Abstract
Some evidence suggests that the cerebellum participates in the complex network processing emotional facial expression. To evaluate the role of the cerebellum in recognizing facial expressions we delivered transcranial direct current stimulation (tDCS) over the cerebellum and prefrontal cortex. A facial emotion recognition task was administered to 21 healthy subjects before and after cerebellar tDCS; we also tested subjects with a visual attention task and a visual analogue scale (VAS) for mood.
Anodal and cathodal cerebellar tDCS both significantly enhanced sensory processing in response to negative facial expressions (anodal tDCS, p=0.0021; cathodal tDCS, p= 0.018), but left positive emotion and neutral facial expressions unchanged (p>0.05). tDCS over the right prefrontal cortex left facial expressions of both negative and positive emotion unchanged.
These findings suggest that the cerebellum is specifically involved in processing facial expressions of negative emotion.
Keywords: Cerebellum, Cerebellar Dysfunction, Prefrontal Cortex, Emotion, Facial Emotion Recognition, Emotional Disturbances, tDCS
INTRODUCTION
Faces are multi-dimensional stimuli conveying many important signals simultaneously, each one having a complex social and motivational meaning. Besides providing distinctive information about a person’s identity, gender, or age, faces also provide more subtle signals related to emotion, trustworthiness, attractiveness, as well as gaze direction or other people’s intentions. Facial expressions of emotions, such as happiness, sadness, anger and fear are universally recognized (Ekman, 1992; Izard, 1994). Each of these expressions has a meaning and targets a specific response (Halberstadt & Niedenthal, 1997). The ability to recognize the emotional content of a specific facial expression is one of the major communication skills in humans and non-human primates. Despite intensive research in this field, considerable debate surrounds how these various dimensions are coded and integrated into a single face percept.
Strong evidence from animal studies shows that facial expressions evoke brain responses in individual neurons (Leonard, Rolls, Wilson, & Baylis, 1985), and behavioral data from brain lesioned patients, and functional neuroimaging studies with positron-emission tomography (PET) and functional magnetic resonance imaging (fMRI) in healthy humans show localized cortical responses (Streit et al., 1999). Facial perception primarily entails recognizing specific patterns and facial structures (those that help defining gender and identity) and dynamic patterns of facial expression (identifying facial expressions such as anger, happiness, sadness, and disgust). The brain processes information for each of these facial perception features through specific neural networks. Behavioral and clinical data show that recognition of specific facial expressions engages various brain structures and therefore involves separate neural systems (Adolphs, 2002; Kesler-West et al., 2001; Loughead, Gur, Elliott, & Gur, 2008). The first processed information is delivered and integrated in a common circuitry in frontal areas. Among the multiple structures that participate in recognizing facial emotions are the occipitotemporal cortex, amygdala, orbitofrontal cortex, basal ganglia, right parietal cortex, left dorsolateral prefrontal cortex, superior temporal sulcus, medial temporal gyrus (Hennenlotter & Schroeder, 2006; Posamentier & Abdi, 2003) and, possibly, cerebellum (Stoodley & Schmahmann, 2009).
Because these structures engage in multiple processes at various points in time, assigning a single function to a specific brain structure is a difficult task. The available neuroimaging data leave unanswered the question whether the cerebellum’s contribution to emotional expression recognition or whether cerebellar activation is just a non-causal epiphenomenon. Some hypotheses about cerebral network phylogenetics suggest that positive and negative emotions relay though different circuits. Because the ability to appreciate positive emotions requires more sophisticated processing of individually personalized stimuli and has features resembling a “higher” cortical process, detecting pleasant features arguably relies on phylogenetically newer circuits that widely involve the prefrontal cortex and cortical executive system (Paradiso et al. 1999). Negative emotions, such as sadness and anger, being crucial for survival and serving to prepare the organism for rapid defense, as part of a defense system designed to protect the organism against threats to acquire valuable resources, activate phylogenetically older circuits including the cerebellum (Stoodley & Schmahmann, 2009).
The concept that the cerebellum intervenes in regulating emotions and mood has gained popularity since the 1970s (Heath, Dempesy, Fontana, & Myers, 1978; Turner et al., 2007). The first study linking the cerebellum to emotions reported the case of a patient who underwent electrical stimulation of the dentate nucleus and superior peduncle and reported experiencing negative feelings (Nashold & Slaughter, 1969). Others then showed that chronic electrical stimulation delivered to the superficial parts of the vermis normalized behavior in severely emotionally disturbed patients (Heath, 1977). Clinical studies then yielded strong evidence for cerebellar abnormalities in emotional disorders such as depression (Schmahmann, 2004; Schmahmann & Sherman, 1998; Turner et al., 2007). More recent findings suggest that high-frequency repetitive transcranial magnetic stimulation (rTMS) to the cerebellum has mood-improving properties, a single session of high-frequency rTMS over the cerebellum benefitted mood in healthy volunteers (Schutter, van Honk, d’Alfonso, Peper, & Panksepp, 2003).
A neuromodulatory tool used for manipulating neural activity thus gathering insights into the cognitive role of the human cerebellum is transcranial direct current stimulation (tDCS) (Ferrucci et al., 2008). tDCS is a simple, inexpensive, non-invasive technique for brain stimulation that induces prolonged functional changes in cerebral cortex (Nitsche & Paulus, 2000; Priori, 2003) and cerebellar cortex (Ferrucci et al., 2008; Galea, Jayaram, Ajagbe, & Celnik, 2009). tDCS essentially consists in delivering a weak direct current for a few minutes over the scalp: the resulting constant electric field penetrates the skull and influences neuronal function. Whereas cathodal tDCS suppresses neuronal function, anodal tDCS increases neuronal function in the underlying cerebral cortex. The prolonged after-effects induced by tDCS probably reflect synaptic (Liebetanz et al. 2002) and non synaptic effects (Ardolino et al. 2005). The changes in cortical excitability induced by tDCS (increase or decrease) lead to corresponding changes in cortical function and activation. In our previous study we showed that cerebellar tDCS modulates performance in a working memory task in healthy subjects (Ferrucci et al., 2008). A subsequent study using a different approach confirmed the effects induced by cerebellar tDCS changes in motor cortical excitability induced by cerebellar TMS (Galea et al., 2009). No study has to our knowledge investigated whether and how cerebellar tDCS modulates the emotional information processing required to recognize facial expressions. Given the cerebellum’s known role in psychiatric illnesses (Andreasen et al. 1996), knowing more about tDCS-induced changes in emotional processing would help in developing new therapeutic applications for tDCS.
Our primary aim in this tDCS study addressing the role of the cerebellum in processing emotional information was to investigate whether the cerebellum intervenes directly in the recognition of facial expression. To do so, in healthy adult volunteers, before cerebellar tDCS began and at 35 min after it ended, we tested recognition of emotion with a facial expression recognition task. To investigate whether the effects of cerebellar tDCS were specific for emotion recognition or reflected changes in arousal or attention, we further tested all subjects before and after cerebellar tDCS with a visual attention task. To check whether tDCS influences mood, we also tested subjects with a 100-mm visual analog scale (VAS) for mood. In additional control experiments, we then investigated the specificity of changes in emotional recognition by delivering tDCS over the right prefrontal cortex.
MATERIALS and METHODS
Participants
Twenty-one healthy right-handed volunteers (aged 20–49 years; 12 women-9 men) participated in the study. All subjects were right-handed (assessed by the Edinburgh Handedness Inventory). All participants gave their informed consent and the procedures had the approval of the hospital ethical committee. The experimental procedure was in accordance with the Declaration of Helsinki. Before and after tDCS, all participants underwent a neurological examination using paper-and-pencil tests of motor-graphics (signature, Archimedes spiral, and horizontal lines test). Subjects had no history of medical, neurological, or psychiatric disorders and none of them were receiving acute or chronic medication affecting the central nervous system.
Facial Emotion Recognition Task
We used 64 facial expressions from the NimStim Face Stimulus Set (www.macbrain.org), these faces consisted of sixteen Caucasian adults (eight men and eight women) expressing anger, happiness, sadness and neutral expression. We generated two alternative sets of pictures consisting of 32 trials (eight faces, four men and four women), the pictures were presented in a random order and each facial expression was shown three times, making a total of 96 trials (24 for each emotion category). Stimulus presentation, timing and data collection were controlled by the E-prime (Psychology Software Tools, Pittsburg, PA) program running on a laptop computer. The stimuli were presented on a computer screen: the software first presented a fixation signal (“+”) in the middle of the screen for 100 ms, followed immediately by a picture of a facial expression, face stimuli were presented for a maximum of 1300 ms and when subjects pressed a key the picture disappeared. Subjects were asked to judge which of the four facial expressions emotions the picture represented (happiness, sadness, anger, or neutral) by pressing the appropriate button on a keypad as quickly and accurately as possible. The subjects were tested individually in a quiet room. They sat in front of a computer screen and the task session lasted 10 min. Reaction times (RTs) and accuracy (number of incorrect responses) were collected and used for further analysis.
Visual Analogue Scale (VAS)
Before the facial expression recognition test, the subjects completed a VAS comprising self-evaluation scales ranging from 0 to 100 designed to assess 2 different mood domains. Each VAS consisted of a horizontal line, 100 mm in length, anchored at each end by word descriptors. The subject marked on the line the point they felt best represented how they perceived their current state. The VAS score was calculated by measuring in millimeters the distance from the left-hand end of the line to the point that the patient marked. We used a VAS for anxiety (0 mm no anxiety and 100 mm the worst anxiety ever) and for mood (0 mm the worst mood and 100 mm the best mood ever).
Visual Attention Task
To investigate whether the effects of tDCS were specific for emotion recognition or reflected changes in arousal or attention, we tested all subjects again before and after cerebellar and prefrontal tDCS with a visual attention task. We used an endogenous cue version of the Posner paradigm (Posner, 1980) for studying attention using a computer-controlled procedure (Wadsworth Publishing, Belmont, CA, USA). In this task, the patients responded to targets that appeared at one of two locations on either side of the fixation mark. Before the target appeared, one of these locations was cued so that subjects focused their attention on this location. The experimental procedure for studying attention required subjects to direct their attention to the type of cue presented: for valid cues, the target appeared on the same side as that indicated by an arrow; for invalid cues, the target appeared on the side opposite to that indicated by an arrow; for neutral cues, the target appeared without a preceding arrow. The control task was a detection task in which every grating stimulus required a right index finger response, independent of its spatial frequency or location. Subjects were instructed to respond quickly and accurately and to maintain central eye fixation during the trials. The total reaction times (RTs) for valid plus invalid and neutral were collected and used for further analysis.
Cerebellar and prefrontal transcranial direct current stimulation
Cerebellar tDCS was delivered by an electrical constant direct current stimulator connected to a pair of a rectangular saline-soaked synthetic sponge electrodes (6×7cm). The active electrode was centered on the median line 2 cm below the inion with its lateral borders about 1 cm medially to the mastoid apophisis (over the cerebellum) and the reference electrode over the right deltoid muscle. At the scalp site where we delivered tDCS the cerebellum is more superficial than, for instance, the motor cortex below the fronto-parietal bone because the occipital bone is thinner than the parietal bone (Axelsson et al., 2005). For prefrontal cortex tDCS, electrodes were placed one over the right prefrontal cortex (between Fp2 and F4) and the other over the right deltoid muscle.
The stimulating current was an anodal or cathodal direct current at 2 mA intensity (current density: 0.06 mA/cm2) delivered for 20 minutes over the cerebellum or the right prefrontal cortex.
After a short-lasting and mild itching sensation at both electrodes in the first 10/20 seconds of stimulation, subjects perceive no other sensation. Because for sham tDCS electrodes were placed as for real stimulation and the stimulator was turned on for 10 to 15 sec the subjects felt the initial itching sensation, as they did during anodal or cathodal tDCS, but thereafter received no current. To avoid confounding biases arising from two electrodes with opposite polarities over the scalp, we used a non-cephalic reference electrode (Ferrucci et al 2008)
Experimental Protocol
The cerebellar tDCS session was always conducted before the prefrontal session, and at least 1 month elapsed between the two sessions. Both sessions tested the same protocol. During the cerebellar and the prefrontal tDCS sessions, subjects were studied three times, once for each stimulation type (anodal, cathodal, sham). One week elapsed between anodal, cathodal and sham stimulation. Stimulations were applied in random order (for instance, 1st week anodal, 2nd week cathodal, 3rd week sham) balanced across subjects, and for each stimulation session subjects performed the task before cerebellar tDCS stimulation and 35 min after it ended. The order of cerebellar or prefrontal tDCS and exposure to the task were counterbalanced. The subjects were blind to the type of tDCS delivered in each session.
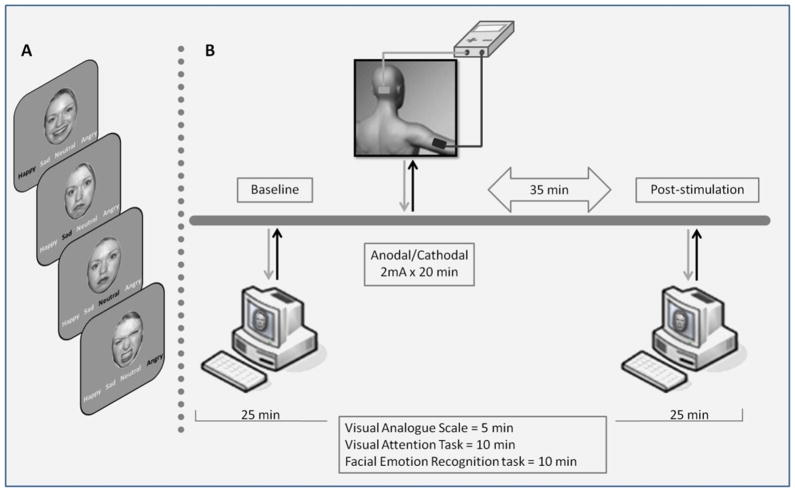
For each experimental session we administered the VAS, visual attention task and recognition task, twice in all subjects, keeping a constant order for all tasks: at baseline and 35 minutes after tDCS ended (post-stimulation) (Fig. 1). The picture set used at baseline differed from the set used for the post-stimulation evaluation, the two sets were randomized between subjects and conditions.
Figure 1.
Experimental protocol. A. Examples of facial stimuli for each of the four facial emotion expressions. B. Schematic diagram of the experimental design during a single session.
Because our previous study showed that the major effect became evident 35 min after cerebellar tDCS ended, we used this time delay for studying the role of the cerebellum in recognizing facial expressions.
The whole session lasted about 90 min, and testing lasted about 25 min (5 min VAS; 10 min visual attention task; and 10 min visual recognition task, always presented in this order).
Statistical Analysis
Because our objective was to evaluate whether the tDCS influences facial emotion recognition processes, presuming that each emotion activates different neuronal circuits (Adolphs, 2002; Kesler-West et al., 2001; Loughead, Gur, Elliott, & Gur, 2008), our analyses tested negative emotions (anger and sadness), positive emotion (happiness) and neutral emotion (neutral). RTs data for negative emotions were tested with a preliminary three-way repeated measures analysis of variance (ANOVA) with main factors “emotion” two levels (anger and sadness); “stimulation” (three levels: anodal, cathodal, and sham) and “time” (two levels: baseline, post-stimulation). The results of this preliminary analysis were used to confirm the correctness of our assumptions.
When analyzing the data for negative emotions we pooled the data for anger and sadness and we tested mean RT values for correct answers and the accuracy (i.e., the number of answer errors).
First, we investigated possible differences between negative, positive and neutral emotions at baseline, analyzing RTs for correct answers and accuracy with a two-way ANOVA, main factors “stimulation” (three levels: anodal, cathodal and sham) and “emotion” (three levels: negative, positive emotions and neutral).
To study whether tDCS influences RTs for correct answers and accuracy we ran a two-way ANOVA for repeated measure with factors “stimulation” (three levels: anodal, cathodal, and sham) and “time” (two levels: baseline, post-stimulation) for negative, positive emotions and neutral.
Baseline data for anxiety and mood visual analogue scales (VASs) and for attention task were tested in a one-way ANOVA with main factors “stimulation” (three levels: anodal, cathodal and sham) for each emotion (negative, positive emotions and neutral). To evaluate changes in arousal, anxiety and mood, we calculated percentage (baseline = 100%) changes in the visual attention task and VAS, after tDCS as (post stimulation-baseline)/(baseline). To compare percentage changes across stimulation types we used a one-way repeated measures ANOVA (factor “stimulation”). Tukey honest significant test was used for post hoc analysis; differences were considered significant at p<0.05.
The same analyses were used for the control experiments to investigate the specificity of changes in emotional recognition by delivering tDCS over the right prefrontal cortex.
RESULTS
Cerebellar tDCS
Because the preliminary three-way ANOVA, performed on the negative emotions, showed that cerebellar tDCS had the same effect on RTs measured for anger and sadness (interaction “emotion” x “stimulation”, p>0.05) and no significant interaction “emotion” x “time” (p>0.05) in subsequent analysis, data for both types of negative emotions were pooled.
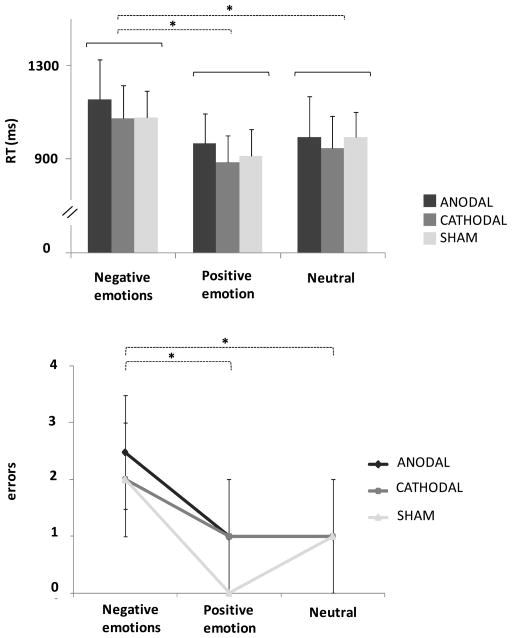
Significant differences were found between participants’ ability to recognize the various emotional expressions at baseline (ANOVA factor emotion, p<0.001), specifically, participants took longer to identify negative facial expressions (post hoc ANOVA, negative emotions vs. positive emotion p<0.001, negative emotions vs. neutral p<0.001) (Fig. 2A; Table 1). At baseline participants also differed in accuracy during the recognition task (ANOVA factor emotion, p<0.001); specifically, they achieved the worst accuracy in identifying negative facial expressions (post hoc ANOVA, negative emotions vs. positive emotion p<0.001, negative emotions vs. neutral p<0.001) (Fig. 2B). All participants achieved a high percentage of correct answers at baseline for all emotions (93%), suggesting that they remained attentive throughout the lengthy scanning session. The worse baseline performance, 83% of correct answers, allowed us to include all participants in the study.
Figure 2. Baseline analysis.
reaction times (RTs) and answer errors for each emotion.
(A) Histograms represent grand average (n=21) of reaction time (RT) for correct answers for each emotion (negative, positive and neutral) and cerebellar transcranial direct current stimulation (anodal cerebellar tDCS, cathodal cerebellar tDCS, sham cerebellar tDCS). y-axis: mean RT values (ms).
(B) Grand average (n=21) of answer errors for each emotion (negative, positive and neutral) and stimulation (anodal cerebellar tDCS, cathodal cerebellar tDCS and sham cerebellar tDCS). y-axis: mean number of errors. Error bars represent the 95% confidence interval of the estimated mean (1.96*standard error). * p<0.05, two-way analysis of variance (ANOVA). Note that, at baseline participants identified sadness with lower speed and worse accuracy than all other emotions. Note that no differences were found between sham cerebellar tDCS, anodal cerebellar tDCS and cathodal cerebellar tDCS in RTs and answer errors.
Table 1.
Data for reaction times (RTs) for the facial emotion recognition task, before and after cerebellar transcranial direct current stimulation (tDCS) and prefrontal tDCS. All values are means ± SEM.
| Cerebellar tDCS | Prefrontal tDCS | ||||
|---|---|---|---|---|---|
|
| |||||
| Total | Baseline | Post-stimulation | Baseline | Post-stimulation | |
| Stimulation | Emotion | RTs (msec) Mean (± SEM) |
RTs (msec) Mean (± SEM) |
RTs (msec) Mean (± SEM) |
RTs (msec) Mean (± SEM) |
| Anodal | Negative | 1156.69 (86.57) | 985.6657 (63.45) | 988.75 (113.60) | 989.00 (122.38) |
| Positive | 967.49 (60.86) | 904.2429 (68.18) | 821.21 (112.39) | 787.94 (88.09) | |
| Neutral | 994.999 (65.12) | 884.1529 (57.09) | 862.15 (83.83) | 903.03 (100.63) | |
| Cathodal | Negative | 1074.359 (72.32) | 935.12476 (52.58) | 966.78 (85.09) | 955.14 (67.88) |
| Positive | 887.82476 (59.06) | 822.7695 (43.77) | 846.26 (86.91) | 816.62 (93.90) | |
| Neutral | 946.621 (58.60) | 858.9486 (45.14) | 851.48 (74.25) | 835.20 (52.48) | |
| Sham | Negative | 1078.006 (45.14) | 1047.606 (61.87) | 1091.89 (96.34) | 1046.88 (100.02) |
| Positive | 915.3463 (57.92) | 852.039 (40.80) | 885.15 (80.22) | 841.19 (59.44) | |
| Neutral | 994.9933 (57.86) | 921.3181 (53.47) | 1060.24 (107.50) | 969.77 (96.60) | |
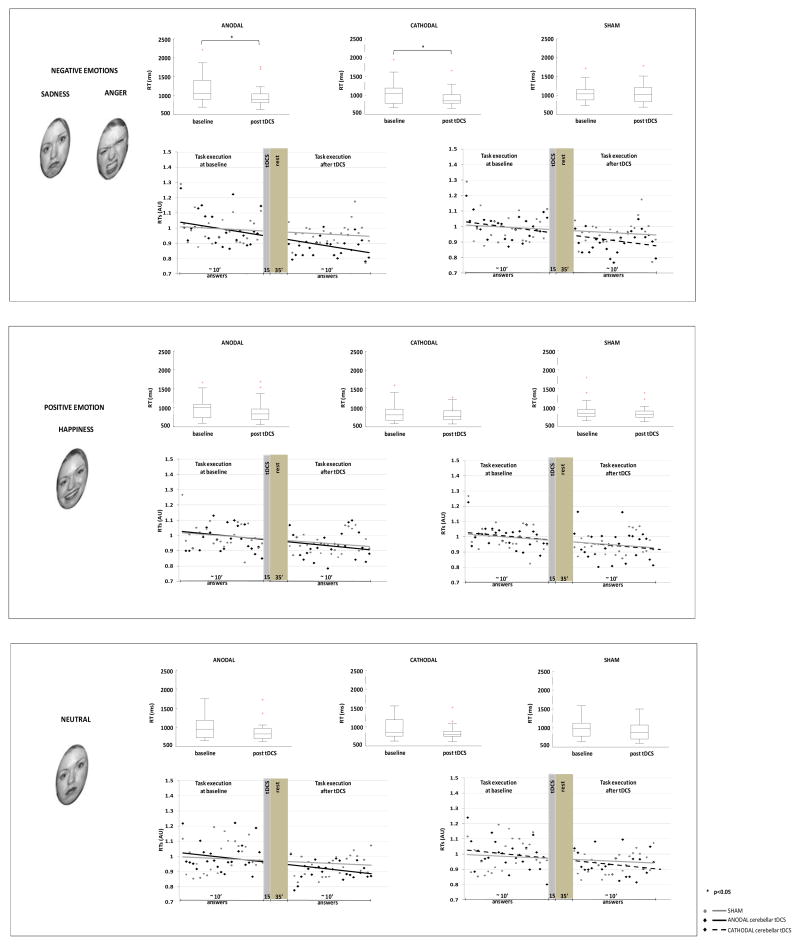
When we tested how cerebellar tDCS affected RTs for each emotion, we found that RTs decreased significantly for all emotions (ANOVA factor time: negative emotions, p<0.001; positive emotion, p<0.001; neutral, p<0.001), but only for negative emotions was the reduction related to cerebellar tDCS (ANOVA interaction “stimulation” x “time”, p<0.05). Post hoc analysis disclosed that anodal and cathodal tDCS both reduced baseline RTs (anodal tDCS, p<0.05; cathodal tDCS, p<0.05).
The data analysis testing the accuracy gave no changes for any of the emotions tested after cerebellar tDCS (p>0.05).
Similarly, ANOVA evaluating the 2-item VAS (assessing anxiety and mood) showed no differences between sham cerebellar tDCS, anodal cerebellar tDCS and cathodal cerebellar tDCS for both items (anxiety, p>0.05; mood, p>0.05). Nor was a difference found in baseline RTs (attention task) between the three stimulation procedures (p>0.05).
The visual attention task disclosed no significant cerebellar tDCS-induced changes. Nor did cathodal cerebellar tDCS or anodal cerebellar tDCS induce specific changes that differed significantly from those after sham cerebellar tDCS (p>0.05) (Table 2).
Table 2.
Data for reaction times (RTs) for the visual attention task, before and after cerebellar transcranial direct current stimulation (tDCS) and prefrontal tDCS. All values are means ± SEM.
| Cerebellar tDCS | Prefrontal tDCS | |||
|---|---|---|---|---|
|
| ||||
| Total | Baseline | Post-stimulation | Baseline | Post-stimulation |
| Stimulation | RTs (msec) Mean (± SEM) |
RTs (msec) Mean (± SEM) |
RTs (msec) Mean (± SEM) |
RTs (msec) Mean (± SEM) |
| Anodal | 358.3 (19.5) | 357.5 (21.5) | 292.6 (15.4) | 295.2 (15.21) |
| Cathodal | 334.6 (22.8) | 322.9 (24.8) | 322.2 (32.52) | 327.2 (31.79) |
| Sham | 338.9 (20.1) | 334.0 (19.6) | 326.0 (20.55) | 325.6 (22.56) |
Similarly, feelings of anxiety and mood as evaluated by VAS remained unchanged after cerebellar tDCS. ANOVA evaluating the 2-item VAS (assessing anxiety and mood) disclosed a non significant interaction (anxiety, p>0.05; mood, p>0.05) (Table 3).
Table 3.
Data for the two items on the visual analogue scale (VAS), before and after cerebellar transcranial direct current stimulation (tDCS) and prefrontal tDCS. All values are means ± SEM.
| Cerebellar tDCS | Prefrontal tDCS | ||||
|---|---|---|---|---|---|
|
| |||||
| Total | Baseline | Post-stimulation | Baseline | Post-stimulation | |
| Stimulation | Emotion | Mean (± SEM) | Mean (± SEM) | Mean (± SEM) | Mean (± SEM) |
| Anodal | Anxiety | 16.7 (5.2) | 9.6 (2.5) | 9.2 (3.7) | 10.5 (4.8) |
| Mood | 22.7 (5.5) | 23.1 (4.9) | 22.5 (8.08) | 24.25 (8.3) | |
| Cathodal | Anxiety | 11.9 (4.0) | 8.4 (3.0) | 12.8 (6.0) | 14.75 (6.5) |
| Mood | 25.0 (5.4) | 24.0 (5.5) | 18.25 (6.2) | 22.75 (7.4) | |
| Sham | Anxiety | 14.5 (4.1) | 11.4 (3.6) | 22.13 (7.2) | 18.00 (6.7) |
| Mood | 24.4 (4.9) | 25.4 (5.0) | 16.88 (5.2) | 15.13 (5.2) | |
Prefrontal tDCS
Additional control experiments to investigate the specificity in recognition of emotion changes by delivering tDCS over the right prefrontal cortex in 8 of the 20 subjects yielded similar mean RT values in all subjects (Table 1). ANOVA showed a significant difference between baseline RTs for emotion (p<0.001). Participants took longer to identify facial expressions than all other emotions (negative emotions vs. positive emotion p<0.001, negative emotions vs. neutral p<0.05). At baseline participants were less accurate in identifying negative facial expressions than the other facial expressions tested (negative emotions vs. positive emotion p<0.001, negative emotions vs. neutral p<0.05, positive emotion vs. neutral p<0.05).
When we tested the effect of prefrontal tDCS on RTs and answer accuracy we found no change in any of the emotions tested (p>0.05). Nor did the visual attention task disclose significant prefrontal tDCS-induced changes (p>0.05). Similarly, feelings of anxiety and mood as evaluated by VAS remained unchanged after prefrontal tDCS (anxiety p>0.05; mood, p>0.05).
DISCUSSION
Our main finding in this study in healthy subjects is that whereas anodal and cathodal cerebellar tDCS both significantly enhanced sensory processing in response to a negative facial expression, it left sensory processing for positive emotion (happiness) and neutral facial expressions unchanged. Another interesting finding is that whereas tDCS delivered over the cerebellum specifically affects recognition of negative facial emotion, tDCS over the right prefrontal cortex leaves it unchanged. This difference agrees with current knowledge that the neuronal circuits, at least those for the negative recognition system involve the cerebellum.
Our finding that tDCS over the cerebellum specifically enhanced the ability to recognize negative facial expression corroborates and extends current knowledge on the cerebellum’s role in emotional information processing (Schmahmann & Sherman, 1998; Schutter & van Honk, 2005; Turner et al., 2007). Our study, for example, implies that cerebellar tDCS could help to enhance awareness and behavioral responses toward emotionally relevant stimuli, especially those having some negative value.
The cerebellar tDCS-induced changes related to negative emotion we describe here therefore fit in well with previous research showing that negative events generally evoke stronger cognitive, emotional, and social responses than do neutral or positive events (Fox et al., 2000).
Our study therefore adds strength to previous proposals suggesting that the cerebellum belongs in a widespread network that determines the meaning of external stimuli and might also mediate facilitatory cortical processes. Recognizing facial expressions permits us to detect another person’s emotional state and provides cues on how to respond in these social situations. Facial expressions are central to non-verbal social exchange as markers of internal states and intentions (Schupp et al., 2004). For example, across cultures the internal state of anger is externally expressed as frowning brows, staring eyes, and a shut mouth with tense lips (Ekman & Friesen, 1975), which in turn signals readiness for a physical or symbolic attack on an observer. Because angry faces signal potential negative consequences to the observer, they are regarded as threatening.
Our healthy subjects’ ability to recognize a negative facial expression also fit in with current evolutionary knowledge suggesting that natural selection resulted in a propensity to react more strongly to negative than to positive stimuli (Fox et al., 2000). This heightened sensitivity to negative information, termed ‘negativity bias,’ is a reliable psychological phenomenon in adults (Morewedge, 2009). By allowing individuals to adapt to the environment it favors survival of the human species.
The cerebellar tDCS-induced changes in recognition of facial expressions we identified in healthy volunteers also fit in well with functional magnetic resonance imaging (fMRI) studies showing that whereas the emotional stimulus happiness activates the middle temporal gyrus, parahippocampal gyrus, hippocampus, claustrum, inferior parietal lobule, cuneus, middle frontal gyrus, inferior frontal gyrus, and anterior cingulate gyrus, a negative emotional stimulus activates the posterior cingulate, fusiform gyrus, and cerebellum (Park et al. 2008).
Given the cerebellum’s known role in emotional information processing, we attribute the specific cerebellar modulation for the recognition of an angry and sad face to the reciprocal cerebellar connections with the amygdala (Turner et al., 2007). After amygdala damage, the most consistently reported impaired emotional function is recognition of anger. Evidence from human lesion studies that damage to the amygdala bilaterally invariably leads to impaired recognition of emotional facial expressions implies that the amygdala is principally involved in processing stimuli related to threat and danger, that it triggers cognitive resources to help resolve ambiguity in the environment, or that the emotions whose recognition depends most on the amygdala are related to behavioral withdrawal (Adolphs, 2002). In line with the findings of Blair et al. (1999) we suggest the involvement of at least two dissociable, but interlocking systems in the processing of negative facial expressions. One system responds to facial stimuli (sad) involved in social conditions; the other system implicates regions involved in behavioral extinction by responding to angry facial expressions.
When we delivered cerebellar tDCS in healthy subjects we observed no changes in recognition of positive facial expressions. Conversely, Shutter and colleagues (2009) showed that high-frequency rTMS over the cerebellum elicited significant increases in masked emotional responses but only in those to happy facial expressions. The discrepancy between our results and those reported by Shutter et al. (2009) could depend on the different kinds of stimulation, different emotional task and different experimental protocol used. In agreement with another rTMS study by Shutter and colleagues (2009) we observed no changes in mood. The fact that the current rTMS findings showed the implicit level of information-processing coincides with the general idea that mood altering drugs such as antidepressants manifest their early effects on implicit and automatic features of information-processing rather than on the conscious experience of mood (Schutter & van Honk, 2009).
Our study may also help to explain impaired recognition of facial expression in mental illnesses such as depression, anxiety and schizophrenia. Emotional information processing comprises evaluative, experiential, and expressive components (Ekman, 1992; Izard, 1994). The evaluation of affect depends on an individual’s ability to identify the emotional valence conveyed by an event or an object and is influenced by mental illness. People with psychiatric disorders lose their ability to distinguish between pleasant and unpleasant experiences and the ability to assign the appropriate emotional valence to these experiences. For example, patients with depression tend to consider positive life events negative or harmful whereas patients with schizophrenia seem unable to extract from a situation or experience the emotional content needed to decide whether the experience is pleasurable or unpleasant. In a study applying TMS over the cerebellum, others provided support for dysfunctional cerebello-cortical connectivity in schizophrenia (Daskalakis, Christensen, Fitzgerald, Fountain, & Chen, 2005). In another study cerebellar TMS applied for 20 minutes in healthy volunteers modified EEG recordings over the prefrontal cortex and increased positive mood and alertness (Schutter, van Honk, d’Alfonso, Peper, & Panksepp, 2003). Hence, using a brain stimulation technique to study emotional processing in healthy humans should help identify the neural mechanisms underlying mental illnesses such as schizophrenia, depression and anxiety.
In our experiments, we found no difference between anodal and cathodal tDCS applied over the cerebellum. This observation agrees with the lack of polarity-specific tDCS-induced changes in cognitive experiments (Marshall et al., 2005). A possible explanation for the lack of polarity specificity of cerebellar tDCS comes from general physiological mechanisms that have been known for years (Lorente De Nò, 1947). The loss of function in any excitable tissue can be obtained with depolarization and with hyperpolarization. For instance, classic neurophysiological experiments demonstrated that axonal conduction can be completely blocked, even for several hours, by depolarization (“depolarizing” block) and by hyperpolarization (“hyperpolarizing” or “anodal” block), both leading to the same decreased excitability and, ultimately, to a loss of function (Lorente De Nò, 1947). This lack of polarity specificity could well apply also to the cerebellum, a brain structure that is theoretically more susceptible to direct current and has a hierarchically superior role in controlling cortical processing.
In conclusion, cerebellar tDCS alters the way healthy subjects recognize specific facial expressions thus showing that the cerebellum plays a direct role in recognition of negative emotions. By providing objective evidence confirming the proposed link between social cognition and the cerebellum our neuromodulatory approach could provide new insights into mental illnesses. Combined with cognitive-behavioral therapy, cerebellar tDCS might be useful in treating patients with psychiatric illnesses thought to involve cerebellar dysfunction and emotional disturbances.
Figure 3. Box plots and trial-to-trial representation showing the effect of cerebellar transcranial direct current stimulation (tDCS) on emotional recognition (negative, positive emotions and neutral).
On each box the central mark is the median, the edges of the box are the 25th and 75th percentiles, the whiskers extend to the most extreme data points not considered outliers, and each outlier is plotted individually (cross). y-axis: RTs (ms). Trial to trial grand average (n=21) of reaction times (RTs) across task stimulus presentation before cerebellar tDCS stimulation (24 answers) and after cerebellar tDCS stimulation (24 answers). x-axis: answers, note that the X axis graphically represents the time elapsing between the end of the task execution before stimulation and the beginning of the task execution after tDCS (15′ tDCS and 35′ rest); y-axis: RTs (arbitrary units, AU). A Note that anodal and cathodal cerebellar tDCS both reduce baseline RTs for negative emotions (two-way ANOVA; *p<0.05). B, C cerebellar tDCS induced no change in RTs from baseline either for positive emotion or neutral emotion. The trial-to-trial representation highlights the finding that anodal and cathodal curves differ from sham curves for negative emotions, but are similar to sham curves for positive and neutral emotions.
Footnotes
Disclosure Statement
Roberta Ferrucci reports no financial interests or potential conflicts of interests Gaia Giannicola reports no financial interests or potential conflicts of interests Manuela Rosa reports no financial interests or potential conflicts of interests Manuela Fumagalli reports no financial interests or potential conflicts of interests Prof. Paulo S Boggio reports no financial interests or potential conflicts of interests Prof. Mark Hallett reports no financial interests or potential conflicts of interests Stefano Zago reports no financial interests or potential conflicts of interests Prof. Alberto Priori reports no financial interests or potential conflicts of interests
* Roberta Ferrucci and Alberto Priori, are stake holders of Newronika s.r.l., a spin-off company of the Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico of Milan and of the Università degli Studi di Milano.
References
- Adolphs R. Neural systems for recognizing emotion. Current Opinion in Neurobiology. 2002;12(2):169–177. doi: 10.1016/s0959-4388(02)00301-x. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, O’Leary DS, Arndt S, Cizadlo T, Hurtig R, Rezai K, et al. Neural substrates of facial recognition. The Journal of Neuropsychiatry and Clinical Neurosciences. 1996;8:139–146. doi: 10.1176/jnp.8.2.139. [DOI] [PubMed] [Google Scholar]
- Ardolino G, Bossi B, Barbieri S, Priori A. Non-synaptic mechanisms underlie the after-effects of cathodal transcutaneous direct current stimulation of the human brain. Journal of Physiology. 2005;568:653–663. doi: 10.1113/jphysiol.2005.088310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelsson S, Kjaer I, Heiberg A, Bjornland T, Storhaug K. Neurocranial morphology and growth in Williams syndrome. European Journal of Orthodontics. 2005;27(1):32–47. doi: 10.1093/ejo/cjh065. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Morris JS, Frith CD, Perrett DI, Dolan RJ. Dissociable neural responses to facial expressions of sadness and anger. Brain. 1999;122(5):883–893. doi: 10.1093/brain/122.5.883. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Christensen BK, Fitzgerald PB, Fountain SI, Chen R. Reduced cerebellar inhibition in schizophrenia: A preliminary study. The American Journal of Psychiatry. 2005;162(6):1203–1205. doi: 10.1176/appi.ajp.162.6.1203. [DOI] [PubMed] [Google Scholar]
- Ekman P. Facial expressions of emotion: An old controversy and new findings. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 1992;335(1273):63–69. doi: 10.1098/rstb.1992.0008. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Unmasking the face. Englewood Cliffs, NJ: Prentice-Hall; 1975. [Google Scholar]
- Ferrucci R, Marceglia S, Vergari M, Cogiamanian F, Mrakic-Sposta S, Mameli F, et al. Cerebellar transcranial direct current stimulation impairs the practice-dependent proficiency increase in working memory. Journal of Cognitive Neuroscience. 2008;20(9):1687–1697. doi: 10.1162/jocn.2008.20112. [DOI] [PubMed] [Google Scholar]
- Fox E, Lester V, Russo R, Bowles RJ, Pichler A, Dutton K. Facial expressions of emotion: Are angry faces detected more efficiently? Cognition and Emotion. 2000;14(1):61–92. doi: 10.1080/026999300378996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea JM, Jayaram G, Ajagbe L, Celnik P. Modulation of cerebellar excitability by polarity-specific noninvasive direct current stimulation. The Journal of Neuroscience. 2009;29(28):9115–9122. doi: 10.1523/JNEUROSCI.2184-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt JB, Niedenthal PM. Emotional state and the use of stimulus dimensions in judgment. The Journal of Personality and Social Psychology. 1997;72(5):1017–1033. doi: 10.1037//0022-3514.72.5.1017. [DOI] [PubMed] [Google Scholar]
- Heath RG. Modulation of emotion with a brain pacemaker: Treatment for intractable psychiatric illness. The Journal of Nervous and Mental Disease. 1977;165(5):300–317. [PubMed] [Google Scholar]
- Heath RG, Dempesy CW, Fontana CJ, Myers WA. Cerebellar stimulation: Effects on septal region, hippocampus, and amygdala of cats and rats. Biological Psychiatry. 1978;13(5):501–529. [PubMed] [Google Scholar]
- Hennenlotter A, Schroeder U. Partly dissociable neural substrates for recognizing basic emotions: A critical review. Progress in Brain Research. 2006;156:443–456. doi: 10.1016/S0079-6123(06)56024-8. [DOI] [PubMed] [Google Scholar]
- Izard CE. Innate and universal facial expressions: Evidence from developmental and crosscultural research. Psychological Bulletin. 1994;115(2):288–299. doi: 10.1037/0033-2909.115.2.288. [DOI] [PubMed] [Google Scholar]
- Kesler-West ML, Andersen AH, Smith CD, Avison MJ, Davis CE, Kryscio RJ, et al. Neural substrates of facial emotion processing using fMRI. Brain Research Cognitive Brain Research. 2001;11(2):213–226. doi: 10.1016/s0926-6410(00)00073-2. [DOI] [PubMed] [Google Scholar]
- Leonard CM, Rolls ET, Wilson FA, Baylis GC. Neurons in the amygdala of the monkey with responses selective for faces. Behavioural Brain Research. 1985;15(2):159–176. doi: 10.1016/0166-4328(85)90062-2. [DOI] [PubMed] [Google Scholar]
- Liebetanz D, Nitsche MA, Tergau F, Paulus W. Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain. 2002;125:2238–2247. doi: 10.1093/brain/awf238. [DOI] [PubMed] [Google Scholar]
- Lorente De Nò R. A study of nerve physiology. New York, NY: Rockefeller Institute for Medical Research; 1947. [PubMed] [Google Scholar]
- Loughead J, Gur RC, Elliott M, Gur RE. Neural circuitry for accurate identification of facial emotions. Brain Research. 2008;1194:37–44. doi: 10.1016/j.brainres.2007.10.105. [DOI] [PubMed] [Google Scholar]
- Marshall L, Molle M, Siebner HR, Born J. Bifrontal transcranial direct current stimulation slows reaction time in a working memory task. BMC Neuroscience. 2005;6(23) doi: 10.1186/1471-2202-6-23.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morewedge CK. Negativity bias in attribution of external agency. Journal of Experimental Psychology: General. 2009;138(4):535–545. doi: 10.1037/a0016796. [DOI] [PubMed] [Google Scholar]
- Nashold BS, Jr, Slaughter DG. Effects of stimulating or destroying the deep cerebellar regions in man. Journal of Neurosurgery. 1969;31(2):172–186. doi: 10.3171/jns.1969.31.2.0172. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. The Journal of Physiology. 2000;527(3):633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradiso S, Johnson DL, Andreasen NC, O’Leary DS, Watkins GL, Boles Ponto LL, et al. Cerebral blood flow changes associated with attribution of emotional valence to pleasant, unpleasant, and neutral visual stimuli in a PET study of normal subjects. American Journal of Psychiatry. 1999;156:1618–1629. doi: 10.1176/ajp.156.10.1618. [DOI] [PubMed] [Google Scholar]
- Park JY, Gu BM, Kang DH, Shin YW, Choi CH, Lee JM, et al. Integration of crossmodal emotional information in the human brain: An fMRI study. Cortex. 2008;46(2):161–169. doi: 10.1016/j.cortex.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Posamentier MT, Abdi H. Processing faces and facial expressions. Neuropsychology Review. 2003;13(3):113–143. doi: 10.1023/a:1025519712569. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Quarterly Journal of Experimental Psychology. 1980;32(1):3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Priori A. Brain polarization in humans: A reappraisal of an old tool for prolonged noninvasive modulation of brain excitability. Clinical Neurophysiology. 2003;114(4):589–595. doi: 10.1016/s1388-2457(02)00437-6. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. Disorders of the cerebellum: Ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. The Journal of Neuropsychiatry and Clinical Neurosciences. 2004;16(3):367–378. doi: 10.1176/jnp.16.3.367. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121(4):561–579. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Öhman A, Junghofer M, Weike AI, Stockburger J, Hamm AO. The facilitated processing of threatening faces: An ERP analysis. Emotion. 2004;4(2):189–200. doi: 10.1037/1528-3542.4.2.189. [DOI] [PubMed] [Google Scholar]
- Schutter DJ, Enter D, Hoppenbrouwers SS. High-frequency repetitive transcranial magnetic stimulation to the cerebellum and implicit processing of happy facial expressions. Journal of Psychiatry & Neuroscience. 2009;34(1):60–65. [PMC free article] [PubMed] [Google Scholar]
- Schutter DJ, van Honk J. The cerebellum on the rise in human emotion. Cerebellum. 2005;4(4):290–294. doi: 10.1080/14734220500348584. [DOI] [PubMed] [Google Scholar]
- Schutter DJ, van Honk J. The cerebellum in emotion regulation: A repetitive transcranial magnetic stimulation study. Cerebellum. 2009;8(1):28–34. doi: 10.1007/s12311-008-0056-6. [DOI] [PubMed] [Google Scholar]
- Schutter DJ, van Honk J, d’Alfonso AA, Peper JS, Panksepp J. High frequency repetitive transcranial magnetic stimulation over the medial cerebellum induces a shift in the prefrontal electroencephalography gamma spectrum: A pilot study in humans. Neuroscience Letters. 2003;336(2):73–76. doi: 10.1016/s0304-3940(02)01077-7. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: A meta-analysis of neuroimaging studies. NeuroImage. 2009;44(2):489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- Streit M, Ioannides AA, Liu L, Wolwer W, Dammers J, Gross J, et al. Neurophysiological correlates of the recognition of facial expressions of emotion as revealed by magnetoencephalography. Brain Research Cognitive Brain Research. 1999;7(4):481–491. doi: 10.1016/s0926-6410(98)00048-2. [DOI] [PubMed] [Google Scholar]
- Turner BM, Paradiso S, Marvel CL, Pierson R, Boles Ponto LL, Hichwa RD, et al. The cerebellum and emotional experience. Neuropsychologia. 2007;45(6):1331–1341. doi: 10.1016/j.neuropsychologia.2006.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]