Abstract
In this article, we first review current knowledge of the AMPA and NMDA glutamate receptors, their physiological properties and functions and their regulation by signaling cascades. We then discuss our hypothesis that the therapeutic effects of monoaminergic antidepressants and ketamine, an NMDA receptor antagonist, may be mediated by increased AMPA to NMDA throughput in critical neuronal circuits. We hypothesize that ketamine mediates this throughput directly, thus resulting in rapid antidepressant effects whereas monoaminergic antidepressants work indirectly and gradually; this may explain, in part, the delayed onset of several weeks to months that is observed with traditional antidepressants.
Introduction
Medications currently used to treat major depression include a variety of antidepressants that are primarily serotonergically and/or noradrenergically based. Although many people suffering from major depression benefit from existing antidepressant medications, these therapies have several limitations including treatment-limiting side effects, and incomplete response and remission rates. Recent data from a large effectiveness study in major depression involving approximately 2876 outpatients found that only 28% of patients achieved remission within 10–14 weeks. However, by 24 weeks 50% eventually did achieve remission with either a switch to another antidepressant or an augmentation strategy [1]. This study suggests that although people with major depression do benefit from antidepressants, it often takes up to 6 months and two antidepressant trials to achieve remission and a significant proportion of patients are still left unwell. The search for more tolerable and effective antidepressants has been underway for several decades. However, an area that has been neglected is the lag in onset of our current therapies. Since antidepressant medications often take weeks to months to achieve their full effects [2], patients remain vulnerable to the risk of self-harm. Sustainable pharmacological strategies with rapid onset profiles of hours or even days would therefore have an enormous impact on public health.
Finding a more direct target for depression
As noted above, a major limitation in the currently available monoaminergic antidepressants is their delayed onset of action. This delay has led to a heuristic `initiation and adaptation' hypothesis, which posits that the delay may be due to the medications exerting their primary effects considerably upstream of their ultimate therapeutic targets. In this context, it is noteworthy that a growing body of data suggests that upon chronic administration, antidepressants attenuate expression and/or function of NMDA receptor subunits. This has led to an interest in glutamatergic-based potential therapeutics [3–5]. Substantiating this interest, recent clinical studies have shown that the NMDA antagonist ketamine exerts rapid antidepressant effects [6,7]. Specifically, robust, rapid and sustained (1–2 weeks) antidepressant effects have been observed after a single intravenous ketamine infusion in patients with major treatment-resistant depression [7]. Understanding ketamine's effects on the glutamatergic system, a drug that has been shown to have rapid antidepressant action, could possibly lead to insights in developing the next generation of more rapid-acting and effective therapies.
The direct antagonist effects of ketamine on the NMDA receptor complex are well known [8]. In addition to being a non-competitive NMDA antagonist, ketamine also induces rapid increases in extracellular levels of glutamate by facilitating the presynaptic release of glutamate [9], a process that is believed to be mediated by NMDA autoreceptors, and/or by GABAergic interneurons.
We hypothesize that the antidepressant properties of ketamine are mediated by the functional interplay of AMPA and NMDA receptors. In our model, ketamine increases presynaptic release of glutamate, which in turn preferentially activates AMPA over NMDA receptors due to NMDA receptor (NMDAR) blockade. The net effect of this action is increased glutamatergic throughput of AMPARs relative to NMDA receptors.
Glutamatergic system – AMPA and NMDA receptor families
Glutamate exerts its function via three classes of ionotropic glutamate receptors, including AMPAR (GluR1-4), NMDAR (NR1, NR2A-D, NR3A-B) and kainate receptors (KA, GluR5–7, KA1-2), as well as a class of metabotropic glutamate receptors (mGluRs, mGluR1–8) [10,11]. Ionotropic glutamate receptors are ion channels that allow sodium, potassium and calcium to flow into or out of the cell upon glutamate stimulation. Pore opening and subsequent influx of ions leads to changes in membrane polarization as well as the direct activation of intracellular signaling pathways. The second group, `metabotropic receptors' are G-protein-coupled receptors that exert their action indirectly via second messenger pathways [10,11].
AMPAR are composed of four homologous subunits (GluR1-4), which assemble into various heteromeric tetramers. In the adult hippocampus, most AMPAR contain GluR1 or GluR3 subunits in combination with GluR2, which confers calcium impermeability [10,11]. The NMDAR is present in the brain primarily as a tetramer, in which two subunits are obligatory glycine-binding NR1 subunits, whereas the remaining two are typically glutamate-binding NR2 subunits (Fig. 1). The NR1 subunit plays a particularly important role in the targeting and transport of NMDARs from their site of assembly in the endoplasmic reticulum to sites on the cell surface. Each subunit consists of an extracellular amino-terminal and ligand-binding domain, three transmembrane domains plus a re-entrant pore loop, and an intracellular carboxyl-terminal domain [12] for all ion channel glutamate receptors.
Figure 1.

Structure of NMDA receptor. NMDAR is a tetramer comprised of two NR1 and two NR2 subunits. This 3D schematic shows a cross-section of the NMDAR through the channel pore. The NMDAR is composed of four domains: amino terminal domain (ATD), agonist binding domain, transmembrane domain and intracellular C-terminal domain (not shown). The NR1 subunit binds glycine or d-serine and the NR2A/B subunit binds glutamate. Activation of the NMDAR requires binding of both glycine (or d-serine) and glutamate, as well as simultaneous depolarization of the ion channel by AMPA or kainate receptors, which relieves the Mg2+ block. Under these conditions, the NMDAR channel will open and permit the entry of both Na+ and Ca2+ and the exit of K+. Within the ion channel, two additional binding sites have been identified: the sigma (σ) site (holding Mg2+) and the phencyclidine (PCP) site. The hallucinogenic drug PCP and ketamine, bind the PCP site and are considered noncompetitive receptor antagonists, which inhibit NMDAR channel function. Several other endogenous ligands interact with the NMDAR at different sites on the molecule and can have modulatory effects on its function. The endogenous polyamine spermine interacts with the NR2B subunit of the NMDAR and potentiates channel opening; paradoxically, it (and other polyamines) can also block the channel, reducing its conductance. Zinc also inhibits the NMDAR in a subtype-specific manner; receptors containing NR2A are inhibited by far lower concentrations of zinc than are those containing NR2B. Receptor activity is also modulated, at least in vitro, by redox state and pH. The 3D structure was cited from Dr Huggins' article [55].
Functional regulation of AMPAR by phosphorylation
Recent studies in cultured neurons and brain slices have shown that the AMPAR subunits (GluR1, GluR2 and GluR4) can be phosphorylated at serine, threonine or tyrosine residues located on the intracellular C-terminal domain (Fig. 2, reviewed in [10]). Protein kinase signaling cascades, such as protein kinase A, protein kinase C, Ca2+/calmodulin-dependent protein kinase II, and tyrosine kinases (Trks; or Src family kinases) are involved in site-specific AMPAR phosphorylation (Fig. 2, Table 1, [13–16]). Other glutamate (NMDAR) and G-protein coupled receptors (mGluRs, dopamine receptors DA1, 5-HT receptors) can also regulate AMPARs through protein phosphorylation and/or dephosphorylation mechanisms (Fig. 2, Table 1, reviewed in [10,11]). For example, stimulation of DA1 regulates GluR1 function via activation of PKA and the subsequent insertion of GluR1 into the neuronal membrane of cortical neurons [17]. It also has been reported that a growing body of evidence shows that protein phosphorylation directly modulates the electrophysiological (channel kinetics), morphological and biochemical (synthesis and subunit composition) properties of the AMPAR, as well as protein-protein interactions between the AMPAR subunits and various intracellular proteins (Table 1, [13–16]). These modulations underlie the major molecular mechanisms that ultimately affect many forms of synaptic plasticity in affective-associated neuronal circuitry [17,18].
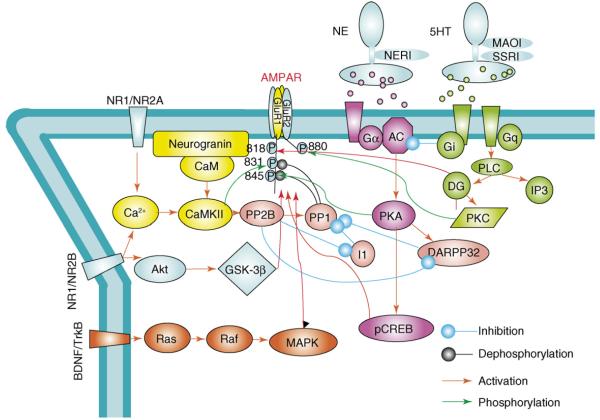
Figure 2.
Regulation of AMPAR trafficking by signaling cascades. AMPAR trafficking and channel properties are regulated by phosphorylation of C-terminal domain and subsequent alteration of protein–protein interactions. (1) Phosphorylation of GluR1at the PKA site S845 facilitates insertion of GluR1 into the neuronal membrane and is often associated with induction of long-term potentiation. Dephosphorylation of GluR1 by protein phosphatases (e.g. calcineurin and PP1) targets GluR1 to recycling endosomes, where re-phosphorylation by PKA can occur, with subsequent reinsertion into the neuronal membrane. (2) CREB has been shown to be important for AMPAR synthesis at the synapse. (3) In response to stimulation, CaMKII translocates to postsynaptic sites from dendrites and phosphorylates GluR1 at Ser831 to enhance AMPAR single channel conductance, as well as to aid in its delivery to the synapse (reviewed in [10]). (4) Ras mediates activity-evoked increases in synaptic GluR1 and GluR4 containing AMPARs via a pathway that requires p42/44 MAPK activation (reviewed in [10]). (5) It was recently shown that GSK-3β mediates NMDAR-induced GluR1/2 internalization (Professor Yu-Tian Wang, pers. commun.). Phosphorylation of Ser880 by PKC on GluR2 causes a switch from receptor retention at the membrane by binding to ABP/GRIP, to receptor internalization by binding to PICK 1 to release the AMPAR from anchoring proteins and initiate receptor internalization (reviewed in [10]).
Table 1.
Phosphorylation of AM PA and NMDA and receptors and regulation of their functions
| Receptor | Phosphosite | Kinase | Functional regulation | Refs |
|---|---|---|---|---|
| AM PA receptors | ||||
| GluR1 | S845 | PKA | Insertion of GluR1 onto the neuronal surface, increasing channel open probability | [13,16] |
| S831 | CAMKII/PKC | Trafficking of GluR1 to the synapses. Increasing single channel conductance | [13,36–38] | |
| S818 | PKC | Promoting GluR1 synaptic incorporation | [14] | |
| GluR2 | S863 | PKC | Regulating GluR2 internalization | [19] |
| Y876 | Src | Tyrosine phosphorylation of GluR2 dissociates GluR2 from GRIP1/2 | [39] | |
| S880 | PKC | Inhibition of the association of GluR2 to GRIP | [40] | |
| GluR4 | T830 | PKC | Unknown | [41] |
| S842 | PKC/PKA/CaMKII | Driving AMPA receptor to the synapses | [41] | |
| NMDA receptors | ||||
| NRI | S890 | PKC α | Dispersion of surface-associated cluster of NRI, membrane localization | [42] |
| S896 | PKC γ | Enhancing NMDAR delivery to the synapses | [42,43] | |
| S897 | PKA | Phosphorylation is significantly reduced in schizophrenia samples | [42,44] | |
| NR2A | Y402 | Pyk-2 | Li inhibits pNR2A Y402 increase after ischemia | [45] |
| Y416 | Src | Li inhibits pNR2A Y416 increase after ischemia | [45] | |
| Y1292 | Src and Fyn | Controlling NMDA receptor current modulation | [46] | |
| Y1325 | Src and Fyn | Unknown | [46] | |
| Y1387 | Src and Fyn | Controlling NMDA receptor current modulation | [46] | |
| S1232 | Cdk5 | Increasing spontaneous NMDA mediated Excitatary Post Synaptic Currents (EPSCs) | [47,48] | |
| S1291 S1312 |
PKC PKC |
Necessary and sufficient for insulin induced potentiation of NR2A receptors | [49] | |
| S1416 | PKC | Inhibiting of αCaMKII-binding and promoting dissociation of alphaCaMKII. NR2A complex | [50] | |
| NR2B | S1303 | PKC CaMKII | Phosphorylation of NR2B at Ser (1303) by CaMKII inhibits glutamate binding and promotes slow dissociation of preformed CaMKII.NR2B complexes | [51] |
| S1323 | PKC | Potentiation of EPSCs | [49] | |
| Y1336 | Fyn | Enhanced by Eupherin B | [52] | |
| Y1472 | Src | Potentiation of EPSCs, NMDA receptor insertion to the synapses; Li inhibits Src and NR2B SI472 phosphorylation | [52,53] | |
| S1480 | CK-2 | Phosphorylation leads to decreased surface expression of NR2B | [54] | |
Modulation of NMDAR functions by phosphorylation
NMDARs are targets for PKC and PKC-dependent tyrosine kinase cascades. There are two ways by which PKC modulates NMDA receptor – indirectly via Src family kinase (SFK) and by an alternative direct path. (1) Indirect path: via CAKβ then SFK cascades, PKC upregulates NMDAR function, by enhancing channel gating properties and increasing the number of receptors at the synapse (Fig. 3) [19,20]. Five members of SFK: Src, Fyn, Yes, Lck and Lyn, have been localized to the post-synaptic density [20]. SFK are important in regulation of NMDAR function through phosphorylation of specific sites on NMDA receptor subunits during synaptic plasticity and CNS development (Fig. 3 and Table 1). (2) Direct path: direct phosphorylation of NMDARs by PKC increases the sensitivity of the NMDAR to inactivation by intracellular calcium. In the hippocampus, PKC is a point of convergence for a variety of G-protein coupled and growth factor receptors at which to maintain control over NMDAR function and synaptic plasticity (Fig. 3 and Table 1) [19].
Figure 3.
Regulation of NMDAR functions by phosphorylation. NMDARs are regulated by several signaling cascades. (1) G-protein coupled receptors interact with and liberate α and βγ subunits, leading to activation of phospholipase C (PLC). PLC activation induces DG and IP3 production, leading to PKC activation and intracellular calcium release. PKC and calcium promote activation of CAKβ, and its association with SFK. Phosphorylation of NMDAR by SFK (indicated by red color) will either enhance NMDAR channel gating or synaptic localization. The phosphorylation sites of SFK are indicated by red color. PKC also directly phosphorylates NMDA receptors (sites marked in green) [56]. (2) Initial calcium influx and subsequent formation of the Ca 2+/calmodulin complex promotes binding of CaMKII to NMDAR. Continuous calcium influx through NMDAR further activates CaMKII, which phosphorylates NMDAR, AMPAR and other proteins important for long-term potentiation [23]. (3) Stimulation of G-protein coupled receptors (such as dopamine receptor 1) leads to cAMP synthesis and activation of PKA. PKA releases the inhibition of Fyn from RACK1 and also prevents STEP from interacting with its substrates to promote tyrosine phosphorylation of NMDAR. (4) Calcium influx and activation of calcineurin (PP2B) leads to dephosphorylation of STEP and thereby activation of STEP, which dephosphorylates NMDAR at their SFK sites [21].
Striatal enriched tyrosine phosphotase (STEP) was recently shown to play an important role in regulation of synaptic function [21]. STEP down-regulates NMDAR, Fyn and MAP kinase activity by dephosphorylation of key activation sites [21]. It participates in several pathways, such as PKA, CaMKII and MAPK pathways in regulating synaptic function. G-protein coupled receptors inactive STEP via PKA, whereas calcium activates STEP through a calcineurin-dependent mechanism (Fig. 3) [21].
Upon glutamate binding, influx of calcium through the NMDAR activates CaMKII. Ca2+/calmodulin then promotes binding of CaMKII to NMDAR. CaMKII binds NR2B by its catalytic site and by the autophosphorylation site-binding pocket (APBP), a non-catalytic site. Mutagenesis of Glu-236, a residue in the APBP of CaMKII that likely interacts with NR2B, influences phosphorylation of NR2B [22]. After CaMKII and NMDAR bind to one another, continued calcium influx through NMDAR further activates CaMKII, which can subsequently phosphorylate AMPAR and other synaptic proteins that can facilitate long-term potentiation (Fig. 3 and Table 1) [23,24]. Subunit-specific regulation of NMDAR phosphorylation was summarized in Table 1.
AMPA/NMDA receptors are targets of antidepressant drugs
Recent data suggest AMPAR involvement in the mechanism of action of effective antidepressants. Studies have shown that chronic antidepressant treatment with norepinephrine and/or serotonin reuptake inhibitors (reboxetine, fluoxetine and desipramine) induce positive regulation of AMPAR surface expression by altering the phosphorylation levels of specific AMPAR subunits [25]. Acute fluoxetine treatment increases GluR1 phosphorylation at Ser831 and Ser845 (reviewed in [10]). In addition, chronic treatment with imipramine enhances hippocampal expression of GluR1 and GluR2/3 and GluR1 Ser 845 phosphorylation in rats [18,26]. Taken together, these results suggest that enhanced AMPA receptor function and/or inhibition of NMDA receptor function is associated with antidepressant effects.
AMPA/NMDA modulators have efficacy in the animal behavioral models for mood disorders
Preclinical studies have shown that drugs exerting positive allosteric modulation of AMPAR, called AMPAkines also display antidepressant-like effects. Several classes of these small benzamide compounds have been developed, including benzylpiperidines (CX-516, CX-546), pyrroliddones (piracetam, aniracetam), benzothiazides (cyclothiazide) and biarylpropylsulfonamides (LY392098, LY404187) [27]. The antidepressant-like efficacy of AMPAkines was shown to be similar to standard antidepressants in animal models of depression [7]. AMPAkines have also been demonstrated to augment the effectiveness of standard antidepressants in animal models of depression [28].
Similar to AMPAkines, chronic treatment with NMDA antagonists induces antidepressant-like effects in different animal models of depression [29]. Ketamine, a noncompetitive NMDAR antagonist, showed significant antidepressant-like effects in animal models (reviewed in [11]). NMDAR antagonists such as MK-801 and AP-7 have been shown to have antidepressant properties in animal models of depression, including the forced swim test (FST), the application of inescapable stressors and tail suspension test (TST), and in the learned helplessness model of depression. The antidepressant effects of NMDA antagonists are comparable to the tricyclic antidepressants (reviewed in [30]). Interestingly, the antidepressant effects of ketamine seem to depend on the activation of AMPA-mediated synaptic activity (discussed in the next section). Similar to chronic treatment with diverse monoa-mine-based antidepressants, AMPAkines were shown to enhance the expression of BDNF, which has been shown to have antidepressant efficacy in animal models of depression [27]. Interestingly, the AMPAR antagonist NBQX inhibited an increase in BDNF levels whereas NMDA receptor blockers did not negatively regulate this neurotrophin, which suggests specific positive regulation induced by AMPAR pathways in the expression and release of BDNF [31]. In summary, an increase in AMPA/NMDA throughput, which indicates AMPA activation and NMDA inhibition, appears to be associated with antidepressant action.
The NMDA antagonist ketamine in treatment-resistant major depression
Preclinical and clinical research indicates that the NMDAR complex may also be involved in the therapeutic mechanisms of antidepressant and possibly in the pathophysiology of depression (reviewed in [30]). In a double-blind placebo-controlled crossover study, we found that a single intravenous infusion of ketamine (0.5 mg/kg over 40 min) in patients with treatment-resistant major depression resulted in a rapid onset of antidepressant effects within hours. Seventy-one percent met response criteria (50% decrease in Hamilton depression rating scales (HDRS) scores), and 29% achieved remission (≤7 HDRS) at 24 h following the infusion of ketamine. Six (35%) subjects maintained response to ketamine for at least 1 week. Mild perceptual disturbances occurred in most patients; overall the study mediation was well tolerated [7].
Although there is much preclinical information on the antidepressant-like properties of AMPA potentiators in depression, to date, there are no published studies with these types of compounds on their efficacy in mood disorders [32]. One trial is currently underwayat NIMH to determine whether an AMPA potentiator (ORG24448) is efficacious in major depression http://clinicaltrials.gov/ct/show/NCT00113022?order=1. We review below our preclinical findings with AMPAR.
Function interplay between NMDA and AMPA receptors: does increasing glutamatergic throughput of AMPA relative to NMDA result in rapid antidepressant response?
As discussed above, we found that a single-intravenous infusion of the non-competitive NMDA antagonist ketamine, resulted in rapid (within 2 h), robust and relatively sustained (1–2 weeks) antidepressant effects in patients with treatment-resistant major depression [7]. Using ketamine as a pharmacological tool could possibly lead to insights in developing the next generation of rapid-onset therapies.
The direct antagonist effects of ketamine on the NMDAR complex are well known [8]. In addition to being a non-competitive NMDA antagonist, ketamine also facilitates the presynaptic release of glutamate [9]. This increase in glutamate release then preferentially favors AMPAR over NMDAR because the NMDARs are blocked by ketamine; the net effect is an increased glutamatergic throughput of AMPA relative to NMDA. Notably antidepressants, upon chronic administration, have also been shown to enhance AMPAR surface levels [18,26]. In addition, AMPA potentiators have antidepressant-like properties in animal models [28]. We have therefore postulated that the therapeutic effects of both monoaminergic antidepressants and ketamine may be mediated by increased AMPA to NMDA throughput in critical neuronal circuits. The difference in the rapidity of onset of ketamine versus monoaminergic-based antidepressant (hours versus weeks/months, respectively) might be owing to the fact that traditional anti-depressants increase AMPA to NMDA throughput indirectly and gradually after a series of downstream signaling changes have occurred while ketamine does this more directly. Indeed, in animal behavioral studies, we found that in the forced swim test (FST), a test that has fairly high predictive validity in identifying antidepressant compounds, ketamine decreased the immobility time as also occurs with standard antidepressants [33,34]. In the same test, NBQX, an AMPA/Kainate antagonist, had no effects in FST when giving alone. When NBQX was giving immediately before ketamine and imipra-mine, it abolished the decrease in immobility time with keta-mine but not imipramine [31,35]. This finding suggests, at least in animal models, that the antidepressant-like properties of ketamine are mediated in part by AMPAR throughput.
Concluding remarks
Although there are many antidepressants available for the treatment of our patients, it is evident that there are limitations in what they offer. Recent preclinical and clinical studies indicate that the functional interplay between AMPA and NMDA receptors in how they modulate glutamatergic throughput may be particularly important to the onset of rapid antidepressant effects. We hypothesize that antidepressant effects are mediated by increased AMPA to NMDA throughput in critical neuronal circuits and that ketamine does this directly – by increasing glutamate release and concurrently blocking postsynaptic NMDA receptors, whereas monoaminergic antidepressants do this indirectly and gradually by producing delayed effects on AMPA and NMDA receptor phosphorylation. Indeed, data by our group supports our hypothesis. Furthermore, more direct targeting of AMPA to NMDA throughput might be a strategy for treatment-resistant depression as this strategy would bypass defects in critical circuits, which monoaminergic antidepressants require for their therapeutic effects. This line of research holds considerable promise and might lead to the next generation of rapid-acting antidepressants, which could help to reduce the initial morbidity and mortality associated with the delay of current treatments.
Acknowledgment
This study was supported by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health, and Department of Health & Human Services, The Stanly Medical Research Institute and NARSAD.
References
- 1.Trivedi MH, et al. Medication augmentation after the failure of SSRIs for depression. N. Engl. J. Med. 2006;354:1243–1252. doi: 10.1056/NEJMoa052964. [DOI] [PubMed] [Google Scholar]
- 2.Entsuah AR, et al. Response and remission rates in different subpopulations with major depressive disorder administered venlafaxine, selective serotonin reuptake inhibitors, or placebo. J. Clin. Psychiatry. 2001;62:869–877. doi: 10.4088/jcp.v62n1106. [DOI] [PubMed] [Google Scholar]
- 3.Mineur YS, et al. Antidepressant-like effects of ceftriaxone in male C57BL/6J mice. Biol. Psychiatry. 2006 doi: 10.1016/j.biopsych.2006.04.037. [DOI] [PubMed] [Google Scholar]
- 4.Sanacora G, et al. Clinical studies implementing glutamate neurotransmission in mood disorders. Ann. N Y Acad. Sci. 2003;1003:292–308. doi: 10.1196/annals.1300.018. [DOI] [PubMed] [Google Scholar]
- 5.Skolnick P. Modulation of glutamate receptors: strategies for the development of novel antidepressants. Amino Acids. 2002;23:153–159. doi: 10.1007/s00726-001-0121-7. [DOI] [PubMed] [Google Scholar]
- 6.Berman RM, et al. Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 7.Zarate CA, Jr, et al. A randomized trial of an N-methyl-d-aspartate antagonist in treatment-resistant major depression. Arch. Gen. Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 8.Krystal JH, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive and neuroendocrine responses. Arch. Gen. Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 9.Moghaddam B, et al. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J. Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du J, et al. Bipolar disorder: involment of signaling cascades and AMPA receptor trafficking at synapses. Neuron Glia Biol. 2004;1:231–243. doi: 10.1017/S1740925X05000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zarate CAJ, et al. Regulation of cellular plasticity cascades in the pathophysiology and treatment of mood disorders: role of the glutamatergic system. Ann. N Y Acad. Sci. 2003;1003:273–291. doi: 10.1196/annals.1300.017. [DOI] [PubMed] [Google Scholar]
- 12.Hollmann M, et al. N-glycosylation site tagging suggests a three transmembrane domain topology for the glutamate receptor GluR1. Neuron. 1994;13:1331–1343. doi: 10.1016/0896-6273(94)90419-7. [DOI] [PubMed] [Google Scholar]
- 13.Roche KW, et al. Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron. 1996;16:1179–1188. doi: 10.1016/s0896-6273(00)80144-0. [DOI] [PubMed] [Google Scholar]
- 14.Boehm J, et al. Synaptic incorporation of AMPA receptors during LTP is controlled by a PKC phosphorylation site on GluR1. Neuron. 2006;51:213–225. doi: 10.1016/j.neuron.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 15.Barria A, et al. Identification of the Ca2+/calmodulin-dependent protein kinase II regulatory phosphorylation site in the alpha-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate-type glutamate receptor. J. Biol. Chem. 1997;272:32727–32730. doi: 10.1074/jbc.272.52.32727. [DOI] [PubMed] [Google Scholar]
- 16.Banke TG, et al. Control of GluR1 AMPA receptor function by cAMP-dependent protein kinase. J. Neurosci. 2000;20:89–102. doi: 10.1523/JNEUROSCI.20-01-00089.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao C, et al. Activation of D1 dopamine receptors increases surface expression of AMPA receptors and facilitates their synaptic incorporation in cultured hippocampal neurons. J. Neurochem. 2006;98:1664–1677. doi: 10.1111/j.1471-4159.2006.03999.x. [DOI] [PubMed] [Google Scholar]
- 18.Du J, et al. Modulation of synaptic plasticity by antimanic agents: the role of AMPA glutamate receptor subunit 1 synaptic expression. J. Neurosci. 2004;24:6578–6589. doi: 10.1523/JNEUROSCI.1258-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDonald BJ, et al. Identification of protein kinase C phosphorylation sites within the AMPA receptor GluR2 subunit. Neuropharmacology. 2001;41:672–679. doi: 10.1016/s0028-3908(01)00129-0. [DOI] [PubMed] [Google Scholar]
- 20.Salter MW, Kalia LV. Src kinases: a hub for NMDA receptor regulation. Nat. Rev. Neurosci. 2004;5:317–328. doi: 10.1038/nrn1368. [DOI] [PubMed] [Google Scholar]
- 21.Braithwaite SP, et al. Synaptic plasticity: one STEP at a time. Trends Neurosci. 2006;29:452–458. doi: 10.1016/j.tins.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Praseeda M, et al. Interaction of peptide substrate outside the active site influences catalysis by CaMKII. Biochem. Biophys. Res. Commun. 2004;313:845–849. doi: 10.1016/j.bbrc.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 23.Merrill MA, et al. Activity-driven postsynaptic translocation of CaMKII. Trends Pharmacol. Sci. 2005;26:645–653. doi: 10.1016/j.tips.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Liu L, et al. Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science. 2004;304:1021–1024. doi: 10.1126/science.1096615. [DOI] [PubMed] [Google Scholar]
- 25.Barbon A, et al. Regulation of editing and expression of glutamate alpha-amino-propionic-acid (AMPA)/kainate receptors by antidepressant drugs. Biol. Psychiatry. 2006;59:713–720. doi: 10.1016/j.biopsych.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 26.Du J, et al. The anticonvulsants lamotrigine, riluzole, and valproate differentially regulate AMPA receptor membrane localization: relationship to clinical effects in mood disorders. Neuropsycophamarcology. 2006 doi: 10.1038/sj.npp.1301178. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 27.Alt A, et al. AMPA receptor potentiators as novel antidepressants. Curr. Pharm. Des. 2005;11:1511–1527. doi: 10.2174/1381612053764814. [DOI] [PubMed] [Google Scholar]
- 28.Li X, et al. Antidepressant-like actions of an AMPA receptor potentiator (LY392098) Neuropharmacology. 2001;40:1028–1033. doi: 10.1016/s0028-3908(00)00194-5. [DOI] [PubMed] [Google Scholar]
- 29.Kendell SF, et al. GABA and glutamate systems as therapeutic targets in depression and mood disorders. Expert Opin. Ther. Targets. 2005;9:153–168. doi: 10.1517/14728222.9.1.153. [DOI] [PubMed] [Google Scholar]
- 30.Zarate CA, et al. Modulators of the glutamatergic system: implications for the development of improved therapeutics in mood disorders. Psychopharmacol. Bull. 2002;36:35–83. [PubMed] [Google Scholar]
- 31.Legutko B, et al. Regulation of BDNF expression in primary neuron culture by LY392098, a novel AMPA receptor potentiator. Neuropharmacology. 2001;40:1019–1027. doi: 10.1016/s0028-3908(01)00006-5. [DOI] [PubMed] [Google Scholar]
- 32.Manji HK, et al. Enhancing neuronal plasticity and cellular resilience to develop novel, improved therapeutics for difficult-to-treat depression. Biol. Psychiatry. 2003;53:707–742. doi: 10.1016/s0006-3223(03)00117-3. [DOI] [PubMed] [Google Scholar]
- 33.Kirby LG, Lucki I. Interaction between the forced swimming test and fluoxetine treatment on extracellular 5-hydroxytryptamine and 5-hydroxyindoleacetic acid in the rat. J. Pharmacol. Exp. Ther. 1997;282:967–976. [PubMed] [Google Scholar]
- 34.Cooper BR, et al. Behavioral and biochemical effects of the antidepressant bupropion (Wellbutrin): evidence for selective blockade of dopamine uptake in vivo. J. Pharmacol. Exp. Ther. 1980;215:127–134. [PubMed] [Google Scholar]
- 35.Maeng S, et al. Cellular mechanisms underlying the antidepressant effects of Ketamine: role of AMPA receptors. Biol. Psychiatry. doi: 10.1016/j.biopsych.2007.05.028. in press. [DOI] [PubMed] [Google Scholar]
- 36.Barria A, et al. Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science. 1997;276:2042–2045. doi: 10.1126/science.276.5321.2042. [DOI] [PubMed] [Google Scholar]
- 37.Lisman JE, Zhabotinsky AM. A model of synaptic memory: a CaMKII/PP1 switch that potentiates transmission by organizing an AMPA receptor anchoring assembly. Neuron. 2001;31:191–201. doi: 10.1016/s0896-6273(01)00364-6. [DOI] [PubMed] [Google Scholar]
- 38.Derkach V, et al. Ca2+/calmodulin-kinase II enhances channel conductance of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate type glutamate receptors. Proc. Natl. Acad. Sci. U S A. 1999;96:3269–3274. doi: 10.1073/pnas.96.6.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hayashi T, Huganir RL. Tyrosine phosphorylation and regulation of the AMPA receptor by SRC family tyrosine kinases. J. Neurosci. 2004;24:6152–6160. doi: 10.1523/JNEUROSCI.0799-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chung HJ, et al. Requirement of AMPA receptor GluR2 phosphorylation for cerebellar long-term depression. Science. 2003;300:1751–1755. doi: 10.1126/science.1082915. [DOI] [PubMed] [Google Scholar]
- 41.Carvalho AL, et al. Characterization of phosphorylation sites on the glutamate receptor 4 subunit of the AMPA receptors. J. Neurosci. 1999;19:4748–4754. doi: 10.1523/JNEUROSCI.19-12-04748.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tingley WG, et al. Characterization of protein kinase A and protein kinase C phosphorylation of the N-methyl-d-aspartate receptor NR1 subunit using phosphorylation site-specific antibodies. J. Biol. Chem. 1997;272:5157–5166. doi: 10.1074/jbc.272.8.5157. [DOI] [PubMed] [Google Scholar]
- 43.Sanchez-Perez AM, Felipo V. Serines 890 and 896 of the NMDA receptor subunit NR1 are differentially phosphorylated by protein kinase C isoforms. Neurochem. Int. 2005;47:84–91. doi: 10.1016/j.neuint.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 44.Emamian ES, et al. Decreased phosphorylation of NMDA receptor type 1 at serine 897 in brains of patients with Schizophrenia. J. Neurosci. 2004;24:1561–1564. doi: 10.1523/JNEUROSCI.4650-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ma J, et al. Lithium suppressed Tyr-402 phosphorylation of proline-rich tyrosine kinase (Pyk2) and interactions of Pyk2 and PSD-95 with NR2A in rat hippocampus following cerebral ischemia. Neurosci. Res. 2004;49:357–362. doi: 10.1016/j.neures.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 46.Yang M, Leonard JP. Identification of mouse NMDA receptor subunit NR2A C-terminal tyrosine sites phosphorylated by coexpression with v-Src. J. Neurochem. 2001;77:580–588. doi: 10.1046/j.1471-4159.2001.00255.x. [DOI] [PubMed] [Google Scholar]
- 47.Wang J, et al. Cdk5 activation induces hippocampal CA1 cell death by directly phosphorylating NMDA receptors. Nat. Neurosci. 2003;6:1039–1047. doi: 10.1038/nn1119. [DOI] [PubMed] [Google Scholar]
- 48.Li BS, et al. Regulation of NMDA receptors by cyclin-dependent kinase-5. Proc. Natl. Acad. Sci. U S A. 2001;98:12742–12747. doi: 10.1073/pnas.211428098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jones ML, Leonard JP. PKC site mutations reveal differential modulation by insulin of NMDA receptors containing NR2A or NR2B subunits. J. Neurochem. 2005;92:1431–1438. doi: 10.1111/j.1471-4159.2004.02985.x. [DOI] [PubMed] [Google Scholar]
- 50.Gardoni F, et al. Protein kinase C activation modulates alpha-calmodulin kinase II binding to NR2A subunit of N-methyl-d-aspartate receptor complex. J. Biol. Chem. 2001;276:7609–7613. doi: 10.1074/jbc.M009922200. [DOI] [PubMed] [Google Scholar]
- 51.Strack S, et al. Mechanism and regulation of calcium/calmodulin-dependent protein kinase II targeting to the NR2B subunit of the N-methyl-d-aspartate receptor. J. Biol. Chem. 2000;275:23798–23806. doi: 10.1074/jbc.M001471200. [DOI] [PubMed] [Google Scholar]
- 52.Nakazawa T, et al. Characterization of Fyn-mediated tyrosine phosphorylation sites on GluR epsilon 2 (NR2B) subunit of the N-methyl-d-aspartate receptor. J. Biol. Chem. 2001;276:693–699. doi: 10.1074/jbc.M008085200. [DOI] [PubMed] [Google Scholar]
- 53.Hashimoto R, et al. Lithium-induced inhibition of Srctyrosine kinase in rat cerebral cortical neurons: a role in neuroprotection against N-methyl-d-aspartate receptor-mediated excitotoxicity. FEBS Lett. 2003;538:145–148. doi: 10.1016/s0014-5793(03)00167-4. [DOI] [PubMed] [Google Scholar]
- 54.Chung HJ, et al. Regulation of the NMDA receptor complex and trafficking by activity-dependent phosphorylation of the NR2B subunit PDZ ligand. J. Neurosci. 2004;24:10248–10259. doi: 10.1523/JNEUROSCI.0546-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huggins DJ, Grant GH. The function of the amino terminal domain in NMDA receptor modulation. J. Mol. Graph Model. 2005;23:381–388. doi: 10.1016/j.jmgm.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 56.Anwyl R. Induction and expression mechanisms of postsynaptic NMDA receptor-independent homosynaptic long-term depression. Prog. Neurobiol. 2006;78:17–37. doi: 10.1016/j.pneurobio.2005.12.001. [DOI] [PubMed] [Google Scholar]