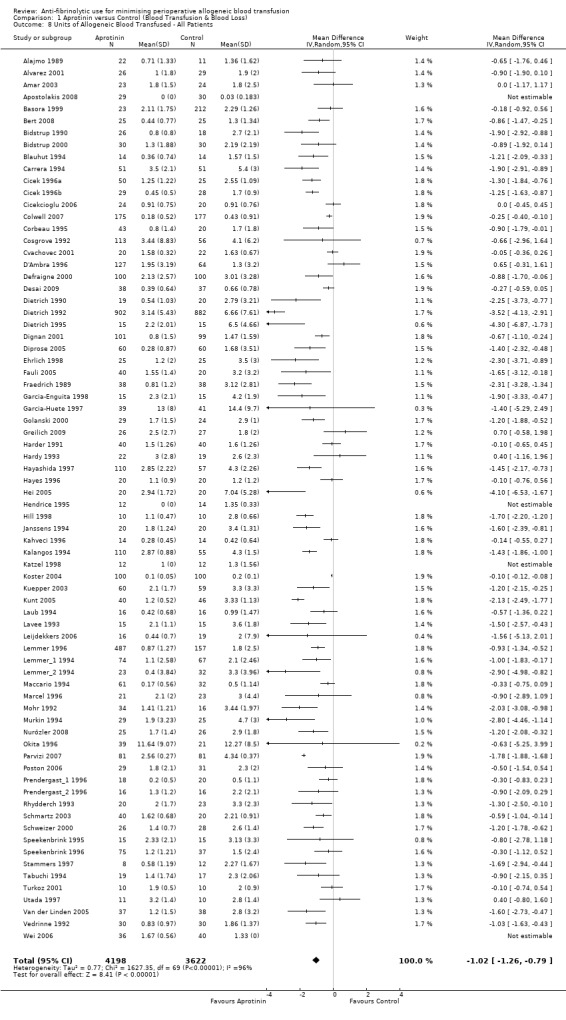
Comparison 1 Aprotinin versus Control (Blood Transfusion & Blood Loss), Outcome 8 Units of Allogeneic Blood Transfused ‐ All Patients.
1.9. Analysis.
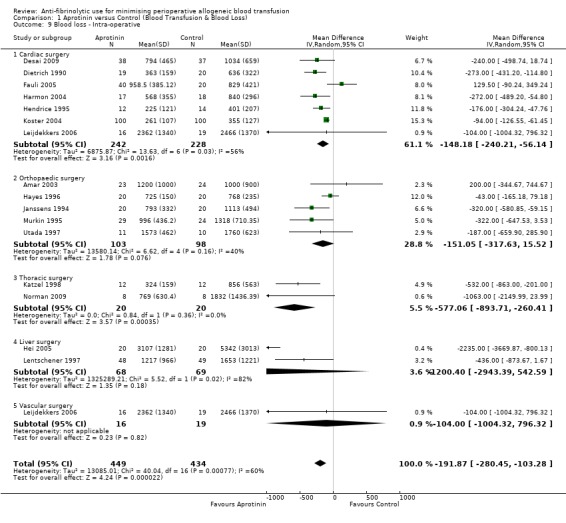
Comparison 1 Aprotinin versus Control (Blood Transfusion & Blood Loss), Outcome 9 Blood loss ‐ Intra‐operative.
1.10. Analysis.
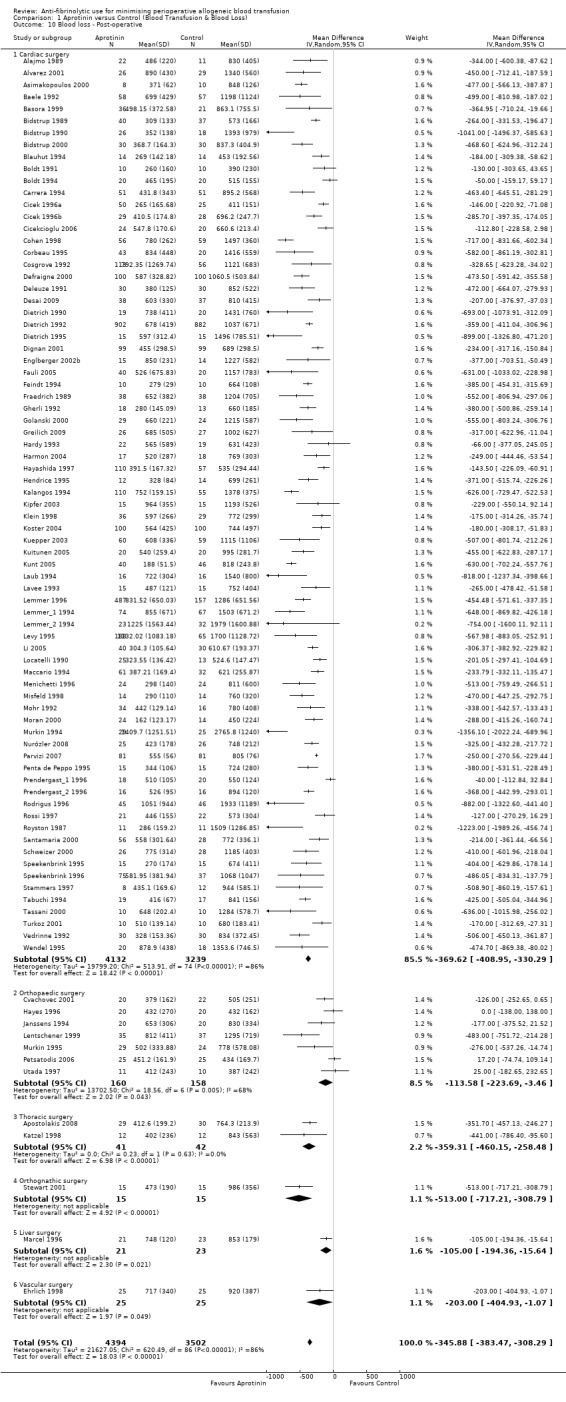
Comparison 1 Aprotinin versus Control (Blood Transfusion & Blood Loss), Outcome 10 Blood loss ‐ Post‐operative.
1.11. Analysis.
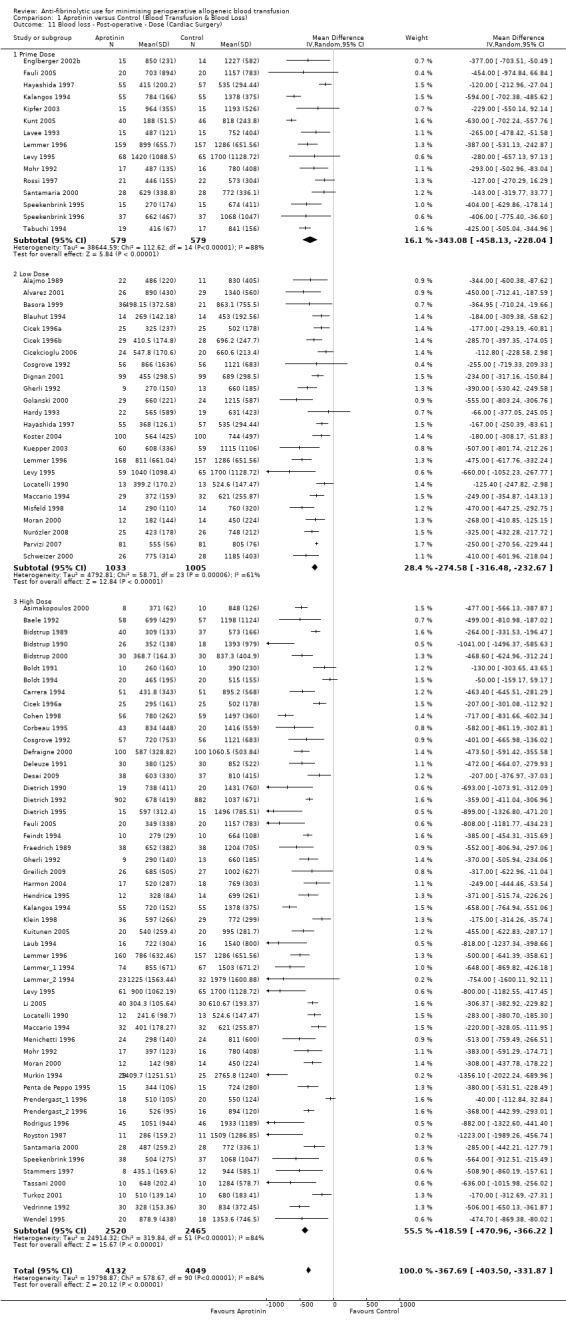
Comparison 1 Aprotinin versus Control (Blood Transfusion & Blood Loss), Outcome 11 Blood loss ‐ Post‐operative ‐ Dose (Cardiac Surgery).
1.12. Analysis.
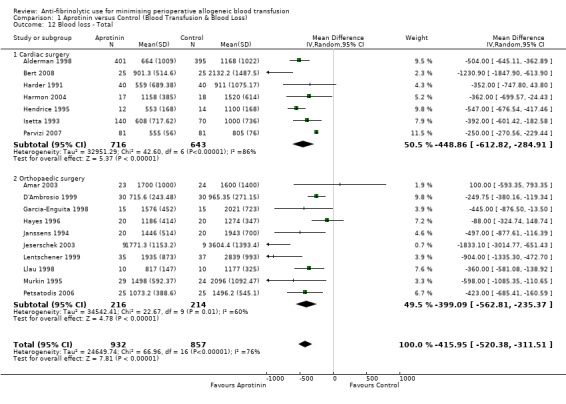
Comparison 1 Aprotinin versus Control (Blood Transfusion & Blood Loss), Outcome 12 Blood loss ‐ Total.
Comparison 2. Tranexamic Acid versus Control (Blood Transfusion & Blood Loss).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 No. Exposed to Allogeneic Blood | 65 | 4842 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.53, 0.70] |
| 2 No. Exposed to Allogeneic Blood ‐ Type of Surgery | 65 | 4842 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.53, 0.70] |
| 2.1 Cardiac surgery | 34 | 3006 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.57, 0.81] |
| 2.2 Orthopaedic surgery | 27 | 1381 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.39, 0.62] |
| 2.3 Liver surgery | 2 | 296 | Risk Ratio (M‐H, Random, 95% CI) | 0.16 [0.00, 32.47] |
| 2.4 Vascular surgery | 1 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.33, 0.96] |
| 2.5 Gynaecological surgery | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 1.5 [0.75, 3.01] |
| 3 No. Exposed to Allogeneic Blood ‐ Transfusion Protocol | 65 | 4842 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.53, 0.70] |
| 3.1 Transfusion Protocol | 56 | 4125 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.48, 0.67] |
| 3.2 No Transfusion Protocol | 9 | 717 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.61, 0.96] |
| 4 No. Exposed to Allogeneic Blood ‐ Dose (Cardiac Surgery) | 36 | 3191 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.58, 0.80] |
| 4.1 Total dose < 2.0 grams | 19 | 1123 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.58, 0.84] |
| 4.2 Total dose 2.0 ‐ 10.0 grams | 18 | 2068 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.52, 0.86] |
| 5 Trial Methodological Quality ‐ Allocation Concealment | 65 | 4842 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.53, 0.70] |
| 5.1 Allocation concealment ‐ Yes | 28 | 2110 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.51, 0.69] |
| 5.2 Allocation concealment ‐ Unclear | 24 | 1503 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.37, 0.76] |
| 5.3 Allocation concealment ‐ No | 13 | 1229 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.62, 0.86] |
| 6 Units Allogeneic Blood Transfused ‐ Transfused Patients | 13 | 481 | Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.80, 0.11] |
| 7 Units of Allogeneic Blood Transfused ‐ All Patients | 23 | 1814 | Mean Difference (IV, Random, 95% CI) | ‐0.87 [‐1.20, ‐0.53] |
| 8 Blood loss ‐ Intra‐operative | 17 | 1173 | Mean Difference (IV, Random, 95% CI) | ‐121.41 [‐180.19, ‐62.63] |
| 8.1 Cardiac surgery | 4 | 244 | Mean Difference (IV, Random, 95% CI) | ‐166.76 [‐331.24, ‐2.27] |
| 8.2 Orthopaedic surgery | 12 | 829 | Mean Difference (IV, Random, 95% CI) | ‐115.52 [‐187.88, ‐43.16] |
| 8.3 Gynaecological surgery | 1 | 100 | Mean Difference (IV, Random, 95% CI) | ‐164.00 [‐366.45, 34.45] |
| 8.4 Head & neck surgery | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Blood loss ‐ Post‐operative | 35 | 2501 | Mean Difference (IV, Random, 95% CI) | ‐247.17 [‐294.76, ‐199.58] |
| 9.1 Cardiac surgery | 22 | 1597 | Mean Difference (IV, Random, 95% CI) | ‐272.87 [‐328.85, ‐216.89] |
| 9.2 Orthopaedic surgery | 12 | 804 | Mean Difference (IV, Random, 95% CI) | ‐228.52 [‐321.76, ‐135.27] |
| 9.3 Gynaecological surgery | 1 | 100 | Mean Difference (IV, Random, 95% CI) | ‐63.0 [‐118.89, ‐7.11] |
| 9.4 Head & neck surgery | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Blood loss ‐ Post‐operative ‐ Dose (Cardiac Surgery) | 22 | 1597 | Mean Difference (IV, Random, 95% CI) | ‐272.87 [‐328.85, ‐216.89] |
| 10.1 Total dose < 2.0 grams | 12 | 619 | Mean Difference (IV, Random, 95% CI) | ‐245.03 [‐329.76, ‐160.29] |
| 10.2 Total dose 2.0 ‐ 10.0 grams | 10 | 978 | Mean Difference (IV, Random, 95% CI) | ‐297.94 [‐364.49, ‐231.39] |
| 11 Blood loss ‐ Total | 28 | 1712 | Mean Difference (IV, Random, 95% CI) | ‐414.06 [‐525.19, ‐302.92] |
| 11.1 Cardiac surgery | 6 | 391 | Mean Difference (IV, Random, 95% CI) | ‐300.47 [‐470.74, ‐130.21] |
| 11.2 Orthopaedic surgery | 20 | 1201 | Mean Difference (IV, Random, 95% CI) | ‐446.19 [‐554.61, ‐337.78] |
| 11.3 Liver surgery | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐6552.0 [‐14329.54, 1225.54] |
| 11.4 Gynaecological surgery | 1 | 100 | Mean Difference (IV, Random, 95% CI) | ‐243.0 [‐460.02, ‐25.98] |
| 11.5 Head & neck surgery | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
2.1. Analysis.
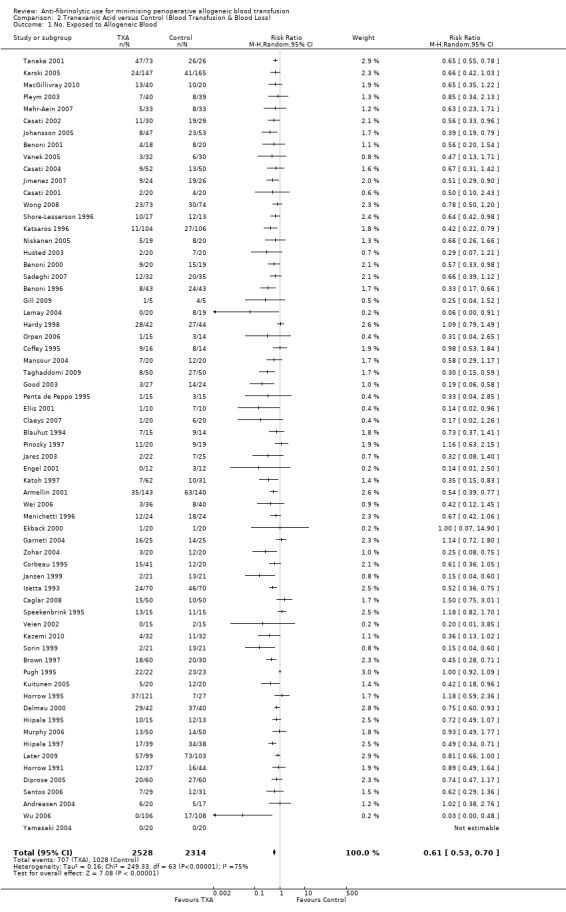
Comparison 2 Tranexamic Acid versus Control (Blood Transfusion & Blood Loss), Outcome 1 No. Exposed to Allogeneic Blood.
2.2. Analysis.
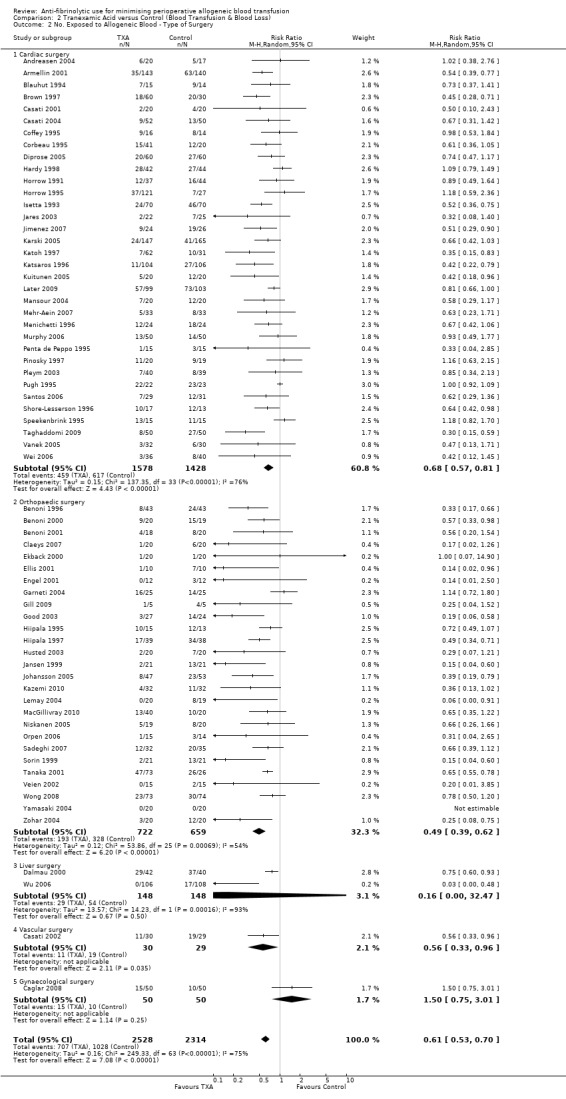
Comparison 2 Tranexamic Acid versus Control (Blood Transfusion & Blood Loss), Outcome 2 No. Exposed to Allogeneic Blood ‐ Type of Surgery.
2.3. Analysis.
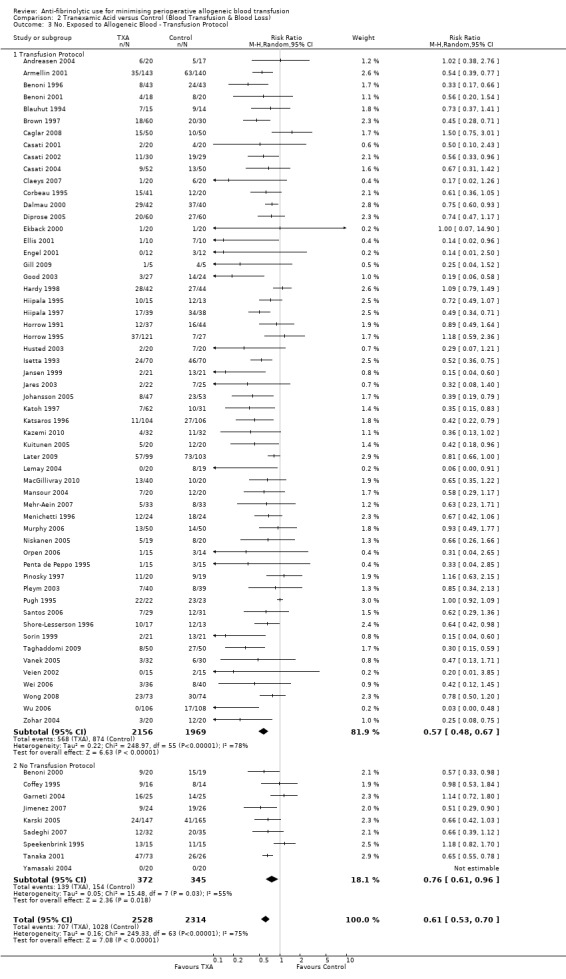
Comparison 2 Tranexamic Acid versus Control (Blood Transfusion & Blood Loss), Outcome 3 No. Exposed to Allogeneic Blood ‐ Transfusion Protocol.
2.4. Analysis.
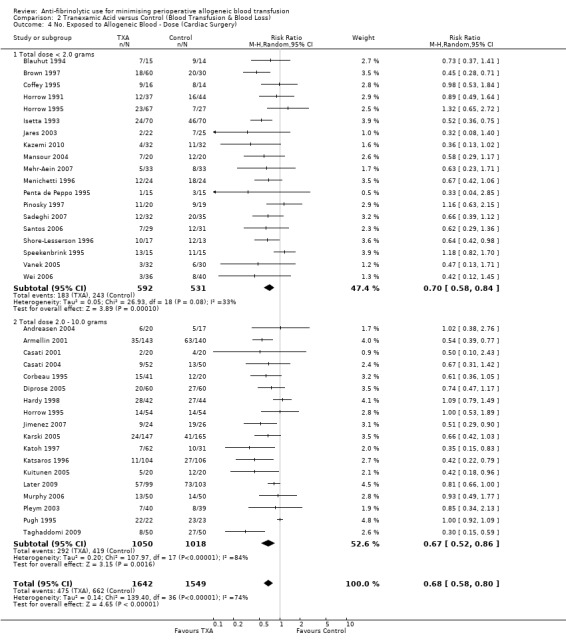
Comparison 2 Tranexamic Acid versus Control (Blood Transfusion & Blood Loss), Outcome 4 No. Exposed to Allogeneic Blood ‐ Dose (Cardiac Surgery).
2.5. Analysis.
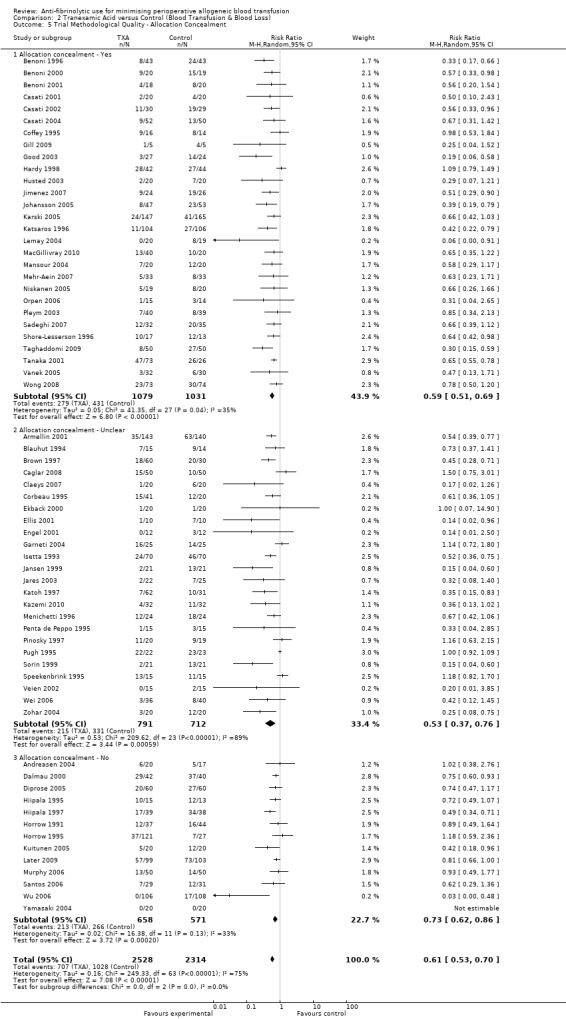
Comparison 2 Tranexamic Acid versus Control (Blood Transfusion & Blood Loss), Outcome 5 Trial Methodological Quality ‐ Allocation Concealment.
2.6. Analysis.
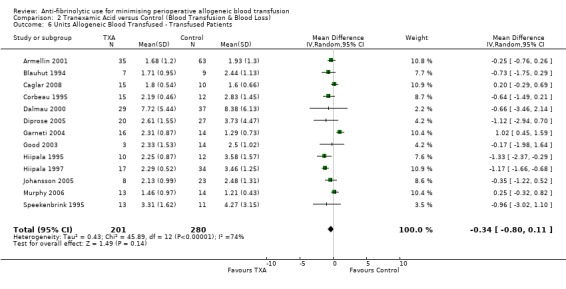
Comparison 2 Tranexamic Acid versus Control (Blood Transfusion & Blood Loss), Outcome 6 Units Allogeneic Blood Transfused ‐ Transfused Patients.
2.7. Analysis.
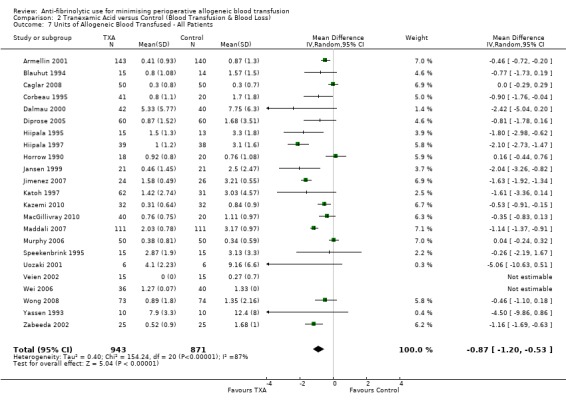
Comparison 2 Tranexamic Acid versus Control (Blood Transfusion & Blood Loss), Outcome 7 Units of Allogeneic Blood Transfused ‐ All Patients.
2.8. Analysis.
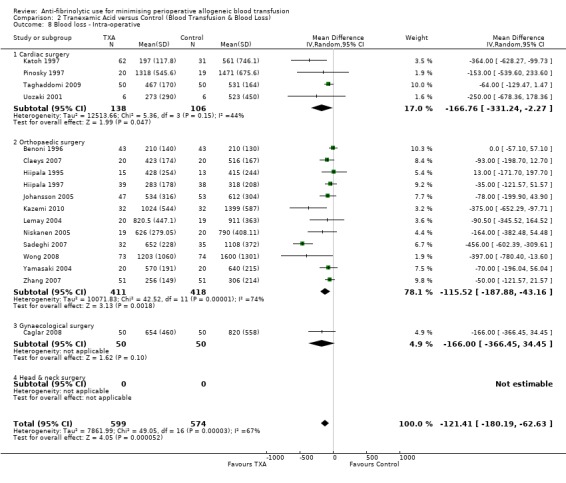
Comparison 2 Tranexamic Acid versus Control (Blood Transfusion & Blood Loss), Outcome 8 Blood loss ‐ Intra‐operative.
2.9. Analysis.
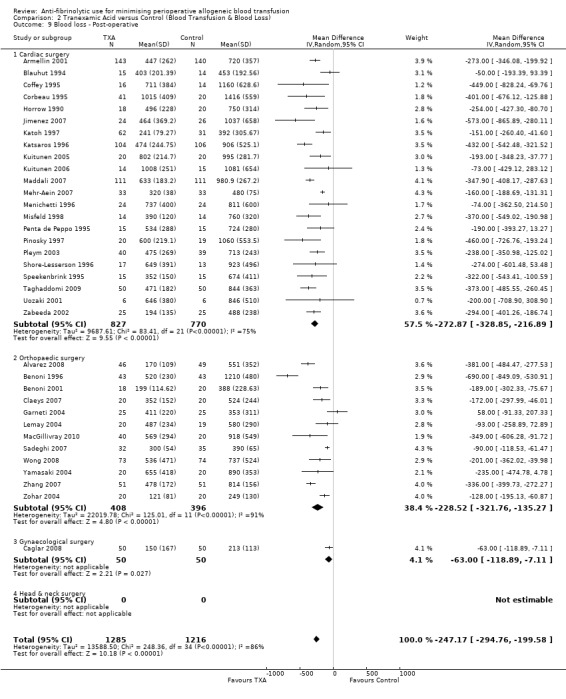
Comparison 2 Tranexamic Acid versus Control (Blood Transfusion & Blood Loss), Outcome 9 Blood loss ‐ Post‐operative.
2.10. Analysis.
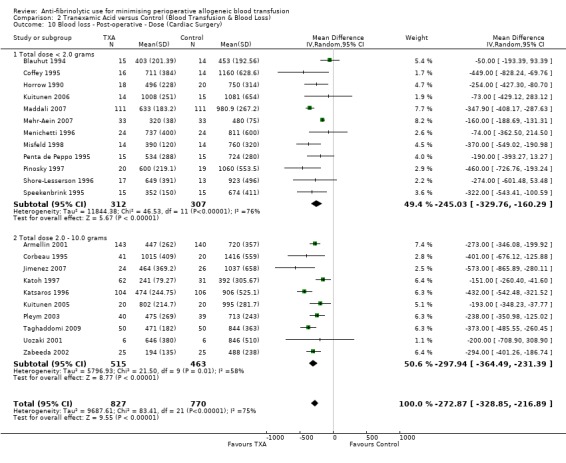
Comparison 2 Tranexamic Acid versus Control (Blood Transfusion & Blood Loss), Outcome 10 Blood loss ‐ Post‐operative ‐ Dose (Cardiac Surgery).
2.11. Analysis.
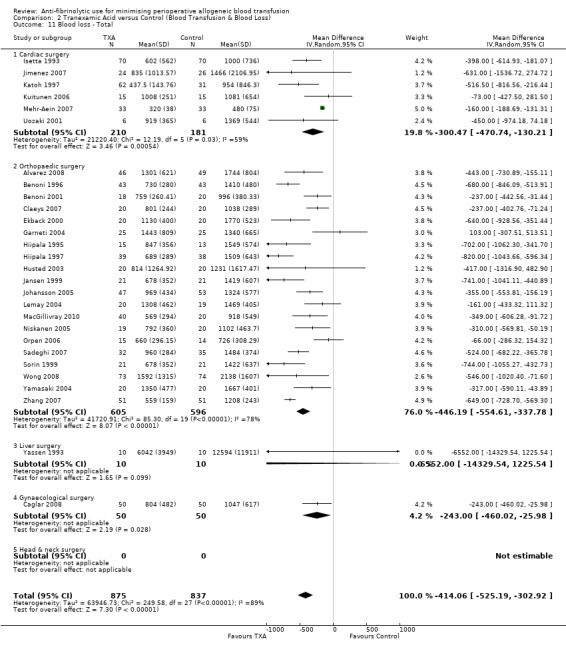
Comparison 2 Tranexamic Acid versus Control (Blood Transfusion & Blood Loss), Outcome 11 Blood loss ‐ Total.
Comparison 3. Epsilon Aminocaproic Acid versus Control (Blood Transfusion & Blood Loss).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 No. Exposed to Allogeneic Blood | 16 | 1035 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.67, 0.99] |
| 2 No. Exposed to Allogeneic Blood ‐ Type of Surgery | 16 | 1035 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.67, 0.99] |
| 2.1 Cardiac Surgery | 11 | 649 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.52, 0.93] |
| 2.2 Orthopaedic Surgery | 4 | 304 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.93, 1.08] |
| 2.3 Liver Surgery | 1 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.80, 1.08] |
| 3 No. Exposed to Allogeneic Blood ‐ Transfusion Protocol | 16 | 1035 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.67, 0.99] |
| 3.1 Transfusion Protocol | 15 | 1005 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.65, 0.98] |
| 3.2 No Transfusion Protocol | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.36, 4.97] |
| 4 Trial Methodological Quality ‐ Allocation Concealment | 16 | 1035 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.67, 0.99] |
| 4.1 Allocation concealment ‐ Yes | 5 | 452 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.58, 1.16] |
| 4.2 Allocation concealment ‐ Unclear | 9 | 455 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.46, 1.03] |
| 4.3 Allocation concealment ‐ No | 2 | 128 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.81, 1.08] |
| 5 Units of Allogeneic Blood Transfused ‐ Transfused Patients | 3 | 119 | Mean Difference (IV, Random, 95% CI) | 0.22 [‐0.34, 0.79] |
| 6 Units of Allogeneic Blood Transfused ‐ All Patients | 6 | 432 | Mean Difference (IV, Random, 95% CI) | ‐1.30 [‐2.14, ‐0.45] |
| 7 Blood loss ‐ Intra‐operative | 5 | 353 | Mean Difference (IV, Random, 95% CI) | ‐156.63 [‐276.92, ‐36.33] |
| 7.1 Cardiac surgery | 2 | 79 | Mean Difference (IV, Random, 95% CI) | ‐213.58 [‐310.03, ‐117.13] |
| 7.2 Orthopaedic surgery | 3 | 274 | Mean Difference (IV, Random, 95% CI) | ‐40.66 [‐236.71, 155.38] |
| 8 Blood loss ‐ Post‐operative | 14 | 1174 | Mean Difference (IV, Random, 95% CI) | ‐207.49 [‐276.43, ‐138.54] |
| 8.1 Cardiac surgery | 12 | 946 | Mean Difference (IV, Random, 95% CI) | ‐200.27 [‐273.44, ‐127.09] |
| 8.2 Orthopaedic surgery | 2 | 228 | Mean Difference (IV, Random, 95% CI) | ‐285.06 [‐452.73, ‐117.39] |
| 9 Blood loss ‐ Total | 2 | 92 | Mean Difference (IV, Random, 95% CI) | ‐299.69 [‐522.54, ‐76.84] |
| 9.1 Orthopaedic surgery | 2 | 92 | Mean Difference (IV, Random, 95% CI) | ‐299.69 [‐522.54, ‐76.84] |
3.1. Analysis.
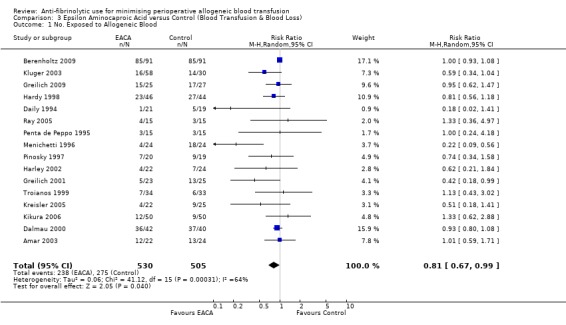
Comparison 3 Epsilon Aminocaproic Acid versus Control (Blood Transfusion & Blood Loss), Outcome 1 No. Exposed to Allogeneic Blood.
3.2. Analysis.
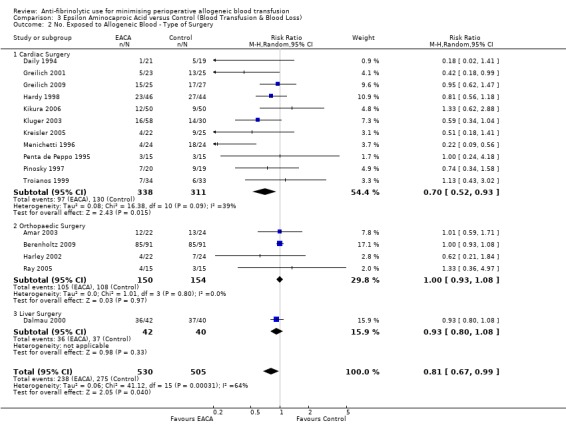
Comparison 3 Epsilon Aminocaproic Acid versus Control (Blood Transfusion & Blood Loss), Outcome 2 No. Exposed to Allogeneic Blood ‐ Type of Surgery.
3.3. Analysis.
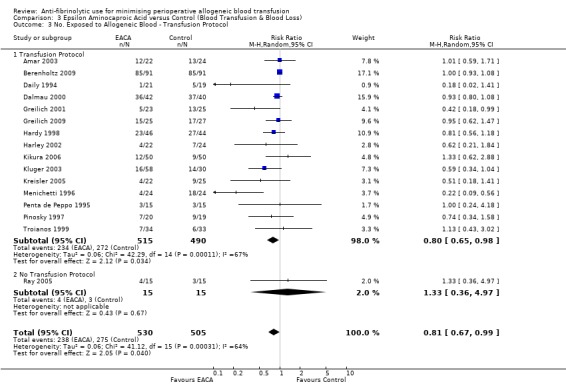
Comparison 3 Epsilon Aminocaproic Acid versus Control (Blood Transfusion & Blood Loss), Outcome 3 No. Exposed to Allogeneic Blood ‐ Transfusion Protocol.
3.4. Analysis.
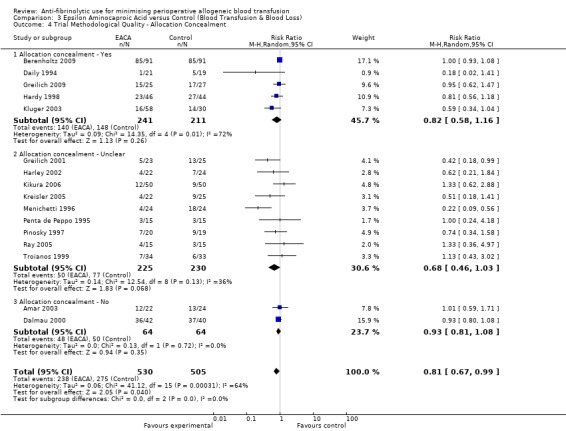
Comparison 3 Epsilon Aminocaproic Acid versus Control (Blood Transfusion & Blood Loss), Outcome 4 Trial Methodological Quality ‐ Allocation Concealment.
3.5. Analysis.
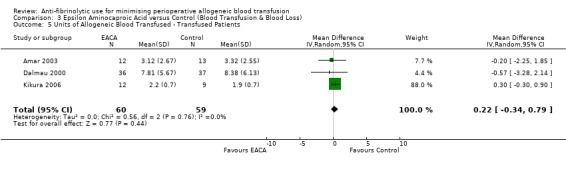
Comparison 3 Epsilon Aminocaproic Acid versus Control (Blood Transfusion & Blood Loss), Outcome 5 Units of Allogeneic Blood Transfused ‐ Transfused Patients.
3.6. Analysis.
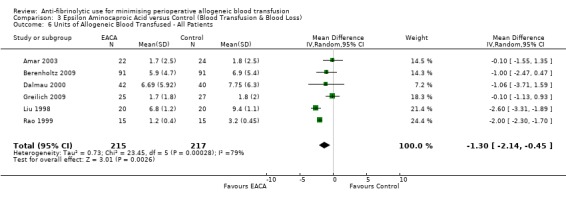
Comparison 3 Epsilon Aminocaproic Acid versus Control (Blood Transfusion & Blood Loss), Outcome 6 Units of Allogeneic Blood Transfused ‐ All Patients.
3.7. Analysis.
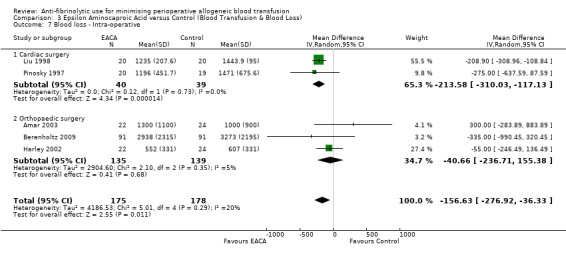
Comparison 3 Epsilon Aminocaproic Acid versus Control (Blood Transfusion & Blood Loss), Outcome 7 Blood loss ‐ Intra‐operative.
3.8. Analysis.
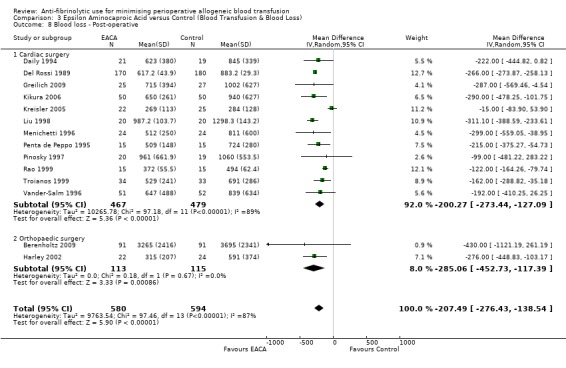
Comparison 3 Epsilon Aminocaproic Acid versus Control (Blood Transfusion & Blood Loss), Outcome 8 Blood loss ‐ Post‐operative.
3.9. Analysis.
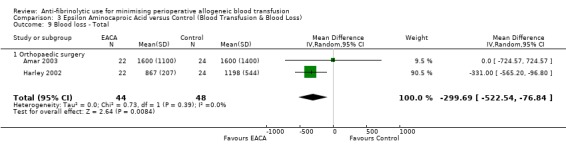
Comparison 3 Epsilon Aminocaproic Acid versus Control (Blood Transfusion & Blood Loss), Outcome 9 Blood loss ‐ Total.
Comparison 4. Aprotinin versus Tranexamic Acid (Blood Transfusion & Blood Loss).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 No. Exposed to Allogeneic Blood | 21 | 4185 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.81, 1.01] |
| 2 No. Exposed to Allogeneic Blood ‐ Type of Surgery | 21 | 4185 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.81, 1.01] |
| 2.1 Cardiac surgery | 18 | 3983 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.76, 0.99] |
| 2.2 Liver surgery | 2 | 178 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.91, 1.11] |
| 2.3 Orthopaedic surgery | 1 | 24 | Risk Ratio (M‐H, Random, 95% CI) | 11.00 [0.67, 179.29] |
| 3 No. Exposed to Allogeneic Blood ‐ Transfusion Protocol | 21 | 4185 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.81, 1.01] |
| 3.1 Transfusion Protocol | 20 | 4155 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.80, 1.01] |
| 3.2 No Transfusion Protocol | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.67, 1.27] |
| 4 Trial Methodological Quality ‐ Allocation Concealment | 21 | 4185 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.81, 1.01] |
| 4.1 Allocation concealment ‐ Yes | 4 | 1871 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.69, 0.92] |
| 4.2 Allocation concealment ‐ Unclear | 13 | 1832 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.88, 1.07] |
| 4.3 Allocation concealment ‐ No | 4 | 482 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.62, 1.39] |
| 5 Units Allogeneic Blood Transfused ‐ Transfused Patients | 6 | 207 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.44, 0.30] |
| 6 Units Allogeneic Blood Transfused ‐ All Patients | 10 | 992 | Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.45, ‐0.04] |
| 7 Blood loss | 14 | 1041 | Mean Difference (IV, Random, 95% CI) | ‐136.44 [‐198.40, ‐74.47] |
| 7.1 Cardiac surgery ‐ Post‐operative | 13 | 831 | Mean Difference (IV, Random, 95% CI) | ‐145.81 [‐209.99, ‐81.62] |
| 7.2 Cardiac surgery ‐ Total | 1 | 210 | Mean Difference (IV, Random, 95% CI) | 6.0 [‐171.38, 183.38] |
4.1. Analysis.
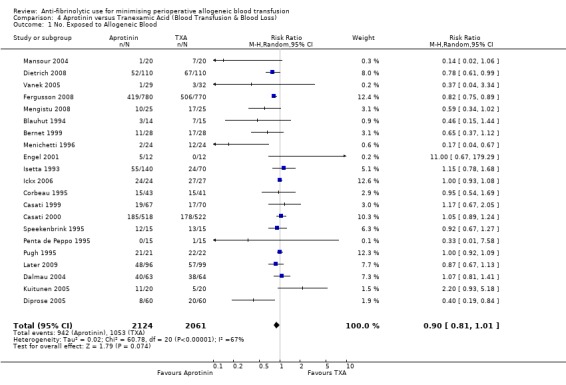
Comparison 4 Aprotinin versus Tranexamic Acid (Blood Transfusion & Blood Loss), Outcome 1 No. Exposed to Allogeneic Blood.
4.2. Analysis.
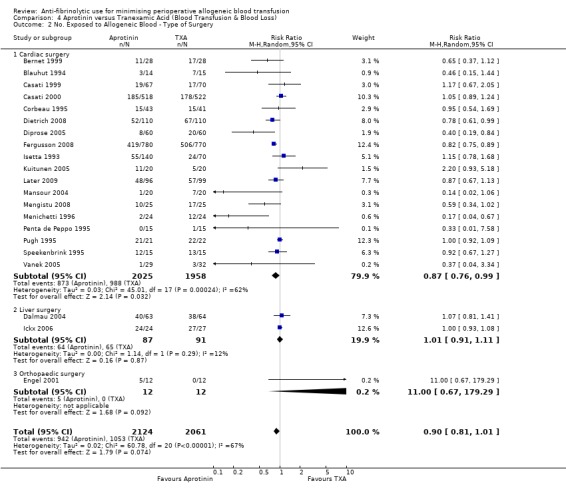
Comparison 4 Aprotinin versus Tranexamic Acid (Blood Transfusion & Blood Loss), Outcome 2 No. Exposed to Allogeneic Blood ‐ Type of Surgery.
4.3. Analysis.
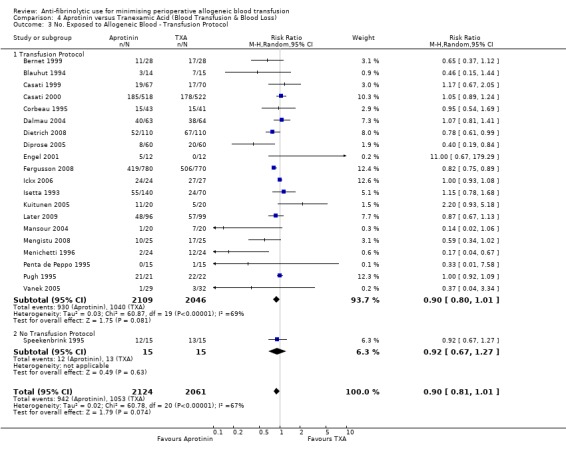
Comparison 4 Aprotinin versus Tranexamic Acid (Blood Transfusion & Blood Loss), Outcome 3 No. Exposed to Allogeneic Blood ‐ Transfusion Protocol.
4.4. Analysis.
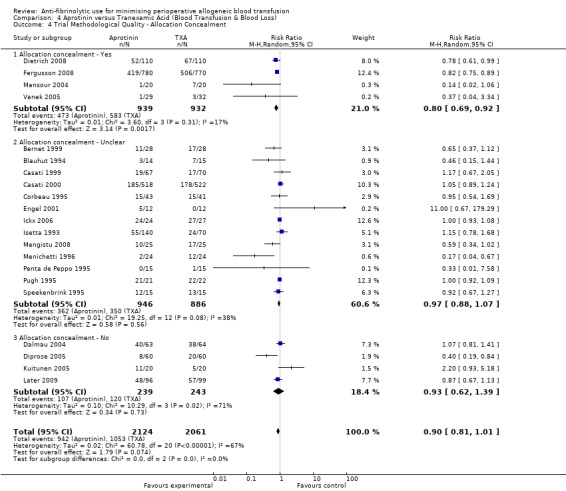
Comparison 4 Aprotinin versus Tranexamic Acid (Blood Transfusion & Blood Loss), Outcome 4 Trial Methodological Quality ‐ Allocation Concealment.
4.5. Analysis.
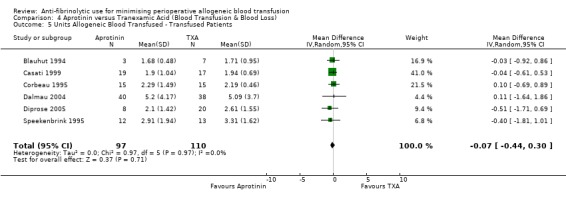
Comparison 4 Aprotinin versus Tranexamic Acid (Blood Transfusion & Blood Loss), Outcome 5 Units Allogeneic Blood Transfused ‐ Transfused Patients.
4.6. Analysis.
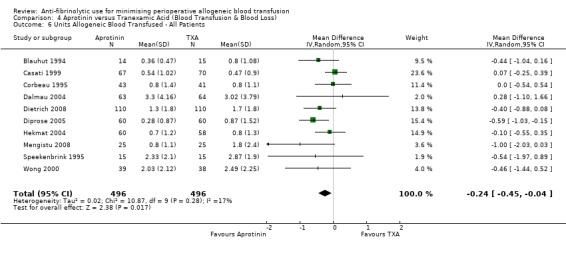
Comparison 4 Aprotinin versus Tranexamic Acid (Blood Transfusion & Blood Loss), Outcome 6 Units Allogeneic Blood Transfused ‐ All Patients.
4.7. Analysis.
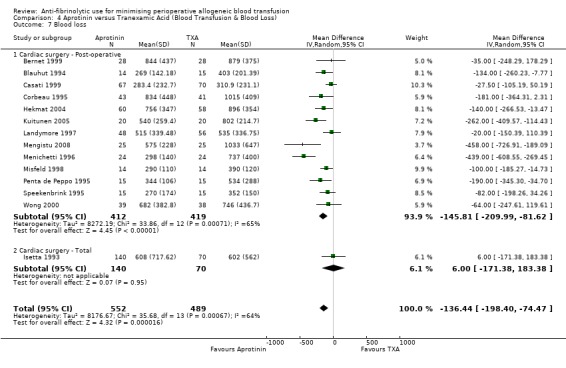
Comparison 4 Aprotinin versus Tranexamic Acid (Blood Transfusion & Blood Loss), Outcome 7 Blood loss.
Comparison 5. Aprotinin versus Epsilon Aminocaproic Acid (Blood Transfusion & Blood Loss).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 No. Exposed to Allogeneic Blood | 12 | 2200 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.76, 0.89] |
| 2 No. Exposed to Allogeneic Blood ‐ Type of Surgery | 12 | 2200 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.76, 0.89] |
| 2.1 Cardiac surgery | 10 | 2125 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.76, 0.89] |
| 2.2 Orthopaedic surgery | 2 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.48, 1.40] |
| 3 No. Exposed to Allogeneic Blood ‐ Transfusion Protocol | 12 | 2200 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.76, 0.89] |
| 3.1 Transfusion Protocol | 9 | 2014 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.76, 0.89] |
| 3.2 No Transfusion Protocol | 3 | 186 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.47, 1.31] |
| 4 Trial Methodological Quality ‐ Allocation Concealment | 12 | 2200 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.76, 0.89] |
| 4.1 Allocation concealment ‐ Yes | 3 | 1651 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.71, 1.05] |
| 4.2 Allocation concealment ‐ Unclear | 8 | 504 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.58, 0.99] |
| 4.3 Allocation concealment ‐ No | 1 | 45 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.49, 1.55] |
| 5 Units of Allogeneic Blood Transfused ‐ Transfused Patients | 2 | 63 | Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.63, 0.28] |
| 6 Units of Allogeneic Blood Transfused ‐ All Patients | 5 | 329 | Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.55, 0.14] |
| 7 Blood loss | 8 | 499 | Mean Difference (IV, Random, 95% CI) | ‐106.01 [‐212.50, 0.47] |
| 7.1 Cardiac surgery ‐ Post‐operative | 7 | 454 | Mean Difference (IV, Random, 95% CI) | ‐111.43 [‐220.64, ‐2.21] |
| 7.2 Orthopaedic surgery ‐ Total | 1 | 45 | Mean Difference (IV, Random, 95% CI) | 100.0 [‐515.06, 715.06] |
5.1. Analysis.
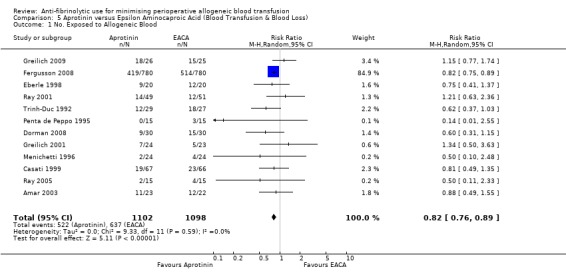
Comparison 5 Aprotinin versus Epsilon Aminocaproic Acid (Blood Transfusion & Blood Loss), Outcome 1 No. Exposed to Allogeneic Blood.
5.2. Analysis.
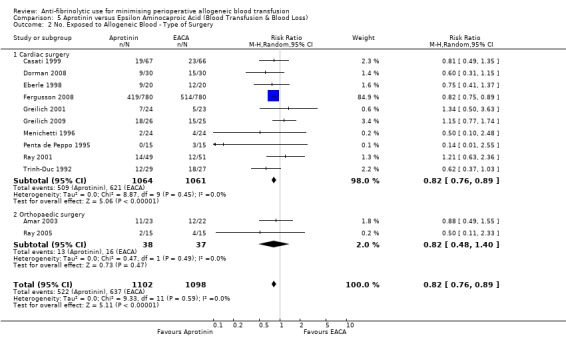
Comparison 5 Aprotinin versus Epsilon Aminocaproic Acid (Blood Transfusion & Blood Loss), Outcome 2 No. Exposed to Allogeneic Blood ‐ Type of Surgery.
5.3. Analysis.
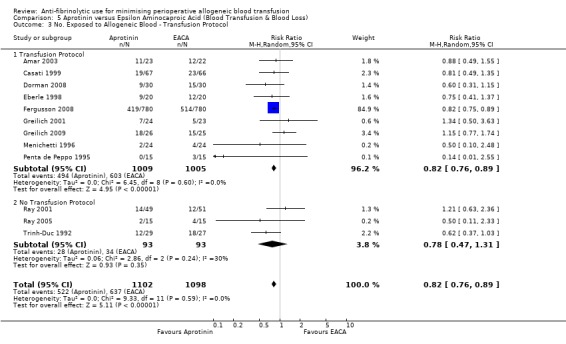
Comparison 5 Aprotinin versus Epsilon Aminocaproic Acid (Blood Transfusion & Blood Loss), Outcome 3 No. Exposed to Allogeneic Blood ‐ Transfusion Protocol.
5.4. Analysis.
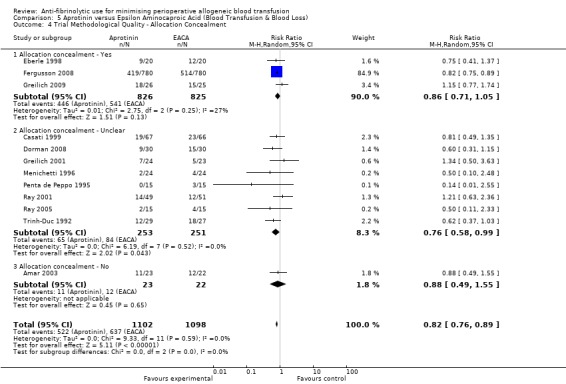
Comparison 5 Aprotinin versus Epsilon Aminocaproic Acid (Blood Transfusion & Blood Loss), Outcome 4 Trial Methodological Quality ‐ Allocation Concealment.
5.5. Analysis.
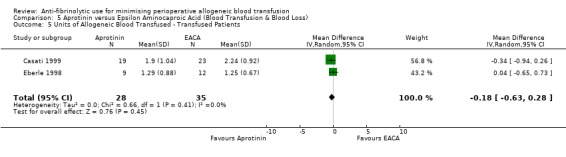
Comparison 5 Aprotinin versus Epsilon Aminocaproic Acid (Blood Transfusion & Blood Loss), Outcome 5 Units of Allogeneic Blood Transfused ‐ Transfused Patients.
5.6. Analysis.
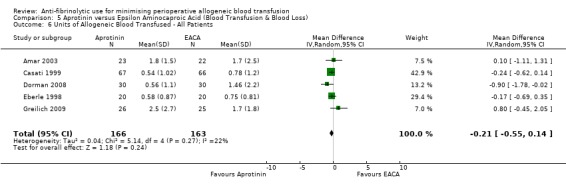
Comparison 5 Aprotinin versus Epsilon Aminocaproic Acid (Blood Transfusion & Blood Loss), Outcome 6 Units of Allogeneic Blood Transfused ‐ All Patients.
5.7. Analysis.
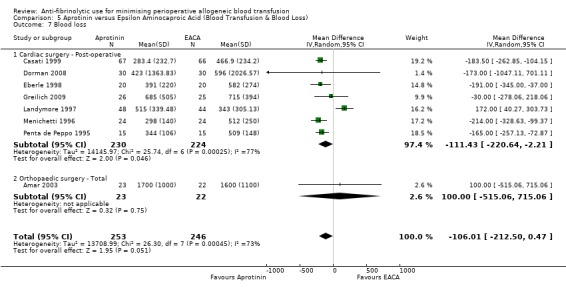
Comparison 5 Aprotinin versus Epsilon Aminocaproic Acid (Blood Transfusion & Blood Loss), Outcome 7 Blood loss.
Comparison 6. Tranexamic Acid versus Epsilon Aminocaproic Acid (Blood Transfusion & Blood Loss).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 No. Exposed to Allogeneic Blood | 8 | 2003 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.77, 1.21] |
| 2 No. Exposed to Allogeneic Blood ‐ Type of Surgery | 8 | 2003 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.77, 1.21] |
| 2.1 Cardiac surgery | 6 | 1852 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.79, 1.46] |
| 2.2 Orthopaedic surgery | 1 | 67 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.03, 1.94] |
| 2.3 Liver surgery | 1 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.64, 1.02] |
| 3 No. Exposed to Allogeneic Blood ‐ Transfusion Protocol | 8 | 2003 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.77, 1.21] |
| 3.1 Transfusion Protocol | 8 | 2003 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.77, 1.21] |
| 4 Trial Methodological Quality ‐ Allocation Concealment | 8 | 2003 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.77, 1.21] |
| 4.1 Allocation concealment ‐ Yes | 1 | 1550 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.93, 1.07] |
| 4.2 Allocation concealment ‐ Unclear | 5 | 302 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.68, 1.98] |
| 4.3 Allocation concealment ‐ No | 2 | 151 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.22, 1.84] |
| 5 Units of Allogeneic Blood Transfused ‐ Transfused Patients | 4 | 133 | Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.74, 0.07] |
| 6 Units of Allogeneic Blood Transfused ‐ All Patients | 3 | 268 | Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.59, 0.03] |
| 7 Blood loss | 7 | 469 | Mean Difference (IV, Random, 95% CI) | ‐4.20 [‐147.29, 138.89] |
| 7.1 Cardiac surgery ‐ Post‐operative | 6 | 402 | Mean Difference (IV, Random, 95% CI) | ‐4.36 [‐163.35, 154.63] |
| 7.2 Orthopaedic surgery ‐ Total | 1 | 67 | Mean Difference (IV, Random, 95% CI) | ‐9.0 [‐270.16, 252.16] |
| 7.3 Gynaecological surgery ‐ Total | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
6.1. Analysis.
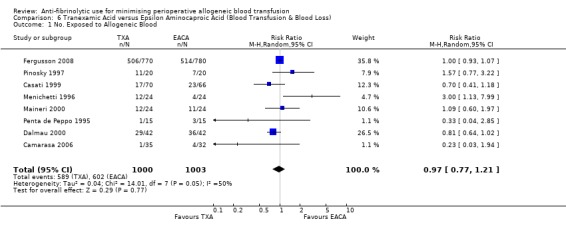
Comparison 6 Tranexamic Acid versus Epsilon Aminocaproic Acid (Blood Transfusion & Blood Loss), Outcome 1 No. Exposed to Allogeneic Blood.
6.2. Analysis.
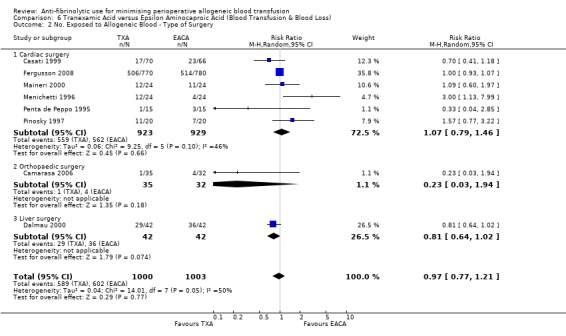
Comparison 6 Tranexamic Acid versus Epsilon Aminocaproic Acid (Blood Transfusion & Blood Loss), Outcome 2 No. Exposed to Allogeneic Blood ‐ Type of Surgery.
6.3. Analysis.
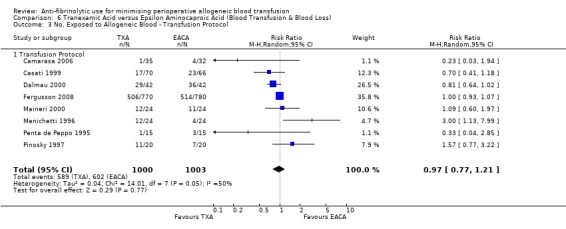
Comparison 6 Tranexamic Acid versus Epsilon Aminocaproic Acid (Blood Transfusion & Blood Loss), Outcome 3 No. Exposed to Allogeneic Blood ‐ Transfusion Protocol.
6.4. Analysis.
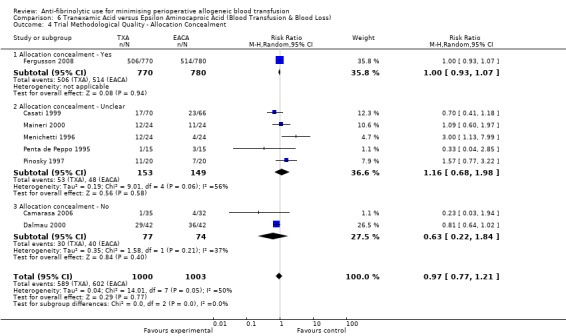
Comparison 6 Tranexamic Acid versus Epsilon Aminocaproic Acid (Blood Transfusion & Blood Loss), Outcome 4 Trial Methodological Quality ‐ Allocation Concealment.
6.5. Analysis.
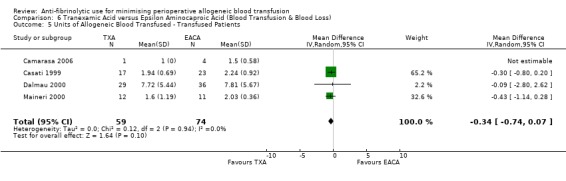
Comparison 6 Tranexamic Acid versus Epsilon Aminocaproic Acid (Blood Transfusion & Blood Loss), Outcome 5 Units of Allogeneic Blood Transfused ‐ Transfused Patients.
6.6. Analysis.
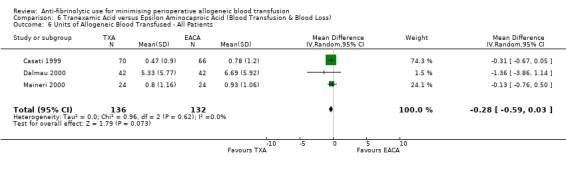
Comparison 6 Tranexamic Acid versus Epsilon Aminocaproic Acid (Blood Transfusion & Blood Loss), Outcome 6 Units of Allogeneic Blood Transfused ‐ All Patients.
6.7. Analysis.
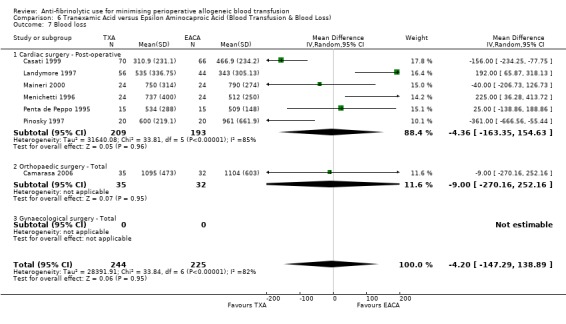
Comparison 6 Tranexamic Acid versus Epsilon Aminocaproic Acid (Blood Transfusion & Blood Loss), Outcome 7 Blood loss.
Comparison 7. Aprotinin versus Lysine Analogues (Blood Transfusion).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 No. Exposed to Allogeneic Blood | 29 | 5566 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.81, 0.99] |
| 2 No. Exposed to Allogeneic Blood ‐ Type of Surgery | 29 | 5469 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.80, 0.98] |
| 2.1 Cardiac surgery | 24 | 5192 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.76, 0.96] |
| 2.2 Orthopaedic surgery | 3 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.32, 3.48] |
| 2.3 Liver surgery | 2 | 178 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.91, 1.11] |
| 3 No. Exposed to Allogeneic Blood ‐ Transfusion Protocol | 29 | 5429 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.81, 0.98] |
| 3.1 Transfusion Protocol | 25 | 5213 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.80, 0.99] |
| 3.2 No Transfusion Protocol | 4 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.65, 1.12] |
| 4 Units of Allogeneic Blood Transfused ‐ Transfused Patients | 7 | 251 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.42, 0.21] |
| 5 Units of Allogeneic Blood Transfused ‐ All Patients | 14 | 1254 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.42, ‐0.09] |
| 6 Trial Methodological Quality ‐ Allocation Concealment | 29 | 5566 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.81, 0.99] |
| 6.1 Allocation concealment ‐ Yes | 6 | 2742 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.71, 0.95] |
| 6.2 Allocation concealment ‐ Unclear | 18 | 2297 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.86, 1.04] |
| 6.3 Allocation concealment ‐ No | 5 | 527 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.67, 1.28] |
7.1. Analysis.
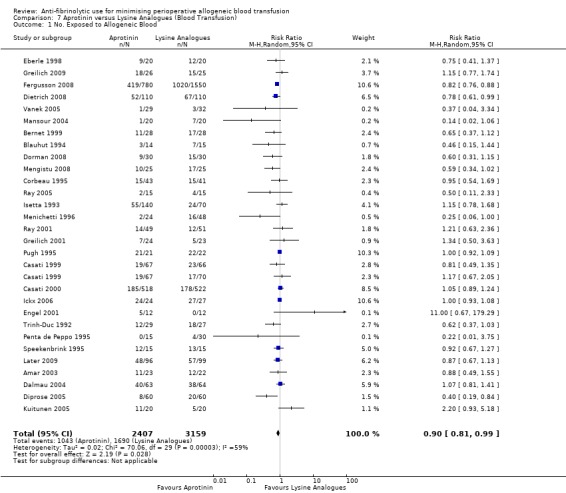
Comparison 7 Aprotinin versus Lysine Analogues (Blood Transfusion), Outcome 1 No. Exposed to Allogeneic Blood.
7.2. Analysis.
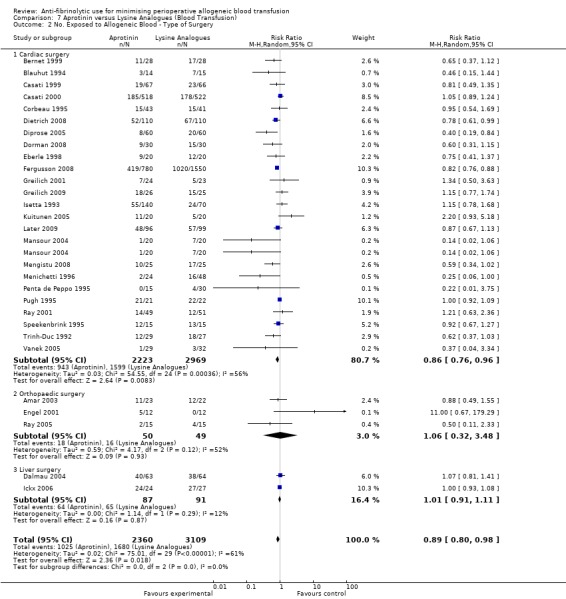
Comparison 7 Aprotinin versus Lysine Analogues (Blood Transfusion), Outcome 2 No. Exposed to Allogeneic Blood ‐ Type of Surgery.
7.3. Analysis.
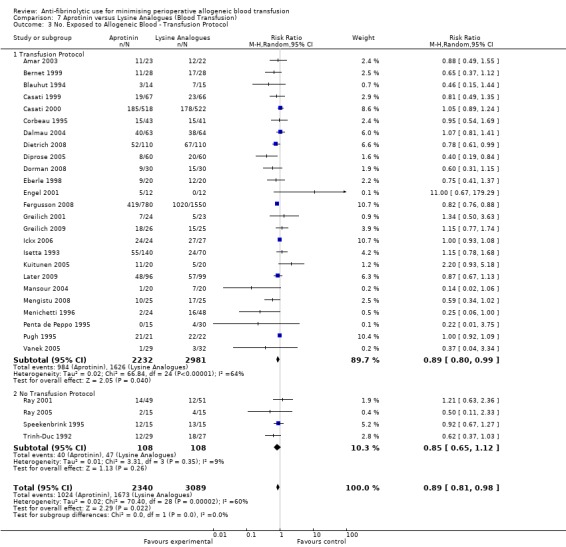
Comparison 7 Aprotinin versus Lysine Analogues (Blood Transfusion), Outcome 3 No. Exposed to Allogeneic Blood ‐ Transfusion Protocol.
7.4. Analysis.
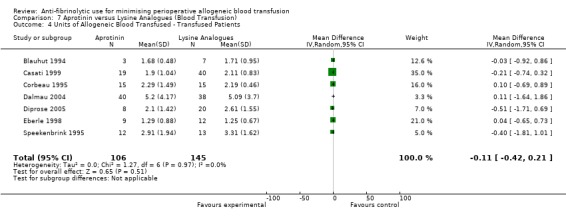
Comparison 7 Aprotinin versus Lysine Analogues (Blood Transfusion), Outcome 4 Units of Allogeneic Blood Transfused ‐ Transfused Patients.
7.5. Analysis.
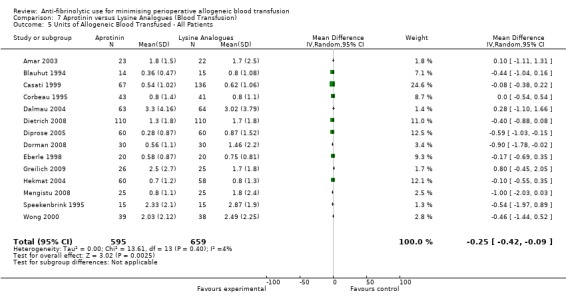
Comparison 7 Aprotinin versus Lysine Analogues (Blood Transfusion), Outcome 5 Units of Allogeneic Blood Transfused ‐ All Patients.
7.6. Analysis.
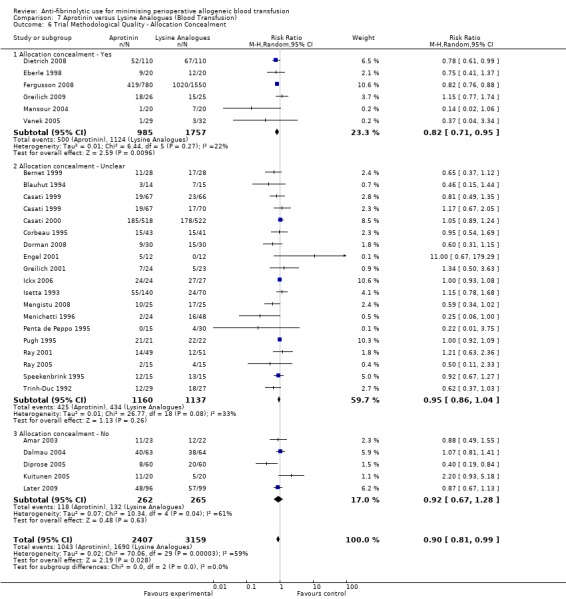
Comparison 7 Aprotinin versus Lysine Analogues (Blood Transfusion), Outcome 6 Trial Methodological Quality ‐ Allocation Concealment.
Comparison 8. Adverse Events and Other Outcomes (Active versus Control).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Re‐operation for bleeding | 85 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Aprotinin versus Control | 61 | 6117 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.34, 0.62] |
| 1.2 Tranexamic Acid versus Control | 27 | 2386 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.55, 1.17] |
| 1.3 Epsilon Aminocaproic Acid versus Control | 8 | 922 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.11, 0.99] |
| 2 Mortality | 92 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Aprotinin versus Control | 63 | 8876 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.63, 1.06] |
| 2.2 Tranexamic Acid versus Control | 30 | 2917 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.33, 1.10] |
| 2.3 Epsilon Aminocaproic Acid versus Control | 8 | 988 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.44, 2.57] |
| 3 Myocardial Infarction (MI) | 71 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Aprotinin versus Control | 49 | 7137 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.69, 1.11] |
| 3.2 Tranexamic Acid versus Control | 21 | 2186 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.41, 1.52] |
| 3.3 Epsilon aminocaproic Acid versus Control | 7 | 896 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.48, 1.63] |
| 4 Stroke (CVA) | 45 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Aprotinin versus Control | 23 | 3122 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.44, 1.52] |
| 4.2 Tranexamic Acid versus Control | 18 | 2027 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.49, 3.07] |
| 4.3 Epsilon Aminocaproic Acid versus Control | 8 | 936 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.16, 2.36] |
| 5 Deep Vein Thrombosis (DVT) | 40 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Aprotinin versus Control | 16 | 1456 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.47, 1.29] |
| 5.2 Tranexamic Acid versus Control | 23 | 1472 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.35, 1.43] |
| 5.3 Epsilon Aminocaproic Acid versus Control | 4 | 304 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.20, 3.03] |
| 6 Pulmonary Embolism (PE) | 20 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Aprotinin versus Control | 4 | 585 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.42, 5.29] |
| 6.2 Tranexamic Acid versus Control | 14 | 1006 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.23, 1.99] |
| 6.3 Epsilon Aminocaproic Acid versus Control | 3 | 274 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.06, 2.13] |
| 7 Other Thrombosis | 19 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Aprotinin versus Control | 9 | 736 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.25, 2.15] |
| 7.2 Tranexamic Acid versus Control | 9 | 484 | Risk Ratio (M‐H, Random, 95% CI) | 2.10 [0.49, 8.99] |
| 7.3 Epsilon Aminocaproic Acid versus Control | 2 | 264 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.15, 1.72] |
| 8 Coronary artery graft occlusion | 2 | 728 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.10, 5.67] |
| 8.1 Aprotinin versus Control | 2 | 728 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.10, 5.67] |
| 9 Renal Failure / Dysfunction | 34 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Aprotinin versus Control | 27 | 5185 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.79, 1.54] |
| 9.2 Tranexamic Acid versus Control | 9 | 912 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.33, 2.37] |
| 9.3 Epsilon Aminocaproic Acid versus control | 2 | 235 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.14, 1.22] |
| 10 Hospital Length of Stay | 31 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10.1 Aprotinin versus Control | 23 | 2017 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.71, 0.20] |
| 10.2 Tranexamic Acid versus Control | 10 | 772 | Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.82, 0.13] |
| 10.3 Epsilon Aminocaproic Acid versus Control | 2 | 228 | Mean Difference (IV, Random, 95% CI) | 0.58 [‐3.17, 4.33] |
8.1. Analysis.
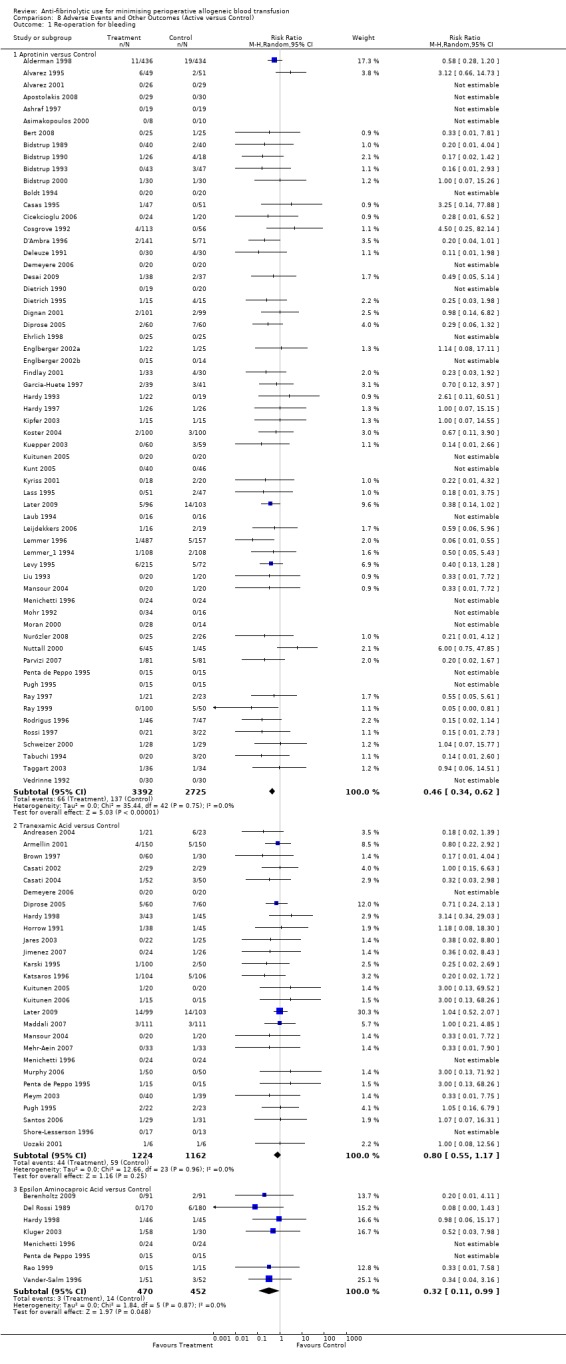
Comparison 8 Adverse Events and Other Outcomes (Active versus Control), Outcome 1 Re‐operation for bleeding.
8.2. Analysis.
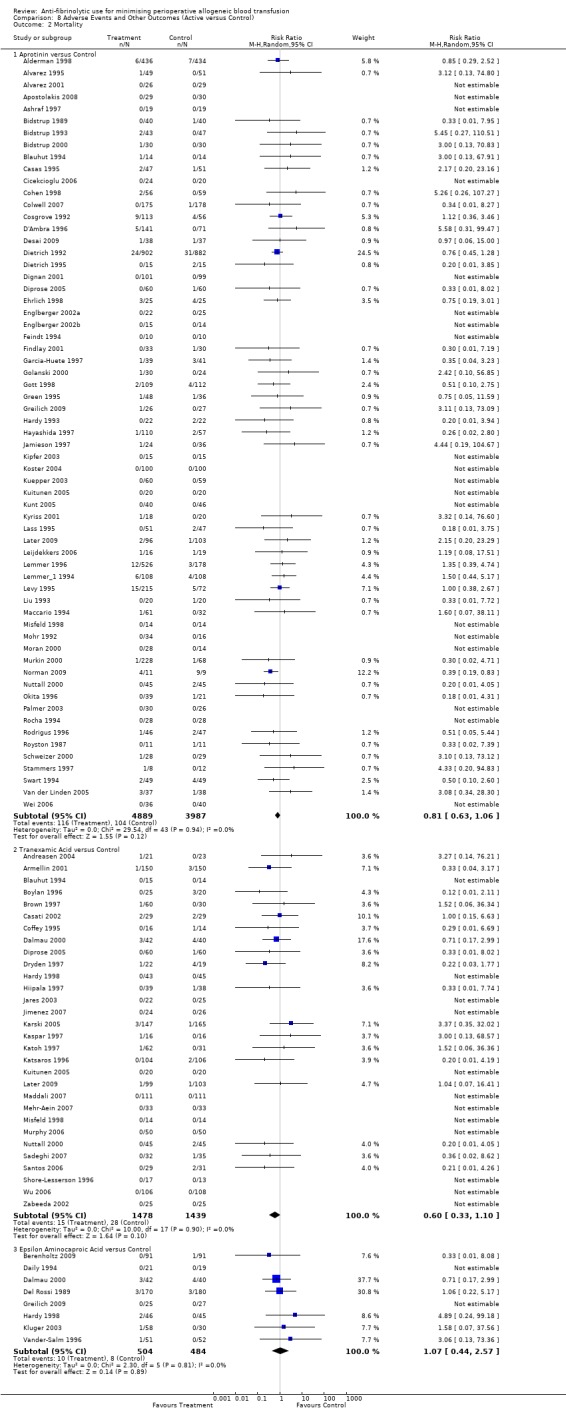
Comparison 8 Adverse Events and Other Outcomes (Active versus Control), Outcome 2 Mortality.
8.3. Analysis.
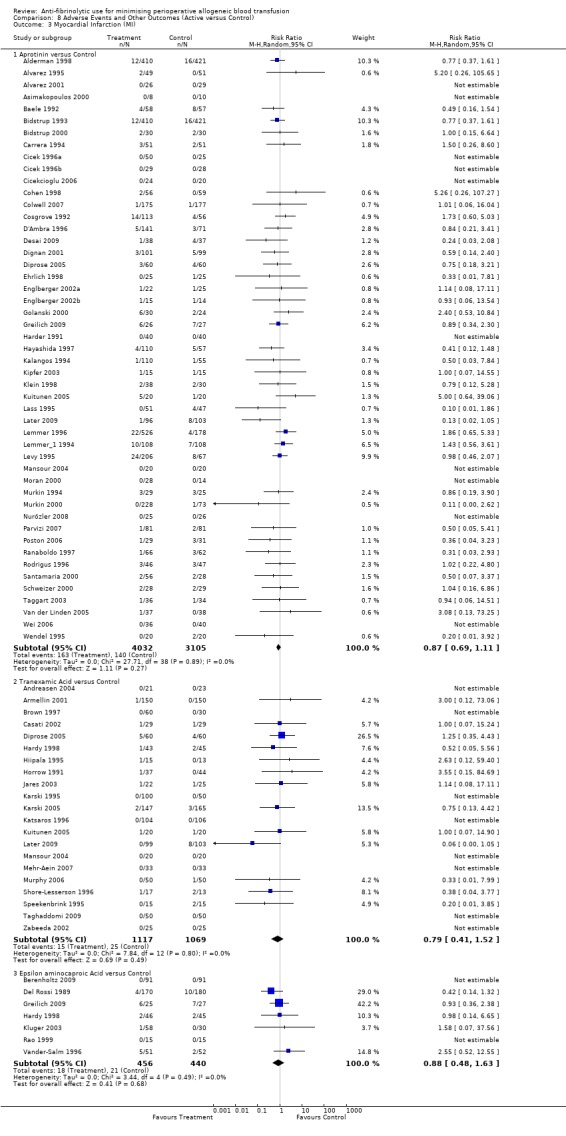
Comparison 8 Adverse Events and Other Outcomes (Active versus Control), Outcome 3 Myocardial Infarction (MI).
8.4. Analysis.
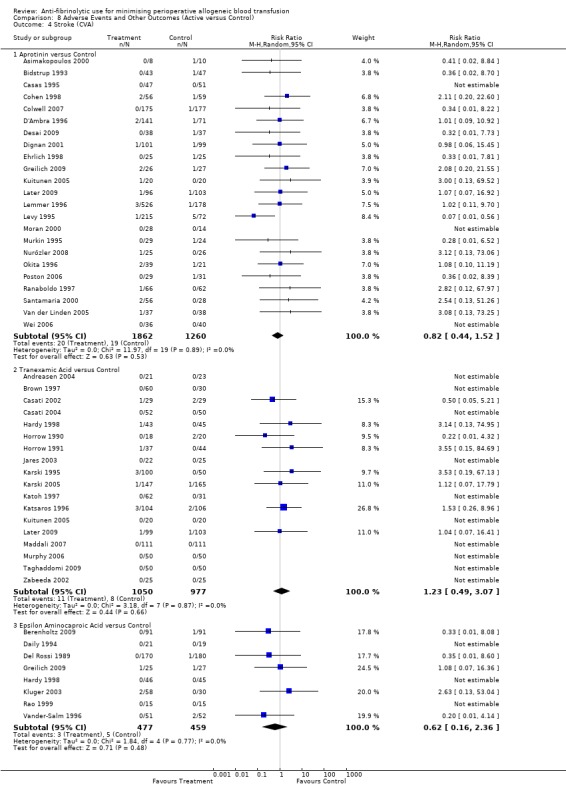
Comparison 8 Adverse Events and Other Outcomes (Active versus Control), Outcome 4 Stroke (CVA).
8.5. Analysis.
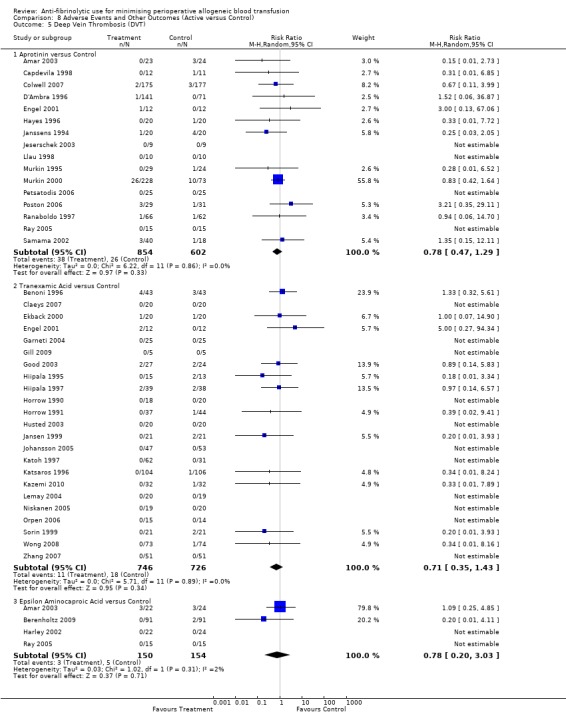
Comparison 8 Adverse Events and Other Outcomes (Active versus Control), Outcome 5 Deep Vein Thrombosis (DVT).
8.6. Analysis.
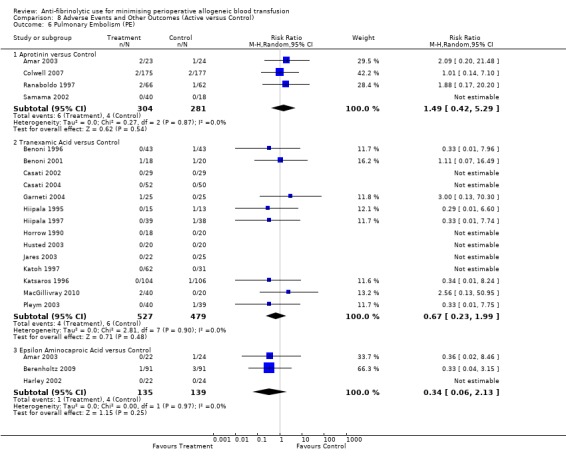
Comparison 8 Adverse Events and Other Outcomes (Active versus Control), Outcome 6 Pulmonary Embolism (PE).
8.7. Analysis.
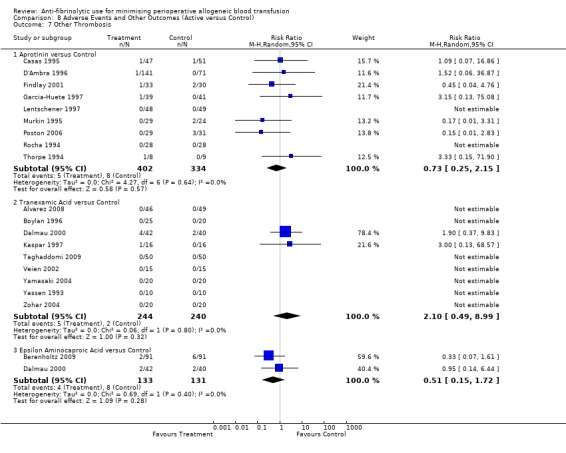
Comparison 8 Adverse Events and Other Outcomes (Active versus Control), Outcome 7 Other Thrombosis.
8.8. Analysis.
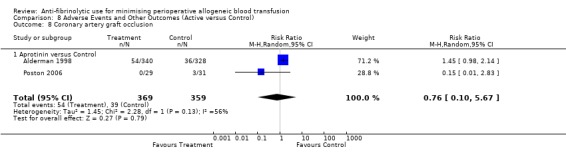
Comparison 8 Adverse Events and Other Outcomes (Active versus Control), Outcome 8 Coronary artery graft occlusion.
8.9. Analysis.
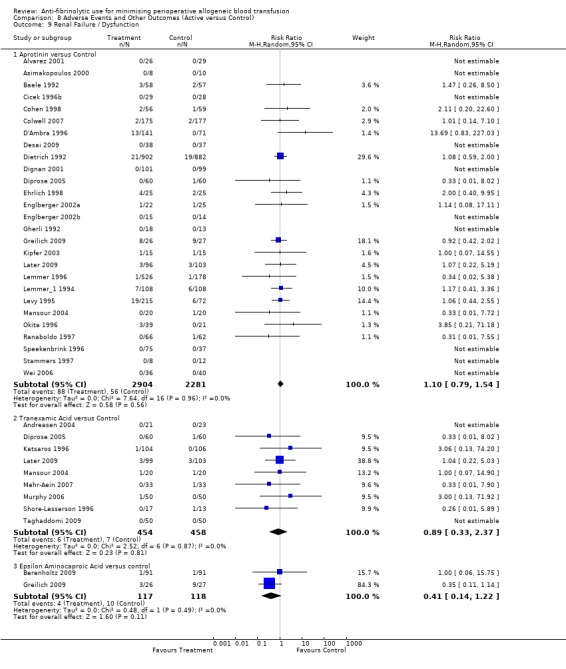
Comparison 8 Adverse Events and Other Outcomes (Active versus Control), Outcome 9 Renal Failure / Dysfunction.
8.10. Analysis.
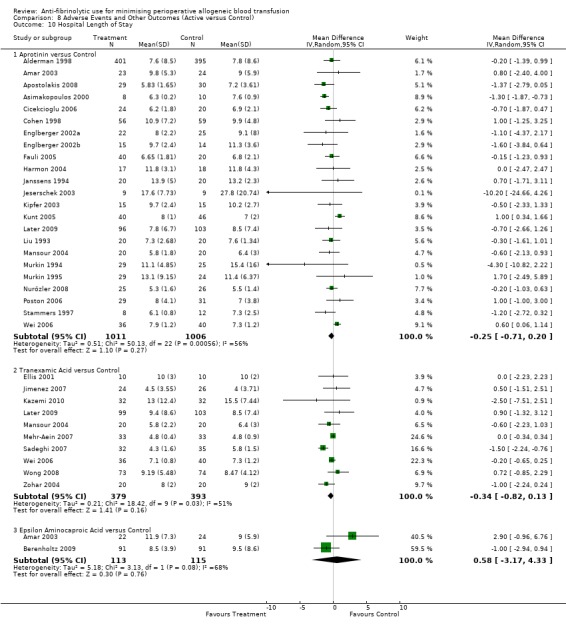
Comparison 8 Adverse Events and Other Outcomes (Active versus Control), Outcome 10 Hospital Length of Stay.
Comparison 9. Adverse Events and Other Outcomes (Active versus Active).
9.1. Analysis.
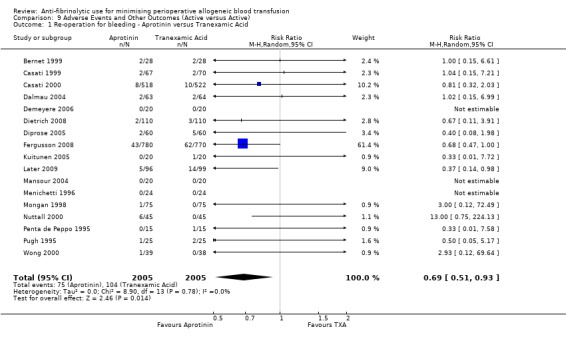
Comparison 9 Adverse Events and Other Outcomes (Active versus Active), Outcome 1 Re‐operation for bleeding ‐ Aprotinin versus Tranexamic Acid.
9.2. Analysis.
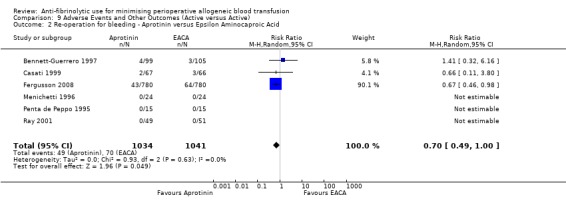
Comparison 9 Adverse Events and Other Outcomes (Active versus Active), Outcome 2 Re‐operation for bleeding ‐ Aprotinin versus Epsilon Aminocaproic Acid.
9.3. Analysis.
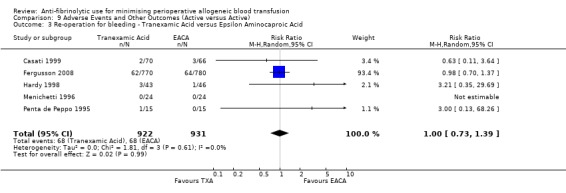
Comparison 9 Adverse Events and Other Outcomes (Active versus Active), Outcome 3 Re‐operation for bleeding ‐ Tranexamic Acid versus Epsilon Aminocaproic Acid.
9.4. Analysis.
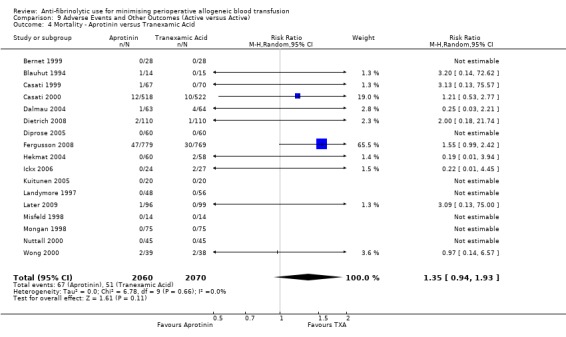
Comparison 9 Adverse Events and Other Outcomes (Active versus Active), Outcome 4 Mortality ‐ Aprotinin versus Tranexamic Acid.
9.5. Analysis.
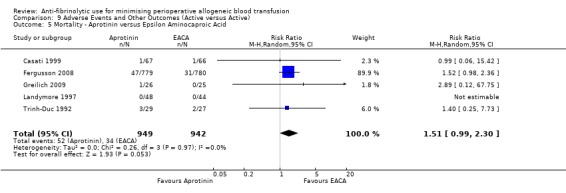
Comparison 9 Adverse Events and Other Outcomes (Active versus Active), Outcome 5 Mortality ‐ Aprotinin versus Epsilon Aminocaproic Acid.
9.6. Analysis.
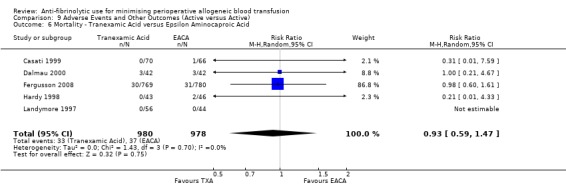
Comparison 9 Adverse Events and Other Outcomes (Active versus Active), Outcome 6 Mortality ‐ Tranexamic Acid versus Epsilon Aminocaproic Acid.
9.7. Analysis.
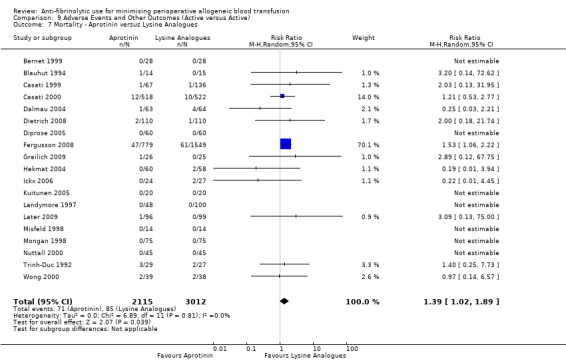
Comparison 9 Adverse Events and Other Outcomes (Active versus Active), Outcome 7 Mortality ‐ Aprotinin versus Lysine Analogues.
9.8. Analysis.
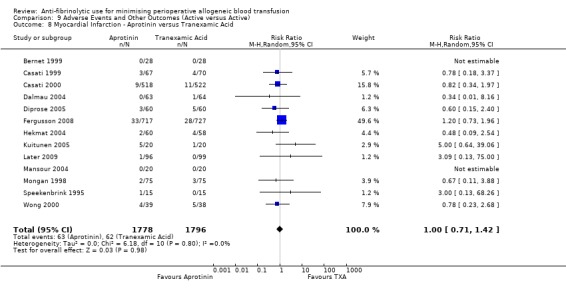
Comparison 9 Adverse Events and Other Outcomes (Active versus Active), Outcome 8 Myocardial Infarction ‐ Aprotinin versus Tranexamic Acid.
9.9. Analysis.
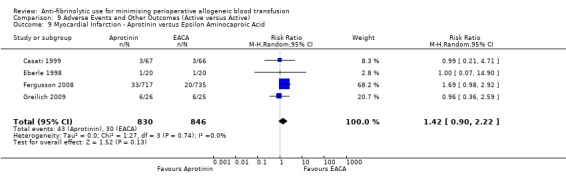
Comparison 9 Adverse Events and Other Outcomes (Active versus Active), Outcome 9 Myocardial Infarction ‐ Aprotinin versus Epsilon Aminocaproic Acid.
9.10. Analysis.
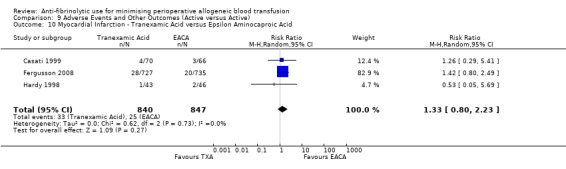
Comparison 9 Adverse Events and Other Outcomes (Active versus Active), Outcome 10 Myocardial Infarction ‐ Tranexamic Acid versus Epsilon Aminocaproic Acid.
9.11. Analysis.
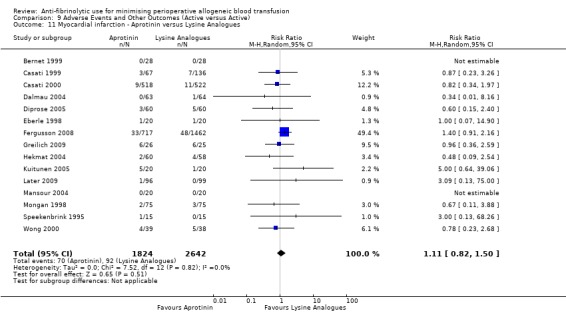
Comparison 9 Adverse Events and Other Outcomes (Active versus Active), Outcome 11 Myocardial infarction ‐ Aprotinin versus Lysine Analogues.
9.12. Analysis.
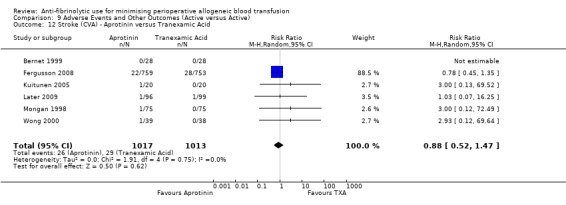
Comparison 9 Adverse Events and Other Outcomes (Active versus Active), Outcome 12 Stroke (CVA) ‐ Aprotinin versus Tranexamic Acid.
9.13. Analysis.
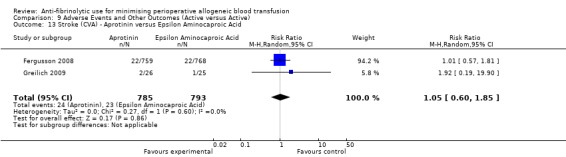
Comparison 9 Adverse Events and Other Outcomes (Active versus Active), Outcome 13 Stroke (CVA) ‐ Aprotinin versus Epsilon Aminocaproic Acid.
9.14. Analysis.
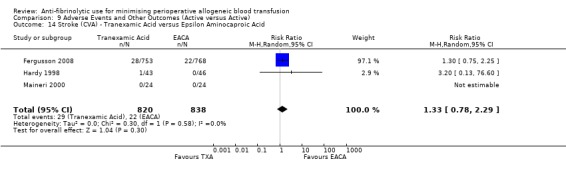
Comparison 9 Adverse Events and Other Outcomes (Active versus Active), Outcome 14 Stroke (CVA) ‐ Tranexamic Acid versus Epsilon Aminocaproic Acid.
9.15. Analysis.
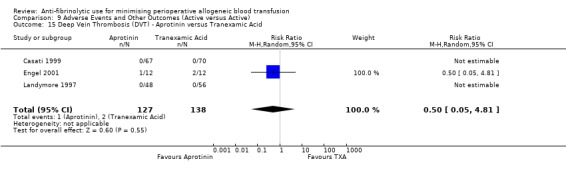
Comparison 9 Adverse Events and Other Outcomes (Active versus Active), Outcome 15 Deep Vein Thrombosis (DVT) ‐ Aprotinin versus Tranexamic Acid.
9.16. Analysis.
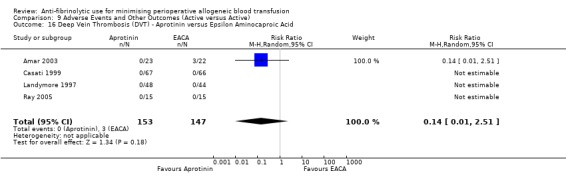
Comparison 9 Adverse Events and Other Outcomes (Active versus Active), Outcome 16 Deep Vein Thrombosis (DVT) ‐ Aprotinin versus Epsilon Aminocaproic Acid.
9.17. Analysis.
Comparison 9 Adverse Events and Other Outcomes (Active versus Active), Outcome 17 Deep Vein Thrombosis (DVT) ‐ Tranexamic Acid versus Epsilon Aminocaproic Acid.
9.18. Analysis.
Comparison 9 Adverse Events and Other Outcomes (Active versus Active), Outcome 18 Pulmonary Embolism (PE) ‐ Aprotinin versus Tranexamic Acid.
9.19. Analysis.
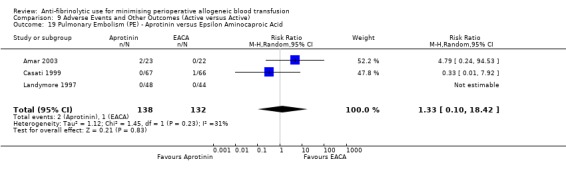
Comparison 9 Adverse Events and Other Outcomes (Active versus Active), Outcome 19 Pulmonary Embolism (PE) ‐ Aprotinin versus Epsilon Aminocaproic Acid.
9.20. Analysis.
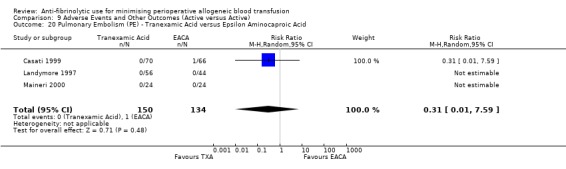
Comparison 9 Adverse Events and Other Outcomes (Active versus Active), Outcome 20 Pulmonary Embolism (PE) ‐ Tranexamic Acid versus Epsilon Aminocaproic Acid.
9.21. Analysis.
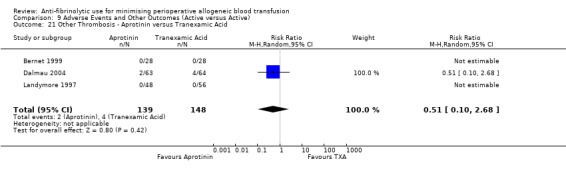
Comparison 9 Adverse Events and Other Outcomes (Active versus Active), Outcome 21 Other Thrombosis ‐ Aprotinin versus Tranexamic Acid.
9.22. Analysis.
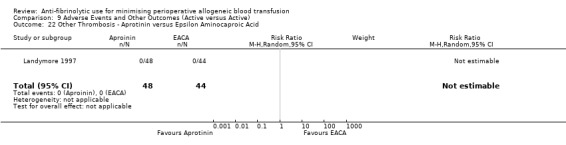
Comparison 9 Adverse Events and Other Outcomes (Active versus Active), Outcome 22 Other Thrombosis ‐ Aprotinin versus Epsilon Aminocaproic Acid.
9.23. Analysis.
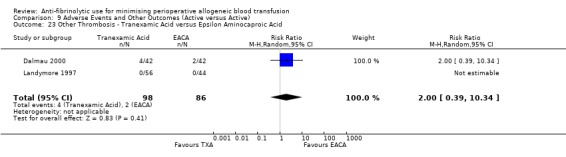
Comparison 9 Adverse Events and Other Outcomes (Active versus Active), Outcome 23 Other Thrombosis ‐ Tranexamic Acid versus Epsilon Aminocaproic Acid.
9.24. Analysis.
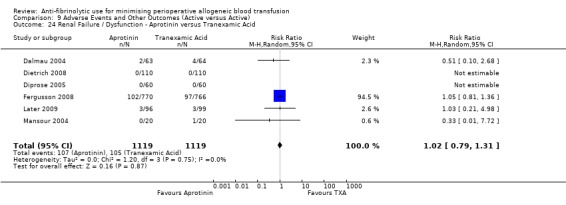
Comparison 9 Adverse Events and Other Outcomes (Active versus Active), Outcome 24 Renal Failure / Dysfunction ‐ Aprotinin versus Tranexamic Acid.
9.25. Analysis.
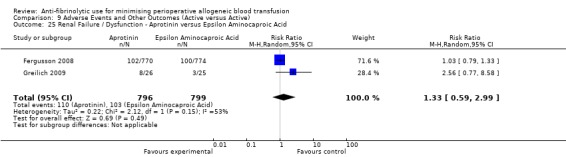
Comparison 9 Adverse Events and Other Outcomes (Active versus Active), Outcome 25 Renal Failure / Dysfunction ‐ Aprotinin versus Epsilon Aminocaproic Acid.
9.26. Analysis.
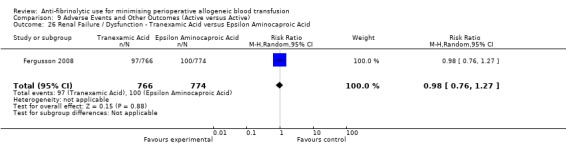
Comparison 9 Adverse Events and Other Outcomes (Active versus Active), Outcome 26 Renal Failure / Dysfunction ‐ Tranexamic Acid versus Epsilon Aminocaproic Acid.
9.27. Analysis.
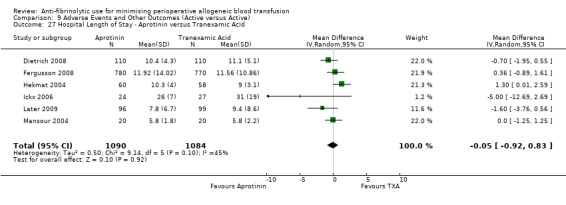
Comparison 9 Adverse Events and Other Outcomes (Active versus Active), Outcome 27 Hospital Length of Stay ‐ Aprotinin versus Tranexamic Acid.
9.28. Analysis.
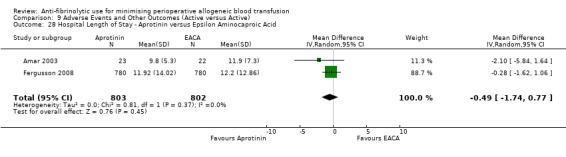
Comparison 9 Adverse Events and Other Outcomes (Active versus Active), Outcome 28 Hospital Length of Stay ‐ Aprotinin versus Epsilon Aminocaproic Acid.
9.29. Analysis.
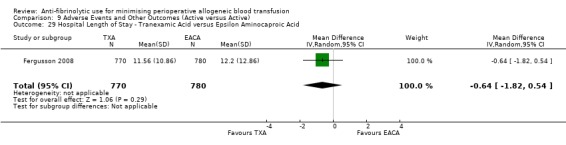
Comparison 9 Adverse Events and Other Outcomes (Active versus Active), Outcome 29 Hospital Length of Stay ‐ Tranexamic Acid versus Epsilon Aminocaproic Acid.
Comparison 10. Adverse Events and Other Outcomes (Active versus Control) ‐ Cardiac Surgery.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Re‐operation for bleeding | 78 | 8895 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.44, 0.71] |
| 1.1 Aprotinin versus Control | 56 | 5827 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.34, 0.63] |
| 1.2 Tranexamic Acid versus Control | 26 | 2328 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.54, 1.17] |
| 1.3 Epsilon Aminocaproic Acid versus Control | 7 | 740 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.11, 1.17] |
| 2 Mortality | 76 | 11240 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.65, 1.07] |
| 2.1 Aprotinin versus Control | 55 | 8174 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.64, 1.10] |
| 2.2 Tranexamic Acid versus Control | 23 | 2342 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.26, 1.28] |
| 2.3 Epsilon Aminocaproic Acid versus Control | 6 | 724 | Risk Ratio (M‐H, Random, 95% CI) | 1.65 [0.50, 5.43] |
| 3 Myocardial Infarction | 65 | 9472 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.71, 1.09] |
| 3.1 Aprotinin versus Control | 46 | 6658 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.71, 1.14] |
| 3.2 Tranexamic Acid versus Control | 19 | 2100 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.37, 1.47] |
| 3.3 Epsilon Aminocaproic Acid versus Control | 6 | 714 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.48, 1.63] |
| 4 Stroke | 38 | 4850 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.55, 1.63] |
| 4.1 Aprotinin versus Control | 18 | 2127 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.40, 1.67] |
| 4.2 Tranexamic Acid versus Control | 17 | 1969 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [0.53, 3.91] |
| 4.3 Epsilon Aminocaproic Acid versus Control | 7 | 754 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.16, 3.10] |
| 5 Deep Vein Thrombosis (DVT) | 7 | 1046 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.31, 2.87] |
| 5.1 Aprotinin versus Control | 3 | 624 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.36, 4.58] |
| 5.2 Tranexamic Acid versus Control | 4 | 422 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.04, 3.47] |
| 6 Pulmonary Embolism | 7 | 921 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.14, 2.74] |
| 6.1 Tranexamic Acid versus Control | 6 | 569 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.04, 3.15] |
| 6.2 Aprotinin versus Control | 1 | 352 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.14, 7.10] |
| 7 Other Thrombosis | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Aprotinin versus Control | 4 | 426 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.11, 3.36] |
| 7.2 Tranexamic Acid versus Control | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Renal Failure / Dysfunction | 30 | 5912 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.71, 1.33] |
| 8.1 Aprotinin versus Control | 24 | 4947 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.76, 1.51] |
| 8.2 Tranexamic Acid versus Control | 9 | 912 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.33, 2.37] |
| 8.3 Epsilon Aminocaproic Acid versus control | 1 | 53 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.11, 1.14] |
| 9 Hospital Length of Stay | 19 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.1 Aprotinin versus Control | 17 | 1756 | Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.73, 0.29] |
| 9.2 Tranexamic Acid versus Control | 5 | 434 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.34, 0.18] |
10.1. Analysis.
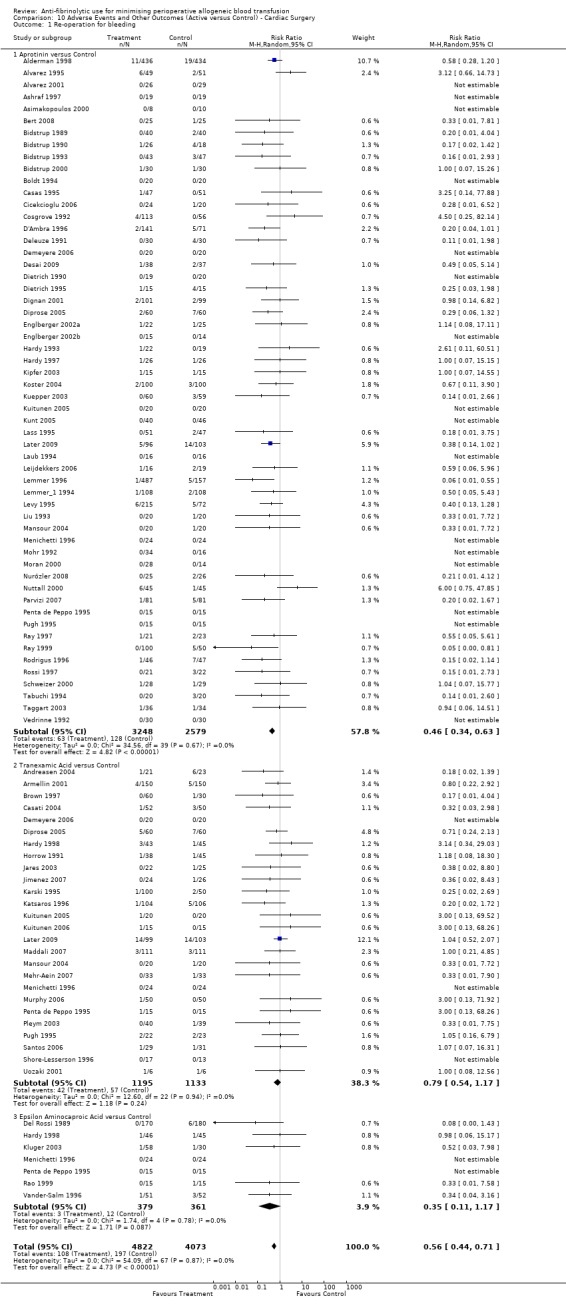
Comparison 10 Adverse Events and Other Outcomes (Active versus Control) ‐ Cardiac Surgery, Outcome 1 Re‐operation for bleeding.
10.2. Analysis.
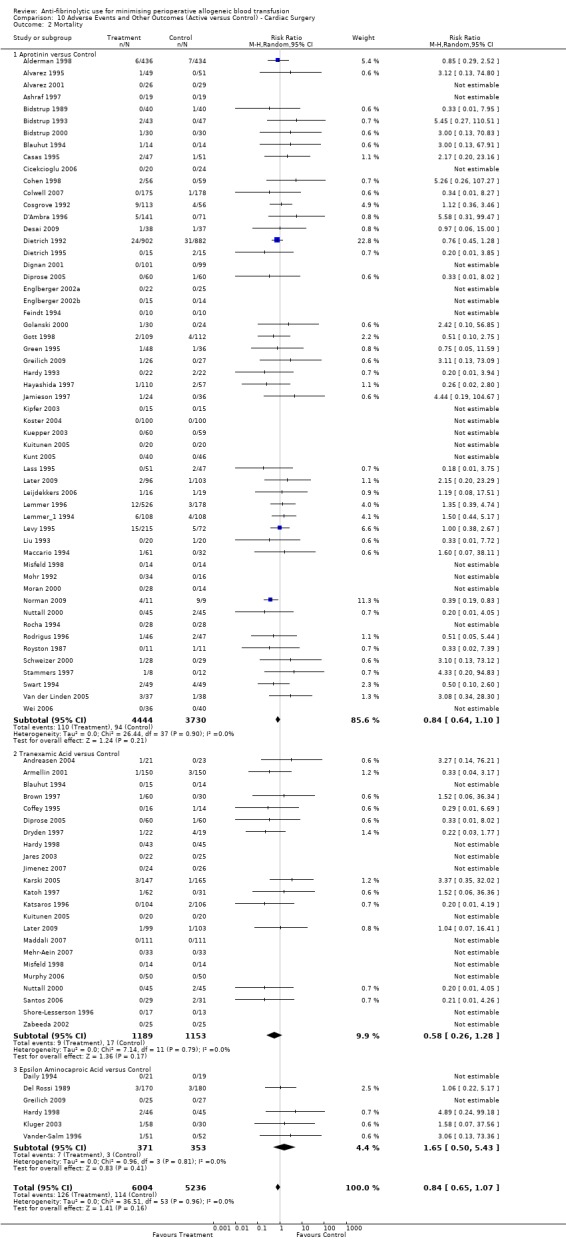
Comparison 10 Adverse Events and Other Outcomes (Active versus Control) ‐ Cardiac Surgery, Outcome 2 Mortality.
10.3. Analysis.
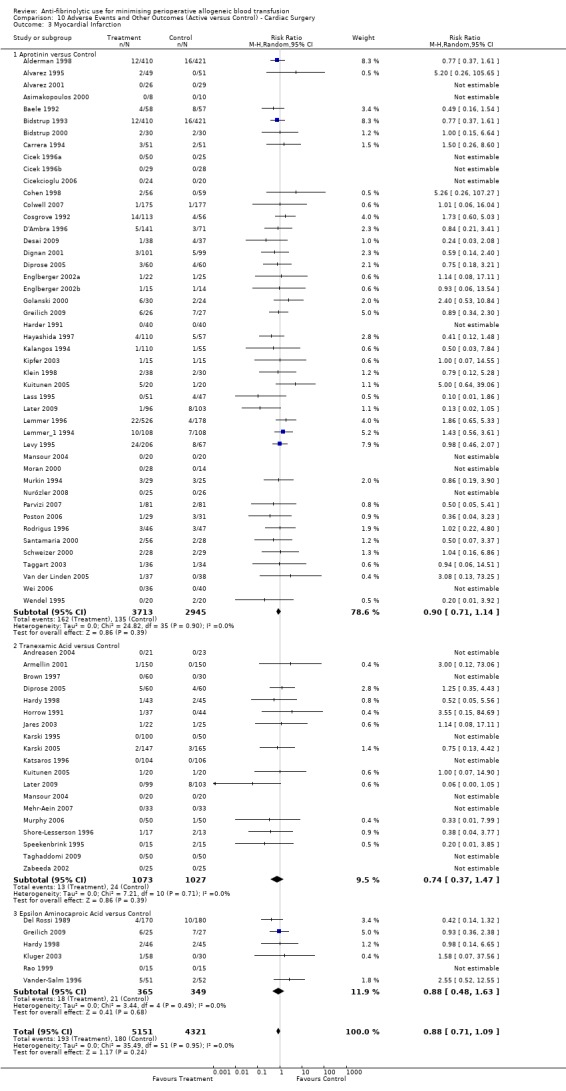
Comparison 10 Adverse Events and Other Outcomes (Active versus Control) ‐ Cardiac Surgery, Outcome 3 Myocardial Infarction.
10.4. Analysis.
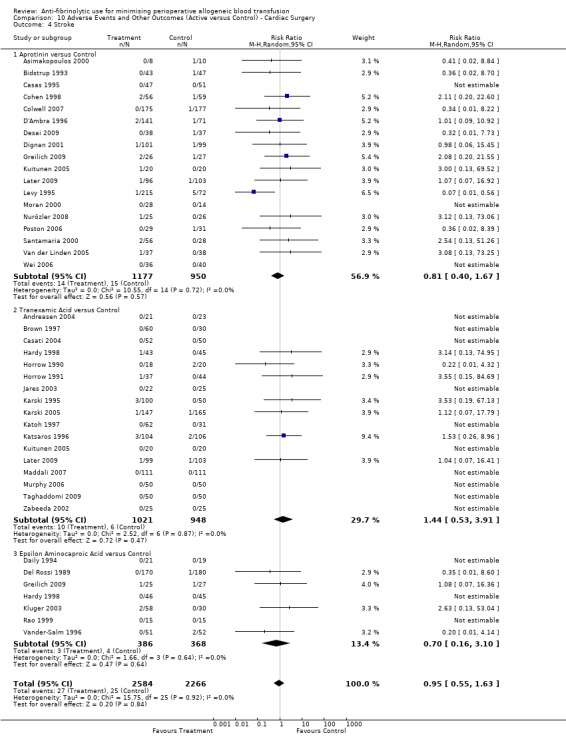
Comparison 10 Adverse Events and Other Outcomes (Active versus Control) ‐ Cardiac Surgery, Outcome 4 Stroke.
10.5. Analysis.
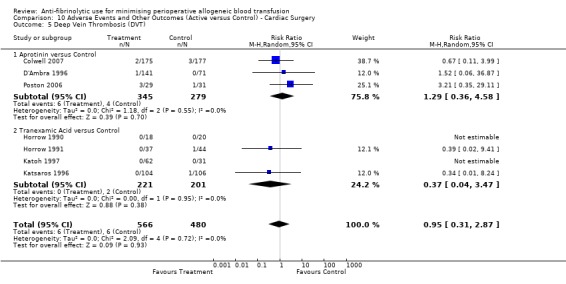
Comparison 10 Adverse Events and Other Outcomes (Active versus Control) ‐ Cardiac Surgery, Outcome 5 Deep Vein Thrombosis (DVT).
10.6. Analysis.
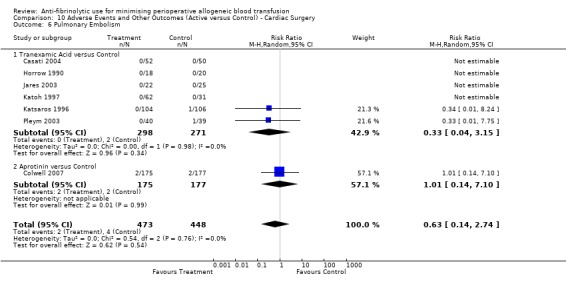
Comparison 10 Adverse Events and Other Outcomes (Active versus Control) ‐ Cardiac Surgery, Outcome 6 Pulmonary Embolism.
10.7. Analysis.
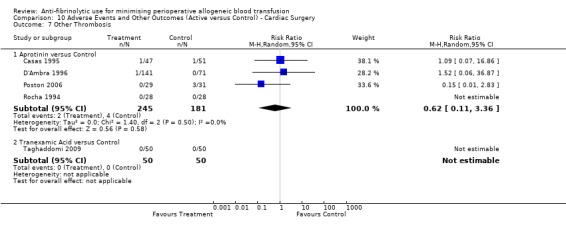
Comparison 10 Adverse Events and Other Outcomes (Active versus Control) ‐ Cardiac Surgery, Outcome 7 Other Thrombosis.
10.8. Analysis.
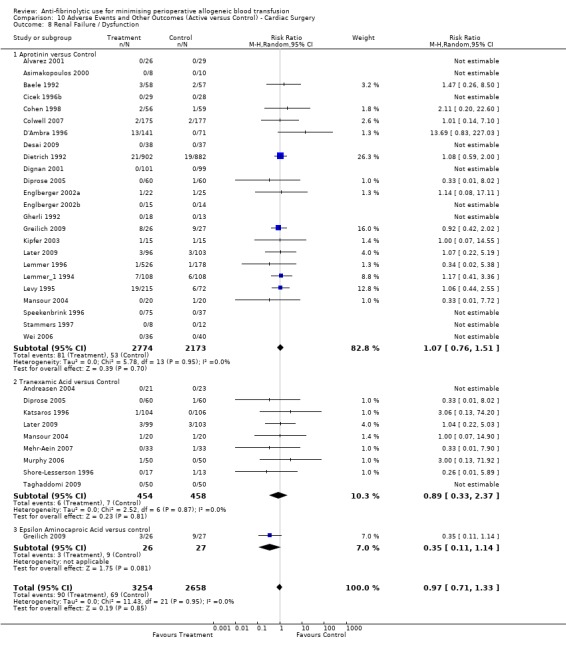
Comparison 10 Adverse Events and Other Outcomes (Active versus Control) ‐ Cardiac Surgery, Outcome 8 Renal Failure / Dysfunction.
10.9. Analysis.
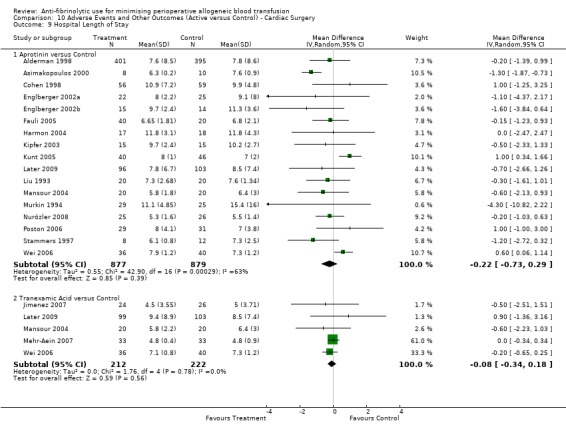
Comparison 10 Adverse Events and Other Outcomes (Active versus Control) ‐ Cardiac Surgery, Outcome 9 Hospital Length of Stay.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alajmo 1989.
| Methods | Patients were randomly divided into two groups according to birth date until an appropriate number of treated patients was reached. Method of blinding and generation of allocation sequences were not described. | |
| Participants | 34 consecutive patients undergoing cardiac operations were randomly divided into two groups:
NB: Possible error in the gender data provided for the aprotinin group. |
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, blood loss, haemoglobin levels, platelet counts | |
| Notes | Quality assessment score (Schulz criteria): 2/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | Patients were randomly allocated into two groups according to birth date until an appropriate number of treated patients was reached |
| Allocation concealment? | High risk | Inadequate |
| Blinding? All outcomes | High risk | |
Alderman 1998.
| Methods | Patients were randomly divided into two groups by random code, generated in blocks with clinical center and stratum. Allocation concealment was not described. | |
| Participants | 870 patients were randomised into two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, blood loss, deaths, myocardial infarction, CABG thrombosis, re‐operation for bleeding. | |
| Notes | Quality assessment score (Schulz criteria): 3/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Random code generated in blocks |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Alvarez 1995.
| Methods | The hospital pharmacy made up identical infusions of the study drugs identifiable only by random number. Patients were prospectively randomised into two groups by sealed envelopes. The method used to generate allocation sequences was not described. | |
| Participants | 100 patients undergoing primary elective cardiac surgery were randomised into one of two groups:
|
|
| Interventions |
NB: Both the intervention and control group were combined with cell salvage. |
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, patients receiving autotransfusion, blood loss, mortality, myocardial infarctions, re‐operation, patients receiving cell salvage. | |
| Notes | Quality assessment score (Schulz criteria): 5/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | High risk | Inadequate ‐ sealed envelopes were used to conceal treatment allocation |
| Blinding? All outcomes | Low risk | Double blind |
Alvarez 2001.
| Methods | Patients were randomised by a computer‐generated random number sequence into either treatment group. All clinical participants were double blinded until the completion of the trial. Placebo and treatment solutions were identical in their appearance and packaging. | |
| Participants | 55 patients undergoing either elective or urgent cardiac surgery were randomised into one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, patients receiving autotransfusion, blood loss, mortality, myocardial infarctions, re‐operation. | |
| Notes | Quality assessment score (Schulz criteria): 7/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated random number sequence |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Alvarez 2008.
| Methods | Patients were allocated according to a computer‐generated randomisation sequence. Allocation was concealed using sealed, numbered envelopes. | |
| Participants | 95 patients undergoing orthopaedic (knee arthroplasty) surgery. Patients were randomly allocated to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients requiring blood transfusion, blood loss, volume of blood transfused, thrombosis. | |
| Notes | Quality assessment score (Schulz criteria): 5/7 Transfusion protocol used |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated random number sequence |
| Allocation concealment? | High risk | Inadequate ‐ randomised assignment was sealed in a numbered envelope |
| Blinding? All outcomes | Low risk | Double blind |
Amar 2003.
| Methods | Randomisation of patients in blocks of 20 were done by the Biostatistics Department and the hospital pharmacy using sealed, opaque treatment code envelopes. | |
| Participants | 69 patients undergoing elective orthopaedic surgery were randomised into one of three groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood units ‐ total includes intra‐operative & 48 hours post‐operative, blood loss ‐ total blood loss = intra‐operative & 48 hours post‐operative, deep venous thrombosis, pulmonary embolus, hospital length of stay (days), wound infection, thrombocytopenia, Haemoglobin levels (pre‐operative & post‐operative). | |
| Notes | Quality assessment score (Schulz criteria): 6/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated random numbers |
| Allocation concealment? | High risk | Inadequate ‐ sealed opaque treatment coded envelopes were used to conceal treatment allocation |
| Blinding? All outcomes | Low risk | Double blind |
Andreasen 2004.
| Methods | Patients were randomised by a random number sequence. The randomisation schedule was provided in sealed envelopes and preparation of the drug or placebo was carried out just prior to anaesthesia by a staff member not involved in the treatment of the patient. | |
| Participants | 46 patients undergoing elective coronary surgery. Patients were randomly allocated to one of two groups:
|
|
| Interventions |
NB: Cell salvage ‐ postoperatively shed mediastinal blood was returned in all patients using a closed autotransfusion system. |
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, blood loss, deaths, myocardial Infarctions, CABG thrombosis, renal insufficiency, re‐operation for bleeding, cell salvage ‐ autotransfusion 6 hrs, transient ischemic attack (30 day), stroke 30 day. | |
| Notes | Quality assessment score (Schulz criteria): 4/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Random number sequence |
| Allocation concealment? | High risk | Inadequate ‐ used sealed envelopes to conceal treatment allocation |
| Blinding? All outcomes | Low risk | Double blind |
Apostolakis 2008.
| Methods | A randomisation table was used to generate the allocation sequence. No information was provided regarding allocation concealment. | |
| Participants | 59 patients undergoing elective thoracic surgery. Patients were randomly allocated to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Blood loss, volume of transfused blood (units), mortality, re‐operation for bleeding, length of hospital stay (days). | |
| Notes | Quality assessment score (Schulz criteria): 5/7 Transfusion protocol used |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Random number table |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Armellin 2001.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 300 patients undergoing elective cardiac surgery were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), blood loss, mortality, myocardial infarction, re‐operation for bleeding, hospital length of stay (days), fresh frozen plasma (FFP), platelets (units). | |
| Notes | Quality assessment score (Schulz criteria): 3/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not reported |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Arom 1994.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 200 patients undergoing cardiac surgery were randomised to one of two groups:
|
|
| Interventions |
NB: Both groups received 0.03ug/kg of intravenous desmopressin (DDAVP) after CPB. |
|
| Outcomes | Outcomes reported: Allogeneic blood usage (units), fresh frozen plasma usage (units), platelet usage (units), cryoprecipitate (units), blood loss (ml). | |
| Notes | Quality assessment score (Schulz criteria): 2/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Unclear risk | Unclear |
Ashraf 1997.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 38 patients undergoing coronary artery bypass graft surgery were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), blood loss 24hrs, mortality, re‐exploration for bleeding, pro‐inflammatory cytokine levels. | |
| Notes | Quality assessment score (Schulz criteria): 2/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Unclear risk | Unclear |
Asimakopoulos 2000.
| Methods | Method of randomisation and allocation concealment not specified. | |
| Participants | 18 adults undergoing elective coronary artery bypass grafting were randomly allocated to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Allogeneic blood (units), blood loss, myocardial infarction, renal failure, re‐operation for bleeding, cerebrovascular accident (stroke), hospital length of stay (days). | |
| Notes | Quality assessment score (Schulz criteria): 4/7 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Baele 1992.
| Methods | Method of randomisation was not described. Allocation concealment was inadequately concealed (sealed envelopes). | |
| Participants | 115 consecutive adults undergoing cardiac surgery were randomly allocated to one of two groups:
|
|
| Interventions |
NB: Both the intervention and control groups were exposed to pre‐operative autologous donation (7 control and 4 intervention patients), acute normovolemic haemodilution (13 patients in each group), and/or cell salvage (data not presented). |
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood (units) blood loss, mortality, myocardial infarction, myocardial ischemia, pericarditis, cardiac failure, pneumonia, renal insufficiency, hemiplegia, re‐operation, allogeneic + autologous blood usage (units), intensive care unit (ICU) length of stay (hrs), hospital length of stay (days). | |
| Notes | Quality assessment score (Schulz criteria): 3/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not reported |
| Allocation concealment? | High risk | Inadequate ‐ sealed envelopes were used to conceal treatment allocation |
| Blinding? All outcomes | Unclear risk | Unclear |
Bailey 1994.
| Methods | Generation of allocation sequences was by a computer generated random number table. One investigator made up all the test solutions; a known volume of sterile 0.9% saline was discarded from 500ml bags and replaced with the same volume of test solution so that all bags contained the same equal volume (500ml). Each set of bags was given a consecutive number. A separate investigator performed all the patient measurements. | |
| Participants | 100 patients scheduled to undergo primary elective cardiac surgery employing cardiopulmonary bypass were consecutively allocated to one of four groups.
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood (units), fresh frozen plasma usage (units), blood loss. | |
| Notes | Quality assessment score (Schulz criteria): 7/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Generation of allocation sequences was by a computer generated random number table |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Basora 1999.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 59 patients undergoing elective cardiac surgery were randomised to one of three groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, blood loss, platelet function. | |
| Notes | Quality assessment score (Schulz criteria): 2/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not reported |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | High risk | Single blind |
Bennett‐Guerrero 1997.
| Methods | Patients were randomised by means of a computer‐generated schedule. Study drug was prepared according to a protocol by hospital pharmacies. | |
| Participants | 204 patients undergoing repeat cardiac surgery were randomised to one of two groups:
|
|
| Interventions |
NB: Both groups were exposed to cell salvage. |
|
| Outcomes | Outcomes reported: Number of participants exposed to allogeneic blood, allogeneic blood usage (units), fresh frozen plasma usage (units), platelet usage (units), blood loss, re‐operation for bleeding. | |
| Notes | Quality assessment score (Schulz criteria): 7/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Patients were randomised by means of a computer‐generated schedule |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Benoni 1996.
| Methods | Randomisation into blocks of 12 was done by an independent pharmacologist who was not otherwise engaged in the study. Pairs of ampoules, each containing 10ml of either the active substance or the placebo were numbered and packed into envelopes which were opened by the anaesthetist before administration. These ampoules could be identified only by their numbers, and the randomisation code was known only to the independent pharmacologist. The code was not broken until the end of the study and until all data had been corrected and included in the database. Ten patients were excluded from the study after randomisation. | |
| Participants | 96 patients undergoing total knee arthroplasty were randomly allocated to one of two groups:
|
|
| Interventions |
NB: 15 patients from the placebo group received an extra dose of TXA for severe post‐operative bleeding, these patients represented the 'placebo‐extra' group. |
|
| Outcomes | Outcomes reported: Number of participants exposed to allogeneic blood, allogeneic blood usage (units), blood loss, deep venous thrombosis, pulmonary embolus, wound haematomas, chest pain, haemoglobin concentrations. | |
| Notes | Quality assessment score (Schulz criteria): 4/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Adequate |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Benoni 2000.
| Methods | Medication was administered using numbered ampoules and the randomisation was performed by a pharmacist not otherwise engaged in the study. | |
| Participants | 40 patients undergoing total hip arthroplasty were randomly assigned to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of participants exposed to allogeneic blood, allogeneic blood usage (units), blood loss, amount of pre‐operative autologous donated blood (2 units), infection. | |
| Notes | Quality assessment score (Schulz criteria): 5/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Benoni 2001.
| Methods | Method of randomisation was not described. Medication was concealed by a code only known by the hospitals chief pharmacist who was not involved in the study. | |
| Participants | 40 patients undergoing total hip arthroplasty were randomly assigned to one of two treatments groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of participants exposed to allogeneic blood, allogeneic blood usage (units), pulmonary embolus. | |
| Notes | Quality assessment score (Schulz criteria): 5/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Low risk | Adequate ‐ medication was concealed by a code only known by the hospitals chief pharmacist who was not involved in the study |
| Blinding? All outcomes | Low risk | Double blind |
Berenholtz 2009.
| Methods | Patients were randomised according to a computer‐generated randomisation schedule. Allocation was concealed through central (pharmacy) allocation. | |
| Participants | 182 patients undergoing orthopaedic surgery were randomly allocated to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients receiving blood transfusion, volume of blood transfused (units), blood loss, mortality, pulmonary embolism, myocardial infarction, renal failure, stroke, thrombosis, deep vein thrombosis, length of hospital stay (days). | |
| Notes | Quality assessment score (Schulz criteria): 5/7 Transfusion protocol used |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Patients were randomised according to a computer‐generated randomisation schedule |
| Allocation concealment? | Low risk | Adequate ‐ allocation was concealed through central (pharmacy) allocation |
| Blinding? All outcomes | Low risk | Double blind |
Bernet 1999.
| Methods | Random code used for randomisation. Drug solutions were prepared by the hospital pharmacy. | |
| Participants | 70 patients were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), fresh frozen plasma (units), platelets (units), blood loss, mortality, myocardial Infarctions, haematocrit levels, stroke, thrombotic complications, re‐exploration for bleeding. | |
| Notes | Quality assessment score (Schulz criteria): 4/7 Transfusion protocol used. All patients received ASA until the day of the operation (100mg/day). All patients received cell salvage (Imed 960) ‐ 8 hours post‐operatively. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Random code used for randomisation |
| Allocation concealment? | Unclear risk | Adequate ‐ drug solutions were prepared by the hospital pharmacy |
| Blinding? All outcomes | Low risk | Double blind |
Bert 2008.
| Methods | A computer‐generated randomisation list was used to generate the allocation sequence. No information was provided regarding allocation concealment. | |
| Participants | 50 patients undergoing elective cardiac surgery were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Blood loss, volume of blood transfused (units), re‐operation for bleeding, inflammatory cytokines. | |
| Notes | Quality assessment score (Schulz criteria): 4/7 Transfusion protocol was not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | A computer‐generated randomisation list was used to generate the allocation sequence |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Unclear risk | Unclear |
Bidstrup 1989.
| Methods | The trial drug was provided by the manufacturer (Bayer AG, Leverkusen) in identical case packs, each of 12 bottles identifiable only by the random number. Method of generating allocation sequences was not described. | |
| Participants | 80 patients undergoing primary aorto‐coronary bypass grafting were randomised to either one of two groups:
|
|
| Interventions |
NB: Both intervention and control received preoperative autologous donation (PAD) |
|
| Outcomes | Outcomes reported: Number of participants exposed to allogeneic blood, allogeneic blood usage (units), blood loss (18 ‐24hrs), mortality. | |
| Notes | Quality assessment score (Schulz criteria): 5/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not reported |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Bidstrup 1990.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 44 patients undergoing aortocoronary artery bypass graft surgery were randomly allocated to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of participants exposed to allogeneic blood, allogeneic blood usage (units), fresh frozen plasma usage (units), platelets (units), blood loss (18‐24hrs), re‐operation for bleeding. | |
| Notes | Quality assessment score (Schulz criteria): 2/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not reported |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Unclear risk | Unclear |
Bidstrup 1993.
| Methods | Patients received aprotinin or placebo (normal saline) from identical bottles supplied by the manufacturer, identifiable only by their random number. Method of randomisation was not described. | |
| Participants | 96 adult male patients undergoing first‐time isolated coronary bypass grafting were randomised to either one of two groups:
NB: Six patients withdrew from the study, four in the aprotinin group and two in the placebo group. |
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Allogeneic blood usage (units), blood loss, haemoglobin levels, platelet counts, haemoglobin loss, activated clotting times, adverse events, graft patency, re‐operation for bleeding, wound infection. | |
| Notes | Quality assessment score (Schulz criteria): 5/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not reported |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Bidstrup 2000.
| Methods | Patients were allocated to receive either placebo or active treatment in accordance with a previously determined randomization schedule in a double blind fashion. Allocation concealment was adequate, active drug and placebo were contained in identical bottles, identifiable only by a random number. | |
| Participants | 60 patients undergoing aortocoronary bypass were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of participants exposed to allogeneic blood, mortality, myocardial infarction, re‐operation for bleeding, wound infection, neurologic disturbance, atrial fibrillation/flutter. | |
| Notes | Quality assessment score (Schulz criteria): 6/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not reported |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Blauhut 1994.
| Methods | Method of randomisation and allocation concealment were not described. No exclusions or loss to follow‐up reported. | |
| Participants | 45 patients undergoing cardiopulmonary bypass for coronary surgery were allocated at random to one of three groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of participants exposed to allogeneic blood, allogeneic blood usage (units), blood loss (24 hrs), mortality, platelet function, coagulation, haematocrit levels. | |
| Notes | Quality assessment score (Schulz criteria): 1/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not reported |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Unclear risk | Unclear |
Boldt 1991.
| Methods | Method of randomisation and allocation concealment were not described. No exclusions or loss to follow‐up reported. | |
| Participants | 30 male patients undergoing elective aortocoronary bypass grafting were randomised to one of three groups:
NB: Control group did not appear to be part of the randomised schedule. Possibly a non‐concurrent or historical control group. |
|
| Interventions |
NB: Only Group 2 and Group 3 were compared. |
|
| Outcomes | Outcomes reported: Number of participants exposed to allogeneic blood, allogeneic blood usage (units), blood loss (24hrs). | |
| Notes | Quality assessment score (Schulz criteria): 2/7 Transfusion protocol used. Study used neurosurgical patients as a control group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Unclear risk | Unclear |
Boldt 1994.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 40 patients undergoing cardiac surgery were randomised to one of two groups:
NB: Gender data were not reported |
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Allogeneic blood usage (units), fresh frozen plasma usage (units), blood loss (24hrs), re‐operation for bleeding, haemoglobin levels. | |
| Notes | Quality assessment score (Schulz criteria): 4/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Boylan 1996.
| Methods | Study agents were prepared by the hospital pharmacy using a randomisation schedule provided in sealed envelopes. The method used to generate allocation sequences was not described. | |
| Participants | 45 patients undergoing primary, isolated orthotopic liver transplantation were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Allogeneic blood usage (units), fresh frozen plasma usage (units), platelet usage (units), blood loss, mortality, portal vein thrombosis, hepatic thrombosis, hospital length of stay, intensive care unit (ICU) length of stay, overall donor exposure. | |
| Notes | Quality assessment score (Schulz criteria):5/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | High risk | Inadequate ‐ randomisation schedule was provided in sealed envelopes |
| Blinding? All outcomes | Low risk | Double blind |
Brown 1997.
| Methods | Method of allocation concealment was not described. Patients were randomised using a computer‐generated random number sequence. | |
| Participants | 91 patients scheduled for elective coronary revascularisation were randomly allocated to one of three groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of participants exposed to allogeneic blood, haematologic/thromboelastographic/coagulation characteristics, mortality, myocardial infarction, stroke, re‐exploration for bleeding, infection. | |
| Notes | Quality assessment score (Schulz criteria): 4/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Adequate ‐ computer‐generated random number sequence |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Caglar 2008.
| Methods | A computer‐generated randomisation list was used to generate the allocation sequence. No information was provided regarding allocation concealment. | |
| Participants | 100 female patients undergoing myomectomy were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: number of patients exposed to allogeneic blood, blood loss, volume of blood transfused. | |
| Notes | Quality assessment score (Schulz criteria): 5/7 Transfusion protocol used |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated randomisation |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Camarasa 2006.
| Methods | Randomisation was achieved by computer‐generated random numbers. The randomised assignment was sealed in an opaque, numbered envelope which was opened only by the nurse who prepared the solutions. This nurse was the only person who knew the patients study groups and did not participate in any other phase of the trial. | |
| Participants | 68 patients undergoing total knee replacement were randomised to one of two groups:
NB: One patient was excluded from the final analysis |
|
| Interventions |
NB: Both groups were exposed to cell salvage and pre‐operative autologous blood donation (PAD). |
|
| Outcomes | Outcomes reported: Number of participants exposed to allogeneic blood, allogeneic blood usage (units), blood loss, deep vein thrombosis. | |
| Notes | Quality assessment score (Schulz criteria): 5/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated random numbers |
| Allocation concealment? | High risk | Inadequate ‐ randomised assignment was sealed in an opaque, numbered envelope |
| Blinding? All outcomes | Low risk | Double blind |
Capdevila 1998.
| Methods | Randomisation was performed using a random number list generated by computer programme. Allocation was adequately concealed (administered fluids were prepared by the hospitals central pharmacy in identical 100‐ml bottles). | |
| Participants | 23 patients scheduled for orthopaedic surgery of the hip, femur or pelvis for sepsis or malignant tumours were randomly allocated to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), fresh frozen plasma usage (units), platelet usage (units), blood loss, haemoglobin and haematocrit levels, coagulation and fibrinolytic pathway explorations, allergic reactions. | |
| Notes | Quality assessment score (Schulz criteria): 7/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation was performed using a random number list generated by computer programme |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Carrera 1994.
| Methods | Randomisation and allocation concealment not specified. No exclusions or loss to follow‐up reported. [Spanish language] | |
| Participants | 102 patients undergoing cardiac surgery were randomly assigned to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of participants exposed to allogeneic blood, allogeneic blood usage, fresh frozen plasma usage, platelet usage, blood loss, myocardial infarction. | |
| Notes | Quality assessment score (Schulz criteria): 2/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Unclear risk | Unclear |
Casas 1995.
| Methods | Generation of allocation sequences was not specified. Allocation concealment was by sealed envelopes. The pharmacy prepared the encoded infusions. | |
| Participants | 149 patients scheduled to undergo either coronary artery bypass grafting (CABG), heart valve replacement or annuloplasty, combined valve replacement and CABG, or closure of atrial septal defects, were randomised to one of three groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), number of patients exposed to fresh frozen plasma, number of patients exposed to platelets, blood loss (24hrs), re‐operation for bleeding, femoral embolism, stroke. | |
| Notes | Quality assessment score (Schulz criteria): 4/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | High risk | Inadequate ‐ allocation concealment was by sealed envelopes |
| Blinding? All outcomes | Unclear risk | Double blind |
Casati 1999.
| Methods | Method ofrandomisation and allocation concealment were not described. | |
| Participants | 210 patients undergoing cardiac surgery were randomly assigned to one of three groups:
|
|
| Interventions |
NB: All groups were exposed to cell salvage. Pre‐operative autologous blood donation use was evenly distributed between groups. |
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units) blood loss, mortality, myocardial infarction, deep venous thrombosis, pulmonary embolus, pre‐operative autologous donation of blood, neurological complications, re‐operation for surgical bleeding. | |
| Notes | Quality assessment score (Schulz criteria): 1/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Unclear risk | Unclear |
Casati 2000.
| Methods | Patients were randomised into treatment groups by means of a computer generated random number sequence. Allocation concealment was not described. Trial was unblinded. | |
| Participants | 1040 patients undergoing primary elective cardiac operations were randomly assigned to one of two groups:
|
|
| Interventions |
NB: Both groups were exposed to cell salvage. |
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), blood loss, mortality, myocardial infarction, fresh frozen plasma usage, (units), platelet usage (units), pulmonary embolus. | |
| Notes | Quality assessment score (Schulz criteria): 3/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated random number sequence |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | High risk | |
Casati 2001.
| Methods | Method of randomisation was not described. Coded infusion syringes were used and prepared by a staff member not directly involved with perioperative clinical treatment. | |
| Participants | 40 patients undergoing elective cardiac surgery were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), blood loss. | |
| Notes | Quality assessment score (Schulz criteria): 6/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Casati 2002.
| Methods | Coded infusion syringes were used to conceal which medication was placebo and which was TXA. Method of randomisation was not described. | |
| Participants | 60 patients undergoing elective thoracic‐aorto surgery were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), number of patients exposed to fresh frozen plasma, number of patients exposed to platelets, blood loss (24hrs), re‐operation for bleeding, mortality, myocardial infarction, pulmonary embolus, pre‐operative aspirin, pre‐operative anticoagulant, stroke, hospital length of stay (days). | |
| Notes | Quality assessment score (Schulz criteria): 5/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Casati 2004.
| Methods | Randomisation was by means of two seperate computer‐generated random number sequences. Coded syringes were used to administer medication. | |
| Participants | 102 patients scheduled for cardiac surgery with either 'on‐pump' or 'off‐pump' procedures were randomised to one of four groups:
|
|
| Interventions |
NB: 'On‐pump' surgery patients (Groups 3 & 4) the remaining blood in the cardiopulmonary bypass (CPB) circuit and that blood aspirated from the surgical field was concentrated with a cell separator and reinfused. For 'Off‐pump' surgery patients only in cases of significant intra‐operative bleeding was the shed blood concentrated in a cell separator and reinfused. No autotransfusion of shed mediastinal blood was performed during the post‐operative period for any group. ONCAB‐Cell Salvage. Only in cases of significant intra‐operative bleeding was the shed blood concentrated in a cell separator and reinfused. No autotransfusion of shed mediastinal blood was performed during the post‐operative period. |
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), number of patients exposed to fresh frozen plasma, number of participants exposed to platelets, blood loss (24hrs), re‐exploration for bleeding, stroke, intra‐operative resternotomy, fresh frozen plasma usage (units), platelet usage (units). | |
| Notes | Quality assessment score (Schulz criteria): 7/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated random number sequences |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Cicek 1996a.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 75 patients scheduled for elective cardiac operations with cardiopulmonary bypass were randomly assigned to one of three groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), blood loss, myocardial infarction. | |
| Notes | Quality assessment score (Schulz criteria): 2/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Unclear risk | Unclear |
Cicek 1996b.
| Methods | Patients were randomised to receive aprotinin or placebo by means of a random numbers table. Method of allocation concealment was not described. | |
| Participants | 57 patients undergoing cardiac operations with cardiopulmonary bypass (CPB) were randomly assigned to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), blood loss. | |
| Notes | Quality assessment score (Schulz criteria): 5/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Random numbers table |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Cicekcioglu 2006.
| Methods | Method of randomisation and allocation concealment were not described. Data were collected in a blinded fashion. | |
| Participants | 44 patients undergoing coronary artery bypass grafting were randomly assigned to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, amount of allogeneic blood transfused (units), blood loss, mortality, length of hospital stay, post‐operative morbidity. | |
| Notes | Quality assessment score (Schulz criteria): 4/7 Transfusion protocol used |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Claeys 2007.
| Methods | Methods of sequence generation and allocation concealment were not described. | |
| Participants | 40 patients undergoing orthopaedic (hip) surgery were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients receiving blood transfusions, blood loss, deep vein thrombosis. | |
| Notes | Quality assessment score (Schulz criteria): 4/7 Transfusion protocol used |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Coffey 1995.
| Methods | Method of randomisation was not described. Pharmacy controlled the randomisation process. Method of allocation concealment was not clear. | |
| Participants | 30 patients undergoing cardiac surgery were randomised to either one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, blood loss, mortality. | |
| Notes | Quality assessment score (Schulz criteria): 6/7 Transfusion protocol not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Cohen 1998.
| Methods | Patients were randomised by the hospital pharmacy after stratification and blocking in groups of six. The pharmacy supplied bags that contained dipyridamole (DIP), aprotinin (APR) or a saline placebo. | |
| Participants | 115 patients undergoing cardiac operations for valve replacement or myocardial revascularization, or a combined procedure were randomised to one of two groups:
|
|
| Interventions |
NB: All patients were administered dipyridamole (DIP) orally (100mg four times daily for three or more doses pre‐operatively) and intravenously (at a rate of 0.24mg/kghr beginning before anaesthesia induction and continuing for 1 hour post‐operatively). Autologous blood shed into sterile cardiotomy reservoirs from chest drains was autotransfused to the patient when drainage exceeded 150ml during the first 4 hours post‐operatively. |
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, blood loss, mortality, myocardial infarction, autologous shed blood transfused, blood loss (24 hrs), renal failure, stroke, intensive care unit length of stay (days), hospital length of stay (days). | |
| Notes | Quality assessment score (Schulz criteria): 5/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Colwell 2007.
| Methods | Patients were randomly assigned on the day of surgery to a treatment group in a 1:1 ration from a computer‐generated list managed by an interactive voice response system. Aprotinin and placebo were provided to the pharmacy in the same packaging and were dispensed by the randomisation assignment, blinding the patient and staff to the actual treatment group. The primary efficacy analysis was performed on the intention‐to‐treat population. | |
| Participants | 359 patients undergoing orthopaedic surgery (hip arthroplasty) were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients receiving blood transfusions, volume of blood transfused (units), blood loss, deep vein thrombosis, renal failure, myocardial infarction, stroke, mortality, pulmonary embolism. | |
| Notes | Quality assessment score (Schulz criteria): 6/7 Transfusion protocol used |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated list |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Corbeau 1995.
| Methods | Method of randomisation and allocation concealment were not described. Two patients were excluded after randomisation. [French language] | |
| Participants | 104 adults undergoing either coronary artery bypass grafting (CABG) or aortic valve replacement (AVR) were randomised to one of three groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), blood loss. | |
| Notes | Quality assessment score (Schulz criteria): 1/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Unclear risk | Unclear |
Cosgrove 1992.
| Methods | Method of randomisation and allocation concealment were not described. All subjects were included in the final analysis. | |
| Participants | 169 patients undergoing isolated re‐operative myocardial revascularisation were randomised to either one of three groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), mortality, myocardial infarction, re‐operation for bleeding, fresh frozen plasma usage (units), platelet usage (units). | |
| Notes | Quality assessment score (Schulz criteria): 4/7 Transfusion protocol not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Cvachovec 2001.
| Methods | Method of randomisation and allocation concealment were not described in the abstract. [Czech Republic] | |
| Participants | 42 patients undergoing total hip arthroplasty were randomly allocated to one of two groups:
NB: No age data were reported |
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), blood loss. | |
| Notes | Foreign language paper Transfusion protocol used |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Unclear risk | Unclear |
D'Ambra 1996.
| Methods | A separate random code, using blocks of six, was generated for each site by the statistical department of Bayer Incorporated. The study medication for each patient was supplied for each patient in a case pack containing 14 vials. The loading dose vials, pump prime vials, and constant infusion vials were separately identified and packaged within the pack for each patient. Investigators were blinded to the identity and lot number of each case pack. | |
| Participants | 213 patients undergoing cardiac surgery were enrolled and randomised at the five sites to one of three groups:
NB: Of the 213 patients enrolled and randomised, 212 were included in the safety analysis and 191 were included in a primary analysis of efficacy. |
|
| Interventions |
NB: Blood conservation measures were used for all groups. These measures included the reinfusion of post‐operative mediastinal shed blood (cell salvage) and the pre‐operative donation of autologous blood (PAD). Epsilon aminocaproic acid (EACA) and desmopressin (DDAVP) were used to treat active bleeding after the reversal of heparin. |
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), fresh frozen plasma usage (units), platelet usage (units), mortality, myocardial infarction, re‐operation, renal dysfunction, deep vein thrombosis, cardiovascular complications, cerebrovascular accident. | |
| Notes | Quality assessment score (Schulz criteria): 6/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Random code generated for each site by the statistical department of Bayer |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
D'Ambrosio 1999.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 60 patients undergoing total hip arthroplasty were randomised into one of four groups: Comparison 1:
Comparison 2:
|
|
| Interventions |
Comparison 1:
Comparison 2:
NB: All subjects were exposed to pre‐operative autologous blood donation and cell savage. |
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, blood loss, allogeneic & autologous blood usage (units). | |
| Notes | Quality assessment score (Schulz criteria): 2/7 No transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Daily 1994.
| Methods | Method of randomisation was not described. Epsilon‐aminocaproic acid (EACA) and placebo were delivered to the operating room in numbered, but otherwise identical vials labelled "study drug". | |
| Participants | 40 patients undergoing first‐time coronary artery bypass grafting without prior sternotomy were randomised to one of two groups:
|
|
| Interventions |
NB: Both groups were exposed to cell salvage. |
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, blood loss (12/24hrs), myocardial infarction, stroke (cerebrovascular accident), use of shed mediastinal blood. | |
| Notes | Quality assessment score (Schulz criteria): 6/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Dalmau 1999.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 124 patients undergoing orthotopic liver transplantation were randomised to one of three groups:
NB: No age or gender data were reported. |
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), fresh frozen plasma usage (units), platelet usage (units), thrombotic events, cryoprecipitate (units). | |
| Notes | Quality assessment score (Schulz criteria): 4/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Dalmau 2000.
| Methods | Drugs were prepared then randomised to patients using a randomisation schedule provided in sealed envelopes. | |
| Participants | 132 patients undergoing orthotopic liver transplantation (OLT) were randomly assigned to one of three groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), fresh frozen plasma (units), platelets (units), other arterial thrombosis, prophylactic DDAVP treatment, DDAVP treatment for bleeding, EACA treatment (clinical fibrolysis). | |
| Notes | Quality assessment score (Schulz criteria): 4/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | High risk | Inadequate ‐ sealed envelopes were used to conceal treatment allocation |
| Blinding? All outcomes | Low risk | Double blind |
Dalmau 2004.
| Methods | Drugs were prepared and then randomised to patients using a randomisation schedule provided in sealed envelopes. Method of randomisation was not described. | |
| Participants | 127 patients undergoing orthotopic liver transplantation were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Allogeneic blood usage (units), fresh frozen plasma (units), platelets (units), mortality, myocardial infarction, DDAVP pre‐operative administration, EACA intra‐operative administration, any thrombosis, re‐operation for bleeding, renal failure. | |
| Notes | Quality assessment score (Schulz criteria): 4/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | High risk | Inadequate ‐ sealed envelopes were used to conceal treatment allocation |
| Blinding? All outcomes | Low risk | Double blind |
Defraigne 2000.
| Methods | Randomisation was accomplished using a random number table. Sealed envelopes were used to conceal treatment allocation. | |
| Participants | 200 patients undergoing cardiac surgery were randomly allocated to one of four groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Allogeneic blood usage (units), fresh frozen plasma usage (units), platelet usage (units), blood loss. | |
| Notes | Quality assessment score (Schulz criteria): 6/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Random number table |
| Allocation concealment? | High risk | Inadequate ‐ sealed envelopes were used to conceal treatment allocation |
| Blinding? All outcomes | High risk | Single blind |
Del Rossi 1989.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 350 patients undergoing elective coronary artery bypass surgery, repair of myocardial aneurysms, valve replacement or combined procedures were randomly assigned to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Allogeneic blood usage (units), blood loss (24hrs), mortality, myocardial infarction, re‐operation for bleeding, stroke, graft failure. | |
| Notes | Quality assessment score (Schulz criteria): 4/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Deleuze 1991.
| Methods | Pharmaceutical company supplied the study drugs in identical bottles, identifiable only by number. The method of generating allocation sequences was not described. [French] | |
| Participants | 60 coronary patients undergoing at least two aorto‐coronary bypass grafts for the first time were randomised to one of two groups:
|
|
| Interventions |
NB: One active flask contained 70mg (500,000 KIU) of aprotinin in 50mls of physiological serum. One placebo flask contained an equivalent quantity of physiological serum. |
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), blood loss (48 hrs), re‐operation. | |
| Notes | Quality assessment score (Schulz criteria): 4/7 Transfusion protocol used French article ‐ translated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Demeyere 2006.
| Methods | Method of sequence generation and allocation concealment were not described. [Poster presentation] | |
| Participants | 60 patients undergoing cardiac surgery were randomised to one of three groups:
NB: Demographic data were not reported. |
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Transfusion of blood products, blood loss, re‐operation for bleeding. | |
| Notes | Quality assessment score (Schulz criteria): 2/7 Transfusion protocol not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Unclear risk | Unclear |
Desai 2009.
| Methods | Methods of sequence generation and allocation concealment were unclear. | |
| Participants | 75 patients undergoing cardiac surgery were randomised to one of two groups:
NB: Demographic data were not reported. |
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients receiving blood transfusion, blood loss, myocardial infarction, renal failure, mortality, re‐operation for bleeding. | |
| Notes | Quality assessment score (Schulz criteria): 3/7 Transfusion protocol used |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Dietrich 1990.
| Methods | Method of randomisation was not described. Aprotinin and placebo were provided by the manufacturer (Bayer AG, Leverkusen, FRG) in identical packages, each containing 12 bottles that could only be identified by the random number. One patient from the aprotinin trial arm was excluded from the final analysis. | |
| Participants | 40 patients scheduled for elective primary myocardial revascularisation were randomised to one of two groups:
NB: Gender data were not reported. |
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic whole blood usage (ml/units), fresh frozen plasma usage (units), platelet usage (units), blood loss, re‐operation for bleeding. | |
| Notes | Quality assessment score (Schulz criteria): 5/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Dietrich 1992.
| Methods | Method of randomisation and allocation concealment were not described. No exclusions reported. | |
| Participants | 1784 adult patients undergoing primary coronary artery bypass grafting, valve replacement (or combined procedures), and cardiac reoperations, were randomly assigned to one of two groups:
|
|
| Interventions |
NB: Both groups were exposed to cell salvage. |
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage, blood loss (36 hrs), mortality, intensive care unit length of stay (days), re‐operation, renal failure, hypotension. | |
| Notes | Quality assessment score (Schulz criteria): 2/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Unclear risk | Unclear |
Dietrich 1995.
| Methods | Patients were independently randomised, using a table of random numbers, to either aprotinin or control group. Aprotinin and placebo were provided by the manufacturer (Bayer AG, Leverkusen, Germany) in identical packages each containing 14 bottles, that could only be identified by the random number. No loss to follow‐up reported. | |
| Participants | 30 male patients scheduled for elective primary coronary revascularisation were randomly assigned to one of two groups:
|
|
| Interventions |
NB: Both groups were exposed to cell salvage. |
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), blood loss, complications, re‐operation, mortality. | |
| Notes | Quality assessment score (Schulz criteria): 7/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Table of random numbers |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Dietrich 2008.
| Methods | Computer‐generated randomisation list and central allocation were used. | |
| Participants | 220 patients undergoing cardiac surgery were randomly allocated to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients receiving blood transfusion, volume of blood transfused (units), mortality, renal failure, length of hospital stay (days). | |
| Notes | Quality assessment score (Schulz criteria): 7/7 Transfusion protocol used |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated randomisation list |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Dignan 2001.
| Methods | Study drug was administered by the anesthesiologist as an infusion in a blinded fashion. Allocation concealment was not described. | |
| Participants | 200 participants undergoing elective cardiac surgery were randomly assigned to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic whole blood usage (units/mls), fresh frozen plasma usage (units), platelet usage (units), blood loss, re‐operation for bleeding, acute renal failure, stroke. | |
| Notes | Quality assessment score (Schulz criteria): 3/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Diprose 2005.
| Methods | Computer generated random numbers determined patient allocation to one of three treatment groups. Sealed envelopes were used to conceal treatment allocation. | |
| Participants | 186 patients undergoing elective cardiac surgery were randomly assigned to one of three groups:
|
|
| Interventions |
NB: All groups received intra‐operative cell salvage (Compact A; Dideco, Sorin Biomedica, Italy) and each group received a test dose of 5ml of study solution. |
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), blood loss, mortality, myocardial infarction, re‐operation for bleeding, renal failure, respiratory failure. | |
| Notes | Quality assessment score (Schulz criteria): 5/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated random numbers determined patient allocation |
| Allocation concealment? | High risk | Inadequate |
| Blinding? All outcomes | Low risk | Double blind |
Dorman 2008.
| Methods | Method of sequence generation and allocation concealment were not described. | |
| Participants | 60 patients undergoing cardiac surgery were randomly allocated to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients receiving blood transfusion, volume blood transfused (units), blood loss. | |
| Notes | Quality assessment score (Schulz criteria): 2/7 Transfusion protocol used |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Unclear risk | Unclear |
Dryden 1997.
| Methods | Method of randomisation was not described. The "study drug" was mixed by independent Intensive Care Unit (ICU) personnel. | |
| Participants | 41 patients undergoing re‐operative cardiac valve surgery were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Allogeneic blood usage (mls), blood loss, total platelets transfused, total plasma transfused, mortality, hospital length of stay (days), hospital complications, re‐operation for active bleeding. | |
| Notes | Quality assessment score (Schulz criteria): 5/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | High risk | Single blind |
Eberle 1998.
| Methods | Different treatment solutions were identical in appearance. Method of randomisation was not described. | |
| Participants | 40 patients undergoing cardiac surgery were randomised to one of three groups:
NB: No gender data were reported. |
|
| Interventions |
NB: Both EACA and aprotinin groups received cell salvage. |
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), blood loss, myocardial Infarction. | |
| Notes | Quality assessment score (Schulz criteria): 4/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Ehrlich 1998.
| Methods | Each bottle of aprotinin provided by the pharmaceutical company was placed in a box with bottles of normal saline solution. These bottles were indistinguishable from one another. An assistant, who was only involved in randomisation of the medication, arranged these bottles into 50 pairs. Each pair consisted of two bottles of aprotinin or two bottles of saline solution. Each of these pairs was randomly assigned a number from 1 to 50. Each patient was randomly assigned a number and then given the corresponding bottles. After the study was completed, the randomisation code was broken and the data were analysed. | |
| Participants | 50 patients undergoing thoracic aortic operations with the use of profound hypothermic circulatory arrest were randomly assigned to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Allogeneic blood usage (units), blood loss, mortality (30‐day), myocardial infarction, renal dysfunction/renal failure, re‐operation for bleeding, neurological deficit, stroke. | |
| Notes | Quality assessment score (Schulz criteria): 6/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Ekback 2000.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 40 patients undergoing elective adult surgery and were randomised to one of two groups:
|
|
| Interventions |
NB: All study participants underwent pre‐operative autologous blood donation (2 units autologous blood donated) on two occasions within a four week period. Both trial arms were equally exposed to cell salvage. |
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, blood loss, deep vein thrombosis. | |
| Notes | Quality assessment score (Schulz criteria): 4/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Ellis 2001.
| Methods | Randomisation was carried out using a computer generated randomisation table. Allocation concealment was not described. | |
| Participants | 30 patients were randomly assigned to one of three groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogenic blood usage (units), hospital length of stay (days). | |
| Notes | Quality assessment score (Schulz criteria): 3/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation was carried out using a computer generated randomisation table |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Engel 2001.
| Methods | Method of randomisation and allocation concealment was not described. | |
| Participants | 36 patients were randomised to one of three groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), blood loss, deep vein thrombosis. | |
| Notes | Quality assessment score (Schulz criteria): 2/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | High risk | Unblinded |
Englberger 2002a.
| Methods | Bottles of aprotinin and saline (placebo solution) were numbered continuously. Blinding of bottles was performed by personal otherwise not involved in the study. Method of randomisation was not described. | |
| Participants | 47 patients undergoing elective 'off‐pump' cardiac surgery were randomly allocated to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, number of patients exposed to fresh frozen plasma, blood loss, mortality, myocardial infarction, re‐operation for bleeding, number of patients exposed to autotransfusion, volume of blood autotransfused, hospital length of stay (days), neurological deficit, renal dysfunction. | |
| Notes | Quality assessment score (Schulz criteria): 5/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Englberger 2002b.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 29 patients undergoing elective cardiac surgery were randomly allocated to one of two groups:
|
|
| Interventions |
NB: Both groups were exposed to cell salvage. |
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), number of patients exposed to fresh frozen plasma, fresh frozen plasma usage (units), blood loss, mortality, myocardial infarction, re‐operation for bleeding, neurological deficit, renal dysfunction, hospital length of stay (days), number of patients exposed to autotransfusion, volume of blood autotransfused. | |
| Notes | Quality assessment score (Schulz criteria): 2/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Unclear risk | Unclear |
Fauli 2005.
| Methods | Method of randomisation was not described. All patients received pre‐prepared infusions of similar volume and appearance provided by the pharmaceutical company. | |
| Participants | 60 patients undergoing cardiac surgery were randomised to one of three groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Allogeneic blood usage (units), blood loss (24hrs), hospital length of stay (days). | |
| Notes | Quality assessment score (Schulz criteria): 6/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Feindt 1994.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 20 patients undergoing aortocoronary bypass surgery were randomly allocated to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood transfusion, post‐operative blood loss, mortality, parameters of thrombin activation and fibrinolysis. | |
| Notes | Quality assessment score (Schulz criteria): 2/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind ‐ study was described as being double blind |
Fergusson 2008.
| Methods | The research pharmacist at each centre randomly assigned patients to receive one of three antifibrinolytic agents with the use of a voice‐activated automated centralised program. An independent biostatistician generated the randomisation scheme using a computer‐generated randomisation list. Researchers, patients, members of the clinical teams, and members of the data and safety monitoring committee were all unaware of study‐group assignment. | |
| Participants | 2331 high‐risk cardiac surgical patients were randomly allocated to one of three groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients transfused allogeneic blood, massive post‐operative bleeding, re‐operation for bleeding, myocardial infarction, stroke, mortality, renal failure, length of stay (days). | |
| Notes | Quality assessment score (Schulz criteria): 7/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated randomisation list |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Findlay 2001.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 63 patients undergoing orthotopic liver transplantation were randomised to one of two groups:
NB: Gender data were not reported. |
|
| Interventions |
NB: Both groups were exposed to cell salvage. |
|
| Outcomes | Outcomes reported: Allogeneic blood usage (units), fresh frozen plasma usage (units), platelet usage (units), mortality, re‐operation for bleeding, hepatic artery thrombosis, hospital length of stay (days), intensive care unit length of stay (hours). | |
| Notes | Quality assessment score (Schulz criteria): 2/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Fraedrich 1989.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 80 male patients undergoing primary coronary bypass surgery were randomised to one of two groups:
NB: Four patients, two from the intervention and two from the control group were excluded. Aprotinin group excluded two patients: one allergic reaction, one severe cardiac failure. Control group excluded two patients: one surgical bleeding, one lethal cardiac failure. |
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Allogeneic blood usage, plasma usage, blood loss, volume of re‐transfused mediastinal blood. | |
| Notes | Quality assessment score (Schulz criteria): 2/7 Transfusion protocol not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | High risk | Single blind |
Garcia‐Enguita 1998.
| Methods | Method of randomisation and allocation concealment were not described. [Abstract only] | |
| Participants | 30 patients undergoing elective orthopaedic surgery were randomly allocated to one of two groups:
NB: Gender and age data were reported. |
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Allogenic blood usage (units), blood loss. | |
| Notes | Quality assessment score (Schulz criteria): 2/7 Transfusion protocol was not used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Unclear risk | Unclear |
Garcia‐Huete 1997.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 80 consecutive patients undergoing elective orthotopic liver transplantation were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), mortality, allergic reactions, re‐operation for bleeding, re‐transplantation. | |
| Notes | Quality assessment score (Schulz criteria): 4/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Garneti 2004.
| Methods | Patients were randomised using a list of random numbers. Allocation concealment was not described. | |
| Participants | 50 patients undergoing total hip arthroplasty were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), blood loss (48hrs), deep vein thrombosis, pulmonary embolus. | |
| Notes | Quality assessment score (Schulz criteria): 5/7 Transfusion protocol not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | List of random numbers |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Gherli 1992.
| Methods | Method of randomisation and allocation concealment were not described.[Italian] | |
| Participants | 31 patients undergoing cardiopulmonary bypass were randomly divided into one of three groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), blood loss, myocardial infarction, renal failure, blood products used, haemoglobin levels. | |
| Notes | Quality assessment score (Schulz criteria): 2/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Unclear risk | Unclear |
Gill 2009.
| Methods | Patients were allocated according to a computer‐generated randomisation schedule. | |
| Participants | 10 patients undergoing orthopaedic (hip) surgery were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients receiving blood transfusion, blood loss, volume blood transfused (units). | |
| Notes | Quality assessment score (Schulz criteria): 5/7 Transfusion protocol used |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated randomisation schedule |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Golanski 2000.
| Methods | Method of randomisation and allocation concealment were not described. [Polish] | |
| Participants | 54 patients undergoing elective cardiac surgery were randomly allocated to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Allogeneic blood usage (units), blood loss, mortality, myocardial infarction. | |
| Notes | Quality assessment score (Schulz criteria): 0/7 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Unclear risk | Unclear |
Good 2003.
| Methods | Coded ampoules of TXA or saline placebo prepared by the drug company were randomised in blocks of 10 (five saline, five TXA) by means of computer generated numbers. Four patients were withdrawn from the final analysis before the randomisation code was broken. | |
| Participants | 55 patients undergoing total knee replacement surgery were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), blood loss, deep vein thrombosis, infection. | |
| Notes | Quality assessment score (Schulz criteria): 6/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated numbers |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Gott 1998.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 400 cardiac surgery patients were randomly allocated to one of three groups:
NB: No demographic data were reported. |
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Total amount of allogeneic blood transfused (units), mortality, length of hospital stay, renal dysfunction, lung function. | |
| Notes | Quality assessment score (Schulz criteria): 0/7 Transfusion protocol not used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Unclear risk | Unclear |
Green 1995.
| Methods | Study was described as an open label randomised controlled trial conducted in two phases. Patients were assigned to groups by means of computer generated table of random numbers. | |
| Participants | 84 consecutive patients undergoing primary coronary artery bypass graft surgery or re‐operations were randomly assigned to one of two groups:
Two aprotinin dose regimens were studied:
|
|
| Interventions |
The study also compared patients who underwent primary operations (n = 60) with those patients who underwent re‐operations (n = 24). NB: Both groups were exposed to cell salvage. |
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, blood loss, overall erythrocyte loss, autotransfusion device erythrocytes, myocardial infarction, mortality, renal function (BUN + creatinine levels), pre‐operative and post‐operative haemoglobin levels. | |
| Notes | Quality assessment score (Schulz criteria): 3/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Patients were assigned to groups by means of computer generated table of random numbers |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | High risk | |
Greilich 2001.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 72 patients undergoing elective cardiac surgery were randomised to one of three groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, blood loss, intensive care unit length of stay (days), mechanical ventilation (hours). | |
| Notes | Quality assessment score (Schulz criteria): 2/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Greilich 2003.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 60 male patients undergoing cardiac surgery were randomised to one of three groups:
|
|
| Interventions |
NB: All groups were exposed to cell salvage. |
|
| Outcomes | Outcomes reported: Allogeneic blood usage (units), platelet usage (units), blood loss, plasma levels of Interleukin‐6 and Interleukin‐8 during and after CPB. | |
| Notes | Quality assessment score (Schulz criteria): 4/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Greilich 2004.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 36 male patients undergoing cardiac surgery were randomised to one of three groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Allogeneic blood usage (units), blood loss (24hrs), biochemical markers of plasmin activity (D‐dimer), biochemical markers of platelet (CD62P), activation, biochemical markers of leukocyte (CD11b) activation, biochemical markers of leukocyte‐platelet conjugate formation. | |
| Notes | Quality assessment score (Schulz criteria):3/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Greilich 2009.
| Methods | A table of random numbers was used to generate the allocation sequence. Central (pharmacy) allocation was used. | |
| Participants | 78 male patients undergoing cardiac surgery were randomised to one of three groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients transfused allogeneic blood, mortality, stroke, myocardial infarction, renal failure, length of hospital stay (days). | |
| Notes | Quality assessment score (Schulz criteria):7/7 Transfusion protocol was used |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | A table of random numbers was used to generate the allocation sequence |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Groh 1993.
| Methods | Method of randomisation and allocation concealment was not described. | |
| Participants | 20 patients undergoing orthotopic liver transplantation were randomised to one of two groups:
NB: Two patients were excluded from the final analysis |
|
| Interventions |
NB: Both groups were exposed to cell salvage. |
|
| Outcomes | Outcomes reported: Allogeneic blood usage (units), fresh frozen plasma usage (units), platelet usage (units) | |
| Notes | Quality assessment score (Schulz criteria):3/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Harder 1991.
| Methods | Method of randomisation was not described. The randomisation code was only known by the hospital pharmacy. Allocation concealment was by means of coded vials collected from the hospital pharmacy in the morning before the operation. No exclusions were reported. | |
| Participants | 80 male patients scheduled for elective coronary artery bypass grafting with cardiopulmonary bypass were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), blood loss. | |
| Notes | Quality assessment score (Schulz criteria): 6/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Hardy 1993.
| Methods | Randomisation was performed by the pharmacy department with each successive block of four patients being randomised (random allocation of two patients to Group A and two patients to Group C). Method used to generate allocation sequences was not described. | |
| Participants | 44 patients scheduled for repeat myocardial revascularisation, repeat value surgery, or a combined procedure (primary or repeat) were randomised to one of two groups:
NB: Three patients in the control group were excluded: one patient died in the operating room and one died upon arrival in the ICU. The third patient was excluded when the surgeon proceeded to a single valve replacement instead of the planned combined procedure. |
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), fresh frozen plasma usage (units), platelet usage (units), mortality, re‐exploration for bleeding. | |
| Notes | Quality assessment score (Schulz criteria): 5/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Hardy 1997.
| Methods | Each successive block of four patients was randomised by the Department of Pharmacy (random allocation of two patients to the treatment group and two patients to the control group). Method used to generate allocation sequences was not described. | |
| Participants | 52 patients undergoing primary or repeat myocardial revascularisation, or repeat valve surgery were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), re‐operation, blood loss, haemoglobin concentrations, coagulation factors. | |
| Notes | Quality assessment score (Schulz criteria): 6/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Hardy 1998.
| Methods | Study participants were randomised by the pharmacy department. Each successive block of nine patients were randomised to ensure a comparable number of patients in all groups and a similar distribution of patients over time. All bags were coded by the Department of Pharmacy and identical volumes of solution were infused. | |
| Participants | 134 patients undergoing scheduled elective coronary artery bypass graft surgery were randomised to one of three groups:
NB: Age data were not reported |
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), fresh frozen plasma usage (units), platelet usage (units), blood loss, mortality, myocardial infarction, cerebrovascular accident, re‐operation for bleeding. | |
| Notes | Quality assessment score (Schulz criteria): 6/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Adequate |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Harley 2002.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 35 patients undergoing elective orthopaedic surgery were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), blood loss, deep vein thrombosis, pulmonary embolus. | |
| Notes | Quality assessment score (Schulz criteria): 3/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Harmon 2004.
| Methods | A computer‐generated randomisation sequence was used to allocate patients. The sequence was concealed (numbered containers) until treatment was assigned. | |
| Participants | 36 patients undergoing cardiac surgery were randomised to one of two groups:
NB: One patient was excluded from the final analysis |
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Cognitive deficit, number of patients exposed to allogeneic blood, blood loss, hospital length of stay (days), serious adverse events. | |
| Notes | Quality assessment score (Schulz criteria): 5/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated randomisation |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | High risk | Single blind |
Havel 1992.
| Methods | Patients were randomly allocated to receive the test compound or a placebo by use of sealed envelopes. Method of randomisation was not described. | |
| Participants | 20 male patients undergoing orthotopic heart transplantation were randomised to one of two groups:
NB: Demographic data not reported. |
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), blood loss (24hrs) | |
| Notes | Quality assessment score (Schulz criteria): 5/7 Transfusion protocol not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | High risk | Inadequate ‐ used sealed envelopes to conceal treatment allocation |
| Blinding? All outcomes | Low risk | Double blind |
Havel 1994.
| Methods | Method used to generate random sequences was not described. Bottles of aprotinin and placebo were indistinguishable from each other. The preparation of each patient was individually packaged with 12 bottles each; each individual bottle, as well as the carton, was marked with a label carrying the patient number (the randomisation number). Each study package contained a total of 12 bottles, of which eight carried the label "Infusion" and four the label "Pump". | |
| Participants | 45 male patients undergoing cardiac surgery were randomised to one of three groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Allogeneic blood usage (units), blood loss, graft patency rates. | |
| Notes | Quality assessment score (Schulz criteria): 6/7 Transfusion protocol not used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Hayashida 1997.
| Methods | Patients were randomised by means of computer‐generated randomisation table. Method of allocation concealment was not described. | |
| Participants | 167 patients undergoing primary isolated coronary artery bypass graft surgery were randomised to one of three groups:
|
|
| Interventions |
NB: All groups were exposed to pre‐operative autologous blood donation (PAD). |
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), fresh frozen plasma usage (units), all blood product requirements (units), blood loss, mortality, myocardial infarction, allergic reaction, parameters of clotting and fibrinolysis, renal function, early graft pattency rates. | |
| Notes | Quality assessment score (Schulz criteria): 4/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated randomisation table |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | High risk | Single blind |
Hayes 1996.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 40 patients scheduled for total hip replacement surgery were randomly allocated to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Allogeneic blood usage (units), blood loss, haemoglobin levels, coagulation parameters, complications. | |
| Notes | Quality assessment score (Schulz criteria): 4/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Hei 2005.
| Methods | Method of randomisation and allocation concealment were not described. [Chinese language] | |
| Participants | 40 patients with severe hepatitis undergoing liver transplantation were randomly assigned to one of two groups:
Demographic data: M/F = 38/2, age range = 31‐67 years. |
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Allogeneic blood usage (units), fresh frozen plasma usage, platelet usage, blood loss. | |
| Notes | Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Unclear risk | Unclear |
Hekmat 2004.
| Methods | Randomisation was based on a computer‐generated code and sealed in sequentially numbered, opaque envelopes. | |
| Participants | 120 patients undergoing elective cardiac surgery were randomly assigned to one of two groups:
|
|
| Interventions |
NB: Cell Salvage was used during surgery in both groups using a cell saver (Brat2, Cobe Cardiovascular Inc, Arvada, CO.). |
|
| Outcomes | Outcomes reported: Allogeneic blood usage (units), fresh frozen plasma usage (units), platelet usage (units), blood loss, mortality, myocardial Infarction, hospital length of stay (days), intensive care unit length of stay (days), intubation time (hours), number of patients requiring Intra‐aortic balloon pump (IABP) therapy. | |
| Notes | Quality assessment score (Schulz criteria): 5/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated code |
| Allocation concealment? | High risk | Inadequate ‐ sealed envelopes |
| Blinding? All outcomes | Low risk | Double blind |
Hendrice 1995.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 26 patients undergoing primary coronary artery bypass surgery were randomly allocated to one of two groups:
Nb: Gender data not provided. |
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Allogeneic blood usage, blood loss (24 hrs), haemoglobin levels, coagulation factors. | |
| Notes | Quality assessment score (Schulz criteria): 2/7 Transfusion protocol not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Unclear risk | Unclear |
Hiipala 1995.
| Methods | Concealment of treatment allocation was by means of a ticket drawn from an envelope containing an equal number of treatment and placebo tickets. The method used to generate allocation sequences was not described. | |
| Participants | 29 patients undergoing total knee arthroplasty were randomly allocated to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), blood loss, myocardial infarction, deep vein thrombosis, pulmonary embolus, minor non‐thromboembolic complications. | |
| Notes | Quality assessment score (Schulz criteria): 4/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | High risk | Inadequate |
| Blinding? All outcomes | Low risk | Double blind |
Hiipala 1997.
| Methods | A ticket indicating the group was drawn and enclosed in an envelope. The envelopes were opened after the study was completed. Injection syringes were prepared by a person outside the surgical team. | |
| Participants | 75 patients scheduled for 77 total knee arthroplasties were randomly allocated to one of two groups:
NB: Three patients were excluded from the final analysis for miscellaneous reasons. |
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), blood loss, mortality, deep vein thrombosis, pulmonary embolus, non‐thrombotic complications. | |
| Notes | Quality assessment score (Schulz criteria): 4/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | High risk | Inadequate |
| Blinding? All outcomes | Low risk | Double blind |
Hill 1998.
| Methods | Patients were randomised according to a computer‐generated sequence. Method of allocation concealment was not described. | |
| Participants | 20 adult patients scheduled for first‐time myocardial revascularisation were randomly assigned to one of two groups:
NB: Gender data not reported. |
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Allogeneic blood usage (units), interleukin‐10 (IL‐10) levels. | |
| Notes | Quality assessment score (Schulz criteria): 3/7 Transfusion protocol not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated sequence |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Unclear risk | Unclear |
Horrow 1990.
| Methods | Patients were randomised using a random number table. Sealed envelopes ensured that only the pharmacist, who prepared the encoded infusions, knew whether a patient received drug or placebo. | |
| Participants | 49 patients undergoing cardiac surgery were randomised to one of two groups:
|
|
| Interventions |
NB: Both groups received cell salvage. |
|
| Outcomes | Outcomes reported: Allogeneic blood usage (units), number of participants exposed to fresh frozen plasma, number of participants exposed to platelets, blood loss (12 hrs), deep vein thrombosis, pulmonary embolus, stroke, number of patients receiving cell salvage, volume of cell salvage autotransfused. | |
| Notes | Quality assessment score (Schulz criteria): 4/7 Transfusion protocol not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Random number table |
| Allocation concealment? | High risk | Inadequate |
| Blinding? All outcomes | Low risk | Double blind |
Horrow 1991.
| Methods | A table of random numbers determined patient allocation to one of four groups. Coded infusion bags and sealed envelopes prepared by a pharmacist not involved in the study provided double blinded conditions. | |
| Participants | 163 adult patients undergoing coronary revascularisation, valve replacement, both procedures, or repair of atrial septal defect, were randomly allocated to one of four groups:
NB: Gender data were not reported. |
|
| Interventions |
NB: All patients received cell salvaged autologous blood if available. |
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), fresh frozen plasma usage (units), platelet usage (units), blood loss (12hrs), myocardial infarction, stroke, deep venous thrombosis, re‐operation for bleeding, rash, ventricular dysfunction, pulmonary oedema, ventricular tachycardia. | |
| Notes | Quality assessment score (Schulz criteria): 4/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Table of random numbers |
| Allocation concealment? | High risk | Inadequate |
| Blinding? All outcomes | High risk | Single blind |
Horrow 1995.
| Methods | Coded infusion bags for both loading and infusion doses and sealed envelopes prepared by a pharmacist provided allocation concealment. Randomisation was determined by a table of random numbers. | |
| Participants | 148 patients undergoing elective cardiac operations were randomised to one of six groups:
|
|
| Interventions |
NB: All groups were exposed to cell salvage. |
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), number of patients exposed to fresh frozen plasma, number of patients exposed to platelets, blood loss (12 hrs), mortality, hypotension. | |
| Notes | Quality assessment score (Schulz criteria): 5/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Table of random numbers |
| Allocation concealment? | High risk | Inadequate |
| Blinding? All outcomes | Low risk | Double blind |
Husted 2003.
| Methods | Randomisation was performed by computer. The drugs were packed in numbered envelopes by a person not connected with the surgical procedure and handled by the anaesthetist. The randomisation code was not broken until the study was completed. | |
| Participants | 40 patients undergoing primary total hip arthroplasty were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), blood loss, deep vein thrombosis, pulmonary embolus, infection, haemoglobin levels. | |
| Notes | Quality assessment score (Schulz criteria): 6/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation was performed by computer |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Ickx 2006.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 51 patients undergoing primary liver transplantation were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, number of patients exposed to fresh frozen plasma, number of patients exposed to platelets, allogeneic blood usage (units), mortality, hospital length of stay (days), intensive care unit length of stay (days). | |
| Notes | Quality assessment score (Schulz criteria): 2/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Unclear risk | Unclear |
Isetta 1993.
| Methods | Method of randomisation and allocation concealment were not described. Exclusions or loss to follow‐up were not reported. [Abstract] | |
| Participants | 240 patients undergoing cardiac surgery were randomised to one of four groups:
NB: Demographic data were not reported. |
|
| Interventions |
NB: All groups were exposed to cell salvage. |
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, blood loss, haematocrit values. | |
| Notes | Quality assessment score (Schulz criteria): 0/7 (Abstract) Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Unclear risk | Unclear |
Jamieson 1997.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 50 patients undergoing re‐operative cardiac surgery were randomised to one of two groups:
|
|
| Interventions |
NB: Both groups were exposed to cell salvage. |
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), fresh frozen plasma usage (units), platelet usage (units), blood loss, mortality, hospital length of stay (days), total blood products transfused. | |
| Notes | Quality assessment score (Schulz criteria): 4/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Jansen 1999.
| Methods | Randomisation was performed using a computer‐generated random number list. Method of allocation concealment was not described. | |
| Participants | 42 patients undergoing unilateral bicondylar cemented total knee arthroplasty were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), blood loss, deep venous thrombosis. | |
| Notes | Quality assessment score (Schulz criteria): 5/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation was performed using a computer‐generated random number list |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Janssens 1994.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 40 patients undergoing primary total hip replacement were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Blood loss, number of patients exposed to allogeneic/autologous blood, allogeneic blood usage (units), autologous blood usage (units), hospital length of stay (days), deep vein thrombosis. | |
| Notes | Quality assessment score (Schulz criteria): 4/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Jares 2003.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 47 patients undergoing 'off pump' coronary artery bypass graft surgery were randomised to one of two groups:
NB: No age data were reported. |
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, blood loss, pulmonary embolus, aspirin use <5 days, re‐operation for bleeding, stroke. | |
| Notes | Quality assessment score (Schulz criteria): 1/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | High risk | No blinding |
Jeserschek 2003.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 18 patients undergoing elective orthopaedic surgery were randomised to one of two groups:
|
|
| Interventions |
NB: Both groups were exposed to intra‐operative cell salvage. |
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), blood loss. | |
| Notes | Quality assessment score (Schulz criteria): 4/7 Transfusion protocol not used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Jimenez 2007.
| Methods | Patients were assigned to treatment group by independent pharmacists using a list of pseudo‐randomised numbers to receive coded infusions of either TXA or placebo. The cose was revealed once recruitment, data collection, and laboratory analyses were completed. | |
| Participants | 50 patients undergoing cardiac surgery were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients receiving blood transfusion, volume of blood transfused (units), blood loss, mortality, hospital length of stay, mechanical ventilation hours, inflammatory response, d‐dimer levels. | |
| Notes | Quality assessment score (Schulz criteria): 7/7 Use of a transfusion protocol was not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomised number list |
| Allocation concealment? | Low risk | Adeaquate |
| Blinding? All outcomes | Low risk | Double blind |
Johansson 2005.
| Methods | Patients were randomised by computer in blocks of 10. Coded ampoules were prepared by the pharmaceutical company. All personnel and patients were blinded as to the treatment until the randomisation code was broken which took place after all patients had been evaluated. | |
| Participants | 119 patients undergoing total hip arthroplasty were randomised to one of two groups:
NB: Before the randomisation code was broken 19 patients were excluded due to violation of the study protocol. |
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), blood loss, deep vein thrombosis. | |
| Notes | Quality assessment score (Schulz criteria): 5/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer randomisation |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Kahveci 1996.
| Methods | Method of randomisation and allocation concealment were not described. [Turkish language] | |
| Participants | 28 patients undergoing coronary artery bypass surgery or cardiac valvular surgery were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Allogeneic blood usage (units), fresh frozen plasma usage (units), blood loss. | |
| Notes | Transfusion protocol not used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Unclear risk | Unclear |
Kalangos 1994.
| Methods | Method of randomisation and allocation concealment were not described. No exclusions were reported. | |
| Participants | 165 adult patients undergoing elective primary aortocoronary bypass operations were randomised to one of three groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), blood loss, myocardial infarction, creatine phosphokinase ‐ myocardial band (CK‐MB) levels. | |
| Notes | Quality assessment score (Schulz criteria): 4/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Karski 1995.
| Methods | Randomisation was performed by the pharmacy department. The method used to generate allocation sequences was not described. | |
| Participants | 150 patients undergoing cardiac operations were randomised to one of three groups:
NB: Gender data were not reported. |
|
| Interventions |
NB: All groups were exposed to cell salvage (autotransfusion). Patients with defined 'excessive bleeding' were treated with 10‐40g of intravenous epsilon aminocaproic acid (EACA) or desmopressin (DDAVP). |
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), blood loss. | |
| Notes | Quality assessment score (Schulz criteria): 4/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | High risk | Single blind |
Karski 2005.
| Methods | A computer‐generated randomisation code in blocks of four was used to assign patients to treatment or control in a double‐blinded fashion. The hospital pharmacy prepared identical bags of solution. | |
| Participants | 312 patients undergoing cardiac surgery were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patinets exposed to allogeneic blood, number of patients exposed to fresh frozen plasma, number of patients exposed to platelets, mortality, myocardial infarction, stroke, cardiac arrest, atrial fibrillation. | |
| Notes | Quality assessment score (Schulz criteria): 7/7 Transfusion protocol not specified NB: Open‐labeled tranexamic acid was administered to 4 patients in the TXA group and 24 patients in the control group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated randomisation code |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Kaspar 1997.
| Methods | Study infusions were prepared by the hospital pharmacy using a computer generated randomisation schedule. | |
| Participants | 32 consecutive patients undergoing orthotopic liver transplantation were randomly allocated to one of two groups:
NB: Demographic data were not reported. |
|
| Interventions |
NB: Both groups were exposed to cell salvage. |
|
| Outcomes | Outcomes reported: Allogeneic blood usage (units), fresh frozen plasma (units), platelets (units), mortality, hepatic arterial thrombosis, epsilon aminocaproic acid 'rescue', cryoprecipitate. | |
| Notes | Quality assessment score (Schulz criteria): 7/7 Transfusion protocol not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated randomisation schedule |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Katoh 1997.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 93 patients undergoing either coronary artery bypass grafting or heart valve operations were randomly divided into one of three groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), blood loss, mortality, myocardial infarction, stroke, pulmonary embolism, deep vein thrombosis. | |
| Notes | Quality assessment score (Schulz criteria): 2/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Unclear risk | Unclear |
Katsaros 1996.
| Methods | Method of randomisation was not described. Allocation concealment was by coded infusions. One patient was eliminated from the study due to improper data collection. | |
| Participants | 211 patients scheduled for open heart operations were randomised to one of two groups:
|
|
| Interventions |
NB: Both groups were exposed to cell salvage. |
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, bood loss (24hrs), mortality, myocardial infarctions, deep vein thrombosis, pulmonary embolus, re‐operation for bleeding, cerebrovascular accident, renal failure, central nervous system complications. | |
| Notes | Quality assessment score (Schulz criteria): 5/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Katzel 1998.
| Methods | Method of randomisation and allocation concealment were not described. [German language] | |
| Participants | 24 male patients undergoing thoracic surgery for malignant lung disease were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, blood loss, transient ischaemic attack. | |
| Notes | Quality assessment score (Schulz criteria): 4/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Kazemi 2010.
| Methods | Methods of sequence generation and allocation concealment were not described. | |
| Participants | 64 patients undergoing orthopaedic surgery were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Volume of blood transfused (units), blood loss, deep vein thrombosis, length of stay (days). | |
| Notes | Transfusion protocol was used. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Kikura 2006.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 100 patients undergoing cardiac surgery were randomised to one of two groups:
|
|
| Interventions |
NB: Both groups were exposed to intra‐operative cell salvage. |
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), number of patients exposed to fresh frozen plasma, number of patients exposed to platelets, blood loss (24hrs). | |
| Notes | Quality assessment score (Schulz criteria): 4/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Kipfer 2003.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 30 adult patients undergoing elective cardiac surgery were randomised into one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), blood loss, fresh frozen plasma, myocardial infarction, mortality, myocardial infarction, retransfused mediastinal shed blood, re‐operation for bleeding, renal dysfunction, neurological deficit, hospital length of stay (days). | |
| Notes | Quality assessment score (Schulz criteria): 2/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Unclear risk | Unclear |
Klein 1998.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 109 patients undergoing elective cardiac surgery were randomised to one of three groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Allogeneic blood usage (units), blood loss, fresh frozen plasma, myocardial infarction. | |
| Notes | Quality assessment score (Schulz criteria): 4/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Kluger 2003.
| Methods | Patients underwent permuted block randomisation using random number tables. All patients received four syringes, labelled A,B,C,and D. Patients, clinicians, and investigators were all blinded to group allocation. | |
| Participants | 90 patients undergoing cardiac surgery were randomised to one of three groups:
NB: Two patients were excluded from the final analysis. |
|
| Interventions |
NB: All groups were exposed to acute normovolaemic haemodilution (ANH). |
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, number of patients exposed to fresh frozen plasma and platelets, mortality, myocardial infarction, re‐operation for bleeding, stroke. | |
| Notes | Quality assessment score (Schulz criteria): 6/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Random number table |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Koster 2004.
| Methods | Allocation of patients was blinded to the surgeon. Method of randomisation was not described. | |
| Participants | 200 patients undergoing elective cardiac surgery were randomised to one of two groups:
|
|
| Interventions |
NB: Both groups were exposed to cell salvage. |
|
| Outcomes | Outcomes reported: Allogeneic blood usage (units), fresh frozen plasma, blood loss, duration of ventilation (hours). | |
| Notes | Quality assessment score (Schulz criteria): 0/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | High risk | Single blind |
Kratzer 1997.
| Methods | Method of randomisation was by means of a random number generator. Method used to conceal treatment allocation was not described. [German language] | |
| Participants | 18 patients undergoing orthotopic liver transplantation were randomised to one of two groups:
NB: Gender data were not reported. |
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Allogeneic blood usage (units), coagulation parameters, blood loss. | |
| Notes | Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Unclear risk | Unclear |
Kreisler 2005.
| Methods | Randomisation was accomplished through the use of a computer‐generated table of random numbers. Method used to conceal treatment allocation was not described. | |
| Participants | 71 patients undergoing cardiac surgery were randomised to one of three groups:
|
|
| Interventions |
NB: All groups were exposed to cell savage. |
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, number of patients exposed to platelets, hospital length of stay (days), intensive care length of stay (hours), cell saver volume autotransfused. | |
| Notes | Quality assessment score (Schulz criteria): 4/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated table of random numbers |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Kuepper 2003.
| Methods | Group assignment was by sealed envelopes. Sealed envelopes were opened after induction of anaesthesia by the unblinded investigator who was not part of the operating team. | |
| Participants | 120 patients undergoing elective cardiac surgery were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Allogeneic blood usage (units), blood loss, fresh frozen plasma, platelets (units), mortality. | |
| Notes | Quality assessment score (Schulz criteria): 3/7 Transfusion threshold for RBC not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | High risk | Inadequate |
| Blinding? All outcomes | High risk | Single blind |
Kuitunen 2005.
| Methods | Study drugs were prepared by the hospital pharmacy. Randomisation was carried out using closed envelopes. | |
| Participants | 60 patients undergoing primary coronary artery bypass graft surgery were randomised to one of three groups:
|
|
| Interventions |
NB: All groups were exposed to cell salvage. |
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, number of patients exposed to fresh frozen plasma, number of participants exposed to platelets, blood loss (16hrs), mortality, myocardial infarction, re‐operation for bleeding, stroke. | |
| Notes | Quality assessment score (Schulz criteria): 5/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | Closed envelopes |
| Allocation concealment? | High risk | Inadequate |
| Blinding? All outcomes | Low risk | Double blind |
Kuitunen 2006.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 30 patients undergoing elective primary cardiac surgery were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood transfusion, number of patients exposed to fresh frozen plasma and platelets, blood loss (24hrs), re‐operation for bleeding | |
| Notes | Transfusion protocol used. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Kunt 2005.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 86 patients undergoing routine cardiac surgery were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Allogeneic blood usage (units), blood loss (24 hrs), mortality, hospital length of stay (days), intensive care unit length of stay (hours), re‐operation for bleeding. | |
| Notes | Quality assessment score (Schulz criteria): 2/7 Transfusion protocol not used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Unclear risk | Unclear |
Kyriss 2001.
| Methods | Randomisation carried out using a computer‐generated random list. Allocation concealment not specified. | |
| Participants | 38 patients undergoing elective thoracic surgery were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), blood loss, fresh frozen plasma, blood loss, mortality, blood loss, re‐operation for bleeding. | |
| Notes | Quality assessment score (Schulz criteria): 5/7 No transfusion protocol | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated random list |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Landymore 1997.
| Methods | Method of randomisation was not described. The study drugs were prepared by pharmacy, given an identification number, and then sent to the operating room. | |
| Participants | 148 patients undergoing primary myocardial revascularisation were randomised to one of three groups:
NB: Demographic data were not reported. |
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Allogeneic blood usage (units), blood loss, thrombosis, deep vein thrombosis, pulmonary embolus. | |
| Notes | Quality assessment score (Schulz criteria):2/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Unclear risk | Unclear |
Lass 1995.
| Methods | Method of randomisation and allocation concealment were not described. Four aprotinin (7.8%) and eight control (14.5%) patients were excluded from the final analysis. | |
| Participants | 110 male patients undergoing elective primary coronary bypass surgery were randomised to one of two groups:
NB: Demographic data were not reported. |
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), graft patency, blood loss, mortality, myocardial infarction, acute heart failure, post‐operative complications, re‐operation. | |
| Notes | Quality assessment score (Schulz criteria): 3/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Later 2009.
| Methods | Allocated according to a computer‐generated randomisation sequence, allocation concealed by use of sealed, opaque envelopes. | |
| Participants | 298 patients undergoing cardiac surgery were randomised to one of three groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patienst exposed to allogeneic blood, myocardial infarction, renal failure, hospital length of stay (days), re‐operation for bleeding. | |
| Notes | Quality assessment score (Schulz criteria): 5/7 Transfusion protocol used. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Computer‐generated randomisation sequence |
| Allocation concealment? | High risk | Inadequate ‐ sealed envelopes |
| Blinding? All outcomes | Low risk | Double blind |
Laub 1994.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 47 patients undergoing isolated coronary revascularisation were randomised to one of two groups:
NB: The study group consisted of 32 patients in total. Fifteen of the originally enrolled patients were not included in the final analysis due to: adverse reactions while receiving the study medication (n = 2), inability to obtain or a technically inadequate CT scan (n = 7), refusal to come for follow‐up examinations (n = 4), or died (n = 2). |
|
| Interventions |
NB: Autologous blood salvage with reinfusion of washed RBCs was used for all patients. Shed mediastinal and pleural blood was filtered and reinfused using an autotransfusion system. |
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, volume of allogeneic blood transfused, blood loss, volume of platelets and fresh frozen plasma, re‐operation for bleeding, post‐operative Hb levels, graft occlusions, any blood product usage, haematologic variables, coagulation profiles, renal function. | |
| Notes | Quality assessment score (Schulz criteria): 2/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Lavee 1993.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 30 patients undergoing various cardiopulmonary bypass procedures were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Allogeneic blood usage (units), blood loss (24hrs), platelets (units), platelet aggregation. | |
| Notes | Quality assessment score (Schulz criteria): 2/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Leijdekkers 2006.
| Methods | Patients were randomised to receive either placebo or aprotinin using a standard randomisation list stored in the pharmacy department, only to be opened after the study was closed for inclusion. | |
| Participants | 35 patients undergoing cardiac surgery were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outocmes reported: Volume blood transfused (units), blood loss, mortality, re‐operation for bleeding. | |
| Notes | Quality assessment score (Schulz criteria): 7/7 Transfusion protocol used. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation list |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Lemay 2004.
| Methods | Method of randomisation was not described. Study drugs were prepared by the hospital pharmacist. Patient caregivers and the investigator collecting the data were blinded to the solution used. | |
| Participants | 40 patients undergoing total hip replacement were randomised to one of two groups:
NB: One patient was excluded from the final analysis. |
|
| Interventions |
NB: Pre‐operative autologous donation of 3 units was offered to all patients. |
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), blood loss, deep vein thrombosis, changes in haemoglobin levels. | |
| Notes | Quality assessment score (Schulz criteria): 5/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Lemmer 1994.
| Methods | Metho used to generate allocation sequences was not described. Aprotinin and an identically appearing placebo was supplied by Bayer AG, Leverkusen, Germany. Enrolled patients were stratified as to whether they were undergoing primary procedures (n = 151 patients: Lemmer_1) or repeat procedures (n = 65 patients: Lemmer_2). | |
| Participants | 151 patients undergoing isolated primary coronary artery bypass graft operations were randomised to one of two groups:
65 patients undergoing repeat coronary artery bypass graft operations were randomised to one of two groups:
|
|
| Interventions |
NB: Both groups were exposed to cell salvage. |
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), fresh frozen plasma (units), platelets (units), blood loss, mortality, myocardial infarction, re‐operation for bleeding, allergic reactions, renal failure, renal failure + dialysis. | |
| Notes | Quality assessment score (Schulz criteria): 5/7 Transfusion protocol used. Of the 151 patients undergoing primary CABG, 141 (74 in the aprotinin treated group and 67 in the placebo treated group) fulfilled the criteria for efficacy analysis. Patients were eliminated from efficacy analysis before the random code was broken. Of the 65 patients undergoing repeat CABG surgery 55 (23 in the aprotinin treated group and 32 in the placebo treated group) fulfilled the criteria for efficacy analysis. Patients were eliminated from efficacy analysis before the random code was broken. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Lemmer 1996.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 704 first time coronary artery bypass grafting patients were randomised to one of four groups:
|
|
| Interventions |
NB: All groups were exposed to cell salvage autotransfusion. |
|
| Outcomes | Outcomes reported: Total blood product exposures per patient, number of patients exposed to allogeneic blood, allogeneic blood usage (units), platelet (units), fresh frozen plasma (units), cryoprecipitate (units), blood loss, re‐operation for diffuse bleeding, myocardial infarction. | |
| Notes | Quality assessment score (Schulz criteria): 2/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Lemmer_1 1994.
| Methods | Refer to Lemmer 1994 | |
| Participants | 151 patients undergoing isolated primary coronary artery bypass graft operations were randomised to one of two groups:
|
|
| Interventions |
NB: Both groups were exposed to cell salvage. |
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), fresh frozen plasma (units), platelets (units), blood loss, mortality, myocardial infarction, re‐operation for bleeding, allergic reactions, renal failure, renal failure + dialysis. | |
| Notes | Quality assessment score (Schulz criteria): 5/7 Transfusion protocol used. Of the 151 patients undergoing primary CABG, 141 (74 in the aprotinin treated group and 67 in the placebo treated group) fulfilled the criteria for efficacy analysis. Patients were eliminated from efficacy analysis before the random code was broken. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Lemmer_2 1994.
| Methods | Refer to Lemmer 1994 | |
| Participants | 65 patients undergoing repeat coronary artery bypass graft operations were randomised to one of two groups:
|
|
| Interventions |
NB: Both groups were exposed to cell salvage. |
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), fresh frozen plasma (units), platelets (units), blood loss, mortality, myocardial infarction, re‐operation for bleeding. | |
| Notes | Quality assessment score (Schulz criteria): 5/7 Transfusion protocol used. Of the 65 patients undergoing repeat CABG surgery 55 (23 in the aprotinin treated group and 32 in the placebo treated group) fulfilled the criteria for efficacy analysis. Patients were eliminated from efficacy analysis before the random code was broken. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Yes |
Lentschener 1997.
| Methods | Generation of allocation sequences was by means of computer‐generated random codes. Method of allocation concealment was not described. | |
| Participants | 97 patients scheduled for elective liver resection performed through subcostal incision were randomly assigned to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Blood loss, number of patients exposed to allogeneic blood transfusion, fresh frozen plasma transfused, platelet units transfused. | |
| Notes | Quality assessment score (Schulz criteria): 3/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated random codes |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Lentschener 1999.
| Methods | Patients were randomised in a double blind fashion by using a computer generated random code. Randomisation was both stratified by the number of fused levels and blocked in groups of four before the induction of anesthesia. Allocation concealment was not described. | |
| Participants | 72 patients undergoing posterior lumbar spine fusion were randomly assigned to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood (units), autologous transfusion, blood loss (24hrs), post‐operative total autologous units (total). | |
| Notes | Quality assessment score (Schulz criteria): 5/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated random code |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Levy 1995.
| Methods | Method of randomisation and allocation concealment were not described. Eleven medical centres participated. Study performed efficacy and safety analysis. Exclusions defined by protocol. | |
| Participants | 287 patients undergoing repeat coronary artery bypass graft surgery were randomly assigned to one of four groups:
|
|
| Interventions |
NB: All groups were exposed to cell salvage. |
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), fresh frozen plasma usage (units), platelet usage (units), blood loss. | |
| Notes | Quality assessment score (Schulz criteria): 2/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Li 2005.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 70 patients undergoing elective cardiac surgery were randomised to one of four groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), number of patients receiving fresh frozen plasma, number of patients receiving platelets, blood loss. | |
| Notes | Quality assessment score (Schulz criteria): 2/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Unclear risk | Unclear |
Liu 1993.
| Methods | Allocation sequences were generated by a computer generated random list. The trial drug and placebo were supplied in identical packs. Exclusions or loss to follow‐up were reported. | |
| Participants | 40 patients undergoing elective myocardial revascularisation were randomised to one of two groups:
|
|
| Interventions |
NB: Both groups were exposed to cell salvage. |
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), fresh frozen plasma, blood loss, total post‐operative autotransfusion from the chest drainage, mortality, re‐operation for bleeding, allergic reaction, hospital length of stay (days). | |
| Notes | Quality assessment score (Schulz criteria): 5/7 Transfusion protocol not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Allocation sequences were generated by a computer generated random list |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Liu 1998.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 60 patients undergoing open heart surgery were randomly assigned to one of three groups:
NB: Gender data were not reported. |
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Allogeneic blood usage (units), fresh frozen plasma usage (units), platelets usage (units), blood loss. | |
| Notes | Quality assessment score (Schulz criteria): 0/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Unclear risk | Unclear |
Llau 1998.
| Methods | Method of randomisation and allocation concealment were not described. [Abstract] | |
| Participants | 20 patients undergoing elective orthopaedic surgery were randomly allocated to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), blood loss, deep vein thrombosis, change in haematocrit levels ‐ baseline to 24 hrs post‐operative, change in haemoglobin levels ‐ baseline to 24 hrs post‐operative. | |
| Notes | Transfusion protocol used. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Unclear risk | Unclear |
Locatelli 1990.
| Methods | Method of randomisation and allocation concealment were not described. [Italian language] | |
| Participants | 38 patients undergoing myocardial revascularisation were randomly allocated to one of three groups:
NB: Demographic data were not reported. |
|
| Interventions |
NB: All groups were exposed to cell salvage. |
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), blood loss (28 hrs), adverse reactions. | |
| Notes | Quality assessment score (Schulz criteria): 2/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Unclear risk | Unclear |
Luo 1998.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 20 patients undergoing cardiac surgery were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: volume of blood transfused, duration of CPB. | |
| Notes | Quality assessment score (Schulz criteria): 2/7 Transfusion protocol not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Unclear risk | Unclear |
Maccario 1994.
| Methods | Method of randomisation and allocation concealment were not described. [Italian language] | |
| Participants | 99 patients undergoing coronary artery bypass graft surgery and valvular cardiac surgery were randomised to one of three groups:
NB: Gender data were not reported. |
|
| Interventions |
NB: All groups were exposed to acute normovolemic haemodilution and cell salvage. |
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), blood loss (24hrs), allergic reactions. | |
| Notes | Quality assessment score (Schulz criteria): 2/7 Transfusion protocol used. Four patients were excluded from the study due to surgical bleeding (one from the control group, one from the high‐dose aprotinin group, and two from the low‐dose aprotinin group). One patient from the low‐dose aprotinin group died and was excluded from analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | High risk | Single blind |
MacGillivray 2010.
| Methods | Tranexamic acid or placebo for infusion was prepared by the institution's pharmacy in two identical 50mL bags (identified only by random number) with the constituents unknown to the administering anesthesiologist or surgeon. Method of randomisation was not described. | |
| Participants | 60 patients undergoing orthopaedic (knee) surgery were randomised to one of three groups:
|
|
| Interventions |
NB: Patients received re‐infusion of autotransfused blood from the intra‐articular drains. |
|
| Outcomes | Outcomes reported: number of patients exposed to allogeneic blood transfusion, blood loss, number of allogeneic units transfused, adverse events. | |
| Notes | Quality assessment score (Schulz criteria): 6/7 Transfusion protocol used. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Maddali 2007.
| Methods | A computer‐generated randomisation code was used to allocate participants. Allocation was concealed by using sequentially‐numbered, sealed opaque envelopes. | |
| Participants | 222 patients undergoing cardiac surgery were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Volume blood transfused, blood loss, mortality, stroke, re‐operation for bleeding. | |
| Notes | Quality assessment score (Schulz criteria): 4/7 Transfusion protocol used |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated randomisation code |
| Allocation concealment? | High risk | Inadequate ‐ sealed envelopes |
| Blinding? All outcomes | Low risk | Double blind |
Maineri 2000.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 48 patients undergoing elective cardiac surgery were randomised to one of two groups:
NB: Gender data were not reported. |
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), blood loss, pulmonary embolus, post ‐operative Hct, stroke. | |
| Notes | Quality assessment score (Schulz criteria): 0/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Unclear risk | Unclear |
Mansour 2004.
| Methods | Randomisation of patients was performed with the help of a computer ‐generated random number sequence programme. To ensure proper blinding the three studied solutions were prepared by the pharmacy as bottles. | |
| Participants | 60 patients undergoing elective cardiac surgery (off pump CABG) were randomly assigned to one of three groups:
|
|
| Interventions |
NB: Loading dose was administered over 20 minutes in all groups. Infusion dose was infused at a rate of 50ml/hr in all groups. |
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), fresh frozen plasma usage (units), platelet usage (units), blood loss (24hrs), myocardial infarction, number of patients exposed to fresh frozen plasma, number of patients exposed to platelets, re‐operation for bleeding, renal dysfunction, hospital length of stay (days), renal dysfunction, neurological deficit. | |
| Notes | Quality assessment score (Schulz criteria): 7/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer ‐generated random number sequence programme |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Marcel 1996.
| Methods | Patients were randomised by a computer program. Method of allocation concealment was not described. | |
| Participants | 44 consecutive patients undergoing orthotopic liver transplantation were randomised to one of two groups:
NB: Demographic data were not reported. |
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Allogeneic blood usage (units), fresh frozen plasma usage (units), platelet usage (units), blood loss (24hrs). | |
| Notes | Quality assessment score (Schulz criteria): 5/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomised by a computer program |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Mehr‐Aein 2007.
| Methods | Method of randomisation was not reported. Concealment of allocation was achieved by using pharmacy prepared coded infusion syringes. | |
| Participants | 66 patients undergoing cardiac surgery were randomised to one of two groups:
NDB: Gender data were not reported. |
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patient exposed to allogeneic blood transfusion, volume of blood transfused (units), blood loss, re‐operation for bleeding, mortality, myocardial infarction, renal failure, length of hospital stay (days). | |
| Notes | Quality assessment score (Schulz criteria): 6/7 Transfusion protocol used |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Mengistu 2008.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 50 patients undergoing cardiac surgery were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: number of patients exposed to allogeneic blood transfusion, volume of allogeneic blood transfused, blood loss. | |
| Notes | Quality assessment score (Schulz criteria): 3/7 Transfusion protocol used |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | High risk | Single blind |
Menichetti 1996.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 96 consecutive patients undergoing coronary artery bypass surgery were randomised to one of four groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, blood loss (24hrs), re‐operation for bleeding, haemoglobin levels, activated clotting times (ACT), prothrombin times, activated partial thromboplastin times (APTT), plasminogen levels, factor VIII levels, TAT complex/values. | |
| Notes | Quality assessment score (Schulz criteria): 2/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Unclear risk | Unclear |
Misfeld 1998.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 42 patients undergoing elective cardiac surgery were randomised to one of three groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), blood loss (6 hrs), mortality, change in haemoglobin levels ‐ baseline to 24 hrs post‐operative. | |
| Notes | Quality assessment score (Schulz criteria): 1/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | High risk | Single blind |
Mohr 1992.
| Methods | Method of randomisation and allocation concealment were not described. No loss to follow‐up reported. | |
| Participants | 50 patients undergoing primary coronary artery bypass graft surgery (CABG), repeat CABG, valve replacement, or valve replacement + CABG surgery were randomised to one of three groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), blood loss (24hrs), re‐operation for bleeding, post‐operative platelet counts, platelet aggregation evaluation by scanning electron microscopy. | |
| Notes | Quality assessment score (Schulz criteria): 4/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Mongan 1998.
| Methods | Patients were randomised using a computer‐generated random sequence. Method used to conceal treatment allocation was not described. | |
| Participants | 150 patients undergoing primary coronary artery bypass graft surgery were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), mortality, myocardial infarction, re‐operation for bleeding, stroke. | |
| Notes | Quality assessment score (Schulz criteria): 5/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated random sequence |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Moran 2000.
| Methods | Patients were randomly assigned by a computer‐generated code. Method used to conceal treatment allocation was not described. | |
| Participants | 42 patients undergoing coronary artery bypass surgery were randomised to one of three groups:
NB: Four patients were excluded from the final efficacy analysis. All 42 patients were included in the safety analysis. |
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, blood loss (24hrs), mortality, myocardial infarction, re‐operation for bleeding, stroke. | |
| Notes | Quality assessment score (Schulz criteria): 4/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated code |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Murkin 1994.
| Methods | Method of allocation concealment was not described. Randomisation was by means of computer‐generated random code. | |
| Participants | 54 patients undergoing first‐time coronary artery bypass or valvular heart operations requiring cardiopulmonary bypass (CPB) were randomised to one of two groups:
NB: Three of the 57 enrolled patients were deemed ineligible because of cancellation of the operation (n = 2) and non‐use of CPB (n = 1). All 54 remaining patients were included for analysis. |
|
| Interventions |
NB: Both groups were exposed to cell salvage. |
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), fresh frozen plasma usage (units), platelet usage (units), blood loss (36hrs), myocardial infarction, pulmonary embolic events, hospital length of stay (days). | |
| Notes | Quality assessment score (Schulz criteria): 4/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated random code |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Murkin 1995.
| Methods | Randomisation was by means of computer generated codes. Method of allocation concealment was not described. | |
| Participants | 53 consecutive patients undergoing revision total hip arthroplasty or primary bilateral total hip arthroplasty were randomly assigned to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), blood loss, cerebrovascular accident, deep vein thrombosis, hospital length of stay (days). | |
| Notes | Quality assessment score (Schulz criteria): 5/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated codes |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Murkin 2000.
| Methods | Labels on all medication vials were the same except for the patient executive number. Patients were stratified on the basis of whether or not pre‐operative autologous blood donations had been made. | |
| Participants | 301 undergoing elective primary unilateral total hip replacement were randomised to one of four groups:
|
|
| Interventions |
NB: Epsilon aminocaproic acid and desmopressin were used if deemed necessary. Data regarding the use of these two drugs to minimise blood loss were not reported. All groups used pre‐operative autologous donation. |
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, blood loss, myocardial infarction, mortality, deep venous thrombosis, pre‐operative autologous blood donation. | |
| Notes | Quality assessment score (Schulz criteria): 6/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Murphy 2006.
| Methods | Allocation was generated by a card system and concealed in sealed, opaque envelopes. | |
| Participants | 100 off‐pump coronary artery bypass grafting surgical patients were randomly allocated to one of two groups:
|
|
| Interventions |
NB: All patients underwent peri‐operative cell salvage with autotransfusion of washed salvaged red blood cells at the completion of the operative procedure. |
|
| Outcomes | Outcomes reported: number of patients exposed to allogeneic blood transfusion, blood loss, mortality, stroke, renal dysfunction, myocardial infarction, length of stay. | |
| Notes | Quality assessment score (Schulz criteria): 5/7 Transfusion protocol used |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | High risk | Inadequate ‐ sealed envelopes |
| Blinding? All outcomes | Low risk | Double blind |
Niskanen 2005.
| Methods | Patients were randomised into two groups by an envelope method in a double‐blind manner. The randomisation and preparation of the drug were done in the absence of other personnel by two anaesthesia nurses not engaged in the study. The code was broken after the last patient had been treated. | |
| Participants | 40 patients undergoing cemented hip arthroplasty were randomised to one of two groups:
NB: One patient was excluded from the final analysis. |
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), deep vein thrombosis. | |
| Notes | Quality assessment score (Schulz criteria): 6/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Norman 2009.
| Methods | Patients were allocated according to a randomisation schedule based on study accession number. Pharmacy controlled allocation. | |
| Participants | 20 undergoing extrapleural pneumonectomy for mesothelioma were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood transfusion, blood loss, mortality. | |
| Notes | Quality assessment score (Schulz criteria): 7/7 Use of transfusion protocol not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation schedule based on study accession number |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Nurözler 2008.
| Methods | Patients were allocated according to a list of random treatment codes. Method used to conceal treatment allocation was not described. | |
| Participants | 51 undergoing cardiac surgery were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood transfusion, volume blood transfused (units), blood loss, re‐operation for bleeding, myocardial infarction, stroke, length of hospital stay (days). | |
| Notes | Quality assessment score (Schulz criteria): 3/7 Transfusion protocol used |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | List of random treatment codes |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Nuttall 2000.
| Methods | Patients were randomly assigned by a computer‐generated random number sequence. Method used to conceal treatment allocation was not described. | |
| Participants | 168 patients undergoing cardiac surgery were randomised to one of four groups:
NB: Eight patients were excluded from the final analysis. |
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), mortality, re‐operation for bleeding. | |
| Notes | Quality assessment score (Schulz criteria): 4/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated random number sequence |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Okita 1996.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 60 patients undergoing aortic surgery under deep hypothermic circulatory arrest were randomly allocated to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage, blood loss (24 hrs), mortality, myocardial infarction, stroke, renal failure, respiratory failure + pneumonia. | |
| Notes | Quality assessment score (Schulz criteria): 2/7 Transfusion protocol not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Unclear risk | Unclear |
Orpen 2006.
| Methods | A pharmacist not involved with the study carried out randomisation in the pharmacy by a sealed envelope method and prepared the contents of the administered solution. The operating team was blinded to the contents of the administered solution for every patient although allowance was made for the code to be broken should an adverse drug reaction occur. | |
| Participants | 30 patients undergoing total knee arthroplasty were randomised to one of two groups:
NB: One patient was excluded from the final analysis. |
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, blood loss, deep vein thrombosis, change in haemoglobin levels. | |
| Notes | Quality assessment score (Schulz criteria): 5/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Palmer 2003.
| Methods | A computer generated randomisation schedule was used to randomly assign patients into the treatment groups. The vials used for each group were only identifiable by the patient study number. | |
| Participants | 95 patients undergoing elective neurological surgery were divided into two subsets: Meningioma subset: n = 56
Vestibular Schwannoma subset: n = 39
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), blood loss, mortality (7‐day & 30‐day). | |
| Notes | Quality assessment score (Schulz criteria): 6/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated randomisation schedule |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Parvizi 2007.
| Methods | Patients were allocated according to a computer‐generated randomisation list. Adequacy of allocation concealment was unclear. | |
| Participants | 162 undergoing cardiac surgery were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood transfusion, volume of blood transfused, blood loss, myocardial infarction, re‐operation for bleeding. | |
| Notes | Quality assessment score (Schulz criteria): 5/7 Transfusion protocol used |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated randomisation list |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Penta de Peppo 1995.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 60 consecutive patients undergoing elective open‐heart surgery were randomised to one of four groups:
|
|
| Interventions |
NB: All groups were exposed to cell salvage. |
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), blood loss, re‐operation for bleeding, | |
| Notes | Quality assessment score (Schulz criteria): 2/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Unclear risk | Unclear |
Petsatodis 2006.
| Methods | Patients were randomised using an envelope technique. Method of allocation concealment was not described. | |
| Participants | 50 patients undergoing total hip arthroplasty were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), blood loss, deep vein thrombosis. | |
| Notes | Quality assessment score (Schulz criteria): 5/7 Transfusion protocol not used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Inadequate ‐ sealed envelopes |
| Blinding? All outcomes | Low risk | Double blind |
Pinosky 1997.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 59 patients undergoing cardiac surgery were randomly assigned to one of three groups:
|
|
| Interventions |
NB: Both groups were exposed to cell salvage. |
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, blood loss, aspirin use, number of patients exposed to platelets and fresh frozen plasma. | |
| Notes | Quality assessment score (Schulz criteria): 2/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Pleym 2003.
| Methods | Randomisation was by mean of a computer programme. Study medications were delivered in identical 50mL syringes. | |
| Participants | 79 patients undergoing elective cardiac surgery were randomised to one of two groups:
|
|
| Interventions |
NB: Both groups were exposed to post‐operative cell salvage, tranexamic acid, and desmopressin. |
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), blood loss, re‐operation for bleeding, fresh frozen plasma usage (units), platelet usage (units), pulmonary embolus, retransfused mediastinal shed blood, post‐operative TXA, post‐operative DDAVP, ASA 75mg/day, ASA 160mg/day. | |
| Notes | Quality assessment score (Schulz criteria): 6/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer programme |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Porte 2000.
| Methods | The trial drug was provided double‐blind by the manufacturer in blocks of 12 identical case packs. Each case pack contained all bottles for one patient, identifiable only by the sequence number. Each block of 12 case packs contained four packs of each dosage group, randomly assigned to the sequence numbers 1 to 12. Patients received the next available case pack of each block. Centres were provided with sealed cards with the randomisation codes to enable an individual patient's code to be broken in an emergency. A separate set of the sealed randomisation cards was kept at the Central Data Centre. At the end of the study all cards with randomisation codes were sent to the Central Data Centre. | |
| Participants | 141 patients undergoing orthotopic liver transplantation were randomised to one of three groups:
NB: Four patients were excluded from the final analysis. |
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Allogeneic blood usage (units), blood loss, number of patients exposed to platelets and cryoprecipitate. | |
| Notes | Quality assessment score (Schulz criteria): 5/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Poston 2006.
| Methods | Study drug or placebo was delivered to the operating room in unlabeled bottles to maintain blinding. Method of randomisation was not specified. | |
| Participants | 70 patients undergoing 'off‐pump' coronary artery bypass graft surgery were randomised to one of two groups:
NB: Demographic data were not reported. |
|
| Interventions |
NB: Both groups were exposed to cell salvage. |
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), fresh frozen plasma usage (units), platelet usage (units), myocardial infarction, deep vein thrombosis, stroke, hospital length of stay (days), Intensive Care Unit length of stay (days). | |
| Notes | Quality assessment score (Schulz criteria): 4/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Prendergast_1 1996.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 38 patients undergoing primary sternotomy for heart transplantation were randomised to one of two groups:
|
|
| Interventions |
NB: Precise dose of aprotinin (KIU or mg) was not reported. |
|
| Outcomes | Outcomes reported: Allogeneic blood usage (units), blood loss (24hrs), haemoglobin levels, creatinine levels. | |
| Notes | Quality assessment score (Schulz criteria): 2/7 Transfusion protocol not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Unclear risk | Unclear |
Prendergast_2 1996.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 32 patients undergoing re‐operative heart transplantation were randomised to one of two groups:
|
|
| Interventions |
NB: Precise dose of aprotinin (KIU or mg) was not reported. |
|
| Outcomes | Outcomes reported: Allogeneic blood usage (units), blood loss (24 hrs), haemoglobin levels, creatinine levels. | |
| Notes | Quality assessment score (Schulz criteria): 2/7 Transfusion protocol not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Unclear risk | Unclear |
Pugh 1995.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 75 patients scheduled for routine primary cardiac surgery were randomly allocated to one of three groups:
NB: Nine patients were withdrawn from the trial: two from the control group, three from the tranexamic acid group, and four from the aprotinin group. |
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), number of patinets exposed to fresh frozen plasma, blood loss, re‐operation for bleeding. | |
| Notes | Quality assessment score (Schulz criteria): 1/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Unclear risk | Unclear |
Ranaboldo 1997.
| Methods | Allocation concealment was by the use of identical coded bottles containing active drug or placebo. The method of randomisation was not described. | |
| Participants | 136 patients undergoing elective aortic surgery were randomised to one of two groups:
NB: Eight patients were excluded from the final analysis. Four deaths occurred within 7 days of surgery (two in each group). Four patients were found at operation not to be suitable for the planned reconstructive surgery. |
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), blood loss (24hrs), mortality (30 day), myocardial infarction, stroke, pulmonary embolus, deep vein thrombosis, chest infection, hepatitis, sepsis, renal failure, urinary tract infection. | |
| Notes | Quality assessment score (Schulz criteria): 4/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Rao 1999.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 30 patients undergoing elective cardiac surgery were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Allogeneic blood usage (units), blood loss (24 hrs), myocardial infarction, fresh frozen plasma usage (units), platelet usage (units), ASA treatment until surgery (185mg), ASA treatment until surgery (375mg), stroke, re‐operation for bleeding. | |
| Notes | Quality assessment score (Schulz criteria): 0/7 Transfusion protocol not used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Unclear risk | Unclear |
Ray 1997.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 106 patients undergoing aortic or mitral valve replacement or both were randomly assigned to one of two groups:
|
|
| Interventions |
NB: Both groups were exposed to cell salvage. |
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, re‐operation, platelet usage (units), fresh frozen plasma usage (units). | |
| Notes | Quality assessment score (Schulz criteria): 3/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Ray 1999.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 150 patients in elective adult cardiac surgery were randomly assigned to one of three groups:
NB: Gender or age data were not reported. |
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, re‐operation for bleeding. | |
| Notes | Quality assessment score (Schulz criteria): 4/7 Transfusion protocol not used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Ray 2001.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 100 patients undergoing elective cardiac surgery were randomly assigned to one of two groups:
NB: Gender or age data were not reported. |
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, re‐operation for bleeding, aspirin use within 10 days, Intensive Care Unit length of stay (hours), neurologic events. | |
| Notes | Quality assessment score (Schulz criteria): 1/7 Transfusion protocol was not used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | High risk | Single blind |
Ray 2005.
| Methods | Method of randomisation and allocation concealment were not described. Allocation of the randomised drug was performed by a nurse not otherwise connected with the study. | |
| Participants | 45 patients undergoing elective primary total hip arthroplasty were randomly allocated to one of three groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), blood loss (24hrs), deep vein thrombosis, pre‐operative apirin use. | |
| Notes | Quality assessment score (Schulz criteria): 2/7 Transfusion protocol not used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Rhydderch 1993.
| Methods | Methods of sequence generation and allocation concealment were unclear. | |
| Participants | 43 undergoing cardiac surgery were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Volume blood transfused, re‐operation for bleeding. | |
| Notes | Quality assessment score (Schulz criteria): 3/7 Transfusion protocol used |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Rocha 1994.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 109 of 122 eligible patients scheduled for coronary artery bypass graft surgery, valvular surgery, or mixed cardiac surgery were randomised to one of four groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage, blood loss (72hrs), mortality, thrombosis. | |
| Notes | Quality assessment score (Schulz criteria): 1/7 Transfusion protocol not used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | High risk | Single blind |
Rodrigus 1996.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 99 adult patients undergoing elective primary coronary artery bypass graft, or valvular surgery, with cardiopulmonary bypass were randomised to one of two groups:
NB: Six of the 99 patients randomised were excluded from the study. Ninety‐three patients remained in the study for analysis. |
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), fresh frozen plasma usage (units), platelet usage (units), blood loss (24hrs), mortality, myocardial infarction [definite & possible], re‐operation for bleeding, atrial fibrillation. | |
| Notes | Quality assessment score (Schulz criteria): 3/7 Transfusion protocol not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Rossi 1997.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 43 patients scheduled for elective primary myocardial revascularisation were randomly allocated to one of two groups:
NB: Gender data were not reported. |
|
| Interventions |
NB: Both groups were exposed to acute normovolaemic haemodilution (ANH). |
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, blood loss (24hrs), re‐operation for bleeding, side effects. | |
| Notes | Quality assessment score (Schulz criteria): 2/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Unclear risk | Unclear |
Royston 1987.
| Methods | Patients were randomly allocated to receive test compound by means of sealed envelopes. Method of randomisation was not described. | |
| Participants | 22 patients undergoing repeat cardiac surgery through previous median sternotomy wound were randomised to one of two groups:
NB: Gender data were not reported. |
|
| Interventions |
NB: Both groups were exposed to acute normovolaemic haemodilution (ANH). |
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), blood loss (24hrs), mortality, total haemoglobin loss, time for wound closure (mins), platelet counts. | |
| Notes | Quality assessment score (Schulz criteria):3/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | High risk | Inadequate ‐ sealed envelopes |
| Blinding? All outcomes | Unclear risk | Unclear |
Sadeghi 2007.
| Methods | Patients were randomised using a random number technique. The correct treatment option was assured by means of coded infusion syringes prepared by hospital pharmacy not involved otherwise in the study. | |
| Participants | 67 undergoing orthopaedic surgery for hip fractures were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood transfusion, mortality, blood loss, volume blood transfused (units), length of hospital stay (days). | |
| Notes | Quality assessment score (Schulz criteria):7/7 Transfusion protocol not used |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Random number technique |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Samama 2002.
| Methods | Centres were provided with sealed envelopes with the randomisation codes to enable an individual patient's code to be broken in an emergency. A separate set of sealed randomisation tables were kept at the central data centre. To maintain masking, all patients received identical volumes of solution and an identical number of bottles for the identical dose and for the continuous infusion, regardless of treatment group. | |
| Participants | 58 patients undergoing elective orthopaedic surgery were randomised to one of three groups:
NB: Gender data were not reported. |
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), blood loss, deep vein thrombosis, pulmonary embolus, trauma cases, cell salvage used, autologous transfusion. | |
| Notes | Quality assessment score (Schulz criteria):6/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation codes |
| Allocation concealment? | High risk | Inadequate ‐ sealed envelopes |
| Blinding? All outcomes | Low risk | Double blind |
Santamaria 2000.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 84 patients undergoing elective coronary artery bypass graft surgery were randomly allocated to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), blood loss, myocardial infarction, stroke, hypertension, A‐V block. | |
| Notes | Quality assessment score (Schulz criteria):4/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Santos 2006.
| Methods | Groups were randomised by means of sequentially numbered sealed envelopes opened by a nurse in the operating room. Only the nurse, who prepared the infusions, knew whether a patient received drug or placebo. Study drugs were delivered in identical volumes. Staff in the operating room and in the intensive care unit were not aware of the treatment. | |
| Participants | 65 patients undergoing primary coronary artery bypass graft surgery were randomised to one of two groups:
NB: Five patients were excluded from the final analysis. |
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), fresh frozen plasma usage (units), blood loss, mortality, re‐operation for bleeding. | |
| Notes | Quality assessment score (Schulz criteria): 4/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | High risk | Inadequate ‐ sealed envelopes |
| Blinding? All outcomes | Low risk | Double blind |
Schmartz 2003.
| Methods | Sixty patients were divided into three groups by means of computerised randomisation. Allocation concealment was not specified. | |
| Participants | 60 male patients undergoing primary elective cardiac surgery were randomised to one of three groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Allogeneic blood usage (units), blood markers of inflammation during and after CPB. | |
| Notes | Quality assessment score (Schulz criteria): 4/7 Transfusion protocol not used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computerised randomisation |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blinding |
Schweizer 2000.
| Methods | Concealment of treatment allocation was not described. Patients were allocated randomly in a double‐blind manner. Method of randomisation was not described. | |
| Participants | 60 patients undergoing elective coronary artery bypass graft surgery, aortic valve replacement and mitral valve replacement and repair were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Allogeneic blood usage (units), blood loss, mortality, myocardial infarction, re‐operation for bleeding. | |
| Notes | Quality assessment score (Schulz criteria):3/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Shore‐Lesserson 1996.
| Methods | Patients were randomly assigned to treatment or placebo by computer generated table. The pharmacist who prepared the infusions knew whether the patient received active treatment or placebo in the event of an adverse response. | |
| Participants | 31 patients undergoing repeat open heart surgery were randomised to one of two groups:
NB: One patient from the placebo group was withdrawn from the study due to excessive post‐operative bleeding and requiring intra‐aortic balloon counter pulsation. |
|
| Interventions |
NB: Both groups were exposed to cell salvage. |
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage, fresh frozen plasma usage, platelet usage, blood loss, mortality, myocardial infarction, pulmonary complications, re‐operation, renal impairment, cerebral ischemia, embolic stroke. | |
| Notes | Quality assessment score (Schulz criteria): 6/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated table |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Sorin 1999.
| Methods | Method of randomisation and allocation concealment were not described. [Abstract] | |
| Participants | 42 patients undergoing total knee replacement were randomly assigned to one of two groups:
NB: Demographic data were not reported. |
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), blood loss, deep vein thrombosis. | |
| Notes | Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Unclear risk | Unclear |
Speekenbrink 1995.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 60 patients scheduled for elective primary coronary artery bypass grafting were randomly assigned to one of four groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), fresh frozen plasma usage (units), blood loss (6hrs), myocardial infarction, haemorrhage from chest. | |
| Notes | Quality assessment score (Schulz criteria): 1/7 Transfusion protocol not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Unclear risk | Unclear |
Speekenbrink 1996.
| Methods | Study medications were supplied in boxes containing 12 bottles with 50mL solution. The randomisation code was kept by supplied. The codes were broken after data acquisition were complete and verified. | |
| Participants | 115 patients scheduled for elective coronary artery bypass graft surgery were randomised to one of three groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), blood loss (24hrs), mortality, myocardial infarction, renal failure, re‐operation for bleeding. | |
| Notes | Quality assessment score (Schulz criteria): 5/7 Transfusion protocol used Three patients were excluded from the final analysis: Two from the placebo group (one for excessive postoperative bleeding caused by a broken suture and one for a small left ventricular aneurysm requiring resection), one from the high dose aprotinin group who had dense pericardial adhesions resembling those found in reoperation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Stammers 1997.
| Methods | Method of randomisation was not described. All drugs were drawn up by a pharmacist and placed in a 500mL glass bottle which was labelled with the patient's name, registration number and date. No other clinician knew of the treatment received by the patient. | |
| Participants | 20 patients undergoing first time coronary artery bypass grafting were randomly assigned to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Allogeneic blood usage, Intensive care ventilator time (hrs), renal failure, neurological injury, hospital length of stay (days), blood loss (24hrs). | |
| Notes | Quality assessment score (Schulz criteria): 6/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Stewart 2001.
| Methods | Randomisation using numbers chosen randomly from a computer generated table. Study drug and placebo bottles were identifiable only by the random number. | |
| Participants | 30 patients undergoing elective orthognathic surgery (maxillary Le Fort I and mandibular sagittal split osteotomies) were randomised to one of two groups:
NB: Gender and age data were not reported. |
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), blood loss. | |
| Notes | Quality assessment score (Schulz criteria): 6/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated table |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Swart 1994.
| Methods | Method of randomisation was not described. Intervention and placebo solutions were supplied by Bayer AG (Germany). | |
| Participants | 50 patients undergoing primary coronary artery bypass surgery and 50 patients undergoing valve surgery were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, blood loss (48hrs), mortality, biochemistry and haematology values. | |
| Notes | Quality assessment score (Schulz criteria): 5/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Tabuchi 1994.
| Methods | Method of randomisation was not described. The study solution was prepared by the pharmacy department according to a randomised code, which was kept blind to all clinicians and investigators until all data were obtained. | |
| Participants | 40 patients undergoing elective coronary artery bypass grafting were randomised to one of two groups:
NB: Gender data were not reported. Four patients were excluded from the final analysis; three from the placebo group for surgical bleeding requiring repeat thoracotomy, and one from the aprotinin group for haemothorax. |
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), fresh frozen plasma usage (units), platelet usage (units), re‐operation for bleeding, haemothorax. | |
| Notes | Quality assessment score (Schulz criteria): 5/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Taggart 2003.
| Methods | A pre‐determined randomisation scheme was generated by the pharmaceutical company supplying the trial drug. Sealed code break cards were available if necessary. The study was analysed on an intention‐to‐treat (ITT) basis and included those patients who received open‐label aprotinin. | |
| Participants | 74 patients undergoing cardiac surgery with total arterial grafting were randomised to one of two groups:
NB: Four patients were excluded from the final analysis. |
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), number of participants exposed to fresh frozen plasma and platelets, blood loss, myocardial infarction, re‐operation for bleeding, hospital length of stay. | |
| Notes | Quality assessment score (Schulz criteria): 3/7 Transfusion protocol used NB: Nine patients in the control group (placebo) received open‐label aprotinin whilst two patients in the aprotinin group received open‐label aprotinin. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | High risk | Single blind |
Taghaddomi 2009.
| Methods | Table of random numbers was used to generate the allocation sequence. An independent anesthesiologist prepared coded infusions with tranexamic acid and placebo and was not directly involved in the clinical treatment of randomised patients. Both operating room staff and the intensive care unit staff were blinded regarding the study group. | |
| Participants | 100 patients undergoing cardiac surgery were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood transfusion, blood loss, stroke, renal failure, myocardial infarction. | |
| Notes | Quality assessment score (Schulz criteria): 6/7 Transfusion protocol used |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Table of random numbers |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Tanaka 2001.
| Methods | Ampoules containing either tranexamic acid or placebo were numbered and placed in envelopes at random by a pharmacologist. | |
| Participants | 99 patients undergoing elective knee arthroplasty were randomised to one of four groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), blood loss. | |
| Notes | Quality assessment score (Schulz criteria): 6/7 Transfusion protocol not used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Tassani 2000.
| Methods | Study performed in a double blind, placebo controlled manner. Method of randomisation and allocation concealment were not described. | |
| Participants | 20 patients undergoing elective cardiac surgery were randomly assigned to one of two groups:
NB: Gender and age data were not reported. |
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), blood loss. | |
| Notes | Quality assessment score (Schulz criteria): 2/7 Transfusion protocol not used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Thorpe 1994.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 17 patients undergoing elective knee replacement surgery were randomly allocated to one of two groups:
NB: Demographic data were not reported. |
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), blood loss, femoral thrombosis. | |
| Notes | Quality assessment score (Schulz criteria): 1/7 Transfusion protocol not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Unclear risk | Unclear |
Trinh‐Duc 1992.
| Methods | Method of randomisation and allocation concealment were not described. [French language] | |
| Participants | 60 patients undergoing cardiac surgery were randomised to one of two groups:
NB: Four patients were excluded from the final analysis. |
|
| Interventions |
NB: Both groups received cell salvage and acute normovolaemic haemodilution (ANH). |
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), fresh frozen plasma usage (units), blood loss (48hrs), mortality, minor stroke, respiratory problems, severe hypotension. | |
| Notes | Transfusion protocol not used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Unclear risk | Unclear |
Troianos 1999.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 72 patients undergoing primary coronary artery bypass surgery were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), number of patients exposed to fresh frozen plasma and platelets, blood loss (6hrs & 48hrs), re‐exploration for bleeding, haemoglobin loss. | |
| Notes | Quality assessment score (Schulz criteria): 3/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Turkoz 2001.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 30 patients undergoing elective cardiac surgery were allocated randomly to one of three groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Allogeneic blood usage (units), blood loss. | |
| Notes | Quality assessment score (Schulz criteria): 2/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Unclear risk | Unclear |
Uozaki 2001.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 14 patients undergoing elective cardiac surgery were allocated to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Allogeneic blood usage (units), blood loss (24hrs), re‐operation for bleeding. | |
| Notes | Quality assessment score (Schulz criteria): 0/7 Transfusion protocol not used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Unclear risk | Unclear |
Urban 2001.
| Methods | Patients were randomised by means of a random number generator. Method used to conceal treatment allocation was not described. | |
| Participants | 60 patients undergoing complex reconstructive spinal surgery were randomised to one of three groups:
NB: Gender data were not reported. Five patients were excluded from the final analysis. |
|
| Interventions |
NB: All groups were exposed to cell salvage and pre‐operative autologous blood donation (PAD). |
|
| Outcomes | Outcomes reported: Allogeneic & autologous blood usage (units), blood loss, respiratory complications. | |
| Notes | Quality assessment score (Schulz criteria): 2/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Random number generator |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Unclear risk | Unclear |
Utada 1997.
| Methods | Method of randomisation and allocation concealment were not described. [Japanese language] | |
| Participants | 21 patients undergoing primary total hip replacement were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Allogeneic blood usage (units), blood loss, changes in haemoglobin levels. | |
| Notes | Transfusion protocol not used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Unclear risk | Unclear |
Van der Linden 2005.
| Methods | Random assignment was conducted using unmasked envelopes, each containing a card indicating treatment with aprotinin or placebo. A nurse, assigned to another department of the hospital was responsible for the preparation of placebo and treatment solutions, which were identical in appearance and packing. | |
| Participants | 75 patients scheduled for urgent or acute isolated coronary artery bypass graft surgery were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), fresh frozen plasma usage (units), platelets usage (units), mortality, myocardial infarction, stroke, atrial fibrillation, number of patients receiving TXA treatment. | |
| Notes | Quality assessment score (Schulz criteria): 4/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | High risk | Inadequate ‐ sealed envelopes |
| Blinding? All outcomes | Low risk | Double blind |
Van Oeveren 1987.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 22 patients undergoing coronary artery bypass graft surgery were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Allogeneic blood usage, blood loss, biochemical markers. | |
| Notes | Quality assessment score (Schulz criteria): 0/7 Transfusion protocol not used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Unclear risk | Unclear |
Vander‐Salm 1996.
| Methods | Random assignment was by means of a random number table and drug preparation was performed by the hospital pharmacy. | |
| Participants | 103 patients undergoing coronary artery bypass graft surgery or valvular surgery were randomly assigned to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), number of patients exposed to fresh frozen plasma, blood loss (12hrs & 24hrs), mortality, cerebrovascular accident, re‐operation for bleeding. | |
| Notes | Quality assessment score (Schulz criteria): 7/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Random number table |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Vanek 2005.
| Methods | An envelope method with random numbers was used to randomise patients. An independent pharmacologist not directly involved in the clinical treatment of randomised patients prepared coded infusions with the study drug and placebo. | |
| Participants | 91 patients undergoing cardiac surgery were randomised to one of three groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, blood loss, number of patients exposed to fresh frozen plasma. | |
| Notes | Quality assessment score (Schulz criteria): 6/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Random numbers |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Vedrinne 1992.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 90 consecutive patients undergoing cardiac surgery were randomly allocated to one of three groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), number of patients exposed to fresh frozen plasma and platelets, blood loss (6hrs & 48hrs), re‐operation for bleeding, haemoglobin loss. | |
| Notes | Quality assessment score (Schulz criteria): 2/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Unclear risk | Unclear |
Veien 2002.
| Methods | Patients were randomised using a computer generated randomisation table to treatment groups. Method of allocation concealment was not described. | |
| Participants | 30 patients undergoing elective orthopaedic surgery were randomly assigned to one of two groups:
|
|
| Interventions |
NB: All groups were exposed to cell salvage. |
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), cell salvage autologous blood returned, thrombo‐embolic events. | |
| Notes | Quality assessment score (Schulz criteria):3/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Computer generated randomisation table |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Unclear risk | Unclear |
Wei 2006.
| Methods | Method of allocation concealment and randomisation were not described. | |
| Participants | 112 patients undergoing 'off‐pump' coronary artery bypass graft surgery were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), fresh frozen plasma usage (units), number of patients exposed to fresh frozen plasma, blood loss (24hrs), hospital length of stay (days), Intensive Care Unit length of stay (days). | |
| Notes | Quality assessment score (Schulz criteria): 4/7 Transfusion protocol used Results for aprotinin versus control and TXA versus control ‐ reported in separate publications. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Wendel 1995.
| Methods | Method of randomisation was not described. Aprotinin and placebo were provided by the manufacturer in identical bottles that differed only in the random numbers on their labels. | |
| Participants | 40 patients undergoing aorto‐coronary artery bypass graft surgery were randomised to one of two groups:
NB: Gender data were not reported. |
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), blood loss, myocardial infarction, infarctional biomarkers. | |
| Notes | Quality assessment score (Schulz criteria): 5/7 Transfusion protocol not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Wong 2000.
| Methods | The randomisation and preparation of study drugs was performed by the hospitals department of pharmacy. There was no attempt to stratify the randomisation process. | |
| Participants | 80 patients undergoing elective cardiac surgery were randomised to one of two groups:
NB: Gender data were not reported. |
|
| Interventions |
NB: Both groups were exposed to cell salvage. |
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), blood loss (24hrs), myocardial infarction, mortality, fresh frozen plasma usage, platelet usage (units), re‐operation for bleeding, stroke. | |
| Notes | Quality assessment score (Schulz criteria): 5/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Wong 2008.
| Methods | A computer‐generated randomisation list was used for sequence generation. The randomisation schedule was kept inaccessible throughout the study period. Patient assignments were placed into sequentially numbered opaque sealed envelopes. A research pharmacist, not involved with care of the patient prepared the placebo and treatment medications that were identical in appearance. The research personnel, anaesthesiologists, surgeons, and operating room staff were blinded to the randomisation. | |
| Participants | 151 patients undergoing orthopaedic (spinal) surgery were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: number of patients exposed to allogeneic blood transfusion, blood loss, volume blood transfused (units), deep vein thrombosis. | |
| Notes | Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated randomisation list |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Low risk | Double blind |
Wu 2006.
| Methods | Method of randomisation was not described. Sealed envelopes were used to conceal treatment allocation. | |
| Participants | 217 patients undergoing liver tumor resection were randomised to one of two groups:
NB: Three patients were excluded from the final analysis. |
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, blood loss, hospital length of stay (days), wound infection. | |
| Notes | Quality assessment score (Schulz criteria): 4/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | High risk | Inadequate ‐ sealed envelopes |
| Blinding? All outcomes | Low risk | Double blind |
Yamasaki 2004.
| Methods | Randomisation was carried out by a person not involved in the operation using a ticket drawn from an envelope containing an equal number of tranexamic acid and placebo tickets. | |
| Participants | 40 patients undergoing cementless total hip arthroplasty were randomised to one of two groups:
|
|
| Interventions |
NB: Both groups received pre‐operatively donated autologous blood (PAD). |
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, blood loss (24hrs), thrombo‐embolic events. | |
| Notes | Quality assessment score (Schulz criteria): 2/7 Transfusion protocol not used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Adequate |
| Allocation concealment? | High risk | Inadequate |
| Blinding? All outcomes | Unclear risk | Unclear |
Yassen 1993.
| Methods | Method of randomisation and allocation concealment were not described. | |
| Participants | 20 patients undergoing orthotopic liver transplantation were randomly allocated to one of two groups:
|
|
| Interventions |
NB: Both groups were exposed to cell salvage. |
|
| Outcomes | Outcomes reported: Allogeneic blood usage (units), fresh frozen plasma usage (units), platelets usage (units), blood loss, any thrombosis. | |
| Notes | Quality assessment score (Schulz criteria): 2/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Zabeeda 2002.
| Methods | Method of randomisation and allocation concealment were not described. The surgeon was blinded with respect to whether tranexamic acid or placebo was infused. | |
| Participants | 50 patients undergoing coronary artery bypass graft surgery were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage (units), blood loss (24hrs), stroke, mediastinal infection, pre‐operative aspirin use. | |
| Notes | Quality assessment score (Schulz criteria): 4/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Low risk | Double blind |
Zhang 2007.
| Methods | Methods of sequence generation and allocation concealment were not described. [Chinese language] | |
| Participants | 102 patients undergoing orthopaedic knee surgery were randomised to one of two groups:
NB: Randomised subjects were aged between 59‐77 years of age. Gender: M/F = 43/59 |
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Volume blood transfused (units), blood loss, deep vein thrombosis. | |
| Notes | Use of transfusion protocol is not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Unclear risk | Unclear |
Zohar 2004.
| Methods | Patients were randomly allocated to treatment groups using a computer generated randomisation table. Method used to conceal treatment allocation was not described. | |
| Participants | 40 patients undergoing total knee replacement were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood, allogeneic blood usage, blood loss (12hrs), thrombo‐embolic events (30‐day), hospital length of stay (days). | |
| Notes | Quality assessment score (Schulz criteria): 1/7 Transfusion protocol used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated randomisation table |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Unclear risk | Unclear |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Fejer 1998 | Study was excluded on the basis there was uncertainty regarding the age of study participants. As the study involved thoracolumbar transpedicular (TLT) fixation of the spine for spondylolisthesis subjects less than 18 years of age may have been included. |
| Langdown 2000 | Study did not report the number of patients randomised to each trial arm rather reported the total number of patients randomised. Study was excluded on the basis there was uncertainty regarding the number of patients in each trial arm. |
| Montesano 1996 | Abstract refers to patients as being randomly selected but methods section of paper states study was retrospective. Study was excluded on the basis there was uncertainty regarding trial design. |
| Zufferey 2010 | Patients undergoing surgery for hip fractures ‐ not elective. |
Characteristics of ongoing studies [ordered by study ID]
Myles 2008.
| Trial name or title | ATACAS trial |
| Methods | Multi‐centre, randomised, blinded 2x2 factorial trial. |
| Participants | N=4600, patients undergoing elective CABG surgery. |
| Interventions | Patients will be allocated to one of four groups (1) Aspirin (2) Tranexamic acid (3) Tranexamic acid plus aspirin (4) Placebo |
| Outcomes | Mortality Myocardial infarction Stroke Pulmonary embolism Renal failure Bowel infarction Re‐operation for bleeding Blood transfusion |
| Starting date | |
| Contact information | |
| Notes |
Verma 2010.
| Trial name or title | |
| Methods | Single‐centre, randomised, double‐blinded control study |
| Participants | Patient undergoing corrective spinal surgery. |
| Interventions | Patients will be allocated to one of three groups (1) Tranexamic acid (2) EACA (3) Saline |
| Outcomes | Perioperative blood loss Renal failure |
| Starting date | |
| Contact information | |
| Notes | ClinicalTrials.gov ID: NCT00958581 |
Contributions of authors
Contributors (names are listed alphabetically)
Paul Carless (University of Newcastle) obtained relevant papers, applied inclusion/ exclusion criteria to retrieved papers, quality assessed trials, extracted data from the trials, entered data into RevMan Analyses, entered study details into Review Manager 4.2.8, and co‐wrote review; Dean Fergusson (ISPOT Coordinator*) co‐conceived the review, performed the original literature searches, data extraction, and analyses; David Henry (University of Newcastle) obtained funding for the study, was involved in study design, screened abstracts and titles for relevant articles, and co‐wrote review; Katharine Ker (London School of Hygiene & Tropical Medicine) performed updated literature searches extracted data and co‐wrote the updated review; Annette Moxey (University of Newcastle) obtained relevant papers, applied inclusion/ exclusion criteria to retrieved papers, quality assessed trials, extracted data from the trials and entered data into MetaView 3.1; Dianne O'Connell (University of Newcastle) provided statistical consultancy for the review, checked data for consistency, analysed and interpreted the results, provided methodological content, and co‐wrote review, Barrie Stokes (University of Newcastle) provided statistical consultancy for the review and performed Bayesian analyses. * ISPOT ‐ International Study of Peri‐Operative Transfusion
Sources of support
Internal sources
Special purpose grant, Hunter Area Pathology Service, Australia.
External sources
Australian Health Ministers' Advisory Committee. National Health and Medical Research Council of Australia, Australia.
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Alajmo 1989 {published data only}
- Alajmo F, Calamai G, Perna AM, Melissano G, Pretelli P, Palmarini MF, et al. High‐dose aprotinin: hemostatic effects in open heart operations. Annals of Thoracic Surgery 1989;48(4):536‐9. [DOI] [PubMed] [Google Scholar]
Alderman 1998 {published data only}
- Alderman EL, Levy JH, Rich JB, Nili M, Vidne B, Schaff H, et al. Analyses of coronary graft patency after aprotinin use: results from the International Multicenter Aprotinin Graft Patency Experience (IMAGE) trial. Journal of Thoracic & Cardiovascular Surgery 1998;116(5):716‐30. [DOI] [PubMed] [Google Scholar]
Alvarez 1995 {published data only}
- Alvarez JM, Quiney NF, McMillan D, Joscelyne K, Connelly T, Brady P, et al. The use of ultra‐low‐dose aprotinin to reduce blood loss in cardiac surgery. Journal of Cardiothoracic & Vascular Anesthesia 1995;9(1):29‐33. [DOI] [PubMed] [Google Scholar]
Alvarez 2001 {published data only}
- Alvarez JM, Jackson LR, Chatwin C, Smolich JJ. Low‐dose postoperative aprotinin reduces mediastinal drainage and blood product use in patients undergoing primary coronary artery bypass grafting who are taking aspirin: a prospective, randomized, double‐blind, placebo‐controlled trial. Journal of Thoracic & Cardiovascular Surgery 2001;122(3):457‐63. [DOI] [PubMed] [Google Scholar]
Alvarez 2008 {published data only}
- Álvarez JC, Santiveri FX, Ramos I, Vela E, Puig L, Escolano F. Tranexamic acid reduce blood transfusion in total knee arthroplasty event when a blood conservation program is applied. Blood Conservation & Transfusion Alternatives 2008;48(3):519‐25. [DOI] [PubMed] [Google Scholar]
Amar 2003 {published data only}
- Amar D, Grant FM, Zhang H, Boland PJ, Leung DH, Healey JA. Antifibrinolytic therapy and perioperative blood loss in cancer patients undergoing major orthopedic surgery. Anesthesiology 2003;98(2):337‐42. [DOI] [PubMed] [Google Scholar]
Andreasen 2004 {published data only}
- Andreasen JJ, Nielsen C. Prophylactic tranexamic acid in elective, primary coronary artery bypass surgery using cardiopulmonary bypass. European Journal of Cardio‐Thoracic Surgery 2004;26(2):311‐7. [DOI] [PubMed] [Google Scholar]
Apostolakis 2008 {published data only}
- Apostolakis E, Panagopoulos N, Koletsis E N, Crockett J, Stamou‐Kouki H, Sourgiadaki E, et al. Influence of ultra‐low dose Aprotinin on thoracic surgical operations: a prospective randomized trial. Journal of cardiothoracic surgery 2008;3:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Armellin 2001 {published data only}
- Armellin G, Casella S, Guzzinati S, Pasini L, Marcassa A, Giron G. Tranexamic acid in aortic valve replacement. Journal of Cardiothoracic & Vascular Anesthesia 2001;15(3):331‐5. [DOI] [PubMed] [Google Scholar]
Arom 1994 {published data only}
- Arom KV, Emery RW. Decreased postoperative drainage with addition of epsilon‐aminocaproic acid before cardiopulmonary bypass. Annals of Thoracic Surgery 1994;57(5):1108‐12. [DOI] [PubMed] [Google Scholar]
Ashraf 1997 {published data only}
- Ashraf S, Tian Y, Cowan D, Nair U, Chatrath R, Saunders NR, et al. "Low‐dose" aprotinin modifies hemostasis but not proinflammatory cytokine release. Annals of Thoracic Surgery 1997;63(1):68‐73. [DOI] [PubMed] [Google Scholar]
Asimakopoulos 2000 {published data only}
- Asimakopoulos G, Kohn A, Stefanou DC, Haskard DO, Landis RC, Talyor KM. Leukocyte integrin expression in patients undergoing cardiopulmonary bypass. Annals of Thoracic Surgery 2000;69:1192‐7. [DOI] [PubMed] [Google Scholar]
Baele 1992 {published data only}
- Baele PL, Ruiz‐Gomez J, Londot C, Sauvage M, Dyck MJ, Robert A. Systematic use of aprotinin in cardiac surgery: influence on total homologous exposure and hospital cost. Acta Anaesthesiologica Belgica 1992;43(2):103‐12. [PubMed] [Google Scholar]
Bailey 1994 {published data only}
- Bailey CR, Kelleher A, Wielogorski AK. Randomised placebo‐controlled double blind study of three aprotinin regimes in primary cardiac surgery. British Journal of Surgery 1994;81:969‐73. [DOI] [PubMed] [Google Scholar]
Basora 1999 {published data only}
- Basora M, Gomar C, Escolar G, Pacheco M, Fita G, Rodriguez E, Ordinas A. Platelet function during cardiac surgery and cardiopulmonary bypass with low‐dose aprotinin. Journal of Cardiothoracic & Vascular Anesthesia 1999;13(4):382‐7. [DOI] [PubMed] [Google Scholar]
Bennett‐Guerrero 1997 {published data only}
- Bennett‐Guerrero E, Sorohan JG, Gurevich ML, Kazanjian PE, Levy RR, Barbera AV, et al. Cost‐benefit and efficacy of aprotinin compared with epsilon‐aminocaproic acid in patients having repeated cardiac operations: a randomized, blinded clinical trial. Anesthesiology 1997;87(6):1373‐80. [DOI] [PubMed] [Google Scholar]
Benoni 1996 {published data only}
- Benoni G, Fredin H. Fibrinolytic inhibition with tranexamic acid reduces blood loss and blood transfusion after knee arthroplasty: a prospective, randomised, double‐blind study of 86 patients. Journal of Bone & Joint Surgery ‐ British Volume 1996;78(3):434‐40. [PubMed] [Google Scholar]
Benoni 2000 {published data only}
- Benoni G, Lethagen S, Nilsson P, Fredin H. Tranexamic acid, given at the end of the operation, does not reduce postoperative blood loss in hip arthroplasty. Acta Orthopaedica Scandinavica 2000;71(3):250‐4. [DOI] [PubMed] [Google Scholar]
Benoni 2001 {published data only}
- Benoni G, Fredin H, Knebel R, Nilsson P. Blood conservation with tranexamic acid in total hip arthroplasty: a randomized, double‐blind study in 40 primary operations. Acta Orthopaedica Scandinavica 2001;72(5):442‐8. [DOI] [PubMed] [Google Scholar]
Berenholtz 2009 {published data only}
- Berenholtz SM, Cuong Pham J, Garrett‐Mayer E, Atchison CW, Kostuik JP, Cohen DB, et al. Effect of Epsilon Aminocaproic Acid on red‐cell transfusion requirements in major spinal surgery. Spine 2009;34(19):2096‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bernet 1999 {published data only}
- Bernet F, Carrel T, Marbet G, Skarvan K, Stulz P. Reduction of blood loss and transfusion requirements after coronary artery bypass grafting: similar efficacy of tranexamic acid and aprotinin in aspirin‐treated patients. Journal of Cardiac Surgery 1999;14(2):92‐7. [DOI] [PubMed] [Google Scholar]
Bert 2008 {published data only}
- Bert C, Buck F, Sergeant P, Hemelrijck J, Kasran A, Duppen V, et al. Aprotinin reduces cardiac troponin I release and inhibits apoptosis of polymorphonuclear cells during off‐pump coronary artery bypass surgery. Journal of cardiothoracic and vascular anesthesia 2008;22(1):16‐22. [DOI] [PubMed] [Google Scholar]
Bidstrup 1989 {published data only}
- Bidstrup BP, Royston D, Sapsford RN, Taylor KM. Reduction in blood loss and blood use after cardiopulmonary bypass with high dose aprotinin (Trasylol). Journal of Thoracic & Cardiovascular Surgery 1989;97(3):364‐72. [PubMed] [Google Scholar]
Bidstrup 1990 {published data only}
- Bidstrup BP, Royston D, McGuinness C, Sapsford RN. Aprotinin in aspirin‐pretreated patients. Perfusion 1990;5:77‐81. [Google Scholar]
Bidstrup 1993 {published data only}
- Bidstrup BP, Underwood SR, Sapsford RN, Streets EM. Effect of aprotinin (Trasylol) on aorta‐coronary bypass graft patency. Journal of Thoracic & Cardiovascular Surgery 1993;105(1):147‐52. [PubMed] [Google Scholar]
Bidstrup 2000 {published data only}
- Bidstrup BP, Hunt BJ, Sheikh S, Parratt RN, Bidstrup JM, Sapsford RN. Amelioration of the bleeding tendency of preoperative aspirin after aortocoronary bypass grafting. Annals of Thoracic Surgery 2000;69(2):541‐7. [DOI] [PubMed] [Google Scholar]
Blauhut 1994 {published data only}
- Blauhut B, Harringer W, Bettelheim P, Doran JE, Spath P, Lundsgaard‐Hansen P. Comparison of the effects of aprotinin and tranexamic acid on blood loss and related variables after cardiopulmonary bypass. Journal of Thoracic & Cardiovascular Surgery 1994;108(6):1083‐91. [PubMed] [Google Scholar]
Boldt 1991 {published data only}
- Boldt J, Zickmann B, Czeke A, Herold C, Dapper F, Hempelmann G. Blood conservation techniques and platelet function in cardiac surgery. Anesthesiology 1991;75:426‐32. [DOI] [PubMed] [Google Scholar]
Boldt 1994 {published data only}
- Boldt J, Schindler E, Knothe C, Hammermann H, Stertmann WA, Hempelmann G. Does aprotinin influence endothelial‐associated coagulation in cardiac surgery?. Journal of Cardiothoracic & Vascular Anesthesia 1994;8(5):527‐31. [DOI] [PubMed] [Google Scholar]
Boylan 1996 {published data only}
- Boylan JF, Klinck JR, Sandler AN, Arellano R, Greig PD, Nierenberg H, et al. Tranexamic acid reduces blood loss, transfusion requirements, and coagulation factor use in primary orthotopic liver transplantation. Anesthesiology 1996;85(5):1043‐8. [DOI] [PubMed] [Google Scholar]
Brown 1997 {published data only}
- Brown RS, Thwaites BK, Mongan PD. Tranexamic acid is effective in decreasing postoperative bleeding and transfusions in primary coronary artery bypass operations: A double‐blind, randomized, placebo‐controlled trial. Anesthesia & Analgesia 1997;85(5):963‐970. [DOI] [PubMed] [Google Scholar]
Caglar 2008 {published data only}
- Caglar G S, Tasci Y, Kayikcioglu F, Haberal A. Intravenous tranexamic acid use in myomectomy: a prospective randomized double‐blind placebo controlled study. European journal of obstetrics, gynecology, and reproductive biology 2008;137(2):227‐31. [DOI] [PubMed] [Google Scholar]
Camarasa 2006 {published data only}
- Camarasa MA, Olle G, Serra‐Prat M, Martin A, Sanchez M, Ricos P, et al. Efficacy of aminocaproic, tranexamic acids in the control of bleeding during total knee replacement: a randomized clinical trial. British Journal of Anaesthesia 2006;96(5):576‐82. [DOI] [PubMed] [Google Scholar]
Capdevila 1998 {published data only}
- Capdevila X, Calvet Y, Biboulet P, Biron C, Rubenovitch J, d'Athis F. Aprotinin decreases blood loss and homologous transfusions in patients undergoing major orthopedic surgery. Anesthesiology 1998;88(1):50‐7. [DOI] [PubMed] [Google Scholar]
Carrera 1994 {published data only}
- Carrera A, Martinez MV, Garcia Guiral M, Herrero E, Peral A, Planas A. Utilizacion de altas dosis de aprotinina en cirugia cardiaca ‐ High doses of aprotinin in cardiac surgery. Revista Espanola de Anestesiologia y Reanimacion 1994;41:13‐9. [PubMed] [Google Scholar]
Casas 1995 {published data only}
- Casas JI, Zuazu‐Jausoro I, Mateo J, Oliver A, Litvan H, Muniz‐Diaz E, et al. Aprotinin versus desmopressin for patients undergoing operations with cardiopulmonary bypass. A double‐blind placebo‐controlled study. Journal of Thoracic & Cardiovascular Surgery 1995;110(4 Pt 1):1107‐17. [DOI] [PubMed] [Google Scholar]
Casati 1999 {published data only}
- Casati V, Guzzon D, Oppizzi M, Cossolini M, Torri G, Calori G, Alfieri O. Hemostatic effects of aprotinin, tranexamic acid and epsilon‐aminocaproic acid in primary cardiac surgery. Annals of Thoracic Surgery 1999;68(6):2252‐6. [DOI] [PubMed] [Google Scholar]
Casati 2000 {published data only}
- Casati V, Guzzon D, Oppizzi M, Bellotti F, Franco A, Gerli C, et al. Tranexamic acid compared with high‐dose aprotinin in primary elective heart operations: effects on perioperative bleeding and allogeneic transfusions. Journal of Thoracic & Cardiovascular Surgery 2000;120(3):520‐7. [DOI] [PubMed] [Google Scholar]
Casati 2001 {published data only}
- Casati V, Gerli C, Franco A, Torri G, D'Angelo A, Benussi S, Alfieri O. Tranexamic acid in off‐pump coronary surgery: a preliminary, randomized, double‐blind, placebo‐controlled study. Annals of Thoracic Surgery 2001;72(2):470‐5. [DOI] [PubMed] [Google Scholar]
Casati 2002 {published data only}
- Casati V, Sandrelli L, Speziali G, Calori G, Grasso MA, Spagnolo S. Hemostatic effects of tranexamic acid in elective thoracic aortic surgery: a prospective, randomized, double‐blind, placebo‐controlled study. Journal of Thoracic & Cardiovascular Surgery 2002;123(6):1084‐91. [DOI] [PubMed] [Google Scholar]
Casati 2004 {published data only}
- Casati V, Della VP, Benussi S, Franco A, Gerli C, Baili P, et al. Effects of tranexamic acid on postoperative bleeding and related hematochemical variables in coronary surgery: Comparison between on‐pump and off‐pump techniques. Journal of Thoracic & Cardiovascular Surgery 2004;128(1):83‐91. [DOI] [PubMed] [Google Scholar]
Cicek 1996a {published data only}
- Cicek S, Demirkilic U, Kuralay E, Ozal E, Tatar H. Postoperative Aprotinin: Effect on blood loss and transfusion requirements in cardiac operations. Annals of Thoracic Surgery 1996;61:1372‐6. [DOI] [PubMed] [Google Scholar]
Cicek 1996b {published data only}
- Cicek S, Demirkilic U, Ozal E, Kuralay E, Bingol H, Tatar H, Ozturk OY. Postoperative use of aprotinin in cardiac operations: An alternative to its prophylactic use. Journal of Thoracic & Cardiovascular Surgery 1996;112(6):1462‐7. [DOI] [PubMed] [Google Scholar]
Cicekcioglu 2006 {published data only}
- Cicekcioglu F, Cagli K, Emir M, Topbas M, Catav Z, Sener E, et al. Effects if minimal dose aprotinin on blood loss and fibrinolytic system‐complement activation in coronary artery bypass grafting. Journal of Cardiac Surgery 2006;21:336‐41. [DOI] [PubMed] [Google Scholar]
Claeys 2007 {published data only}
- Claeys M A, Vermeersch N, Haentjens P. Reduction of blood loss with tranexamic acid in primary total hip replacement surgery. Acta chirurgica Belgica 2007;107(4):397‐401. [DOI] [PubMed] [Google Scholar]
Coffey 1995 {published data only}
- Coffey A, Pittmam J, Halbrook H, Fehrenbacher J, Beckman D, Hormuth D, et al. The use of tranexamic acid to reduce postoperative bleeding following cardiac surgery: A double‐blind randomized trial. American Surgeon 1995;61(7):566‐8. [PubMed] [Google Scholar]
Cohen 1998 {published data only}
- Cohen G, Ivanov J, Weisel RD, Rao V, Mohabeer MK, Mickle DA. Aprotinin and dipyridamole for the safe reduction of postoperative blood loss. Annals of Thoracic Surgery 1998;65(3):674‐83. [DOI] [PubMed] [Google Scholar]
Colwell 2007 {published data only}
- Colwell CW, Chelly JE, Murkin JM, Stevens D, O'Keefe TJ, Hall R, et al. Randomized study of Aprotinin effect in transfusions and blood loss in primary THA. Clinical Orthopaedics & Relation Research 2007;465:189‐95. [DOI] [PubMed] [Google Scholar]
Corbeau 1995 {published data only}
- Corbeau JJ, Monrigal JP, Jacob JP, Cottineau C, Moreau X, Bukowski JG, et al. Comparaison des effets de l'aprotinine et de l'acide tranexamique sur le saignment en chirurgie cardiaque ‐ Comparative effects of aprotinin and tranexamic acid on blood loss in cardiac surgery. Annales Francaises d'Anesthesie et de Reanimation 1995;14:154‐61. [PubMed] [Google Scholar]
Cosgrove 1992 {published data only}
- Cosgrove DM, 3d, Heric B, Lytle BW, Taylor PC, Novoa R, Golding LA, et al. Aprotinin therapy for reoperative myocardial revascularization: a placebo‐controlled study. Annals of Thoracic Surgery 1992;54(6):1031‐6. [DOI] [PubMed] [Google Scholar]
Cvachovec 2001 {published data only}
- Cvachovec K, Sechovska H, Berousek J, Popelka S, Hladikova M. Aprotinin (Antilysin Spofa)‐‐its effect on decreasing hemorrhage in the postoperative period in hip arthroplasty [Czech]. Acta Chirurgiae Orthopaedicae et Traumatologiae Cechoslovaca 2000;68(5):311‐4. [PubMed] [Google Scholar]
D'Ambra 1996 {published data only}
- D'Ambra MN, Akins CW, Blackstone EH, Bonney SL, Cohn LH, Cosgrove DM, et al. Aprotinin in primary valve replacement and reconstruction: a multicenter, double‐blind, placebo‐controlled trial. Journal of Thoracic & Cardiovascular Surgery 1996;112(4):1081‐9. [DOI] [PubMed] [Google Scholar]
- D'Ambra MN, Lynch KE, Boccagno J, et al. The effect of perioperative administration of recombinant human erythropoietin (R‐HuEPO) in CABG patients: A double blind, placebo‐controlled trial. Anesthesiology: ASA Abstracts 1992;77(3A):A159. [Google Scholar]
D'Ambrosio 1999 {published data only}
- D'Ambrosio A, Borghi B, Damato A, D'Amato G, Antonacci D, Valeri F. Reducing perioperative blood loss in patients undergoing total hip arthroplasty. International Journal of Artificial Organs 1999;22(1):47‐51. [PubMed] [Google Scholar]
Daily 1994 {published data only}
- Daily PO, Lamphere JA, Dembitsky WP, Adamson RM, Dans NF. Effect of prophylactic epsilon‐aminocaproic acid on blood loss and transfusion requirements in patients undergoing first‐time coronary artery bypass grafting. A randomized, prospective, double‐blind study. Journal of Thoracic & Cardiovascular Surgery 1994;108(1):99‐106. [PubMed] [Google Scholar]
Dalmau 1999 {published data only}
- Dalmau A, Sabate A, Acosta F, Garcia‐Huete L, Koo M, Reche M, et al. Comparative study of antifibrinolytic drugs in orthotopic liver transplantation. Transplantation Proceedings 1999;31(6):2361‐2. [DOI] [PubMed] [Google Scholar]
Dalmau 2000 {published data only}
- Dalmau A, Sabate A, Acosta F, Garcia‐Huete L, Koo M, Sansano T, et al. Tranexamic acid reduces red cell transfusion better than epsilon‐aminocaproic acid or placebo in liver transplantation. Anesthesia & Analgesia 2000;91(1):29‐34. [DOI] [PubMed] [Google Scholar]
Dalmau 2004 {published data only}
- Dalmau A, Sabate A, Koo M, Bartolome C, Rafecas A, Figueras J, et al. The prophylactic use of tranexamic acid and aprotinin in orthotopic liver transplantation: a comparative study. Liver Transplantation 2004;10(2):279‐84. [DOI] [PubMed] [Google Scholar]
Defraigne 2000 {published data only}
- Defraigne JO, Pincemail J, Larbuisson R, Blaffart F, Limet R. Cytokine release and neutrophil activation are not prevented by heparin‐ coated circuits and aprotinin administration. Annals of Thoracic Surgery 2000;69(4):1084‐91. [DOI] [PubMed] [Google Scholar]
Del Rossi 1989 {published data only}
- DelRossi AJ, Cernaianu AC, Botros S, Lemole GM, Moore R. Prophylactic treatment of postperfusion bleeding using EACA. Chest 1989;96(1):27‐30. [DOI] [PubMed] [Google Scholar]
Deleuze 1991 {published data only}
- Deleuze P, Loisance DY, Feliz A, Hillion ML, Castanie JB, Richemond J, et al. Reduction of per‐ and postoperative blood loss with aprotinin (Trasylol) during extracorporeal circulation [French]. Archives des Maladies du Coeur et des Vaisseaux 1991;84(12):1797‐802. [PubMed] [Google Scholar]
Demeyere 2006 {published data only}
- Demeyere R, Bosteels A, Arnout J, Gasthuisberg UV. Comparison of the effects of tranexamic acid, aprotinin and placebo on blood conservation, fibrinolysis and platelet function with extensive heart surgery. Critical Care 2006;10(Suppl 1):S67. [Google Scholar]
Desai 2009 {published data only}
- Desai P H, Kurian D, Thirumavalavan N, Desai S P, Ziu P, Grant M, et al. A randomized clinical trial investigating the relationship between aprotinin and hypercoagulability in off‐pump coronary surgery. Anesthesia and analgesia 2009;109(5):1387‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dietrich 1990 {published data only}
- Dietrich W, Spannagl M, Jochum M, Wendt P, Schramm W, Barankay A, Sebening F, Richter JA. Influence of high‐dose aprotinin treatment on blood loss and coagulation patterns in patients undergoing myocardial revascularization. Anesthesiology 1990;73(6):1119‐26. [DOI] [PubMed] [Google Scholar]
Dietrich 1992 {published data only}
- Dietrich W, Barankay A, Hahnel C, Richter JA. High‐dose aprotinin in cardiac surgery: three years' experience in 1,784 patients. Journal of Cardiothoracic & Vascular Anesthesia 1992;6(3):324‐7. [DOI] [PubMed] [Google Scholar]
Dietrich 1995 {published data only}
- Dietrich W, Dilthey G, Spannagl M, Jochum M, Braun SL, Richter JA. Influence of high‐dose aprotinin on anticoagulation, heparin requirement, and celite‐ and kaolin‐activated clotting time in heparin‐pretreated patients undergoing open‐heart surgery. A double‐blind, placebo‐controlled study. Anesthesiology 1995;83(4):679‐89. [DOI] [PubMed] [Google Scholar]
Dietrich 2008 {published data only}
- Dietrich W, Spannagl M, Boehm J, Hauner K, Braun S, Schuster T, et al. Tranexamic acid and aprotinin in primary cardiac operations: an analysis of 220 cardiac surgical patients treated with tranexamic acid or aprotinin. Anesthesia and analgesia 2008;107(5):1469‐78. [DOI] [PubMed] [Google Scholar]
Dignan 2001 {published data only}
- Dignan RJ, Law DW, Seah PW, Manganas CW, Newman DC, Grant PW, et al. Ultra‐low dose aprotinin decreases transfusion requirements and is cost effective in coronary operations. Annals of Thoracic Surgery 2001;71(1):158‐63. [DOI] [PubMed] [Google Scholar]
Diprose 2005 {published data only}
- Diprose P, Herbertson MJ, O'Shaughnessy D, Deakin CD, Gill RS. Reducing allogeneic transfusion in cardiac surgery: a randomized double‐blind placebo‐controlled trial of antifibrinolytic therapies used in addition to intra‐operative cell salvage. British Journal of Anaesthesia 2005;94(3):271‐8. [DOI] [PubMed] [Google Scholar]
Dorman 2008 {published data only}
- Dorman B H, Stroud R E, Wyckoff M M, Zellner J L, Botta D, Leonardi A H, et al. Differential effects of epsilon‐aminocaproic acid and aprotinin on matrix metalloproteinase release in patients following cardiopulmonary bypass. Journal of cardiovascular pharmacology 2008;51(4):418‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dryden 1997 {published data only}
- Dryden PJ, O'Connor JP, Jamieson WR, Reid I, Ansley D, Sadeghi H, et al. Tranexamic acid reduces blood loss and transfusion in reoperative cardiac surgery. Canadian Journal of Anaesthesia 1997;44(9):934‐41. [DOI] [PubMed] [Google Scholar]
Eberle 1998 {published data only}
- Eberle B, Mayer E, Hafner G, Heinermann J, Dahm M, Prellwitz W, et al. High‐dose epsilon‐aminocaproic acid versus aprotinin: antifibrinolytic efficacy in first‐time coronary operations. Annals of Thoracic Surgery 1998;65(3):667‐73. [DOI] [PubMed] [Google Scholar]
Ehrlich 1998 {published data only}
- Ehrlich M, Grabenwoger M, Cartes‐Zumelzu F, Luckner D, Kovarik J, Laufer G, et al. Operations on the thoracic aorta and hypothermic circulatory arrest: is aprotinin safe?. Journal of Thoracic & Cardiovascular Surgery 1998;115(1):220‐5. [DOI] [PubMed] [Google Scholar]
Ekback 2000 {published data only}
- Ekback G, Axelsson K, Ryttberg L, Edlund B, Kjellberg J, Weckstrom J, et al. Tranexamic acid reduces blood loss in total hip replacement surgery. Anesthesia & Analgesia 2000;91(5):1124‐30. [DOI] [PubMed] [Google Scholar]
Ellis 2001 {published data only}
- Ellis MH, Fredman B, Zohar E, Ifrach N, Jedeikin R. The effect of tourniquet application, tranexamic acid, and desmopressin on the procoagulant and fibrinolytic systems during total knee replacement. Journal of Clinical Anesthesia 2001;13(7):509‐13. [DOI] [PubMed] [Google Scholar]
Engel 2001 {published data only}
- Engel JM, Hohaus T, Ruwoldt R, Menges T, Jurgensen I, Hempelmann G. Regional hemostatic status and blood requirements after total knee arthroplasty with and without tranexamic acid or aprotinin. Anesthesia & Analgesia 2001;92(3):775‐80. [DOI] [PubMed] [Google Scholar]
Englberger 2002a {published data only}
- Englberger L, Markart P, Eckstein FS, Immer FF, Berdat PA, Carrel TP. Aprotinin reduces blood loss in off‐pump coronary artery bypass (OPCAB) surgery. European Journal of Cardio‐Thoracic Surgery 2002;22(4):545‐51. [DOI] [PubMed] [Google Scholar]
Englberger 2002b {published data only}
- Englberger L, Kipfer B, Berdat PA, Nydegger UE, Carrel TP. Aprotinin in coronary operation with cardiopulmonary bypass: does "low‐dose" aprotinin inhibit the inflammatory response?. Annals of Thoracic Surgery 2002;73(6):1897‐904. [DOI] [PubMed] [Google Scholar]
Fauli 2005 {published data only}
- Fauli A, Gomar C, Campistol JM, Alvarez L, Manig AM, Matute P. Kidney‐specific proteins in patients receiving aprotinin at high‐ and low‐dose regimens during coronary artery bypass graft with cardiopulmonary bypass. European Journal of Anaesthesiology 2005;22(9):666‐71. [DOI] [PubMed] [Google Scholar]
Feindt 1994 {published data only}
- Feindt P, Seyfert U, Volkmer I, Huwer H, Kalweit G, Gams E. Is there a phase of hypercoagulability when aprotinin is used in cardiac surgery?. European Journal of Cardio‐thoracic Surgery 1994;8:308‐14. [DOI] [PubMed] [Google Scholar]
Fergusson 2008 {published data only}
- Fergusson DA, Hébert PC, Mazer CD, Fremes S, MacAdams C, Murkin JM, et al. A comparison of aprotinin and lysine analogues in high‐risk cardiac surgery. The New England Journal of Medicine 2008;358(22):2319‐31. [DOI] [PubMed] [Google Scholar]
Findlay 2001 {published data only}
- Findlay JY, Rettke SR, Ereth MH, Plevak DJ, Krom RA, Kufner RP. Aprotinin reduces red blood cell transfusion in orthotopic liver transplantation: a prospective, randomized, double‐blind study. Liver Transplantation 2001;7(9):802‐7. [DOI] [PubMed] [Google Scholar]
Fraedrich 1989 {published data only}
- Fraedrich G, Weber C, Bernard C, Hettwer A, Schlosser V. Reduction of blood transfusion requirement in open heart surgery by administration of high doses of aprotinin ‐ preliminary results. Thoracic & Cardiovascular Surgeon 1989;37(2):89‐91. [DOI] [PubMed] [Google Scholar]
Garcia‐Enguita 1998 {published data only}
- Garcia‐Enguita MA, Ortega JP, Gomez C, Rasal S, Pascual A, Urieta SA. High dose aprotinin in complex total hip arthroplasty [abstract]. British Journal of Anaesthesia 1998;80(Suppl 1):80. [Google Scholar]
Garcia‐Huete 1997 {published data only}
- Garcia‐Huete L, Domenech P, Sabate A, Martinez‐Brotons F, Jaurrieta E, Figueras J. The prophylactic effect of aprotinin on intraoperative bleeding in liver transplantation: A randomized clinical study. Hepatology 1997;26(5):1143‐8. [DOI] [PubMed] [Google Scholar]
Garneti 2004 {published data only}
- Garneti N, Field J. Bone bleeding during total hip arthroplasty after administration of tranexamic acid. Journal of Arthroplasty 2004;19(4):488‐92. [DOI] [PubMed] [Google Scholar]
Gherli 1992 {published data only}
- Gherli T, Porcu A, Padua G, Marongiu GM, Antona C, Alamanni F, Bacciu PP. Reducing bleeding during extracorporeal circulation interventions by high doses of aprotinin [Italian]. Minerva Cardioangiologica 1992;40(4):121‐6. [PubMed] [Google Scholar]
Gill 2009 {published data only}
- Gill JB, Chase E, Rosenstein AD. The use of tranexamic acid in revision total hip arthroplasty: a pilot study. Current Orthopaedic Practice 2009;20(2):152‐6. [Google Scholar]
Golanski 2000 {published data only}
- Golanski R, Golanski J, Chizynski K, Iwaszkiewicz A, Zaslonka J, Pietrucha T, et al. Low doses of aprotinin in aortocoronary bypass surgery ‐ advantages and disadvantages. Medical Science Monitor 2000;6(4):722‐8. [PubMed] [Google Scholar]
Good 2003 {published data only}
- Good L, Peterson E, Lisander B. Tranexamic acid decreases external blood loss but not hidden blood loss in total knee replacement. British Journal of Anaesthesia 2003;90(5):596‐9. [DOI] [PubMed] [Google Scholar]
Gott 1998 {published data only}
- Gott JP, Cooper WA, Schmidt FE, Brown WM, Wright CE, Merlino JD, et al. Modifying risk for extracorporeal circulation: trial of four antiinflammatory strategies. Annals of Thoracic Surgery 1998;66:747‐54. [DOI] [PubMed] [Google Scholar]
Green 1995 {published data only}
- Green D, Sanders J, Eiken M, Wong CA, Frederiksen J, Joob A, et al. Recombinant aprotinin in coronary artery bypass graft operations. Journal of Thoracic & Cardiovascular Surgery 1995;110(4 Pt 1):963‐70. [DOI] [PubMed] [Google Scholar]
Greilich 2001 {published data only}
- Greilich PE, Okada K, Latham P, Kumar RR, Jessen ME. Aprotinin but not epsilon‐aminocaproic acid decreases interleukin‐10 after cardiac surgery with extracorporeal circulation: randomized, double‐blind, placebo‐controlled study in patients receiving aprotinin and epsilon‐aminocaproic acid. Circulation 2001;104(12 Suppl 1):265‐9. [DOI] [PubMed] [Google Scholar]
Greilich 2003 {published data only}
- Greilich PE, Brouse CF, Whitten CW, Chi L, Dimaio JM, Jessen ME. Antifibrinolytic therapy during cardiopulmonary bypass reduces proinflammatory cytokine levels: a randomized, double‐blind, placebo‐controlled study of epsilon‐aminocaproic acid and aprotinin. Journal of Thoracic & Cardiovascular Surgery 2003;126(5):1498‐503. [DOI] [PubMed] [Google Scholar]
Greilich 2004 {published data only}
- Greilich PE, Brouse CF, Rinder CS, Smith BR, Sandoval BA, Rinder HM, et al. Effects of epsilon‐aminocaproic acid and aprotinin on leukocyte‐platelet adhesion in patients undergoing cardiac surgery. Anesthesiology 2004;100(2):225‐33. [DOI] [PubMed] [Google Scholar]
Greilich 2009 {published data only}
- Greilich PE, Jessen ME, Satyanarayana N, Whitten CW, Nuttall GA, Beckham GA, et al. The effects of Epsilon‐Aminocaproic Acid and Aprotinin on fibrinolysis and blood loss in patients undergoing primary, isolated coronary artery bypass surgery: a randomized double‐blind, placebo‐controlled, noninferiority trial. Anesthesia & Analgesia 2009;109(1):15‐24. [DOI] [PubMed] [Google Scholar]
Groh 1993 {published data only}
- Groh J, Welte M, Azad SC, Anthuber M, Haller M, Kratzer MA. Does aprotinin really reduce blood loss in orthotopic liver transplantation?. Seminars in Thrombosis & Hemostasis 1993;19(3):306‐8. [DOI] [PubMed] [Google Scholar]
Harder 1991 {published data only}
- Harder MP, Eijsman L, Roozendaal KJ, Oeveren W, Wildevuur CR. Aprotinin reduces intraoperative and postoperative blood loss in membrane oxygenator cardiopulmonary bypass. Annals of Thoracic Surgery 1991;51(6):936‐41. [DOI] [PubMed] [Google Scholar]
Hardy 1993 {published data only}
- Hardy JF, Desroches J, Belisle S, Perrault J, Carrier M, Robitaille D. Low‐dose aprotinin infusion is not clinically useful to reduce bleeding and transfusion of homologous blood products in high‐risk cardiac surgical patients. Canadian Journal of Anaesthesia 1993;40(7):625‐31. [DOI] [PubMed] [Google Scholar]
Hardy 1997 {published data only}
- Hardy JF, Belisle S, Couturier A, Robitaille D. Randomized, placebo‐controlled, double‐blind study of an ultra‐low‐dose aprotinin regimen in reoperative and/or complex cardiac operations. Journal of Cardiac Surgery 1997;12(1):15‐22. [DOI] [PubMed] [Google Scholar]
Hardy 1998 {published data only}
- Hardy JF, Belisle S, Dupont C, Harel F, Robitaille D, Roy M, et al. Prophylactic tranexamic acid and epsilon‐aminocaproic acid for primary myocardial revascularization. Annals of Thoracic Surgery 1998;65(2):371‐6. [DOI] [PubMed] [Google Scholar]
Harley 2002 {published data only}
- Harley BJ, Beaupre LA, Jones CA, Cinats JG, Guenther CR. The effect of epsilon aminocaproic acid on blood loss in patients who undergo primary total hip replacement: a pilot study. Canadian Journal of Surgery 2002;45(3):185‐90. [PMC free article] [PubMed] [Google Scholar]
Harmon 2004 {published data only}
- Harmon DC, Ghori KG, Eustace NP, O'Callaghan SJ, O'Donnell AP, Shorten GD. Aprotinin decreases the incidence of cognitive deficit following CABG and cardiopulmonary bypass: a pilot randomized controlled study. Canadian Journal of Anaesthesia 2004;51(10):1002‐9. [DOI] [PubMed] [Google Scholar]
Havel 1992 {published data only}
- Havel M, Owen AN, Simon P, Teufelsbauer H, Zwoelfer W, Laczkovics A, et al. Decreasing use of donated blood and reduction of bleeding after orthotopic heart transplantation by use of aprotinin. Journal of Heart & Lung Transplantation 1992;11(2 Pt 1):348‐9. [PubMed] [Google Scholar]
Havel 1994 {published data only}
- Havel M, Grabenwoger F, Schneider J, Laufer G, Wollenek, G, Owen A, et al. Aprotinin does not decrease early graft patency after coronary artery bypass grafting despite reducing postoperative bleeding and use of donated blood. Journal of Thoracic & Cardiovascular Surgery 1994;107(3):807‐10. [PubMed] [Google Scholar]
Hayashida 1997 {published data only}
- Hayashida N, Isomura T, Sato T, Maruyama H, Kosuga K, Aoyagi S. Effects of minimal‐dose aprotinin on coronary artery bypass grafting. Journal of Thoracic & Cardiovascular Surgery 1997;114(2):261‐9. [DOI] [PubMed] [Google Scholar]
Hayes 1996 {published data only}
- Hayes A, Murphy DB, McCarroll M. The efficacy of single‐dose aprotinin 2 million KIU in reducing blood loss and its impact on the incidence of deep venous thrombosis in patients undergoing total hip replacement surgery. Journal of Clinical Anesthesia 1996;8(5):357‐60. [DOI] [PubMed] [Google Scholar]
Hei 2005 {published data only}
- Hei ZQ, Luo CF, Li SR, Ma WH, Luo GJ, Chi XJ. Effect of aprotinin on perioperative requirements of blood and liquid in severe hepatisis patients undergoing liver transplantation. Chinese Pharmaceutical Journal 2005;40(18):1432‐5. [Google Scholar]
Hekmat 2004 {published data only}
- Hekmat K, Zimmermann T, Kampe S, Kasper SM, Weber HJ, Geissler HJ, et al. Impact of tranexamic acid vs. aprotinin on blood loss and transfusion requirements after cardiopulmonary bypass: a prospective, randomised, double‐blind trial. Current Medical Research & Opinion 2004;20(1):121‐6. [DOI] [PubMed] [Google Scholar]
Hendrice 1995 {published data only}
- Hendrice C, Schmartz D, Pradier O, Coron T, Kucharcezwski C, d'Hollander A, et al. Effects of aprotinin on blood loss, heparin monitoring tests, and heparin doses in patients undergoing coronary artery bypass surgery. Journal of Cardiothoracic & Vascular Anesthesia 1995;9(3):245‐9. [DOI] [PubMed] [Google Scholar]
Hiipala 1995 {published data only}
- Hiippala S, Strid L, Wennerstrand M, Arvela V, Mantyla S, Ylinen J, et al. Tranexamic acid (Cyklokapron) reduces perioperative blood loss associated with total knee arthroplasty. British Journal of Anaesthesia 1995;74(5):534‐7. [DOI] [PubMed] [Google Scholar]
Hiipala 1997 {published data only}
- Hiippala ST, Strid LJ, Wennerstrand MI, Arvela JVV, Niemela HM, Mantyla SK, et al. Tranexamic acid radically decreases blood loss and transfusions associated with total knee arthroplasty. Anesthesia & Analgesia 1997;84(4):839‐44. [DOI] [PubMed] [Google Scholar]
Hill 1998 {published data only}
- Hill GE, Diego RP, Stammers AH, Huffman SM, Pohorecki R. Aprotinin enhances the endogenous release of interleukin‐10 after cardiac operations. Annals of Thoracic Surgery 1998;65(1):66‐9. [DOI] [PubMed] [Google Scholar]
Horrow 1990 {published data only}
- Horrow JC, Hlavacek J, Strong MD, Collier W, Brodsky I, Goldman SM, Goel IP. Prophylactic tranexamic acid decreases bleeding after cardiac operations. Journal of Thoracic & Cardiovascular Surgery 1990;99(1):70‐4. [PubMed] [Google Scholar]
Horrow 1991 {published data only}
- Horrow JC, Riper DF, Strong MD, Brodsky I, Parmet JL. Hemostatic effects of tranexamic acid and desmopressin during cardiac surgery. Circulation 1991;84(5):2063‐70. [DOI] [PubMed] [Google Scholar]
Horrow 1995 {published data only}
- Horrow JC, Riper DF, Strong MD, Grunewald KE, Parmet JL. The dose‐response relationship of tranexamic acid. Anesthesiology 1995;82(2):383‐92. [DOI] [PubMed] [Google Scholar]
Husted 2003 {published data only}
- Husted H, Blond L, Sonne‐Holm S, Holm G, Jacobsen TW, Gebuhr P. Tranexamic acid reduces blood loss and blood transfusions in primary total hip arthroplasty: a prospective randomized double‐blind study in 40 patients. Acta Orthopaedica Scandinavica 2003;74(6):665‐9. [DOI] [PubMed] [Google Scholar]
Ickx 2006 {published data only}
- Ickx BE, Linden PJ, Melot C, Wijns W, Pauw L, Vandestadt J, et al. Comparison of the effects of aprotinin and tranexamic acid on blood loss and red blood cell transfusion requirements during the late stages of liver transplantation. Transfusion 2006;46(4):595‐605. [DOI] [PubMed] [Google Scholar]
Isetta 1993 {published data only}
- Isetta C, Gunness TK, Samat C, Paolini G, Lugrin D, Sanchez B, et al. Antifibrinolytic Treatment and Homologeous Transfusion in Cardiac Surgery. European Heart Journal 1993;14(Suppl):424. [Google Scholar]
Jamieson 1997 {published data only}
- Jamieson WRE, Dryden PJ, O'Connor JP, Sadeghi H, Ansley DM, Merrick PM, et al. Beneficial effect of both tranexamic acid and aprotinin on blood loss reduction in reoperative valve replacement surgery. Circulation 1997;96(9 SUPPL):II96‐101. [PubMed] [Google Scholar]
Jansen 1999 {published data only}
- Jansen AJ, Andreica S, Claeys M, D'Haese J, Camu F, Jochmans K. Use of tranexamic acid for an effective blood conservation strategy after total knee arthroplasty. British Journal of Anaesthesia 1999;83(4):596‐601. [DOI] [PubMed] [Google Scholar]
Janssens 1994 {published data only}
- Janssens M, Joris J, David JL, Lemaire R, Lamy M. High‐dose aprotinin reduces blood loss in patients undergoing total hip replacement surgery. [see comments]. Anesthesiology 1994;80(1):23‐9. [DOI] [PubMed] [Google Scholar]
Jares 2003 {published data only}
- Jares M, Vanek T, Straka Z, Brucek P. Tranexamic acid reduces bleeding after off‐pump coronary artery bypass grafting. Journal of Cardiovascular Surgery 2003;44(2):205‐8. [PubMed] [Google Scholar]
Jeserschek 2003 {published data only}
- Jeserschek R, Clar H, Aigner C, Rehak P, Primus B, Windhager R. Reduction of blood loss using high‐dose aprotinin in major orthopaedic surgery: a prospective, double‐blind, randomised and placebo‐controlled study. Journal of Bone & Joint Surgery ‐ British Volume 2003;85(2):174‐7. [DOI] [PubMed] [Google Scholar]
Jimenez 2007 {published data only}
- Jimenez J J, Iribarren J L, Lorente L, Rodriguez J M, Hernandez D, Nassar I, et al. Tranexamic acid attenuates inflammatory response in cardiopulmonary bypass surgery through blockade of fibrinolysis: a case control study followed by a randomized double‐blind controlled trial. Critical care (London, England) 2007;11(6):R117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez Rivera J, Iribarren Sarrias J, Nassar I, Rodriguez J, Raya J, Garrido P, et al. Tranexamic acid decreased postoperative bleeding and systemic inflammatory response syndrome associated with cardiopulmonary bypass: a prospective, randomized, double‐blind controlled study. Critical Care 2006;10(Suppl 1):S95. [Google Scholar]
Johansson 2005 {published data only}
- Johansson T, Pettersson LG, Lisander B. Tranexamic acid in total hip arthroplasty saves blood and money: a randomized, double‐blind study in 100 patients. Acta Orthopaedica 2005;76(3):314‐9. [PubMed] [Google Scholar]
Kahveci 1996 {published data only}
- Kahveci SF, Ozkan U, Korfali G, Sahin S. Effects of intraoperative aprotinin administration on hemostatic parameters in patients undergoing cardiac surgery [Turkish]. Turk Anesteziyoloji Ve Reanimasyon 1996;24(9):404‐7. [Google Scholar]
Kalangos 1994 {published data only}
- Kalangos A, Tayyareci G, Pretre R, Dio P, Sezerman O. Influence of aprotinin on early graft thrombosis in patients undergoing myocardial revascularization. European Journal of Cardiothoracic Surgery 1994;8(12):651‐6. [DOI] [PubMed] [Google Scholar]
Karski 1995 {published data only}
- Karski JM, Teasdale SJ, Norman P, Carroll J, VanKessel K, Wong P, et al. Prevention of bleeding after cardiopulmonary bypass with high‐dose tranexamic acid. Double‐blind, randomized clinical trial. Journal of Thoracic & Cardiovascular Surgery 1995;110(3):835‐42. [DOI] [PubMed] [Google Scholar]
Karski 2005 {published data only}
- Karski J, Djaiani G, Carroll J, Iwanochko M, Seneviratne P, Liu P, et al. Tranexamic acid and early saphenous vein graft patency in conventional coronary artery bypass graft surgery: a prospective randomized controlled clinical trial. Journal of Thoracic & Cardiovascular Surgery 2005;130(2):309‐14. [DOI] [PubMed] [Google Scholar]
Kaspar 1997 {published data only}
- Kaspar M, Ramsay MAE, Nguyen AT, Cogswell M, Hurst G, Ramsay KJ. Continuous small‐dose tranexamic acid reduces fibrinolysis but not transfusion requirements during orthotopic liver transplantation. Anesthesia & Analgesia 1997;85(2):281‐5. [DOI] [PubMed] [Google Scholar]
Katoh 1997 {published data only}
- Katoh J, Tsuchiya K, Sato W, Nakajima M, Iida Y. Additional postbypass administration of tranexamic acid reduces blood loss after cardiac operations. Journal of Thoracic & Cardiovascular Surgery 1997;113(4):802‐4. [DOI] [PubMed] [Google Scholar]
Katsaros 1996 {published data only}
- Katsaros D, Petricevic M, Snow NJ, Woodhall DD, Bergen R. Tranexamic acid reduces postbypass blood use: a double‐blinded, prospective, randomized study of 210 patients. Annals of Thoracic Surgery 1996;61(4):1131‐5. [DOI] [PubMed] [Google Scholar]
Katzel 1998 {published data only}
- Katzel R, Keuper H, Wiedemann B, Brethner L, Voigt B. Effects of aprotinin application on perioperative changes in hemostasis and fibrinolysis and its consequence for blood loss and transfusion requirement in lung surgery. [German]. Infusionstherapie und Transfusionsmedizin 1998;25(4):236‐245. [Google Scholar]
Kazemi 2010 {published data only}
- Kazemi SM, Mosaffa F, Eajazi A, Kaffashi M, Daftari Besheli L, Reza Bigdeli M, et al. The effects of tranexamic acid on reducing blood loss in cementless total hip arthroplasty under epidural anesthesia. Orthopedics 2010;33(1):17. [DOI] [PubMed] [Google Scholar]
Kikura 2006 {published data only}
- Kikura M, Levy JH, Tanaka KA, Ramsay JG. A double‐blind, placebo‐controlled trial of epsilon‐aminocaproic acid for reducing blood loss in coronary artery bypass grafting surgery. Journal of the American College of Surgeons 2006;202(2):216‐222. [DOI] [PubMed] [Google Scholar]
Kipfer 2003 {published data only}
- Kipfer B, Englberger L, Gygax E, Nydegger U, Carrel T. Is reduced systemic heparinization justified with heparin‐bonded bypass circuits in cardiac surgery? ‐ Experience with and without aprotinin. Transfusion & Apheresis Science 2003;29(1):17‐24. [DOI] [PubMed] [Google Scholar]
Klein 1998 {published data only}
- Klein M, Keith PR, Dauben HP, Schulte HD, Beckmann H, Mayer G, Elert O, Gams E. Aprotinin counterbalances an increased risk of peri‐operative hemorrhage in CABG patients pre‐treated with Aspirin. European Journal of Cardio‐Thoracic Surgery 1998;14(4):360‐6. [DOI] [PubMed] [Google Scholar]
Kluger 2003 {published data only}
- Kluger R, Olive DJ, Stewart AB, Blyth CM. Epsilon‐aminocaproic acid in coronary artery bypass graft surgery: preincision or postheparin?. Anesthesiology 2003;99(6):1263‐9. [DOI] [PubMed] [Google Scholar]
Koster 2004 {published data only}
- Koster A, Huebler S, Merkle F, Hentschel T, Grundel M, Krabatsch T, Tambeur L, Praus M, Habazettl H, Kuebler WM, Kuppe H. Heparin‐level‐based anticoagulation management during cardiopulmonary bypass: a pilot investigation on the effects of a half‐dose aprotinin protocol on postoperative blood loss and hemostatic activation and inflammatory response. Anesthesia & Analgesia 2004;98(2):285‐90. [DOI] [PubMed] [Google Scholar]
Kratzer 1997 {published data only}
- Kratzer MAA, Azad SC, Groh J, Welte M, Haller M, Pratschke E. Effect of aprotinin on blood loss and coagulation parameters in orthotopic liver transplantation. [German]. Anaesthesist 1997;46(4):294‐302. [DOI] [PubMed] [Google Scholar]
Kreisler 2005 {published data only}
- Kreisler KR, Vance RA, Cruzzavala J, Mahnken JD. Heparin‐bonded cardiopulmonary bypass circuits reduce the rate of red blood cell transfusion during elective coronary artery bypass surgery. Journal of Cardiothoracic & Vascular Anesthesia 2005;19(5):608‐11. [DOI] [PubMed] [Google Scholar]
Kuepper 2003 {published data only}
- Kuepper F, Dangas G, Mueller‐Chorus A, Kulka PM, Zenz M, Wiebalck A. Fibrinolytic activity and bleeding after cardiac surgery with cardiopulmonary bypass and low‐dose aprotinin therapy. Blood Coagulation & Fibrinolysis 2003;14(2):147‐53. [DOI] [PubMed] [Google Scholar]
Kuitunen 2005 {published data only}
- Kuitunen A, Hiippala S, Vahtera E, Rasi V, Salmenpera M. The effects of aprotinin and tranexamic acid on thrombin generation and fibrinolytic response after cardiac surgery. Acta Anaesthesiologica Scandinavica 2005;49(9):1272‐9. [DOI] [PubMed] [Google Scholar]
Kuitunen 2006 {published data only}
- Kuitunen AH, Suojaranta‐Ylinen RT, Kukkonen SI, Niemi TT. Tranexamic acid does not correct the haemostatic impairment caused by hydroxyethyl starch (200 kDa/0.5) after cardiac surgery. Blood Coagulation and Fibrinolysis 2006;17:639‐45. [DOI] [PubMed] [Google Scholar]
Kunt 2005 {published data only}
- Kunt AS, Darcin OT, Aydin S, Demir D, Selli C, Andac MH. Mini‐dose pump‐prime aprotinin inhibited enhanced fibrinolytic activity and reduced blood loss and transfusion requirements after coronary artery bypass surgery. Journal of Thrombosis & Thrombolysis 2005;19(3):197‐200. [DOI] [PubMed] [Google Scholar]
Kyriss 2001 {published data only}
- Kyriss T, Wurst H, Friedel G, Jaki R, Toomes H. Reduced blood loss by aprotinin in thoracic surgical operations associated with high risk of bleeding. A placebo‐controlled, randomized phase IV study. European Journal of Cardio‐Thoracic Surgery 2001;20(1):38‐41. [DOI] [PubMed] [Google Scholar]
Landymore 1997 {published data only}
- Landymore RW, Murphy JT, Lummis H, Carter C. The use of low‐dose aprotinin, epsilon‐aminocaproic acid or tranexamic acid for prevention of mediastinal bleeding in patients receiving aspirin before coronary artery bypass operations.. European Journal of Cardio‐Thoracic Surgery 1997;11:798‐800. [DOI] [PubMed] [Google Scholar]
Lass 1995 {published data only}
- Lass M, Simic O, Ostermeyer J. Re‐Graft patency and clinical efficacy of aprotinin in elective bypass surgery. Cardiovascular Surgery 1997;5(6):604‐7. [DOI] [PubMed] [Google Scholar]
- Lass M, Welz A, Kochs M, Mayer G, Schwandt M, Hannekum A. Aprotinin in elective primary bypass surgery. European Journal of Cardio‐thoracic Surgery 1995;9:206‐10. [DOI] [PubMed] [Google Scholar]
Later 2009 {published data only}
- Later A F, Maas J J, Engbers F H, Versteegh M I, Bruggemans E F, Dion R A, et al. Tranexamic acid and aprotinin in low‐ and intermediate‐risk cardiac surgery: a non‐sponsored, double‐blind, randomised, placebo‐controlled trial. European journal of cardio‐thoracic surgery : official journal of the European Association for Cardio‐thoracic Surgery 2009;36(2):322‐9. [DOI] [PubMed] [Google Scholar]
Laub 1994 {published data only}
- Laub GW, Riebman JB, Chen C, Adkins MS, Anderson WA, Fernandez J, McGrath LB. The impact of aprotinin on coronary artery bypass graft patency. Chest 1994;106(5):1370‐5. [DOI] [PubMed] [Google Scholar]
Lavee 1993 {published data only}
- Lavee J, Raviv Z, Smolinsky A, Savion N, Varon D, Goor DA, Mohr R. Platelet protection by low‐dose aprotinin in cardiopulmonary bypass: electron microscopic study. Annals of Thoracic Surgery 1993;55(1):114‐9. [DOI] [PubMed] [Google Scholar]
Leijdekkers 2006 {published data only}
- Leijdekkers VJ, Vahl AC, Mackaay AJC, Huijgens PC, Rauwerda JA. Aprotinin does not diminish blood loss in elective operations for infrarenal abdominal aneurysms: a randomized double‐blind controlled trial. Annals of Vascular Surgery 2006;20:322‐9. [DOI] [PubMed] [Google Scholar]
Lemay 2004 {published data only}
- Lemay E, Guay J, Cote C, Roy A. Tranexamic acid reduces the need for allogenic red blood cell transfusions in patients undergoing total hip replacement. Canadian Journal of Anaesthesia 2004;51(1):31‐7. [DOI] [PubMed] [Google Scholar]
Lemmer 1994 {published data only}
- Lemmer JH, Jr, Stanford W, Bonney SL, Breen JF, Chomka EV, Eldredge WJ, Holt WW, Karp RB, Laub GW, Lipton MJ, et al. Aprotinin for coronary bypass operations: efficacy, safety, and influence on early saphenous vein graft patency. A multicenter, randomized, double‐blind, placebo‐controlled study. Journal of Thoracic & Cardiovascular Surgery 1994;107(2):543‐51. [PubMed] [Google Scholar]
Lemmer 1996 {published data only}
- Lemmer JH, Jr, Dilling EW, Morton JR, Rich JB, Robicsek F, Bricker DL, Hantler CB, Copeland JG, 3rd, Ochsner JL, Daily PO, et al. Aprotinin for primary coronary artery bypass grafting: a multicenter trial of three dose regimens. Annals of Thoracic Surgery 1996;62(6):1659‐67. [DOI] [PubMed] [Google Scholar]
Lemmer_1 1994 {published data only}
- Lemmer JH, Jr, Stanford W, Bonney SL, Breen JF, Chomka EV, Eldredge WJ, Holt WW, Karp RB, Laub GW, Lipton MJ, et al. Aprotinin for coronary bypass operations: efficacy, safety, and influence on early saphenous vein graft patency. A multicenter, randomized, double‐blind, placebo‐controlled study. Journal of Thoracic & Cardiovascular Surgery 1994;107(2):543‐51. [PubMed] [Google Scholar]
- Lemmer JH, Jr, Stanford W, Bonney SL, Chomka EV, Karp RB, Laub GW, Rumberger JA, Schaff HV. Aprotinin for coronary artery bypass grafting: effect on postoperative renal function. Annals of Thoracic Surgery 1995;59(1):132‐6. [DOI] [PubMed] [Google Scholar]
Lemmer_2 1994 {published data only}
- Lemmer JH, Jr, Stanford W, Bonney SL, Breen JF, Chomka EV, Eldredge WJ, Holt WW, Karp RB, Laub GW, Lipton MJ, et al. Aprotinin for coronary bypass operations: efficacy, safety, and influence on early saphenous vein graft patency. A multicenter, randomized, double‐blind, placebo‐controlled study. Journal of Thoracic & Cardiovascular Surgery 1994;107(2):543‐51. [PubMed] [Google Scholar]
- Lemmer JH, Jr, Stanford W, Bonney SL, Chomka EV, Karp RB, Laub GW, Rumberger JA, Schaff HV. Aprotinin for coronary artery bypass grafting: effect on postoperative renal function. Annals of Thoracic Surgery 1995;59(1):132‐6. [DOI] [PubMed] [Google Scholar]
Lentschener 1997 {published data only}
- Lentschener C, Benhamou D, Mercier FJ, Boyer‐Neumann C, Naveau S, Smadja C, Wolf M, Franco D. Aprotinin reduces blood loss in patients undergoing elective liver resection. Anesthesia & Analgesia 1997;84(4):875‐81. [DOI] [PubMed] [Google Scholar]
Lentschener 1999 {published data only}
- Lentschener C, Cottin P, Bouaziz H, Mercier FJ, Wolf M, Aljabi Y, et al. Reduction of blood loss and transfusion requirement by aprotinin in posterior lumbar spine fusion. Anesthesia & Analgesia 1999;89(3):590‐7. [DOI] [PubMed] [Google Scholar]
Levy 1995 {published data only}
- Levy JH, Pifarre R, Schaff HV, Horrow JC, Albus R, Spiess B, Rosengart TK, Murray J, Clark RE, Smith P. A multicenter, double‐blind, placebo‐controlled trial of aprotinin for reducing blood loss and the requirement for donor‐blood transfusion in patients undergoing repeat coronary artery bypass grafting. Circulation 1995;92(8):2236‐44. [DOI] [PubMed] [Google Scholar]
Li 2005 {published data only}
- Li S, Ji H, Lin J, Lenehan E, Ji B, Liu J, et al. Combination of acute preoperative plateletpheresis, cell salvage, and aprotinin minimizes blood loss and requirement during cardiac surgery. Journal of Extra‐Corporeal Technology 2005;37(1):9‐14. [PMC free article] [PubMed] [Google Scholar]
Liu 1993 {published data only}
- Liu B, Belboul A, Radberg G, Tengborn L, Dernevik L, Roberts D, William‐Olsson G. Effect of reduced aprotinin dosage on blood loss and use of blood products in patients undergoing cardiopulmonary bypass. Scandinavian Journal of Thoracic & Cardiovascular Surgery 1993;27(3‐4):149‐55. [DOI] [PubMed] [Google Scholar]
- Liu B, Tengborn L, Larson G, Radberg LO, Belboul A, Dernevik L, Roberts D. Half‐dose aprotinin preserves hemostatic function in patients undergoing bypass operations. Annals of Thoracic Surgery 1995;59(6):1534‐40. [DOI] [PubMed] [Google Scholar]
Liu 1998 {published data only}
- Liu YC, Tsai TP. The effect of coagulation protection with combination of epsilon aminocaproic acid and plasma saver in open‐heart surgery. Acta Anaesthesiologica Sinica 1998;36(3):149‐154. [PubMed] [Google Scholar]
Llau 1998 {published data only}
- Llau JV, Aguilar G, Soliveres J, Minguez MF, Saz T, Belda FJ. Aprotinin administration reduces blood loss and transfusion requirements in patients undergoing total hip arthroplasty. [abstract]. British Journal of Anaesthesia 1998;80(Suppl 1):80. [Google Scholar]
Locatelli 1990 {published data only}
- Locatelli A, Bertollo D, Bianchi T, et al. Aprotinin in cardiosurgery: a randomized prospective study with different protocols for use. [Italian]. Minerva Anestesiologica 1990;56:973‐5. [PubMed] [Google Scholar]
Luo 1998 {published data only}
- Luo J, Huang Y, Lan H. Effect of Aprotinin on the red cell immunity in cardiopulmonary bypass. Journal of Tongi Medical University 1998;18(2):97‐100. [DOI] [PubMed] [Google Scholar]
Maccario 1994 {published data only}
- Maccario M, Fumagalli C, Deangelis R, Delfino R, Pergolo A, Dottori V, Barberis L. Comparison between low and high doses of aprotinin in heart surgery. [Italian]. Minerva Anestesiologica 1994;60(6):315‐20. [PubMed] [Google Scholar]
MacGillivray 2010 {published data only}
- MacGillivray RG, Tarabichi SB, Hawari MF, Raoof NT. Tranexamic acid to reduce blood loss after bilateral total knee arthroplasty. The Journal of Arthroplasty 2010;[In press]. [DOI] [PubMed] [Google Scholar]
Maddali 2007 {published data only}
- Maddali M M, Rajakumar M C. Tranexamic acid and primary coronary artery bypass surgery: a prospective study. Asian cardiovascular & thoracic annals 2007;15(4):313‐9. [DOI] [PubMed] [Google Scholar]
Maineri 2000 {published data only}
- Maineri P, Covaia G, Realini M, Caccia G, Ucussich E, Luraschi M, Crosta A, Foresti B, Chiaranda M. Postoperative bleeding after coronary revascularization. Comparison between tranexamic acid and epsilon‐aminocaproic acid. Minerva Cardioangiologica 2000;48(6):155‐60. [PubMed] [Google Scholar]
Mansour 2004 {published data only}
- Mansour EE, Mustafa B. Aprotinin versus tranexamic acid in patients receiving aspirin and undergoing off‐pump coronary artery bypass. Egyptian Journal of Anaesthesia 2004;20(3):229‐36. [Google Scholar]
Marcel 1996 {published data only}
- Marcel RJ, Stegall WC, Suit CT, Arnold JC, Vera RL, Ramsay MA, O'Donnell MB, Swygert TH, Hein HA, Whitten CW. Continuous small‐dose aprotinin controls fibrinolysis during orthotopic liver transplantation. Anesthesia & Analgesia 1996;82(6):1122‐5. [DOI] [PubMed] [Google Scholar]
Mehr‐Aein 2007 {published data only}
- Mehr‐Aein A, Sadeghi M, Madani‐civi M. Does tranexamic acid reduce blood loss in off‐pump coronary artery bypass?. Asian cardiovascular & thoracic annals 2007;15(4):285‐9. [DOI] [PubMed] [Google Scholar]
Mengistu 2008 {published data only}
- Mengistu A M, Röhm K D, Boldt J, Mayer J, Suttner S W, Piper S N. The influence of aprotinin and tranexamic acid on platelet function and postoperative blood loss in cardiac surgery. Anesthesia and analgesia 2008;107(2):391‐7. [DOI] [PubMed] [Google Scholar]
Menichetti 1996 {published data only}
- Menichetti A, Tritapepe L, Ruvolo G, Speziale G, Cogliati A, Giovann C, Pacilli M, Criniti A. Changes in coagulation patterns, blood loss and blood use after cardiopulmonary bypass: aprotinin vs tranexamic acid vs epsilon aminocaproic acid. Journal of Cardiovascular Surgery 1996;37(4):401‐7. [PubMed] [Google Scholar]
Misfeld 1998 {published data only}
- Misfeld M, Dubbert S, Eleftheriadis S, Siemens HJ, Wagner T, Sievers HH. Fibrinolysis‐adjusted perioperative low‐dose aprotinin reduces blood loss in bypass operations. Annals of Thoracic Surgery 1998;66(3):792‐9. [DOI] [PubMed] [Google Scholar]
Mohr 1992 {published data only}
- Mohr R, Goor DA, Lusky A, Lavee J. Aprotinin prevents cardiopulmonary bypass‐induced platelet dysfunction. A scanning electron microscope study. Circulation 1992;86(5:Suppl):II405‐9. [PubMed] [Google Scholar]
Mongan 1998 {published data only}
- Mongan PD, Brown RS, Thwaites BK. Tranexamic acid and aprotinin reduce postoperative bleeding and transfusions during primary coronary revascularization. Anesthesia & Analgesia 1998;87(2):258‐65. [DOI] [PubMed] [Google Scholar]
Moran 2000 {published data only}
- Moran SV, Lema G, Medel J, Irarrazaval MJ, Zalaquett R, Garayar B, Flaskamp R. Comparison of two doses of aprotinin in patients receiving aspirin before coronary bypass surgery. Perfusion 2000;15(2):105‐10. [DOI] [PubMed] [Google Scholar]
Murkin 1994 {published data only}
- Murkin JM, Lux J, Shannon NA, Guiraudon GM, Menkis AH, McKenzie FN, Novick RJ. Aprotinin significantly decreases bleeding and transfusion requirements in patients receiving aspirin and undergoing cardiac operations. Journal of Thoracic & Cardiovascular Surgery 1994;107(2):554‐61. [PubMed] [Google Scholar]
Murkin 1995 {published data only}
- Murkin JM, Shannon NA, Bourne RB, Rorabeck CH, Cruickshank M, Wyile G. Aprotinin decreases blood loss in patients undergoing revision or bilateral total hip arthroplasty. Anesthesia & Analgesia 1995;80(2):343‐8. [DOI] [PubMed] [Google Scholar]
Murkin 2000 {published data only}
- Murkin JM, Haig GM, Beer KJ, Cicutti N, McCutchen J, Comunale ME, Hall R, Ruzicka BB. Aprotinin decreases exposure to allogeneic blood during primary unilateral total hip replacement. Journal of Bone & Joint Surgery 2000;82(5):675‐84. [DOI] [PubMed] [Google Scholar]
Murphy 2006 {published data only}
- Murphy GJ, Mango E, Lucchetti V, Battaglia F, Catapano D, Rogers CA, et al. A randomized trial of tranexamic acid in combination with cell salvage plus a meta‐analysis of randomized trials evaluating tranexamic acid in off‐pump coronary artery bypass grafting. Cardiopulmonary & Physiology 2006;132(3):475‐80. [DOI] [PubMed] [Google Scholar]
Niskanen 2005 {published data only}
- Niskanen RO, Korkala OL. Tranexamic acid reduces blood loss in cemented hip arthroplasty: a randomized, double‐blind study of 39 patients with osteoarthritis. Acta Orthopaedica 2005;76(6):829‐32. [DOI] [PubMed] [Google Scholar]
Norman 2009 {published data only}
- Norman P H, Thall P F, Purugganan R V, Riedel B J, Thakar D R, Rice D C, et al. A possible association between aprotinin and improved survival after radical surgery for mesothelioma. Cancer 2009;115(4):833‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nurözler 2008 {published data only}
- Nurözler F, Kutlu T, Küçük G. Aprotinin for patients exposed to clopidogrel before off‐pump coronary bypass. Asian cardiovascular & thoracic annals 2008;16(6):483‐7. [DOI] [PubMed] [Google Scholar]
Nuttall 2000 {published data only}
- Nuttall GA, Oliver WC, Ereth MH, Santrach PJ, Bryant SC, Orszulak TA, Schaff HV. Comparison of blood‐conservation strategies in cardiac surgery patients at high risk for bleeding. Anesthesiology 2000;92(3):674‐82. [DOI] [PubMed] [Google Scholar]
Okita 1996 {published data only}
- Okita Y, Takamoto S, Ando M, Morota T, Yamaki F, Kawashima Y. Is use of aprotinin safe with deep hypothermic circulatory arrest in aortic surgery? Investigations on blood coagulation. Circulation 1996;94(Suppl):II177‐81. [PubMed] [Google Scholar]
Orpen 2006 {published data only}
- Orpen NM, Little C, Walker G, Crawfurd EJP. Tranexamic acid reduces early post‐operative blood loss after total knee arthroplasty: A prospective randomised controlled trial of 29 patients. Knee 2006;13(2):106‐10. [DOI] [PubMed] [Google Scholar]
Palmer 2003 {published data only}
- Palmer JD, Francis JL, Pickard JD, Iannotti F. The efficacy and safety of aprotinin for hemostasis during intracranial surgery. Journal of Neurosurgery 2003;98(6):1208‐16. [DOI] [PubMed] [Google Scholar]
Parvizi 2007 {published data only}
- Parvizi R, Azarfarin R, Hassanzadeh S. Ultra‐low dose aprotinin effects on reducing the need for blood transfusion in cardiac surgery. Saudi Medical Journal 2007;28(1):49‐53. [PubMed] [Google Scholar]
Penta de Peppo 1995 {published data only}
- Penta de Peppo A, Pierri MD, Scafuri A, Paulis R, Colantuono G, Caprara E, Tomai F, Chiariello L. Intraoperative antifibrinolysis and blood‐saving techniques in cardiac surgery. Prospective trial of 3 antifibrinolytic drugs. Texas Heart Institute Journal 1995;22(3):231‐6. [PMC free article] [PubMed] [Google Scholar]
Petsatodis 2006 {published data only}
- Petsatodis G, Samoladas E, Christodoulou A, Hatzokos I, Pournaras I. Does aprotinin reduce blood loss in total hip arthroplasty?. Orthopedics 2006;29(1):75‐7. [DOI] [PubMed] [Google Scholar]
Pinosky 1997 {published data only}
- Pinosky ML, Kennedy DJ, Fishman RL, Reeves ST, Alpert CC, Ecklund J, Kribbs S, Spinale FG, Kratz JM, Crawford R, Gravlee GP, Dorman BH. Tranexamic acid reduces bleeding after cardiopulmonary bypass when compared to epsilon aminocaproic acid and placebo. Journal of Cardiac Surgery 1997;12(5):330‐8. [DOI] [PubMed] [Google Scholar]
Pleym 2003 {published data only}
- Pleym H, Stenseth R, Wahba A, Bjella L, Karevold A, Dale O. Single‐dose tranexamic acid reduces postoperative bleeding after coronary surgery in patients treated with aspirin until surgery. Anesthesia & Analgesia 2003;96(4):923‐8. [DOI] [PubMed] [Google Scholar]
Porte 2000 {published data only}
- Porte RJ, Molenaar IQ, Begliomini B, Groenland THN, Januszkiewicz A, Lindgren L, Palareti G, Hermans J, Terpstra OT. Aprotinin and transfusion requirements in orthotopic liver transplantation: a multicentre randomised double‐blind study. Lancet 2000;355(9212):1303‐9. [DOI] [PubMed] [Google Scholar]
Poston 2006 {published data only}
- Poston RS, White C, Gu J, Brown J, Gammie J, Pierson RN, et al. Aprotinin shows both hemostatic and antithrombotic effects during off‐pump coronary artery bypass grafting. Annals of Thoracic Surgery 2006;81(1):104‐11. [DOI] [PubMed] [Google Scholar]
Prendergast_1 1996 {published data only}
- Prendergast TW, Furukawa S, Beyer AJ, 3rd, Eisen HJ, McClurken JB, Jeevanandam V. Defining the role of aprotinin in heart transplantation. Annals of Thoracic Surgery 1996;62(3):670‐4. [PubMed] [Google Scholar]
Prendergast_2 1996 {published data only}
- Prendergast TW, Furukawa S, Beyer AJ, 3rd, Eisen HJ, McClurken JB, Jeevanandam V. Defining the role of aprotinin in heart transplantation. Annals of Thoracic Surgery 1996;62(3):670‐4. [PubMed] [Google Scholar]
Pugh 1995 {published data only}
- Pugh SC, Wielogorski AK. A comparison of the effects of tranexamic acid and low‐dose aprotinin on blood loss and homologous blood usage in patients undergoing cardiac surgery. Journal of Cardiothoracic & Vascular Anesthesia 1995;9:240‐4. [DOI] [PubMed] [Google Scholar]
Ranaboldo 1997 {published data only}
- Ranaboldo CJ, Thompson JF, Davies JN, Shutt AM, Francis JN, Roath OS, Webster JHH, Chant ADB. Prospective randomized placebo‐controlled trial of aprotinin for elective aortic reconstruction. British Journal of Surgery 1997;84(8):1110‐3. [PubMed] [Google Scholar]
Rao 1999 {published data only}
- Rao BH, Saxena N, Chauhan S, Sashikanth M. Use of E‐Aminocaproic acid in the management of aspirin related postoperative bleeding in patients undergoing coronary revascularization. Journal of Anaesthesiology Clinical Pharmacology 1999;15(3):261‐4. [Google Scholar]
Ray 1997 {published data only}
- Ray MJ, Marsh NA, Just SJ, Perrin EJ, O'Brien MF, Hawson GA. Preoperative platelet dysfunction increases the benefit of aprotinin in cardiopulmonary bypass. Annals of Thoracic Surgery 1997;63(1):57‐63. [DOI] [PubMed] [Google Scholar]
Ray 1999 {published data only}
- Ray MJ, Brown KF, Burrows CA, O'Brien MF. Economic evaluation of high‐dose and low‐dose aprotinin therapy during cardiopulmonary bypass. Annals of Thoracic Surgery 1999;68(3):940‐5. [DOI] [PubMed] [Google Scholar]
Ray 2001 {published data only}
- Ray MJ, O'Brien MF. Comparison of epsilon aminocaproic acid and low‐dose aprotinin in cardiopulmonary bypass: efficiency, safety and cost. Annals of Thoracic Surgery 2001;71(3):838‐43. [DOI] [PubMed] [Google Scholar]
Ray 2005 {published data only}
- Ray M, Hatcher S, Whitehouse SL, Crawford S, Crawford R. Aprotinin and epsilon aminocaproic acid are effective in reducing blood loss after primary total hip arthroplasty‐‐a prospective randomized double‐blind placebo‐controlled study. Journal of Thrombosis & Haemostasis 2005;3(7):1421‐7. [Google Scholar]
Rhydderch 1993 {published data only}
- Rhydderch RD, Khan B, Saleh A. Single dose Aprotinin in routine cardiac surgery. Middle East Journal of Anesthesiology 1993;12(3):287‐97. [PubMed] [Google Scholar]
Rocha 1994 {published data only}
- Rocha E, Hidalgo F, Llorens R, Melero JM, Arroyo JL, Paramo JA. Randomized study of aprotinin and DDAVP to reduce postoperative bleeding after cardiopulmonary bypass surgery. Circulation 1994;90(2):921‐7. [DOI] [PubMed] [Google Scholar]
Rodrigus 1996 {published data only}
- Rodrigus IE, Vermeyen KM, Hert SG, Amsel BJ, Walter PJ. Efficacy and safety of aprotinin in aortocoronary bypass and valve replacement operations: a placebo‐controlled randomized double‐blind study. Perfusion 1996;11(4):313‐8. [DOI] [PubMed] [Google Scholar]
Rossi 1997 {published data only}
- Rossi M, Storti S, Martinelli L, Varano C, Marra R, Zamparelli R, Possati G, Schiavello R. A pump‐prime aprotinin dose in cardiac surgery: appraisal of its effects on the hemostatic system. Journal of Cardiothoracic & Vascular Anesthesia 1997;11(7):835‐9. [DOI] [PubMed] [Google Scholar]
Royston 1987 {published data only}
- Royston D, Bidstrup BP, Taylor KM, Sapsford RN. Effect of aprotinin on need for blood transfusion after repeat open‐heart surgery. Lancet 1987;2(8571):1289‐91. [DOI] [PubMed] [Google Scholar]
Sadeghi 2007 {published data only}
- Sadeghi M, Mehr‐Aein A. Does a single bolus dose of tranexamic acid reduce blood loss and transfusion requirements during hip fracture surgery? A prospective randomized double blind study in 67 patients. Acta Medica Iranica 2007;45(6):437‐42. [Google Scholar]
Samama 2002 {published data only}
- Samama CM, Langeron O, Rosencher N, Capdevila X, Rouche P, Pegoix M, Berniere J, Coriat P. Aprotinin versus placebo in major orthopedic surgery: a randomized, double‐blinded, dose‐ranging study. Anesthesia & Analgesia 2002;95(2):287‐93. [DOI] [PubMed] [Google Scholar]
Santamaria 2000 {published data only}
- Santamaria A, Mateo J, Oliver A, Litvan H, Murillo J, Souto JC, Fontcuberta J. The effect of two different doses of aprotinin on hemostasis in cardiopulmonary bypass surgery: similar transfusion requirements and blood loss. Haematologica 2000;85(12):1277‐84. [PubMed] [Google Scholar]
Santos 2006 {published data only}
- Santos ATL, Kalil RAK, Bauemann C, Pereira JB, Nesralla IA. A randomized, double‐blind, and placebo‐controlled study with tranexamic acid of bleeding and fibrinolytic activity after primary coronary artery bypass grafting. Brazilian Journal of Medical & Biological Research 2006;39(1):63‐9. [DOI] [PubMed] [Google Scholar]
Schmartz 2003 {published data only}
- Schmartz D, Tabardel Y, Preiser JC, Barvais L, d'Hollander A, Duchateau J, Vincent JL. Does aprotinin influence the inflammatory response to cardiopulmonary bypass in patients?. Journal of Thoracic & Cardiovascular Surgery 2003;125(1):184‐90. [DOI] [PubMed] [Google Scholar]
Schweizer 2000 {published data only}
- Schweizer A, Hohn L, Morel DR, Kalangos A, Licker M. Aprotinin does not impair renal haemodynamics and function after cardiac surgery. British Journal of Anaesthesia 2000;84(1):16‐22. [DOI] [PubMed] [Google Scholar]
Shore‐Lesserson 1996 {published data only}
- Shore‐Lesserson L, Reich DL, Vela‐Cantos F, Ammar T, Ergin MA. Tranexamic acid reduces transfusions and mediastinal drainage in repeat cardiac surgery. Anesthesia & Analgesia 1996;83(1):18‐26. [DOI] [PubMed] [Google Scholar]
Sorin 1999 {published data only}
- Sorin A, Claeys MA, Jansen A, D'Haese J, Camu F. Reduction of blood loss by tranexamic acid in total knee replacement. Journal of Bone & Joint Surgery ‐ British Volume. 81‐B 1999; Vol. 81‐B, issue Suppl.II:234.
Speekenbrink 1995 {published data only}
- Speekenbrink RG, Vonk AB, Wildevuur CR, Eijsman L. Hemostatic efficacy of dipyridamole, tranexamic acid, and aprotinin in coronary bypass grafting. Annals of Thoracic Surgery 1995;59(2):438‐42. [DOI] [PubMed] [Google Scholar]
Speekenbrink 1996 {published data only}
- Speekenbrink RGH, Wildevuur CRH, Sturk A, Eijsman L. Low‐dose and high‐dose aprotinin improve hemostasis in coronary operations. Journal of Thoracic & Cardiovascular Surgery 1996;112(2):523‐30. [DOI] [PubMed] [Google Scholar]
Stammers 1997 {published data only}
- Stammers AH, Huffman S, Alonso A, Fristoe LW, Hill G, Casebeer D, Diego RP, Song Z. The antiinflammatory effects of aprotinin in patients undergoing cardiac surgery with cardiopulmonary bypass. Journal of Extra‐Corporeal Technology 1997;29(3):114‐22. [Google Scholar]
Stewart 2001 {published data only}
- Stewart A, Newman L, Sneddon K, Harris M. Aprotinin reduces blood loss and the need for transfusion in orthognathic surgery. British Journal of Oral & Maxillofacial Surgery 2001;39(5):365‐70. [DOI] [PubMed] [Google Scholar]
Swart 1994 {published data only}
- Swart MJ, Gordon PC, Hayse‐Gregson PB, Dyer RA, Swanepoel AL, Buckels NJ, Schall R, Odell JA. High‐dose aprotinin in cardiac surgery‐‐a prospective, randomized study. Anaesthesia & Intensive Care 1994;22(5):529‐33. [DOI] [PubMed] [Google Scholar]
Tabuchi 1994 {published data only}
- Tabuchi N, Huet RC, Sturk A, Eijsman L, Wildevuur CR. Aprotinin preserves hemostasis in aspirin‐treated patients undergoing cardiopulmonary bypass. Annals of Thoracic Surgery 1994;58(4):1036‐9. [DOI] [PubMed] [Google Scholar]
Taggart 2003 {published data only}
- Taggart DP, Djapardy V, Naik M, Davies A. A randomized trial of aprotinin (Trasylol) on blood loss, blood product requirement, and myocardial injury in total arterial grafting. Journal of Thoracic & Cardiovascular Surgery 2003;126(4):1087‐94. [DOI] [PubMed] [Google Scholar]
Taghaddomi 2009 {published data only}
- Taghaddomi RJ Mirzaee A Attar AS Shirdel A. Tranexamic Acid Reduces Blood Loss in Off‐Pump Coronary Artery Bypass Surgery. Journal of Cardiothoracic and Vascular Anesthesia 2009;23(3):312‐5. [DOI] [PubMed] [Google Scholar]
Tanaka 2001 {published data only}
- Tanaka N, Sakahashi H, Sato E, Hirose K, Ishima T, Ishii S. Timing of the administration of tranexamic acid for maximum reduction in blood loss in arthroplasty of the knee. Journal of Bone & Joint Surgery ‐ British Volume 2001;83(5):702‐5. [DOI] [PubMed] [Google Scholar]
Tassani 2000 {published data only}
- Tassani P, Augustin N, Barankay A, Braun SL, Zaccaria F, Richter JA. High‐dose aprotinin modulates the balance between proinflammatory and anti‐inflammatory responses during coronary artery bypass graft surgery. Journal of Cardiothoracic & Vascular Anesthesia 2000;14(6):682‐6. [DOI] [PubMed] [Google Scholar]
Thorpe 1994 {published data only}
- Thorpe CM, Murphy WG, Logan M. Use of aprotinin in knee replacement surgery. British Journal of Anaesthesia 1994;73(3):408‐10. [DOI] [PubMed] [Google Scholar]
Trinh‐Duc 1992 {published data only}
- Trinh‐Duc P, Wintrebert P, Boulfroy D, Albat B, Thevenet A, Roquefeuil B. Comparison of the effects of epsilon‐aminocaproic acid and aprotinin on intra‐ and postoperative bleeding in heart surgery. [French]. Annales de Chirurgie 1992;46(8):677‐683. [PubMed] [Google Scholar]
Troianos 1999 {published data only}
- Troianos CA, Sypula RW, Lucas DM, D'Amico F, Mathie TB, Desai M, Pasqual RT, Pellegrini RV, Newfeld ML. The effect of prophylactic epsilon‐aminocaproic acid on bleeding, transfusions, platelet function and fibrinolysis during coronary artery bypass grafting. Anesthesiology 1999;91(2):430‐5. [DOI] [PubMed] [Google Scholar]
Turkoz 2001 {published data only}
- Turkoz A, Cigli A, But K, Sezgin N, Turkoz R, Gulcan O, Ersoy MO. The effects of aprotinin and steroids on generation of cytokines during coronary artery surgery. Journal of Cardiothoracic & Vascular Anesthesia 2001;15(5):603‐10. [DOI] [PubMed] [Google Scholar]
Uozaki 2001 {published data only}
- Uozaki Y, Watanabe G, Kotou K, Ueyama K, Doi Y, Misaki T. Effect of tranexamic acid on blood loss reduction after cardiopulmonary bypass. Japanese Journal of Thoracic & Cardiovascular Surgery 2001;49(5):273‐8. [DOI] [PubMed] [Google Scholar]
Urban 2001 {published data only}
- Urban MK, Beckman J, Gordon M, Urquhart B, Boachie‐Adjei O. The efficacy of antifibrinolytics in the reduction of blood loss during complex adult reconstructive spine surgery. Spine 2001;26(10):1152‐6. [DOI] [PubMed] [Google Scholar]
Utada 1997 {published data only}
- Utada K, Matayoshi Y, Sumi C, Itaya M, Miyawaki H, Ito M, Tamura H, Mitsukuji T. Aprotinin 2 million KIU reduces perioperative blood loss in patients undergoing primary total hip replacement. [Japanese]. Masui ‐ Japanese Journal of Anesthesiology 1997;46(1):77‐82. [PubMed] [Google Scholar]
Van der Linden 2005 {published data only}
- Linden J, Lindvall G, Sartipy U. Aprotinin decreases postoperative bleeding and number of transfusions in patients on clopidogrel undergoing coronary artery bypass graft surgery: A double‐blind, placebo‐controlled, randomized clinical trial. Circulation 2005;112(Suppl I):I‐276‐80. [DOI] [PubMed] [Google Scholar]
Van Oeveren 1987 {published data only}
- Oeveren W, Jansen NJ, Bidstrup BP, Royston D, Westaby S, Neuhof H, Wildevuur CR. Effects of aprotinin on hemostatic mechanisms during cardiopulmonary bypass. Annals of Thoracic Surgery 1987;44(6):640‐5. [DOI] [PubMed] [Google Scholar]
Vander‐Salm 1996 {published data only}
- Vander Salm TJ, Kaur S, Lancey RA, Okike ON, Pezzella AT, Stahl RF, Leone L, Li J‐M, Valeri CR, Michelson AD. Reduction of bleeding after heart operations through the prophylactic use of epsilon‐aminocaproic acid. Journal of Thoracic & Cardiovascular Surgery 1996;112(4):1098‐107. [DOI] [PubMed] [Google Scholar]
Vanek 2005 {published data only}
- Vanek T, Jares M, Fajt R, Straka Z, Jirasek K, Kolesar M, Brucek P, Maly M. Fibrinolytic inhibitors in off‐pump coronary surgery: A prospective, randomized, double‐blind TAP study (tranexamic acid, aprotinin, placebo). European Journal of Cardio‐Thoracic Surgery 2005;28(4):563‐8. [DOI] [PubMed] [Google Scholar]
Vedrinne 1992 {published data only}
- Vedrinne C, Girard C, Jegaden O, Blanc P, Bouvier H, Ffrench P, Mikaeloff P, Estanove S. Reduction in blood loss and blood use after cardiopulmonary bypass with high‐dose aprotinin versus autologous fresh whole blood transfusion. Journal of Cardiothoracic & Vascular Anesthesia 1992;6(3):319‐23. [DOI] [PubMed] [Google Scholar]
Veien 2002 {published data only}
- Veien M, Sorensen JV, Madsen F, Juelsgaard P. Tranexamic acid given intraoperatively reduces blood loss after total knee replacement: a randomized, controlled study. Acta Anaesthesiologica Scandinavica 2002;46(10):1206‐11. [DOI] [PubMed] [Google Scholar]
Wei 2006 {published data only}
- Wei M, Jian K, Guo Z, Wang L, Jiang D, Zhang L, Tarkka M. Tranexamic acid reduces postoperative bleeding in off‐pump coronary artery bypass grafting. Scandinavian Cardiovascular Journal 2006;40(2):105‐9. [DOI] [PubMed] [Google Scholar]
Wendel 1995 {published data only}
- Wendel HP, Heller W, Michel J, Mayer G, Ochsenfahrt C, Graeter U, Schulze J, Hoffmeister HM, Hoffmeister HE. Lower cardiac troponin T levels in patients undergoing cardiopulmonary bypass and receiving high‐dose aprotinin therapy indicate reduction of perioperative myocardial damage. Journal of Thoracic & Cardiovascular Surgery 1995;109(6):1164‐72. [DOI] [PubMed] [Google Scholar]
Wong 2000 {published data only}
- Wong BI, McLean RF, Fremes SE, Deemar KA, Harrington EM, Christakis GT, Goldman BS. Aprotinin and tranexamic acid for high transfusion risk cardiac surgery. Annals of Thoracic Surgery 2000;69(3):808‐16. [DOI] [PubMed] [Google Scholar]
Wong 2008 {published data only}
- Wong J, Beheiry H, Rampersaud Y R, Lewis S, Ahn H, Silva Y, et al. Tranexamic Acid reduces perioperative blood loss in adult patients having spinal fusion surgery. Anesthesia and analgesia 2008;107(5):1479‐86. [DOI] [PubMed] [Google Scholar]
- Wong J, El‐Beheiry H, Rampersaud R, Lewis S, Fehlings M, Chung F. Tranexamic acid reduces blood loss and transfusion in adult patients having spinal fusion surgery. Canadian Journal of Surgery 2007;50(3 Suppl):S12‐3. [Google Scholar]
Wu 2006 {published data only}
- Wu CC, Ho WM, Cheng SB, Yeh DC, Wen MC, Liu TJ, et al. Perioperative parenteral tranexamic acid in liver tumor resection: a prospective randomized trial toward a "blood transfusion"‐free hepatectomy. Annals of Surgery 2006;243(2):173‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yamasaki 2004 {published data only}
- Yamasaki S, Masuhara K, Fuji T. Tranexamic acid reduces blood loss after cementless total hip arthroplasty‐prospective randomized study in 40 cases. International Orthopaedics 2004;28(2):69‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yassen 1993 {published data only}
- Yassen K, Bellamy MC, Sadek SA, Webster NR. Tranexamic acid reduces blood loss during orthotopic liver transplantation. Clinical Transplantation 1993;7(5):453‐8. [Google Scholar]
Zabeeda 2002 {published data only}
- Zabeeda D, Medalion B, Sverdlov M, Ezra S, Schachner A, Ezri T, Cohen AJ. Tranexamic acid reduces bleeding and the need for blood transfusion in primary myocardial revascularization. Annals of Thoracic Surgery 2002;74(3):733‐8. [DOI] [PubMed] [Google Scholar]
Zhang 2007 {published data only}
- Zhang F, Gao Z, Yu J. [Clinical comparative studies on effect of tranexamic acid on blood loss associated with total knee arthroplasty]. [Chinese]. Chinese Journal of Reparative & Reconstructive Surgery 2007;21(12):1302‐4. [PubMed] [Google Scholar]
Zohar 2004 {published data only}
- Zohar E, Ellis M, Ifrach N, Stern A, Sapir O, Fredman B. The postoperative blood‐sparing efficacy of oral versus intravenous tranexamic acid after total knee replacement. Anesthesia & Analgesia 2004;99(6):1679‐83. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Fejer 1998 {published data only}
- Fejer C, Mezofi M, Varga PP. Use of high‐dose aprotinin during spinal surgery results in a reduction in both blood loss and the amount of blood transfused. [abstract]. British Journal of Anaesthesia 1998;80(Suppl 1):80. [Google Scholar]
Langdown 2000 {published data only}
- Langdown AJ, Field J, Grote J, Himayat H. Aprotinin (Trasylol) does not reduce bleeding in primary total hip arthroplasty. Journal of Arthroplasty 2000;15(8):1009‐12. [DOI] [PubMed] [Google Scholar]
Montesano 1996 {published data only}
- Montesano RM, Gustafson PA, Palanzo DA, Manley NJ, Sadr FS. The effect of low‐dose epsilon‐aminocaproic acid on patients following coronary artery bypass surgery. Perfusion 1996;11(1):53‐6. [DOI] [PubMed] [Google Scholar]
Zufferey 2010 {published data only}
- Zufferey P J, Miquet M, Quenet S, Laporte S, Martin P, Chambefort V, et al. Does tranexamic acid decrease erythrocyte transfusion in patients undergoing hip fracture surgery with fondaaparinux for prevention of venous thromboembolism?. http://www isth2007 com/ 2007.
- Zufferey PJ Miquet M Quenet S Martin P Adam P Albaladejo P Mismetti P Molliex S. Tranexamic acid in hip fracture surgery: A randomized controlled trial. British Journal of Anaesthesia 2010;104(1):23‐30. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Myles 2008 {published data only}
- Myles P S, Smith J, Knight J, Cooper D J, Silbert B, McNeil J, et al. Aspirin and Tranexamic Acid for Coronary Artery Surgery (ATACAS) Trial: rationale and design. American heart journal 2008;155(2):224‐30. [DOI] [PubMed] [Google Scholar]
Verma 2010 {published data only}
- Verma K, Errico TJ, Vaz KM, Lonner BS. A prospective, randomized, double‐blinded single‐site control study comparing blood loss prevention of tranexamic acid (TXA) to epsilon aminocaproic acid (EACA) for corrective spinal surgery. BMC Surgery 2010;10(13). [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Beattie 2006
- Beattie WS, Karkouti K. Con: Aprotinin has a good efficacy and safety profile relative to other alternatives for prevention of bleeding in cardiac surgery.[comment]. Anesthesia & Analgesia 2006;103(6):1360‐4. [DOI] [PubMed] [Google Scholar]
Bidstrup 2006
- Bidstrup BP. Aprotinin and tranexamic acid in high‐transfusion‐risk cardiac surgery. Transfusion 2006;46(12):2208‐10. [DOI] [PubMed] [Google Scholar]
Blumberg 1997
- Blumberg N. Allogeneic transfusion and infection: economic and clinical implications. Seminars in Hematology 1997;34(3 Suppl 2):34‐40. [PubMed] [Google Scholar]
Body 2006
- Body SC, Mazer CD. Pro: Aprotinin has a good efficacy and safety profile relative to other alternatives for prevention of bleeding in cardiac surgery. Anesthesia & Analgesia 2006;103(6):1354‐9. [DOI] [PubMed] [Google Scholar]
Boldt 2009
- Boldt J, Suttner S, Brosch C, Lehmann A, Roehm K, Mengitsu A. Cardiopulminary bypass priming using a high dose of a balanced hydroxyethyl starch versus an albumin‐based priming strategy. Anesthesia & Analgesia 2009;109:1752‐62. [DOI] [PubMed] [Google Scholar]
Bryson 1998
- Bryson GL, Laupacis A, Wells GA. Does acute normovolemic hemodilution reduce perioperative allogeneic transfusion? A meta‐analysis. The International Study of Perioperative Transfusion. Anesthesia & Analgesia 1998;86(1):9‐15. [DOI] [PubMed] [Google Scholar]
Carless 2005
- Carless PA, Moxey AJ, Stokes BJ, Henry DA. Are antifibrinolytic drugs equivalent in reducing blood loss and transfusion in cardiac surgery? A meta‐analysis of randomized head‐to‐head trials. BMC Cardiovascular Disorders 2005;5:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carson 1998
- Carson JL, Terrin ML, Barton FB, Aaron R, Greenburg AG, Heck DA, Magaziner J, Merlino FE, Bunce G, McClelland B, et al. A pilot randomized trial comparing symptomatic vs. hemoglobin‐level‐driven red blood cell transfusions following hip fracture. Transfusion 1998;38(6):522‐9. [DOI] [PubMed] [Google Scholar]
Coyle 1999
- Coyle D, Lee KM, Fergusson DA, Laupacis A. Economic analysis of erythropoietin use in orthopaedic surgery. Transfusion Medicine 1999;9(1):21‐30. [DOI] [PubMed] [Google Scholar]
CRASH‐2 2010
- Shakur H, Roberts I, Bautista R, Caballero J, Coats T, Dewan Y, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH‐2): a randomised, placebo‐controlled trial. Lancet 2010;376(9734):23‐32. [PUBMED: 20554319] [DOI] [PubMed] [Google Scholar]
Der Simonian 1986
- Simonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. [DOI] [PubMed] [Google Scholar]
Faught 1998
- Faught C, Wells P, Fergusson D, Laupacis A. Adverse effects of methods for minimizing perioperative allogeneic transfusion: a critical review of the literature. Transfusion Medicine Reviews 1998;12(3):206‐25. [DOI] [PubMed] [Google Scholar]
Ferguson 2007
- Ferguson TB, Jr. Aprotinin‐‐are there lessons learned?. JAMA 2007;297(5):527‐9. [DOI] [PubMed] [Google Scholar]
Fergusson 1999
- Fergusson D, Walraven C, Coyle D, Laupacis A. Economic evaluations of technologies to minimize perioperative transfusion: a systematic review of published studies. International Study of Peri‐operative Transfusion (ISPOT) investigators. Transfusion Medicine Reviews 1999;13(2):106‐17. [DOI] [PubMed] [Google Scholar]
Forgie 1998
- Forgie MA, Wells PS, Laupacis A, Fergusson D. Pre‐operative Autologous Donation deceases allogeneic transfusion but increases exposure to all red cell transfusion. Results of a Meta‐Analysis. Archives of Internal Medicine 1998;158(6):610‐16. [DOI] [PubMed] [Google Scholar]
Fritz 1983
- Fritz H, Wunderer G. Biochemistry and applications of aprotinin, the kallikrein inhibitor from bovine organs. Arzneimittel‐Forschung 1983;33(4):479‐94. [PubMed] [Google Scholar]
Hebert 1999
- Hebert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. New England Journal of Medicine 1999;340(6):409‐17. [DOI] [PubMed] [Google Scholar]
Hebert 2005
- Hebert P, Fergusson DA, BART Investigators. Blood conservation using antifibrinolytics: A randomized trial in a cardiac surgery population ‐ the BART study. (http://www.controlled‐trials.com/ISRCTN15166455/BART) 2005.
Henry 1999
- Henry DA, Moxey AJ, Carless PA, O'Connell D, McClelland B, Henderson KM, et al. Anti‐fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane Database of Systematic Reviews 1999, Issue 4. [DOI: 10.1002/14651858.CD001886.pub2] [DOI] [PubMed] [Google Scholar]
Henry 2007
- Henry DA, Carless PA, Moxey AJ, O'Connell D, Stokes BJ, McClelland B, et al. Anti‐fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane Database of Systematic Reviews 2007, Issue 4. Art. No.: CD001886. DOI: 10.1002/14651858.CD001886.pub2.. [PUBMED: 17943760] [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2009
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration. Chichester, UK: Wiley & Sons, 2009. [Google Scholar]
Huet 1999
- Huet C, Salmi LR, Fergusson D, Koopman‐van Gemert AW, Rubens F, Laupacis A. A meta‐analysis of the effectiveness of cell salvage to minimize perioperative allogeneic blood transfusion in cardiac and orthopedic surgery. International Study of Perioperative Transfusion (ISPOT) Investigators. Anesthesia & Analgesia 1999;89(4):861‐9. [DOI] [PubMed] [Google Scholar]
Karkouti 2004
- Karkouti K, Wijeysundera DN, Yau TM, Beattie WS, Abdelnaem E, McCluskey SA, et al. The independent association of massive blood loss with mortality in cardiac surgery. Transfusion 2004;44(10):1453‐62. [DOI] [PubMed] [Google Scholar]
Karkouti 2006
- Karkouti K, Beattie WS, Dattilo KM, McCluskey SA, Ghannam M, Hamdy A, et al. A propensity score case‐control comparison of aprotinin and tranexamic acid in high‐transfusion‐risk cardiac surgery. Transfusion 2006;46(3):327‐38. [DOI] [PubMed] [Google Scholar]
Kimball 1995
- Kimball AM, Berkley S, Ngugi E, Gayle H. International aspects of the AIDS/HIV epidemic. Annual Review of Public Health 1995;16:253‐82. [DOI] [PubMed] [Google Scholar]
Laupacis 1997
- Laupacis A, Fergusson D. Drugs to minimize perioperative blood loss in cardiac surgery: meta‐analyses using perioperative blood transfusion as the outcome. The International Study of Peri‐operative Transfusion (ISPOT) Investigators. Anesthesia & Analgesia 1997;85(6):1258‐67. [DOI] [PubMed] [Google Scholar]
Levi 1999
- Levi M, Cromheecke ME, Jonge E, Prins MH, Mol BJM, Briet E, et al. Pharmacological strategies to decrease excessive blood loss in cardiac surgery: a meta‐analysis of clinically relevant endpoints. Lancet 1999;354(9194):1940‐7. [DOI] [PubMed] [Google Scholar]
Levy 2006
- Levy JH, Ramsay JG, Guyton RA. Aprotinin in cardiac surgery. New England Journal of Medicine 2006; Vol. 354, issue 18:1953‐7. [PubMed]
Mangano 2006
- Mangano DT, Tudor IC, Dietzel C, Multicenter Study of Perioperative Ischemia Research Group, Ischemia Research and Education Foundation. The risk associated with aprotinin in cardiac surgery. New England Journal of Medicine 2006;354(4):353‐65. [DOI] [PubMed] [Google Scholar]
Mangano 2007
- Mangano DT, Miao Y, Vuylsteke A, Tudor IC, Juneja R, Filipescu D, et al. Investigators of The Multicenter Study of Perioperative Ischemia Research Group, Ischemia Research and Education Foundation. Mortality associated with aprotinin during 5 years following coronary artery bypass graft surgery. JAMA 2007;297(5):471‐9. [DOI] [PubMed] [Google Scholar]
Mannucci 1998
- Mannucci PM. Hemostatic drugs. New England Journal of Medicine 1998;339(4):245‐53. [DOI] [PubMed] [Google Scholar]
McFarland 1997
- McFarland W, Mvere D, Shandera W, Reingold A. Epidemiology and prevention of transfusion‐associated human immunodeficiency virus transmission in sub‐Saharan Africa. Vox Sanguinis 1997;72(2):85‐92. [DOI] [PubMed] [Google Scholar]
Munoz 1999
- Munoz JJ, Birkmeyer NJ, Birkmeyer JD, O'Connor GT, Dacey LJ. Is epsilon‐aminocaproic acid as effective as aprotinin in reducing bleeding with cardiac surgery?: a meta‐analysis. Circulation 1999;99(1):81‐9. [DOI] [PubMed] [Google Scholar]
O'Brien 2002
- O'Brien MF, Harrocks S, Clarke A, Garlick B, Barnett AG. How to do safe sternal reentry and the risk factors of redo cardiac surgery: a 21‐year review with zero major cardiac injury. Journal of Cardiac Surgery 2002;17(1):4‐13. [DOI] [PubMed] [Google Scholar]
Reinhart 2011
- Reinhart K, Takala J. Hydroxyethyl Starches: What Do We Still Know?. Anesthesia and Analgesia 2011;112(3):507‐511. [DOI] [PubMed] [Google Scholar]
Review Manager [Computer program]
- The Nordic Cochrane Centre. Review Manager. Version 4.2.8. Copenhagen: The Cochrane Collaboration, 2003.
Royston 1998
- Royston D. Aprotinin versus lysine analogues: the debate continues. Annals of Thoracic Surgery 1998;65(4):S9‐19. [DOI] [PubMed] [Google Scholar]
Schneeweiss 2008
- Schneeweiss S, Seeger JD, Landon J, Walker AM. Aprotinin during coronary‐artery bypass grafting and risk of death. The New England journal of medicine 2008;358(8):771‐83. [PUBMED: 18287600] [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DJ. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Sedrakyan 2004
- Sedrakyan A, Treasure T, Elefteriades JA. Effect of aprotinin on clinical outcomes in coronary artery bypass graft surgery: a systematic review and meta‐analysis of randomized clinical trials. Journal of Thoracic & Cardiovascular Surgery 2004;128(3):442‐8. [DOI] [PubMed] [Google Scholar]
Shafer 2011
- Shafer SL. Shadow of Doubt. Anesthesia and Analgesia 2011;112(3):498‐500. [DOI] [PubMed] [Google Scholar]
Smith 1998
- Smith CR. Management of bleeding complications in redo cardiac operations. Annals of Thoracic Surgery 1998;65(4):S2‐8; discussion S27‐8. [DOI] [PubMed] [Google Scholar]
Vamvakas 2001
- Vamvakas EC, Blajchman MA. Deleterious clinical effects of transfusion‐associated immunomodulation: fact or fiction?. Blood 2001;97(5):1180‐95. [DOI] [PubMed] [Google Scholar]
Vamvakas 2004
- Vamvakas EC. White‐blood‐cell‐containing allogeneic blood transfusion, postoperative infection and mortality: a meta‐analysis of observational 'before‐and‐after' studies. Vox Sanguinis 2004;86(2):111‐9. [DOI] [PubMed] [Google Scholar]
Whyte 1997
- Whyte GS, Savoia HF. The risk of transmitting HCV, HBV or HIV by blood transfusion in Victoria. Medical Journal of Australia 1997;166(11):584‐6. [DOI] [PubMed] [Google Scholar]


