Abstract
The development of effective treatment strategies for most forms of acute myeloid leukemia (AML) has languished for the past several decades. There are a number of reasons for this, but key among them is the considerable heterogeneity of this disease and the paucity of molecular markers that can be used to predict clinical outcomes and responsiveness to different therapies. The recent large-scale sequencing of AML genomes is now providing opportunities for patient stratification and personalized approaches to treatment that are based on individual mutational profiles. It is particularly notable that studies by The Cancer Genome Atlas and others have determined that 44% of patients with AML exhibit mutations in genes that regulate methylation of genomic DNA. In particular, frequent mutation has been observed in the genes encoding DNA methyltransferase 3A (DNMT3A), isocitrate dehydrogenase 1 (IDH1) and isocitrate dehydrogenase 2 (IDH2), as well as Tet oncogene family member 2. This review will summarize the incidence of these mutations, their impact on biochemical functions including epigenetic modification of genomic DNA and their potential usefulness as prognostic indicators. Importantly, the presence of DNMT3A, IDH1 or IDH2 mutations may confer sensitivity to novel therapeutic approaches, including the use of demethylating agents. Therefore, the clinical experience with decitabine and azacitidine in the treatment of patients harboring these mutations will be reviewed. Overall, we propose that understanding the role of these mutations in AML biology will lead to more rational therapeutic approaches targeting molecularly defined subtypes of the disease.
CURRENT TREATMENT PARADIGMS FOR AML
Acute myeloid leukemia (AML) is a heterogeneous clonal disorder of myeloid precursors and the most common acute leukemia diagnosed in adults.1 The median age at diagnosis is 68 years.2 Although some progress has been made in characterizing AML at diagnosis, substantial advances in initial therapy, prevention of relapse and improvement of overall survival (OS) have been limited.
The goal of induction chemotherapy in AML is complete remission (CR) with restoration of normal hematopoiesis.3 Achievement of CR is an important first goal, as CR is closely associated with improved survival. The combination of an anthracycline (that is, daunorubicin or idarubicin) with cytarabine has been the cornerstone of initial induction therapy for adult AML for the past four decades. CR can be expected in 60–70% of newly diagnosed AML patients with current induction regimens. However, without additional therapy, most patients relapse. The use of consolidation therapy with high-dose cytarabine or allogeneic hematopoietic cell transplantation (HCT) improves outcomes in AML patients. For patients under the age of 60, cure rates have continued to rise and now approach 50%. However, for individuals over the age of 60, the cure rates for AML remain unacceptably low.4,5
The value of risk stratification by cytogenetic abnormality in AML has been demonstrated by analyses of patients enrolled in prospective clinical trials.6,7 In addition, a few recurring gene mutations and overexpressed genes with prognostic relevance in AML have been identified and have been incorporated into current prognostication models.8 Table 1 indicates the cytogenetic and molecular abnormalities used in current clinical practice to provide prognostication for AML at initial diagnosis. Key among the molecular alterations are mutations in the genes encoding FLT3, NPM1 and c-Kit.
Table 1.
Risk status based on cytogenetic and molecular abnormalities
| Risk status | Cytogenetics | Molecular abnormalities | |
|---|---|---|---|
| Favorable risk | inv(16) or t(16;16) t(8;21) t(15;17) |
OR | Normal cytogenetics with NPM1 mutation in the absence of FLT3-ITD |
| Intermediate risk | Normal cytogenetics +8 t(9;11) |
OR | t(8;21), inv(16), t(16;16) with c-Kit mutation |
| Poor risk | Complex (≥3 clonal chromosomal abnormalities) −5, 5q-, −7, 7q- 11q23–non t(9;11) inv(3), t(3;3) t(6;9) t(9;22) |
OR | Normal cytogenetics with FLT3-ITD mutation |
Abbreviation: OR, overall remission.
FLT3 is a receptor tyrosine kinase that spans the plasma membrane and has an important role in proliferation, survival and differentiation of hematopoietic progenitor cells. FLT3-mutant proteins with internal tandem duplications (ITDs) in the juxta-membrane region (FLT3-ITD mutants) exhibit constitutive or enhanced tyrosine kinase activity.9 The incidence of FLT3-ITD mutations in AML is ~25% (28–34% in patients with normal cytogenetics), varying somewhat according to age and clinical risk, and being less common in pediatric AML and in AML arising from an antecedent myelodysplastic syndrome (MDS). When treated with conventional chemotherapy, the prognosis for AML harboring FLT3-ITD mutations is significantly worse compared with AML without FLT3 mutations. Several FLT3 inhibitors are in various stages of development. Increasing evidence indicates that allogeneic HCT is of benefit for patients with FLT3-ITD mutations.
Nucleophosmin (NPM1) is one of the most frequently mutated genes in AML.8 Mutations in the NPM1 protein are found in 25–35% of adult AML and are particularly frequent in cytogenetically normal AML (45–64%). AML-associated mutations in NPM1 result in disruption of a carboxy-terminal nuclear localization signal and generation of a new nuclear export signal, leading to aberrant cytoplasmic localization of the protein. Patients with NPM1 mutations, particularly those without concurrent FLT3-ITD mutations, consistently exhibit a superior outcome. The favorable prognostic impact of NPM1 mutation applies to both younger and older patients.
The identification of the above mutations and their incorporation into risk stratification along with cytogenetic data has advanced our treatment approaches for patients with newly diagnosed AML. However, additional mutations have recently been discovered and our knowledge of their prognostic impact continues to evolve. In particular, a new class of mutations that impact epigenetic mechanisms has emerged. In this review, we highlight these mutations, their impact on prognosis, and their potential role in prognostication and treatment approaches.
MUTATIONS IMPACTING EPIGENETIC MECHANISMS
Whole-genome and -exome sequencing efforts have led to the identification of a broader panel of recurrently mutated genes in AML. Many of these mutations occur in genes that are involved in epigenetic regulation of transcription. Mutations in the genes encoding DNA methyltransferase 3A (DNMT3A), isocitrate dehydrogenase 1 (IDH1) and isocitrate dehydrogenase 2 (IDH2), in particular, result in alterations in DNA methylation. Mutations in DNMT3A and IDH1/2 are among the most commonly occurring mutations found in AML, especially in the intermediate- or normal cytogenetic risk category.10 The Cancer Genome Atlas Research Network has revealed that 44% of AML primary specimens contain a nonsynonymous mutation in DNA methylation-related genes.11 These mutations may have both mechanistic and prognostic implications, as studies of large AML patient cohorts have determined that aberrant DNA methylation is a hallmark feature of AML and is a likely mechanism of carcinogenesis.12,13 Moreover, different AML subtypes exhibit different and specific patterns of DNA methylation, with distinct differences in regulation of gene expression.12,14 Thus, there has been interest in elucidating the role of mutations in epigenetic modifying enzymes in the pathogenesis of AML.
DNMT3A
DNA methylation is an essential epigenetic modification of the genome that is involved in the regulation of gene expression. DNA methylation occurs primarily at the 5 position of cytosine, in particular in the context of CpG islands, which occur in and regulate gene promoters. Cancer genomes are most commonly characterized by global DNA hypomethylation. However, cancer cells also typically exhibit distinct regions of DNA hypermethylation, which are particularly well characterized in the CpG islands of promoter regions of tumor-suppressor genes. Methylation of histones, as well as histone acetylation, is other epigenetic modification by which gene transcription is regulated. Methylation of DNA and histones alters chromatin compaction and alters recruitment of co-activators and co-repressors. Hypermethylation of CpG islands and histones, and deacetylation of histones, leads to inactivation of transcription and, hence, silencing of these tumor-suppressor genes.
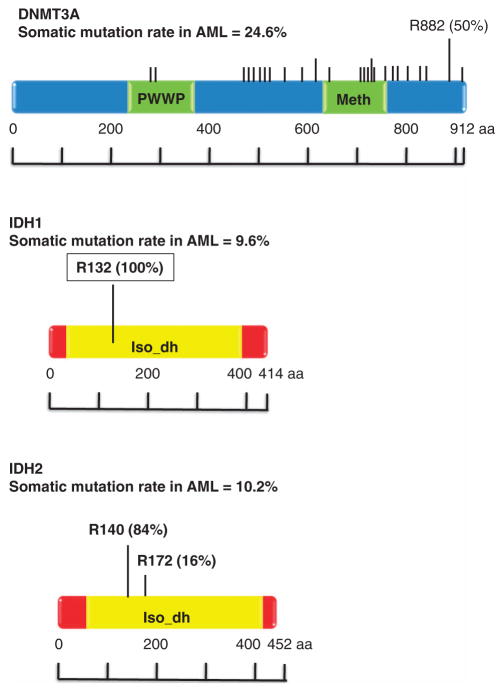
DNMT3A belongs to a family of DNA methyltransferases including DNMT1, DNMT3A and DNMT3B, whose role is to catalyze the addition of methyl groups to cytosine residues of CpG nucleotides.15 DNMT3A is specifically involved in de novo DNA methylation, and functions independently of replication. Somatic mutations in DNMT3A were initially identified by three groups of investigators, who observed these mutations in 4–22% of AML patients.16–18 The majority of DNMT3A mutations are missense mutations that occur at residue R882 near the carboxyl terminus of the DNMT3A protein (Figure 1). However, a number of less common nonsense, frameshift and splice site mutations have been found throughout the DNMT3A coding sequence (Figure 1). Thus, mutations in DNMT3A are often classified as R882 versus non-R882 mutations. To date, no clinical difference has been described separating these two classes and the reason for the high prevalence of R882 mutations is unclear.
Figure 1.
Location and frequency of DNMT3A, IDH1 and IDH2 mutations as determined by The Cancer Genome Atlas. Mutation data was obtained from the cBio portal.101
The function and biological consequences of DNMT3A mutations have yet to be fully elucidated. These mutations are almost always heterozygous, which suggests a gain of function effect, although this remains controversial. Yan et al. have reported that DNMT3A mutated at R882 exhibits substantially reduced enzymatic activity in vitro compared with wild-type DNMT3A.17 The non-R882 DNMT3A mutations result in expression of a truncated protein, and thus are believed to disrupt enzymatic function. Challen et al.19 sought to examine the effects of DNMT3A deficiency on hematopoiesis in DNMT3A knockout mice, and found that in comparison with wild-type hematopoietic stem cells (HSCs), serial transplantation of the DNMT3A-null HSCs resulted in loss of peripheral blood differentiation and expansion of the HSC population in the bone marrow. Moreover, loss of DNMT3A resulted in areas of increased, and areas of decreased, DNA methylation at distinct loci in the DNMT3A-null HSCs, as well as incomplete repression of HSC-specific genes. Hypomethylation was observed in genes that are commonly overexpressed in AML, such as Runx1, Erg, Myc, Smad3 and so on, concomitant with upregulation of genes commonly expressed in HSCs and downregulation of differentiation factors. Interestingly, recipients of DNMT3A-null HSCs did not develop apparent myeloproliferative neoplasms (MPN), indicating that additional cooperating mutations may be required for disease development.
Despite the apparent biological consequences of DNMT3A loss in normal hematopoietic cells, the impact of DNMT3A mutations on DNA methylation and specific gene expression in AML remains controversial. Ley et al.16 have reported that there are no differences in the total expression levels of DNMT3A protein or the mean 5-methylcytosine content of cells harboring mutant DNMT3A versus wild-type DNMT3A. This study also reported that although AML patients with mutated DNMT3A contained genomic regions with significantly different levels of methylation, there was no correlation between any of the differentially methylated regions and altered expression of nearby genes. Moreover, there were no clearly defined gene expression patterns that were associated with DNMT3A mutation status. Other studies have supported the lack of correlation between DNA methylation and DNMT3A mutational status, and have also reported an inability of gene and microRNA expression signatures to predict for DNMT3A mutational status.20,21 In contrast, mRNA expression profiling of primary AML specimens by Yan et al.18 determined that reduction of DNMT3A enzymatic activity as a result of mutation leads to enhanced expression of several genes in the HOX family, a family of genes that have important roles in normal hematopoiesis and are dysregulated in AML. In addition, several other studies have demonstrated a clear correlation between DNA methylation and DNMT3A mutational status. Hájková et al.22 found significantly lower levels of global DNA methylation and simultaneous hypermethylation of specific promoter sequences in patients with mutated DNMT3A. The lower levels of DNA methylation correlated with higher relapse rates and worse OS in the patients with mutant DNMT3A. Lower levels of HOX gene methylation were also observed, similar to the findings of Yan et al. Additional studies by Ribeiro et al.,23 although failing to find a predictive methylation or gene expression signature associated with DNMT3A mutation, identified a single methylation cluster that was enriched in DNMT3A-mutated cases. Specifically, this methylation clustered was observed primarily in patients with NPM1 and FLT3-ITD mutations, which were also characterized by HOX gene overexpression. Methylation of the tumor-suppressor gene growth arrest and DNA-damage-inducible alpha (GADD45A) has also been observed in AML with DNMT3A mutations.24 These discordant results may be due to differences in the patient populations or differences in methodologies for assessing DNA methylation. DNA methylation assays are still highly varied and the conflicts highlighted here are likely to be clarified, as more robust and uniform assays are applied to these questions.
IDH1/2
IDH1 and its mitochondrial homolog, IDH2, are enzymes involved in citrate metabolism, a critical step in the Krebs cycle.25 Mutations in the IDH1/2 genes are well described in lower-grade gliomas (grade II and III astrocytomas and oligodendrogliomas) and secondary glioblastomas, where the mutations have an incidence of more than 70%.26 These gliomas are characterized by distinctive genetic and clinical characteristics. IDH1 and IDH2 normally function to catalyze the oxidative decarboxylation of isocitrate, producing α-ketoglutarate (α-KG) in an nicotinamide adenine dinucleotide phosphate-dependent manner. In AML, IDH1/2 mutations result in amino acid changes that are highly restricted, occurring primarily at residue R132 in IDH1 and R140 or R172 in IDH2 (Figure 1).27 IDH1/2 mutations are heterozygous, suggesting that the mutations result in an enzymatic gain of function. In fact, IDH1/2 mutations give rise to proteins with newly acquired and distinct enzyme activity, where the new activity of the enzymes is able to catalyze nicotinamide adenine dinucleotide phosphate hydrogen-dependent reduction of α-KG to 2-hydroxyglutarate (2HG).28,29 This results in a decrease in α-KG and an increase in 2HG, with 2HG subsequently acting as a competitive inhibitor of α-KG-dependent reactions. Accumulation of this putative oncogenic metabolite has been observed in malignant gliomas and may be related to the pathogenesis of malignant brain tumors.30 In AML, increased cellular 2HG levels contribute to epigenetic mechanisms of pathogenesis by inhibiting α-KG-dependent enzymes that are important for normal DNA methylation.
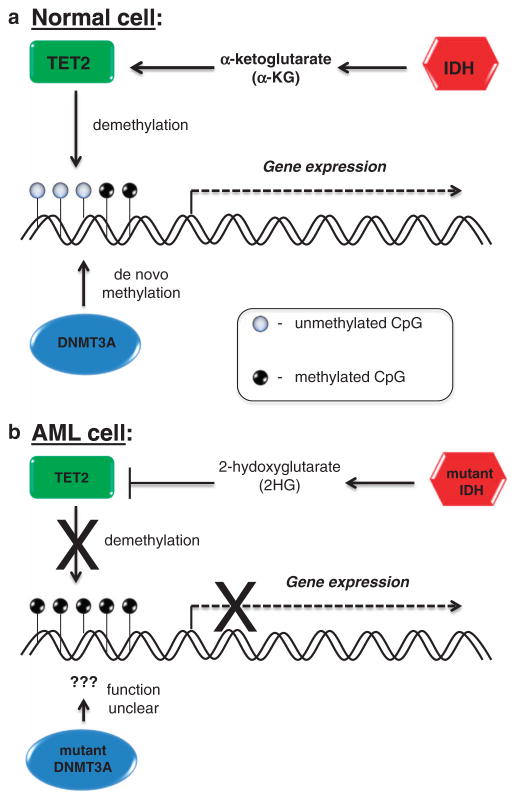
An important clue regarding the role of IDH1/2 mutation and 2HG overproduction in the modulation of DNA methylation lies in the relationship of the IDH enzymes with Tet oncogene family member 2 (TET2; Figure 2). TET2 is an α-KG-utilizing enzyme that hydroxylates 5-methylcytosine as an important step in the demethylation of DNA.31 TET2 mutations, which lead to loss of function, and IDH1/2 mutations appear to be mutually exclusive in AML, suggesting an overlapping biologic effect.25 The lack of α-KG and the presence of the competitive inhibitor 2HG in cells expressing mutant IDH1 or IDH2 serve to attenuate TET2 activity and, thereby, TET2-dependent demethylation of genomic DNA.32 In fact, Figueroa et al.25 have shown that TET2 loss of function mutations and IDH1/2 mutations are associated with similar epigenetic defects, and expression of IDH1/2-mutant proteins leads to impaired TET2 catalytic function in cells. In addition, expression of either IDH1/2- or TET2-mutant proteins impairs myeloid differentiation and increases the expression of stem/progenitor cell markers, suggesting a role for these mutations in AML differentiation blockade.
Figure 2.
(a) Normal function of IDH1/2, TET2 and DNMT3A enzymes in DNA methylation and gene expression. (b) Pathogenic mechanism of mutated IDH1/2 and DNMT3A in gene silencing in AML.
In contrast to AML associated with mutated DNMT3A, AML with mutations in IDH1/2 has been clearly associated with a distinct methylation profile. The Cancer Genome Atlas data has revealed extensive gains of methylation in primary AML samples containing IDH1/2 mutations.11 In addition, studies by Figueroa et al.25 have shown that AML samples with IDH1/2 mutations display global DNA hypermethylation and a specific hypermethylation signature. These studies also showed that IDH1- and IDH2-mutated AML samples displayed similar DNA methylation profiles, consistent with the observation that these are mutually exclusive mutations and likely have a similar biologic effect. Moreover, expression of IDH1/2-mutant proteins in established and primary hematopoietic cells resulted in decreased expression of GATA1 (involved in myeloid differentiation), upregulation of c-Kit and impaired myeloid differentiation with an increase in stem/progenitor cells. Of note, the effects of mutated IDH proteins may not be limited to DNA methylation, as it has also been shown that IDH1/2 mutants capable of producing 2HG act to inhibit the histone demethylation that is necessary for terminal differentiation of lineage-specific progenitor cells.33 Moreover, mutant IDH1 cooperates with HoxA9 gene to promote leukemogenesis in a mouse model.34 Collectively, these, and other, studies confirm that expression of mutant IDH1 or IDH2 proteins leads to DNA hypermethylation, and that this epigenetic effect contributes to AML pathogenesis, in part, through impairment of hematopoietic differentiation.
INCIDENCE AND PROGNOSTIC IMPACT OF DNMT3A AND IDH1/2 MUTATIONS IN AML
Emerging evidence has linked the presence of DNMT3A and IDH1/2 mutations with clinical outcomes, particularly in cytogenetically normal AML, and it is likely that this will have a significant impact on risk stratification in the future. Subsequent to the initial descriptions of DNMT3A mutations in AML, multiple studies have retrospectively evaluated DNMT3A mutations in different patient populations (Table 2).11,17,19–22,35–46 Overall, the incidence of DNMT3A mutations ranges from 12 to 35%, with mutations more commonly found in cytogenetically normal AML, and almost never described in AML with favorable cytogenetics. Older age, higher white blood cell and platelet counts, normal cytogenetics and the presence of NPM1, FLT3-ITD and IDH1 mutations have been found to be more common in patients with DNMT3A mutations versus wild-type DNMT3A in the majority of studies.
Table 2.
Incidence and prognosis of DNMT3A and IDH1/2 mutations in AML, MDS and myeloproliferative neoplasms
| Study | DNMT3A | IDH1 | IDH2 | Association of mutation with prognosis | Reference |
|---|---|---|---|---|---|
| AML | |||||
| Mardis et al. (n =80) | ND | 16% in NC | ND | IDH1: no association with OS; possible worse OS in AML with NC and wild-type NPM1 | 55 |
| Marcucci et al. (n =358) | ND | 14% | 19% | IDH1: worse DFS IDH2 (R172): lower CR |
49 |
| Wagner et al. (n =275) | ND | R132: 10.9% | ND | IDH1 R132: no association with prognosis NPM1/mutated FLT3 |
52 |
| Paschka et al. (n =805) | ND | 7.6% | 8.7% | IDH1/2: worse RFS and OS in NC with mutated NPM1/wild-type FLT3 | 50 |
| Abbas et al. (n =893) | ND | 6% | 11% | IDH1/2: worse OS in NC with wild-type NPM1/wild-type FLT3 |
47 |
| Caramazza et al. (n =26) | ND | IDH1/2: 19% in AML with trisomy 8 | IDH1/2: 19% in AML with trisomy 8 | ND | 53 |
| Figueroa et al. (n =385) | ND | 6.2% | 8.6% (IDH1/2: 27.1% in NC) | ND | 25 |
| Ley et al. (n =281) | 22.1% (33.7% in NC) | ND | ND | DNMT3A: worse OS | 16 |
| Patel et al. (n =199) | ND | 6% | 2% (IDH1/2: 11.8% in NC) | ND | 56 |
| Chou et al. (n =446) | ND | 6.1% | 12.1% | IDH1: no association with prognosis IDH2: better prognosis, but worse OS in mutated FLT3 |
27 |
| Yan et al. (n =112) | 20.5% | ND | ND | DNMT3A: worse IS, time to treatment failure and lower CR | 18 |
| Thol et al. (n =489) | 17.8% | ND | ND | DNMT3A: worse OS overall; worse OS and CR in NC; worse OS, RFS and lower CR in NC with wild-type NPM1/wild-type FLT3 |
20 |
| Shen et al. (n =605) | 12.3% | 9.3% | 9.8% | DNMT3A: worse OS, EFS and lower CR IDH1/2: no association with prognosis |
36 |
| Hou et al. (n =500) | 14% (22.9% in NC) | ND | ND | DNMT3A: worse OS and RFS | 39 |
| Markova et al. (n =226) | 29.6% in NC | ND | ND | DNMT3A: higher relapse rate, especially in mutated FLT3 | 41 |
| Chotirat et al. (n =230) | ND | 8.7% | 10.4% | IDH1/2: no association with prognosis | 54 |
| Marcucci et al. (n =415) | 35.3% in age<60; 33.3% in age≥60 | ND | ND | DNMT3A: worse DFS, trend toward worse OS Younger patients with non-R882 mutations: worse DFS and OS Older patients with R882 mutations: shorter DFS and OS |
21 |
| Patel et al. (n =398) | 23% | 7% | 8% | DNMT3A: worse OS in mutated FLT3; predictive of response to high-dose daunorubicin IDH1/2: better OS in mutated NPM1 IDH2: better OS |
43 |
| Ravandi et al. (n =170) | ND | IDH1/2: 30% | IDH1/2: 30% | IDH1/2: no association with prognosis; worse OS in mutated NPM1/wild-type FLT3 | 51 |
| Renneville et al. (n =123) | 29% in NC | ND | ND | DNMT3A: worse OS and EFS | 44 |
| Ribeiro et al. (n =415) | 23.1% | ND | ND | DNMT3A: worse OS and RFS, especially in wild-type NPM1/wild-type FLT3 | 23 |
| Fernandez-Mercado et al. (n =84) | 16.7% in NC | 12.2% in NC | 12.2% in NC | ND | 37 |
| Hájková et al. (n =79) | 41% | ND | ND | ND | 22 |
| Westman et al. (n =11) | ND | IDH1/2: 12% | IDH1/2: 12% | ND | 59 |
| Ostronoff et al. (n =191) | 19% | ND | ND | DNMT3A: worse OS, EFS and RFS | 42 |
| Koszarska et al. (n =376) | ND | 8.5% | 7.5% | IDH1/2: no association with prognosis IDH1 R132H: worse OS |
48 |
| The Cancer Genome Atlas Research Network (n =200) | 26% | IDH1/2: 20% | IDH1/2: 20% | ND | 11 |
| Gaidzik et al. (n =1770) | 20.9% | ND | ND | DNMT3A: no association with prognosis R882: worse RFS non-R882: better OS |
38 |
| Roller et al. (n =194) | 41% in NC | ND | ND | ND | 45 |
| Hou et al. (n =318) | 14.9% (19.5% in NC) | ND | ND | DNMT3A: worse OS and EFS | 40 |
| Haferlach et al. (n =1291) | 30.9% | 8.8% | 16.4% | ND | 46 |
| MDS | |||||
| Kosmider et al. (n =100) | ND | 2% | 3% | IDH1/2: no impact on survival | 69 |
| Patnaik et al. (n =88) | ND | 0% | 0% | ND | 72 |
| Rocquain et al. (n =65) | ND | 3% | 5% | ND | 102 |
| Thol et al. (n =193) | ND | 4% | 0% | IDH1: associated with inferior OS and higher rate of transformation to AML | 71 |
| Ewalt et al. (n =100) | 5% | ND | ND | ND | 66 |
| Brecqueville et al. (n =66) | 6% | IDH1/2: 5% | IDH1/2: 5% | ND | 65 |
| Walter et al. (n =150) | 8.7% | ND | ND | DNMT3A: worse OS and increased progression to AML | 68 |
| Lin et al. (n =51) | 7.8% | ND | ND | DNMT3A: worse OS | 64 |
| Thol et al. (n =193) | 2.6% | ND | ND | DNMT3A: increased progression to AML | 67 |
| Patnaik et al. (n =277) | ND | 3% | 9% | IDH1: associated with inferior survival | 70 |
| Roller et al. (n =115) | 13% | ND | ND | DNMT3A: no impact on survival | 45 |
| Myeloproliferative Neoplasms | |||||
| Green et al. (n =180) | ND | 0% | 0% | ND | 77 |
| Kosmider et al. (n =100 CMML) | ND | 0% | 8% | IDH1/2: no impact on survival | 69 |
| Pardanani et al. (n =166) | ND | IDH1/2: 3% | IDH1/2: 3% | ND | 78 |
| Tefferi et al. (n =1422) | ND | 1% | 1% | IDH1/2: no impact on survival in 111 patients with primary myelofibrosis | 80 |
| Brecqueville et al. (n =135) | 1% | 0% | 0% | ND | 65 |
| Abdel-Wahab et al. (n =68 MPN; 15 CMML) | 4% (MPN) 0% (CMML) |
ND | ND | DNMT3A: no patients underwent leukemic transformation in follow-up time | 74 |
| Stegelmann et al. (n =80) | 5% | ND | ND | ND | 76 |
| Jankowski et al. (n =52 CMML) | 4% | ND | ND | DNMT3A: no impact on survival | 75 |
| Tefferi et al. (n =130 PMF) | ND | 4% | 5% | IDH1/2: inferior OS and LFS, more pronounced in presence of JAK2V617F | 79 |
| Vannucchi et al. (n =879 PMF) | 5.7% | IDH1/2: 2.6% | IDH1/2: 2.6% | IDH1/2: inferior LFS | 81 |
Abbreviations: AML, acute myeloid leukemia; CMML, chronic myelomonocytic leukemia; CR, complete remission; DFS, disease-free survival; EFS, event-free survival; LFS, leukemia-free survival; MDS, myelodysplastic syndrome; MPN, myeloproliferative neoplasms; NC, normal cytogenetics; ND, not determined; OS, overall survival; PMF, primary myelofibrosis; RFS, relapse-free survival.
Although the exact biochemical effects of DNMT3A mutations are still being elucidated, their impact on prognosis is more evident. Most studies have shown a negative impact on outcomes such as CR rates, relapse rates, event-free survival, relapse-free survival and OS. The negative impact on prognosis has been shown to be even more pronounced in specific populations, such as older patients, patients with normal cytogenetics and patients in a ‘molecular high-risk’ group, variably defined but including FLT3-ITD mutations or wild-type NPM1.25,27,47–52 However, not all studies have demonstrated a negative impact on clinical outcomes, perhaps due to different baseline characteristics or treatment courses.38,45 Prospective studies on the impact of DNMT3A mutations in specific, well-defined patient populations are warranted, as well as studies evaluating the ability of specific therapies to abrogate the negative prognosis. Furthermore, the pattern of association of DNMT3A mutations with other molecular abnormalities such as NPM1, FLT3 and IDH mutations suggests an interplay in the pathogenesis of AML that has yet to be fully delineated.
In contrast to DNMT3A mutations, more is known about the biochemical impact of IDH1/2 mutations (particularly as it pertains to elevated production of 2HG), but the impact on prognosis is less clear. The frequency of IDH mutations in AML is well described, with an incidence of 6–16% for IDH1 and 8–19% for IDH2 (Table 2).11,27,28,34,43,45–59 As with DNMT3A, the higher incidences are seen in AML populations with normal cytogenetics. The clinical characteristics that are commonly found with IDH1/2 mutations compared with wild-type are older age, higher platelet levels, normal cytogenetics and the presence of NPM1 mutations. In addition, as stated above, IDH1/2 and TET2 mutations are known to be mutually exclusive. The frequent association with NPM1 mutation is again suggestive of an interaction in the pathogenesis of AML.
The prognosis of patients with gliomas that harbor IDH1/2 mutations has been shown to be significantly better than the prognosis of patients with wild-type IDH1/2.26 However, the data in AML has been conflicting. Different studies, comprised of large patient cohorts, have shown that IDH1/2 mutations are associated with a worse prognosis, a better prognosis or have no association at all (see Table 2). It appears likely that the impact of IDH1/2 mutations on clinical outcomes may depend on the specific patient population. For example, in a study by Patel et al.,43 mutational analyses were performed on 398 patients enrolled in an Eastern Cooperative Oncology Group clinical trial, evaluating high- versus low-dose daunorubicin for patients with AML. It was shown that patients with IDH1/2 mutations had a better OS if they also had concurrent NPM1 mutations. On the other hand, results from Mardis et al.,55 Paschka et al.50 and Ravandi et al.51 have suggested that IDH1/2 mutations confer a worse prognosis in cytogenetically normal AML, and this was observed in patients with NPM1 mutations as well. Wagner et al.52 have reported that mutations of R132 in IDH1, the most common IDH mutation, did not have an impact on survival. Koszarska et al.48 have reported that IDH1/2 mutations overall are not prognostic for survival, although the IDH1 R132H mutation is associated with a worse OS. For now, the impact of IDH1/2 mutations on clinical outcomes remains unclear.
It is interesting that serum levels of 2HG have recently been shown to predict the presence of IDH mutations.60,61 As noted, 2HG is the oncometabolite produced by leukemic cells expressing mutant IDH. It is released into the serum, although little is known about regulation of 2HG steady-state levels in the serum. Interestingly, serum 2HG levels were found to be associated with shorter OS and response to treatment in patients with IDH mutations.60,61 Thus, measurement of serum 2HG levels may have significant value for screening and prognostic purposes. This may become particularly relevant as mutant IDH inhibitors are brought into clinical applications.
Prognostic models for AML that are based on molecular mutations have been proposed by different groups.36,39,40,43,62,63 These models provide better understanding of the impact of gene mutations in the context of coexisting gene mutations and may allow for comprehensive prognostication. For example, Patel et al.43 proposed a prognostic stratification model based on findings that IDH1/2 mutation was associated with improved OS in patients with mutated NPM1, and in patients who were FLT3-ITD positive, while DNMT3A mutations led to worse OS than in those who were DNMT3A wild type. Using this revised model, the intermediate-risk/normal cytogenetic risk group could be further divided into six distinct risk groups, each of which were described as molecularly favorable, intermediate or unfavorable. Other groups have proposed alternative models and there is not yet a consensus as to how to integrate the growing number of molecular abnormalities associated with AML into a single robust prognostication schema. Clearly, mutational profiling as a means to redefine prognostic risk stratification need to be studied and validated prospectively, but increasing evidence suggests that integration of molecular profiling with cytogenetic risk factors will lead to an improved risk stratification approach for AML patients.
INCIDENCE AND PROGNOSTIC IMPACT OF DNMT3A AND IDH1/2 MUTATIONS IN MDS AND MPN
Myelodysplastic syndrome
Following the discovery of DNMT3A and IDH1/2 mutations in AML, the mutational status of these genes has been evaluated in MDS. DNMT3A mutations occur in 2.6–13% of MDS cases, and like AML, the most common mutation is a heterozygous mutation at the R882 location that occurs most frequently in patients with normal cytogenetics (Table 2).45,64–68 The presence of mutant DNMT3A in MDS appears to confer an inferior survival and a higher likelihood of progression to secondary AML compared with wild-type DNMT3A patients.64,67,68 This has led to speculation that DNMT3A mutations may be involved in leukemic transformation in patients with MDS, although it is important to note that the observed DNMT3A mutations occurred in all French-American-British subtypes and across a range of International prognostic scoring system risk categories.68
Similarly, IDH1/2 mutations have been detected in 4–12% of MDS cases in several studies.65,69–71 One series of 88 patients with isolated 5q deletion MDS failed to identify any cases with IDH1 or IDH2 mutations.72 The two largest studies of patients with MDS who were analyzed for IDH mutations identified IDH1 but not IDH2 mutations as inferring a worse prognosis with a higher rate of transformation to AML.70,71 These findings raise the possibility that IDH mutations may be involved in the mechanism of progression to AML.
Myeloproliferative neoplasms
MPN are known to harbor JAK2, MPL and TET2 mutations.73 More recently, mutations in DNMT3A and IDH1/2, as well as other genes, have been discovered. DNMT3A mutations occur at a low frequency (1–5%) in chronic phases of MPN, but at significantly higher frequencies (17–20%) in MPN-derived AMLs. Heterozygous mutations in the R882 location are again the most frequent DNMT3A mutations found in MPN (Table 2).65,74–76
IDH1/2 mutations are also more common in MPN-derived AML (21–31%), but occur in the chronic phase as well (2–4%), mostly in patients with primary myelofibrosis.69,77–80 Although the presence of DNMT3A mutations has not been determined to have prognostic value in chronic phase MPN, it appears that IDH mutations may help to identify patients more likely to develop leukemic transformation.79 Indeed, in the largest cohort of primary myelofibrosis patients to date, including both a European cohort and a cohort of patients from the Mayo Clinic, patients with mutant IDH1 (and IDH2 in the European cohort) but not DNMT3A had a shorter leukemia-free survival than those without IDH mutations.81 Interestingly, Tefferi et al. recently reported the outcomes from a large cohort of patients with primary myelofibrosis and determined that IDH mutation was associated with increased leukemic transformation in patients with JAK2V617F mutation compared with patients without this mutation. This suggests potential cooperation of JAK2V617F and IDH mutations in promoting leukemia development.79
APPLICATION OF EPIGENETIC MODIFIERS IN AML WITH DNMT3A OR IDH1/2 MUTATIONS: FUTURE DIRECTIONS
Although molecular mutations provide valuable prognostic information, they may also predict responsiveness to different therapies or may represent potential targets for novel therapeutic agents. As mutations in DNMT3A and IDH1/2 affect DNA methylation, there is particular interest in evaluating the impact of hypomethylating agents in AML patients harboring these mutations. Table 3 summarizes clinical trials in which the presence of DNMT3A and IDH1/2 mutations has been assessed for association with response to hypomethylating agents.
Table 3.
Impact of DNMT3A and IDH1/2 mutations in response to hypomethylating agents
| Study | Treatment | Response rate overall | Impact of mutation in response to hypomethylating agents | Reference |
|---|---|---|---|---|
| Blum et al. (n =53) | Decitabine 20 mg/m2 × 10 days | CR 47% | Responders had a trend for lower pretreatment DNMT3A compared with non-responders Higher level of pretreatment miR-29b associated with clinical response. |
82 |
| Metzeler et al. (n =46) | Decitabine or decitabine +vorinostat | CR 41% | CR 75% in mutated DNMT3A versus CR 34% in wild-type DNMT3A; DNMT3A mutation was predictive of response TET2 and IDH1/2 mutations were not associated with CR |
92 |
| Traina et al. (n =92) | Decitabine, azacitidine or both | ORR 24% | TET2 and DNMT3A mutations were predictive of response TET2 and DNMT3A independently prognostic for better PFS |
91 |
| DiNardo et al. (n =56) | Decitabine, azacitidine or combination therapies | CR 25% | Neither DNMT3A nor IDH1/2 were associated with CR Neither DNMT3A nor IDH1/2 were associated with EFS or OS |
90 |
Abbreviations: CR, complete remission; EFS, event-free survival; OS, overall survival; ORR, overall remission rate; PFS, progression-free survival.
Application of hypomethylating agents in AML
Hypomethylating agents, such as decitabine and azacitidine, are DNA methyltransferase inhibitors, and FDA approved agents for treatment of high-risk MDS. Decitabine is a deoxycytidine analog that is incorporated into DNA during S-phase of the cell cycle and binds to DNA methyltransferase, rendering it inactive. Azacitidine is a cytidine analog that primarily is incorporated into RNA, inhibiting RNA processing and function. To a lesser extent, it is incorporated into DNA, similarly to decitabine. The use of these agents for treatment of patients with AML who are unfit for standard induction chemotherapy has been an area of great interest. Decitabine as a single agent has been evaluated in single-arm phase II and randomized controlled phase III studies in older patients with AML who were not candidates for intensive chemotherapy, and has shown CR rates of 18–47%, with a median survival of 7.7–12.6 months.82–84 Azacitidine was shown to be effective in a specific population of older AML patients with 20–30% bone marrow blasts, with a CR rate of 18% and median survival of 24.5 months.85 By comparison, older patients who did not receive treatment have an estimated median survival of 1–4 months. As there is no standard of care for older patients who are unfit for induction chemotherapy, decitabine and azacitidine are now commonly used agents in this challenging patient population.
Relationship between response to hypomethylating agents and the status of DNMT3A and IDH1/2
A few studies have retrospectively assessed the impact of DNMT3A and IDH1/2 mutations in response to hypomethylating agents. In a single-arm phase II trial evaluating the use of decitabine alone as upfront therapy in elderly AML, Blum et al.82 described a CR rate of 47%, and found that higher pretreatment levels of miR-29b, a microRNA that targets DNA methyltransferases, were associated with clinical response. Moreover, responders exhibited a trend toward lower pretreatment levels of DNMT3A mRNA compared with non-responders. Methylation and gene expression analyses revealed that treatment with decitabine significantly reduced global DNA methylation, with significant concentration of the hypomethylated regions in chromosome subtelomeric regions. This suggests differential activity of decitabine in distinct chromosome regions.86 The concentrated impact of decitabine on specific chromosomal regions, including regions that are important for regulation of hematopoietic cell differentiation, has been seen in preclinical studies as well.87 Further evidence supporting an inverse relationship between miR-29b and DNMT3A levels in AML has been provided by Garzon et al.88 who showed that enforced expression of miR-29b in AML cell lines resulted in marked reduction of DNMT3A mRNA and protein levels, which in turn led to global hypomethylation. This suggests that strategies to increase miR-29b levels, such as the use of synthetic miR-29b oligonucleotides, may represent a viable therapeutic option in AML expressing either wild-type or, particularly, mutant DNMT3A. In this regard, treatment with the histone deacetylator AR-42 has been shown to increase miR-29b levels and downregulate DNMT3A in leukemia cell lines.89 Sequential treatment with AR-42 followed by decitabine generated a stronger anti-leukemic effect than either agent alone, both in vitro and in vivo (nonobese diabetic/severe combined immunodeficiency mice), although reduced potency was seen using the reverse sequence of administration.
DiNardo et al.90 have evaluated the mutational status of DNMT3A and IDH1/2 in 68 older patients treated with decitabine alone, azacitidine alone or a combination regimen incorporating one of these agents in front-line therapy. Overall, a CR rate of 25% was seen among all of the regimens. Patients with DNMT3A mutations had a 40% CR versus 22% in wild-type patients, although this was not a statistically significant difference; no association between IDH1/2 mutation and response was observed. Interestingly, the presence of neither IDH1/2 nor DNMT3A mutations was associated with event-free survival or OS. One hypothesis for the absence of prognostic impact of DNMT3A mutations in this study is that the use of hypomethylating agents may have abrogated the poor prognosis that is usually seen in patients with DNMT3A mutations. Supporting this hypothesis, Traina et al.91 evaluated DNMT3A and TET2 mutations in 92 MDS patients treated with decitabine or azacitidine, and found that not only was the presence of mutations predictive of CR to these agents, but that patients with these mutations had a better PFS than wild-type patients. Again, this suggests that the poor prognosis conferred by these mutations may be lessened by the use of epigenetic therapies. Finally, Metzeler et al.92 evaluated 46 elderly patients with AML treated with decitabine with or without the histone deacetylase inhibitor vorinostat, and found a significantly higher CR rate in patients with DNMT3A mutations (75%) versus wild-type DNMT3A (34%). No association was observed between TET2 and IDH1/2 mutations and response, similar to that observed by DiNardo et al.90 Collectively, these studies, although small in number, suggest a strong predictive value of DNMT3A mutations, but an unclear predictive value of IDH1/2 mutations, for response to hypomethylating agents. They support the need for larger studies conducted in a prospective manner.
Strategies for targeting mutant IDH1/2 proteins
The newly acquired and distinct enzyme activity gained on mutation of IDH1 and IDH2 proteins provides an attractive and novel therapeutic target. A number of IDH inhibitors are in various stages of development and have been evaluated in preclinical studies. These inhibitors have been shown to reverse hyper-methylation of both histones and DNA in IDH-mutant-expressing leukemia cell lines, and also lower 2HG levels by >90% in xenograft models.93 Rohle et al.94 have shown that AGI-5198, a selective R132H-IDH1 inhibitor, blocks the ability of mutant IDH1 to produce 2HG, and in turn impairs the growth of mutant, but not wild-type, glioma cells in vitro. Other studies have shown that AGI-5198 reverses DNA methylation induced by mutant IDH1, resulting in re-expression of genes involved in glioma cell differentiation, and can block IDH mutant production of 2HG, leading to restored differentiation and regulation of proliferation in IDH-mutated leukemia cell lines.95,96 These effects were not seen in cells expressing only wild-type IDH1. Another small molecule inhibitor of mutant IDH1, HMS-101, induces apoptosis and decreases colony formation of human bone marrow cells expressing mutant IDH1.34 A small molecule inhibitor of IDH2/R140Q (AGI-6780) has also been developed that promotes differentiation of TF-1 erythroleukemia and primary human AML cells in vitro.97 Indirect inhibition of IDH mutations has been studied as well. For example, growth inhibition of IDH-mutant primary AML cells has been demonstrated by inhibition of glutaminase, the enzyme responsible for production of glutamine, and the primary source of α-KG and 2HG in IDH-mutated AML.98 Future testing is needed to determine whether inhibitors targeting mutant IDH1/2 proteins will demonstrate clinical benefit. In this regard, it is of note that AG-221 is currently in Phase I clinical trials in patients with IDH2-mutant AML (clinicaltrials.gov NCT01915498). Results of this and other ongoing studies should quickly provide insight into how such compounds will be tolerated in the clinic.
Perspectives
The role of specific gene mutations in AML continues to be an area of intense interest, and it is becoming increasingly evident that mutations impacting epigenetic mechanisms, such as mutations in DNMT3A and IDH1/2, are likely to dictate both prognosis and potential therapeutic responsiveness in intermediate-risk AML. Although there is still much to elucidate about the mechanisms of pathogenesis in DNMT3A-mutated AML and the specific prognostic role of DNMT3A and IDH1/2 mutations, there is an abundance of data to support different hypotheses for study in prospective clinical trials. From current evidence, it is possible to foresee a refinement of the intermediate cytogenetic risk group, where the presence or absence of mutations will stratify patients into unique risk categories and aid in determining treatment plans. The impact of hypomethylating agents in DNMT3A-mutated AML is intriguing and merits focused investigation. The design of treatment regimens that incorporate hypomethylating agents with standard chemotherapy may also be warranted for younger patients with DNMT3A mutations, a patient population where hypomethylating agents are typically not considered. In addition, being able to define a subset of older patients with AML who might have improved responses and survival with hypomethylating agents may help to better refine our current treatment approach in this challenging patient population with a particularly poor prognosis and limited treatment options. Moreover, aside from the potential predictive utility of DNMT3A mutations in the use of hypomethylating agents, the presence of these mutations may be predictive of responsiveness to other types of drugs. For example, the presence of DNMT3A mutations has been shown to be predictive of response in patients receiving high-dose daunorubicin as an induction regimen.26 Regarding allogeneic HCT, the clinical benefit of HCT in first CR for patients with DNMT3A mutations has not been clearly established, but is an important question for future studies. Even dietary compounds such as curcumin (turmeric), genistein (soybean), tea polyphenols (green tea), resveratrol (grapes) and sulforaphane (cruciferous vegetables) have been found to alter DNA methylation and histone modifications, and there is interest in evaluating the impact of these compounds on cancer prevention and treatment.99,100 Prospective determination of the predictive value of DNMT3A mutations for benefit from hypomethylating agents, specific chemotherapeutic regimens or from allogeneic HCT may provide an important step forward for creating individualized therapies based on the molecular profile of individual patients. Finally, as novel therapies such as newer hypomethylating agents (for example, sapacitibine and SGI-110) and specific targeted IDH1/2 inhibitors continue to be developed, molecular ‘fingerprinting’ of AML for these and other mutations may help to guide the use of both conventional and novel agents in this disease. It is to be hoped that such improved subclassification systems for AML will lead to more improvements in AML therapy in the decades ahead than have been seen in recent years.
Acknowledgments
We thank Dr Robert Redner for critical reading of the manuscript.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Dores GM, Devesa SS, Curtis RE, Linet MS, Morton LM. Acute leukemia incidence and patient survival among children and adults in the United States, 2001–2007. Blood. 2012;119:34–43. doi: 10.1182/blood-2011-04-347872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dohner H, Estey EH, Amadori S, Appelbaum FR, Buchner T, Burnett AK, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 4.Gupta V, Tallman MS, Weisdorf DJ. Allogeneic hematopoietic cell transplantation for adults with acute myeloid leukemia: myths, controversies, and unknowns. Blood. 2011;117:2307–2318. doi: 10.1182/blood-2010-10-265603. [DOI] [PubMed] [Google Scholar]
- 5.Mayer RJ, Davis RB, Schiffer CA, Berg DT, Powell BL, Schulman P, et al. Intensive postremission chemotherapy in adults with acute myeloid leukemia. Cancer and Leukemia Group B. N Engl J Med. 1994;331:896–903. doi: 10.1056/NEJM199410063311402. [DOI] [PubMed] [Google Scholar]
- 6.Byrd JC, Mrozek K, Dodge RK, Carroll AJ, Edwards CG, Arthur DC, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461) Blood. 2002;100:4325–4336. doi: 10.1182/blood-2002-03-0772. [DOI] [PubMed] [Google Scholar]
- 7.Slovak ML, Kopecky KJ, Cassileth PA, Harrington DH, Theil KS, Mohamed A, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood. 2000;96:4075–4083. [PubMed] [Google Scholar]
- 8.Marcucci G, Haferlach T, Dohner H. Molecular genetics of adult acute myeloid leukemia: prognostic and therapeutic implications. J Clin Oncol. 2011;29:475–486. doi: 10.1200/JCO.2010.30.2554. [DOI] [PubMed] [Google Scholar]
- 9.Levis M. FLT3 mutations in acute myeloid leukemia: what is the best approach in 2013? Hematology Am Soc Hematol Educ Prog. 2013;2013:220–226. doi: 10.1182/asheducation-2013.1.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abdel-Wahab O, Levine RL. Mutations in epigenetic modifiers in the pathogenesis and therapy of acute myeloid leukemia. Blood. 2013;121:3563–3572. doi: 10.1182/blood-2013-01-451781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cancer Genome Atlas Research Network. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013;368:2059–2074. doi: 10.1056/NEJMoa1301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Figueroa ME, Lugthart S, Li Y, Erpelinck-Verschueren C, Deng X, Christos PJ, et al. DNA methylation signatures identify biologically distinct subtypes in acute myeloid leukemia. Cancer cell. 2010;17:13–27. doi: 10.1016/j.ccr.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schoofs T, Berdel WE, Muller-Tidow C. Origins of aberrant DNA methylation in acute myeloid leukemia. Leukemia. 2013;28:1–14. doi: 10.1038/leu.2013.242. [DOI] [PubMed] [Google Scholar]
- 14.Akalin A, Garrett-Bakelman FE, Kormaksson M, Busuttil J, Zhang L, Khrebtukova I, et al. Base-pair resolution DNA methylation sequencing reveals profoundly divergent epigenetic landscapes in acute myeloid leukemia. PLoS Genet. 2012;8:e1002781. doi: 10.1371/journal.pgen.1002781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan SM, Majeti R. Role of DNMT3A, TET2, and IDH1/2 mutations in pre-leukemic stem cells in acute myeloid leukemia. Int J Hematol. 2013;98:648–657. doi: 10.1007/s12185-013-1407-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ley TJ, Ding L, Walter MJ, McLellan MD, Lamprecht T, Larson DE, et al. DNMT3A mutations in acute myeloid leukemia. N Engl J Med. 2010;363:2424–2433. doi: 10.1056/NEJMoa1005143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamashita Y, Yuan J, Suetake I, Suzuki H, Ishikawa Y, Choi YL, et al. Array-based genomic resequencing of human leukemia. Oncogene. 2010;29:3723–3731. doi: 10.1038/onc.2010.117. [DOI] [PubMed] [Google Scholar]
- 18.Yan XJ, Xu J, Gu ZH, Pan CM, Lu G, Shen Y, et al. Exome sequencing identifies somatic mutations of DNA methyltransferase gene DNMT3A in acute monocytic leukemia. Nat Genet. 2011;43:309–315. doi: 10.1038/ng.788. [DOI] [PubMed] [Google Scholar]
- 19.Challen GA, Sun D, Jeong M, Luo M, Jelinek J, Berg JS, et al. Dnmt3a is essential for hematopoietic stem cell differentiation. Nat Genet. 2012;44:23–31. doi: 10.1038/ng.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thol F, Damm F, Ludeking A, Winschel C, Wagner K, Morgan M, et al. Incidence and prognostic influence of DNMT3A mutations in acute myeloid leukemia. J Clin Oncol. 2011;29:2889–2896. doi: 10.1200/JCO.2011.35.4894. [DOI] [PubMed] [Google Scholar]
- 21.Marcucci G, Metzeler KH, Schwind S, Becker H, Maharry K, Mrozek K, et al. Age-related prognostic impact of different types of DNMT3A mutations in adults with primary cytogenetically normal acute myeloid leukemia. J Clin Oncol. 2012;30:742–750. doi: 10.1200/JCO.2011.39.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hajkova H, Markova J, Haskovec C, Sarova I, Fuchs O, Kostecka A, et al. Decreased DNA methylation in acute myeloid leukemia patients with DNMT3A mutations and prognostic implications of DNA methylation. Leuk Res. 2012;36:1128–1133. doi: 10.1016/j.leukres.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 23.Ribeiro AF, Pratcorona M, Erpelinck-Verschueren C, Rockova V, Sanders M, Abbas S, et al. Mutant DNMT3A: a marker of poor prognosis in acute myeloid leukemia. Blood. 2012;119:5824–5831. doi: 10.1182/blood-2011-07-367961. [DOI] [PubMed] [Google Scholar]
- 24.Perugini M, Iarossi DG, Kok CH, Cummings N, Diakiw SM, Brown AL, et al. GADD45A methylation predicts poor overall survival in acute myeloid leukemia and is associated with IDH1/2 and DNMT3A mutations. Leukemia. 2013;27:1588–1592. doi: 10.1038/leu.2012.346. [DOI] [PubMed] [Google Scholar]
- 25.Figueroa ME, Abdel-Wahab O, Lu C, Ward PS, Patel J, Shih A, et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer cell. 2010;18:553–567. doi: 10.1016/j.ccr.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360:765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chou WC, Lei WC, Ko BS, Hou HA, Chen CY, Tang JL, et al. The prognostic impact and stability of Isocitrate dehydrogenase 2 mutation in adult patients with acute myeloid leukemia. Leukemia. 2011;25:246–253. doi: 10.1038/leu.2010.267. [DOI] [PubMed] [Google Scholar]
- 28.Dang L, White DW, Gross S, Bennett BD, Bittinger MA, Driggers EM, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462:739–744. doi: 10.1038/nature08617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ward PS, Patel J, Wise DR, Abdel-Wahab O, Bennett BD, Coller HA, et al. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer cell. 2010;17:225–234. doi: 10.1016/j.ccr.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dang L, Jin S, Su SM. IDH mutations in glioma and acute myeloid leukemia. Trends Mol Med. 2010;16:387–397. doi: 10.1016/j.molmed.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 31.Rakheja D, Konoplev S, Medeiros LJ, Chen W. IDH mutations in acute myeloid leukemia. Hum Pathol. 2012;43:1541–1551. doi: 10.1016/j.humpath.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 32.Pfeifer GP, Kadam S, Jin SG. 5-hydroxymethylcytosine and its potential roles in development and cancer. Epigenetics Chromatin. 2013;6:10. doi: 10.1186/1756-8935-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu C, Ward PS, Kapoor GS, Rohle D, Turcan S, Abdel-Wahab O, et al. IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature. 2012;483:474–478. doi: 10.1038/nature10860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chaturvedi A, Araujo Cruz MM, Jyotsana N, Sharma A, Yun H, Gorlich K, et al. Mutant IDH1 promotes leukemogenesis in vivo and can be specifically targeted in human AML. Blood. 2013;122:2877–2887. doi: 10.1182/blood-2013-03-491571. [DOI] [PubMed] [Google Scholar]
- 35.Esteller M. Epigenetics in cancer. N Engl J Med. 2008;358:1148–1159. doi: 10.1056/NEJMra072067. [DOI] [PubMed] [Google Scholar]
- 36.Shen Y, Zhu YM, Fan X, Shi JY, Wang QR, Yan XJ, et al. Gene mutation patterns and their prognostic impact in a cohort of 1185 patients with acute myeloid leukemia. Blood. 2011;118:5593–5603. doi: 10.1182/blood-2011-03-343988. [DOI] [PubMed] [Google Scholar]
- 37.Fernandez-Mercado M, Yip BH, Pellagatti A, Davies C, Larrayoz MJ, Kondo T, et al. Mutation patterns of 16 genes in primary and secondary acute myeloid leukemia (AML) with normal cytogenetics. PLoS One. 2012;7:e42334. doi: 10.1371/journal.pone.0042334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gaidzik VI, Schlenk RF, Paschka P, Stolzle A, Spath D, Kuendgen A, et al. Clinical impact of DNMT3A mutations in younger adult patients with acute myeloid leukemia: results of the AML Study Group (AMLSG) Blood. 2013;121:4769–4777. doi: 10.1182/blood-2012-10-461624. [DOI] [PubMed] [Google Scholar]
- 39.Hou HA, Kuo YY, Liu CY, Chou WC, Lee MC, Chen CY, et al. DNMT3A mutations in acute myeloid leukemia: stability during disease evolution and clinical implications. Blood. 2012;119:559–568. doi: 10.1182/blood-2011-07-369934. [DOI] [PubMed] [Google Scholar]
- 40.Hou HA, Lin CC, Chou WC, Liu CY, Chen CY, Tang JL, et al. Integration of cytogenetic and molecular alterations in risk stratification of 318 patients with de novo non-M3 acute myeloid leukemia. Leukemia. 2013;28:50–58. doi: 10.1038/leu.2013.236. [DOI] [PubMed] [Google Scholar]
- 41.Markova J, Michkova P, Burckova K, Brezinova J, Michalova K, Dohnalova A, et al. Prognostic impact of DNMT3A mutations in patients with intermediate cytogenetic risk profile acute myeloid leukemia. Eur J Haematol. 2012;88:128–135. doi: 10.1111/j.1600-0609.2011.01716.x. [DOI] [PubMed] [Google Scholar]
- 42.Ostronoff F, Othus M, Ho PA, Kutny M, Geraghty DE, Petersdorf SH, et al. Mutations in the DNMT3A exon 23 independently predict poor outcome in older patients with acute myeloid leukemia: a SWOG report. Leukemia. 2013;27:238–241. doi: 10.1038/leu.2012.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patel JP, Gonen M, Figueroa ME, Fernandez H, Sun Z, Racevskis J, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med. 2012;366:1079–1089. doi: 10.1056/NEJMoa1112304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Renneville A, Boissel N, Nibourel O, Berthon C, Helevaut N, Gardin C, et al. Prognostic significance of DNA methyltransferase 3A mutations in cyto-genetically normal acute myeloid leukemia: a study by the Acute Leukemia French Association. Leukemia. 2012;26:1247–1254. doi: 10.1038/leu.2011.382. [DOI] [PubMed] [Google Scholar]
- 45.Roller A, Grossmann V, Bacher U, Poetzinger F, Weissmann S, Nadarajah N, et al. Landmark analysis of DNMT3A mutations in hematological malignancies. Leukemia. 2013;27:1573–1578. doi: 10.1038/leu.2013.65. [DOI] [PubMed] [Google Scholar]
- 46.Haferlach T, Bacher U, Alpermann T, Kern W, Kohlmann A, Schnittger S, et al. Further Insights Into The Molecular Landscape of De Novo Acute Myeloid Leukemia (AML) Investigating 1291 Patients. 55th ASH Annual Meeting December; 2013; New Orleans, GA, USA. 2013. [Google Scholar]
- 47.Abbas S, Lugthart S, Kavelaars FG, Schelen A, Koenders JE, Zeilemaker A, et al. Acquired mutations in the genes encoding IDH1 and IDH2 both are recurrent aberrations in acute myeloid leukemia: prevalence and prognostic value. Blood. 2010;116:2122–2126. doi: 10.1182/blood-2009-11-250878. [DOI] [PubMed] [Google Scholar]
- 48.Koszarska M, Bors A, Feczko A, Meggyesi N, Batai A, Csomor J, et al. Type and location of isocitrate dehydrogenase mutations influence clinical characteristics and disease outcome of acute myeloid leukemia. Leuk Lymphoma. 2013;54:1028–1035. doi: 10.3109/10428194.2012.736981. [DOI] [PubMed] [Google Scholar]
- 49.Marcucci G, Maharry K, Wu YZ, Radmacher MD, Mrozek K, Margeson D, et al. IDH1 and IDH2 gene mutations identify novel molecular subsets within de novo cytogenetically normal acute myeloid leukemia: a Cancer and Leukemia Group B study. J Clin Oncol. 2010;28:2348–2355. doi: 10.1200/JCO.2009.27.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paschka P, Schlenk RF, Gaidzik VI, Habdank M, Kronke J, Bullinger L, et al. IDH1 and IDH2 mutations are frequent genetic alterations in acute myeloid leukemia and confer adverse prognosis in cytogenetically normal acute myeloid leukemia with NPM1 mutation without FLT3 internal tandem duplication. J Clin Oncol. 2010;28:3636–3643. doi: 10.1200/JCO.2010.28.3762. [DOI] [PubMed] [Google Scholar]
- 51.Ravandi F, Patel K, Luthra R, Faderl S, Konopleva M, Kadia T, et al. Prognostic significance of alterations in IDH enzyme isoforms in patients with AML treated with high-dose cytarabine and idarubicin. Cancer. 2012;118:2665–2673. doi: 10.1002/cncr.26580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wagner K, Damm F, Gohring G, Gorlich K, Heuser M, Schafer I, et al. Impact of IDH1 R132 mutations and an IDH1 single nucleotide polymorphism in cytogenetically normal acute myeloid leukemia: SNP rs11554137 is an adverse prognostic factor. J Clin Oncol. 2010;28:2356–2364. doi: 10.1200/JCO.2009.27.6899. [DOI] [PubMed] [Google Scholar]
- 53.Caramazza D, Lasho TL, Finke CM, Gangat N, Dingli D, Knudson RA, et al. IDH mutations and trisomy 8 in myelodysplastic syndromes and acute myeloid leukemia. Leukemia. 2010;24:2120–2122. doi: 10.1038/leu.2010.213. [DOI] [PubMed] [Google Scholar]
- 54.Chotirat S, Thongnoppakhun W, Promsuwicha O, Boonthimat C, Auewarakul CU. Molecular alterations of isocitrate dehydrogenase 1 and 2 (IDH1 and IDH2) metabolic genes and additional genetic mutations in newly diagnosed acute myeloid leukemia patients. J Hematol Oncol. 2012;5:5. doi: 10.1186/1756-8722-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mardis ER, Ding L, Dooling DJ, Larson DE, McLellan MD, Chen K, et al. Recurring mutations found by sequencing an acute myeloid leukemia genome. N Engl J Med. 2009;361:1058–1066. doi: 10.1056/NEJMoa0903840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Patel KP, Ravandi F, Ma D, Paladugu A, Barkoh BA, Medeiros LJ, et al. Acute myeloid leukemia with IDH1 or IDH2 mutation: frequency and clinicopathologic features. Am J Clin Pathol. 2011;135:35–45. doi: 10.1309/AJCPD7NR2RMNQDVF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Andersson AK, Miller DW, Lynch JA, Lemoff AS, Cai Z, Pounds SB, et al. IDH1 and IDH2 mutations in pediatric acute leukemia. Leukemia. 2011;25:1570–1577. doi: 10.1038/leu.2011.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Damm F, Thol F, Hollink I, Zimmermann M, Reinhardt K, van den Heuvel-Eibrink MM, et al. Prevalence and prognostic value of IDH1 and IDH2 mutations in childhood AML: a study of the AML-BFM and DCOG study groups. Leukemia. 2011;25:1704–1710. doi: 10.1038/leu.2011.142. [DOI] [PubMed] [Google Scholar]
- 59.Westman MK, Pedersen-Bjergaard J, Andersen MT, Andersen MK. IDH1 and IDH2 mutations in therapy-related myelodysplastic syndrome and acute myeloid leukemia are associated with a normal karyotype and with der(1;7)(q10;p10) Leukemia. 2013;27:957–959. doi: 10.1038/leu.2012.347. [DOI] [PubMed] [Google Scholar]
- 60.DiNardo CD, Propert KJ, Loren AW, Paietta E, Sun Z, Levine RL, et al. Serum 2-hydroxyglutarate levels predict isocitrate dehydrogenase mutations and clinical outcome in acute myeloid leukemia. Blood. 2013;121:4917–4924. doi: 10.1182/blood-2013-03-493197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fathi AT, Sadrzadeh H, Borger DR, Ballen KK, Amrein PC, Attar EC, et al. Prospective serial evaluation of 2-hydroxyglutarate, during treatment of newly diagnosed acute myeloid leukemia, to assess disease activity and therapeutic response. Blood. 2012;120:4649–4652. doi: 10.1182/blood-2012-06-438267. [DOI] [PubMed] [Google Scholar]
- 62.Grossmann V, Schnittger S, Kohlmann A, Eder C, Roller A, Dicker F, et al. A novel hierarchical prognostic model of AML solely based on molecular mutations. Blood. 2012;120:2963–2972. doi: 10.1182/blood-2012-03-419622. [DOI] [PubMed] [Google Scholar]
- 63.Schlenk RF, Dohner K, Krauter J, Frohling S, Corbacioglu A, Bullinger L, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358:1909–1918. doi: 10.1056/NEJMoa074306. [DOI] [PubMed] [Google Scholar]
- 64.Lin J, Yao DM, Qian J, Chen Q, Qian W, Li Y, et al. Recurrent DNMT3A R882 mutations in Chinese patients with acute myeloid leukemia and myelodysplastic syndrome. PLoS One. 2011;6:e26906. doi: 10.1371/journal.pone.0026906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brecqueville M, Cervera N, Gelsi-Boyer V, Murati A, Adelaide J, Chaffanet M, et al. Rare mutations in DNMT3A in myeloproliferative neoplasms and myelodysplastic syndromes. Blood Cancer J. 2011;1:e18. doi: 10.1038/bcj.2011.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ewalt M, Galili NG, Mumtaz M, Churchill M, Rivera S, Borot F, et al. DNMT3a mutations in high-risk myelodysplastic syndrome parallel those found in acute myeloid leukemia. Blood Cancer J. 2011;1:e9. doi: 10.1038/bcj.2011.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thol F, Winschel C, Ludeking A, Yun H, Friesen I, Damm F, et al. Rare occurrence of DNMT3A mutations in myelodysplastic syndromes. Haematologica. 2011;96:1870–1873. doi: 10.3324/haematol.2011.045559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Walter MJ, Ding L, Shen D, Shao J, Grillot M, McLellan M, et al. Recurrent DNMT3A mutations in patients with myelodysplastic syndromes. Leukemia. 2011;25:1153–1158. doi: 10.1038/leu.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kosmider O, Gelsi-Boyer V, Slama L, Dreyfus F, Beyne-Rauzy O, Quesnel B, et al. Mutations of IDH1 and IDH2 genes in early and accelerated phases of myelodysplastic syndromes and MDS/myeloproliferative neoplasms. Leukemia. 2010;24:1094–1096. doi: 10.1038/leu.2010.52. [DOI] [PubMed] [Google Scholar]
- 70.Patnaik MM, Hanson CA, Hodnefield JM, Lasho TL, Finke CM, Knudson RA, et al. Differential prognostic effect of IDH1 versus IDH2 mutations in myelodysplastic syndromes: a Mayo Clinic study of 277 patients. Leukemia. 2012;26:101–105. doi: 10.1038/leu.2011.298. [DOI] [PubMed] [Google Scholar]
- 71.Thol F, Weissinger EM, Krauter J, Wagner K, Damm F, Wichmann M, et al. IDH1 mutations in patients with myelodysplastic syndromes are associated with an unfavorable prognosis. Haematologica. 2010;95:1668–1674. doi: 10.3324/haematol.2010.025494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Patnaik MM, Lasho TL, Finke CM, Gangat N, Caramazza D, Holtan SG, et al. WHO-defined ’myelodysplastic syndrome with isolated del(5q)’ in 88 consecutive patients: survival data, leukemic transformation rates and prevalence of JAK2, MPL and IDH mutations. Leukemia. 2010;24:1283–1289. doi: 10.1038/leu.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tefferi A. Novel mutations and their functional and clinical relevance in myeloproliferative neoplasms: JAK2, MPL, TET2, ASXL1, CBL, IDH and IKZF1. Leukemia. 2010;24:1128–1138. doi: 10.1038/leu.2010.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abdel-Wahab O, Pardanani A, Rampal R, Lasho TL, Levine RL, Tefferi A. DNMT3A mutational analysis in primary myelofibrosis, chronic myelomonocytic leukemia and advanced phases of myeloproliferative neoplasms. Leukemia. 2011;25:1219–1220. doi: 10.1038/leu.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jankowska AM, Makishima H, Tiu RV, Szpurka H, Huang Y, Traina F, et al. Mutational spectrum analysis of chronic myelomonocytic leukemia includes genes associated with epigenetic regulation: UTX, EZH2, and DNMT3A. Blood. 2011;118:3932–3941. doi: 10.1182/blood-2010-10-311019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stegelmann F, Bullinger L, Schlenk RF, Paschka P, Griesshammer M, Blersch C, et al. DNMT3A mutations in myeloproliferative neoplasms. Leukemia. 2011;25:1217–1219. doi: 10.1038/leu.2011.77. [DOI] [PubMed] [Google Scholar]
- 77.Green A, Beer P. Somatic mutations of IDH1 and IDH2 in the leukemic transformation of myeloproliferative neoplasms. N Engl J Med. 2010;362:369–370. doi: 10.1056/NEJMc0910063. [DOI] [PubMed] [Google Scholar]
- 78.Pardanani A, Lasho TL, Finke CM, Mai M, McClure RF, Tefferi A. IDH1 and IDH2 mutation analysis in chronic- and blast-phase myeloproliferative neoplasms. Leukemia. 2010;24:1146–1151. doi: 10.1038/leu.2010.77. [DOI] [PubMed] [Google Scholar]
- 79.Tefferi A, Jimma T, Sulai NH, Lasho TL, Finke CM, Knudson RA, et al. IDH mutations in primary myelofibrosis predict leukemic transformation and shortened survival: clinical evidence for leukemogenic collaboration with JAK2V617F. Leukemia. 2012;26:475–480. doi: 10.1038/leu.2011.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tefferi A, Lasho TL, Abdel-Wahab O, Guglielmelli P, Patel J, Caramazza D, et al. IDH1 and IDH2 mutation studies in 1473 patients with chronic-, fibrotic- or blast-phase essential thrombocythemia, polycythemia vera or myelofibrosis. Leukemia. 2010;24:1302–1309. doi: 10.1038/leu.2010.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vannucchi AM, Lasho TL, Guglielmelli P, Biamonte F, Pardanani A, Pereira A, et al. Mutations and prognosis in primary myelofibrosis. Leukemia. 2013;27:1861–1869. doi: 10.1038/leu.2013.119. [DOI] [PubMed] [Google Scholar]
- 82.Blum W, Garzon R, Klisovic RB, Schwind S, Walker A, Geyer S, et al. Clinical response and miR-29b predictive significance in older AML patients treated with a 10-day schedule of decitabine. Proc Natl Acad Sci USA. 2010;107:7473–7478. doi: 10.1073/pnas.1002650107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cashen AF, Schiller GJ, O’Donnell MR, DiPersio JF. Multicenter, phase II study of decitabine for the first-line treatment of older patients with acute myeloid leukemia. J Clin Oncol. 2010;28:556–561. doi: 10.1200/JCO.2009.23.9178. [DOI] [PubMed] [Google Scholar]
- 84.Kantarjian HM, Thomas XG, Dmoszynska A, Wierzbowska A, Mazur G, Mayer J, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2012;30:2670–2677. doi: 10.1200/JCO.2011.38.9429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Gattermann N, Germing U, et al. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J Clin Oncol. 2010;28:562–569. doi: 10.1200/JCO.2009.23.8329. [DOI] [PubMed] [Google Scholar]
- 86.Yan P, Frankhouser D, Murphy M, Tam HH, Rodriguez B, Curfman J, et al. Genome-wide methylation profiling in decitabine-treated patients with acute myeloid leukemia. Blood. 2012;120:2466–2474. doi: 10.1182/blood-2012-05-429175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Negrotto S, Ng KP, Jankowska AM, Bodo J, Gopalan B, Guinta K, et al. CpG methylation patterns and decitabine treatment response in acute myeloid leukemia cells and normal hematopoietic precursors. Leukemia. 2012;26:244–254. doi: 10.1038/leu.2011.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Garzon R, Liu S, Fabbri M, Liu Z, Heaphy CE, Callegari E, et al. MicroRNA-29b induces global DNA hypomethylation and tumor suppressor gene reexpression in acute myeloid leukemia by targeting directly DNMT3A and 3B and indirectly DNMT1. Blood. 2009;113:6411–6418. doi: 10.1182/blood-2008-07-170589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mims A, Walker AR, Huang X, Sun J, Wang H, Santhanam R, et al. Increased anti-leukemic activity of decitabine via AR-42-induced upregulation of miR-29b: a novel epigenetic-targeting approach in acute myeloid leukemia. Leukemia. 2013;27:871–878. doi: 10.1038/leu.2012.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dinardo CD, Patel KP, Garcia-Manero G, Luthra R, Pierce S, Borthakur G, et al. Lack of association of IDH1, IDH2, and DNMT3A mutations with outcome in older patients with AML treated with hypomethylating agents. Leuk Lymphoma. 2014 doi: 10.3109/10428194.2013.855309. e-pub ahead of print 4 February 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Traina F, Visconte V, Elson P, Tabarroki A, Jankowska AM, Hasrouni E, et al. Impact of molecular mutations on treatment response to DNMT inhibitors in myelo-dysplasia and related neoplasms. Leukemia. 2013;28:78–87. doi: 10.1038/leu.2013.269. [DOI] [PubMed] [Google Scholar]
- 92.Metzeler KH, Walker A, Geyer S, Garzon R, Klisovic RB, Bloomfield CD, et al. DNMT3A mutations and response to the hypomethylating agent decitabine in acute myeloid leukemia. Leukemia. 2012;26:1106–1107. doi: 10.1038/leu.2011.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Popovici-Muller J, Shipps GW, Jr, Rosner KE, Deng Y, Wang T, Curran PJ, et al. Pyrazolo[1,5-a]pyrimidine-based inhibitors of HCV polymerase. Bioorg Med Chem Lett. 2009;19:6331–6336. doi: 10.1016/j.bmcl.2009.09.087. [DOI] [PubMed] [Google Scholar]
- 94.Rohle D, Popovici-Muller J, Palaskas N, Turcan S, Grommes C, Campos C, et al. An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells. Science. 2013;340:626–630. doi: 10.1126/science.1236062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Turcan S, Fabius AW, Borodovsky A, Pedraza A, Brennan C, Huse J, et al. Efficient induction of differentiation and growth inhibition in IDH1 mutant glioma cells by the DNMT Inhibitor Decitabine. Oncotarget. 2013;4:1729–1736. doi: 10.18632/oncotarget.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Losman JA, Looper RE, Koivunen P, Lee S, Schneider RK, McMahon C, et al. (R)-2-hydroxyglutarate is sufficient to promote leukemogenesis and its effects are reversible. Science. 2013;339:1621–1625. doi: 10.1126/science.1231677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang F, Travins J, DeLaBarre B, Penard-Lacronique V, Schalm S, Hansen E, et al. Targeted inhibition of mutant IDH2 in leukemia cells induces cellular differentiation. Science. 2013;340:622–626. doi: 10.1126/science.1234769. [DOI] [PubMed] [Google Scholar]
- 98.Emadi A, Jun SA, Tsukamoto T, Fathi AT, Minden MD, Dang CV. Inhibition of glutaminase selectively suppresses the growth of primary AML cells with IDH mutations. Exp Hematol. 2013 doi: 10.1016/j.exphem.2013.12.001. pii: S0301-472X(13)00926-0. [DOI] [PubMed] [Google Scholar]
- 99.Khan SI, Aumsuwan P, Khan IA, Walker LA, Dasmahapatra AK. Epigenetic events associated with breast cancer and their prevention by dietary components targeting the epigenome. Chem Res Toxicol. 2012;25:61–73. doi: 10.1021/tx200378c. [DOI] [PubMed] [Google Scholar]
- 100.Meeran SM, Ahmed A, Tollefsbol TO. Epigenetic targets of bioactive dietary components for cancer prevention and therapy. Clin Epigenetics. 2010;1:101–116. doi: 10.1007/s13148-010-0011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rocquain J, Carbuccia N, Trouplin V, Raynaud S, Murati A, Nezri M, et al. Combined mutations of ASXL1, CBL, FLT3, IDH1, IDH2, JAK2, KRAS, NPM1, NRAS, RUNX1, TET2, and WT1 genes in myelodysplastic syndromes and acute myeloid leukemias. BMC Cancer. 2010;10:401. doi: 10.1186/1471-2407-10-401. [DOI] [PMC free article] [PubMed] [Google Scholar]