Abstract
Cytogenetically normal acute myeloid leukemia (CN-AML) is a heterogeneous disease with variable clinical outcomes. Emerging data has identified molecular markers that provide additional prognostic information to better classify these patients into those with a more favorable prognosis and those with an unfavorable prognosis who may require more aggressive or investigational therapies. Markers such as mutations in nucleophosmin 1 gene and CCAAT/enhancer binding protein alpha gene have been associated with a more favorable prognosis in CN-AML. In contrast, FMS-related tyrosine kinase 3 mutations, partial tandem duplication of mixed-lineage leukemia gene and overexpression of brain and acute leukemia, cytoplasmic gene are associated with inferior clinical outcomes. In this article, the authors discuss the classical clinical features of AML and the importance of cytogenetics that predict prognosis in AML. They review the best-described molecular markers in CN-AML and their significance to clinical decision making in CN-AML.
Key Indexing Terms: Acute myeloid leukemia, AML, Prognosis, Molecular markers, Cytogenetics
Acute myeloid leukemia (AML) is a heterogeneous disease with variable clinical outcomes. It is estimated that nearly 13,000 new cases will be diagnosed in the United States this year and 9,000 patients will die from AML.1 In the 1970s, the French-American-British classification of AML described 8 subtypes of AML (M0–M7) based primarily on morphology, cytochemical stains and cell surface markers.2 In 1999, the World Health Organization classification incorporated additional features including karyotype, molecular markers and morphologic evidence for dysplasia into its AML classification, resulting in a total of 17 subclasses of AML.3 Further revisions in 2008 incorporated emerging data regarding the significance of specific gene mutations.4 Remission and survival rates for patients with AML differ when stratified by either clinical features or biologic markers. Historically, clinical features were used to predict the probability of attaining a complete remission to induction chemotherapy and long-term disease-free survival. The most significant unfavorable clinical features include advanced age, poor performance status, white blood cell (WBC) count greater than 30,000/microliter, history of prior cytotoxic or radiation therapy and history of antecedent hematologic disorder (ie, myelodysplasia or myeloproliferative disorders) (Table 1). More important than clinical features, cytogenetic analysis of a patient’s leukemic blasts remains the most significant predictor of remission rate and survival.5–7 This information often guides the selection of postremission therapy, with the most aggressive treatments, including bone marrow transplantation, traditionally reserved for those patients with the least favorable prognosis. AML karyotypes are most commonly classified into 3 prognostic categories (Table 2) with differing median survivals as noted by data from the Cancer and Leukemia Group B and other published reports: favorable risk, 7.6 years; intermediate risk, 1.3 years; and poor risk, 0.5 years.7
TABLE 1.
Clinical features associated with adverse prognosis
| White blood cell count >30,000/microliter |
| Extramedullary leukemia |
| Advanced age |
| Poor performance status |
| AML arising from a prior bone marrow disorder |
| Therapy-related AML (chemotherapy or radiation) |
TABLE 2.
Prognostic categories and associated cytogenetic abnormalities in AML
| Risk status | Karyotype |
|---|---|
| Favorable | Inversion 16 or t(16;16) |
| t(8;21) | |
| t(15;17) | |
| Intermediate | Normal cytogenetics |
| +8 | |
| t(9;11) | |
| Other nondefined | |
| Poor | Complex (≥3 abnormal clones) |
| -5, -5q, -7, -7q | |
| 11q23 | |
| Inversion 3 or t(3;3) | |
| t(6;9) | |
| t(9;22) |
Clinicians caring for patients with AML are often challenged to determine the best course of therapy when patients do not have specific prognostic markers. The best example of this is seen in patients with normal cytogenetics that fall into an intermediate-risk category. Clinical experience with cytogenetically normal AML (CN-AML) suggests that some patients do better than “intermediate” whereas others clearly do much worse. Within CN-AML, gene mutations and gene expression profiles have begun to reveal new markers that seem to suggest which patients with normal karyotype have better or worse prognosis.8–10 These include mutations or activation of FMS-like tyrosine kinase 3 gene (FLT3), mutations in the nucleophosmin 1 gene (NPM1) and mutations in the myeloid transcription factor CCAAT/enhancer binding protein alpha (CEBPA), as well as others.11–13 The additional molecular markers may shift patients with CN-AML from intermediate risk to better risk [in the case of NPM1 mutation or isolated CEBPA mutation in the absence of a FLT3-internal tandem repeat (ITD) mutation] or from intermediate risk to poor risk (in the case of FLT3-ITD mutation in the absence of NPM1 mutation).14 Nearly 50% of patients with AML have normal cytogenetics, and these emerging prognostic markers may help guide treatment decisions and suggest targets for novel therapies. In fact, in 2008, the World Health Organization revised their classification of AML to include molecular markers such as FLT3 and NPM1, recognizing their clinical significance as a distinct entity within CN-AML.4 In this review, we will discuss gene mutations and gene expression profiles in CN-AML and how their discovery impacts clinical management. We present a case of a young woman with newly diagnosed CN-AML and conflicting prognostic markers.
CASE REPORT
A 29 year-old woman was transferred to our hospital with a suspected diagnosis of AML. Approximately 2 months before her presentation, she complained of symptoms of fatigue and low-grade fevers. Several weeks before admission, she was seen by her primary physician with new onset of a cough. She was treated with antibiotics for presumed bronchitis and was noted to have some bleeding of her gums. With antibiotics, the cough improved, but her fatigue worsened. She went on to develop lightheadedness and heart palpitations. She was seen by her physician, and a complete blood count at that time revealed a hematocrit of 18% and WBC count of 21,000/microliter with 37% blasts. She was admitted to her local hospital, and a diagnostic evaluation for leukemia was begun. The bone marrow was inaspirable, and touch preparation from the bone marrow biopsy showed blasts consistent with acute leukemia. She was transferred to our hospital for further evaluation and management.
At the time of transfer, her WBC count measured 28,000/microliter, hematocrit 28% and platelets of 75,000. There were 59% circulating blasts morphologically. Coagulation studies were normal. Repeat bone marrow aspirate and biopsy showed a markedly hypercellular marrow with >80% blasts identified by their increased size and high nuclear to cytoplasmic ratio, fine chromatin and prominent nucleoli (Figure 1). By flow cytometry, the blasts had higher than expected side scatter and marked with bright CD33, dim CD71, CD13, CD34, CD38, CD117 and CD15. They also had partial CD14 and CD64 expression suggestive of AML with monocytic differentiation. There were no Auer rods seen. Diagnostic testing, including traditional bone marrow cytogenetics, fluorescence in situ hybridization for common AML karyotypic abnormalities and molecular testing for FLT3 and NPM1 mutations was performed by our laboratory on the bone marrow aspirate.
FIGURE 1.
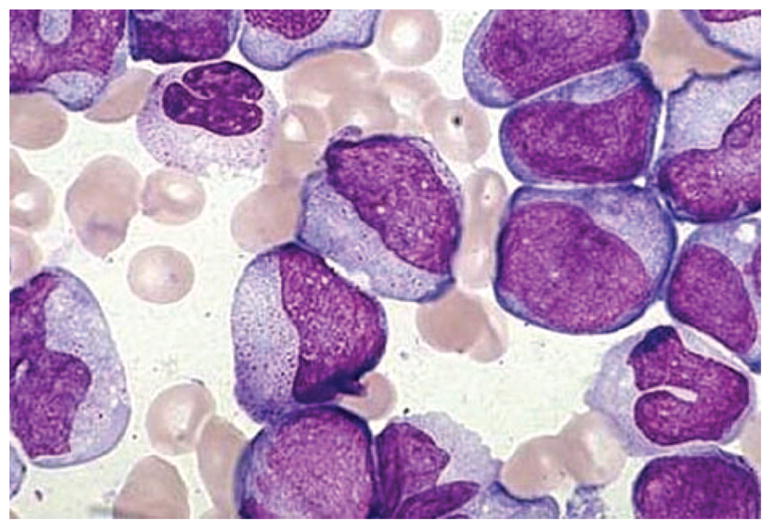
Bone marrow biopsy showing leukemic blasts. Bone marrow biopsy showing a uniform population of large, immature cells with prominent nucleoli.
Induction chemotherapy for AML was initiated per our institutional standard timed sequential therapy [consisting of daunorubicin 45 mg/m2 intravenous (IV) push (days 1, 2 and 3) + cytarabine 2 g/m2 continuous IV infusion (days 1, 2 and 3) + etoposide 400 mg/m2 IV over 6 hours (days 8, 9 and10)]. Classical cytogenetics and fluorescence in situ hybridization testing revealed a normal karyotype; however, molecular studies confirmed the presence of both an internal tandem duplication of FLT3 (FLT3-ITD) and an NPM1 mutation. A bone marrow aspirate and biopsy on count recovery showed a morphologic remission with 2% blasts by flow cytometry. Treatment options contemplated at this time included proceeding with a chemotherapy-based consolidation regimen versus the addition of a FLT3-targeted agent versus moving directly to an allogeneic stem cell transplant. Based on the FLT3-ITD mutation, our team favored an allogeneic stem cell transplant as there were no available FLT3 inhibitor trials at our institution. Unfortunately, neither of the patients’ 2 siblings were HLA matches. Based on these limits to her treatment options, the patient went on to a single course of high-dose cytarabine (3 g/m2 every 12 hours on days 1, 3 and 5) while efforts to find an alternative donor were undertaken. Based on her rare HLA typing, there were no matching donors in the national or in the international transplant registries. Although there are little transplant outcomes data for FLT3-ITD AML using alternative donors (including the use of cord blood stem cells), our institutional program offered her an allogeneic transplant using a haploidentical sibling donor on a high-risk protocol. The patient underwent an allogeneic stem cell transplant with minimal residual disease after recovery from a single course of high-dose cytarabine using a myeloablative preparative regimen and a haploidentical donor.
DISCUSSION
Cytogenetics remains the best prognostic marker for AML, and, unfortunately, with CN-AML accounting for nearly 50% of all cases of adult AML, many patients receive an “intermediate” prognosis. More recently, molecular discoveries have identified additional abnormalities, many present within CN-AML, which offer additional prognostic information. These emerging biologic markers are now becoming vital in distinguishing patients with CN-AML into those with a better prognosis from those with potentially very poor prognosis. It is now becoming commonplace for physicians to use these results to help guide therapeutic decisions. We will briefly review the most well described of these molecular markers of prognosis (Table 3).
TABLE 3.
Genetic markers in CN-AML associated with favorable and unfavorable prognosis
| Markers with favorable prognosis | Markers with unfavorable prognosis |
|---|---|
| NPM1 mutation | FLT3 mutation |
| CEBPA mutation | MLL-PTD |
| BAALC overexpression | |
| ERG overexpression | |
| EVI1 overexpression | |
| IDH1 mutation | |
| IDH2 mutation | |
| ABCG2 overexpression |
Favorable Markers
Nucleophosmin 1 Gene
Mutations of the NPM1 are the most frequently observed genetic abnormality in CN-AML occurring in approximately 50% of cases.11,15–17 More than 50 different mutations have been described in exon 12,11 and because they are readily identified, this test has become routinely available in many diagnostic laboratories. In addition, these mutations seem to be stable throughout disease evolution, suggesting a possible role in monitoring minimal residual disease. NPM1 normally functions as a molecular chaperone to regulate processing and transport of preribosomal particles through the nucleus to the cytoplasm. When mutated, NPM1 aberrantly localizes to the cytoplasm and disrupts this transport pathway. Other known functions of NPM1 include regulation of the ARF-p53 tumor suppressor pathway and interactions with CDK2-cyclin E.11 Clinical features associated with NPM1 mutated CN-AML include female sex,15,16 higher WBC count at presentation15–17 and increased bone marrow blasts.16,17 Analyses confirmed in several series that the presence of NPM1 as an isolated abnormality predicted improved rates of complete response,16,17 disease-free survival16 and overall survival.16,17 Interestingly, NPM1 mutations appear commonly along with other mutations, including the poor-risk FLT3-ITD mutation.15–17 It remains unclear how such mutations may interact, but early analyses suggest that there is little advantage provided by the NPM1 mutation and outcomes are similar to those of an isolated FLT3 mutation.15
CCAAT/Enhancer Binding Protein Alpha
The CCAAT/enhancer binding protein alpha (CEBPA) is critical to granulocyte maturation. N-terminus mutations result in a truncated protein, and C-terminus mutations affect dimerization and DNA binding.18 In several large series, 15% to 19% of CN-AML harbor CEBPA mutations, either homozygous or biallelic compound heterozygous mutations.8,12 Clinically, these patients often present with higher circulating blasts in the peripheral blood and lower platelet counts.12 There was no difference seen in rates of complete remission regardless of CEBPA mutation status. Mutations in CEBPA have independent prognostic significance in CN-AML, and they predict a more favorable outcome in terms of disease-free and overall survivals.8,12 Patients with CN-AML with biallelic mutations in CEBPA and unmutated FLT3 have a prognosis similar to those with cytogenetic abnormalities classified as favorable.12 In contrast to the growing availability of diagnostic testing for NPM1 mutations, the diversity of mutations seen in CEBPA has made the development of a diagnostic test more complicated, and at this time, CEBPA mutation testing is not routinely available.
Unfavorable Markers
FMS-Related Tyrosine Kinase 3
FLT3 gene mutations are the most well-characterized mutation in CN-AML. FLT3 is a receptor tyrosine kinase normally expressed on hematopoietic progenitors, and it critically regulates cell survival and maturation. ITD in the juxtamembrane domain are the most common mutations, occurring in up to one third of patients,13,19,20 and point mutations may occur in the tyrosine kinase domain (FLT3-TKD) or juxtamembrane domain. These mutations all result in a constitutively active FLT3 receptor, leading to abnormal downstream activation of signaling pathways of cell proliferation and survival. FLT3-ITD mutations are significantly associated with inferior outcome including worse disease-free, event-free and overall survivals.13,19,20 Studies looking at the clinical impact of FLT3 point mutations in CN-AML have not clearly demonstrated a worse prognosis compared with wild-type FLT3 status.13 It is clear that patients with CN-AML and FLT3-ITD mutations present with a reproducible pattern of not only high WBC but also higher peripheral blood blast percentages and a myelomonocytic/monocytic morphology.13 Several small molecule inhibitors of FLT3 tyrosine kinase are being actively studied in both early-phase clinical trials and in later-phase trials in combination with chemotherapy.21,22 Given the poor prognosis conferred by FLT3-ITD mutations, patients are encouraged to participate in clinical trials or pursue aggressive therapy including stem cell transplant as consolidation. This is not the case with the less common FLT3-TKD mutations (approximately 5% of patients with AML). In a recent series of >3000 patients with AML, the clinical and prognostic characteristics of AML with FLT3-TKD mutations were described. There were no significant differences seen in age or sex of the patients when correlated with FLT3-TKD status; however, patients with FLT3-TKD mutations had significantly higher WBC counts versus patients with either wild-type FLT3 or FLT3-ITD mutations. There were no significant differences in either event-free or overall survival seen with the FLT3-TKD mutations. These data suggest thatFLT3-ITD and FLT3-TKD mutations in FLT3 represent distinct biological entities and that FLT3-TKD mutations do not seem to confer a worse prognosis in patients with CN-AML.23 Additional studies to clarify the prognostic significance, if any, of FLT3-TKD mutations are needed, as well as data regarding the effects of FLT3 inhibitors in patients with both types of FLT3 mutations.
Mixed-Lineage Leukemia Gene—Partial Tandem Duplications
Mixed-lineage leukemia gene (MLL) mutations are often seen as chromosomal rearrangements involving the MLL gene (at band 11q23) and are commonly seen in secondary AML related to the use of cytotoxic drugs with topoisomerase inhibitory activity. Within CN-AML, however, there are partial tandem duplications of the MLL gene (MLL-PTD) in approximately 8% of patients.24,25 Patients with MLL-PTD do not vary in their clinical presentation from other patients with CN-AML. Although studies have not demonstrated differences in rates of complete remission or overall survival, duration of complete remission, event-free survival and relapse-free survival were significantly shorter in patients with MLL-PTD.24 Histone deactylase inhibitors have been shown to restore wild-type MLL activity in vitro, resulting in apoptosis of leukemic blasts,26 suggesting a potential therapeutic strategy. Importantly, up to 40% of patients with MLL-PTD may also have FLT3-ITD mutations,25 and aggressive treatment with allogeneic transplant or clinical trials is strongly recommended.
Overexpression of Brain and Acute Leukemia, Cytoplasmic Gene
Brain and acute leukemia, cytoplasmic gene (BAALC) overexpression is another biomarker associated with inferior outcomes in CN-AML. Normally expressed in CD34 positive hematopoietic progenitors and neural tissues, BAALC seems to function in the cytoskeleton.27 Expression of BAALC was associated with a statistically significant difference in presenting WBC count and morphology (lower BAALC expression associated with higher WBC count and M5 morphology), but otherwise patients did not differ in terms of other blood counts, blast percentage, age, sex, race or presence of extramedullary disease.28 BAALC overexpression is significantly associated with decreased remission duration and overall survival.8,28,29 These clinical findings are also seen in patients with both BAALC overexpression and FLT3-ITD mutations.29 A recent report suggests that monitoring levels of BAALC may be valuable in the setting of minimal residual disease.30
Other Proposed Prognostic Markers
Wilms tumor 1 (WT1) gene mutations are seen approximately in 10% of CN-AML.31,32 WTI is a transcription factor that regulates genes of cell metabolism; its role in normal hematopoiesis is unclear. The use of WT1 mutation status to measure minimal residual disease remains of interest as these are readily identifiable at low levels from blood or marrow in patients with AML.33 Prognostic significance for WT1 mutations or overexpression in CN-AML remains under investigation as conflicting studies alternately suggest that it confers a poor prognosis versus others showing no significant difference in clinical outcome.31,32 Conversely, Damm et al34 suggest improved outcomes for CN-AML with a specific single nucleotide polymorphism rs16754 in WT1 and that this favorable outcome was more pronounced in NPM1 unmutated/FLT3-ITD mutated patients. Further studies of WT1 in larger cohorts of CN-AML are needed to clarify its prognostic significance.
In addition to FLT3-ITD mutations and overexpression of MLL-PTD and BAALC, other molecular markers have been identified as conferring a poor prognosis in CN-AML. These include overexpression of v-ets erythroblastosis virus E26 oncogene homolog avian, ERG,35 meningioma 1gene, MN1,36 ecotropic virus integration-1 gene, EVI1,37 and ATP-binding cassette, subfamily G, member 2, ABCG2,38 and mutations in isocitrate dehydrogenase 1, IDH1, and 2, IDH2.39 These preliminary reports suggest markers for further investigations in larger cohorts of patients with CN-AML.
CN-AML has quite varied clinical outcomes, and emerging molecular data suggest many of these specific abnormalities confer important prognostic information. Identification and correlation of these markers with prognosis has been accomplished using tissues from multicenter studies supported by large cooperative groups and the biospecimens collected for such studies. Continued prospective studies to identify additional biologic markers are ongoing, and further clinical trials of targeted agents in those patients with inferior prognosis are needed to improve survival for patients with CN-AML. Developing therapies to counteract the impact of these biomarkers remains the challenge of many clinical researchers. For example, clinical trials of biologically active FLT3 inhibitors have yet to offer meaningful outcomes data, and questions remain on how to best incorporate them into treatment schemas. For example, should such agents be included as part of initial induction chemotherapy, used in the postremission period as maintenance or saved for patients who relapse? The optimal timing of such agents also comes into question when combining them with cytotoxic chemotherapy. In our patient, the presence of a FLT3-ITD mutation, the minimal residual disease after induction therapy and the lack of an available trial studying FLT3 inhibitors prompted an aggressive treatment course using an alternative donor stem cell transplant. Although it remains unclear if the use of such transplants can ultimately overcome the poor risk nature of FLT3-ITD mutations, their use has been advocated when other options are limited for patients.10,40
Current treatment recommendations for AML now include risk-adapted strategies according to NPM1, FLT3 and CEBPA status, as these tests are becoming routinely available,14 but these have not yet replaced the traditional clinical markers of inferior prognosis. Patients with CN-AML and markers conferring worse prognosis may best benefit from aggressive treatments including bone marrow transplant or the use of investigational agents, as conventional chemotherapy is unlikely to result in long-term survival. Identifying prognostic molecular markers and understanding their biology are the first steps toward developing novel, personalized therapies for each patient with CN-AML.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Bennett J, Catovsky D, Daniel M, et al. Proposals for the classification of the acute leukaemias. French-American-British (FAB) cooperative group. Br J Haematol. 1976;33:451–8. doi: 10.1111/j.1365-2141.1976.tb03563.x. [DOI] [PubMed] [Google Scholar]
- 3.Harris N, Jaffe E, Diebold J, et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting-Airlie House, Virginia, November 1997. J Clin Oncol. 1999;17:3835–49. doi: 10.1200/JCO.1999.17.12.3835. [DOI] [PubMed] [Google Scholar]
- 4.Vardiman J, Thiele J, Arber D, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114:937–51. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 5.Keating M, Smith T, Kantarjian H, et al. Cytogenetic pattern in acute myelogenous leukemia: a major reproducible determinant of outcome. Leukemia. 1988;2:403–12. [PubMed] [Google Scholar]
- 6.Grimwade D, Walker H, Oliver F, et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children’s Leukaemia Working Parties. Blood. 1998;92:2322–33. [PubMed] [Google Scholar]
- 7.Byrd J, Mrózek K, Dodge R, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461) Blood. 2002;100:4325–36. doi: 10.1182/blood-2002-03-0772. [DOI] [PubMed] [Google Scholar]
- 8.Bienz M, Ludwig M, Leibundgut E, et al. Risk assessment in patients with acute myeloid leukemia and a normal karyotype. Clin Cancer Res. 2005;11:1416–24. doi: 10.1158/1078-0432.CCR-04-1552. [DOI] [PubMed] [Google Scholar]
- 9.Mrózek K, Marcucci G, Paschka P, et al. Clinical relevance of mutations and gene-expression changes in adult acute myeloid leukemia with normal cytogenetics: are we ready for a prognostically prioritized molecular classification? Blood. 2007;109:431–48. doi: 10.1182/blood-2006-06-001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schlenk R, Döhner K, Krauter J, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358:1909–18. doi: 10.1056/NEJMoa074306. [DOI] [PubMed] [Google Scholar]
- 11.Falini B, Nicoletti I, Martelli M, et al. Acute myeloid leukemia carrying cytoplasmic/mutated nucleophosmin (NPMc+ AML): biologic and clinical features. Blood. 2007;109:874–85. doi: 10.1182/blood-2006-07-012252. [DOI] [PubMed] [Google Scholar]
- 12.Fröhling S, Schlenk R, Stolze I, et al. CEBPA mutations in younger adults with acute myeloid leukemia and normal cytogenetics: prognostic relevance and analysis of cooperating mutations. J Clin Oncol. 2004;22:624–33. doi: 10.1200/JCO.2004.06.060. [DOI] [PubMed] [Google Scholar]
- 13.Fröhling S, Schlenk R, Breitruck J, et al. Prognostic significance of activating FLT3 mutations in younger adults (16 to 60 years) with acute myeloid leukemia and normal cytogenetics: a study of the AML Study Group Ulm. Blood. 2002;100:4372–80. doi: 10.1182/blood-2002-05-1440. [DOI] [PubMed] [Google Scholar]
- 14.Members NAMLP. Acute Myeloid Leukemia. Vol. 2010. Fort Washington (PA): NCCN; 2010. NCCN Practice Guidelines in Oncology version 2.2010. [Google Scholar]
- 15.Schnittger S, Schoch C, Kern W, et al. Nucleophosmin gene mutations are predictors of favorable prognosis in acute myelogenous leukemia with a normal karyotype. Blood. 2005;106:3733–9. doi: 10.1182/blood-2005-06-2248. [DOI] [PubMed] [Google Scholar]
- 16.Thiede C, Koch S, Creutzig E, et al. Prevalence and prognostic impact of NPM1 mutations in 1485 adult patients with acute myeloid leukemia (AML) Blood. 2006;107:4011–20. doi: 10.1182/blood-2005-08-3167. [DOI] [PubMed] [Google Scholar]
- 17.Döhner K, Schlenk R, Habdank M, et al. Mutant nucleophosmin (NPM1) predicts favorable prognosis in younger adults with acute myeloid leukemia and normal cytogenetics: interaction with other gene mutations. Blood. 2005;106:3740–6. doi: 10.1182/blood-2005-05-2164. [DOI] [PubMed] [Google Scholar]
- 18.Pabst T, Mueller B, Zhang P, et al. Dominant-negative mutations of CEBPA, encoding CCAAT/enhancer binding protein-alpha (C/EBPalpha), in acute myeloid leukemia. Nat Genet. 2001;27:263–70. doi: 10.1038/85820. [DOI] [PubMed] [Google Scholar]
- 19.Kottaridis P, Gale R, Frew M, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood. 2001;98:1752–9. doi: 10.1182/blood.v98.6.1752. [DOI] [PubMed] [Google Scholar]
- 20.Whitman S, Archer K, Feng L, et al. Absence of the wild-type allele predicts poor prognosis in adult de novo acute myeloid leukemia with normal cytogenetics and the internal tandem duplication of FLT3: a cancer and leukemia group B study. Cancer Res. 2001;61:7233–9. [PubMed] [Google Scholar]
- 21.Smith B, Levis M, Beran M, et al. Single-agent CEP-701, a novel FLT3 inhibitor, shows biologic and clinical activity in patients with relapsed or refractory acute myeloid leukemia. Blood. 2004;103:3669–76. doi: 10.1182/blood-2003-11-3775. [DOI] [PubMed] [Google Scholar]
- 22.Stone R, DeAngelo D, Klimek V, et al. Patients with acute myeloid leukemia and an activating mutation in FLT3 respond to a small-molecule FLT3 tyrosine kinase inhibitor, PKC412. Blood. 2005;105:54–60. doi: 10.1182/blood-2004-03-0891. [DOI] [PubMed] [Google Scholar]
- 23.Bacher U, Haferlach C, Kern W, et al. Prognostic relevance of FLT3-TKD mutations in AML: the combination matters—an analysis of 3082 patients. Blood. 2008;111:2527–37. doi: 10.1182/blood-2007-05-091215. [DOI] [PubMed] [Google Scholar]
- 24.Döhner K, Tobis K, Ulrich R, et al. Prognostic significance of partial tandem duplications of the MLL gene in adult patients 16 to 60 years old with acute myeloid leukemia and normal cytogenetics: a study of the Acute Myeloid Leukemia Study Group Ulm. J Clin Oncol. 2002;20:3254–61. doi: 10.1200/JCO.2002.09.088. [DOI] [PubMed] [Google Scholar]
- 25.Steudel C, Wermke M, Schaich M, et al. Comparative analysis of MLL partial tandem duplication and FLT3 internal tandem duplication mutations in 956 adult patients with acute myeloid leukemia. Genes Chromosomes Cancer. 2003;37:237–51. doi: 10.1002/gcc.10219. [DOI] [PubMed] [Google Scholar]
- 26.Whitman S, Liu S, Vukosavljevic T, et al. The MLL partial tandem duplication: evidence for recessive gain-of-function in acute myeloid leukemia identifies a novel patient subgroup for molecular-targeted therapy. Blood. 2005;106:345–52. doi: 10.1182/blood-2005-01-0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanner S, Austin J, Leone G, et al. BAALC, the human member of a novel mammalian neuroectoderm gene lineage, is implicated in hematopoiesis and acute leukemia. Proc Natl Acad Sci U S A. 2001;98:13901–6. doi: 10.1073/pnas.241525498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baldus C, Tanner S, Ruppert A, et al. BAALC expression predicts clinical outcome of de novo acute myeloid leukemia patients with normal cytogenetics: a Cancer and Leukemia Group B Study. Blood. 2003;102:1613–8. doi: 10.1182/blood-2003-02-0359. [DOI] [PubMed] [Google Scholar]
- 29.Baldus C, Thiede C, Soucek S, et al. BAALC expression and FLT3 internal tandem duplication mutations in acute myeloid leukemia patients with normal cytogenetics: prognostic implications. J Clin Oncol. 2006;24:790–7. doi: 10.1200/JCO.2005.01.6253. [DOI] [PubMed] [Google Scholar]
- 30.Najima Y, Ohashi K, Kawamura M, et al. Molecular monitoring of BAALC expression in patients with CD34-positive acute leukemia. Int J Hematol. 2010;91:636–45. doi: 10.1007/s12185-010-0550-8. [DOI] [PubMed] [Google Scholar]
- 31.Paschka P, Marcucci G, Ruppert A, et al. Wilms’ tumor 1 gene mutations independently predict poor outcome in adults with cytogenetically normal acute myeloid leukemia: a cancer and leukemia group B study. J Clin Oncol. 2008;26:4595–602. doi: 10.1200/JCO.2007.15.2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Becker H, Marcucci G, Maharry K, et al. Mutations of the Wilms tumor 1 gene (WT1) in older patients with primary cytogenetically normal acute myeloid leukemia: a Cancer and Leukemia Group B study. Blood. 2010;116:788–92. doi: 10.1182/blood-2010-01-262543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cilloni D, Renneville A, Hermitte F, et al. Real-time quantitative polymerase chain reaction detection of minimal residual disease by standardized WT1 assay to enhance risk stratification in acute myeloid leukemia: a European Leukemia Net study. J Clin Oncol. 2009;27:5195–201. doi: 10.1200/JCO.2009.22.4865. [DOI] [PubMed] [Google Scholar]
- 34.Damm F, Heuser M, Morgan M, et al. Single nucleotide polymorphism in the mutational hotspot of WT1 predicts a favorable outcome in patients with cytogenetically normal acute myeloid leukemia. J Clin Oncol. 2010;28:578–85. doi: 10.1200/JCO.2009.23.0342. [DOI] [PubMed] [Google Scholar]
- 35.Marcucci G, Maharry K, Whitman S, et al. High expression levels of the ETS-related gene, ERG, predict adverse outcome and improve molecular risk-based classification of cytogenetically normal acute myeloid leukemia: a Cancer and Leukemia Group B Study. J Clin Oncol. 2007;25:3337–43. doi: 10.1200/JCO.2007.10.8720. [DOI] [PubMed] [Google Scholar]
- 36.Heuser M, Beutel G, Krauter J, et al. High meningioma 1 (MN1) expression as a predictor for poor outcome in acute myeloid leukemia with normal cytogenetics. Blood. 2006;108:3898–905. doi: 10.1182/blood-2006-04-014845. [DOI] [PubMed] [Google Scholar]
- 37.Gröschel S, Lugthart S, Schlenk R, et al. High EVI1 expression predicts outcome in younger adult patients with acute myeloid leukemia and is associated with distinct cytogenetic abnormalities. J Clin Oncol. 2010;28:2101–7. doi: 10.1200/JCO.2009.26.0646. [DOI] [PubMed] [Google Scholar]
- 38.Damiani D, Tiribelli M, Calistri E, et al. The prognostic value of P-glycoprotein (ABCB) and breast cancer resistance protein (ABCG2) in adults with de novo acute myeloid leukemia with normal karyotype. Haematologica. 2006;91:825–8. [PubMed] [Google Scholar]
- 39.Marcucci G, Maharry K, Wu Y, et al. IDH1 and IDH2 gene mutations identify novel molecular subsets within de novo cytogenetically normal acute myeloid leukemia: a Cancer and Leukemia Group B Study. J Clin Oncol. 2010;28:2348–55. doi: 10.1200/JCO.2009.27.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bornhäuser M, Illmer T, Schaich M, et al. Improved outcome after stem-cell transplantation in FLT3/ITD-positive AML. Blood. 2007;109:2264–5. doi: 10.1182/blood-2006-09-047225. author reply 2265. [DOI] [PubMed] [Google Scholar]


