Abstract
The reaction between dilute fluorine gas and cresolsufonphthalein in acetic acid was investigated. The mono-, di-, and trifluorinated cresolsufonphthalein derivatives were isolated and characterized. These compounds possessed the properties of pH indicators with biologically relevant pKa values (6.4-7.5) and the absorption maxima of the basic forms at 582-592 nm. This method can be used for synthesis of positron-emitting 18F-labeled pH indicators with potential application for non-invasive in vivo pH measurement in biological objects.
Keywords: Fluorine, Cresol Purple, electrophilic fluorination, pH measurement
Introduction
Direct fluorination of complex organic molecules is a useful, although quite rare, method for synthesis of F-18 labeled PET compounds [1]. Recently we reported a new reaction of this type, a direct fluorination of phenolsulfonphthalein (Phenol Red) [2]. The fluorine-containing products of the reaction act as pH indicators with potential application for direct in vivo measurement of hydrogen ion concentration in biological objects. However, fluorinated derivatives of phenolsulfonphthalein can not be considered as ideal biological pH probes, because their absorption maxima are located in the range of 564-572 nm overlapping with absorption of porphyrin-based biomolecules. In order to obtain compounds with optimal properties we should be able to synthesize fluorinated derivatives of other indicator molecules with higher absorption wavelengths. Herein we report the results of direct fluorination of meta-cresolsulfonphthalein, also known as Cresol Purple (CP). The reaction products and their indicator properties were characterized using HPLC, NMR, optical absorption, and mass-spectroscopy.
Materials and methods
All reagents were purchased from Sigma-Aldrich and used as received. Fluorination of Cresol Purple was performed using conditions suitable for direct fluorination with [18F]-F2 gas in acidic solutions [2-4]. The reaction was carried out by bubbling 0.1% F2 in Ne (Matheson Tri-Gas Inc, Twinsburg, OH) for 5 minutes through freshly prepared solutions of the disodium salt of meta-cresolsulfonphthalein in glacial acetic acid (2 mg/mL). The ratio between the reagents was varied by changing of the amount of CP solution. The reaction mixture was evaporated in vacuum at 120°C, redissolved in 2 mL of water, injected into a semi-preparative HPLC column (Phenomenex, Synergi 4 μm Hydro-RP 80 Å 10 × 250 mm) and eluted with 25 % ethanol-water buffer at a rate of 2 mL/min and detection of absorption at 430 nm. Under these conditions, elution of the products of interest occurred between 20 and 30 min. Because separation of individual compounds on the basis of absorbance was not possible due to the very high extinction coefficients of the indicators, we collected consecutive 4 mL fractions and used them for further analysis.
All collected fractions were analyzed by HPLC, UV-VIS, mass-spectroscopy, and 1H and 19F NMR. Analytical HPLC (4.6 × 250 mm column of the same type using ethanol-water and an acetonitrile-water gradient) was used to determine qualitative and quantitative composition of each fraction. Absorption maxima and pKa values were determined by UV-VIS spectroscopy (Beckman-Coulter DU 530) using acetate, phosphate, TRIZMA, and borate buffers for different pH. The concentration of the compounds were determined from the absorption of their basic solutions (pH=10) at the wavelength of maximum absorption (582-592 nm). We assumed that the extinction coefficients for CP and its fluorinated derivatives in the maximum have the same value, which was found to be ε=29,000 cm−1M−1 at 578 nm for CP. This assumption was based on the fact, that size of fluorine atom is comparable with hydrogen and therefore it should not cause any structural distortion. Subsequently, introduction of fluorine atom into molecule of CP should not cause a drastic change of its optical properties. Also, several structurally similar trialylmethane indicators have quite close extinction coefficients: 3·104 for phenolphthalein [5] and 3.16·104 for Phenol Red (Sigma-Aldrich product information), justifying using of the value εmax =2.9·104 for fluorinated derivatives of CP.
In order to characterize individual fluorinated derivatives of Cresol Purple, we collected corresponding fractions from five preparations and repeatedly purified them by semi-preparative HPLC. Molecular mass of the compounds was determined by mass-spectroscopy using a Thermo Fisher Scientific (Bremen, Germany) Orbitrap Exactive. Electrospray Ionization (ESI) was performed, introducing the sample into the instrument at a flow rate of 5 μL/min. The experiment was done in both positive and negative modes with a capillary temperature of 275 °C. In positive-ion mode, the electrospray voltage was set to 4.2 kV, the capillary voltage to 50 V, and the tube lens offset to 145 V. The nitrogen sheath gas flow was optimized at 10 arbitrary units.
In addition, Solvent-Assisted Inlet Ionization (SAII) on the Orbitrap Exactive was used to verify the ESI results in both positive and negative mode. SAII is a novel ionization technique, in which the ion source is removed, a fused silica capillary tube without the coating is placed in the inlet of the mass spectrometer and the sample is pulled by vacuum through the fused silica tube into a heater orifice into the mass spectrometer [6]. This was performed in both positive and negative mode with a capillary temperature of 325 °C, capillary voltage ±90 V and tube lens voltage ±120 V. The data was controlled and analyzed using Xcalibur software (Thermo Fisher Scientific) for both ionization techniques.
1H and 19F NMR spectra were obtained on a Bruker DMX-360 spectrometer using basic forms (disodium salts) in deuterated methanol solvent.
Results
The reaction between dilute fluorine gas and Cresol Purple in acidic solution was found to be similar to the recently described fluorination of Phenol Red [2]. Analytical HPLC of the reaction mixture shows presence of the starting compound and three major fluorine-containing products. These compounds demonstrate increasing retention time on the reverse-phase column allowing their separation by semi-preparative HPLC similarly to fluorinated derivatives of phenolsulfonphthalein.
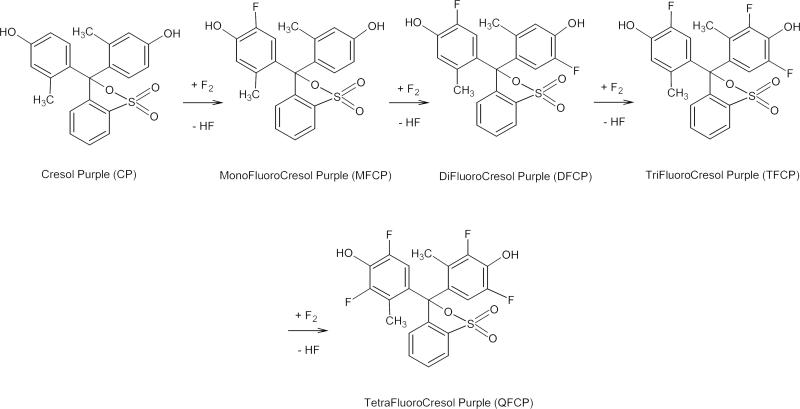
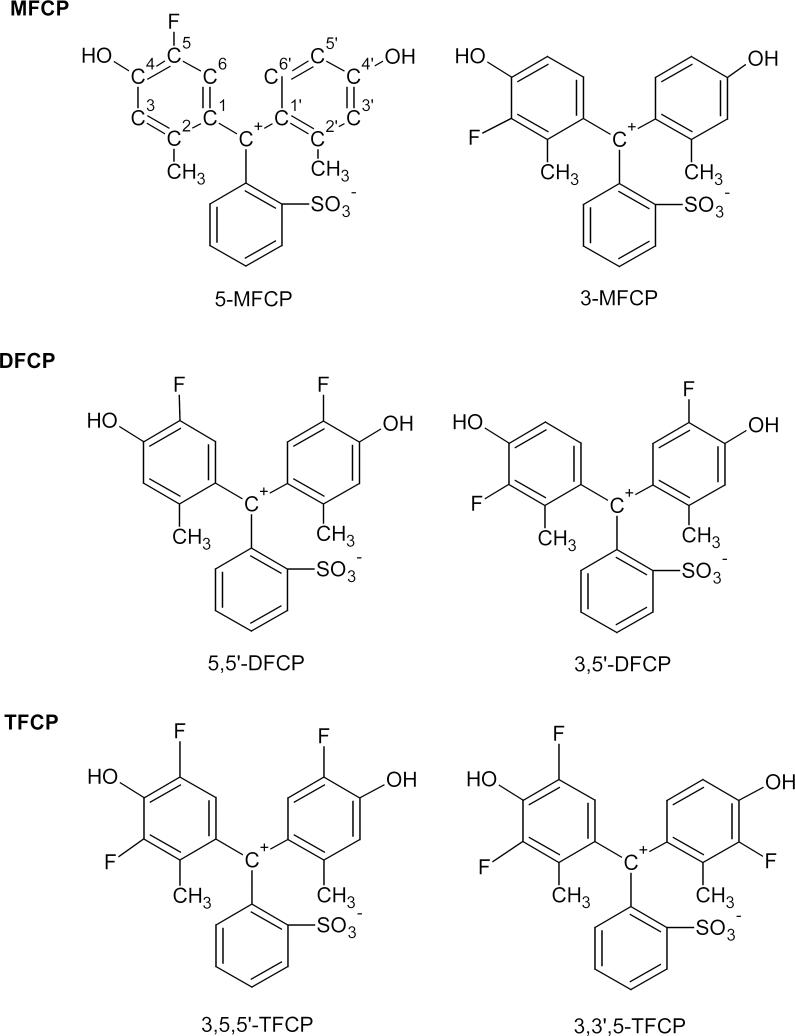
Mass-spectrometric investigation of the fractions containing three fluorination products shows main peaks at m/z 399.070, 417.061, and 435.051 for negative mode and m/z 401.090, 419.081, and 437.071 for positive mode. These numbers correspond to the calculated values for the deprotonated and protonated forms of mono-, di-, and trifluorinated derivatives of Cresol Purple, abbreviated as MFCP, DFCP, and TFCP on Figure 1.
Figure 1.
Proposed reaction of the fluorination of Cresol Purple in acidic media. The formulas represent main reaction products shown as cyclic lactonoid forms.
The yield of the reaction products is variable and depends on the ratio of the reagents. Equimolar quantities of reagents resulted in the production of 9-10% MFCP, 5-6% DFCP, and 1-2% TFCP. An excess of CP over F2 increases efficacy of fluorine incorporation, yielding 13% MFCP, 10% DFCP, and 1% TFCP (relative to the amount of fluorine) at double CP excess. An excess of fluorine increases the fraction of the more fluorinated products, but the overall yields drop due to destruction of the molecules. For example, 50% excess of fluorine leads to production of 4% MFCP, 6.5% DFCP, and 2.5% TFCP. More then a double excess of fluorine over CP caused destruction of the most of the reaction products. In all the cases about 10% of the initial compound remains in the reaction mixture.
Semi-preparative HPLC also showed a UV absorbing peak with retention time higher than TFCP. We could not collect enough of this compound for optical and NMR analysis, but its mass spectra contain distinct signals at m/z 453.042 for negative mode and 455.061 for positive mode, suggesting the synthesis of a tetrafluorinated derivative of CP.
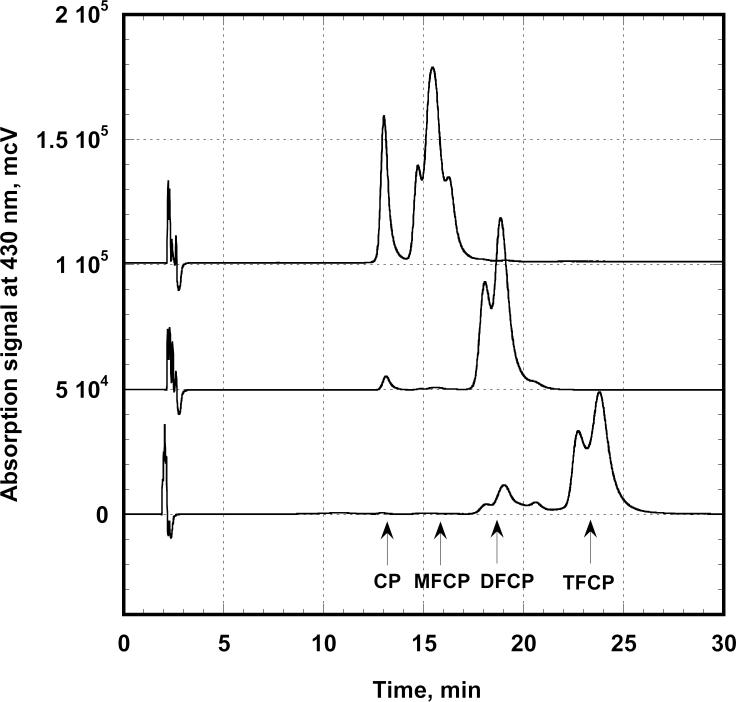
Analytical HPLC of the reaction products reveals an unusual feature for the fluorinated derivatives of cresolsulfonphthalein. For every preparation their HPLC signals were split into very close peaks (Figure 2). This effect did not depend on solvent or the pH of the eluting buffer, suggesting the presence of different compounds with similar properties rather than the existence of an acid-base or tautomeric equilibrium in solution. To identify these products we collected corresponding fractions from analytical HPLC and analyzed them by mass-spectroscopy. The mass spectra were found to be identical for all the peaks from each group, suggesting the existence of the structural isomers for each fluorinated compound.
Figure 2.
Analytical HPLC of three fractions containing fluorinated derivatives of Cresol Purple. The precursor (CP) is represented as a single peak on the upper curve, while other maxima are splitting into several closely located peaks, corresponding to different structural isomers of the products.
Comparison of NMR spectra of cresolsulfonphthalein and its fluorinated derivatives indicate the positions of the molecule fluorination. The NMR spectrum of CP has three groups of peaks: a sharp signal from two methyl groups at δ 1.86 and 1.92, four multiplets from the sulfonated ring: δ 7.01, 7.45, 7.53, and 8.09; and three signals from phenol rings: δ 6.26, 6.35, 6.39, 6.87, and δ 6.89. In the spectrum of fluorinated derivatives of Cresol Purple the peaks from methyl groups are turned into complex multiplets, while peaks from protons of sulfonated ring are slightly shifted, but the integral intensities of the protons are not altered in both groups of peaks. These results indicate that fluorine atoms in fluorinated derivatives of CP do not substitute hydrogen atoms in methyl group and sulfonated ring. On the contrary, the spectra in the range of phenol rings (δ 6-7) are significantly changed. The peaks are represented as set of several difficult to interpret multiplets. Integration of the signals clearly shows the gradual decreasing of the numbers of the protons of this type, which is proportional to number of added fluorine atoms.
These results clearly indicate that fluorine atoms in fluorinated derivatives of CP do not substitute hydrogen atoms in methyl group and sulfonated ring, suggesting fluorination of the molecule into the phenol rings. The complicated proton NMR spectra in this range is not a surprise, since they can not be interpreted adequately even for as simple compound as fluorobenzene [7]. Additional complications may be associated with signal splitting and overlapping due to existence of structural isomers, which are visible on analytical HPLC. Presence of the isomers also explains a complexity of 19F NMR spectra, which contain four signals for MFCP and eight signals for DFCP. Still, all these signals are located in the range from δ -138 to δ -145, which is specific for fluorine atoms in ortho-positions in fluorophenols and excludes possibility of their meta-location with δ -113 [7].
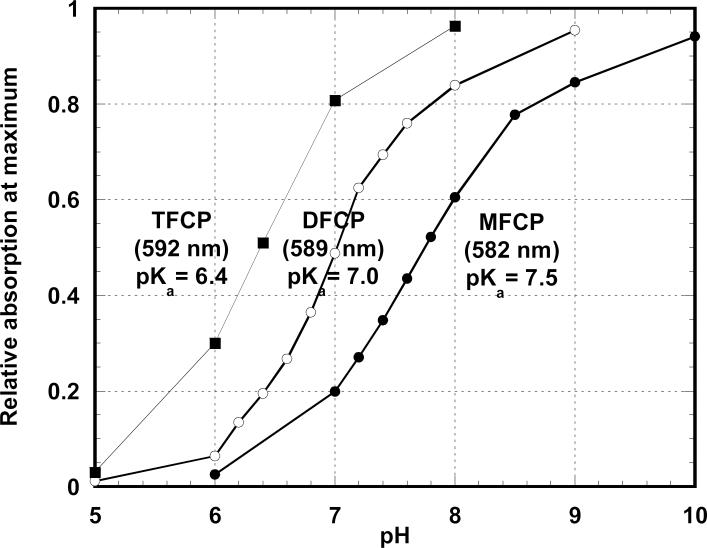
Introduction of fluorine atoms into the Cresol Purple molecule causes alterations of its pH indicator properties in water solution. The pKa values of the fluorinated derivatives of CP were determined from absorbance of the purified fractions containing individual compounds. We compared the intensity of absorption of the basic forms, because they have higher extinction coefficients and are located in a more convenient area of the spectrum. The measurements were taken at the absorption maxima, which were found to be at 582 nm, 589 nm, and 592 nm for MFCP, DFCP, and TFCP, respectively. Titration of the fluorinated indicators showed that the introduction of fluorine atoms into the molecule of CP causes a decrease in pKa from 8.3 for CP [8] to 7.5 for MFCP, 7.0 for DFCP, and 6.4 for TFCP (Figure 3).
Figure 3.
Determination of pKa of fluorinated derivatives of CP from absorption of their basic forms at maxima demonstrating increasing wavelength and decreasing pKa as a result of successive addition of fluorine atoms.
Summary of experimental results
Cresolsulfonphthalein (Cresol Purple): 1H (360 MHz, CD3OD) δ 1.86 (s, 3H); δ 1.92 (s, 3H); δ 6.257 (dd, J = 2.2, 9.0 Hz, 2H); δ 6.349 (dd, J = 0.7, 2.2 Hz, 1H); δ 6.388 (dd, J = 0.7, 2.2 Hz, 1H); δ 6.872 (d, J = 9.0 Hz, 1H); δ 6.886 (d, J = 9.0 Hz, 1H); δ 7.012 (dd, J = 1.2, 7.6 Hz, 1H); δ 7.453 (dt, J = 1.2, 7.6 Hz, 1H); δ 7.532 (dt, J = 1.2, 7.6 Hz, 1H); δ 8.094 (dd, J = 1.2, 7.6 Hz, 1H).
Monofluororesolsulfonphthalein (MFCP): m/z 399.070 (negative), 401.090 (positive); pKa = 7.5, λbasic = 582 nm; 1H (360 MHz, CD3OD): δ 1.7-2.0 (m, 6H); δ 6.15-6.95 (m, 5H), δ 7.009 (dm, J = 7.7 Hz, 1H); δ 7.462 (tm, J = 7.7 Hz, 1H); δ 7.534 (tm, J = 7.7 Hz, 1H); δ 8.096 (dm, J = 7.7 Hz, 1H); 19F (338 MHz, CD3OD): δ -140.581 (dd, J = 3.4, 8.5 Hz, 1F); δ -141.095 (dd, J = 3.4, 8.5 Hz, 0.84F); δ -144.255 (dd, J = 8.8, 13.6 Hz, 0.36F); δ -144.626 (dd, J = 8.8, 13.6 Hz, 0.36F).
Difluororesolsulfonphthalein (DFCP): m/z 417.061 (negative), 419.081 (positive); pKa = 7.0, λbasic = 589 nm; 1H (360 MHz, CD3OD): δ 1.76-1.94 (m, 6H); δ 6.29-6.40 (m, 1H), δ 6.41-6.51 (m, 1H), δ 6.60-6.68 (m, 1H), δ 6.71-6.81 (m, 1H),δ 7.020 (dm, J = 7.6 Hz, 1H); δ 7.491 (tm, J = 7.7 Hz, 1H); δ 7.558 (tm, J = 7.7 Hz, 1H); δ 8.113 (dm, J = 7.7 Hz, 1H); 19F (338 MHz, CD3OD): δ -138.543 (dd, J = 3.7, 8.3 Hz, 1F); δ -139.160 (dd, J = 3.7, 8.3 Hz, 1F) δ -139.618 (dd, J = 3.7, 8.3 Hz, 1F) δ -140.059 (dd, J = 3.7, 8.3 Hz, 1F) δ -143.581 (dt, J = 8,8, 13.6 Hz, 2F); δ -143.830 (dd, J = 8.8, 13.6 Hz, 1F); δ -144.140 (dd, J = 8.8, 13.6 Hz, 1F).
Trifluororesolsulfonphthalein (TFCP): m/z 435.051 (negative), 437.071 (positive); pKa = 6.4, λbasic = 592 nm; 1H (360 MHz, CD3OD): δ 1.80-2.10 (m, 6H); δ 6.1-6.9 (m, 3H), δ 7.06 (m, 1H); δ 7.62 (m, 2H); δ 7.81 (m, 1H).
Discussion
Reactions between elementary fluorine and complex organic molecules usually result in multiple nonspecific processes [9] due to the very high enthalpy of C-H bond fluorination, which exceeds the energy of carbon-based single bonds and causes a destruction of the target molecule. A successful electrophilic fluorination of the phenolsulfonphthalein aromatic system [2] was possible due to the existence of several favorable factors: presence of only low reactive aromatic protons, a positive charge on the target part of molecule in acidic conditions [2,10], and the use of acetic acid as the solvent. Acidic media can promote electrophilic fluorination of aromatic system rather then non-specific radical decomposition [11] or act as a moderating agent by converting fluorine into the less aggressive acetyl hypofluorite CH3COOF [12]. These factors decreased the overall reaction energy, promoting a specific substitution of the hydrogen atom by fluorine rather than destruction of the molecule.
Introduction of a methyl group into the aromatic system increases its activity in the substitution reaction. In addition, a hydrogen atom from the methyl group adjacent to an aromatic system is more reactive in comparison with hydrogen atoms in hydrocarbons. Both these effects are quite undesirable for our purposes and can act as negative factors promoting nonspecific fluorination and destruction of substituted phenolsulfonphthaleins by fluorine molecule. Nevertheless, our results show that fluorination of meta-cresolsufonphthalein occurs in the same moderate way, producing three compounds with consecutively increased numbers of fluorine atoms. This suggests that other similar triarylmethane dyes can also survive direct fluorination in acidic conditions and will be useful precursors for new fluorinated pH indicators.
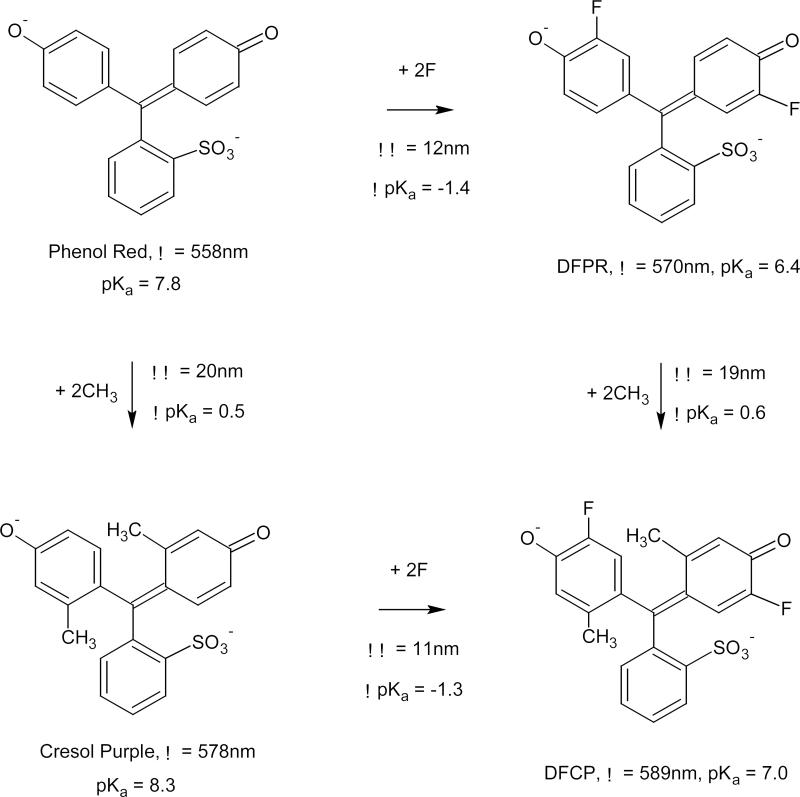
Incorporation of the fluorine atoms into cresolsulfonphthalein molecule alters its indicator properties in the same way as it was observed for phenolsulfonphthalein [2]. Each fluorine atom causes a decrease in pKa value by 0.5-0.8 units and a bathochromic shift of the absorption maxima of the indicator basic form by 3-7 nm. Introduction of methyl groups and fluorine atoms into the phenolsulfonphthalein molecule shifts pKa value and absorption wavelength of its basic form in an independent and additive manner (Figure 4). This kind of relationship allows a prediction of the acid-base and absorption properties of fluorinated phenolsulfonphthalein derivatives and can be useful for synthesis of the compounds with required characteristics. It also presumes the same position of fluorination for both Phenol Red and Cresol Purple.
Figure 4.
Effects of addition of two methyl groups and two fluorine atoms on pKa wavelengths of absorption of basic form of phenolsulfonphthalein are independent and additive, suggesting the same fluorination positions for both DFPR and DFCP and allowing prediction of pKa and absorption maxima of other substituted phenolsulfonphthaleins.
Proton NMR shows that fluorine atoms are not incorporated into methyl groups and the sulfonated ring of cresolsulfonphthalein, locating only in the phenol rings. The location of the fluorine atoms in ortho-position to hydroxyl group is evident from 19F NMR data and is consistent with general rules of electrophilic substitution. The central carbon atom at C1 (Figure 5) has a partial positive charge and promotes meta-orientation into positions C3 and C5, deactivating the C6 atom. Other substitute groups 2-methyl and 4-hydroxyl are both ortho-/para-directing groups. All the three groups are located in coordinated positions, promoting substitution of hydrogen into the 3rd or 5th position and prohibiting fluorine incorporation into 6th position. These results are consistent with a literature data on fluorination of phenols with electron withdrawing groups in para-position in acidic conditions, which resulted in electrophilic substitution exclusively into ortho-position [13].
Figure 5.
Structure of main isomers of MFCP, DFCP, and TFCP is shown for the most symmetrical zwitterion form existing in strong acidic conditions. The upper left formula reflects a numbering of carbon atoms in phenol rings used in the text.
Substitution of the hydrogen by the fluorine atom at the 3rd position seems to be less preferable due to steric hinderance by adjacent methyl and hydroxyl groups. Another potential restriction against fluorination of the molecule at C3 may possibly appear if the reaction mechanism involves intermediate generation of acetyl hypofluorite [12]. Reaction of the aromatic ring with this by-product requires the availability of two adjacent non-substituted carbon atoms [14], which is possible only for the C5–C6 couple and will result in fluorine incorporation strictly into 5th position. Subsequently, we have shown the compounds with fluorination into the 5th positions as the main reaction products in Figure 1. However, the presence of isomers of fluorinated cresolsulfonphthalein, as well as the fact of existence of the trifluorinated derivative with all three fluorine atoms in the phenol rings, indicates the possibility of fluorination into both C3 and C5 positions. This result indicates a minor (if any) role of intermediate acetyl hypofluorite CH3COOF in the reaction.
The availability of both positions for fluorination results in the appearance of different isomers of fluorinated cresolsulfonphthaleins. The existence of these isomers would explain the complicated character of the NMR spectra of these compounds. The isomers are slightly separable only by analytical HPLC, but the preparation of large quantities in this way is much harder and rather impractical. However, the indicator properties of the isomers (both pKa and absorption wavelengths) should be very close, allowing their application as pH indicators without further separation. The proposed structure of main isomers is presented in Figure 5. It has to be mentioned that DFCP could have two other isomers (3,5-DFCP and 3,3’-DFCP). A small peak between the major signals of DFCP and TFCP (located at 20-21 min in two lower curves on Fig. 2) was collected and analyzed by mass-spectroscopy. It was found to have the same mass as DFCP and most likely represents one (or both) of these minor isomers.
Successful fluorination of cresolsulfonphthalein is possible only for the electron-deficient zwitterion form of the molecule, which exists as the non-charged form of the indicator [15] present in strong acidic conditions. This requirement justifies the use of acetic acid as a reaction solvent. However, the acidic form of cresolsulfonphthalein is not soluble in most of solvents, including glacial acetic acid. On the other hand, both the monosodium (yellow) and disodium (purple) forms of the compound can be dissolved in acidic acid, where they are immediately converted into the red zwitterion form. This form precipitates from the solution, but this process occurs quite slowly, allowing several minutes for conducting the fluorination reaction. Because of this, the reaction has to be performed immediately using a freshly prepared solution of the sodium salt in acetic acid, and the product contains a significant (up to 10%) amount of the precursor even with excessive amounts of fluorine. These peculiar conditions could explain why the direct fluorination of phenolsulfonphthalein and its derivatives has not been observed previously.
Conclusion
Direct fluorination of cresolsulfonphthalein in acidic conditions results in production of its mono-, di- and trifluorinated derivatives at mild yields. Successful introduction of fluorine atoms into the molecule of cresolsulfonphthalein sustains its indicator properties, gradually decreasing pKa and increasing wavelength of absorption under basic conditions. These fluorine-containing pH indicators can be prepared in 18F-labeled form and used for non-invasive in vivo pH measurement in biological objects.
Highlights.
We performed a reaction between 0.1% F2 and cresolsufonphthalein in acidic media.
We observed a specific fluorination into ortho positions to hydroxyl groups.
All the fluorinated derivatives of cresolsufonphthalein act like pH indicators.
They have biologically relevant pKa 6.4-7.5 and absorption maxima at 582-592 nm.
The reaction fits for synthesis of 18F-labeled indicators for in vivo pH detection.
Acknowledgements
Supported by the Institute for Translational Medicine and Therapeutics (ITMAT) Transdisciplinary Awards Program in Translational Medicine and Therapeutics Translational Biomedical Imaging Core Grant (TAPITMAT-TBIC). The authors would like to thank Charles N. McEwen at the University of the Sciences for providing mass-spectroscopy equipment and Dr. Wenchao Qu from University of Pennsylvania for his help with NMR spectra.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature
- 1.Miller PW, Long NJ, Vilar R, Gee AD. Synthesis of 11C, 18F, 15O, and 13N Radiolabels for Positron Emission Tomography. Angew. Chem. Int. Ed. 2008;47:8998–9033. doi: 10.1002/anie.200800222. [DOI] [PubMed] [Google Scholar]
- 2.Kachur AV, Popov AV, Karp JS, Delikatny EJ. Direct Fluorination of Phenolsulfonphthalein: a Method for Synthesis of Positron-Emitting Indicators for In Vivo pH Measurement. Cell Biochemistry and Biophysics, accepted. 2012 doi: 10.1007/s12013-012-9390-x. 10.1007/s12013-012-9390-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Visser GWM, Boele S, v. Halteren BW, Knops GHJN, Herscheid JDM, Brinkman GA, Hoekstra A. Mechanism and Stereochemistry of the Fluorination of Uracil and Cytosine Using Fluorine and Acetyl Hypofluorite. J. Org. Chem. 1986;51:1466–1471. [Google Scholar]
- 4.Dolbier WR, Li A-R, Koch CJ, Shiue C-Y, Kachur AV. [18F]-EF5 - a Marker for PET Detection of Hypoxia: Synthesis of Precursor and a New Fluorination Procedure. Appl. Radiat. Isot. 2001;53:73–80. doi: 10.1016/s0969-8043(00)00102-0. [DOI] [PubMed] [Google Scholar]
- 5.Barnes MD, Lamer VK. Kinetics and Equilibria of the Carbinol Formation of Phenolphthalein. J. Am. Chem. Soc. 1942;64:2312–2316. [Google Scholar]
- 6.Chubatyi N, Pagnotti VS, McEwen CN. Solvent Assisted Inlet Ionization: An Ultrasensitive New Liquid Introduction Ionization Method for Mass Spectrometry. Anal. Chem. 2011;83:3981–3985. doi: 10.1021/ac200556z. [DOI] [PubMed] [Google Scholar]
- 7.Dolbier WR. Guide to Fluorine NMR for Organic Chemists. J Wilex and son Ed.; Hoboken, NJ: 2009. [Google Scholar]
- 8.Kulichenko SA, Fesenko SA, Fesenko NI. Color Indicator System for Acid–Base Titration in Aqueous Micellar Solutions of the Cationic Surfactant Tridecylpyridinium Bromide. J. Anal. Chem. 2001;56:1002–1006. [Google Scholar]
- 9.Purrington ST, Kagen BS, Patrick TB. The Application of Elemental Fluorine in Organic Synthesis. Chem. Rev. 1986;86:997–1018. [Google Scholar]
- 10.Kachur AV, Dolbier WR, Xu W, Koch CJ. Catalysis of Fluorine Addition to Double Bond: an Improvement of Method for Synthesis of 18F PET Agents. Appl. Radiat. Isot. 2010;68:293–296. doi: 10.1016/j.apradiso.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chambers RD, Skinner CJ, Hutchinson J, Thomson J. Elemental fluorine. Part 1. Synthesis of fluoroaromatic Compounds. J. Chem. Soc. Perkin Trans. 1996;1:605–609. [Google Scholar]
- 12.Lerman O, Rozen S. Acetyl hypofluorite, a new moderating carrier of elemental fluorine and its use in fluorination of 1,3-dicarbonyl derivatives. J. Org. Chem. 1983;48:724–727. [Google Scholar]
- 13.Sandford G. Elemental fluorine in organic chemistry. J. Fluorine Chem. 2007;128:90–104. [Google Scholar]
- 14.Shiue CY, Wolf AP, Friedkin M. Syntheses of 5’-Deoxy-5-[F-18]Fluorouridine and Related Compounds as Probes for Measuring Tissue Proliferation In Vivo. J. Labelled Compd. Radiopharm. 1984;21:865–873. [Google Scholar]
- 15.Tamura Z, Maeda M. Differences Between Phthaleins and Sulfonphthaleins. Yakugaku Zasshi. 1997;117:764–770. doi: 10.1248/yakushi1947.117.10-11_764. [DOI] [PubMed] [Google Scholar]