Abstract
Over the past six decades, the drug resistance of Plasmodium falciparum has become an issue of utmost concern. Despite the remarkable progress that has been made in recent years in reducing the mortality rate to about 30% with the scaling-up of vector control, introduction of artemisinin-based combination therapies and other malaria control strategies, the confirmation of artemisinin resistance on the Cambodia–Thailand border threatened all the previous success. This review addresses the global scenario of antimalarial resistance and factors associated with it, with the main emphasis on futuristic approaches like nanotechnology and stem cell therapy that may impede resistant malaria, along with novel medications which are preparing to enter the global antimalarial market. These novel studies are likely to escalate over the coming years and will hopefully help to reduce the burden of malaria.
Keywords: Malaria, Drug Resistance, Nanotechnology, RNAi, Stem cell, Peptides
Abstract
Au cours des six dernières décennies, la résistance aux médicaments de Plasmodium falciparum est devenue une question extrêmement préoccupante. Malgré des progrès remarquables accomplis ces dernières années pour réduire le taux de mortalité à environ 30 %, avec l’intensification de la lutte antivectorielle, l’introduction des thérapies combinées basées sur l’artémisinine et d’autres stratégies de lutte contre le paludisme, la confirmation de la résistance à l’artémisinine sur la frontière Cambodge-Thaïlande a menacé tous les succès précédents. Cette synthèse porte sur le scénario global de diverses résistances antipaludiques et les facteurs qui y sont associés, en soulignant les approches futuristes comme la nanotechnologie et la thérapie par les cellules souches, qui peuvent entraver le paludisme résistant, et les nouveaux médicaments qui vont bientôt entrer sur le marché antipaludéen mondial. Ces nouvelles études vont s’intensifier au cours des prochaines années et, nous l’espérons, contribuer à réduire la charge du paludisme.
Introduction
Malaria has been one of the most extensively studied parasitic infectious diseases for millennia. In 2012, there were around 627,000 malaria deaths worldwide, 90% of which were in the African Region, followed by Southeast Asia (7%) and the Eastern Mediterranean (3%). About 482,000 malaria deaths were estimated to occur in children under 5 years of age, constituting 77% of the global total. Most of these deaths were due to Plasmodium falciparum. However, Plasmodium vivax is now increasingly recognized as a cause of severe malaria and death [139]. For decades, drug resistance has been one of the greatest obstacles in fighting malaria. To date, drug resistance has been reported in three of the five Plasmodium species that is, P. falciparum, P. vivax and in P. malariae which are the causative agents for human malaria [45]. Drug resistance was initially outlined by WHO in 1967 as the ability of a parasite strain to survive or reproduce regardless of the administration and absorption of a drug when it is given in doses that are equal to or higher than those usually recommended but within the tolerance range of the given subject [137]. This was later modified by Bruce-Chwatt et al. [20] to include “the amount of the drug which is active against a given parasite must be able to gain access to the parasite or the infected erythrocyte for the length of the time necessary for its natural reaction”. Drug resistance usually leads to a delay or failure to clear asexual parasites from the peripheral blood that eventually enable production of gametocytes which are responsible for transmission of the resistant genotype. After the official recommendation by the WHO in 2001 [132] for use of artemisinin-based combination therapies (ACTs) as the first-line treatment of P. falciparum malaria, it was seen after 2005 that there was a substantial decline in outbreak of this disease [134]. However, parasites that are drug resistant to artemisinin and its derivatives have recently emerged in various parts of Southeast Asia, which threaten, all prior success of malaria control strategies, treatment and elimination efforts [30, 38]. At present, current antimalarial drugs act on a limited number of biological targets [124]. Therefore, the next challenge is to identify new classes of drugs that will attack novel molecular targets, with sufficient therapeutic lifespans that will not be compromised by the rapid development of resistance, and to develop novel technologies, that will effectively clear the parasite with maximum precision, thus minimising the risk of drug resistance [25]. This review summarises current scenarios, along with existing therapies and novel on-going approaches to curb drug-resistant malaria.
Current scenario of drug resistance
Of the various antimalarial drugs available, the aminoquinoline chloroquine was the agent of choice for many decades because of its safety, efficacy and affordability. However, parasite resistance to this drug was initially observed in Thailand in 1957 and then on the border of Colombia and Venezuela in 1959. By the late 1970s, resistance reached East Africa and by the mid-1980s had become a major problem in several areas of the continent [128]. At present, chloroquine remains effective only in some parts of Central America, where clinical studies have confirmed it as an effective drug [69]. However, recent data on the prevalence of chloroquine-resistant genotypes in these areas present an alarming situation for health officials [36]. Amodiaquine has been observed to be more effective than chloroquine mainly in areas of persistent chloroquine resistance. As a result, amodiaquine in combination with artesunate was adopted as the first-line treatment by several countries. Parasite strains that are highly resistant to amodiaquine have however been reported in Tanzania, which may additionally compromise the use of artesunate-amodiaquine in Africa [106]. Another antimalarial, sulfadoxine-pyrimethamine, has been widely used by several countries to treat chloroquine-resistant malaria. Nonetheless, the treatment failure rate of this combination has been found to be low in several countries of South America and Central and Middle East Asia, as compared to the failure rate in eastern Africa (52.8%) [45]. Presently, resistance to mefloquine continues to be a concern in the Greater Mekong sub-region, in particular in Thailand and Cambodia, where artesunate-mefloquine is still used as first-line treatment [108]. In order to maximise the effectiveness of artemisinin and its derivatives and to protect them from the development of resistance, WHO has repeatedly recommended that they can be combined with other drugs that have different mechanisms of action and longer half-lives. As a result, five combinations are currently recommended: artemether-lumefantrine, artesunate-amodiaquine, artesunate-mefloquine, artesunate-sulfadoxine-pyrimethamine and dihydroartemisinin-piperaquine (WHO, 2010) [138]. However, remarkable failure rates of these combinations have been observed in several African countries where resistance to one drug has been previously encountered, like in the case of artemether-lumefantrine. Artemether-lumefantrine remains highly effective in most parts of the world, with the exception of Cambodia. This combination mostly shows failure rates less than 10% [45]. However, resistance to most of these combinations will probably lead to a global epidemic outbreak of malaria. To overcome this concern, GlaxoSmithKline (GSK) along with the PATH Malaria Vaccine Initiative (MVI), with a grant from the Bill & Melinda Gates Foundation, have developed RTS, S/AS01, the most advanced candidate, which has proven its protective efficacy in children with a range of 30–50% [2, 3] and is believed to represent the first-generation malaria vaccine (WHO recommendation expected by 2015). Nevertheless, with data from Phase III trials indicating that the leading malaria vaccine candidate, RTS, S, has limited efficacy, it is important to consider new approaches for the development of a vaccine that is capable of inducing long-term protection [112].
Reasons leading to antimalarial resistance
Various factors lead to the occurrence and massive spread of resistance. Genetic mutations that confer antimalarial drug resistance mostly occur in nature and are independent of drug effect and are considered spontaneous mutations. The onset of resistance is thought to occur in two phases. In the first phase, an initial genetic event produces a resistant mutant (de novo mutation) in which a new genetic trait gives the parasite a survival advantage against the drug. In the second phase, the resistant parasites are then selected and start to multiply, which finally ends with a parasite population no longer being susceptible to treatment. For a few drugs, to confer resistance there is only involvement of single point mutation, however for various other drugs multiple site mutation is required. The acquired mutations allow the survival or reproduction of the resistant parasite whereas drug pressure will eliminate susceptible ones [90]. Antimalarial drug resistance typically arises when there are spontaneous mutations that are selected by different concentrations of anti-malarial drug that impart deferential inhibition to distinct genetic parasite types, i.e. the drug concentrations are sufficient to reduce the susceptible parasite population, but can either not inhibit multiplication or cause less inhibition of the mutants [92]. Drug resistance to several antimalarials is sometimes either due to changes in drug accumulation or efflux mechanisms (chloroquine, amodiaquine, quinine, halofantrine, mefloquine resistance) or due to decreased affinity of the drug target which may result from point mutations in the respective genes that encode these targets (pyrimethamine, cycloguanil, sulphonamide, atovaquone, artemisinin resistance) [6, 42, 75, 113, 125], shown in Figure 1.
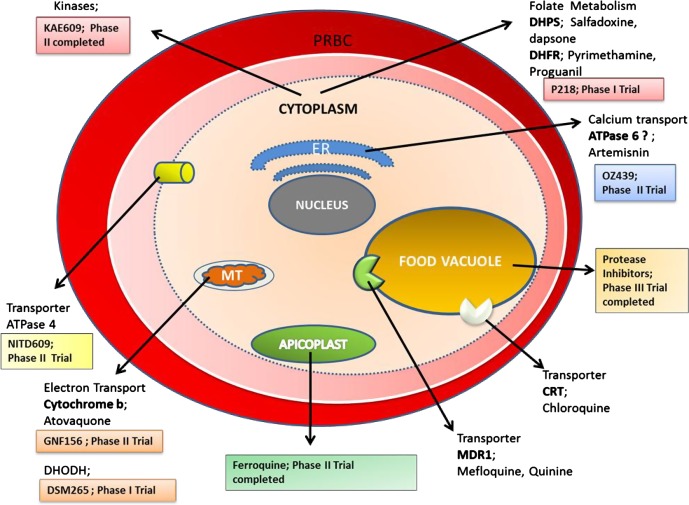
Figure 1.
Different proteins present inside the parasitic organelle that contribute to drug resistance in malaria under selective drug pressure and new drugs in development, targeting the same pathway to rescue resistance. PRBC: parasitized red blood cell, ER: endoplasmic reticulum, MT: mitochondria, DHPS: dihydropterate synthetase, DHFR: dihydrofolate reductase, ATPase6: sacro/endoplasmic reticulum calcium dependent ATPase orthologue, CRT: chloroquine resistance transporter, MDR1: multidrug resistance.
A limited number of genes involved or potentially involved in P. falciparum antimalarial drug resistance have been identified: the genes encoding dihydropteroate synthase (Pfdhps), dihydrofolate reductase (Pfdhfr), the chloroquine resistance transporter (Pfcrt), the multidrug resistance 1 protein (Pfmdr1), Na+/H+ exchanger (Pfnhe-1) and cytochrome b, shown in Table 1.
Table 1.
First reported resistance to antimalarial drugs and molecular markers for drug resistance.
| Antimalarial drug | Introduction date | First reported resistance | Molecular marker | References |
|---|---|---|---|---|
| Quinine | 1632 | 1910 | pfnhe: microsatellite ms4670 | [40, 53, 87, 91] |
| Chloroquine | 1945 | 1957 | crt: C72S, M74I, N75D/E, K76T, A220S, Q271E mdr1: N86Y, Y184F, S1034C, N1042D, D1246Y | [19, 29, 33, 128] |
| Proguanil | 1948 | 1949 | dhfr: A16V, S108T or N51I, C59R, S108N, I164L | [49, 54, 91] |
| Sulfadoxine + Pyrimethamine | 1967 | 1967 | dhps: S436A/F, A437G, K540E, A581G, A613S/T dhfr: N51I, C59R, S108N, I164L or C50R, N51I, S108N, I164L | [16, 91] |
| Mefloquine | 1977 | 1982 | Deamplification of Pfmdr1 copy | [55, 88] |
| Halofantrine | 1988 | 1993 | Changes in Pfmdr1 copy number | [18, 123, 129] |
| Atovaquone | 1996 | 1996 | cyt b: Y268S/N | [60, 70, 135] |
| Artemisinin | 1971 | 1980 | Amplification of Pfmdr1 copy numbers, Mutation of PfATPase6 and Pfubp-1. Recently, mutation in K13-Propeller Domain has been confirmed. | [35, 75, 103, 113, 133] |
| Artesunate | 1975 | 2008 | NA | [86] |
| Artesunate + Mefloquine | 2000 | 2009 | Deamplification of Pfmdr1 copy | [31, 73, 103] |
Drug resistance is complicated by cross-resistance, which mostly occurs among the groups of drugs which belong to a similar chemical family or which have the same mechanism of action, for example, development of cross-resistance between halofantrine and mefloquine [101]. Furthermore, multiple drug resistance of P. falciparum has been seen when the parasite is resistant to more than two operational antimalarial compounds of different chemical classes and modes of action.
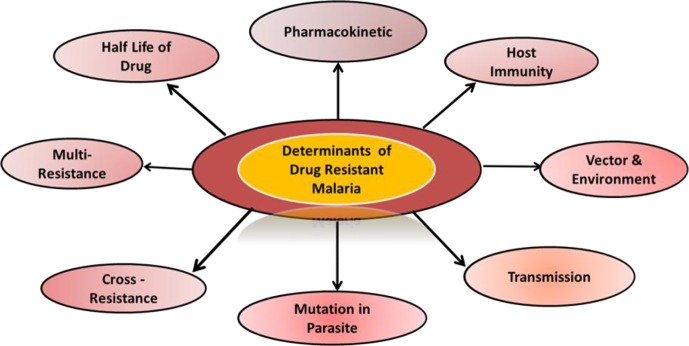
Recently, the role of pharmacokinetics in determining antimalarial efficacy and in promoting the emergence and spread of drug resistance has gained far more attention [10]. In the past, plasma levels of drugs were rarely measured, so it was thought that all episodes leading to clinical treatment failure were due to inherent parasite resistance. The dose selected is usually the lowest dose that achieves a good response so as to minimise adverse effects. However, during expansion of resistance, it has been found that relatively low amounts of drug allow remarkable spread of resistant parasites because the therapeutic level that is needed to clear partially resistant parasites is usually higher than the level which is required to eliminate the fully susceptible ones [11]. Therefore, incomplete understanding of pharmacokinetic factors may shorten the useful life of antimalarial drugs and may hasten the spread of resistance.
The subsequent spread of resistant mutant malaria parasites is facilitated by administration of drugs with longer elimination phases. The remaining antimalarial activity which persists during the post-treatment period acts as a “selective filter”, that found to be preventive in case of infection by sensitive parasites, but enables infection by resistant parasites. Drugs like chloroquine, mefloquine and piperaquine, which persist for longer durations in the blood plasma, provide a selective filter long after administration has ended [140]. The longer the terminal elimination half-life of the drug, the greater is the chance that any freshly acquired parasite can encounter partially effective drug concentrations [51, 126, 127]. The duration of the terminal elimination half-life is therefore a vital determinant of the propensity for an antimalarial drug to become ineffective because of the development of resistance. The prolonged presence of drugs like mefloquine, piperaquine and chloroquine in the host’s blood provides a lengthy exposure time during which resistant parasites may be selected [131] (Fig. 2).
Figure 2.
Different parameters that contribute to antimalarial resistance.
The host immune response to malaria infection likely influences the speed of spread of drug resistance and the extent to which drug resistance translates into clinical drug failure [52]. Host defence contains a major antiparasitic impact, and any drug-resistant mutant Plasmodium that generated spontaneously should contend not only with the antimalarial drug concentrations but also with host immunity that kills parasites irrespective of their antimalarial resistance and reduces the likelihood of parasite survival (independently of drugs) at almost all stages of the transmission cycle. Immunity in case of Plasmodium infection acts by non-specifically eliminating erythrocytic-stage parasites which includes rare de novo resistant mutants, and found to improve curative rates, even with the failing drugs, hence diminishing the comparative transmission advantage taken by the resistant parasites. If a resistant mutant survives the initial drug regimens and is able to proliferate, this will then often result in sufficient gametocytes which finally increase the disease transmission rate. However this frequency of transmission can be decreased if there is immunity against the asexual stage (which reduces the multiplication rate and lowers the density at which the infection is controlled) and to the sexual stage.
Epidemiological studies have implicated low-transmission settings as the primary origin of drug resistance [102]. This is most likely attributable to the fact that in areas of low-transmission intensity, most of the malaria infections are symptomatic and so, proportionately lots of people receive treatment, which ultimately generates higher chances for selection. However, there is less chance of emergence of drug resistance in areas with high-transmission intensity primarily because most malaria infections are asymptomatic and infections are acquired repeatedly throughout life. Also, in high-transmission areas, malaria-experienced populations slowly acquire partial immunity (“premunition”), and therefore the infection is controlled, sometimes at levels below those that cause people to develop symptoms.
Finally, vector and environmental factors may influence the proliferation of resistant parasites [129]. It is likely that drug-resistant parasites are less fit compared to their wild type. As such, there is a possibility of disappearance of resistant parasites by removing the drug pressure [8]. Recently, Lewis et al. studied the re-emergence of a chloroquine-sensitive strain in a wild population by demonstrating that development of chloroquine resistance is mainly due to a defect in the ability of the parasite to degrade haemoglobin, which inhibits active reproduction compared to sensitive ones [67].
Novel on-going approaches to impede drug resistant malaria
The treatment regimens of malaria are directly correlated to parasite drug resistance and dictated by government political strategies of prevention and control of morbidity and mortality due to the disease [9]. Considering the few number of new drugs or innovative antimalarial medicines approved since 1990, the search for more potent and less toxic antimalarials, the development of a successful vaccine, antimalarial peptides and the design of nanotechnology-based delivery systems applied to drugs and antigens and other approaches like RNA interference (RNAi) and stem cell therapy, are likely to be the main strategies in combating this disease. However, the main drawbacks associated with conventional malaria chemotherapy are the development of single or multiple drug resistance and the non-specific targeting to intracellular parasites which results in high dose requirements and subsequent intolerable toxicity that provides a new vision to apply novel approaches in disease treatment.
RNA interference (RNAi)
RNA interference (RNAi) is a method of interrupting gene expression that acts as a post-transcriptional event specifically degrading targeted mRNA that results in decreased synthesis of specific proteins [41]. RNAi is now emerging as a powerful technology with vast applications for genomics, elucidation of molecular signalling pathways, and target identification in drug discovery. Presently, RNAi studies reveal the clinical potential of small interfering RNAs (siRNAs) in metabolic diseases, AIDS, cancer, malaria, dental diseases, neurodegenerative disorders and various other illnesses. Recent studies have shown that the tiny RNA molecules, either endogenously made as microRNAs (miRNAs) or exogenously administered as synthetic double-stranded RNAs (dsRNAs) could efficiently activate a selective gene in a sequence specific manner despite silencing it [96]. The recent discovery of RNAi and its possible adaptation to mosquitoes is now contributing as a crucial tool for understanding vector-parasite interactions as well as providing insight to analyse different aspects of mosquito biology that could influence vectorial capacity. At present, two RNAi approaches have been well-established in mosquitoes. Firstly, transient gene silencing achieved by direct delivery of double-stranded RNA, and secondly, steady and stable expression of hairpin RNAs from the transgenes that integrated in the genome [21]. Few studies has been reported about the role of RNAi in Plasmodium and then taken as a tool to understand their genetic function. Although these studies [72, 74] reveal the down-regulation of gene expression after application of dsRNA targeting specific proteins in P. falciparum, they were unable to prove that these introduced dsRNA were ultimately processed to true siRNA inside Plasmodium. Mohammed et al. also reported specific down-regulation of P. berghei cysteine protease berghepain when targeted with siRNAs in Plasmodium berghei-infected mice and resulted in only about 0.01% of the siRNA which are being internalised inside the parasite that leads to 40–50% reduction in berghepain mRNA levels. However, the experiment did not alter the level of parasitaemia in infected mice treated with siRNA. In addition, others have reported the successful application of RNAi for silencing genes at the blood stage of Plasmodium [77]. In this context, various experiments have been carried out by employing the technique of electroporation to introduce long dsRNAs within infected erythrocytes. Kumar et al. successfully applied siRNA to confirm the essential role of P. falciparum serine/threonine phosphatase (PP) in Plasmodium growth as it was found to be expressed all along the erythrocytic stages [63]. Gissot et al. performed gene silencing experiments by using pfmyb1 double-stranded RNA (dsRNA) to interfere with the cognate messenger expression which results in 40% reduction in parasite growth as compared to untreated culture [44]. P. falciparum signal peptidase was also found to be essential for the intra-erythrocytic growth of the parasite, as implication of PfSP21 dsRNA specifically leads to inhibition in the growth of P. falciparum [118]. Tuteja and Pradhan elucidated the application of RNAi by targeting P. falciparum translation initiation complex eIF4F with the specific dsRNA corresponding to each complex for revealing the importance of these initiation complexes in parasite multiplication, and shows approximate 45% reduction in parasite growth [119]. In a recent study, P. falciparum UvrD helicase revealed that N-terminal UvrD (PfUDN) dsRNA shows inhibition in the growth of the parasite particularly during earlier phase of schizont development. The study reveals that the growth of parasite was inhibited by approximately 40% by adding PfUDN dsRNA in culture [114]. Another study observed transcriptional down-regulation of the hypusinated form of either eIF-5A or DHS upon transfection of P. berghei ANKA merozoites with eIF-5A-shRNA and DHS-shRNA, respectively, as hypusination of eIF-5A is important for cell proliferation of the parasite. This study provided evidence for a noncanonical RNAi-related pathway [109]. However, other studies have revealed the absence of RNAi genes [13]. Taken together, the presence of RNAi in Plasmodium is still quite contradictory. The experimental identification and validation of the P. falciparum small antisense nonprotein-coding RNA (npcRNA) transcriptome may provide an alternative to the classical RNAi pathway [97].
RNAi technology is one of the most significant advances to elucidate the genetic function of an organism, but its clinical implication in malarial parasites is limited. However, two major organelles, having their own genomes, i.e. Plasmodial apicoplast and mitochondria, are consider as a potent drug target with respect to their function. These organelles might be explored clinically in coming decades for their particular function in parasite growth by applying RNAi technology [80]. Looking into Plasmodial apicoplast reveals homology with respect to plant and algal chloroplast that share various metabolic pathways found to be unique to plants like biosynthesis of isoprenoids, fatty acid and heme synthesis. But at the same time, Plasmodial apicoplast has lost its photosynthetic function. This apicoplast is also involved in housekeeping functions which are found to be the same as bacterial housekeeping. Therefore, non-existence of these pathways in humans suggests apicoplast as a major drug target. Moreover, the functional activity of apicoplast like replication, transcription and translation suggests it as an immense and effective target for various anti-apicomplexan drugs [71]. Studies have inferred the uses of reverse genetic techniques to get a clear picture of apicoplast function along with its various processes that helps in parasite growth and survival [47]. Furthermore, Plasmodial mitochondria are the second major organelle regulating various metabolic processes all along the Plasmodium life cycle [85]. The active electron transport chain of Plasmodium contains distinct dehydrogenases compared to human mitochondria so the driving metabolic energy is also quite different from the mammalian host [117] which shows Plasmodial mitochondria as a valuable drug target and it can also be exploited for applying RNAi clinically.
There are numerous advantages of RNAi for studying gene function in P. falciparum: the assay can be conducted in a few days compared with months required for gene inactivation, multiple genes can be analysed simultaneously for genome screening purposes, the cost is considerably less than the synthesis of modified antisense oligonucleotides, and the transient nature of the assay may be an advantage for investigating essential genes. Disadvantages with the current methodology include the dependence on the electroporation efficiency and the lack of a marker phenotype following manipulation of this organism. Both areas of optimisation are currently under investigation. Moreover, effective delivery of RNAi is the biggest concern in Plasmodium as it has to cross the erythrocyte membrane, parasitophorous vacuolar membrane, parasite cytoplasm membrane and the parasite nuclear membrane in order to reach the Plasmodium nuclei [141].
Techniques used for genetic interference in Plasmodium are found to be refractory especially for the genes that are involved in the blood stage development of the parasite. So, the transformation of an RNAi deficient Plasmodium into RNAi possessing one with the lowest set of RNAi transgene machinery might be the foremost challenge for implication of RNAi technology [32]. Thus, more studies will be needed to elucidate the mechanisms of gene silencing observed in Plasmodium and to assess the therapeutic potential of RNAi in this important parasite. In future, practical implications of RNAi in gene silencing will provide us powerful means for developing novel therapeutics [120].
Nanotechnology
The development of drug resistance by malaria parasites may also be due to the use of ineffective pharmaceutical dosage forms of antimalarials. In pharmaceutical sciences, nanotechnology has made dissolution rates remarkably faster and higher, increased the bioavailability of many drugs, and improved the stability of sensitive agents. To our current concern, nanosized carriers are now receiving special attention with the aim of minimising the side effects that arises from conventional drug therapy, like inappropriate bioavailability and least selectivity of drugs. These nanocarriers have now been implicated for malaria diagnosis [110, 136] and treatment [43] and in vaccine formulation [4]. Malaria parasites frequently develop drug resistance due to the administration of low drug concentrations in the presence of a high parasitic count [83]. Furthermore, nanotechnology has the potential to restore the use of old and toxic drugs by modifying their bio-distribution and reducing toxicity [43]. This advantage is particularly important in malaria therapy, since the development of new dosage forms for delivering drugs to parasite infected cells is urgently needed, especially for the antimalarials in clinical use. Nanocarriers may not only allow the use of potentially toxic antimalarials [26], but also increase the efficacy of immune response in vaccine formulations [89].
Several nanosized delivery systems have previously been proved in terms of their effectiveness in experimental models for the treatment and prophylaxis of malaria. For example, a rapid test for malaria diagnosis was developed by the Udomsangpetch group, based on agglutination of sensitive polystyrene particles [94], in order to overcome prior limitations which are associated with the high cost of currently available rapid diagnostic kits. This test includes aggregation of nanoparticles with antigen or antibody-called latex-antigen (or antibody) conjugates under in vitro conditions, in the presence of malaria specific antibody (or antigen). The assay was successfully evaluated for P. falciparum at an outpatient malaria clinic (Mae Sot, Thailand) and claimed to be the quickest and easiest test that can be performed in the field. Plasmodium throughout its intra-erythrocytic phase modifies the host RBC plasma membrane, which has made lipid-based nanocarrier as the most promising carrier for targeting the infected RBCs [26]. This includes encapsulated liposomes which have been used for targeted delivery of antimalarials in vivo [95]. Liposomal nanovessels containing amino acid sequences of P. berghei have been used to target the hepatocyte stage of Plasmodium [100]. Others formulated PEGylated liposomes containing artemisinin, which is mainly a long circulating vesicle representing an efficient nanocarrier that can be used therapeutically in parasitic infection as well as in tumours [56]. However, despite these appreciable results, liposomal-based delivery was not adopted in disease rescue programmes mainly due to its non-selectivity towards parasitised RBCs. In this context, Urbán et al. [121] developed a nanovector for targeted delivery of antimalarials to human parasitized RBCs. At the same time, these lipid-based nano-delivery systems provide a frame to re-formulate existing and toxic antimalarials for achieving better and appreciable pharmacokinetic profiles, and biodistribution, along with appropriate targetability [57]. To this end, transferrin-modified-artemether lipid nanospheres have also been developed as targeted drug delivery systems against tumour cell lines [37]. Significant results shows its might be beneficial in targeting parasitised RBCs. Earlier, Tayade and Nagarsenker formulated microemulsions of artemether which have 1.5 times better antimalarial activity than the marketed one, Larither®, which was mainly due to quick release of drug from their formulation [115].
In addition, the most important property of a nanocarrier in the context of malaria is its ability to remain for a longer time in the blood stream in order to improve the interaction with infected RBCs and parasite membranes [78]. Furthermore, other features are protection against unstable drugs, properties of cell adhesion, as well as the potential to be modified at the surface after being conjugated by specific ligands [59]. Remarkably, during treatment of cerebral malaria, most of these potential benefits can be achieved by colloidal nanocarriers that can be administered intravenously. In case of uncomplicated malaria, the non-parenteral routes are mainly preferred, but this reduces the spectrum of possibilities in terms of the use of drug nanocarriers. Various strategies have been made to make it possible to implement this technology to curb malaria [23, 26, 50, 66]. There are two main strategies for targeting antimalarial drugs to the infected erythrocytes and occasionally the hepatocytes using nanocarriers by the intravenous route: passive and active targeting. Passive targeting is mostly achieved using conventional nanocarriers (e.g. liposomes, hydrophobic polymeric nanoparticles) [12] or surface-modified long-circulating nanocarriers (e.g. PEGylated) [48, 65]. On the contrary, active targeting is achieved by means of nanocarriers surface-modified with specific ligands such as carbohydrates, proteins, peptides or antibodies [12]. Recently, polymer-based nanoaggregates, i.e. polyamidoamine (PAA)-derived polymer, have been used for administering antimalarials into pRBCs. However, after encapsulation, in vitro efficacy of the drugs was found to be moderate [122]. Afterwards, Movellan et al. developed Janus dendrimer based on 2,2-bis(hydroxymethyl)propionic acid (bis-MPA) monomers for encapsulating two antimalarial, i.e. chloroquine and primaquine and their results show significant reduction in in vitro IC50 compared to free drug. Also, encapsulation of primaquine was found to be promising as compared to free primaquine which causes haemolysis in patients having deficiency of glucose-6-phosphate dehydrogenase (G6PD) [79]. The wide range of modulations of the surface properties of these nanometric carriers aimed at improving antimalarial selectivity in the recently-discovered parasite targets has been little exploited to date. From this study it emerged that nanotechnology applied to malaria therapy is a domain that is still in its infancy [107].
Apart from nanocarriers, nano/microfluidic technologies are also emerging as methods that could address the challenges imposed by other conventional diagnostic devices [24, 81]. These approaches enable real-time monitoring of infectious diseases from a small volume of bodily fluids [35], and can be used to integrate various assays into a single device [28, 64, 111, 130], and have the ability to deliver each sample to specific reaction chambers in a systematic manner [58]. Among these technologies, nanofluidics have been highlighted by the recent advent of nanoscience and nanotechnology since the rise of microfluidics in the 1990s. Generally, nanofluidics can be defined as the field of study in fluid flow in and around nanoscale objects [34]. For example, the RDT strip chip can detect proteins derived from the blood of malaria parasites in a microfluidic format and can also be realised as commercialized products. This chip enables the generation of a series of visible lines to indicate the presence of specific antigens in blood that are clearly visible to the naked eye when antibody is accumulated at the test line. Rathod et al. developed microfluidic channels to study malaria pathogenesis related complex interactions between host cell ligands and parasitised erythrocytes [5]. Since the microfluidic channels successfully mimic the sizes and shapes of capillary blood vessels, they could observe host-parasite interaction and malaria-infected red blood cells in a capillary environment. The malaria diagnostic device is inexpensive and handheld for on-site analysis of patient samples and only requires microliter sample volume. Therefore, it has the potential to be widely used at field sites for more accurate malaria diagnosis. Nanotechnology systems may therefore afford a better therapeutic outcome by targeting drugs specifically to their site of action.
Stem cells
Stem cells are unspecialised cells found in embryos during the blastocyst stage and in various tissues of adults. They have the typical characteristic of dividing mitotically in order to self-renew and differentiate into various types of cells under appropriate conditions for each specific function. They also serve as cell reservoirs for fulfilling the purpose of repair of damaged tissues inside the body. Recent studies suggests that stem cells, especially the mesenchymal stem cells, have immuno-modulatory characteristics and due to this property many trials are being conducted by transplanting these mesenchymal stem cells in disease conditions which are thought to arise from immunological abuse.
Severe destruction of red blood cells causes anaemia, thus posing pressure on bone marrow to meet the requirements of myeloid cells. Scientists from the National Institute for Medical Research, UK, have identified an atypical progenitor cell from malaria infected mice which can give rise to a lineage of cells capable of fighting this disease [14]. Transplantation of these specific cells into mice with severe malaria was found to help these infected mice in recovering from the disease. Other reports also support stem cell therapy for malaria treatment [105]. Manipulation in stem cells can also produce erythrocytes with modified haemoglobin variants that are associated with protection from malaria. Thakur et al. [99] identified recruitment of MSCs as a novel host protective mechanism adopted by the host to combat malaria by modulating Treg-cell responses. A massive accumulation of Sca-1+CD44+CD29+CD34− cells (where Sca-1 is stem cell antigen-1), a phenotype consistent with mesenchymal stem cells (MSCs) but not conventional stem cells, was found in this study. Infusion of purified MSCs from infected animals to naive animals dramatically protected against infection by Plasmodium berghei (Pb). Furthermore, prior infusion of MSCs from infected mice prevented splenomegaly, infiltration of NKT cells, and haemozoin accumulation. This was accompanied by a profound reduction in inhibitory cytokines such as IL-10 and up-regulation of inflammatory cytokines such as IL-12 and IL-1β. In addition, these animals had reduced levels of Treg cells that were able to dampen antigen specific protective immune responses. Taken together, the study identified accumulation of MSCs as a novel host response to combat malaria by inhibiting haemozoin and anti-inflammatory cytokine production, and by reducing Treg-cell accumulation in the spleen. In addition, multipotent haematopoietic stem cells were reported to play an important role in the host’s defence mechanisms against Plasmodium berghei infection [7]. Based on these studies, it is believed that although stem cell therapy is at initial stages, it will soon be a real therapeutic option for various parasitic diseases and with continuous effort along with vast knowledge of the present subject will lead to new experimental models, appropriate type and number of stem cells, route of administration and similar disease conditions that will possibly be beneficial for the treatment of the patient with a parasitic infection [142]. Therefore, approaches may differ from disease to disease but stem cells are always in focus to treat several diseases including malaria.
Miscellaneous approaches
Peptides
Besides these novel approaches, peptides that has been isolated from various natural sources like plant, fungi and bacteria or derived synthetically are often widely explored novel molecules that have large chemotherapeutic potential and can serve various diseases including malaria [62]. They are basically secondary metabolites displaying huge amounts of heterogeneity in their primary as well as secondary structures. However, they share some common features that reveal their cytotoxic activity, such as amphipathic along with net positive charge [17]. Although their exact mechanism of action has not been completely studied, most are considered to have cytocidal effects by disintegrating the membrane structure [15].
In addition to various antimalarial drug classes, a number of promising antimalarial peptides of natural or synthetic origin have been reported previously and are listed in Table 2.
Table 2.
Antimalarial peptides with their native source and mechanism of action.
| Peptides | Sources | Mechanism of action | References |
|---|---|---|---|
| Apicidin** | Fusarium pallidoroseum (Fungal Metabolite) | Inhibits protozoan histone deacetylase (HAD) | [27] |
| Dermaseptin S4* (ALWMTLLKKVLKAAAKAALNAVLVGANA) | Frog skin | Inhibits the parasite’s ability to incorporate [3H] hypoxanthine | [46] |
| Dermaseptin S3* (ALWKNMLKGIGKLAGKAALGAVKKLVGAES) | Frog skin | Inhibits the parasite’s ability to incorporate [3H] hypoxanthine | [46] |
| Beauvericin | Paecilo mycestenuipes (Insect pathogenic fungus) | NA | [84] |
| Jasplakinolide* | Jaspis sp. (marine sponge) | Interfere with erythrocyte invasion by the merozoites | [76] |
| Dolastatin 10* | Dolabella auricularia (Sea hare) | Microtubule inhibitor | [39] |
| CEL-1000 (DGQEEKAGVVSTGLIGGG)** | b-chain of the human major histocompatibility complex class II molecule | Elicited Immune response | [22] |
| Hirsutellic acid A* | Hirsutella sp. BCC 1528 (Entomopathogenic fungus) | NA | [116] |
| Venturamide* | Cyanobacterium oscillatoria | NA | [68] |
| Antiamoebin I* | Emericellopsis poonensis (Fungus) | Inhibitors of mitochondrial activity | [82] |
| Efrapeptin* | Tolypocladium niveum (Fungus) | Inhibitors of mitochondrial activity | [82] |
| Zervamicin* | Emericellopsis salmosynnemata (Fungus) | Inhibitors of mitochondrial activity | [82] |
| Tyrothricin* | Bacillus brevis (Bacteria) | Exert its parasitic inhibition by rapid and selective lysis of infected erythrocytes | [98] |
| Isariins* | Isaria (Fungus) | Exact mechanism not known, inhibitory effect on the intra-erythrocytic growth of Plasmodium | [104] |
| Peptide IDR-1018 (Immune defence regulator)** | Host defence peptides (Synthetic) | Ability to modulate inflammatory responses | [1] |
| Benzyloxycarbonyl Z-Phe-Arg-CH2F* | Synthetic | Inhibit haemoglobin degradation by acting on cysteine proteinase | [103] |
| Phe-Orn-Phe-Orn* | Synthetic | NA | [93] |
| Lys-Phe-Phe-Orn* | Synthetic | NA | [93] |
In vitro study.
In vitro and vivo study.
NA: Not available.
Antimalarials under development
For diseases like malaria, there is an urgent need for active drug candidates that combat developing resistance mechanisms of Plasmodium. To address this concern, various lead molecules and vaccines are in the pipeline of drug discovery that can target Plasmodium at its different life cycle stages, Pre-erythrocytic (liver stage), Erythrocytic (blood stage), or Post-erythrocytic (gametocytic stage) to prevent relapses and transmission. They are listed in Table 3.
Table 3.
Antimalarial compounds and clinical trial phases.
| Product name | Clinical trial phase | Target stage/site |
|---|---|---|
| Malaria vaccine 257049 | Phase III | Pre-erythrocytic stages, Erythrocytic stages |
| FMP011/AS01B | Phase I/II trial | Pre-erythrocytic stages, Erythrocytic stages |
| FMP2.1/AS02A | Phase II | Pre-erythrocytic stages, Erythrocytic stages |
| FMP1/AS02A | Phase II completed | Pre-erythrocytic stages, Erythrocytic stages |
| RTS, S/AS02D | Phase II completed | Pre-erythrocytic stages, Erythrocytic stages |
| Recombinant hybrid GMZ 2 [GLURP + MSP 3] | Phase I | Erythrocytic stage |
Malaria vaccine candidates (VAC045)
|
Phase I | Pre-erythrocytic stage |
| Phase II completed | ||
| Plasmodium falciparum malaria protein 010 (FMP010) | Phase I completed | Erythrocytic stage |
| Falciparum malaria protein (FMP012), E. Coli-expressed PfCelTOS | Phase I completed | Pre-erythrocytic stage |
| Peptides MSP3 long synthetic peptide 30 micrograms of MSP3 LSP | Phase II | Erythrocytic stage |
|
Phase III completed | Erythrocytic stage |
| OZ439 (second generation endoperoxide) | Phase II | Erythrocytic stage |
| KAE609 | Phase II completed | Post-erythrocytic stage |
| NITD609 (spiroindolone class, plant Product) | Phase II | Post-erythrocytic stage |
| Ferroquine (SSR-97193, FQ), | Phase II completed | Erythrocytic stage |
| Trioxaquine SAR116242 | Preclinical | Erythrocytic stage |
| MK4815 | Preclinical | Erythrocytic stage |
| GNF156; an imidazolopiperazine | Phase II | Pre-erythrocytic, Erythrocytic Post-erythrocytic stage |
| DSM265 selective triazolopyrimidine-based inhibitor (first compound to target DHODH) | Phase I | Erythrocytic stage |
| P218 inhibitor of DHFR | Phase I | Erythrocytic stage |
| CDRI97/98 | Phase I | Erythrocytic stage |
| CDRI9778 | Phase I | Erythrocytic stage |
| Methylene blue | Phase II | Erythrocytic stage |
| Argemone mexicana | Phase II | NA |
| Rapid diagnostic test for malaria | Phase IV completed | Based on Immuno-detection of HRP2, pLDH |
Source: http://www.clinicaltrials.gov/
Conclusion
Currently, the biggest concern all over the globe is to treat patients with safe and effective medications and to avoid the emergence of drug-resistant malaria parasites. However, the emergence of vector resistance to widely used insecticides and parasite resistance to first-line drugs including artemisinin combination therapy has resulted in a rise in malaria incidence in many endemic areas, which has called for development of new therapeutic and technology approaches to combat the disease and impede drug resistance. However, more progress and better understanding in terms of scientific research and innovation is needed to develop these novel technologies as tools to reduce the occurrence of malaria.
Conflict of interest
Authors have no conflict of interest.
Cite this article as: Sinha S, Medhi B & Sehgal R: Challenges of drug-resistant malaria. Parasite, 2014, 21, 61.
References
- 1. Achtman AH, Pilat S, Law CW, Lynn DJ, Janot L, Mayer ML, Ma S, Kindrachuk J, Finlay BB, Brinkman FS, Symth GK, Hancock RE, Schofield L. 2012. Effective adjunctive therapy by an innate defense regulatory peptide in a preclinical model of severe malaria. Science Translational Medicine, 4, 135ra64. [DOI] [PubMed] [Google Scholar]
- 2. Agnandji ST, Lell B, Fernandes JF, Abossolo BP, Methogo BG, Kabwende AL, Adegnika AA, Kabwende AL, Mordmüller B, Issifou S, Kremsner PG, Sacarlal J, Aide P, Lanaspa M, Aponte JJ, Machevo S, Acacio S, Bulo H, Sigauque B, Macete E, Alonso P, Abdulla S, Salim N, Minja R, Mpina M, Ahmed S, Ali AM, Mtoro AT, Hamad AS, Mutani P, Tanner M, Tinto H, D’Alessandro U, Sorgho H, Valea I, Bihoun B, Guiraud I, Kaboré B, Sombié O, Guiguemdé RT, Ouédraogo JB, Hamel MJ, Kariuki S, Oneko M, Odero C, Otieno K, Awino N, McMorrow M, Muturi-Kioi V, Laserson KF, Slutsker L, Otieno W, Otieno L, Otsyula N, Gondi S, Otieno A, Owira V, Oguk E, Odongo G, Woods JB, Ogutu B, Njuguna P, Chilengi R, Akoo P, Kerubo C, Maingi C, Lang T, Olotu A, Bejon P, Marsh K, Mwambingu G, Owusu-Agyei S, Asante KP, Osei-Kwakye K, Boahen O, Dosoo D, Asante I, Adjei G, Kwara E, Chandramohan D, Greenwood B, Lusingu J, Gesase S, Malabeja A, Abdul O, Mahende C, Liheluka E, Malle L, Lemnge M, Theander TG, Drakeley C, Ansong D, Agbenyega T, Adjei S, Boateng HO, Rettig T, Bawa J, Sylverken J, Sambian D, Sarfo A, Agyekum A, Martinson F, Hoffman I, Mvalo T, Kamthunzi P, Nkomo R, Tembo T, Tegha G, Tsidya M, Kilembe J, Chawinga C, Ballou WR, Cohen J, Guerra Y, Jongert E, Lapierre D, Leach A, Lievens M, Ofori-Anyinam O, Olivier A, Vekemans J, Carter T, Kaslow D, Leboulleux D, Loucq C, Radford A, Savarese B, Schellenberg D, Sillman M, Vansadia P. 2012. A phase 3 trial of RTS, S/AS01 malaria vaccine in African infants. New England Journal of Medicine, 367, 2284–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aide P, Aponte JJ, Renom M, Nhampossa T, Sacarlal J, Mandomando I, Bassat Q, Manaca MN, Leach A, Lievens M, Vekemans J, Dubois MC, Loucq C, Ballou WR, Cohen J, Alonso PL. 2010. Safety, immunogenicity and duration of protection of the RTS, S/AS02(D) malaria vaccine: one year follow-up of a randomized controlled phase I/IIb trial. PLoS One, 5, e13838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alving CR. 2002. Design and selection of vaccine adjuvants: animal models and human trials. Vaccine, 20, S56–S64. [DOI] [PubMed] [Google Scholar]
- 5. Antia M, Herricks T, Rathod PK. 2007. Microfluidic modeling of cell-cell interactions in malaria pathogenesis. PLoS Pathogen, 3, 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ariey F, Witkowski B, Amaratunga C, Beghain J, Langlois AC, Khim N, Kim S, Duru V, Bouchier C, Ma L, Lim P, Leang R, Duong S, Sreng S, Suon S, Chuor CM, Bout DM, Ménard S, Rogers WO, Genton B, Fandeur T, Miotto O, Ringwald P, Le Bras J, Berry A, Barale JC, Fairhurst RM, Benoit-Vical F, Mercereau-Puijalon O, Ménard D. 2014. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature, 505, 50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Asami M, Owhashi M, Abe T, Nawa Y. 1991. Susceptibility of multipotent haemopoietic stem cell deficient W/Wv mice to Plasmodium berghei-infection. Immunology and Cell Biology, 69, 355–360. [DOI] [PubMed] [Google Scholar]
- 8. Babiker HA, Hastings IM, Swedberg G. 2009. Impaired fitness of drug-resistant malaria parasites: evidence and implication on drug-deployment policies. Expert Review of Anti-infective Therapy, 7, 581–593. [DOI] [PubMed] [Google Scholar]
- 9. Bacon DJ, Jambou R, Fandeur T, Le Bras J, Wongsrichanalai C, Fukuda MM, Ringwald P, Sibley CH, Kyle DE. 2007. World antimalarial resistance network (WARN)II: in vitro antimalarial drug susceptibility. Malaria Journal, 6, 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barnes KI, Lindegardh N, Ogundahunsi O, Olliaro P, Plowe CV, Randrianarivelojosia M, Gbotosho GO, Watkins WM, Sibley CH, White NJ. 2007. World Antimalarial Resistance Network (WARN) IV: clinical pharmacology. Malaria Journal, 6, 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barnes KI, Watkins WM, White NJ. 2008. Antimalarial dosing regimens and drug resistance. Trends in Parasitology, 24, 127–134. [DOI] [PubMed] [Google Scholar]
- 12. Barratt G. 2003. Colloidal drug carriers: achievements and perspectives. Cellular and Molecular Life Sciences, 60, 21–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baum J, Papenfuss AT, Mair GR, Janse CJ, Vlachou D, Waters AP, Cowman AF, Crabb BS, de Koning-Ward TF. 2009. Molecular genetics and comparative genomics reveal RNAi is not functional in malaria parasites. Nucleic Acids Research, 37, 3788–3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Belyaev NN, Brown DE, Diaz AI, Rae A, Jarra W, Thompson J, Langhorne J, Potocnik AJ. 2010. Induction of an IL7-R(+)c-Kit(hi) myelolymphoid progenitor critically dependent on IFN-gamma signaling during acute malaria. Nature Immunology, 11, 477–485. [DOI] [PubMed] [Google Scholar]
- 15. Bessalle R, Gorea A, Shalit I, Metzger JW, Dass C, Desiderio DM, Fridkin M. 1993. Structure-function study of amphiphilic peptides. Journal of Medicinal Chemistry, 36, 1203–1209. [DOI] [PubMed] [Google Scholar]
- 16. Bjorkman A, Phillips-Howard PA. 1990. The epidemiology of drug-resistant malaria. Transactions of Royal Society Tropical Medicine and Hygiene, 84, 177–180. [DOI] [PubMed] [Google Scholar]
- 17. Blondelle SE, Houghten RA. 1992. Design of model peptides having potent antimicrobial activities. Biochemistry, 31, 12688–12694. [DOI] [PubMed] [Google Scholar]
- 18. Bouchaud O, Imbert P, Touze JE, Dodoo AN, Danis M, Legros F. 2009. Fatal cardiotoxicity related to halofantrine: a review based on a worldwide safety data base. Malaria Journal, 8, 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bray PG, Martin RE, Tilley L, Ward SA, Kirk K, Fidock DA. 2005. Defining the role of PfCRT in Plasmodium falciparum chloroquine resistance. Molecular Microbiology, 56, 323–333. [DOI] [PubMed] [Google Scholar]
- 20. Bruce-Chwatt LJ, Black RH, Canfield CJ, Clyde DF, Peters W, Wernsdorfer WH. 1986. Chemotherapy of malaria. Revised 2nd ed., World Health Organization: Geneva. [Google Scholar]
- 21. Catteruccia F, Levashina EA. 2009. RNAi in the malaria vector, Anopheles gambiae. Methods in Molecular Biology, 555, 63–75. [DOI] [PubMed] [Google Scholar]
- 22. Charoenvit Y, Brice GT, Bacon D, Majam V, Williams J, Abot E, Ganeshan H, Sedegah M, Doolan DL, Carucci DJ, Zimmerman DH. 2004. A small peptide (CEL-1000) derived from the beta-chain of the human major histocompatibility complex class II molecule induces complete protection against malaria in an antigen-independent manner. Antimicrobial Agents and Chemotherapy, 48, 2455–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chimanuka B, Gabriëls M, Detaevernier MR, Plaizier-Vercammen JA. 2002. Preparation of beta-artemether liposomes, their HPLC-UV evaluation and relevance for clearing recrudescent parasitaemia in Plasmodium chabaudi malaria-infected mice. Journal of Pharmaceutical and Biomedical Analysis, 28, 13–22. [DOI] [PubMed] [Google Scholar]
- 24. Chin CD, Linder V, Sia SK. 2007. Lab-on-a-chip devices for global health: past studies and future opportunities. Lab on a Chip, 7, 41–57. [DOI] [PubMed] [Google Scholar]
- 25. Cowman AF, Baldi DL, Healer J, Mills KE, O’Donnell RA, Reed MB, Triglia T, Wickham ME, Crabb BS. 2000. Functional analysis of proteins involved in Plasmodium falciparum merozoite invasion of red blood cells. FEBS Letters, 476, 84–88. [DOI] [PubMed] [Google Scholar]
- 26. Date AA, Joshi MD, Patravale VB. 2007. Parasitic diseases: liposomes and polymeric nanoparticles versus lipid nanoparticles. Advance Drug Delivery Reviews, 59, 505–521. [DOI] [PubMed] [Google Scholar]
- 27. Darkin-Rattray SJ, Gurnett AM, Myers RW, Dulski PM, Crumley TM, Allocco JJ, Cannova C, Meinke PT, Colletti SL, Bednarek MA, Singh SB, Goetz MA, Dombrowski AW, Polishook JD, Schmatz DM. 1996. Apicidin: a novel antiprotozoal agent that inhibits parasite histone deacetylase. Proceedings of the National Academy of Sciences of the United States of America, 93, 13143–13147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dittrich PS, Manz A. 2006. Lab-on-a-chip: microfluidics in drug discovery. Nature Reviews Drug Discovery, 5, 210–218. [DOI] [PubMed] [Google Scholar]
- 29. Djimdé A, Doumbo OK, Cortese JF, Kayentao K, Doumbo S, Diourté Y, Coulibaly D, Dicko A, Su XZ, Nomura T, Fidock DA, Wellems TE, Plowe CV. 2001. A molecular marker for chloroquine-resistant falciparum malaria. New England Journal of Medicine, 344, 257–263. [DOI] [PubMed] [Google Scholar]
- 30. Dondorp AM, Fairhurst RM, Slutsker L, Macarthur JR, Breman JG, Guerin PJ, Wellems TE, Ringwald P, Newman RD, Plowe CV. 2011. The threat of artemisinin-resistant malaria. New England Journal of Medicine, 365, 1073–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, Lwin KM, Ariey F, Hanpithakpong W, Lee SJ, Ringwald P, Silamut K, Imwong M, Chotivanich K, Lim P, Herdman T, An SS, Yeung S, Singhasivanon P, Day NP, Lindegardh N, Socheat D, White NJ. 2009. Artemisinin resistance in Plasmodium falciparum malaria. New England Journal of Medicine, 361, 455–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Drinnenberg IA, Weinberg DE, Xie KT, Mower JP, Wolfe KH, Fink GR, Bartel DP. 2009. RNAi in budding yeast. Science, 23, 544–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Duraisingh MT, Cowman AF. 2005. Contribution of the Pfmdr1 gene to antimalarial drug resistance. Acta Tropica, 94, 181–190. [DOI] [PubMed] [Google Scholar]
- 34. Eijkel JCT, Van den Berg A. 2005. Nanofluidics: what is it and what can we expect from it? Microfluids and Nanofluidics, 1, 249–267. [Google Scholar]
- 35. El-Ali J, Sorger PK, Jensen KF. 2006. Cells on chips. Nature, 442, 403–411. [DOI] [PubMed] [Google Scholar]
- 36. Elbadry MA, Existe A, Victor YS, Memnon G, Fukuda M, Dame JB, Yowell CA, Okech BA. 2013. Survey of Plasmodium falciparum multidrug resistance-1 and chloroquine resistance transporter alleles in Haiti. Malaria Journal, 12, 426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Eltayeb SE, Su Z, Shi Y, Li S, Xiao Y, Ping Q. 2013. Preparation and optimization of transferrin-modified-artemether lipid nanospheres based on the orthogonal design of emulsion formulation and physically electrostatic adsorption. International Journal of Pharmaceutics, 452, 321–332. [DOI] [PubMed] [Google Scholar]
- 38. Enserink M. 2010. Malaria’s drug miracle in danger. Science, 328, 844–846. [DOI] [PubMed] [Google Scholar]
- 39. Fennell BJ, Carolan S, Pettit GR, Bell A. 2003. Effects of the antimitotic natural product dolastatin 10, and related peptides, on the human malarial parasite Plasmodium falciparum. Journal of Antimicrobial Chemotherapy, 51, 833–841. [DOI] [PubMed] [Google Scholar]
- 40. Ferdig MT, Cooper RA, Mu J, Deng B, Joy DA, Su XZ, Wellems TE. 2004. Dissecting the loci of low-level quinine resistance in malaria parasites. Molecular Microbiology, 52, 985–997. [DOI] [PubMed] [Google Scholar]
- 41. Fire A. 1999. RNA-triggered gene silencing. Trends in Genetics, 15, 358–363. [DOI] [PubMed] [Google Scholar]
- 42. Foote SJ, Cowman AF. 1994. The mode of action and the mechanism of resistance to antimalarial drugs. Acta Tropica, 56, 157–171. [DOI] [PubMed] [Google Scholar]
- 43. Forrest ML, Kwon GS. 2008. Clinical developments in drug delivery nanotechnology. Advance Drug Delivery Reviews, 60, 861–862. [DOI] [PubMed] [Google Scholar]
- 44. Gissot M, Briquet S, Refour P, Boschet C, Vaquero C. 2005. PfMyb1, a Plasmodium falciparum transcription factor, is required for intra-erythrocytic growth and controls key genes for cell cycle regulation. Journal of Molecular Biology, 346, 29–42. [DOI] [PubMed] [Google Scholar]
- 45. Global report on Antimalarial Drug efficacy and Drug Resistance: World Health Organization. 2000–2010.
- 46. Gosh JK, Shaool D, Guillaud P, Cicéron L, Mazier D, Kustanovich I, Shai Y, Mor A. 1997. Selective cytotoxicity of dermaseptin S3 toward intraerythrocytic Plasmodium falciparum and the underlying molecular basis. Journal of Biological Chemistry, 272, 31609–31616. [DOI] [PubMed] [Google Scholar]
- 47. Goodman CD, McFadden GI. 2013. Targeting apicoplasts in malaria parasites. Expert Opinion on Therapeutic Targets, 17, 167–170. [DOI] [PubMed] [Google Scholar]
- 48. Gref R, Domb A, Quellec P, Blunk T, Muller RH, Verbavatz JM, Langer R. 2012. The controlled intravenous delivery of drugs using PEG-coated sterically stabilized nanospheres. Advance Drug Delivery Reviews, 64, 316–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gregson A, Plowe CV. 2005. Mechanisms of resistance of malaria parasites to antifolates. Pharmacological Reviews, 57, 117–145. [DOI] [PubMed] [Google Scholar]
- 50. Gupta Y, Jain A, Jain SK. 2007. Transferrin-conjugated solid lipid nanoparticles for enhanced delivery of quinine dihydrochloride to the brain. Journal of Pharmacy and Pharmacology, 59, 935–940. [DOI] [PubMed] [Google Scholar]
- 51. Hastings I, Watkins WM, White NJ. 2002. Pharmacokinetic parameters affecting the evolution of drug-resistance in malaria: the role of the terminal elimination half-life. Philosophical Transactions of the Royal Society B: Biological Science, 357, 505–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hastings IM. 2006. Complex dynamics and stability of resistance to antimalarial drugs. Parasitology, 132, 615–624. [DOI] [PubMed] [Google Scholar]
- 53. Henry M, Briolant S, Zettor A, Pelleau S, Baragatti M, Baret E, Mosnier J, Amalvict R, Fusai T, Rogier C, Pradines B. 2009. Plasmodium falciparum Na+/H+ exchanger 1 transporter is involved in reduced susceptibility to quinine. Antimicrobial Agents and Chemotherapy, 53, 1926–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hyde JE. 2002. Mechanisms of resistance of Plasmodium falciparum to antimalarial drugs. Microbes and Infection, 4, 165–174. [DOI] [PubMed] [Google Scholar]
- 55. Imwong M, Dondorp AM, Nosten F, Yi P, Mungthin M, Hanchana S, Das D, Phyo AP, Lwin KM, Pukrittayakamee S, Lee SJ, Saisung S, Koecharoen K, Nguon C, Day NP, Socheat D, White NJ. 2010. Exploring the contribution of candidate genes to artemisinin resistance in Plasmodium falciparum. Antimicrobial Agents and Chemotherapy, 54, 2886–2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Isacchi B, Arrigucci S, La Marca G, Bergonzi MC, Vannucchi MG, Novelli A, Bilia AR. 2011. Conventional and long-circulating liposomes of artemisinin: preparation, characterization, and pharmacokinetic profile in mice. Journal of Liposome Research, 21, 237–244. [DOI] [PubMed] [Google Scholar]
- 57. Jain K, Sood S, Gowthamarajan K. 2014. Lipid nanocarriers and molecular targets for malaria chemotherapy. Current Drug Targets, 15, 292–312. [DOI] [PubMed] [Google Scholar]
- 58. Kang L, Chung BG, Langer R, Khademhosseini A. 2008. Microfluidics for drug discovery and development: from target selection to product lifecycle management. Drug Discovery, 13, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kayser O, Kiderlen AF. 2003. Delivery strategies for antiparasitics. Expert Opinion on Investigational Drugs, 12, 197–207. [DOI] [PubMed] [Google Scholar]
- 60. Korsinczky M, Chen N, Kotecka B, Saul A, Rieckmann K, Cheng Q. 2000. Mutations in Plasmodium falciparum cytochrome b that are associated with atovaquone resistance are located at a putative drug-binding site. Antimicrobial Agents and Chemotherapy, 44, 2100–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Krishna S, Pulcini S, Moore CM, Teo B, Staines HM. 2014. Pumped up: reflections on PfATP6 as the target for artemisinins. Trends in Pharmacological Sciences, 35, 4–11. [DOI] [PubMed] [Google Scholar]
- 62. Krugliak M, Feder R, Zolotarev VY. 2000. Antimalarial activities of Dermaseptin S4 derivatives. Antimicrobial Agents and Chemotherapy, 44, 2442–2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kumar R, Adams B, Oldenburg A, Musiyenko A, Barik S. 2002. Characterisation and expression of a PP1 serine/threonine protein phosphatase (PfPP1) from the malaria parasite, Plasmodium falciparum: demonstration of its essential role using RNA interference. Malaria Journal, 1, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lagally ET, Scherer JR, Blazej RG, Toriello NM, Diep BA, Ramchandani M, Sensabaugh GF, Riley LW, Mathies RA. 2004. Integrated portable genetic analysis microsystem for pathogen/infectious disease detection. Analytical Chemistry, 76, 3162–3170. [DOI] [PubMed] [Google Scholar]
- 65. Lasic DD, Martin FJ, Mayhew E. 1991. Stealth liposomes. Annals of Biomedical Engineering, 91, 594. [Google Scholar]
- 66. Legrand P, Mosqueira V, Loiseau P, Bories C, Barratt G. 2003. Long circulating nanocapsules: interest in the treatment of severe malaria with halofantrine. Annales Pharmaceutiques Françaises, 61, 196–202. [PubMed] [Google Scholar]
- 67. Lewis IA, Wacker M, Olszewski KL, Cobbold SA, Baska KS, Tan A, Ferdig MT, Llinás M. 2014. Metabolic QTL analysis links chloroquine resistance in Plasmodium falciparum to impaired hemoglobin catabolism. PLoS Genetics, 10, 1004085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Linington RG, Gonzalez J, Ureña LD, Romero LI, Ortega-Barría E, Gerwick WH. 2007. Venturamides A and B: antimalarial constituents of the panamanian marine cyanobacterium Oscillatoria sp. Journal of Natural Products, 70, 397–400. [DOI] [PubMed] [Google Scholar]
- 69. Londono BL, Eisele TP, Keating J, Bennett A, Chattopadhyay C, Heyliger G, Mack B, Rawson I, Vely JF, Désinor O, Krogstad DJ. 2009. Chloroquine-resistant haplotype Plasmodium falciparum parasites, Haiti. Emerging Infectious Diseases, 15, 735–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Looareesuwan S, Viravan C, Webster HK, Kyle DE, Hutchinson DB, Canfield CJ. 1996. Clinical studies of atovaquone, alone or in combination with other antimalarial drugs, for treatment of acute uncomplicated malaria in Thailand. American Journal of Tropical Medicine and Hygiene, 54, 62–66. [DOI] [PubMed] [Google Scholar]
- 71. MacRae JI, Maréchal E, Biot C, Botté CY. 2012. The apicoplast: a key target to cure malaria. Current Pharmaceutical Design, 18, 3490–3504. [PubMed] [Google Scholar]
- 72. Malhotra P, Dasaradhi PV, Kumar A, Mohmmed A, Agrawal N, Bhatnagar RK, Chauhan VS. 2002. Double-stranded RNA-mediated gene silencing of cysteine proteases (falcipain-1 and -2) of Plasmodium falciparum. Molecular Microbiology, 45, 1245–1254. [DOI] [PubMed] [Google Scholar]
- 73. Maude RJ, Pontavornpinyo W, Saralamba S, Aguas R, Yeung S, Dondorp AM, Day NP, White NJ, White LJ. 2009. The last man standing is the most resistant: eliminating artemisinin-resistant malaria in Cambodia. Malaria Journal, 8, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. McRobert L, McConkey GA. 2002. RNA interference (RNAi) inhibits growth of Plasmodium falciparum. Molecular and Biochemical Parasitology, 119, 273–278. [DOI] [PubMed] [Google Scholar]
- 75. Miotto O, Almagro-Garcia J, Manske M, Macinnis B, Campino S, Rockett KA, Amaratunga C, Lim P, Suon S, Sreng S, Anderson JM, Duong S, Nguon C, Chuor CM, Saunders D, Se Y, Lon C, Fukuda MM, Amenga-Etego L, Hodgson AV, Asoala V, Imwong M, Takala-Harrison S, Nosten F, Su XZ, Ringwald P, Ariey F, Dolecek C, Hien TT, Boni MF, Thai CQ, Amambua-Ngwa A, Conway DJ, Djimdé AA, Doumbo OK, Zongo I, Ouedraogo JB, Alcock D, Drury E, Auburn S, Koch O, Sanders M, Hubbart C, Maslen G, Ruano-Rubio V, Jyothi D, Miles A, O’Brien J, Gamble C, Oyola SO, Rayner JC, Newbold CI, Berriman M, Spencer CC, McVean G, Day NP, White NJ, Bethell D, Dondorp AM, Plowe CV, Fairhurst RM, Kwiatkowski DP. 2013. Multiple populations of artemisinin-resistant Plasmodium falciparum in Cambodia. Nature Genetics, 45, 648–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Mizuno Y, Makioka A, Kawazu S, Kano S, Kawai S, Akaki M, Aikawa M, Ohtomo H. 2002. Effect of jasplakinolide on the growth, invasion, and actin cytoskeleton of Plasmodium falciparum. Parasitology Research, 88, 844–848. [DOI] [PubMed] [Google Scholar]
- 77. Mohmmed A, Dasaradhi PV, Bhatnagar RK, Chauhan VS, Malhotra P. 2003. In vivo gene silencing in Plasmodium berghei – a mouse malaria model. Biochemical and Biophysical Research Communications, 309, 506–511. [DOI] [PubMed] [Google Scholar]
- 78. Mosqueira VC, Loiseau PM, Bories C, Legrand P, Devissaguet JP, Barratt G. 2004. Efficacy and pharmacokinetics of intravenous nanocapsule formulations of halofantrine in Plasmodium berghei-infected mice. Antimicrobial Agents of Chemotherapy, 48, 1222–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Movellan J, Urbán P, Moles E, de la Fuente JM, Sierra T, Serrano JL, Fernàndez-Busquets X. 2014. Amphiphilic dendritic derivatives as nanocarriers for the targeted delivery of antimalarial drugs. Biomaterials, 35, 7940–7950. [DOI] [PubMed] [Google Scholar]
- 80. Mueller AK, Hammerschmidt-Kamper C, Kaiser A. 2014. RNAi in Plasmodium. Current Pharmaceutical Design, 20, 278–283. [DOI] [PubMed] [Google Scholar]
- 81. Myers FB, Lee LP. 2008. Innovations in optical microfluidic technologies for point-of-care diagnostics. Lab on a Chip, 8, 2015–2031. [DOI] [PubMed] [Google Scholar]
- 82. Nagaraj G, Uma MV, Shivayogi MS, Balaram H. 2001. Antibiotics isolated from fungi antimalarial activities of peptide. Antimicrobial Agents and Chemotherapy, 45, 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Newton PN, Ward S, Angus BJ. 2006. Early treatment failure in severe malaria resulting from abnormally low plasma quinine concentrations. Transactions of the Royal Society of Tropical Medicine and Hygiene, 100, 184–186. [DOI] [PubMed] [Google Scholar]
- 84. Nilanonta C, Isaka M, Kittakoop P, Palittapongarnpim P, Kamchonwongpaisan S, Pittayakhajonwut D, Tanticharoen M, et al. 2000. Antimycobacterial and antiplasmodial Cyclodepsipeptides from the insect pathogenic fungus Paecilomyces tenuipes BCC 1614. Planta Medica, 66, 756–758. [DOI] [PubMed] [Google Scholar]
- 85. Nixon GL, Pidathala C, Shone AE, Antoine T, Fisher N, O’Neill PM, Ward SA, Biagini GA. 2013. Targeting the mitochondrial electron transport chain of Plasmodium falciparum: new strategies towards the development of improved antimalarials for the elimination era. Future Medicinal Chemistry, 5, 1573–1591. [DOI] [PubMed] [Google Scholar]
- 86. Noedl H, Se Y, Schaecher K, Socheat D, Fukuda MM. 2008. Evidence of artemisinin-resistant malaria in western Cambodia. New England Journal of Medicine, 359, 2619–2620. [DOI] [PubMed] [Google Scholar]
- 87. Okombo J, Kiara SM, Rono J, Mwai L, Pole L, Ohuma E, Borrmann S, Ochola LI, Nzila A. 2010. In vitro activities of quinine and other antimalarials and pfnhe polymorphisms in Plasmodium isolates from Kenya. Antimicrobial Agents and Chemotherapy, 54, 3302–3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ongsrichanalai C, Sirichaisinthop J, Karwacki JJ, Congpuong K, Miller RS, Pang L, Thimasarn K. 2001. Drug resistant malaria on the Thai-Myanmar and Thai-Cambodian borders. Southeast Asian Journal of Tropical Medicine and Public Health, 32, 41–49. [PubMed] [Google Scholar]
- 89. Peek LJ, Middaugh CR, Berkland C. 2008. Nanotechnology in vaccine delivery. Advance Drug Delivery Reviews, 60, 915–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Bloland PB. 2001. Drug resistance in malaria. World Health Organization: Geneva, WHO/CDS/CSR/DRS/2001.4. [Google Scholar]
- 91. Peters W. 1987. Resistance of human malaria I, III and IV, in Chemotherapy and drug resistance in malaria, 2nd ed., Academic Press: London: p. 543–568, 593–658, 659–786. [Google Scholar]
- 92. Peters W. 1990. The prevention of antimalarial drug resistance. Pharmacology Therapy, 47, 499–508. [DOI] [PubMed] [Google Scholar]
- 93. Pérez-Picaso L, Velasco-Bejarano B, Aguilar-Guadarrama AB, Argotte-Ramos R, Rios MY. 2009. Antimalarial activity of ultra-short peptides. Molecules, 14, 5103–5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Polpanich D, Tangboriboonrat P, Elaissari A, Udomsangpetch R. 2007. Detection of malaria infection via latex agglutination assay. Analytical Chemistry, 79, 4690–4695. [DOI] [PubMed] [Google Scholar]
- 95. Postma NS, Crommelin DJ, Eling WM, Zuidema J. 1999. Treatment with liposome-bound recombinant human tumor necrosis factor-alpha suppresses parasitemia and protects against Plasmodium berghei k173-induced experimental cerebral malaria in mice. Journal of Pharmacology and Experimental Therapeutics, 288, 114–120. [PubMed] [Google Scholar]
- 96. Pushparaj PN, Aarthi JJ, Kumar SD, Manikandan J. 2008. RNAi and RNAa – the Yin and Yang of RNAome. Bioinformation, 2, 235–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Raabe CA, Sanchez CP, Randau G, Robeck T, Skryabin BV, Chinni SV, Kube M, Reinhardt R, Ng GH, Manickam R, Kuryshev VY, Lanzer M, Brosius J, Tang TH, Rozhdestvensky TS. 2010. A global view of the nonprotein-coding transcriptome in Plasmodium falciparum. Nucleic Acids Research, 38(2), 608–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Rautenbach M, Vlok NM, Stander M, Hoppe HC. 2007. Inhibition of malaria parasite blood stages by tyrocidines, membrane-active cyclic peptide antibiotics from Bacillus brevis. Biochimica et Biophysica Acta, 1768, 1488–1497. [DOI] [PubMed] [Google Scholar]
- 99. Thakur RS, Sultan T, Sanyal A, Atul PK, Punia P, Das J. 2013. Mesenchymal stem cells play an important role in host protective immune responses against malaria by modulating regulatory T cells. European Journal of Immunology, 43, 2070–2077. [DOI] [PubMed] [Google Scholar]
- 100. Robertson RT, Baratta JL, Haynes SM, Longmuir KJ. 2008. Liposomes incorporating a Plasmodium amino acid sequence target heparan sulfate binding sites in liver. Journal of Pharmaceutical Sciences, 97, 3257–3273. [DOI] [PubMed] [Google Scholar]
- 101. Rojas-Rivero L, Gay F, Bustos MD, Ciceron L, Pichet C, Danis M, Gentilini M. 1992. Mefloquine-halofantrine cross-resistance in Plasmodium falciparum induced by intermittent mefloquine pressure. American Journal of Tropical Medicine and Hygiene, 47, 372–377. [DOI] [PubMed] [Google Scholar]
- 102. Roper C, Pearce R, Nair S, Sharp B, Nosten F, Anderson T. 2004. Intercontinental spread of pyrimethamine-resistant malaria. Science, 305, 1124. [DOI] [PubMed] [Google Scholar]
- 103. Rosenthal PJ, Wollish WS, Palmer JT, Rasnick D. 1991. Antimalarial effects of peptide inhibitors of a Plasmodium falciparum cysteine proteinase. Journal of Clinical Investigation, 88, 1467–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Sabareesh V, Ranganayaki RS, Raghothama S, Bopanna MP, Balaram H, Srinivasan MC, Balaram P. 2007. Identification and characterization of a library of microheterogeneous cyclohexadepsipeptides from the fungus Isaria. Journal of Natural Products, 70, 715–729. [DOI] [PubMed] [Google Scholar]
- 105. Saei AA, Ahmadian S. 2009. Stem cell engineering might be protective against severe malaria. Bioscience Hypothesis, 2, 48–49. [Google Scholar]
- 106. Sá JM, Twu O, Hayton K, Reyes S, Fay MP, Ringwald P, Wellems TE. 2009. Geographic patterns of Plasmodium falciparum drug resistance distinguished by differential responses to amodiaquine and chloroquine. Proceedings of the National Academy of Sciences of the United States of America, 106, 18883–18889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Santos-Magalhães NS, Mosqueira VC. 2010. Nanotechnology applied to the treatment of malaria. Advance Drug Delivery Reviews, 62, 560–575. [DOI] [PubMed] [Google Scholar]
- 108. Satimai W, Sudathip P, Vijaykadga S, Khamsiriwatchara A, Sawang S, Potithavoranan T, Sangvicheanet A, Delacollette C, Singhasivanon P, Kaewkungwal J, Lawpoolsri S. 2012. Artemisinin resistance containment project in Thailand. II: Responses to mefloquine-artesunate combination therapy among falciparum malaria patients in provinces bordering Cambodia. Malaria Journal, 11, 300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Schwentke A, Krepstakies M, Müller AK, Hammerschmidt-Kamper C, Motaal BA, Bernhard T, Hauber J, Kaiser A. 2012. In vitro and in vivo silencing of plasmodial dhs and eIF-5A genes in a putative, noncanonical RNAi-related pathway. BMC Microbiology, 12, 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Sharma MK, Rao VK, Agarwal GS, Rai GP, Gopalan N, Prakash S, Sharma SK, Vijayaraghavan R. 2008. Highly sensitive amperometric immunosensor for detection of Plasmodium falciparum histidine-rich protein 2 in serum of humans with malaria: comparison with a commercial kit. Journal of Clinical Microbiology, 46, 3759–3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Song YS, Moon S, Hulli L, Hasan SK, Kayaalp E, Demirci U. 2009. Microfluidics for cryopreservation. Lab on a Chip, 9, 1874–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Stanisic DI, Barry AE, Good MF. 2013. Escaping the immune system: how the malaria parasite makes vaccine development a challenge. Trends in Parasitology, 29, 612–622. [DOI] [PubMed] [Google Scholar]
- 113. Takala-Harrison S, Jacob CG, Arze C, Cummings MP, Silva JC, Dondorp AM, Fukuda MM, Hien TT, Mayxay M, Noedl H, Nosten F, Kyaw MP, Nhien NT, Imwong M, Bethell D, Se Y, Lon C, Tyner SD, Saunders DL, Ariey F, Mercereau-Puijalon O, Menard D, Newton PN, Khanthavong M, Hongvanthong B, Starzengruber P, Fuehrer HP, Swoboda P, Khan WA, Phyo AP, Nyunt MM, Nyunt MH, Brown TS, Adams M, Pepin CS, Bailey J, Tan JC, Ferdig MT, Clark TG, Miotto O, MacInnis B, Kwiatkowski DP, White NJ, Ringwald P, Plowe CV. 2014. Independent emergence of Plasmodium falciparum artemisinin resistance mutations in Southeast Asia. Journal of Infectious Diseases [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Tarique M, Tabassum F, Ahmad M, Tuteja R. 2014. Plasmodium falciparum UvrD activities are downregulated by DNA-interacting compounds and its dsRNA inhibits malaria parasite growth. BMC Biochemistry, 15, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Tayade NG, Nagarsenker MS. 2010. Development and evaluation of artemether parenteral microemulsion. Indian Journal of Pharmaceutical Sciences, 72, 637–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Thongtan J, Saenboonrueng J, Rachtawee P, Isaka M. 2006. An antimalarial tetrapeptide from the entomopathogenic fungus Hirsutella sp. BCC 1528. Journal of Natural Products, 69, 713–714. [DOI] [PubMed] [Google Scholar]
- 117. Torrentino-Madamet M, Desplans J, Travaillé C, James Y, Parzy D. 2010. Microaerophilic respiratory metabolism of Plasmodium falciparum mitochondrion as a drug target. Current Molecular Medicine, 10, 29–46. [DOI] [PubMed] [Google Scholar]
- 118. Tuteja R, Pradhan A, Sharma S. 2008. Plasmodium falciparum signal peptidase is regulated by phosphorylation and required for intra-erythrocytic growth. Molecular and Biochemical Parasitology, 157, 137–147. [DOI] [PubMed] [Google Scholar]
- 119. Tuteja R, Pradhan A. 2010. Using RNA interference assay we show that PfeIF4A and PfeIF4A (PfH45) are essentially required for the growth and survival of the parasite. PfeIF4E and PfeIF4A colocalize and their double-stranded RNA inhibits Plasmodium falciparum proliferation. Communicative & Integrative Biology, 3, 611–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Ullu E, Tschudi C, Chakraborty T. 2004. RNA interference in protozoan parasites. Cell Microbiology, 6, 509–519. [DOI] [PubMed] [Google Scholar]
- 121. Urbán P, Estelrich J, Cortés A, Fernàndez-Busquets X. 2011. A nanovector with complete discrimination for targeted delivery to Plasmodium falciparum-infected versus non-infected red blood cells in vitro. Journal of Control Release, 151, 202–211. [DOI] [PubMed] [Google Scholar]
- 122. Urban P, Valle-Delgado JJ, Mauro N, Marques J, Manfredi A, Rottmann M, et al. 2014. Use of poly(amidoamine) drug conjugates for the delivery of antimalarials to Plasmodium. Journal of Control Release, 177, 84–95. [DOI] [PubMed] [Google Scholar]
- 123. Van Tyne D, Park DJ, Schaffner SF, Neafsey DE, Angelino E, Cortese JF, Barnes KG, Rosen DM, Lukens AK, Daniels RF, Milner DA Jr, Johnson CA, Shlyakhter I, Grossman SR, Becker JS, Yamins D, Karlsson EK, Ndiaye D, Sarr O, Mboup S, Happi C, Furlotte NA, Eskin E, Kang HM, Hartl DL, Birren BW, Wiegand RC, Lander ES, Wirth DF, Volkman SK, Sabeti PC. 2011. Identification and functional validation of the novel antimalarial resistance locus PF10_0355 in Plasmodium falciparum. PLoS Genetics, 7, e1001383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Vial H, Taramelli D, Boulton IC, Ward SA, Doerig C, Chibale K. 2013. CRIMALDDI: platform technologies and novel anti-malarial drug targets. Malaria Journal, 12, 396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Ward SA, Bray PG, Mungthin M, Hawley SR. 1995. Current views on the mechanisms of resistance to quinoline-containing drugs in Plasmodium falciparum. Annals of Tropical Medicine and Parasitology, 89, 121–124. [DOI] [PubMed] [Google Scholar]
- 126. Watkins WM, Mosobo M. 1993. Treatment of Plasmodium falciparum malaria with pyrimethamine-sulphadoxine: selective pressure for resistance is a function of long elimination half-life. Transactions of the Royal Society of Tropical Medicine and Hygiene, 87, 75–78. [DOI] [PubMed] [Google Scholar]
- 127. Watkins WM, Mberu EK, Winstanley PA, Plowe CV. 1999. More on the efficacy of antifolate antimalarial combinations in Africa. Parasitology Today, 15, 131–132. [DOI] [PubMed] [Google Scholar]
- 128. Wernsdorfer WH, Payne D. 1991. The dynamics of drug resistance in Plasmodium falciparum. Pharmacology Therapy, 50, 95–121. [DOI] [PubMed] [Google Scholar]
- 129. Wernsdorfer WH. 1994. Epidemiology of drug resistance in malaria. Acta Tropica, 56, 143–156. [DOI] [PubMed] [Google Scholar]
- 130. West J, Becker M, Tombrink S, Tombrink S, Manz A. 2008. Micro total analysis systems: latest achievements. Analytical Chemistry, 80, 4403–4419. [DOI] [PubMed] [Google Scholar]
- 131. White NJ. 1999. Antimalarial drug resistance and combination chemotherapy. Philosophical Transactions of the Royal Society B: Biological Science, 354, 739–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. WHO. 2001. Antimalarial drug combination therapy. World Health Organization: Geneva. [Google Scholar]
- 133. WHO. 2006. WHO calls for an immediate halt to provision of single-drug artemisinin malaria pills. New malaria treatment guidelines issued by WHO. Saudi Medical Journal, 27, 574–575. [PubMed] [Google Scholar]
- 134. WHO. 2012. World malaria report 2012. World Health Organization: Geneva. [Google Scholar]
- 135. Wichmann O, Muehlberger N, Jelinek T, Alifrangis M, Peyerl-Hoffmann G, Muhlen M, Grobusch MP, Gascon J, Matteelli A, Laferl H, Bisoffi Z, Ehrhardt S, Cuadros J, Hatz C, Gjorup I, McWhinney P, Beran J, da Cunha S, Schulze M, Kollaritsch H, Kern P, Fry G, Richter J. 2000. Screening for mutations related to atovaquone/proguanil resistance in treatment failures and other imported isolates of Plasmodium falciparum in Europe. Journal of Infectious Diseases, 190, 1541–1546. [DOI] [PubMed] [Google Scholar]
- 136. Wiwanitkit V. 2009. Alternative tools for field analysis on malarial infection: a reappraisal. Clinical Therapeutics, 160, 83–85. [PubMed] [Google Scholar]
- 137. WHO. 1967. World Malaria Report. World Health Organization: Geneva. [Google Scholar]
- 138. WHO. 2010. World Malaria Report. World Health Organization: Geneva. [Google Scholar]
- 139. WHO. 2013. World Malaria Report. World Health Organization: Geneva. [Google Scholar]
- 140. Yeung S, Pongtavornpinyo W, Hastings IM, Mills AJ, White NJ. 2004. Antimalarial drug resistance, artemisinin-based combination therapy, and the contribution of modeling to elucidating policy choices. American Journal of Tropical Medicine and Hygiene, 71, 179–186. [PubMed] [Google Scholar]
- 141. Zhang C, Xiao B, Jiang Y, Zhao Y, Li Z, Gao H, Ling Y, Wei J, Li S, Lu M, Su XZ, Cui H, Yuan J. 2014. Efficient editing of malaria parasite genome using the CRISPR/Cas9 system. Molecular Biology, 5, e01414-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Zhang Y, Mi JY, Rui YJ, Xu YL, Wang W. 2014. Stem cell therapy for the treatment of parasitic infections: is it far away. Parasitology Research, 113, 607–612. [DOI] [PubMed] [Google Scholar]