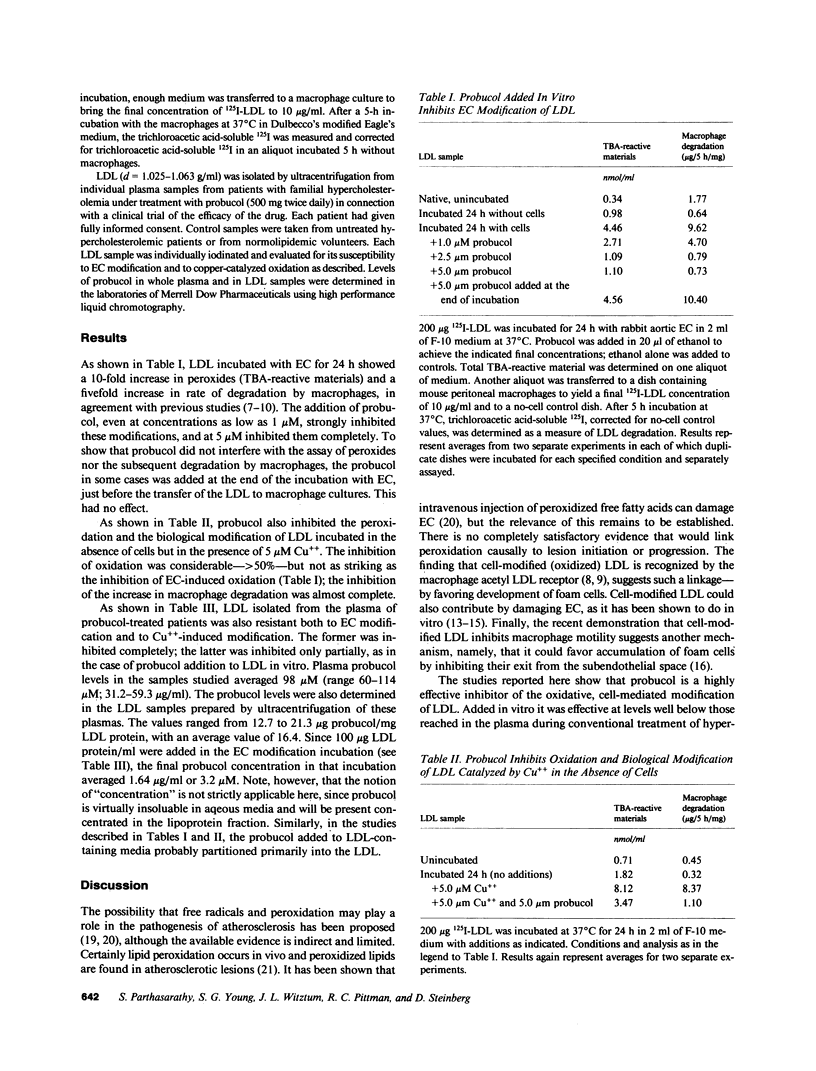
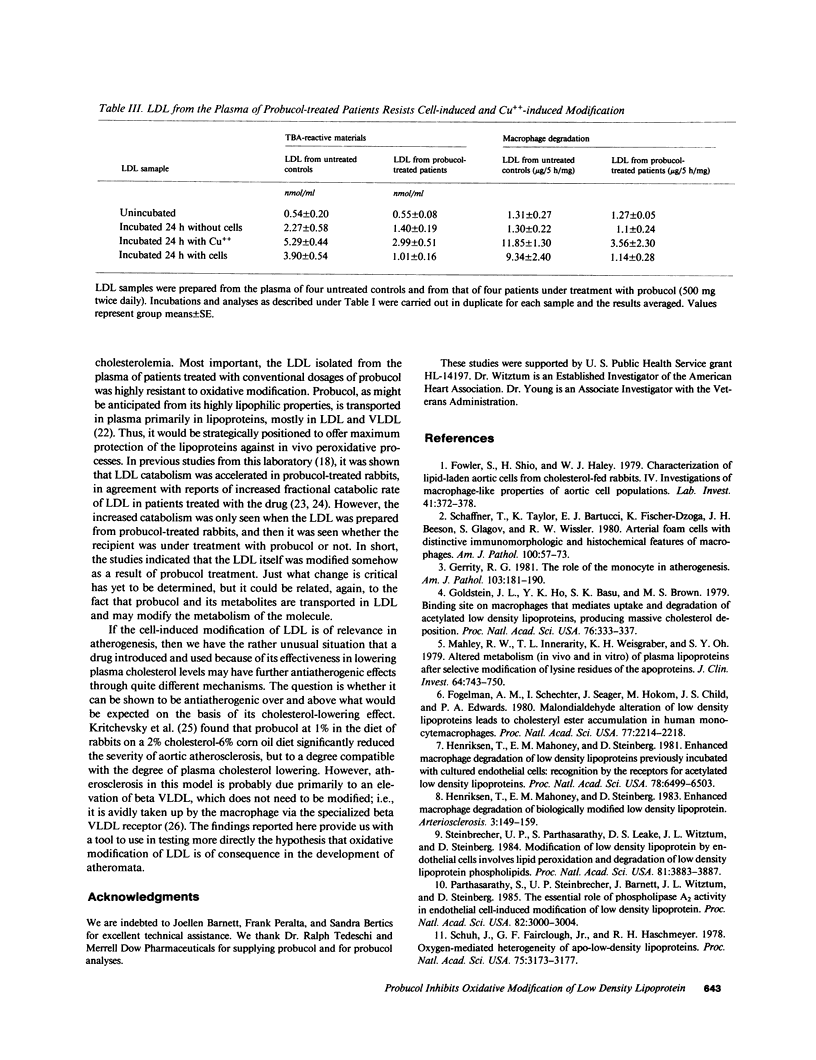
Abstract
Previous studies have established that low density lipoprotein (LDL) incubated with endothelial cells (EC) undergoes extensive oxidative modification in structure and that the modified LDL is specifically recognized by the acetyl LDL receptor of the macrophage. Thus, in principle, EC-modified LDL could contribute to foam cell formation during atherogenesis. Oxidatively modified LDL is also potentially toxic to EC. The present studies show that addition of probucol during the incubation of LDL with EC prevents the increase in the electrophoretic mobility, the increase in peroxides, and the increase in subsequent susceptibility to macrophage degradation. It has also been shown that oxidation of LDL catalyzed by cupric ion induces many of the same changes occurring during EC modification. Addition of probucol (5 microM) also prevented this copper-catalyzed modification of LDL. Most importantly, samples of LDL isolated from plasma of hypercholesterolemic patients under treatment with conventional dosages of probucol were shown to be highly resistant to oxidative modification either by incubation with endothelial cells or by cupric ion in the absence of cells. The findings suggest the hypothetical but intriguing possibility that probucol, in addition to its recognized effects on plasma LDL levels, may inhibit atherogenesis by limiting oxidative LDL modification and thus foam cell formation and/or EC injury. Other compounds with antioxidant properties might behave similarly.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fogelman A. M., Shechter I., Seager J., Hokom M., Child J. S., Edwards P. A. Malondialdehyde alteration of low density lipoproteins leads to cholesteryl ester accumulation in human monocyte-macrophages. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2214–2218. doi: 10.1073/pnas.77.4.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler S., Shio H., Haley N. J. Characterization of lipid-laden aortic cells from cholesterol-fed rabbits. IV. Investigation of macrophage-like properties of aortic cell populations. Lab Invest. 1979 Oct;41(4):372–378. [PubMed] [Google Scholar]
- GLAVIND J., HARTMANN S., CLEMMESEN J., JESSEN K. E., DAM H. Studies on the role of lipoperoxides in human pathology. II. The presence of peroxidized lipids in the atherosclerotic aorta. Acta Pathol Microbiol Scand. 1952;30(1):1–6. doi: 10.1111/j.1699-0463.1952.tb00157.x. [DOI] [PubMed] [Google Scholar]
- Gerrity R. G. The role of the monocyte in atherogenesis: I. Transition of blood-borne monocytes into foam cells in fatty lesions. Am J Pathol. 1981 May;103(2):181–190. [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Ho Y. K., Basu S. K., Brown M. S. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc Natl Acad Sci U S A. 1979 Jan;76(1):333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen T., Evensen S. A., Carlander B. Injury to human endothelial cells in culture induced by low density lipoproteins. Scand J Clin Lab Invest. 1979 Jun;39(4):361–368. doi: 10.3109/00365517909106120. [DOI] [PubMed] [Google Scholar]
- Henriksen T., Mahoney E. M., Steinberg D. Enhanced macrophage degradation of biologically modified low density lipoprotein. Arteriosclerosis. 1983 Mar-Apr;3(2):149–159. doi: 10.1161/01.atv.3.2.149. [DOI] [PubMed] [Google Scholar]
- Henriksen T., Mahoney E. M., Steinberg D. Enhanced macrophage degradation of low density lipoprotein previously incubated with cultured endothelial cells: recognition by receptors for acetylated low density lipoproteins. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6499–6503. doi: 10.1073/pnas.78.10.6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessler J. R., Robertson A. L., Jr, Chisolm G. M., 3rd LDL-induced cytotoxicity and its inhibition by HDL in human vascular smooth muscle and endothelial cells in culture. Atherosclerosis. 1979 Mar;32(3):213–229. doi: 10.1016/0021-9150(79)90166-7. [DOI] [PubMed] [Google Scholar]
- Kesäniemi Y. A., Grundy S. M. Influence of probucol on cholesterol and lipoprotein metabolism in man. J Lipid Res. 1984 Aug;25(8):780–790. [PubMed] [Google Scholar]
- Kritchevsky D., Kim H. K., Tepper S. A. Influence of 4,4'-(isopropylidenedithio)bis(2,6-di-t-butylphenol) (DH-581) on experimental atherosclerosis in rabbits. Proc Soc Exp Biol Med. 1971 Apr;136(4):1216–1221. doi: 10.3181/00379727-136-35461. [DOI] [PubMed] [Google Scholar]
- Lee D. M. Malondialdehyde formation in stored plasma. Biochem Biophys Res Commun. 1980 Aug 29;95(4):1663–1672. doi: 10.1016/s0006-291x(80)80090-8. [DOI] [PubMed] [Google Scholar]
- Mahley R. W., Innerarity T. L., Weisgraber K. B., Oh S. Y. Altered metabolism (in vivo and in vitro) of plasma lipoproteins after selective chemical modification of lysine residues of the apoproteins. J Clin Invest. 1979 Sep;64(3):743–750. doi: 10.1172/JCI109518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall F. N. Pharmacology and toxicology of probucol. Artery. 1982;10(1):7–21. [PubMed] [Google Scholar]
- Morel D. W., Hessler J. R., Chisolm G. M. Low density lipoprotein cytotoxicity induced by free radical peroxidation of lipid. J Lipid Res. 1983 Aug;24(8):1070–1076. [PubMed] [Google Scholar]
- Naruszewicz M., Carew T. E., Pittman R. C., Witztum J. L., Steinberg D. A novel mechanism by which probucol lowers low density lipoprotein levels demonstrated in the LDL receptor-deficient rabbit. J Lipid Res. 1984 Nov;25(11):1206–1213. [PubMed] [Google Scholar]
- Nestel P. J., Billington T. Effects of probucol on low density lipoprotein removal and high density lipoprotein synthesis. Atherosclerosis. 1981 Jan-Feb;38(1-2):203–209. doi: 10.1016/0021-9150(81)90117-9. [DOI] [PubMed] [Google Scholar]
- Parthasarathy S., Steinbrecher U. P., Barnett J., Witztum J. L., Steinberg D. Essential role of phospholipase A2 activity in endothelial cell-induced modification of low density lipoprotein. Proc Natl Acad Sci U S A. 1985 May;82(9):3000–3004. doi: 10.1073/pnas.82.9.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn M. T., Parthasarathy S., Steinberg D. Endothelial cell-derived chemotactic activity for mouse peritoneal macrophages and the effects of modified forms of low density lipoprotein. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5949–5953. doi: 10.1073/pnas.82.17.5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner T., Taylor K., Bartucci E. J., Fischer-Dzoga K., Beeson J. H., Glagov S., Wissler R. W. Arterial foam cells with distinctive immunomorphologic and histochemical features of macrophages. Am J Pathol. 1980 Jul;100(1):57–80. [PMC free article] [PubMed] [Google Scholar]
- Schuh J., Fairclough G. F., Jr, Haschemeyer R. H. Oxygen-mediated heterogeneity of apo-low-density lipoprotein. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3173–3177. doi: 10.1073/pnas.75.7.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbrecher U. P., Parthasarathy S., Leake D. S., Witztum J. L., Steinberg D. Modification of low density lipoprotein by endothelial cells involves lipid peroxidation and degradation of low density lipoprotein phospholipids. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3883–3887. doi: 10.1073/pnas.81.12.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein D. B., Carew T. E., Steinberg D. Uptake and degradation of low density lipoprotein by swine arterial smoot muscle cells with inhibition of cholesterol biosynthesis. Biochim Biophys Acta. 1976 Mar 26;424(3):404–421. doi: 10.1016/0005-2760(76)90030-8. [DOI] [PubMed] [Google Scholar]


