Abstract
Purpose of Review: The purpose of this review is to provide an evidence-based update on the neurostimulation options available for patients with drug-resistant epilepsy in the United States and in European countries.
Recent Findings: The field of neurostimulation for epilepsy has grown dramatically since 1997, when vagus nerve stimulation became the first device to be approved for epilepsy by the US Food and Drug Administration (FDA). New data from recently completed randomized controlled trials are available for deep brain stimulation of the anterior thalamus, responsive neurostimulation, and trigeminal nerve stimulation. Although vagus nerve stimulation is the only device currently approved in the United States, deep brain stimulation and responsive neurostimulation devices are awaiting FDA approval. Deep brain stimulation, trigeminal nerve stimulation, and transcutaneous vagus nerve stimulation are now approved for epilepsy in the European Union. In this article, the mechanisms of action, safety, and efficacy of new neurostimulation devices are reviewed, and the key advantages and disadvantages of each are discussed.
Summary: The exponential growth of the field of neuromodulation for epilepsy is an exciting development; these new devices provide physicians with new options for patients with drug-resistant epilepsy.
INTRODUCTION
The Drug-Resistant Patient
Drug-resistant epilepsy is a serious medical condition characterized by uncontrolled seizures after exposure to two or more appropriate antiepileptic drugs (AEDs) at therapeutic doses.1,2 Although 47% of patients become seizure free after exposure to the first AED, only 10% become seizure free after exposure to subsequent AEDs.1 There is a sizable body of evidence that resective epilepsy surgery for suitable candidates is the most effective therapy for drug-resistant epiplepsy.1,3 Level I evidence indicates that for patients who are candidates fortemporal lobectomy, epilepsy surgery is superior to medical therapy.1,3 In a landmark study, 80 subjects with temporal lobe epilepsy were randomized to surgery or medical therapy for 1 year.3 At 1 year, 58% of surgically treated patients were seizure free versus only 8% in the medically treated group (P=.001).3 Despite evidence and growing consensus, epilepsy surgery is underutilized.1,4 To increase physician awareness of this curative therapy, a new online tool has been developed to assist the physician in decision making; this helpful tool can be found at www.epilepsycases.com.5
While epilepsy surgery is the most effective therapy for properly selected patients with drug-resistant epilepsy, especially temporal lobe epilepsy, many patients are not surgical candidates. Twenty percent to 40% of drug-resistant patients may not have temporal lobe epilepsy. In patients with frontal, parietal, or occipital onset seizures, surgical outcomes are generally inferior to temporal lobe epilepsy. In addition, many patients with drug-resistant epilepsy have bitemporal, bilateral, or poorly localized epilepsy, or epilepsy arising from critical or eloquent cortex; in these patients, a neurostimulation device approved by the US Food and Drug Administration (FDA)—currently, vagus nerve stimulation (VNS)—may be considered.
NEUROSTIMULATION FOR EPILEPSY
Neurostimulation has emerged as a potential alternative to failed antiepileptic drug therapy for patients with drug-resistant epilepsy. Since VNS became the first device approved by the FDA for epilepsy, several new therapies have been developed. These include deep brain stimulation of the anterior nucleus of the thalamus (DBS), responsive neurostimulation (RNS), trigeminal nerve stimulation (TNS), and transcutaneous vagus nerve stimulation (t-VNS).6–12 At this writing, only VNS is FDA-approved for use in the United States, although applications for approval of DBS and RNS devices are pending before the FDA. In the European Union, DBS, external TNS (eTNS), and t-VNS (in addition to VNS) each received European Conformity (or CE, for Conformité Européenne) marking, meaning that these therapies are or will be available for the estimated 4 million people with epilepsy throughout the European Union. Thus, neurologists in Europe will soon have access to four devices for the patient with drug-resistant epilepsy. A randomized trial of t-VNS is in progress for which results are not yet available.
Vagus Nerve Stimulation
The vagus nerve is one of the largest cranial nerves, with both afferent (80% to 90%) and efferent fibers (10% to 20%).12,13 Afferent fibers project from gastric and pulmonary structures and project to the nucleus solitarius in the medulla.12,13 Efferent fibers project primarily from two subnuclei: (1) the dorsal motor nucleus of the vagus nerve, which projects to the pharynx, larynx, and gastrointestinal tract, and (2) the nucleus ambiguous, which modifies heart rate and heart-rate variability, providing parasympathetic control of the heart.12,13 Cardiac branches of the left vagus nerve innervate the atrioventricular (AV) node, and cardiac branches from the right vagus nerve innervate the sinoatrial (SA) node. It has been assumed that right VNS or bilateral VNS may cause bradycardia because of the direct innervation of the SA node. However, although bradycardia could occur rarely with left VNS, the settings approved by the FDA have been designed into the programming software to minimize or eliminate any clinically significant adverse effects on the heart. In fact, VNS has had an excellent track record of safety, with very rare cardiac side effects.6–8 The vagus nerve is accessed by the surgeon in the midcervical portion of the neck where branching is minimal and the nerve lies beneath and between the carotid artery and the jugular vein. Implantation of the distal portion of the lead electrode requires incision of the fascia of the carotid sheath and reflection of the jugular vein laterally and carotid artery medially. The vagus nerve is best mobilized by placing the electrode under the vagus nerve trunk, then attaching the electrode contacts carefully to avoid excessive manipulation of the nerve. The nerve has a rich but delicate network of small vessels surrounding the epineuria; damage from excessive manipulation or traction could cause local ischemia to the nerve and lead to vocal cord paralysis, which may result in swallowing dysfunction or hoarseness.7,8
Since its approval in the United States in 1997, and in Europe and Canada in 2001, over 100,000 patients have been implanted through December 2012 with a VNS device.14 The overwhelming majority of those patients have drug-resistant epilepsy; a small number had treatment-resistant depression. The evidence for efficacy was demonstrated by two randomized, double-blind, active-controlled trials.6,7 The VNS pivotal study randomized 196 subjects to treatment with VNS at 30 seconds on, 5 minutes off at a stimulus frequency of 30 Hz. Active-control settings were 30 seconds on, 180 minutes off, at 1 Hz.7 Subjects with drug-resistant epilepsy with six or more seizures per month entered a 3-month baseline and were then implanted and followed for 3 months of double-blind treatment.7 VNS was well tolerated, with voice alteration or hoarseness (66%) and excessive cough (45%) being the most common adverse side effects.7 Vocal cord paralysis occurred in two patients (1%), and device infection occurred in three patients (1.5%).7 The results demonstrated that VNS was superior to active control, with a median reduction of seizure frequency of 28% in the treatment group compared with 15% in the active-control group (P=.04).7 The 50% responder rate, defined as the percentage of patients with a 50% or greater reduction in seizures, was 23% for the treatment group versus 15.7% for the active control group.7 At 1 year, the 50% responder rate improved to 35%, and the median reduction in seizures increased from 28% to 45%.8
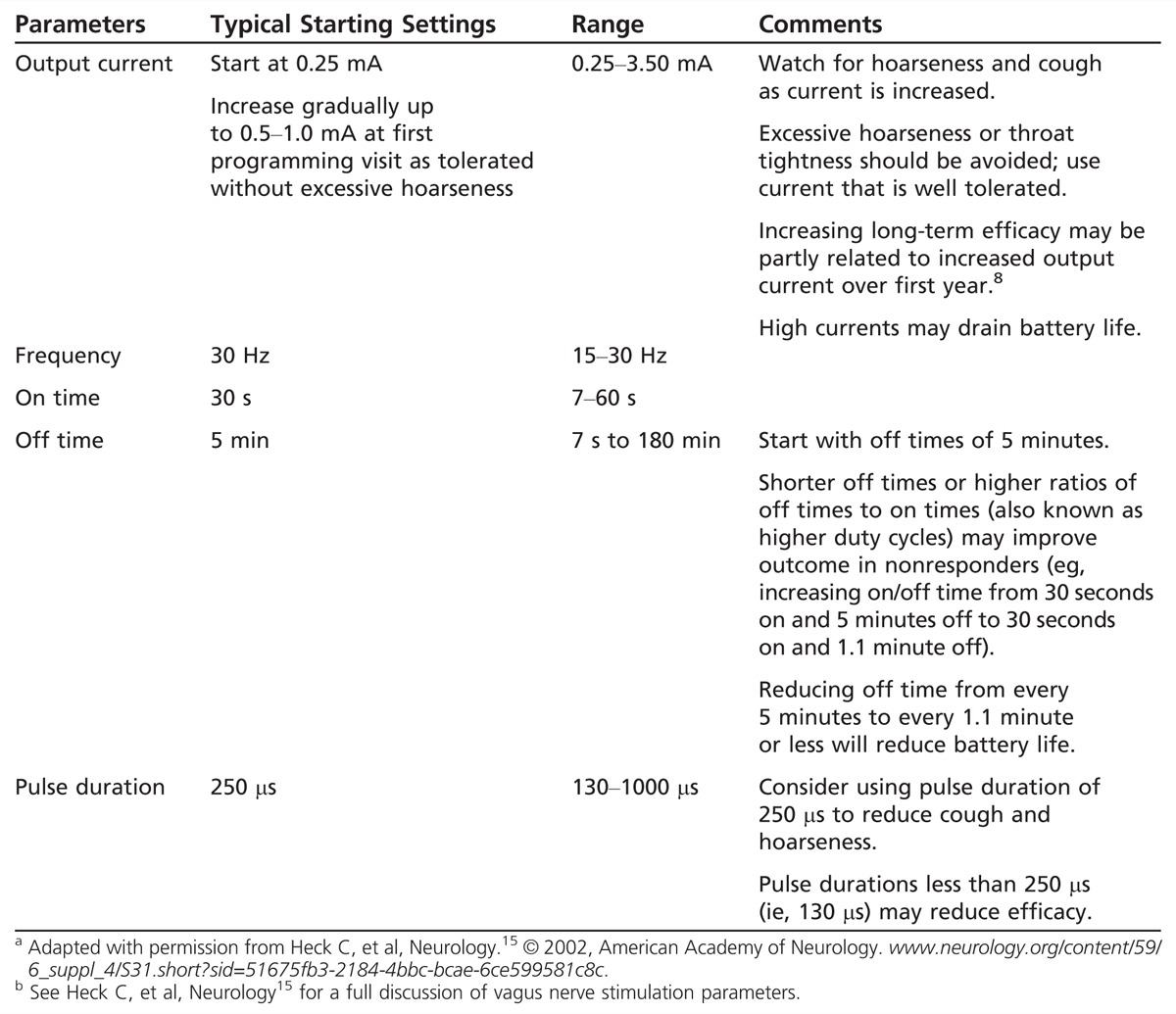
Starting a patient on VNS therapy involves the programming of several parameters, including current, pulse duration, frequency, on and off time, and magnet current. The clinician may be tempted to adjust these settings too high at first in an attempt to improve response, or to use high currents or on/off cycles to improve efficacy rapidly. A measured approach based on years of experience and published data is shown in Table 10-1,15 which provides a suggested protocol for the initiation and titration of VNS. There is little evidence that the use of very high currents or duty cycles early in therapy improves response. It may take several weeks or a few months for a patient to respond to a change in device settings (Case 10-1). Avoid using excessive currents or pulse width that may cause discomfort, severe throat tightness, or shortness of breath. Always aim to use settings that promote patient comfort and safety. For physician information and a full discussion of safety and adjustment of parameters, go to us.cyberonics.com/en/vns-therapy-for-epilepsy/healthcare-professionals. Note that VNS may be MRI compatible under special circumstances that require strict adherence to specific protocols. These protocols can be found at the above website. Per the manufacturer, transmit and receive radio frequency (RF) head coils may be used under specific conditions, but an MRI should be performed only after careful review and preparation in consideration of these guidelines.
Table 10-1.
Possible Vagus Nerve Stimulation Parameters and Initial Programming Settings for Vagus Nerve Stimulationa,b
Case 10-1
A 32-year-old woman with Lennox-Gastaut syndrome and psychosis had severe drop attacks, which were poorly controlled despite treatment with multiple antiepileptic drugs. The patient became nearly seizure free when exposed to oxcarbazepine; however, she experienced severe worsening of her psychosis, possibly due to induction via the cytochrome P450 enzyme system. When oxcarbazepine was discontinued, her behavior improved, but both generalized tonic-clonic and drop attacks worsened. A vagus nerve stimulation (VNS) system was implanted. Postoperatively, the patient reported severe hoarseness and difficulty swallowing. Evaluation of her vocal cords demonstrated complete paralysis of the left vocal cord, and a swallowing study confirmed aspiration with thin fluids. A dysphagia diet and swallow therapy was initiated. Over the next year, the swallowing dysfunction improved, but vocal cord paresis continued; however, over that same year, the frequency of drop and generalized tonic-clonic seizures gradually improved, total seizure frequency decreased over 50%, and drop attack frequency was reduced over 75%.
Comment. The efficacy of VNS may improve over the first year of therapy. Vocal cord paralysis and swallowing dysfunction are important complications of VNS for which the physician should be vigilant. Fortunately, hoarseness and swallowing dysfunction secondary to vocal cord paralysis may improve over time.
Recently, a noninvasive form of VNS called t-VNS received approval in the European Union. T-VNS involves unilateral external transcutaneous stimulation of the auricular branch of the vagus nerve using an external pulse generator and external lead placed in the pinna.16 A pilot feasibility trial of 10 subjects was recently published, using low-frequency stimulation at 10 Hz for 1 hour 3 times daily for 9 months.16 Three patients exited the study because of adverse effects: hoarseness, headache, and obstipation (constipation) were the principal side effects reported.16 Although no patients experienced a 50% reduction in seizures, one subject experienced a 45% reduction in seizures, and another subject reported a 48% reduction.16 A European multicenter study of t-VNS is in process to better define the safety and efficacy of t-VNS for drug-resistant epilepsy.
Deep Brain Stimulation of the Anterior Nucleus of the Thalamus
The anterior nucleus of the thalamus is a core component of the Papez circuit, the network formed by the amygdala, hippocampus, fornix, mammillary body, anterior nucleus of the thalamus, and the cingulate gyrus.17 Electrical outflow from the amygdala and hippocampus projects through this circuit, including the anterior thalamus. The anterior thalamus is thought to serve as a relay station, which can amplify and synchronize seizure discharges from the hippocampus and the central and posterior thalamus.17 Since the anterior thalamus may serve to enhance seizure activity in this circuit, inhibition of the anterior thalamus by electrical stimulation can prevent and abort seizures.9,17–22 In a seminal study published in 1972, it was noted that lesions of the ventral anterior thalamus significantly reduced the frequency and duration of electrographic seizures induced by local injection of tungstic acid gel.18 Similarly, lesions of the anterior thalamus inhibited clinical seizures induced by the alumina cream model in primates. The evidence supports a key regulatory role of the anterior nucleus in the propagation and regulation of seizures.17,19 In addition to lesions, bilateral high-frequency stimulation in animal models inhibits seizures, likely through inhibition of the thalamus.20 Recent evidence indicates that low-frequency stimulation, when bilateral, can also reduce seizures in an amygdala-kindling model, with theoretically lower risk of worsening seizures.17
Stimulation of the anterior nucleus in humans involves the surgical implantation of multicontact depth electrode leads bilaterally into the anterior nucleus using a stereotactic approach. Axial and coronal T1-weighted three-dimensional magnetic resonance images are used for targeting. The anterior nucleus is easily visualized in coronal or sagittal sections as a protrusion on the floor of the lateral ventricles. The target for the lead tips is generally 5 to 6 mm lateral to the midline, 3 mm posterior to the midcommissural plane, and 12 mm superior to the intercommissural line. These coordinates correspond to the posterior and inferior portion of the anterior nucleus, allowing at least two contacts to be located within the boundaries of the anterior nucleus (Figure 10-1). In contrast to DBS surgeries for movement disorders, anterior nucleus implantations can be performed under general anesthesia because intraoperative stimulation of the anterior nucleus and surrounding regions is imperceptible by most patients. After the target has been defined, the guide cannula for the lead is lowered into place. Because the anterior nucleus lies just beneath the floor of the lateral ventricle, it is important to advance the cannula all the way to the target. Leads that are advanced to the target from cannula tips located within the lateral ventricle are prone to misplacement because of a tendency for the flexible lead to be diverted off-course by the ventricle’s relatively dense ependymal surface. This ependymal surface can often be felt when the cannula is lowered by hand. The cannula can be withdrawn once the lead has been lowered into place. Intraoperative fluoroscopy is useful in confirming that the lead has penetrated the ventricle and is appropriately placed in the anterior nucleus. In the pivotal DBS study, four-contact leads were used because of the relatively large size of the anterior nucleus.9 Both right and left leads were tunneled subcutaneously and connected to a dual-channel pulse generator located in a subcutaneous pocket formed in the right subclavicular region of the chest. Since DBS produces little to no observable effects on patients when initial programming is performed, selection of proper contacts relies heavily on postoperative imaging. Because the average height of the human anterior nucleus is 6 mm, one or two contacts will typically be located entirely within the anterior nucleus of the thalamus bilaterally, right and left.
Figure 10-1.
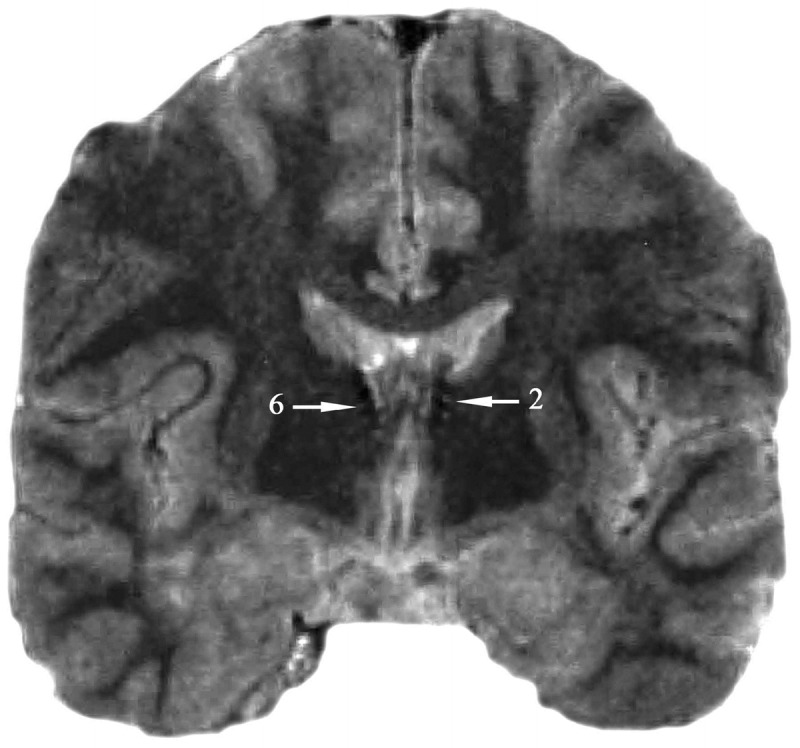
Deep brain stimulation: anterior nucleus of the thalamus. Sample T2-weighted MRI of deep brain stimulation leads placed bilaterally into the anterior nucleus of the thalamus. Contact 6 is located on the electrode placed in the right anterior thalamic nucleus, and contact 2 is located on the electrode placed in the left anterior thalamic nucleus.
DBS: Multicenter randomized controlled trial. The pivotal DBS trial was designed to provide randomized controlled evidence of the safety and efficacy of DBS for epilepsy.9 One hundred nine subjects aged 18 to 65 with six or more simple, complex, or generalized tonic-clonic seizures were included for study.9 The subjects were highly refractory, averaged 19 seizures per month, and had an average duration of epilepsy of 22 years. Prior treatment with VNS had failed in 45% of the subjects.9 Subjects who met inclusion and exclusion criteria entered a 3-month baseline period, after which they underwent implantation, were randomized to receive active treatment or no stimulation (placebo), and then entered a 3-month double-blind treatment period. During the double-blind treatment period, active-treatment patients received the following stimulation settings (output is set in voltage, not current): output = 5 V, pulse-width = 90 μs, stimulation frequency = 145 Hz, on time = 1 minute, off time = 5 minutes. At the end of the double-blind period, all subjects were treated at these settings for at least 3 months, and then parameters could be titrated upward to either 185 Hz or 7.5 V. An intermittent on/off cycle was utilized to preserve battery life (similar to the VNS study), with stimulation at 1 minute on and 5 minutes off.9
DBS was well tolerated.9 Depression (14.8% in the treatment group versus 1.8% in the control group) and memory impairment (13% in the active-treatment group versus 1.8% in controls) were the most common adverse events and occurred significantly more often in the treatment group compared with controls.9 Asymptomatic hemorrhage occurred in 4.5% of subjects overall, but no disability or death was attributed to the hemorrhages.9 Device infection occurred in 12.7% (n = 14) of all subjects, leading to hardware removal in nine subjects but reimplantation in three.9 Death occurred in 4.5% of subjects with a mean follow-up of 3 years (n = 5), primarily due to sudden unexpected death in epilepsy (n = 3), one before implantation; drowning (n = 1); and suicide (n = 1).9 No patient died directly because of the device, and none died during the acute treatment period.9
During the DBS pivotal trial, efficacy tended to increase from 1 month to 3 months for the active-treatment group. The median percent reduction in seizures improved from 22% postoperatively to 33.9% at 1 month, 42.1% at 2 months, and 40.4% at the end of the double-blind period. For the entire acute study period, the median reduction in seizures was 38.8% for the treatment group versus 22.8% for the sham control group.9 In the last month of the blinded phase, a median seizure reduction of 40% was achieved for the treatment group, compared to 14% for the control group. Interestingly, efficacy depended largely on the region of seizure origin. Temporal lobe seizures responded best to DBS.9 Subjects with temporal lobe epilepsy experienced a 44% reduction in seizures in the active treatment group versus 22% in controls, while there was no significant difference between the two groups in the frequency of seizures originating in the frontal, parietal, or occipital regions.9 The authors speculate that DBS may have a greater effect on seizures of temporal origin because of the role of the anterior nucleus role in modulating outflow from the amygdala and hippocampus via the Papez circuit.9
Responsive Neurostimulation
Penfield first demonstrated that stimulation of electrodes placed over the cerebral cortex during electrocorticography could abort electrical seizure activity. Since then, it has become widely reported in both animals and humans that electrical stimulation can abort electrical and clinical seizures. The NeuroPace Responsive Neurostimulation System consists of a pulse generator, seizure detection software, and recording and stimulating intracranial electrodes.11,23,24 The depth or subdural electrodes are placed in close proximity to the seizure focus, which must be clearly defined before implantation by surface or depth electrode video-EEG evaluation. Unlike other systems, which regularly deliver preprogrammed stimulation designed primarily to prevent seizures, the NeuroPace RNS system works by detecting a seizure and then delivering a stimulus designed to disrupt and terminate the electrical component of the seizure at its point of origin (Figure 10-2).11,23,24
Figure 10-2.
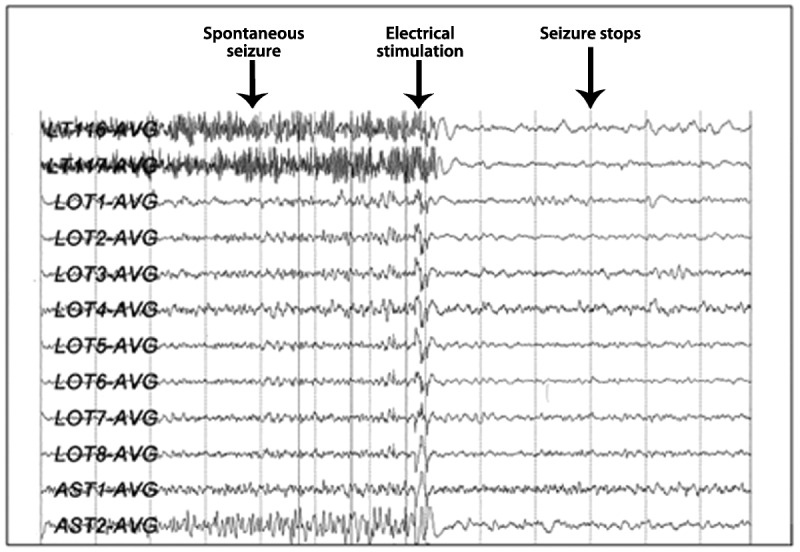
Stimulation with responsive neurostimulation showing rapid termination of a seizure with an electrical pulse. Reprinted with permission from Martha Morrell, MD, FAAN, NeuroPace, Inc.
The RNS system is currently an investigational device (approval pending before FDA). The device is implanted by an experienced team, which determines that the epileptic focus is either bilateral or cannot be safely resected surgically (eg, eloquent cortex or motor cortex). Key to successful implantation is identification of one or two seizure foci on surface or intracranial video-EEG monitoring. In the pivotal trial of RNS, 59% of subjects underwent prior intracranial video-EEG monitoring, and 41% underwent surface EEG monitoring exclusively, indicating that many candidates can be identified using surface video-EEG telemetry.11
In 2011, the results of the randomized double-blind controlled clinical trial of RNS were reported.11 The 240 subjects enrolled were aged 18 to 70 with three or more disabling partial seizures per month (ie, simple partial, complex partial, or secondarily generalized seizures).11 Of these, 191 subjects were implanted with an RNS device. Of all subjects, 50% had temporal lobe epilepsy, 55% had two discrete electrical foci, and 34% had undergone prior VNS; 97 subjects were randomized and implanted with active RNS treatment, and 94 subjects were randomized to sham RNS.11 Sham subjects were all implanted with an RNS device, but the device was not activated. Subjects first entered a 3-month pretreatment baseline and were subsequently implanted with the RNS device. After a 4-week perioperative period, subjects underwent a 4-week titration of stimulation and stimulus optimization, followed by a 12-week double-blind treatment period. The results are summarized in Figure 10-3.11 For the sham group, the initial percent reduction in seizure frequency was 25% at month 1. This reduction was transient, however, and the percent reduction progressively deteriorated to 17% at month 2 and 9% at month 3.11 In contrast, the active-treatment group demonstrated a steady and significant improvement in seizures, from a reduction of 34% at month 1, to 39% at month 2, and to 41.5% at month 3.11 The between-group improvements for active versus sham were significant at month 2 and month 3. The total seizure reduction for the entire treatment period was 37.9% for the active-treatment group versus 17.3% for the sham group.11 Overall, the between-group changes in seizure frequency met the pre hoc primary outcome measure for change in seizures in the 3-month double-blind treatment period and were significant at the 0.01 level (see Figure 10-3).11 The results from this trial are currently being evaluated by the FDA as part of the application for approval of the NeuroPace RNS system for use in patients with epilepsy in the United States.
Figure 10-3.
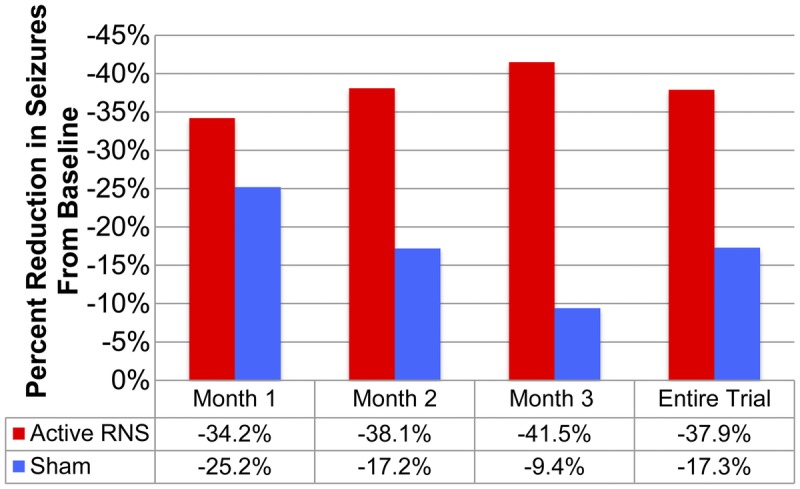
Responsive NeuroStimulation. Active versus sham stimulation during the pivotal randomized controlled trial.RNS = responsive neurostimulation.Data from Morrell MJ, Neurology.11 www.neurology.org/content/77/13/1295.short?sid=56ee15ee-e3c1-457f-9776-95b26cd33fe8.
Trigeminal Nerve Stimulation
TNS is the newest neuromodulation device for epilepsy. ETNS received a CE mark in September 2012 and is approved for adults and children aged 9 and older as adjunctive therapy for either epilepsy or depression in the European Union. In the United States, phase I and phase II clinical trials have been completed, and the manufacturer is in the process of submitting an investigational device exemption to the FDA for a pivotal trial.10,253
Basic science and mechanism of action. The trigeminal nerve is the largest cranial nerve, which projects to key brainstem structures associated with inhibition of seizures (ie, nucleus tractus solitarius, vagus nerve nuclei), and the thalamus and cerebral cortex, both involved with the regulation and genesis of seizures.10,16,26–29 The trigeminal nerve also projects to important structures related to mood and attention, specifically the medial frontal cortex and cingulate gyrus.27 Animal data indicate that stimulation of the trigeminal nerve and its related structures inhibit seizures.30 In a series of studies, a rat pentylenetetrazole seizure model was used to demonstrate that infraorbital TNS results in reductions in seizure activity.30,31 These studies concluded that the magnitude of the seizure-reduction effect increases as the amplitude and frequency of stimulation increase, and that bilateral stimulation is more effective than unilateral stimulation.30,31 More recently, Fanselow and colleagues measured cortical local field potentials with unilateral stimulation of the trigeminal nerve in a rat model and confirmed that TNS results in direct inhibition of pyramidal cortical neurons contralateral to the side of stimulation.31 This provides further support for the antiepileptic effect of TNS and may explain why bilateral stimulation is more effective than unilateral stimulation.30,31
In a pilot feasibility trial, 14 subjects with severe epilepsy were enrolled in an open-label feasibility study of eTNS.10 Subjects entered a 1-month pretreatment baseline and were thereafter treated with eTNS for a minimum of 12 hours daily; they were allowed to control the amplitude of current delivered and were instructed to use the maximum comfortable level. ETNS was well tolerated during the initial 3-month treatment period.10 Side effects were generally mild and included skin irritation, tingling, forehead pressure, and headache. At 3 months, the mean seizure frequency was reduced by 66% (P=.05), and in long-term follow-up, five subjects experienced greater than 50% reduction at 6 and 12 months (responder rate of 42%, based on intent-to-treat analysis.10
Based on these positive results, a double-blind, randomized controlled trial of eTNS in 50 subjects with drug-resistant epilepsy was initiated.25 ETNS was well tolerated, and adverse events were generally mild. Headache (4%), anxiety (4%), and skin irritation (14%) were the most common side effects. Results of the responder analysis are shown in Figure 10-4. The responder rate, defined as the percentage of individual subjects who had a 50% or greater reduction in seizure frequency, increased significantly for the active-treatment group to 40.5% at 18 weeks. For the entire 18-week treatment period, the responder rate for the entire study was 30.2% for the treatment group versus 21.1% for the control group.25 Mood (as measured by the Beck Depression Inventory) demonstrated significant improvements within and between groups, with greater than 50% improvements in mood scores for the active treatment group.
Figure 10-4.
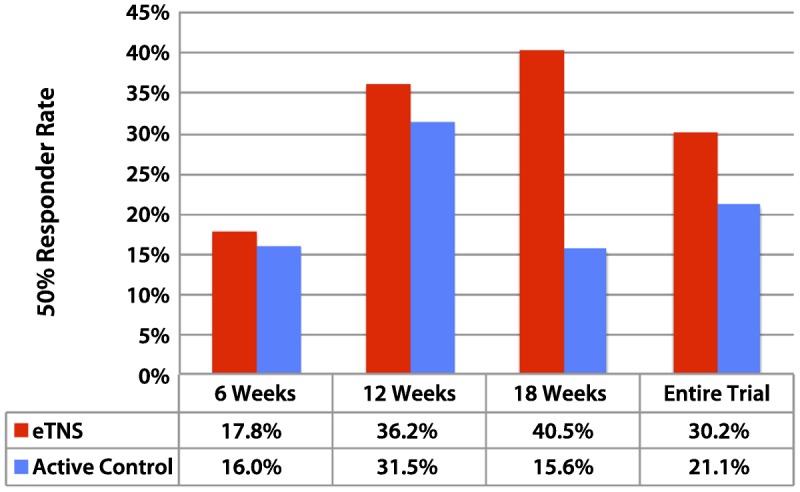
Trigeminal nerve stimulation: active treatment versus control. Randomized controlled trial in 50 subjects with drug-resistant epilepsy. Responder analysis after 18 weeks: 40.5% of patients in the treatment group were responders versus 15.6% in the active control (P=.078, general estimating equation [GEE]). The increase in responders within the treatment group from week 6 to week18 (the end of the trial) was highly significant (P=.01, GEE). eTNS = external trigeminal nerve stimulation.Data from DeGiorgio CM, et al, Neurology.25 www.neurology.org/content/80/9/786.short?sid=f1f4e081-f993-45ff-9c24-b7a28d4590f1.
Summary
As a group, these neurostimulation devices offer the neurologist an ever-evolving armamentarium to address the problem of drug-resistant epilepsy. Results from the key randomized clinical trials published to date for DBS, eTNS, RNS, and VNS show that the responder rate for each of these devices ranges from 23% to 30% for the acute double-blind trial period, and 35% to 43% at 1 year.32 These long-term responder rates reveal that the efficacy of neurostimulation generally tends to increase over time, which may be a function of long-term inhibition or potentiation of the key neural networks involved.
As new neurostimulation therapies come online in the European Union and the United States, it may be helpful to consider the relative advantages and disadvantages of each device. The role of these devices will evolve as physicians and epilepsy centers develop more experience. Noninvasive devices are currently approved for use only in the European Union but offer the advantage of low cost, safety, and the ability to assess efficacy without a surgical procedure. Implantable systems (ie, VNS, DBS, RNS) offer the advantage of 24-hour stimulation without the need for application of an external electrode or daily adjustment of an external pulse generator. VNS may be a good option in patients who are not good resection surgery candidates (poorly localized epilepsy). VNS can be implanted in an outpatient procedure without the risk of intracranial hemorrhage or infection. DBS (currently approved in the European Union only) is useful when the seizure focus is bilateral or poorly defined, but it carries the risk of intracranial hemorrhage and infection. RNS has similar risks to DBS but offers significant flexibility and the ability to stimulate bilaterally or over eloquent or motor cortex.
The online tool at www.epilepsycases.com can help the practicing physician determine whether the patient is a resective surgery candidate.
CONCLUSION
New devices for drug-resistant epilepsy are rapidly evolving and offer physicians and patients new options. Both noninvasive and invasive devices are now available in the European Union. While VNS is the only device currently approved in the United States, applications for approval of DBS and RNS are pending before the FDA.
KEY POINTS
Failure of the first or second antiepileptic drug in a patient is a predictor of drug-resistant epilepsy.
Physicians can go to www.epilepsycases.com to help them determine whether the patient is a candidate for epilepsy surgery.
Deep brain stimulation of the anterior thalamus is approved in the European Union for epilepsy. It is not yet approved in the United States.
Deep brain stimulation of the anterior thalamus and responsive neurostimulation are awaiting approval by the US Food and Drug Administration.
Deep brain stimulation of the anterior thalamus, external trigeminal nerve stimulation, transcutaneous vagus nerve stimulation, and vagus nerve stimulation are approved for the treatment of epilepsy.
Vagus nerve stimulation is the first device for epilepsy approved by the US Food and Drug Administration; it is also approved by the European Union.
Vagus nerve stimulation is generally well tolerated. Serious side effects (eg, vocal cord paralysis, device infection) occur in 1% to 1.5% of patients.
Vagus nerve stimulation is associated with a long-term responder rate of approximately 35%.
Side effects of deep brain stimulation of the anterior thalamus include depression (14.8%), memory problems (13%), hemorrhage (4.5%), and infection (12.7%).
No deaths due to hemorrhage or infection occurred in the pivotal trial of deep brain stimulation of the anterior thalamus.
In a phase III randomized controlled trial of deep brain stimulation of the anterior thalamus, the active-treatment group experienced a 38.8% reduction in seizures versus 22.8% in the control group.
In the phase III randomized controlled trial of responsive neurostimulation, the treatment group had a 37.9% reduction in seizures versus 17.3% seizure reduction in controls.
External trigeminal nerve stimulation is approved in the European Union for adults and children aged 9 and older with epilepsy and depression.
External trigeminal nerve stimulation is not approved and is investigational in the United States.
Side effects of external trigeminal nerve stimulation include skin irritation, headache, and anxiety.
In a phase II randomized controlled trial of external trigeminal nerve stimulation, the responder rate was 30.2% overall.
REFERENCES
- 1. Kwan P, Schachter S, Brodie M. Drug-resistant epilepsy. N Engl J Med 2011; 365 (10): 919– 926. [DOI] [PubMed] [Google Scholar]
- 2. Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med 2000; 342 (5): 314– 319. [DOI] [PubMed] [Google Scholar]
- 3. Weibe S, Blume WT, Girvin JP, et al. A randomized, controlled trial of surgery for temporal lobe epilepsy. N Engl J Med 2001; 345 (5): 311– 318. [DOI] [PubMed] [Google Scholar]
- 4. Englot DJ, Ouyang D, Garcia PA, et al. Epilepsy surgery trends in the United States, 1990-2008. Neurology 2012; 78 (16): 1200– 1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Canadian Appropriateness Study of Epilepsy Surgery. C.A.S.E.S. web site. www.epilepsycases.com. Accessed January 14, 2013.
- 6.The Vagus Nerve Stimulation Study Group. A randomized controlled trial of chronic vagus nerve stimulation for treatment of medically intractable seizures. Neurology 1995; 45 (2): 224– 230. [DOI] [PubMed] [Google Scholar]
- 7. Handforth A, DeGiorgio C, Schachter S. Vagus nerve stimulation therapy for partial-onset seizures: a randomized active-control trial. Neurology 1998; 51 (1): 48– 55. [DOI] [PubMed] [Google Scholar]
- 8. DeGiorgio CM, Schachter SC, Handforth A, et al. Prospective long-term study of vagus nerve stimulation for the treatment of refractory seizures. Epilepsia 2000; 41 (9): 1195– 2000. [DOI] [PubMed] [Google Scholar]
- 9. Fisher R, Salanova V, Witt T, et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia 2010; 51 (5): 899– 908. [DOI] [PubMed] [Google Scholar]
- 10. DeGiorgio C, Murray D, Markovic D, Whitehurst T. Trigeminal nerve stimulation for epilepsy: long-term feasibility and efficacy. Neurology 2009; 72 (10): 936– 938. [DOI] [PubMed] [Google Scholar]
- 11. Morrell MJ. RNS System in Epilepsy Study Group. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology 2011; 77 (13): 1295– 1304. [DOI] [PubMed] [Google Scholar]
- 12. Berthoud H, Neuhuber W. functional and chemical anatomy of the afferent vagal system. Auton Neurosci 2000; 85 (1–3): 1– 17. [DOI] [PubMed] [Google Scholar]
- 13. Gilman S, Newman S. Essentials of clinical neuroanatomy and neurophysiology. 10th ed Philadelphia, PA: F.A. Davis Company, 2003. [Google Scholar]
- 14.Cyberonics announces 100,000th patient implant of VNS therapy, ir.cyberonics.com/releasedetail.cfm?ReleaseID=728198. Published December 20, 2012. Accessed February 20, 2013.
- 15. Heck C, Helmers SL, DeGiorgio CM. Vagus nerve stimulation therapy, epilepsy and device parameters: scientific basis and recommendations for use. Neurology. 2002; 59 (6 suppl 4): S31– S37. [DOI] [PubMed] [Google Scholar]
- 16. Stefan H, Kreiselmeyer G, Kerling F, et al. Transcutaneous vagus nerve stimulation (t-VNS) in pharmacoresistant epilepsies: a proof of concept trial. Epilepsia 2012; 53 (7): e115– e118. [DOI] [PubMed] [Google Scholar]
- 17. Zhong XL, Lv KR, Zhang Q, et al. Low-frequency stimulation of bilateral anterior nucleus of thalamus inhibits amygdale-kindled seizures in rats. Brain Res Bull 2011; 86 (5-6): 422– 427. [DOI] [PubMed] [Google Scholar]
- 18. Kusske JA, Ojemann GA, Ward AA., Jr Effects of lesions in ventral anterior thalamus on experimental focal epilepsy. Exp Neurol 1972; 34 (2): 279– 290. [DOI] [PubMed] [Google Scholar]
- 19. Mirski MA, Ferrendelli JA. Anterior thalamic medication of generalized pentylenetetrazole seizures. Brain Res 1986; 399 (2): 212– 223. [DOI] [PubMed] [Google Scholar]
- 20. Mirski MA, Fisher RS. Electrical stimulation of the mammillary nuclei increases seizure threshold to pentylenetetrazole in rats. Epilepsia 1994; 35 (6): 1309– 1316. [DOI] [PubMed] [Google Scholar]
- 21. Mirski MA, Rossell LA, Terry JB, Fisher RS. Anticonvulsant effect of anterior thalamic high frequency electrical stimulation in the rat. Epilepsy Res 1997; 28 (2): 89– 100. [DOI] [PubMed] [Google Scholar]
- 22. Kerrigan JF, Litt B, Fisher RS, et al. Electrical stimulation of the anterior nucleus of the thalamus for the treatment of intractable epilepsy. Epilepsia 2004; 45 (4): 346– 354. [DOI] [PubMed] [Google Scholar]
- 23. Skarpaas TL, Morrell MJ. Intracranial stimulation therapy for epilepsy. Neurotherapeutics 2009; 6 (2): 238– 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Morrell M. Brain stimulation for epilepsy: can scheduled or responsive neurostimulation stop seizures? Curr Opin Neurol 2006; 19 (2): 164– 168. [DOI] [PubMed] [Google Scholar]
- 25. DeGiorgio C, Soss J, Cook I. Randomized controlled trial of trigeminal nerve stimulation for drug-resistant epilepsy. Neurology 2013;80:786–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Caous CA, de Sousa Buck H, Lindsey CJ. Neuronal connections of the paratrigeminal nucleus: a topographic analysis of neurons projecting to bulbar, pontine and thalamic nuclei related to cardiovascular, respiratory and sensory functions. Auton Neurosci 2001; 94 (1–2): 14– 24. [DOI] [PubMed] [Google Scholar]
- 27. Iwata K, Miyachi S, Imanishi M, et al. Ascending multisynaptic pathways from the trigeminal ganglion to the anterior cingulate cortex. Exp Neurol 2011; 227 (1): 69– 78. [DOI] [PubMed] [Google Scholar]
- 28. Haines D. Neuroanatomy: an atlas of structures, sections, and systems. 8th ed, Philadelphia, PA: Lippincott Williams & Wilkins; 2012. [Google Scholar]
- 29. Schaller B, Cornelius JF, Prabhakar H, et al. The trigemino-cardiac reflex: an update of the current knowledge. J Neurosurg Anesthesiol 2009; 21 (3): 187– 195. [DOI] [PubMed] [Google Scholar]
- 30. Fanselow EE, Reid A, Nicolelis AL. Reduction of pentylenetetrazole induced seizure activity in awake rats by seizure-triggered trigeminal nerve stimulation. J Neuroscience 2000; 20 (21): 8160– 8168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fanselow E. Central mechanisms of cranial nerve stimulation for epilepsy. Surg Neurol Int 2012; 3 (suppl 4): S247– S254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rolston JD, Englot DJ, Wang DD, et al. Comparison of seizure control outcomes and the safety of vagus nerve, thalamic deep brain, and responsive neurostimulation: evidence from randomized controlled trials. Neurosurg Focus 2012; 32 (3): E14. [DOI] [PubMed] [Google Scholar]