Abstract
Exogenous aldehydes can cause pain in animal models, suggesting that aldehyde dehydrogenase 2 (ALDH2), which metabolizes many aldehydes, may regulate nociception. To test this hypothesis, we generated a knock-in mouse with an inactivating point mutation in ALDH2 (ALDH2*2), which is also present in human ALDH2 of ~540 million East Asians. The ALDH2*1/*2 heterozygotic mice exhibited a larger response to painful stimuli than their wild-type littermates, and this heightened nociception was inhibited by an ALDH2-selective activator (Alda-1). No effect on inflammation per se was observed. Using a rat model, we then showed that nociception tightly correlated with ALDH activity (R2=0.90) and that reduced nociception was associated with less early growth response protein 1 (EGR1) in the spinal cord and less reactive aldehyde accumulation at the insult site (including acetaldehyde and 4-hydroxynonenal). Further, acetaldehyde and formalin-induced nociceptive behavior was greater in the ALDH2*1/*2 mice than wild-type mice. Finally, Alda-1 treatment was also beneficial when given even after the inflammatory agent was administered. Our data in rodent models suggest that the mitochondrial enzyme ALDH2 regulates nociception and could serve as a molecular target for pain control, with ALDH2 activators, such as Alda-1, as potential non-narcotic cardiac-safe analgesics. Furthermore, our results suggest a possible genetic basis for East Asians’ apparent lower pain tolerance.
Introduction
Pain is an international health problem, affecting approximately 1 in every 5 individuals (1). Approximately 200 million opioid prescriptions are written annually in the United States, and in 2013 vicodin was the overall number one prescribed medication (2). According to the National Center for Health Statistics data recently released for 2010, opioid drugs caused 75% of all drug-induced deaths and were responsible for 16,651 fatalities (3). Secondary health complications, including opioid abuse and dependence, afflict 2.1 million people (4). In addition, non-steroidal anti-inflammatory pain medications (NSAIDs) are used by over 30 million people each day in the United States for analgesia (5). Yet, NSAIDs and cyclooxygenase-2 inhibitors also increase the risk of gastrointestinal bleeding and cardiac events, and their safety has been questioned for certain patient populations, such as those with cardiac disease (6, 7). Thus, new molecular targets that regulate pain are needed to develop therapeutics for pain control with fewer deleterious addictive and cardiovascular effects.
Reactive aldehydes, including 4-hydroxynonenal (4-HNE), formaldehyde and acetaldehyde, cause pain when directly applied in rodents (8–10). We therefore determined whether altering the enzymatic activity of the mitochondrial aldehyde dehydrogenase-2 (ALDH2), which catalyzes removal of these reactive aldehydes, alters pain responses. We were also interested in this question because a common inactivating point mutation in mitochondrial aldehyde dehydrogenase 2 (ALDH2; Glu487 to Lys487), found in 36% of Han Chinese, affects approximately 8% of the world population (11). The ALDH2*2 in the Han Chinese codes for a dominant negative variant reducing ALDH2 enzymatic activity by ~60–80% in heterozygotes (ALDH2*1/*2) and by ~95% in homozygotes (ALDH2*2/*2) compared to wild type ALDH2*1/*1 (11). The ALDH2*2 variant causes flushing after ethanol consumption, a result of acetaldehyde accumulation (11). The ALDH2*2 inactivating mutation also causes reduced metabolism of other reactive aldehydes, including malondialdehyde and 4-HNE(12), and the rate of formaldehyde metabolism in human mitochondrial liver fractions from ALDH2*1/*2 subjects is ~3 times slower than in those from ALDH2*1/*1 subjects (13).
Our laboratory has developed a small molecule that selectively enhances the activity of ALDH2, Alda-1 (N-(1,3-benzodioxol-5-ylmethyl)-2,6-dichlorobenzamide) (14). Alda-1 corrects the structural defect in the mutant ALDH2*2, thereby increasing ALDH2*2 activity (15). Here we determined whether ALDH2 enzymatic activity modulates acute inflammatory-induced hyperalgesia and whether the ALDH2 activator Alda-1 could be a potential drug to reduce pain.
Results
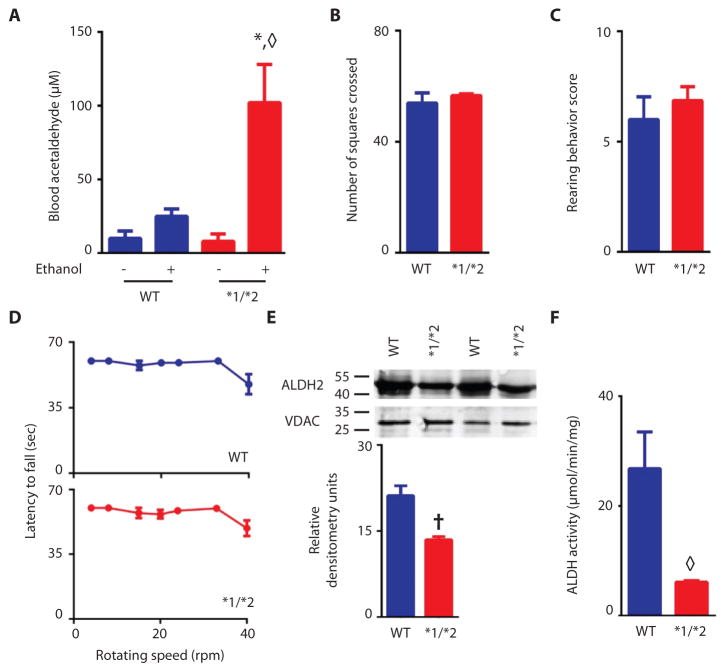
We generated knock-in mice carrying the inactivating Lys487 point mutation in ALDH2, identical to the mutation found in Han Chinese [(11); denoted ALDH2*2] (Fig. S1). To confirm that the mutant mice mimic the human phenotype, we challenged them with ethanol and determined blood acetaldehyde levels. Similar to human heterozygotes (16), heterozygote mice accumulated 5 times higher blood acetaldehyde concentrations than did wild type ALDH2 (ALDH2*1/*1) mice (Fig. 1A). Prior to nociceptive testing, we performed behavioral tests to confirm that the ALDH2 inactivating mutation did not affect mouse gross motor skills. No differences were detected in the mean number of squares crossed in an open field (Fig. 1B) or in rearing behavior (Fig. 1C) between wild type and ALDH2*1/*2 mice. Similarly, in a rotarod test, no differences were seen between wild type and ALDH2*1/*2 mice (Fig. 1D and S2). When assessed in liver tissue homogenate, the abundance of ALDH2*1/*2 protein was approximately 44% lower than that in wild type mice (Fig. 1E), and ALDH activity in the heterozygotic mice was 20% that of wild type animals (Fig. 1F). These characteristics of the ALDH2*1/*2 protein in the heterozygotic mice were similar to those described for human heterozygotes (17).
Figure 1. Characterization of heterozygotic ALDH2*1/*2 mice and ALDH2*1/*1 wild type mice.
A. Wild type (WT) or heterozygous ALDH2*1/*2 (*1/*2) mice were given 4g/kg ethanol or water of equal volume by gavage, and acetaldehyde blood levels were measured 60 minutes later (n=4 animals/treatment group). B–D. Motor skill tests of wild type ALDH2*1/*1 (blue bars) and heterozygous ALDH2*1/*2 mice (red bars). Mean number of squares crossed in an open field test in (B); number of rearing events in 3 minutes in (C); and latency to fall from a rotating rod (D)(for B and C n=6–7 animals/group, for D n=10 animals/group), tested once per animal. E. Western blot quantitation of ALDH2 protein in wild type and heterozygotic ALDH2*1/*2 knock-in mice. (50μg liver protein homogenate was loaded per lane) (n=3 biological replicates/group). F. ALDH enzyme activity in wild type and ALDH2*1/*2 mice, expressed as μmol NADH per minute per mg of protein (n=3 biological replicates/group). All data are mean±SEM; *P<0.01 compared to wild type given water, ◇P<0.01 compared to all other groups, †P<0.05 compared to wild type, as assessed by t-test (B, C, E, and F), one-way ANOVA (A), and two-way ANOVA (D).
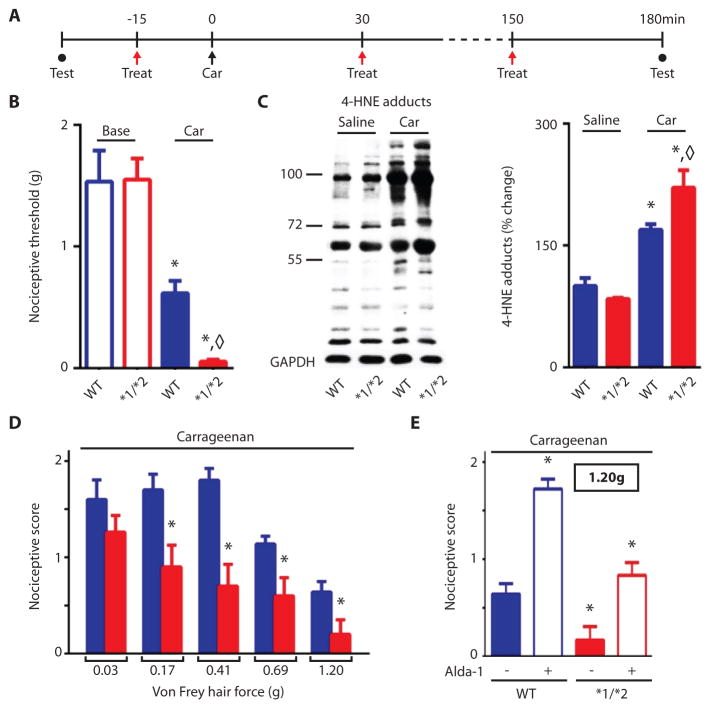
We next assessed nociception in ALDH2*1/*2 mice that had received carrageenan injections in the hind paw and compared their responses to those of wild-type littermates (Fig. 2A). The ALDH2*1/*2 untreated mice had lower ALDH2 levels in their paws than wild type mice (Fig. S3A), similar to the liver homogenates assessed (Fig. 1E). Mouse pain behavior, assessed by an observer blinded to the experimental conditions, was measured by applying von Frey filaments at known force to elicit paw withdrawal, by an “up-down” method (details in methods section). We observed no differences in baseline nociceptive behavior between wild type and ALDH2*1/*2 mice (Fig. 2B). After carrageenan injection to induce acute inflammatory pain, the ALDH2*1/*2 mice exhibited a larger hyperalgesia response than did wild type mice (Fig. 2B). Carrageenan administration also increased the accumulation of 4-HNE adducts in the paw, with the ALDH2*2 mice having higher 4-HNE adduct levels than the wild type mice (Fig. 2C). When we used anociceptive response scoring method for quantification of nociceptive pain responses (18), we also saw that the ALDH2*1/*2 mice exhibited reduced hyperalgesia in response to each von Frey hair tested (Fig. 2D and Fig. S3B). Administration of the ALDH2 activator Alda-1 (2mg/kg per treatment) reduced the response to the nociceptive stimulus in the heterozygous ALDH2*1/*2 mice for each von Frey hair tested (Fig. 2E, S4A–S4E; baseline (untreated) values are provided in S4A). Moreover, Alda-1 reversed the behavioral response to the carrageenan-induced insult, restoring thresholds to baseline values for both wild type and ALDH2*1/*2 mice, when assessed by the up-down method (Fig. S4F baseline: wild type, 1.5±0.1 and ALDH2*1/*2, 1.6±0.2; 3 hours after carrageenan: wild type, 1.7±0.4 and ALDH2*1/*2, 1.3±0.2, values in grams, mean ± SEM, n=8/group). When Alda-1 was given to wild type mice alone, without an inflammatory insult, no changes were noted in behavioral response (Fig. S4G). We confirmed Alda-1 selectivity for ALDH2 by testing in vitro its effect on the activity of five other ALDH enzymes (14) (Table. S1).
Fig. 2. Experimental protocol and nociceptive behavioral results for ALDH2*1/*2 mice (red bars) and wild type mice (blue bars).
A. Experimental protocol: Nociceptive threshold testing (closed circles) using von Frey hairs performed at baseline and 3 hours after induction of the inflammatory insult. Red arrows indicate treatment with either vehicle or with the ALDH2 activator Alda-1 (2mg/kg, subcutaneously). At time zero (black arrow), carrageenan (100μg) was injected to the right hind paw. B. Nociceptive threshold in mice assessed at baseline and 3 hours after carrageenan-induced inflammatory insult using the up-down technique (n=8 animals/group, tested once per animal). C. A representative Western blot and % change in 4-HNE protein adducts in proteins extracted from paws treated with saline or with carrageenan from wild type and heterozygous ALDH2*1/*2 mice (n=8 animals/group, GAPDH used as loading control). D. In a separate subset of mice, nociceptive threshold was assessed with a scoring scale 3 hours after the inflammatory insult (2=no response, 1= withdrawal from stimulus, 0= immediate withdrawal from stimulus with licking or flinching) (n=6–7 animals/group, repeated 6 times per animal). The observers were blinded to the experimental conditions. Baseline measurements for D. are provided in Fig. S3. E. Nociceptive threshold for wild type and ADLH2*1/*2 mice after administration of Alda-1 (2mg/kg per treatment) as assessed by the scoring technique used in (D) (n=8 animals/group, repeated 6 times per animal). Additional data for other von Frey hairs tested is provided in Fig. S3. All data are expressed as mean±SEM, *P<0.05 compared to wild type, ◇P<0.05 compared to all other groups, as assessed by ANOVA with Tukey’s correction; behavioral analyses were carried out by observers blinded to the experimental conditions.
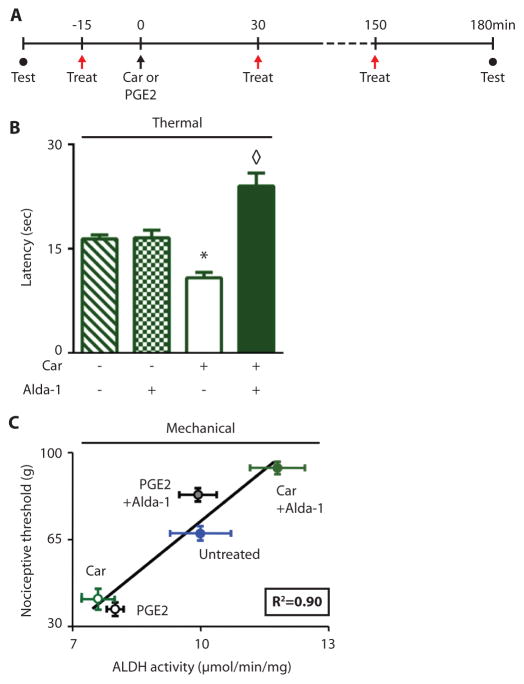
To further investigate how ALDH2 activity may regulate nociception, we used a rat model of inflammatory pain, by measuring a rat nociceptive response after injection of an inflammatory stimulus in the hind paw (Protocol; Fig. 3A). When given alone without an inflammatory stimulus, Alda-1 had no effects on response to thermal stimuli (defined as time lapsed after a change in thermal stimulus, or thermal latency) (Fig. 3B). Similarly, Alda-1 did not affect basal sensitivity to mechanical stimulus as assessed by incrementally increasing the amount of weight in grams applied to the rat paw, by performing the Randall-Selitto test (70±2g versus 73±1g, with and without Alda-1, respectively; n=10 Alda-1 group, n=6 vehicle group). However, when carrageenan was injected in one paw, Alda-1 doubled the latency time to thermal stimulation of that paw (Fig. 3B). Additionally, Alda-1 doubled the nociceptive threshold to mechanical stimulation: rats withdrew their inflamed paw at 45 grams of pressure in the absence of Alda-1, after Alda-1 treatment, they withdrew their paw only after 90 grams of pressure was applied (Fig. 3C, y-axis values). Similar data were observed when prostaglandin E2, instead of carrageenan was used to induce nociception (Fig. 3C, y-axis values). In each condition, ALDH activity in the paw correlated with nociceptive threshold (R2=0.90, Fig. 3C).
Figure 3. Experimental protocol and nociceptive behavior in rats.
A. Experimental protocol: Nociceptive threshold testing (closed circles) assessed by Randall-Selitto at baseline and 3 hours after inflammatory insult. Red arrows indicate treatment with either vehicle or with the ALDH2 activator, Alda-1 (2mg/kg, subcutaneously). At time zero (black arrow) the inflammatory insults (carrageenan or prostaglandin E2; PGE2) were given to the right hind paw. B. Latency to thermal stimulation for rat hind paw withdrawal with and without treatment with Alda-1 C. Nociceptive threshold in rats subjected to carrageenan or prostaglandin E2 pro-inflammatory insults. All data expressed as mean±SEM, *P<0.01 compared to untreated and Alda-1 alone group, ◇P<0.01 compared to all other groups assessed by ANOVA with Tukey’s correction (B) and linear regression (C), n=6–8 animals/group, tested once per animal; the observers were blinded to the experimental conditions.
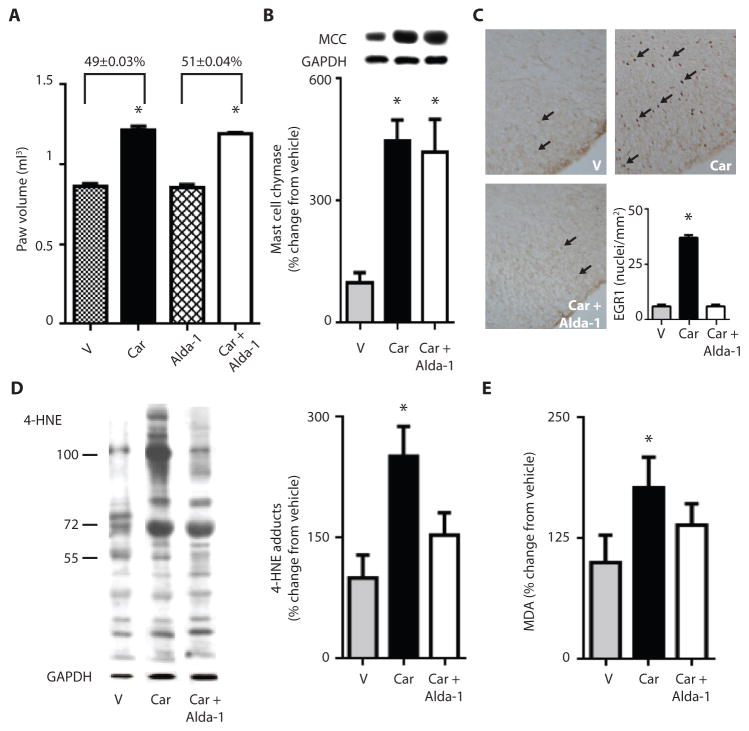
To test whether the Alda-1-reduction in nociception was a result of an anti-inflammatory effect, we measured carrageenan-induced edema of the paw and found that inflammation was unaltered by Alda-1 treatment (Fig. 4A and B). Carrageenan administration caused a ~50% increase in paw volume over the untreated contralateral paw of vehicle-treated rats, and this increase was unaffected by Alda-1 treatment (Fig. 4A). Further, markers of inflammation, including mast cell chymase (Fig. 4B) and neutrophil elastase (267±61 versus 220±26, relative units determined by Western blot, Fig. S5), increased equally in the vehicle-treated and Alda-1-treated carrageenan groups. In contrast, early growth response protein 1 (EGR1) levels, a central nervous system marker of hyperalgesia (19), increased ~5 fold in the L4-6 spinal cord dorsal horn superficial laminae, and the increase in EGR1 levels was brought back to basal levels by Alda-1 treatment (Fig. 4C). We also found that 4-HNE-protein adducts and malondialdehyde (MDA) levels were elevated in the inflamed paw and that these levels declined after Alda-1 treatment (Fig. 4D and 4E). Thus, inflammation-induced aldehydic load, but not the inflammation response, was decreased by Alda-1 induced inhibition of ALDH2.
Figure 4. Effect of ALDH2 activation by Alda-1 on aldehydic load and hyperalgesia.
For A–E, rats were subjected to the protocol described in Fig. 3A, and all analyses were carried out 3 hours after carrageenan-induced insult by an observer blinded to the experimental conditions. A. Paw volume 3 hr after carrageenan injection. B. Mast cell chymase in the paw after carrageenan injection. C. Histological analysis of slices stained for EGR1, a marker of hyperalgesia in the dorsal horn superficial laminae for L4-L6 regions of the spinal cord. (n=6 animals/group, expressed as number of positive nuclei counted per mm2). D. A representative Western blot for 4-HNE protein adducts in the paws of vehicle- (V), carrageenan- (Car) and carrageenan + Alda-1- (Car+ Alda-1) treated groups. (n=5–6 biological replicates/group). E. Malondialdehyde (MDA) levels assessed as percent change relative to naïve rats as assessed by TBARs assay. (n=6 biological replicates/group). All data are expressed as mean±SEM, *P<0.01 versus vehicle, †P<0.05 compared to vehicle assessed by ANOVA with Tukey’s correction.
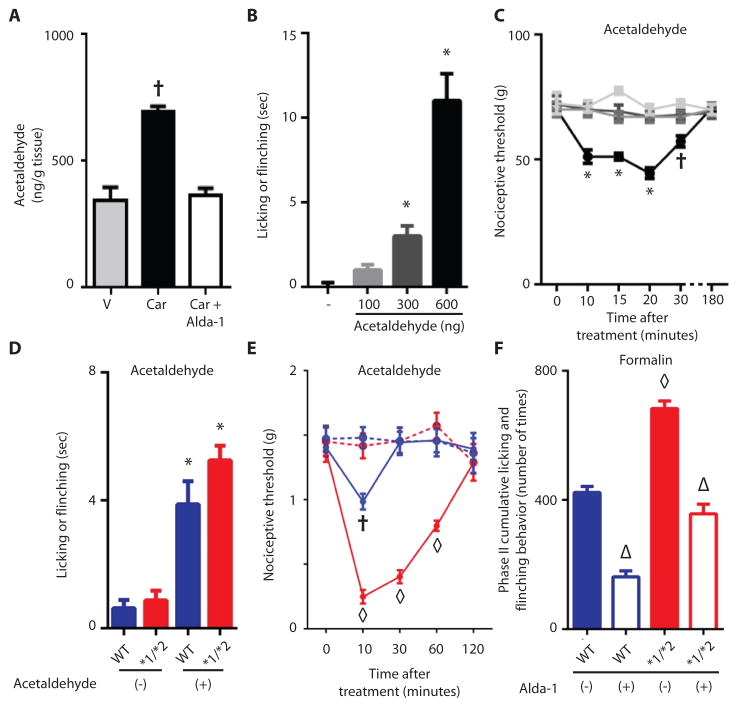
ALDH2 metabolizes acetaldehyde, so we therefore measured this aldehyde in the paws of our animals. Unexpectedly, acetaldehyde was detected in the paws, and carrageenan doubled its levels (Fig. 5A). Alda-1 treatment decreased acetaldehyde to basal values (Fig. 5A). Since acetaldehyde accumulated during the carrageenan-induced inflammatory insult, we determined whether direct administration of acetaldehyde to the paw induced nociception. Acetaldehyde caused an immediate effect on pain behavior, as assessed by paw flinching and licking, in a dose-dependent manner (Fig. 5B). Further, injection of acetaldehyde (600 ng), but not vehicle, in the paw decreased the nociceptive threshold, an effect that declined by 30 minutes and dissipated by 3 hours after injection (Fig. 5C). The ALDH2*1/*2 mice spent slightly more time flinching and licking after acetaldehyde injection compared to the wild type mice, but this was likely biologically insignificant, since the difference was about 1 second (Fig. 5D). More important, the acetaldehyde injection lowered the nociceptive threshold in the ALDH2*1/*2 mice and extended the duration of nociceptive behavior compared to the wild type ALDH2*1/*1 mice (Fig. 5E).
Figure 5. ALDH2 involvement in acetaldehyde and formaldehyde-induced acute nociception.
A. Quantification of acetaldehyde in carrageenan-induced inflamed paw. (n=3 biological replicates/group, repeated 3 times for each replicate). B. Acetaldehyde at the amounts measured in the paw (in A.) was injected into the hind paw, and licking and flinching were measured. C. Nociceptive threshold after administration of 600 ng of acetaldehyde (n=4–7/ animals group, tested once for each animal). D. A single acetaldehyde (100ng) injection was administered to the paw and licking and flinching were measured (n=8/group, tested once per animal). E. Using the up-down technique, the intensity and duration of the acetaldehyde-induced nociceptive behavior was measured in ALDH2*1/*2 mice and wild type ALDH2*1/*1 mice (n=8/group, tested once per animal). F. Phase II cumulative flinching and licking for wild type ALDH2*1/*1 mice and ALDH2*1/*2 mice. (n=8/group, tested once per animal). All data are expressed as mean±SEM, *P<0.001 versus vehicle, †P<0.05 versus all other groups, ◇P<0.001 compared to all other groups, △P<0.001 compared to respective vehicle treated groups, assessed by ANOVA with Tukey’s correction, blue data points and bars= wild type ALDH2*1/*1 mice, red data points and bars= ALDH2*1/*2 mice.
We next subjected both wild type ALDH2*1/*1 and heterozygous ALDH2*1/*2 mice to nociception induced by formalin (2%, aqueous formaldehyde) injection into the paw. The formalin-induced nociceptive response occurs in two phases: An immediate phase (0–9 minutes) and a more sustained phase II (10). After injection, we noted extended phase II flinching and licking behavior (Fig. 5F) but no differences in phase I (Fig. S6A, S6C) between ALDH2*1/*2 mice and ALDH2*1/*1 wild type mice, with phase II lasting 30 minutes longer in ALDH2*1/*2 mice than in formalin-injected wild type mice (Fig. S6A). The increase in cumulative flinching and licking behavior in phase II was largely a result of changes in IIb (Fig. S6D). The formalin-induced flinching during phase II was markedly reduced by Alda-1 treatment in both groups of mice (Fig. 5F, S6D).
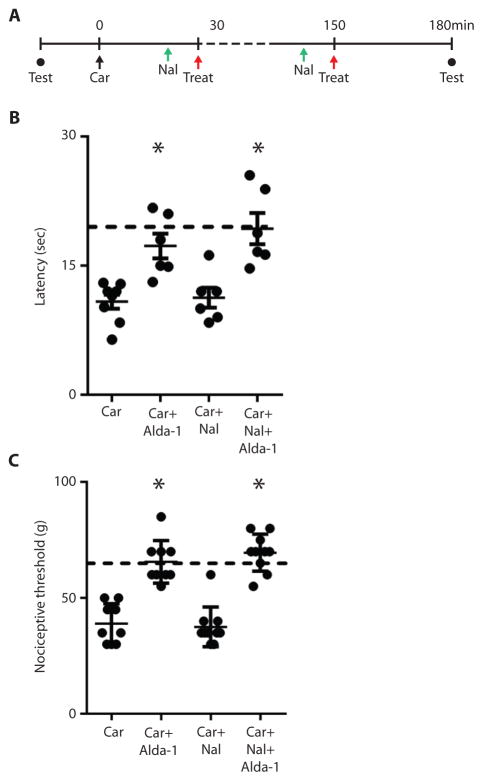
We next determined whether Alda-1 treatment was beneficial when given to the mice after the inflammatory condition was established (Fig. 6A). We found that Alda-1 increased the thermal latency and the nociceptive threshold even when injected after the inflammation-inducing carrageenan stimulus was administered (Fig. 6B and 6C). Thus, the beneficial effect of ALDH2 activators was not limited to prophylactic use. We also found that the anti-nociceptive effect of Alda-1 was not blunted in the presence of the opioid receptor antagonist, naloxone (1mg/kg per injection, given 10 minutes prior to Alda-1 treatment) (Fig. 6B and 6C), suggesting that it does not involve an opiate pathway.
Figure 6. Effect of Alda-1 after the inflammatory insult on nociceptive threshold.
A. Nociceptive threshold testing (closed circles) assessed by either Hargreaves or Randall-Selitto at baseline and 3 hours after inflammatory insult. Red arrows, treatment either of vehicle or the ALDH2 activator, Alda-1 (2mg/kg, subcutaneously). Naloxone (Nal, 1mg/kg) or vehicle was given subcutaneously (green arrows) 10 minutes before each Alda-1 treatment. At time zero (black arrow) the inflammatory insult (carrageenan) was given to the right hind paw. B. and C. Alda-1 was given 30 and 150 minutes after carrageenan (n=9/group, tested once per animal *P<0.0001 assessed by ANOVA followed by Tukey’s correction). Error bars represent mean±SEM. The observers were blinded to the experimental conditions.
Discussion
Our study demonstrates that activation of ALDH2 with Alda-1 in two rodent models reduces nociception and that the ALDH2*2 inactivating mutation in mice, which mimics the human genotype present in 8% of the world population, increases the nociceptive response. We have previously found that activation of ALDH2 reduces cardiac injury after ischemic insult (14) and improves cardiac function in a rodent model of heart failure(20). Here we also showed that the reduced nociception by Alda treatment is insensitive to opiate receptor antagonists. Together, our data suggest that ALDH2 activators, such as Alda-1, may represent a non-narcotic, cardiac-safe class of therapeutic agents for acute inflammatory-induced pain.
Pain is a subjective feeling that can only be measured in animal models as a behavioral response to nociceptive stimulus. We used two different techniques to assess nociception in mice: a scoring system used by some East Asian laboratories (18) and the up-down technique used by the Western world (21) to study pain behavior in rodents. Further, due to the small paw volume in a mouse and time required to breed a number of mice for our behavioral studies, we used a second model of pain in rats and performed more extensive studies in that model.
Reactive aldehydes, including MDA and 4-HNE, induce pain responses (22, 23). These aldehydes are produced by reactive oxygen species-induced peroxidation of exogenous and endogenous polyunsaturated fatty acids. Blood levels of 4-HNE in healthy individuals (~0.5μM) can increase 10–100 fold under oxidative stress (24, 25). Similar increases in MDA levels after oxidative stress have also been reported (26). Although its main source is ethanol metabolism (27, 28), acetaldehyde may be formed by pyruvate dehydrogenase in the mitochondria and the accumulation of aldehydes impairs mitochondrial ALDH2 activity (14); these sources may have contributed to the acetaldehyde accumulation that we observed. Regardless, our data demonstrate the importance of ALDH2 in controlling the levels of reactive aldehydes, including 4-HNE, MDA and acetaldehyde, and reducing pain sensation.
Although additional transgenic mice with alterations in ALDH2 exist, including a knockout ALDH2 mouse (29, 30) and an overexpressing ALDH2*2 mouse (31), the ALDH2*2 mice that we have generated are particularly suitable for pre-clinical and translational studies because they mimic the human ALDH2*2 point mutation. Like humans carrying the ALDH2*2 mutation, no noticeable gross motor differences were identified in mice with this mutation. In addition, ALDH2 protein levels and enzymatic activity in the heterozygotic mice were decreased similarly to these values in heterozygotic humans (17).
A number of limitations to our study should be noted, including that we only studied ALDH2*1/*2 heterozygotic mice. We also did not demonstrate whether the beneficial effect of Alda-1 treatment results from central or peripheral action, nor did we determine Alda-1 efficacy in chronic pain and arthritis Nevertheless, because mice with this mutation exhibited reduced response to nociception after Alda-1 treatment, subjects with the ALDH2*2 variant may experience more severe inflammatory pain and benefit from drugs that activate ALDH2.
Known genetic mutations regulating pain sensation are limited to small familial subsets of the human population (32, 33) and to rare point mutations in ion channels (34, 35). Our findings in rodents suggest the ALDH2 point mutation, ALDH2*2, present in 8% of the world population, might also contribute to lower pain thresholds in humans. The prevalence of the ALDH2*2 point mutation, which is unique to East Asian ethnicity, is ~36% (11) and in some regions as high as 45% (36). Asians are reported to have a lower pain tolerance than other ethnicities (37–40) and are more responsive to painful stimuli such as skin irritation (39), capsaicin (40), heat (41), cold (42) or mechanical stimulus (37) compared with other ethnic populations. Although these differences are ascribed primarily to social or cultural factors (38), it is possible that increased pain sensitivity among Asians can be attributed, at least in part, to the ALDH2*2 mutation. Further evidence supporting a role for ALDH2 in pain sensation are case reports suggesting the ALDH2 inhibitor disulfiram may cause painful peripheral neuropathy, in a dose-dependent and reversible manner (43). Nevertheless, our findings in rodents indicating that ALDH2 activity attenuates nociception remain to be confirmed in humans.
Our finding that Alda-1 corrects ALDH2 inactivity due to the mutation in ALDH2*2 (15), suggest that compounds like Alda-1 may benefit East Asian and other populations in reducing inflammatory pain. The apparent non-opioid dependent mechanism for Alda-1 would circumvent the potential for addiction and abuse seen with opioids used for analgesia (44). Moreover, Alda-1 is beneficial when given during ischemic injury (14) and reduces ischemia-induced cardiac arrhythmias (45). Although more studies are required, this non-opioid, cardiac-safe profile for Alda-1 may be particularly useful for patients with cardiac risk factors and known cardiac disease since the use of some traditional NSAIDs or cyclooxygenase-2 (COX-2) inhibitors was confirmed in a large meta-analysis to increase cardiovascular risk (7).
In summary, our study identified ALDH2 as a regulator of acute nociception in rodents. Although confirmatory studies are needed, our rodent studies suggest that the ALDH2*2 mutation may underlie differences in pain behavior observed in humans. We showed heterozygous mice with an ALDH2 inactivating mutation, ALDH2*1/*2, have increased reactive aldehyde-induced adducts and increased nociception when compared to wild type mice; these effects were corrected by Alda-1 treatment. Increasing the activity of mitochondrial ALDH2 with Alda-1 reduces aldehydic load, limits changes in a marker of hyperalgesia, EGR1, and provides analgesia. Our data suggest activators of ALDH2, such as Alda-1, may represent a promising therapeutic drug class to reduce pain likely independent of opioid-receptor signaling and without increasing cardiovascular risk.
Materials and Methods
Study design
All procedures were in accordance with the guidelines of the International Association for the Study of Pain; approved by Stanford University (#25505) and the Butantan Institute, Sao Paulo, Brazil (CEUAIB #838).
The study objective tested whether ALDH2 regulated inflammatory pain in rodent models. We tested our hypothesis by using 1) ALDH2*1/*2 knock-in mice litter--matched to C57/BL6 wild type ALDH2 mice, 2) Wistar rats, 3) an ALDH2 activator, Alda-1, which also corrects the decreased enzymatic activity of the ALDH2*1/*2 mutation, and 4) inflammatory pain models by administering either carrageenan, PGE2, acetaldehyde or formalin to the rodent paw. We used both mechanical and thermal methods of nociception detection, in addition to biochemical analysis.
Based upon our previous experience, a minimum of 6 rodents per group is required to obtain statistically meaningful behavioral data. An experimental group size of 6 or greater animals is necessary to achieve at least a 20% minimal difference in behavior for a power of 95% with α<0.05 and β<20%. All behavioral tests were performed between 9:00 am and 4:00 pm by observers blinded to the treatment groups. Male Wistar rats (170–190 grams) and male mice (18–21 grams) were housed in a temperature and light-controlled room for at least 3 days before use. All animals were randomized and assigned to testing groups to generate biological replicates for each group and the experimenter was blinded as to genotypes and drug treatments given.
Drugs
0.1 ml saline (control), carrageenan (200μg for rats, 100μg for mice; Marine Colloids), prostaglandin E2 (100ng, Sigma) or formalin (2%, Sigma) was administered by intraplantar injection into the right hind paw to induce nociception. These doses were chosen based on previous studies by our laboratory groups (46) and published literature(47).
Alda-1 dose (2 mg/kg/per injection, dissolved in 50% PEG/50% DMSO) was established in preliminary studies and given in multiple doses because of its estimated short half-life. Alda-1 was injected subcutaneously to the dorsal side of the neck 15 minutes before carrageenan or saline, and again at 30 and 150 minutes after carrageenan, prostaglandin E2 or saline injection. One subset of animals received Alda-1 only 30 and 150 minutes after carrageenan injection. Where indicated, naloxone (1mg/kg per injection, Sigma) or vehicle (saline) was given 10 minutes prior to each Alda-1 treatment to the dorsal side of the neck.
Knock-in mice generation (see also Fig. S1)
ALDH2*2 knock-in mice with a C57/BL6 background were generated by homologous recombination. The 8.0-kb genomic fragment encompassing the mouse ALDH2 locus was cloned to the gene-targeting vector pPNT to introduce a single amino acid substitution. Site-directed mutagenesis introduced a single nucleotide substitution (G to C) within exon 12 of the ALDH2 genomic fragment corresponding to the position of human E487K mutation. Plasmid DNA from the constructed pPNT vector was electroporated to embryonic stem (ES) cells. Positive ES clones were selected on the basis of neomycin and thymine kinase markers and confirmed by PCR, RFLP and by DNA sequencing. Positive ES cells were microinjected into C57/BL6 blastocytes and implanted into pseudopregnant females.
Germ-line transmission of the ALDH2 E487K mutation by homologous recombination was derived from selected ES cell lines from founder mice. The genotype of the E487K mutation was confirmed by direct genomic DNA sequencing of PCR fragments from amplified genomic DNA. The specific primers EG475 (TACTGTCAAAGTGCCACAGAAGAACTCGTAA), EG460 (AACCTGCGTGCAATCCATCTTGTTCAATGG) and EG399 (TTGGCCTTCCACTGGGA GTGGGTCCCTCTGTC) were used for the amplification of a 1.3 kb fragment from exon 13 to the 3’ untranslated region of neomycin marker and a 3.0 kb fragment from exon 13 to downstream of the neomycin marker, respectively, for the presence of the mutated allele. In contrast, for the wild type allele, a 1.4 kb fragment devoid of the neomycin marker was amplified with EG475 and EG399 primers. We confirmed the expression of the mutant ALDH2 E487K mutant protein, which exhibited a change in charge as a result of the glutamate to lysine substitution, by isoelectric focusing gel electrophoresis and Western blot using an ALDH2-specific antibody (Santa Cruz Biotechnology, 1:500). The founder mice were back-crossed to the C57BL/6 background for at least 7 generations to achieve a homogeneous genetic background, and the E487K mutation was transmitted as a single Mendelian gene.
Nociception assessment
The experimental performer was blinded to both genotype and/or treatments. Nociceptive response was assessed by testing mechanical and thermal hypersensitivity. Mechanical sensitivity was measured by the von Frey method for mice and the Randall-Selitto method for rats immediately before and 180 minutes after carrageenan or PGE2 injection. Thermal sensitivity was measured by the Hargreaves method. Both mice and rats were acclimated to the testing equipment one day before baseline testing.
To measure mechanical sensitivity, mice were placed individually in a plastic cage with a wire mesh bottom for testing. After acclimation to the plastic cage for at least 15 min, a von Frey hair was pressed perpendicularly against the plantar skin surface and held for approximately 5 seconds until the hair slightly buckled (36, 50). Mice were randomly separated into two testing groups, one scored with the up-down technique and the other with a pain scoring system.
The up-down technique was performed as follows
Prior to and after carrageenan administration, von Frey hairs of increasing force were applied to the mice hind paw until the paw was withdrawn. Once a response was identified, the next lower von Frey hair was applied. For each positive response (paw withdrawal) the next lesser filament was tested. For each negative response, the next higher filament was tested. The sequence of positive and negative responses were recorded and incorporated in an established curve-fitting algorithm, to determine mechanical sensitivity (48).
The scoring system technique was performed as follows: Hind paw nociception was assessed with six different von Frey hairs (0.03, 0.17, 0.41, 0.69, 1.20 and 1.48 g). Using the same stimulation intensity as the 0.03 g von Frey hair, each individual hair was applied six times to each hind paw at intervals of several seconds. Since the response to each von Frey stimulus is fairly subjective, we used a method of objectifying these nociceptive responses by assigning a specific number to the response. The scoring system consisted of: 2= no response, 1= withdraws paw from hair and 0= immediate flinching or hind paw licking. This method was previously shown by others to provide a reliable, objective assessment of pain response using von Frey hairs (18, 49).
Nociceptive threshold was also determined with the Randall-Selitto paw pressure test (Ugo-Basile), as described (50). The baseline values varied between 65–75g of force. Animals with baseline out of this range (10%) were excluded prior to initiating the study in order to achieve uniform testing.
Thermal sensitivity, as assessed by hind paw withdrawal threshold was measured by Hargreaves method (51). Rats were placed in the apparatus to acclimate for 10 minutes. A light source was then applied to the hind paw, and the time until the rat withdrew the paw from the light source was assessed. A 30 second exposure time was used to limit thermal damage to the paw region, with light source intensity adjusted in preliminary studies to cause a latency response of 15 seconds in control rats.
Edema assessment
Inflammation-induced edema of the injected paw was assessed with a plethysmometer (Ugo Basile) (52).
Evaluation of general motor activity (open field and rotarod)
General activity of ALDH2*1/*2 knock-in and wild type mice was assessed in an open-field arena (Ugo-Basile). Each animal was individually placed in the center zone, and behavioral parameters were recorded for 3 min (50). Hand-operated counters were used to tally the number of squares crossed and rearing behavior (number of times standing on hind legs). For rotarod testing, mice were trained at a fixed speed of 10 rpm for 3 consecutive days. Mice were then tested at the different speeds, and latency to fall was measured. For the acceleration task, the rotarod was accelerated from 5 to 40 rpm over 5 min. The fall latency was recorded.
Blood acetaldehyde determination
Blood samples (50–100μl) were drawn from the retro-orbital sinus under anesthesia. Levels of acetaldehyde were determined by a fluorescence-based HPLC method, as described previously (53).
Tissue acetaldehyde Measurements
Paw samples were quickly sealed in microcentrifuge tubes, weighed and 3 volumes (v/w) of 0.75mg/mL Liberase TL (Roche, Indianapolis, IN) was added for overnight digestion at 37°C. Acetaldehyde standard solutions (100–5000 ng/mL) and tissue samples were reacted with saturated 2,4-dinitrophenylhydrazine solution. To establish LC-MS/MS conditions, a standard of acetaldehyde 2,4-dinitrophenyl-hydrazine (DNPH) was used. Chromatograms of standards established characteristic retention times of acetaldehyde, and verified that the MS signal was linear over the range of 0.1–5μg/mL. The acetaldehyde peak area derivatives were calculated and plotted against the concentration of the calibration standards with Analyst1.5.1 software.
Aliquots (30 μL) were taken from each paw homogenate sample. Acetaldehyde was extracted from samples of paw homogenate by adding methanol containing 403g/mL DNPH (150 μL). Both unknown samples and standards were vortexed and centrifuged to separate the precipitate. Ten microliters of sample extracts were injected into LC-MS/MS for analysis.
Automatic peak detection, integration and data processing were performed by the AB SCIEX Analyst 1.5.1 software package. Concentrations of acetaldehyde were calculated by plotting peak area of unknown samples against a standard curve. The data were normalized to the paw volume obtained by plesthysmography.
Acetaldehyde experiments
Rats were acclimated to the test environment 15 minutes prior to experiments. Acetaldehyde (100, 300 and 600 ng/g of paw tissue) was diluted in saline containing 0.5% Tween 80 and injected intraplantarly. Hindpaw licking and flicking behavior was quantified for 10 minutes. Immediately after this test, the paw threshold was assessed at 10, 15, 20, 30 and 180 minutes after acetaldehyde injection, using Randall-Selitto as described. For mice, acetaldehyde (100 ng/g of paw tissue) was injected intraplantarly, and licking and flicking behavior was quantified for 10 minutes. Immediately afterward, the paw threshold was assessed at 10, 30, 60 and 120 minutes, using von Frey test as described.
Formalin experiments
Mice were placed individually in a plastic cage. After acclimation for ~15 min, formalin (2%, 20μL, in saline) was injected, and flinches as well as time spent licking the injected hindpaw were recorded. Behavior was divided as follows: phase I (0–10 minutes), phase II (10–90 minutes), with phase II divided into phase IIa (10 to 40 min) and phase IIb (40–90 minutes) as described(10). For a subset of experiments, Alda-1 was given once, five minutes before formalin was administered.
Biochemical studies for rats and mice
180 minutes after treatment, rat paw tissue was homogenized, centrifuged at 700g for 5 min at 4°C and the pellet discarded. Protein concentration in the supernatant was determined by Bradford assay.
Aldehyde dehydrogenase enzymatic measurement
ALDH enzymatic activity was determined as described (14).
Western blot analysis
Western blot was performed as described (14). For 4-HNE protein adduct detection, an antibody against the reductively stabilized 4-HNE amino acid adduct (Alpha Diagnostic Intl, 1:1000) was used. For mast cell chymase and neutrophil elastase detection antibodies from Santa Cruz Biotechnology (1:500) were used. Quantification analysis of blots was performed with Image J software, using GAPDH as a loading control.
Thiobarbituric acid-reactive substance (TBARS) assay
TBARS, which measures malondialdehyde, was performed with an Oxi-Tek TBARS assay kit (ZeptoMetrix). The concentration of TBARS was expressed in pmol/mg protein by using a standard curve of malondialdehyde at 0–200 pmol concentrations and expressed as percent control.
Immunohistochemistry
Animals were anesthetized with ketamine (90mg/kg) and xylazine (15mg/kg) after 3 hours of the experimental protocol. Subsequently, animals were perfused with 4% paraformaldehyde (PFA) in 0.1M phosphate buffer (pH 7.4). Spinal cord sections (L4-L6) were removed, post-fixed for 4h in PFA (4%), and transferred to a 30% sucrose solution in phosphate buffer to ensure cryoprotection. Spinal cords were sectioned (10 μm) on a cryostat and displayed on histological slides. The sections were immunostained for early growth response-1 gene (EGR1) expression, using a rabbit polyclonal antibody (Santa Cruz Biotechnology 1:500) in phosphate buffercontaining 0.3% Triton X-100 and 5% normal donkey serum overnight at 24°C. The sections were then washed in phosphate buffer and incubated with a biotinylated donkey anti-rabbit serum (Vector, 1:200) in phosphate buffer for 2h at 24°C. The sections were washed again in phosphate buffer and incubated with the avidin–biotin–peroxidase complex (ABC Elite, Vector Labs). The sections were then reacted with 0.05% 3,3-diaminobenzidine and 0.01% hydrogen peroxide in phosphate buffer. Intensification was conducted with 0.05% osmium tetroxide in water. The sections were dehydrated, cleared and mounted on a cover-slip. Normal rat serum served as control. Digital images of light microscopy were collected and cells with positive EGR were counted.
Statistical analysis
Data are expressed as mean±SEM. Analysis of variance (ANOVA) with Tukey post-hoc analysis was used for behavioral pain studies and biochemical analysis. When only two groups were analyzed and compared, an unpaired t-test was performed. For Fig. 3C, linear regression analysis and Pearson’s correlation testing were used to assess concordance. A value of at least P<0.05 was considered significant. GraphPad Prism software was used for all statistical analysis.
Supplementary Material
Fig S1. Scheme for generation of ALDH2*2 (E487K) knock-in mice.
Fig S2. Rotarod data.
Fig S3. ALDH2 protein level in mouse paw and additional nociceptive data.
Fig S4. Nociceptive testing.
Fig S5. Neutrophil elastase protein levels.
Fig S6. Formalin-induced pain model.
Table S1. ALDH activation by Alda-1.
Acknowledgments
We thank Drs. Hong Zeng and Yanru Chen-Tsai for their help in creating the ALDH2*2 knock-in mice at the Stanford University Transgenic Research Center, Marina Fridlib with acetaldehyde measurement help at the Stanford University BioADD Center, and Bryce Small for proof reading and artistic assistance.
Funding: Fellowship support was provided for ERG and VOZ by HL-109212 and 2011/08873-8, respectively. YC is supported by FAPESP 2012/05035-4 and 2013/07467-1. All work from this manuscript was supported by NIH MERIT award AA11147 to DM-R.
Footnotes
Author Contributions: DM-R and VOZ conceived the project. VOZ, ERG, C-HC, VPG designed and performed the experiments and carried out data analysis. ERG and DM-R wrote the manuscript with input from VOZ and YC. VOZ and ERG contributed to this project equally.
Data and materials availability: All data for this study is provided in the manuscript or supplemental material. Materials used for this manuscript are available upon request.
Competing Interests: DM-R and C-HC are the founders of ALDEA Pharmaceuticals and C-HC is a consultant for the company. However, none of the research in the academic laboratory is supported by or is reported to the company. DM-R, C-HC and VOZ hold a patent on “Methods and Compositions for Treating Pain”. The other authors have no disclosures.
References
- 1.Goldberg DS, McGee SJ. Pain as a global public health priority. BMC Public Health. 2011;11:770. doi: 10.1186/1471-2458-11-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Volkow ND, McLellan TA, Cotto JH, Karithanom M, Weiss SR. Characteristics of opioid prescriptions in 2009. JAMA. 2011;305:1299. doi: 10.1001/jama.2011.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones CM, Mack KA, Paulozzi LJ. Pharmaceutical overdose deaths, United States, 2010. JAMA. 2013;309:657. doi: 10.1001/jama.2013.272. [DOI] [PubMed] [Google Scholar]
- 4.Substance abuse and mental health serivces administration. NSDUH Series H-46, HHS Publication No. (SMA) 13-4795. Rockville, MD: 2013. Results from the 2012 National Survey on Drug Use and Health. [Google Scholar]
- 5.American Gastroenterological Association. Study shows long-term use of NSAIDs causes severe intestinal damage. Science Daily. 2014 Jan 16; http:/www.sciencedaily.com/releases/2005/01/050111123706.htm retrieved.
- 6.Mukherjee D, Nissen SE, Topol EJ. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA. 2001;286:954. doi: 10.1001/jama.286.8.954. [DOI] [PubMed] [Google Scholar]
- 7.Bhala N, Emberson J, Merhi A, Abramson S, Arber N, Baron JA, Bombardier C, Cannon C, Farkouh ME, FitzGerald GA, Goss P, Halls H, Hawk E, Wilson K, Wittes J, Baigent C. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet. 2013;382:769. doi: 10.1016/S0140-6736(13)60900-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bang S, Kim KY, Yoo S, Kim YG, Hwang SW. Transient receptor potential A1 mediates acetaldehyde-evoked pain sensation. The European Journal of Neuroscience. 2007;26:2516. doi: 10.1111/j.1460-9568.2007.05882.x. [DOI] [PubMed] [Google Scholar]
- 9.Trevisani M, Siemens J, Materazzi S, Bautista DM, Nassini R, Campi B, Imamachi N, Andre E, Patacchini R, Cottrell GS, Gatti R, Basbaum AI, Bunnett NW, Julius D, Geppetti P. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. PNAS. 2007;104:13519. doi: 10.1073/pnas.0705923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, Hayward NJ, Chong JA, Julius D, Moran MM, Fanger CM. TRPA1 mediates formalin-induced pain. PNAS. 2007;104:13525. doi: 10.1073/pnas.0705924104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brooks PJ, Enoch MA, Goldman D, Li TK, Yokoyama A. The alcohol flushing response: an unrecognized risk factor for esophageal cancer from alcohol consumption. PLoS Med. 2009;6:e50. doi: 10.1371/journal.pmed.1000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen CH, Ferreira JC, Gross ER, Mochly-Rosen D. Targeting aldehyde dehydrogenase 2: new therapeutic opportunities. Physiological Reviews. 2014;94:1. doi: 10.1152/physrev.00017.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang RS, Nakajima T, Kawamoto T, Honma T. Effects of aldehyde dehydrogenase-2 genetic polymorphisms on metabolism of structurally different aldehydes in human liver. Drug Metabolism and Disposition. 2002;30:69. doi: 10.1124/dmd.30.1.69. [DOI] [PubMed] [Google Scholar]
- 14.Chen CH, Budas GR, Churchill EN, Disatnik MH, Hurley TD, Mochly-Rosen D. Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart. Science. 2008;321:1493. doi: 10.1126/science.1158554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perez-Miller S, Younus H, Vanam R, Chen CH, Mochly-Rosen D, Hurley TD. Alda-1 is an agonist and chemical chaperone for the common human aldehyde dehydrogenase 2 variant. Nat Struct Mol Biol. 2010;17:159. doi: 10.1038/nsmb.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yokoyama A, Tsutsumi E, Imazeki H, Suwa Y, Nakamura C, Mizukami T, Yokoyama T. Salivary acetaldehyde concentration according to alcoholic beverage consumed and aldehyde dehydrogenase-2 genotype. Alcoholism, Clinical and Experimental Research. 2008;32:1607. doi: 10.1111/j.1530-0277.2008.00739.x. [DOI] [PubMed] [Google Scholar]
- 17.Lai CL, Yao CT, Chau GY, Yang LF, Kuo TY, Chiang CP, Yin SJ. Dominance of the Inactive Asian Variant Over Activity and Protein Contents of Mitochondrial Aldehyde Dehydrogenase 2 in Human Liver. Alcoholism, Clinical and Experimental Research. 2013 doi: 10.1111/acer.12215. [DOI] [PubMed] [Google Scholar]
- 18.Takasaki I, Andoh T, Shiraki K, Kuraishi Y. Allodynia and hyperalgesia induced by herpes simplex virus type-1 infection in mice. Pain. 2000;86:95. doi: 10.1016/s0304-3959(00)00240-2. [DOI] [PubMed] [Google Scholar]
- 19.Rahman OI, Terayama R, Ikeda T, Koganemaru M, Nakamura T, Shiba R, Nishimori T. Differential effects of NMDA and AMPA/KA receptor antagonists on c-Fos or Zif/268 expression in the rat spinal dorsal horn induced by noxious thermal or mechanical stimulation, or formalin injection. Neurosci Res. 2002;43:389. doi: 10.1016/s0168-0102(02)00067-6. [DOI] [PubMed] [Google Scholar]
- 20.Gomes KM, Campos JC, Bechara LR, Queliconi B, Lima VM, Disatnik MH, Magno P, Chen CH, Brum PC, Kowaltowski AJ, Mochly-Rosen D, Ferreira JC. Aldehyde dehydrogenase 2 activation in heart failure restores mitochondrial function and improves ventricular function and remodelling. Cardiovascular Research. 2014 doi: 10.1093/cvr/cvu125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun Y, Sahbaie P, Liang DY, Li WW, Li XQ, Shi XY, Clark JD. Epigenetic regulation of spinal CXCR2 signaling in incisional hypersensitivity in mice. Anesthesiology. 2013;119:1198. doi: 10.1097/ALN.0b013e31829ce340. [DOI] [PubMed] [Google Scholar]
- 22.Riahi Y, Cohen G, Shamni O, Sasson S. Signaling and cytotoxic functions of 4-hydroxyalkenals. American Journal of Physiology Endocrinology and metabolism. 2010;299:E879. doi: 10.1152/ajpendo.00508.2010. [DOI] [PubMed] [Google Scholar]
- 23.Patwardhan AM, Akopian AN, Ruparel NB, Diogenes A, Weintraub ST, Uhlson C, Murphy RC, Hargreaves KM. Heat generates oxidized linoleic acid metabolites that activate TRPV1 and produce pain in rodents. The Journal of Clinical Investigation. 2010;120:1617. doi: 10.1172/JCI41678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 25.Strohmaier H, Hinghofer-Szalkay H, Schaur RJ. Detection of 4-hydroxynonenal (HNE) as a physiological component in human plasma. Journal of Lipid Mediators and Cell Signalling. 1995;11:51. doi: 10.1016/0929-7855(94)00027-a. [DOI] [PubMed] [Google Scholar]
- 26.Nielsen F, Mikkelsen BB, Nielsen JB, Andersen HR, Grandjean P. Plasma malondialdehyde as biomarker for oxidative stress: reference interval and effects of life-style factors. Clinical Chemistry. 1997;43:1209. [PubMed] [Google Scholar]
- 27.Parrilla R, Okawa K, Lindros KO, Zimmerman UJ, Kobayashi K, Williamson JR. Functional compartmentation of acetaldehyde oxidation in rat liver. JBC. 1974;249:4926. [PubMed] [Google Scholar]
- 28.Eriksson CJ, Marselos M, Koivula T. Role of cytosolic rat liver aldehyde dehydrogenase in the oxidation of acetaldehyde during ethanol metabolism in vivo. Biochem J. 1975;152:709. doi: 10.1042/bj1520709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fernandez E, Koek W, Ran Q, Gerhardt GA, France CP, Strong R. Monoamine metabolism and behavioral responses to ethanol in mitochondrial aldehyde dehydrogenase knockout mice. Alcoholism, Clinical and Experimental Research. 2006;30:1650. doi: 10.1111/j.1530-0277.2006.00200.x. [DOI] [PubMed] [Google Scholar]
- 30.Kitagawa K, Kawamoto T, Kunugita N, Tsukiyama T, Okamoto K, Yoshida A, Nakayama K, Nakayama K. Aldehyde dehydrogenase (ALDH) 2 associates with oxidation of methoxyacetaldehyde; in vitro analysis with liver subcellular fraction derived from human and Aldh2 gene targeting mouse. FEBS letters. 2000;476:306. doi: 10.1016/s0014-5793(00)01710-5. [DOI] [PubMed] [Google Scholar]
- 31.Ohsawa I, Nishimaki K, Murakami Y, Suzuki Y, Ishikawa M, Ohta S. Age-dependent neurodegeneration accompanying memory loss in transgenic mice defective in mitochondrial aldehyde dehydrogenase 2 activity. Journal of Neuroscience. 2008;28:6239. doi: 10.1523/JNEUROSCI.4956-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cox JJ, Reimann F, Nicholas AK, Thornton G, Roberts E, Springell K, Karbani G, Jafri H, Mannan J, Raashid Y, Al-Gazali L, Hamamy H, Valente EM, Gorman S, Williams R, McHale DP, Wood JN, Gribble FM, Woods CG. An SCN9A channelopathy causes congenital inability to experience pain. Nature. 2006;444:894. doi: 10.1038/nature05413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kremeyer B, Lopera F, Cox JJ, Momin A, Rugiero F, Marsh S, Woods CG, Jones NG, Paterson KJ, Fricker FR, Villegas A, Acosta N, Pineda-Trujillo NG, Ramirez JD, Zea J, Burley MW, Bedoya G, Bennett DL, Wood JN, Ruiz-Linares A. A gain-of-function mutation in TRPA1 causes familial episodic pain syndrome. Neuron. 2010;66:671. doi: 10.1016/j.neuron.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sorge RE, Trang T, Dorfman R, Smith SB, Beggs S, Ritchie J, Austin JS, Zaykin DV, Vander Meulen H, Costigan M, Herbert TA, Yarkoni-Abitbul M, Tichauer D, Livneh J, Gershon E, Zheng M, Tan K, John SL, Slade GD, Jordan J, Woolf CJ, Peltz G, Maixner W, Diatchenko L, Seltzer Z, Salter MW, Mogil JS. Genetically determined P2X7 receptor pore formation regulates variability in chronic pain sensitivity. Nature Medicine. 2012;18:595. doi: 10.1038/nm.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Emery EC, Young GT, Berrocoso EM, Chen L, McNaughton PA. HCN2 ion channels play a central role in inflammatory and neuropathic pain. Science. 2011;333:1462. doi: 10.1126/science.1206243. [DOI] [PubMed] [Google Scholar]
- 36.Enomoto N, Takada A, Date T. Genotyping of the aldehyde dehydrogenase 2 (ALDH2) gene using the polymerase chain reaction: evidence for single point mutation in the ALDH2 gene of ALDH2-deficiency. Gastroenterologia Japonica. 1991;26:440. doi: 10.1007/BF02782812. [DOI] [PubMed] [Google Scholar]
- 37.Woodrow KM, Friedman GD, Siegelaub AB, Collen MF. Pain tolerance: differences according to age, sex and race. Psychosom Med. 1972;34:548. doi: 10.1097/00006842-197211000-00007. [DOI] [PubMed] [Google Scholar]
- 38.Chan MY, Hamamura T, Janschewitz K. Ethnic differences in physical pain sensitivity: Role of acculturation. Pain. 2013;154:119. doi: 10.1016/j.pain.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 39.Rowell LN, Mechlin B, Ji E, Addamo M, Girdler SS. Asians differ from non-Hispanic Whites in experimental pain sensitivity. Eur J Pain. 2011;15:764. doi: 10.1016/j.ejpain.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang H, Papoiu AD, Coghill RC, Patel T, Wang N, Yosipovitch G. Ethnic differences in pain, itch and thermal detection in response to topical capsaicin: African Americans display a notably limited hyperalgesia and neurogenic inflammation. Br J Dermatol. 2010;162:1023. doi: 10.1111/j.1365-2133.2009.09628.x. [DOI] [PubMed] [Google Scholar]
- 41.Watson PJ, Latif RK, Rowbotham DJ. Ethnic differences in thermal pain responses: a comparison of South Asian and White British healthy males. Pain. 2005;118:194. doi: 10.1016/j.pain.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 42.Hsieh AY, Tripp DA, Ji LJ, Sullivan MJ. Comparisons of catastrophizing, pain attitudes, and cold-pressor pain experience between Chinese and European Canadian young adults. The Journal of Pain. 2010;11:1187. doi: 10.1016/j.jpain.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 43.Frisoni GB, Di Monda V. Disulfiram neuropathy: a review (1971–1988) and report of a case. Alcohol and Alcoholism. 1989;24:429. [PubMed] [Google Scholar]
- 44.Okie S. A flood of opioids, a rising tide of deaths. The New England Journal of Medicine. 2010;363:1981. doi: 10.1056/NEJMp1011512. [DOI] [PubMed] [Google Scholar]
- 45.Koda K, Salazar-Rodriguez M, Corti F, Chan NY, Estephan R, Silver RB, Mochly-Rosen D, Levi R. Aldehyde dehydrogenase activation prevents reperfusion arrhythmias by inhibiting local renin release from cardiac mast cells. Circulation. 2010;122:771. doi: 10.1161/CIRCULATIONAHA.110.952481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zambelli VO, Fernandes AC, Gutierrez VP, Ferreira JC, Parada CA, Mochly-Rosen D, Cury Y. Peripheral sensitization increases opioid receptor expression and activation by crotalphine in rats. PloS one. 2014;9:e90576. doi: 10.1371/journal.pone.0090576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shields SD, Cavanaugh DJ, Lee H, Anderson DJ, Basbaum AI. Pain behavior in the formalin test persists after ablation of the great majority of C-fiber nociceptors. Pain. 2010;151:422. doi: 10.1016/j.pain.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Poree LR, Guo TZ, Kingery WS, Maze M. The analgesic potency of dexmedetomidine is enhanced after nerve injury: a possible role for peripheral alpha2-adrenoceptors. Anesthesia and Analgesia. 1998;87:941. doi: 10.1097/00000539-199810000-00037. [DOI] [PubMed] [Google Scholar]
- 49.Dale CS, Altier C, Cenac N, Giorgi R, Juliano MA, Juliano L, Zamponi GW, Vergnolle N. Analgesic properties of S100A9 C-terminal domain: a mechanism dependent on calcium channel inhibition. Fundamental & Clinical Pharmacology. 2009;23:427. doi: 10.1111/j.1472-8206.2009.00686.x. [DOI] [PubMed] [Google Scholar]
- 50.Gutierrez VP, Konno K, Chacur M, Sampaio SC, Picolo G, Brigatte P, Zambelli VO, Cury Y. Crotalphine induces potent antinociception in neuropathic pain by acting at peripheral opioid receptors. European Journal of Pharmacology. 2008;594:84. doi: 10.1016/j.ejphar.2008.07.053. [DOI] [PubMed] [Google Scholar]
- 51.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 52.Clapper JR, Moreno-Sanz G, Russo R, Guijarro A, Vacondio F, Duranti A, Tontini A, Sanchini S, Sciolino NR, Spradley JM, Hohmann AG, Calignano A, Mor M, Tarzia G, Piomelli D. Anandamide suppresses pain initiation through a peripheral endocannabinoid mechanism. Nature Neuroscience. 2010;13:1265. doi: 10.1038/nn.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peng GS, Wang MF, Chen CY, Luu SU, Chou HC, Li TK, Yin SJ. Involvement of acetaldehyde for full protection against alcoholism by homozygosity of the variant allele of mitochondrial aldehyde dehydrogenase gene in Asians. Pharmacogenetics. 1999;9:463. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1. Scheme for generation of ALDH2*2 (E487K) knock-in mice.
Fig S2. Rotarod data.
Fig S3. ALDH2 protein level in mouse paw and additional nociceptive data.
Fig S4. Nociceptive testing.
Fig S5. Neutrophil elastase protein levels.
Fig S6. Formalin-induced pain model.
Table S1. ALDH activation by Alda-1.