Abstract
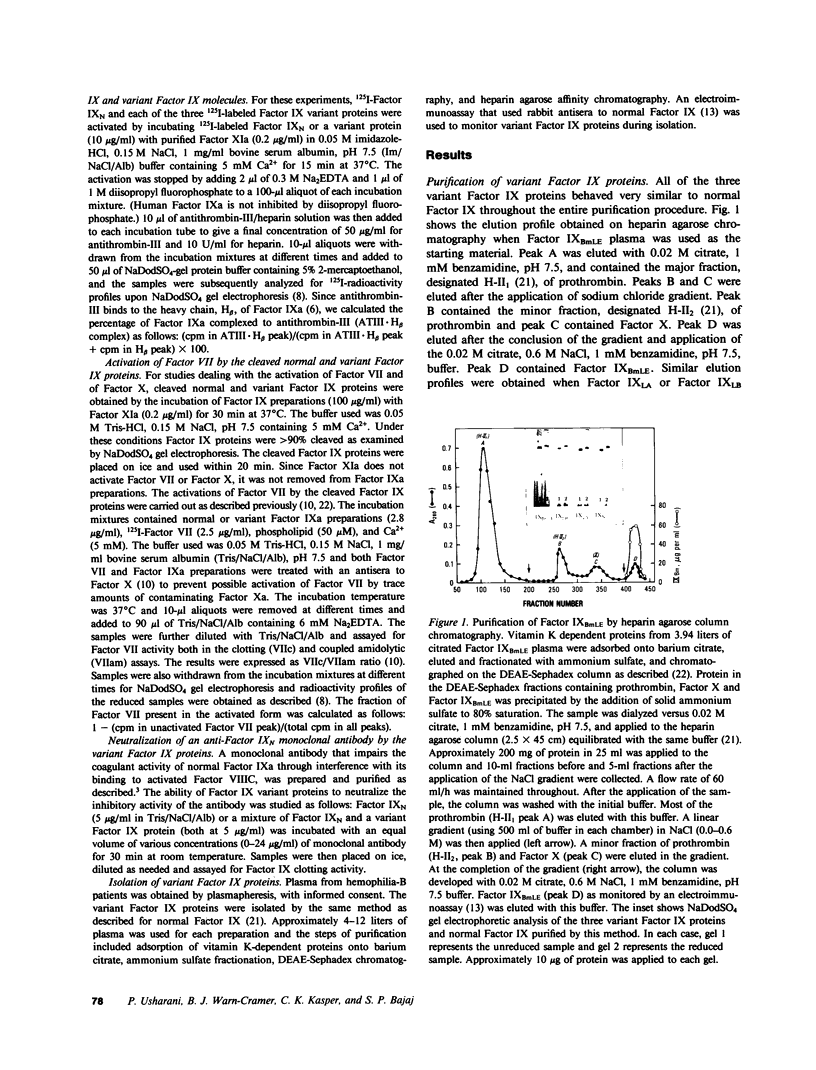
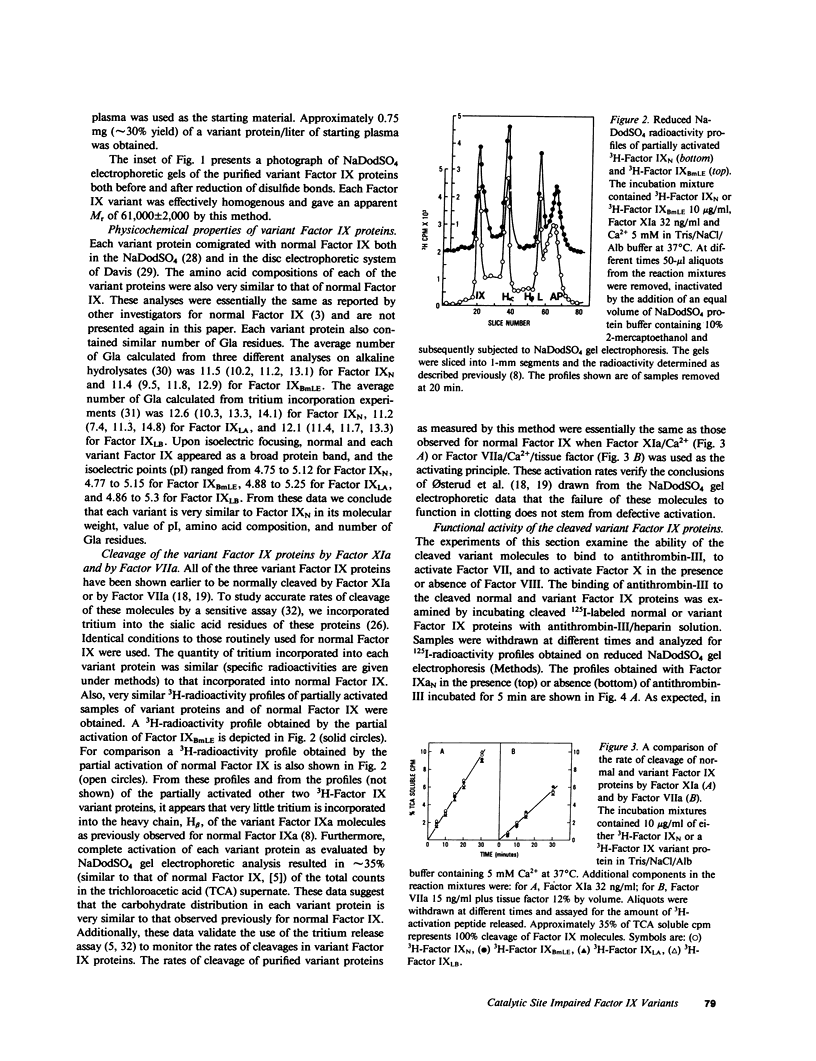
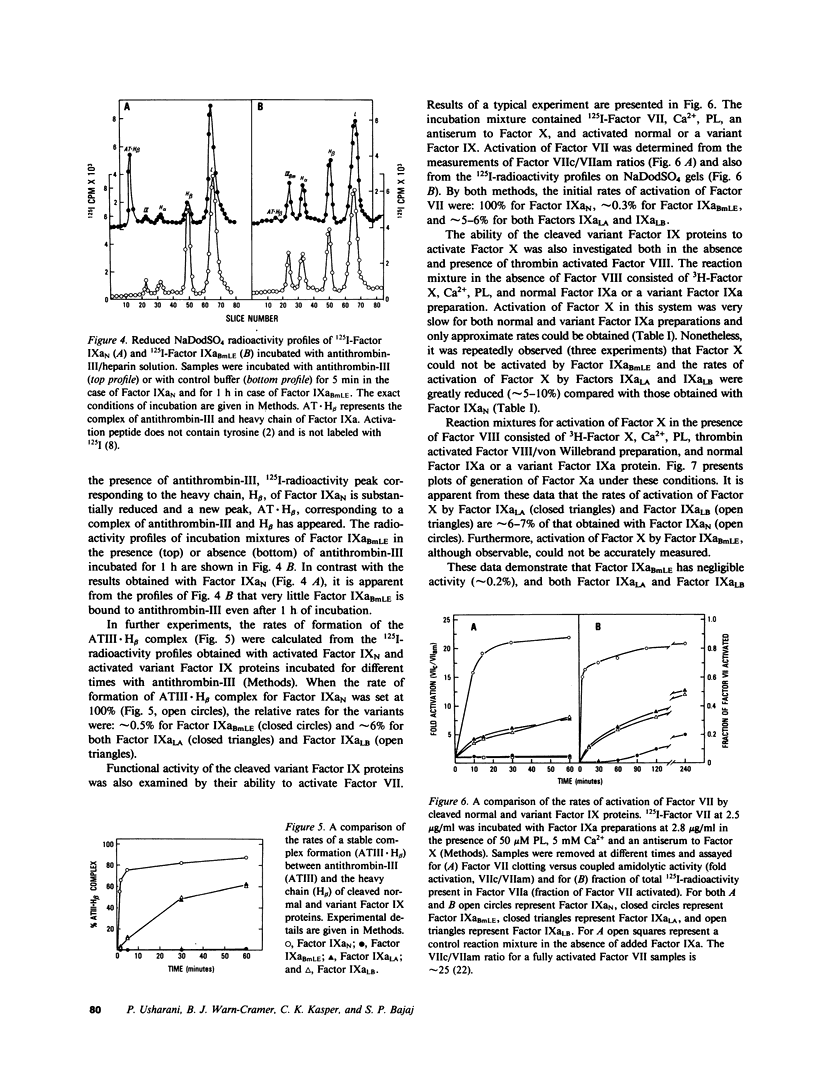
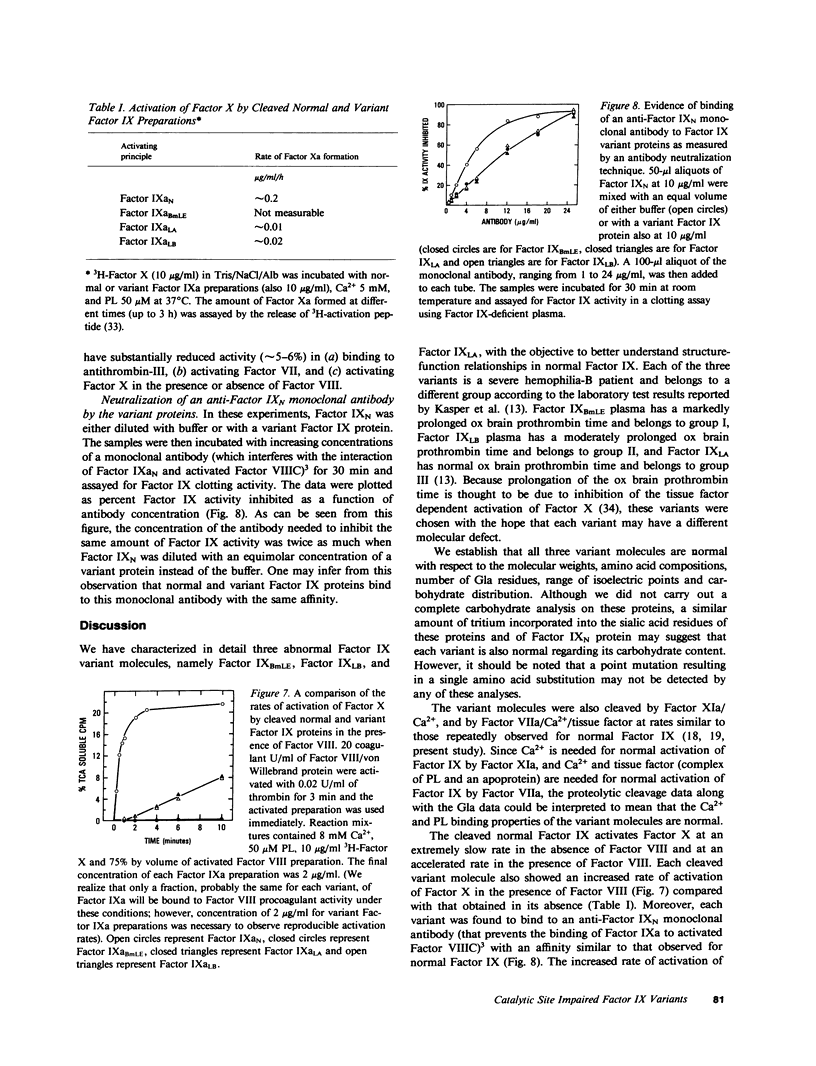
Abnormal factor IX variant proteins were isolated from the plasmas of three unrelated severe hemophilia-B families that had been previously shown to contain functionally impaired molecules immunologically similar to normal factor IX. The families studied were: (1) a patient with markedly prolonged ox brain prothrombin time, designated factor IX Bm Lake Elsinore (IXBmLE); (b) three patients (brothers) with moderately prolonged ox brain prothrombin time, designated factor IX Long Beach (IXLB); and (c) a patient with normal ox brain prothrombin time designated factor IX Los Angeles (IXLA). Each variant molecule comigrates with normal factor IX (IXN) both in the sodium dodecyl sulfate and in the nondenaturing alkaline gel electrophoresis. All three variant proteins are indistinguishable from IXN in their amino acid compositions, isoelectric points, carbohydrate distributions and number of gamma-carboxyglutamic acid residues. Each variant protein undergoes a similar pattern of cleavage by factor XIa/Ca2+ and by factor VIIa/Ca2+/tissue factor, and is activated at a rate similar to that observed for IXN. All of the three variant proteins also react with an anti-IXN monoclonal antibody that interferes with the binding of activated IXN(IXaN) to thrombin-treated factor VIIIC. However, in contrast to IXaN, the cleaved IXBmLE has negligible activity (approximately 0.2%), and cleaved forms of IXLA and IXLB have significantly reduced activity (approximately 5-6%) in binding to antithrombin-III/heparin, and in activating factor VII (plus Ca2+ and phospholipid) or factor X (plus Ca2+ and phospholipid) +/- factor VIII. These data, taken together, strongly indicate that the defect in these three variant proteins resides near or within the latent catalytic site. This results in virtually a complete loss of catalytic activity of the cleaved IXBmLE molecule and approximately 95% loss of catalytic activity of the cleaved IXLA and IXLB molecules.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amphlett G. W., Kisiel W., Castellino F. J. The interaction of Ca2+ with human Factor IX. Arch Biochem Biophys. 1981 May;208(2):576–585. doi: 10.1016/0003-9861(81)90546-4. [DOI] [PubMed] [Google Scholar]
- Bajaj S. P. Cooperative Ca2+ binding to human factor IX. Effects of Ca2+ on the kinetic parameters of the activation of factor IX by factor XIa. J Biol Chem. 1982 Apr 25;257(8):4127–4132. [PubMed] [Google Scholar]
- Bajaj S. P., Rapaport S. I., Brown S. F. Isolation and characterization of human factor VII. Activation of factor VII by factor Xa. J Biol Chem. 1981 Jan 10;256(1):253–259. [PubMed] [Google Scholar]
- Bajaj S. P., Rapaport S. I., Prodanos C. A simplified procedure for purification of human prothrombin, factor IX and factor X. Prep Biochem. 1981;11(4):397–412. doi: 10.1080/00327488108065531. [DOI] [PubMed] [Google Scholar]
- Bajaj S. P., Rapaport S. I., Prodonos C., Russell W. A. Heterogeneity in human prothrombin: analysis of cause. Blood. 1981 Nov;58(5):886–891. [PubMed] [Google Scholar]
- Bajaj S. P., Rapaport S. I., Russell W. A. Redetermination of the rate-limiting step in the activation of factor IX by factor XIa and by factor VIIa/tissue factor. Explanation for different electrophoretic radioactivity profiles obtained on activation of 3H- and 125I-labeled factor IX. Biochemistry. 1983 Aug 16;22(17):4047–4053. doi: 10.1021/bi00286a009. [DOI] [PubMed] [Google Scholar]
- Bertina R. M., Van Der Linden I. K. Factor IX Zutphen. A genetic variant of blood coagulation factor IX with an abnormally high molecular weight. J Lab Clin Med. 1982 Nov;100(5):695–704. [PubMed] [Google Scholar]
- Bertina R. M., van der Linden I. K. Factor IX Deventer-evidence for the heterogeneity of hemophilia BM. Thromb Haemost. 1982 Apr 30;47(2):136–140. [PubMed] [Google Scholar]
- Braunstein K. M., Noyes C. M., Griffith M. J., Lundblad R. L., Roberts H. R. Characterization of the defect in activation of factor IX Chapel Hill by human factor XIa. J Clin Invest. 1981 Dec;68(6):1420–1426. doi: 10.1172/JCI110393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P. E., Hougie C., Roberts H. R. The genetic heterogeneity of hemophilia B. N Engl J Med. 1970 Jul 9;283(2):61–64. doi: 10.1056/NEJM197007092830203. [DOI] [PubMed] [Google Scholar]
- Chung K. S., Madar D. A., Goldsmith J. C., Kingdon H. S., Roberts H. R. Purification and characterization of an abnormal factor IX (Christmas factor) molecule. Factor IX Chapel Hill. J Clin Invest. 1978 Nov;62(5):1078–1085. doi: 10.1172/JCI109213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Denson K. W., Biggs R., Mannucci P. M. An investigation of three patients with Christmas disease due to an abnormal type of factor IX. J Clin Pathol. 1968 Mar;21(2):160–165. doi: 10.1136/jcp.21.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Scipio R. G., Hermodson M. A., Yates S. G., Davie E. W. A comparison of human prothrombin, factor IX (Christmas factor), factor X (Stuart factor), and protein S. Biochemistry. 1977 Feb 22;16(4):698–706. doi: 10.1021/bi00623a022. [DOI] [PubMed] [Google Scholar]
- Di Scipio R. G., Kurachi K., Davie E. W. Activation of human factor IX (Christmas factor). J Clin Invest. 1978 Jun;61(6):1528–1538. doi: 10.1172/JCI109073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hougie C., Twomey J. J. Haemophilia Bm: a new type of factor-IX deficiency. Lancet. 1967 Apr 1;1(7492):698–700. doi: 10.1016/s0140-6736(67)92179-4. [DOI] [PubMed] [Google Scholar]
- Hultin M. B., Nemerson Y. Activation of factor X by factors IXa and VIII; a specific assay for factor IXa in the presence of thrombin-activated factor VIII. Blood. 1978 Nov;52(5):928–940. [PubMed] [Google Scholar]
- Jackson C. M., Nemerson Y. Blood coagulation. Annu Rev Biochem. 1980;49:765–811. doi: 10.1146/annurev.bi.49.070180.004001. [DOI] [PubMed] [Google Scholar]
- Kasper C. K., Osterud B., Minami J. Y., Shonick W., Rapaport S. I. Hemophilia B: characterization of genetic variants and detection of carriers. Blood. 1977 Sep;50(3):351–366. [PubMed] [Google Scholar]
- Kurachi K., Davie E. W. Activation of human factor XI (plasma thromboplastin antecedent) by factor XIIa (activated Hageman factor). Biochemistry. 1977 Dec 27;16(26):5831–5839. doi: 10.1021/bi00645a030. [DOI] [PubMed] [Google Scholar]
- Kurachi K., Davie E. W. Isolation and characterization of a cDNA coding for human factor IX. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6461–6464. doi: 10.1073/pnas.79.21.6461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masys D. R., Bajaj S. P., Rapaport S. I. Activation of human factor VII by activated factors IX and X. Blood. 1982 Nov;60(5):1143–1150. [PubMed] [Google Scholar]
- McMullen B. A., Fujikawa K., Kisiel W. The occurrence of beta-hydroxyaspartic acid in the vitamin K-dependent blood coagulation zymogens. Biochem Biophys Res Commun. 1983 Aug 30;115(1):8–14. doi: 10.1016/0006-291x(83)90961-0. [DOI] [PubMed] [Google Scholar]
- Morita T., Isaacs B. S., Esmon C. T., Johnson A. E. Derivatives of blood coagulation factor IX contain a high affinity Ca2+-binding site that lacks gamma-carboxyglutamic acid. J Biol Chem. 1984 May 10;259(9):5698–5704. [PubMed] [Google Scholar]
- Noyes C. M., Griffith M. J., Roberts H. R., Lundblad R. L. Identification of the molecular defect in factor IX Chapel Hill: substitution of histidine for arginine at position 145. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4200–4202. doi: 10.1073/pnas.80.14.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterud B., Kasper C. K., Lavine K. K., Prodanos C., Rapaport S. I. Purification and properties of an abnormal blood coagulation factor IX (factor IXBm)/kinetics of its inhibition of factor X activation by factor VII and bovine tissue factor. Thromb Haemost. 1981 Feb 23;45(1):55–59. [PubMed] [Google Scholar]
- Osterud B., Kasper C. K., Prodanos C. Factor IX variants of hemophilia B. The effect of activated factor XI and the reaction product of factor VII and tissue factor on the abnormal factor IX molecules. Thromb Res. 1979;15(1-2):235–243. doi: 10.1016/0049-3848(79)90069-0. [DOI] [PubMed] [Google Scholar]
- Osterud B., Rapaport S. I. Activation of factor IX by the reaction product of tissue factor and factor VII: additional pathway for initiating blood coagulation. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5260–5264. doi: 10.1073/pnas.74.12.5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh V. R., Mannucci P. M., Ruggeri Z. M. Immunological heterogeneity of haemophilia B: a multicentre study of 98 kindreds. Br J Haematol. 1978 Dec;40(4):643–655. doi: 10.1111/j.1365-2141.1978.tb05840.x. [DOI] [PubMed] [Google Scholar]
- Price P. A., Otsuka A. A., Poser J. W., Kristaponis J., Raman N. Characterization of a gamma-carboxyglutamic acid-containing protein from bone. Proc Natl Acad Sci U S A. 1976 May;73(5):1447–1451. doi: 10.1073/pnas.73.5.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price P. A., Williamson M. K., Epstein D. J. Specific tritium incorporation into gamma-carboxyglutamic acid in proteins. The pH dependence of gamma-proton exchange. J Biol Chem. 1981 Feb 10;256(3):1172–1176. [PubMed] [Google Scholar]
- Rao L. V., Bajaj S. P. Purification of human factor VII utilizing O-(diethylaminoethyl)-Sephadex and Sulfopropyl-Sephadex chromatography. Anal Biochem. 1984 Feb;136(2):357–361. doi: 10.1016/0003-2697(84)90230-6. [DOI] [PubMed] [Google Scholar]
- Roberts H. R., Grizzle J. E., McLester W. D., Penick G. D. Genetic variants of hemophilia B: detection by means of a specific PTC inhibitor. J Clin Invest. 1968 Feb;47(2):360–365. doi: 10.1172/JCI105732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligsohn U., Osterud B., Rapaport S. I. Coupled amidolytic assay for factor VII: its use with a clotting assay to determine the activity state of factor VII. Blood. 1978 Nov;52(5):978–988. [PubMed] [Google Scholar]
- Silverberg S. A., Nemerson Y., Zur M. Kinetics of the activation of bovine coagulation factor X by components of the extrinsic pathway. Kinetic behavior of two-chain factor VII in the presence and absence of tissue factor. J Biol Chem. 1977 Dec 10;252(23):8481–8488. [PubMed] [Google Scholar]
- Van Lenten L., Ashwell G. Studies on the chemical and enzymatic modification of glycoproteins. A general method for the tritiation of sialic acid-containing glycoproteins. J Biol Chem. 1971 Mar 25;246(6):1889–1894. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Zur M., Nemerson Y. Kinetics of factor IX activation via the extrinsic pathway. Dependence of Km on tissue factor. J Biol Chem. 1980 Jun 25;255(12):5703–5707. [PubMed] [Google Scholar]




