Abstract
The cooperation of B lymphocytes with other antigen presenting cells (APCs) is often necessary in the efficient processing and presentation of antigen. Herein, we describe a mechanism by which B cells physically interact with dendritic cells (DCs) resulting in the transfer of B cell receptor (BCR)-enriched antigen to these APCs. Antigen transfer involves direct contact between the two cells followed by the capture of B cell derived membrane and intracellular components. Strikingly, DCs acquire greater amounts of antigen by transfer from B cells than by endocytosis of free antigen. Blocking scavenger receptor A, a DC surface receptor involved in membrane acquisition, abrogates these events. We propose that antigen transfer from B cells to DCs results in a more focused immunologic response due to the selective editing of Ag by the BCR.
Keywords: Antigen Presentation/Processing, B Cells, Dendritic Cells, Scavenger Receptor A
1. Introduction
Antigen presentation plays a critical role in directing immune responses to a variety of foreign antigens and under pathological conditions, to self-antigens. It is the requisite function of several different cell types of the immune system, notably macrophages, dendritic cells, and B cells, to capture, process and present antigens. Individually, these antigen presenting cells (APCs) have been shown to induce T cell responses, whether immunogenic or tolerogenic, depending on the context in which the antigen is presented (Crowley et al., 1990; Inaba et al., 1990; Jong et al., 2006; Lanzavecchia, 1990; Mannhalter et al., 1983; Roth et al., 1997). Indeed, autologous dendritic cell based therapies have become a part of the landscape of tumor treatment. However, the cooperation and/or direct interaction between similar or different types of APCs can promote a more effective and robust immune response (Balázs et al., 2002; Diaz-de-Durana et al., 2006; Kleindienst and Brocker, 2005; Wykes et al., 1998; Yan et al., 2006). For instance, antigen specific T cell activation and expansion is much greater in transgenic mice that selectively expressed the T cell restricting MHC II molecule on both B cells and dendritic cells as compared to transgenic mice that expressed the MHC II in a single APC population (Kleindienst and Brocker, 2005). Mice individually deficient in B cell APCs, macrophage or dendritic cells do not activate T cells to the same magnitude observed in wt hosts (Chan and Shlomchik, 1998; Duffield et al., 2005; Probst and Broek, 2005).
We have previously demonstrated that antigen specific B cells can induce a T cell response to soluble antigen earlier than other APCs, due to their ability to rapidly capture antigen efficiently through the B cell receptor (BCR) (Lanzavecchia, 1990; Pape et al., 2007; Yan et al., 2006). However, the B cell mediated APC function is short-lived. Long-lived APC functions are best performed by dendritic cells indicating that cooperation between these two APCs is necessary to achieve a productive immune response. Our studies and that of Kleindienst and Brocker highlight the importance of the collaboration between dendritic cells and B cells in immunity (Kleindienst and Brocker, 2005; Yan et al., 2006). Both of these APCs have been shown to modulate the function of the other through cytokine production and antigen presentation (Bayry et al., 2005; Dubois et al., 2001; Kilmon et al., 2005). Furthermore, the direct interaction of B cells with DCs has been clearly demonstrated in vitro as well as in vivo (Huang et al., 2005; Qi et al., 2006). In both of these studies, antigen bearing dendritic cells contact and activate antigen specific B cells. Additional studies have illustrated that DCs can provide antigen directly to B cells by unknown pathways (Balázs et al., 2002; Bergtold et al., 2005; Wykes et al., 1998). Conversely, several studies have implied that the reverse may also occur in that B cells can transfer antigen to DCs (Ferguson et al., 2004; Valdez et al., 2002); however, direct evidence of this pathway has been lacking.
Previously, we have shown using fluorescently labeled antigen that antigen specific B cells can transfer antigen to macrophages and that this process can activate a T cell response both in vitro and in vivo (Harvey et al., 2007; Harvey et al., 2008). Here we demonstrate that human B cells can transfer BCR-targeted antigen to human dendritic cells and that direct interaction between the two APCs is necessary for this event to occur. The predominant mechanism of antigen transfer described herein involves the capture of B cell derived membrane and/or intracellular proteins by the recipient DCs in a process known as trogocytosis. Furthermore, we have identified scavenger receptor A as a key surface receptor on the human dendritic cells that mediate the exchange of cell membrane components along with BCR-enriched antigen. Recipient DCs appear to carry processed forms of antigen. Therefore, antigen transfer could enable the presentation of antigen to T cells by the dendritic cells and thus, induce an immunologic response. We propose that BCR-mediated sequestration and subsequent transfer of specific antigens to other APCs such as dendritic cells leads to a more focused immune response by discriminating a particular set of antigens from a diverse array of potential targets.
2. Materials and methods
2.1 Isolation and tissue culturing of cells
Human PBMCs were isolated from leukopacks (New York Blood Center, Long Island City, NY) by Ficoll-Hypaque method previously described (Bennett and Cohn, 1966). Lineage marker specific cells (Lin1+: CD3, CD14, CD16, CD19 and CD56) were separated from DCs by positive selection using magnetic beads (StemCell Technologies). The negatively selected population was stained with Lin1-FITC, anti-HLA-DR-PE, CD11c-PECy5 (BD Pharmingen) and CD123-APC (Miltenyi Biotech) antibodies and sorted on a FacsAria (Becton Dickinson) for HLA-DR+:CD11c+:CD123− primary myeloid DCs (MoDCs). MoDCs were cultured in RPMI with 10% heat-inactivated human male AB sera (Sigma) and used immediately. Human monocyte derived DCs (MdDCs: StemCell Technologies) were cultured in the same medium as above with addition of 50 ng/ml recombinant human GM-CSF and IL-4 (R&D Systems) for 24 hrs prior to use. Primary human B cells were isolated from PBMC by negative selection using magnetic beads (StemCell Technologies) and cultured in same medium as dendritic cells. Human B cell lines B-LCL and BJAB were maintained in 10% FBS RPMI 1640 medium.
2.2 Preparation of fluorescent antigen
Anti-human IgG/IgM F(ab′)2 antibody fragments (aIg; Jackson ImmunoResearch Laboratories) were conjugated with Alexa Fluor® 488 (AF488; Molecular Probes) at a 1:6 molar ratio, respectively, using the succinimidyl ester form. Antibody was separated from unreacted fluorophore by centrifugation through concentrator (Millipore) and resuspended in PBS. The double conjugated antigen of aIg with AF488 and the pH-sensitive fluorogenic dye pHrodo™ (Molecular Probes) (aIg-AF488/pHrodo) was generated as above with molar ratio of 1:3:3, respectively.
2.3 Uptake of antigen by B lymphocytes
B-LCL or BJAB cells were cultured for 15 min in presence of 10% human serum RPMI 1640 medium and 1 mg/ml human Ig (Sigma) to block Fc receptors. Cells were washed twice in pre-warmed HBSS and once in 10% FBS RPMI medium to remove excess Ig. For various time points, B cells (2 ×107 cells/ml) were pulsed with 10 μg/ml of either aIg or anti-FITC Ig conjugated with AF488 (non-specific antibody; Molecular Probes) at 37°C/5% CO2 followed by 4 washes with ice-cold HBSS and a wash with 10% human serum RPMI 1640 medium. Level of antigen uptake was determined by fluorescence microscopy of wet mounts and by flow cytometry after anti-CD19-PE (BD Pharmingen) staining. Optimal incubation time of B cells with antigen was found to be 60 min. Primary human B cells were pulsed with antigen as described except the Fc receptor-blocking step was omitted.
2.4 Antigen transfer assays with human dendritic cells
Dendritic cells (1 ×106 cells/well) were co-cultured for 18 hr with B cells (2 × 106 cells/well) that had been pulsed with one of the following: no antigen, non-specific antibody or aIg. All cells were harvested and then stained for flow cytometry with anti-CD11c-PECy5 (for dendritic cells) as well as biotinylated anti-CD19 (BD Pharmingen) and anti-CD14 (Invitrogen-Caltag) antibodies followed by streptavidin-AF647 to identify B cells and monocytes (contaminating population), respectively. Level of antigen (AF488+) was evaluated for CD19−:CD14−:CD11c+ gated population that was derived from non-B lymphocyte FSC:SSC gated cells. With co-cultures of DCs and primary B cells, antigen levels were assessed for the same gated population as above except this gate was derived from FSC:SSC gate that included both cell types since these cells are nearly equivalent in size. For co-cultures with soluble antigen, DCs were cultured for 18 hrs under the following conditions: no antigen, 5 ug/ml soluble aIg (no B cells), or BJAB cells pulsed with either non-specific Ab or aIg.
2.5 Live cell imaging of antigen transfer by confocal microscopy
MoDCs (1 ×106 cells per dish) were cultured in glass bottom (1.5 mm thickness) dishes (Mattek) 24 hr prior to use. Both monocyte derived and primary myeloid DCs were labeled with 0.5 uM CellTrace™ Far Red (CTFR) (DDAO-SE; Molecular Probes) for 8 min in HBSS (no phenol red) and the reaction stopped with 5 fold excess of FBS. Cells were then washed twice in HBSS and resuspended in culture medium. BJAB cells were labeled with 5 uM CellTracker™ Orange (CTO) (CMRA; Molecular Probes) and pulsed with antigen. Each DC population (1 ×106 cells) was co-cultured separately with antigen pulsed B cells (2 × 106) in glass bottom dishes. After 18 hr, live cell cultures were examined by confocal microscopy (LSM510meta; Zeiss) at 600x magnification and 2x zoom.
2.6 Assay for antigen transfer from apoptotic B cells
BJAB cells (2 × 106 cells) or primary B cells were pulsed with antigen and induced to undergo apoptosis by hyperthermia (30 min at 65°C) as previously described (Zhao et al., 2006). Induction of apoptosis was confirmed by flow cytometry using the vital dye TO-PRO-3 (Molecular Probes). Given that 15% of BJAB cells (3 × 105 cells of 2 million cells) undergo apoptosis/necrosis when cultured alone (B. Harvey and M. Mamula, unpublished data), primary myeloid dendritic were co-cultured with 3 × 105 apoptotic or 2 × 106 viable B cells for 18 hr prior to being harvested.
2.7 Preventing cell contact between antigen donor and recipient by transwell
Primary DCs (1 × 106 cells/well) were seeded into 24 well plate. Antigen pulsed B cells were added to transwell (1, 3, or 8 μm; BD Falcon) above wells containing DCs and cultured for 18 hr. Cells in transwell were collected and analyzed by flow cytometry separately from those within plate wells.
2.8 Membrane/cytosolic protein exchange assay
BJAB cells and primary myeloid DCs were labeled with CellTracker™ Orange (CTO) and CellTrace™ Far Red (CTFR), respectively, as described previously. B cells were then pulsed with antigen as indicated earlier. The labeled DCs were treated in the absence (untreated; UT) or presence of either 50 μl anti-SRA anti-sera (aSR-A; Chemicon) or 25 μg/ml anti-CD36 (aSR-B) antibody (Harshyne et al., 2003) (Immunotech-Coulter) for 1 hr prior to co-culture with antigen pulsed B cells for 18 hr. All cells were harvested and analyzed by flow cytometry.
2.9 Assay for processed antigen
BJAB cells were pulsed with the following antigens: non-specific antibody, aIg-AF488 or aIg-AF488/pHrodo. These cells were then co-cultured with MoDCs for 18 hr. Presence of processed antigen was determined for CD19−:CD14−:CD11c+ gated population as described above.
3. Results
3.1 Human B cells transfer antigen to primary myeloid dendritic cells
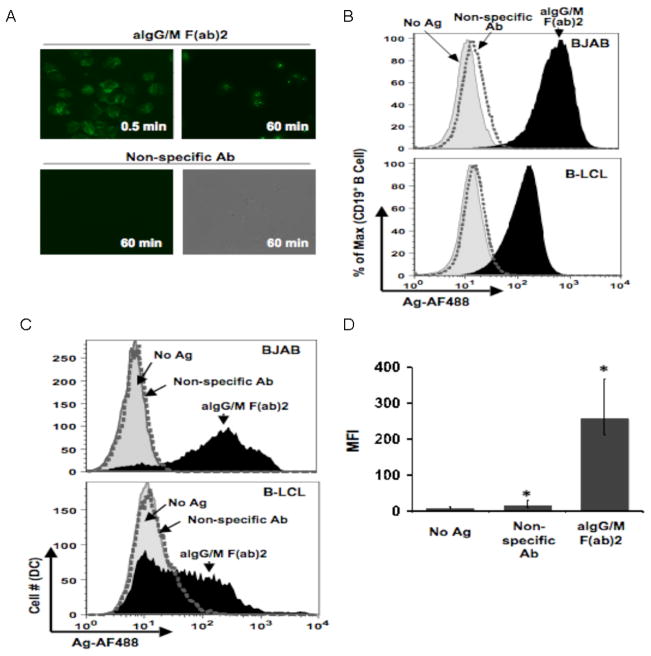
We have previously demonstrated that immunoglobulin Tg B cells can transfer antigen to macrophages and that this event can induce an antigen specific T cell proliferative response (Harvey et al., 2007, Harvey et al., 2008). In our current investigation, we sought to determine whether human B cells could transfer antigen to human dendritic cells using an in vitro system to monitor the exchange of fluorescently labeled antigen between the two types of APCs. For these studies, human B cells (EBV-transformed B cell line, B-LCL, and EBV-negative B cell lymphoma cell line BJAB) were pulsed with the BCR-targeted antigen (anti-human IgG/M F(ab′)2-AF488 antibodies; hereafter referred to as aIg) after which the cells were extensively washed to remove unbound antigen. In a manner as we have previously examined in other systems by fluorescence microscopy (Fig. 1A), B-LCLs were found to bind specific antigen as early as 30 seconds and acquire large amounts of antigen within 1 hr, whereas non-specific antibody binding was undetectable even after 60 min (Harvey, et al., 2008). Following an overnight culture, BJAB cells as well as B-LCLs retained fluorescent antigen as determined by flow cytometry (Fig. 1B) indicating that the antigen persists in B cells over time.
Fig. 1.
Human B cells acquire and transfer antigen to dendritic cells. (a) Kinetics of antigen uptake by B cells. B-LCL pulsed with either aIg (upper panels) or non-specific Ab (lower panels) for indicated time points, washed and observed by fluorescence microscopy (450X magnification). (b) Antigen uptake by various B cell lines. BJAB (top panel) and B-LCL (lower panel) were pulsed with no antigen (gray curve), non-specific Ab (dotted curve) or aIg (black curve) for 60 min and stained with anti-CD19 antibody for flow cytometry. (c) Various B cell lines transfer antigen to dendritic cells. Primary myeloid DCs were co-cultured for 18 hr with BJAB (top panel) or B-LCL (lower panel) pulsed with no antigen (gray shaded curve), non-specific Ab (dotted curve) or aIg (black curve). Antigen levels (AF488) were assessed for the CD19−:CD14−:CD11c+ gated population. (d) Levels of transferred antigen are significant. MFI values for transferred antigen (AF488) are given for 3 independent experiments with BJAB cells as antigen donors (* p< 0.001; n=3; thin bars represent value range).
To determine whether B cells could transfer the fluorescent antigen to primary human myeloid DCs (MoDCs), antigen pulsed BJAB cells and B-LCLs were separately co-cultured for 18 hr with MoDCs isolated directly from PBMCs. Only DCs incubated with aIg pulsed B cells, whether BJAB or B-LCLs, were positive for fluorescent antigen (AF488; Fig. 1C), and the level of antigen in this DC population was significantly greater as compared to those cultured with B cells pulsed with non-specific antibody (Fig 1D). Given that both types of B cell lines were able to function as antigen donors with virtually identical kinetics, we will refer to them collectively throughout the text as B cells unless otherwise specified. Overall, these results indicate that human B cells can transfer a considerable amount of BCR-specific antigen to primary MoDCs in vitro.
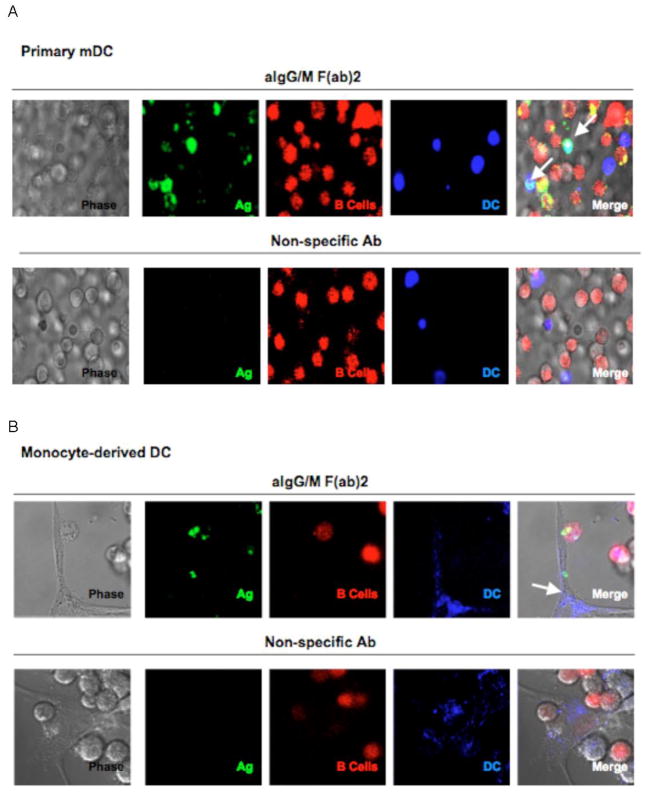
Since our flow cytometry analysis could potentially include a couplet population of B lymphocytes attached to dendritic cells in a manner similar to that described by Deola et al. (Deola et al., 2008), we sought to verify the existence of antigen bearing DCs within live cell cultures using confocal microscopy. For these studies, B lymphocytes were labeled with CellTracker™ Orange (CTO; red) and then pulsed with antigen, whereas the dendritic cells, whether primary MoDC or monocyte-derived DC (MdDC), were labeled with CellTrace™ Far Red (CTFR; blue). The labeled B cells were co-cultured with each of the DC populations for 18 hr prior to live cell imaging. Within the primary MoDC (Fig. 2A) as well as the MdDC (Fig. 2B) populations, co-cultured with Bcells pulsed with BCR-targeted Ag, we could identify individual dendritic cells that had acquired antigen. Interestingly, DCs did not acquire Ag when co-cultured with B cells pulsed with Ag that does not bind BCR. Moreover, using live-imaging microscopy, we captured the direct interaction of a B cell with the veil of a monocyte-derived DC (Fig 2B, upper panel) that had acquired antigen, suggesting that contact between the antigen donor and recipient cell is necessary for antigen transfer. This interaction likely includes the exchange of membrane along with antigen, given that many of the antigen-bearing DCs appeared to also contain B cell derived (red/CTO) components. These live-cell images confirm our flow cytometry findings that DCs are acquiring antigen directly from the B cells and suggest that the mechanism of transfer may include the acquition of B cell membrane and/or intracellular proteins by the DC0.
Fig. 2.
Live cell images depict antigen transfer from B cells to dendritic cells. BJAB cells were labeled with CTO and pulsed with antigen. Primary myeloid DCs (a) or monocyte derived DCs (b) were labeled with CTFR and then co-cultured with either aIg (upper panels) or non-specific Ab (lower panels) pulsed B cells in glass bottom dishes. After 18 hr, live cell images were taken by confocal microscopy (600X, 2x zoom; arrows point to DCs that bear antigen).
3.2 Dendritic cells acquire antigen more efficiently through antigen transfer than endocytosis
The majority of MoDC “freshly isolated” from human blood exhibit an immature phenotype in that these DCs are smaller and lack the dendritic morphology associated with their mature counterparts (O’Doherty et al., 1993). Immature dendritic cells acquire antigen by various mechanisms; the most common of which is the macropinocytosis of soluble antigen from the surrounding environment (Hart, 1997). To determine whether antigen transfer to MoDCs occurs at a different or equivalent level of efficiency as macropinocytosis, MoDCs were either co-cultured with antigen pulsed B cells or cultured alone in the presence of 5 ug/ml soluble aIg for 18 hr prior to being harvested for flow cytometry. Interestingly, a large proportion of DCs (53%) that were co-cultured with aIg pulsed B lymphocytes acquired a greater amount of antigen than those incubated alone with the soluble form of antigen (Fig. 3) indicating that DCs can acquire antigen more efficiently through antigen transfer from B cells than by simple macropinocytosis of free antigen. As described earlier, this Ag does not enter the DCs via Fc receptor-mediated uptake.
Fig. 3.
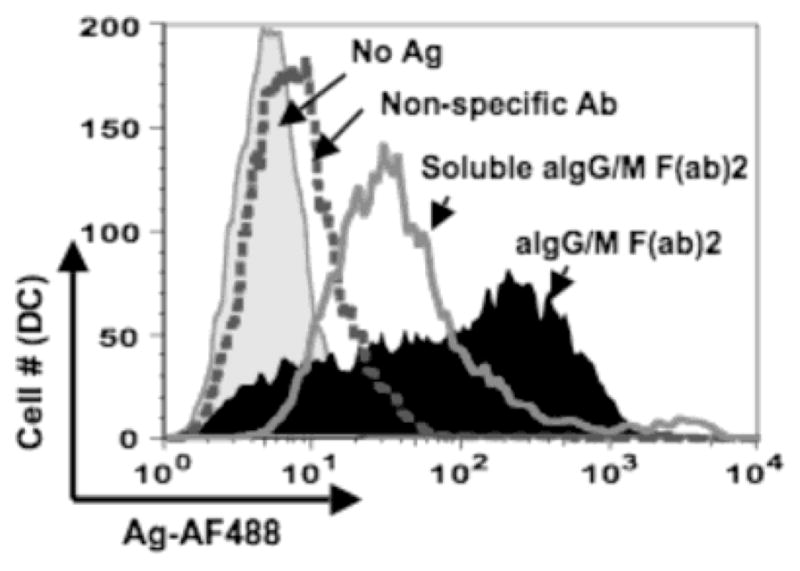
Dendritic cells acquire greater antigen by transfer from B cells than by endocytosis. Primary myeloid DCs were cultured for 18 hrs with the following: no antigen (shaded gray curve), 5 ug/ml soluble Ag alone (light gray curve), or BJAB cells pulsed with either non-specific Ab (dotted curve) or aIg (black curve). Cells were stained for flow cytometry and levels of transferred antigen were assessed for the CD19−:CD14−:CD11c+ gated population.
3.3 Antigen transfer requires direct contact between antigen donor and recipient cells
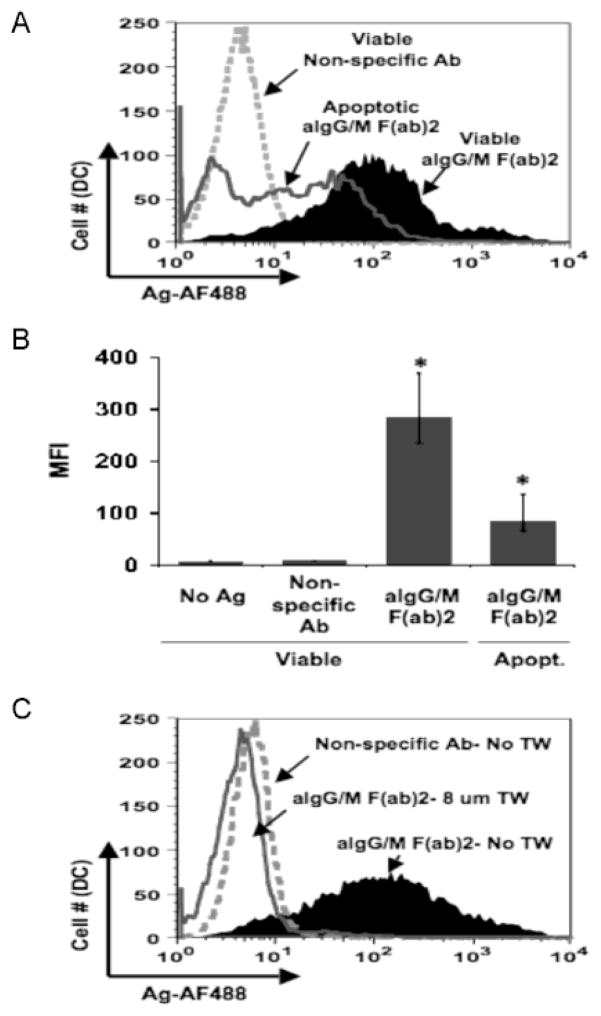
In the live cell imaging studies, we observed that MoDCs were acquiring B cell derived components along with antigen (see figure 2A). This observation could be explained by the findings of Inaba et al. (Inaba et al., 1998) that immature DCs can acquire antigen from either necrotic or apoptotic B blasts at levels sufficient enough to induce a T cell response. To assess whether antigen transfer was being mediated by the uptake of apoptotic bodies from the B cell culture, MoDCs were co-cultured with antigen pulsed B cells that were either viable (1×106 cells: standard cell number) or apoptotic/necrotic (3×105ce lls: number of cells corresponding to the fraction of apoptotic cells within the typical B cell culture). Antigen transfer to MoDCs was more efficient with viable B cells than with apoptotic lymphocytes (Fig. 4A) and this was reflected in the significantly higher level of transferred antigen (Fig. 4B; p=0.001). Although MoDCs were acquiring antigen from the apoptotic B cells as previously reported (Inaba et al., 1998), the levels attributable to this fraction of antigen pulsed B cells could not account for the amount of antigen transfer typically observed in our previous experiments with viable B cells. Therefore, apoptotic bodies do not significantly contribute to the transfer of antigen from antigen specific B cells to MoDCs.
Fig. 4.
Direct interaction between live B cells and dendritic cells is required for antigen transfer. (a) Contribution of apoptotic B cells to antigen transfer is trivial. Apoptosis of antigen pulsed B-LCLs was induced by hyperthermia. Primary DCs were co-cultured for 18 hr with viable (1 ×106 cells) B cells pulsed with non-specific Ab (dotted curve) or aIg (black solid curve) or with apoptotic B cells (3 ×105 cells; number of apoptotic cells within viable population) pulsed with aIg (dark gray curve). Levels of antigen were assessed for the CD19−:CD14−:CD11c+ gated population. (b) Antigen transfer from apoptotic B cells was significantly less compared to viable cells. MFI values for transferred antigen (AF488) are given for 4 independent experiments (* p= 0.001; n=4; thin bars represent value range). (c) Antigen transfer is abrogated upon separation of B cells from dendritic cells. Primary DCs were co-cultured in the absence of transwell (No TW) with B cells pulsed with non-specific Ab (dotted curve) or in the absence (black curve) or presence (8 um TW) (dark gray line) of transwell with B cells pulsed with aIg.
With the exclusion of apoptotic cells as sole mediators of antigen transfer, we considered the possibility that contact between antigen donor and recipient cells was necessary for antigen exchange. Our confocal microscopy studies with MdDCs (see figure 2B) suggest that the two APCs may be interacting with one another during antigen transfer. To confirm the importance of direct cell contact, antigen pulsed B cells were separated from MoDCs by transwells of various pore sizes during an 18 hr co-culture. Even with a pore size of 8 μm, DCs were unable to acquire antigen from B cells (Fig. 4C) (similar results were found with 1 and 3 μm transwells; data not shown) indicating that contact between the two APCs is required for antigen transfer to proceed. Furthermore, this data suggests that the DCs are not acquiring soluble antigen from the B cells within the co-cultures since the 8 μm pore size transwell is permeable to antigens the size of immune complexes.
3.4 Scavenger receptor A mediates antigen transfer through the exchange of membrane
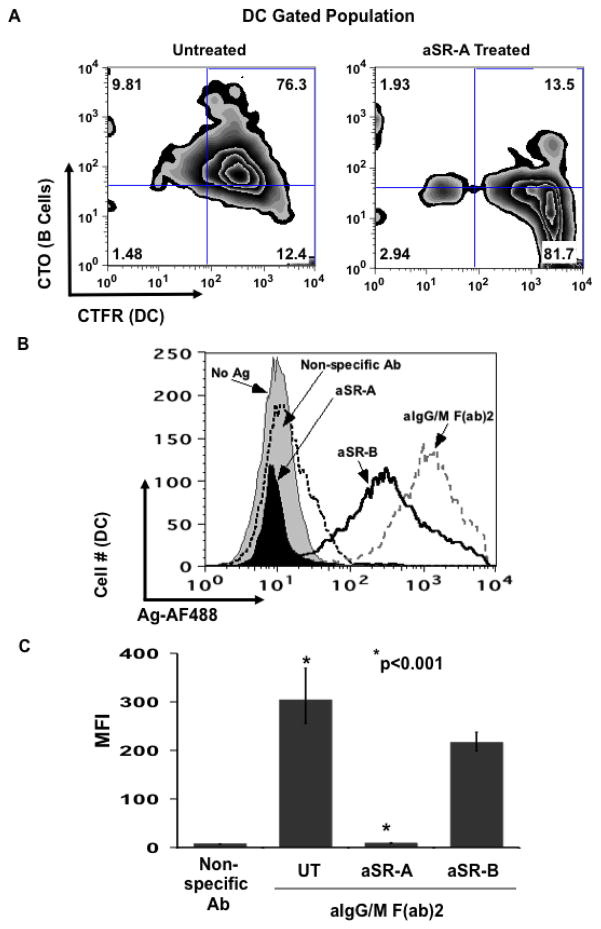
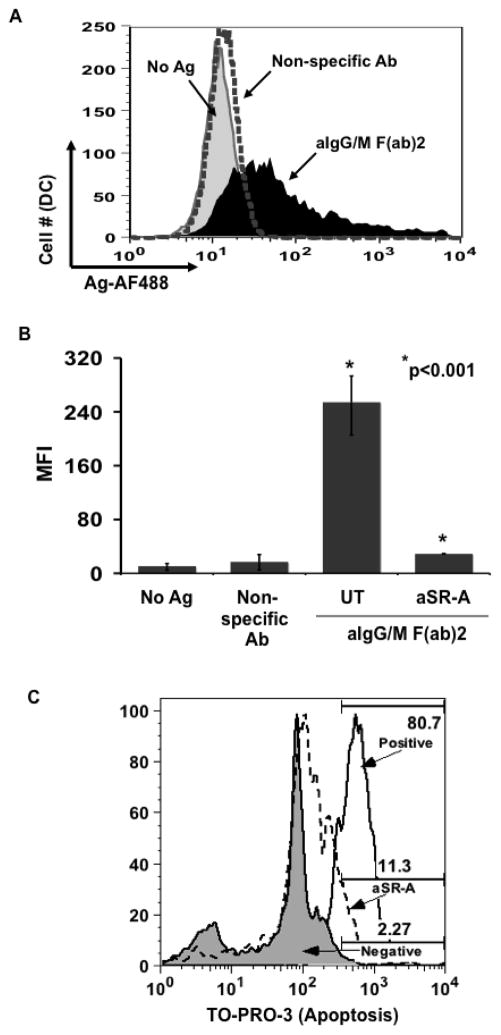
According to our live cell imaging studies, DCs appear to be capturing B cell derived membrane and/or intracellular proteins along with antigen. Several recent studies have demonstrated that scavenger receptor A (SR-A) mediates the exchange of cell membrane and/or cytosolic components between cells following contact (Harshyne et al., 2001; Harshyne et al., 2003). For this reason, we sought to determine by flow cytometry whether the DCs are capturing B cell membrane and/or cytosolic components along with antigen and also whether SR-A is mediating this process. Human B cells were labeled with CTO and pulsed with antigen. MoDCs were labeled with CTFR and then left untreated (UT) or exposed to either anti-SR-A (aSR-A) or anti-SR-B blocking antibodies prior to being co-cultured with the B lymphocytes. As seen in figure 5A, a large proportion (76%) of the DCs were positive for B cell-specific label (CTO) and the percentage was reduced by 63% following treatment with aSR-A blocking antibody indicating that the DCs are capturing B cell derived membrane by an SR-A mediated mechanism. Concomitant with the reduction in B cell membrane capture, there was a decrease in antigen transfer in the aSR-A treated DC population as compared to those left untreated (Fig. 5B) and the level of transferred antigen was significantly less, respectively (Fig. 5C). Blocking SR-B did cause a slight (but insignificant; p=0.1) decrease in the acquisition of antigen that may be due to the inability of the DCs to capture apoptotic bodies since SR-B has been shown to mediate this process (Daviet and McGregor, 1997). Interestingly, antigen transfer to monocyte-derived DCs was equally inhibited by pre-treatment with aSR-A antibody (data not shown). Taken together, these results demonstrate that antigen transfer from B cells to DCs involves an SR-A mediated capturing of B lymphocyte membrane and/or intracellular proteins by the recipient APC.
Fig. 5.
Dendritic cells acquire B cell derived membrane and/or intracellular proteins through an SR-A dependent mechanism. BJAB cells were labeled with CTO and pulsed with antigen. Primary DCs were labeled with CTFR and then left untreated (UT) or treated with either aSR-A or aSR-B blocking antibodies for 1 hr. These DCs were co-cultured for 18 hr in the presence of inhibitors with Ag-pulsed B cells. Cells were harvested and analyzed by flow cytometry. (a) B cell specific fluorescence is transferred to dendritic cells and membrane transfer is blocked by aSR-A. Level of B cell derived label (CTO) was determined for the untreated DCs (CTFR) (left panel) and aSR-A Ab treated (right panel). (b) Antigen transfer to DCs is dependent on SR-A. Levels of transferred antigen (AF488) were assessed for the CTFR+ gated DC cells that were left untreated (UT) and cultured with B cells pulsed with either non-specific Ab (dotted curve), aIg (black curve) and those DCs that were treated with aSR-A Ab (solid black curve) or aSR-B Ab (black line) and cultured with aIg pulsed B cells (gray curve). (c) Blocking SR-A but not SR-B significantly inhibits antigen transfer. MFI values for transferred antigen (AF488) are given for 4 independent experiments (* p<0.001; n=4; thin bars represent value range).
3.5 Primary B cells transfer antigen to dendritic cells through SR-A mediated mechanism
Our current understanding of antigen transfer from B cells to MoDCs is based on human B cell lines. As such, our findings may be a consequence of the transformed properties of these cells instead of the normal physiologic function of B lymphocytes. To assess whether this process was limited to transformed B cells, primary B cells were isolated from PBMCs, pulsed with antigen and co-cultured with MoDCs. The primary B cells were able to transfer antigen to the dendritic cells only when carrying BCR-specific antigen (Fig. 6A and 6B) as we have observed with either BJAB cells or B-LCLs (see Fig. 1C). Following pre-treatment of the MoDCs with a-SR-A blocking antibody, the level of transferred antigen within the DCs was significantly reduced (Fig. 6B), suggesting that the transfer of antigen from primary human B cells to MoDCs was mediated by the same SR-A dependent mechanism as seen with the B cell lines. Although we already determined that apoptotic B cells (BJAB or B-LCL) are a poor source of antigen for DCs (demonstrated in Fig. 4), we wanted to ensure that the primary B cells being used were viable through the 18hr time course of the experiment. Compared to positive control primary B cells induced to apoptosis by hyperthermia, after 18hrs of incubation with the anti-SR-A antibody, ~11% of the primary B cells were positive for the apoptotic marker TO-PRO-3 (Fig. 6C). These results indicate that primary B cells are viable, and recapitulates prior results from our laboratory (Harvey, et al., 2008).
Fig. 6.
Primary B cells transfer antigen to dendritic cells by an SR-A mediated mechanism. Primary DCs were either left untreated (UT) or treated with aSR-A Ab for 1 hr prior to being co-cultured with primary B cells pulsed with the following: no Ag (gray curve) non-specific Ab (dotted curve) or aIg (black curve). Cells were harvested and stained for flow cytometry. (a) Primary B cells are capable of transferring antigen. Antigen levels (AF488) were assessed for CD19−:CD14−:CD11c+ gated population for untreated DC co-cultures. (b) Anti-SR-A Ab significantly inhibits antigen transfer from primary B cells. MFI values for transferred antigen (AF488) were determined for DCs either left untreated or treated with aSR-A Ab from 3 independent experiments (* p<0.001; n=3; thin bars represent value range). (c) Primary B cells (gated by CD19) were assayed for degree of apoptosis by TO-PRO-3. Negative (untreated) primary B cells, aSR-A treated primary B cells, and B cells triggered for apoptosis by incubation at 65°C for 30 min. are illustrated.
3.6 Recipient dendritic cells carry processed antigen following transfer
Effective presentation of antigen by APCs to cognate T cells requires the processing of antigen prior to being presented on MHC. To assess whether recipient DCs contained processed antigen, B cells were pulsed with aIg conjugated with both AF488 and pHrodo™, a dye that fluoresces only under conditions of low pH such as in lysosomes. These B cells were then co-cultured with MdDCs for 18 hr to allow antigen transfer. Of the DCs cultured with B cells pulsed with double conjugated antigen, a large proportion of them (39%) contained only unprocessed antigen (AF488+ alone) whereas a smaller proportion (13%) carried antigen (pHrodo+:AF488+) processed from a low pH compartment (Fig. 7). Interestingly, not every DC degraded acquired antigen, suggesting that a specific subset of DC either captured processed antigen from the B lymphocytes or processed its Ag following transfer. Nevertheless, antigen transfer from B cells resulted in dendritic cells carrying processed antigen, suggesting that these APCs could present the transferred antigen to T cells to induce an immunologic response.
Fig. 7.
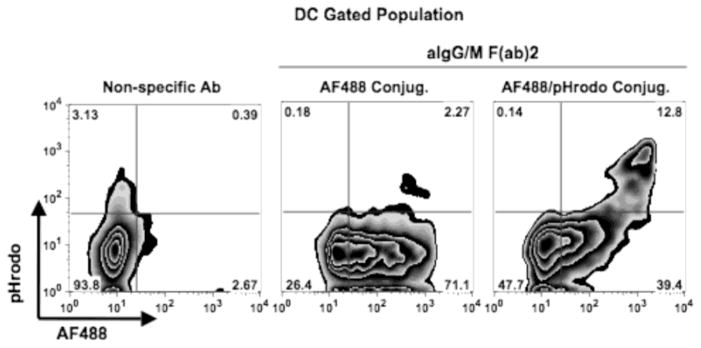
Dendritic cells possess processed antigen following transfer. Monocyte derived DCs were co-cultured for 18 hr with BJAB cells pulsed with the following: non-specific Ab (left panel), aIg-AF488 (middle panel) or aIg-AF488/pHrodo (right panel). Levels of AF488 and pHrodo from transferred antigen were assessed for the CD19−:CD14−:CD11c+ gated populations.
4. Discussion
Although B cells and DCs are unique and effective APCs independently, their interactions and/or synergy lead to a more robust immune response (Kleindienst and Brocker, 2005). We have demonstrated previously that B cells can initiate an antigen specific immune response earlier than other APCs; however, longer term immunity to the same antigen requires dendritic cells to activate T cells as well (Yan et al., 2006). As such, our laboratory investigated how B cells were interacting with other APCs in promoting immunity to a specific antigen. In a recent study, we found that Ag-specific B cells can transfer antigen to murine or human macrophages and that the transferred antigen can be presented by the macrophages to induce an antigen specific T cell response (Harvey et al., 2007, Harvey, et al., 2008). Human macrophages are capable of receiving antigen from B cells in a manner that utilizes surface Scavenger Receptor A (SRA; Harvey et al., 2007, Harvey, et al., 2008). Here we demonstrate that human B lymphocytes are able to transfer BCR-enriched antigen to primary myeloid DCs and moreover, we elucidate the mechanism by which DCs acquire the antigen from B cells.
Antigen transfer has been characterized for various immune and non-immune cell types (Girvan et al., 2003; Harshyne et al., 2003; Marañón et al., 2004; Neijssen et al., 2005). The most relevant to our investigation include transfer between B cells and other APCs such as macrophages and dendritic cells (Bergtold et al., 2005; Bickham et al., 2003; Carrasco and Batista, 2007; Denzer et al., 2000; Harvey et al., 2007; Inaba et al., 1998; Phan et al., 2007; Wykes et al., 1998). The type of antigen transferred as well as the mechanism employed varies depending on the cell types and directionality of transfer involved. Elegant work of Carrasco and Batista illustrate that circulating antigen can be trapped by macrophage sheets in the subcapsular spaces of lymph nodes for acquisition by B cells. The B cells attach to clusters of particulate antigen resembling an immunological synapse (Carrasco and Batista, 2007). DCs can acquire and transfer non-degraded antigen to B cells in an Fc receptor mediated process (Bergtold et al., 2005; Carrasco and Batista, 2007). These collections of studies illustrate how B cells load antigen from other professional APCs. Fewer studies have examined the transport of antigen from B cells to macrophages or DCs.
As for B lymphocytes, these APCs have been found to transfer antigen to DCs either as apoptotic bodies that are endocytosed by the DC or by the formation of cellular exosomes (Bickham et al., 2003; Denzer et al., 2000; Inaba et al., 1998). Unlike our current investigation in which BCR-targeted antigen was utilized, these studies did not discriminate as to whether the transferred antigen was captured by the BCR indicating that antigen specificity was not addressed.
A common feature associated with antigen transfer is the requirement for direct contact between the antigen donor and recipient cells (Denzer et al., 2000; Harshyne et al., 2003; Phan et al., 2007; Wykes et al., 1998). Previous studies have demonstrated the direct interaction of B lymphocytes with DCs, and that this interaction is required for dendritic cells to transfer unprocessed antigen to B lymphocytes (Huang et al., 2005; Qi et al., 2006; Wykes et al., 1998). In our current investigation, confocal microscopy of live cell cultures confirmed that direct contact between B cells and antigen-bearing DCs is a pre-requisite for antigen transfer. Moreover, separation of the two populations by a transwell disrupted the transfer of antigen from the B lymphocytes to the DCs. The results of our flow cytometry analysis of co-cultures (Fig. 5A) indicated that B cell membrane and/or intracellular proteins are being captured by human DCs together with BCR-enriched antigen. In contrast to other studies, the apoptotic fraction of the B cell population did not account for antigen transfer (Fig. 4A) or for the acquisition of B cell derived components by the DCs.
Scavenger receptor A is a member of a larger family of proteins with various biological properties, including pathogen pattern recognition, cellular adhesion, and effects on immune cell activation (reviewed by Jordo, et al., 2011). SR-A has recently been identified as a cell surface receptor that mediates the capture of target cell membrane by DCs (referred to as “nibbling”) (Harshyne et al., 2003; Marañón et al., 2004). We, therefore, examined whether SR-A plays a role in antigen transfer. SR-A is expressed on macrophages as well as dendritic cells and is involved in a variety of functions ranging from clearance of lipoproteins, phagocytosis of bacteria and intercellular adhesion which interestingly includes the binding of activated B cells to macrophages (Dunne et al., 1994; Fraser et al., 1993; Goldstein et al., 1979; Harshyne et al., 2003; Yokota et al., 1998). Indeed, we demonstrated that the capture of B cell derived membrane as well as the acquisition of antigen from B lymphocytes by DCs required SR-A in a manner that resembles acquisition of antigen by macrophages (Harvey et al., 2007, Harvey, et al., 2008) and was not dependent on other scavenger receptor surface proteins such as SR-B. Taken together, these findings suggest that SR-A mediates antigen transfer to the DCs through the capture of B cell membrane and/or cytosolic proteins rather than the phagocytosis of apoptotic bodies.
The importance of SR-A in antigen transfer could extend beyond its role in the acquisition of B cell membrane. This receptor has been shown to be involved in the recognition and internalization of the endoplasmic reticulum heat shock proteins (HSP) gp96/GRP94 and calreticulin (Berwin et al., 2003). Peptides bound to these HSPs have been found to be represented on MHC by APCs following internalization (Binder et al., 2000). Berwin et al. demonstrated that SR-A can direct gp96/peptide ligands to endosomal compartments involved in an antigen cross-presentation pathway (Berwin et al., 2003). We present data herein demonstrating that dendritic cells possess processed antigen following SR-A dependent acquisition from B cells and that transferred antigen may also be shuttled into the re-presentation pathway.
Our findings have broad implications in understanding the immunological events leading to a focused immune response to a select group of antigens, whether foreign or self. First, antigen specific B lymphocytes can capture and present antigen at concentrations 1,000 times lower than other APCs in activating T cells (Lanzavecchia, 1990). Secondly, primary myeloid dendritic cells can acquire antigen more efficiently by antigen transfer from B cells than by endocytosis of free antigen. Taken together, these findings suggest that antigen transfer from antigen specific B cells to dendritic cells would lead to a synergistic effort in presenting the same antigen to T cells whereby a more targeted immunologic response would ensue. We suspect that a single B cell is capable of both Ag transfer to DCs and in direct activation of T cells. Studies already illustrate the importance of B cells in the development and maintenance of follicular T helper cells (Mamula, 2013; Xu, et al., 2013). Herein, we provide evidence for a mechanism by which antigen specific B cells could modulate the immune response through the transfer of BCR-enriched antigen to another APC, the dendritic cell. Antigen transfer requires direct contact between the two APCs followed by the capture of B cell derived components by the recipient DC. Moreover, we have identified SR-A as a key surface receptor on human DCs that mediates the acquisition of antigen from B cells. Depending on the immunological response initiated by antigen transfer, whether tolerogenic or immunogenic, SR-A could be a target for therapeutic intervention for chronic autoimmune diseases that are characterized by the presence of autoantibodies against select autoantigens.
Acknowledgments
These studies were supported by National Institutes of Health grants AI-48120 and AR-41032 and the Alliance for Lupus Research. B.P. Harvey was supported by an Arthritis Foundation postdoctoral fellowship. The authors thank Dr. Mark J. Shlomchik for contributing significantly to the intellectual aspects of our investigation.
Abbreviations
- MoDC
myeloid DC
- MdDC
monocyte derived DC
- aIg
anti-human IgG/M F(ab′)2
- SR-A
class A scavenger receptor
- IC
immune complex
- B-LCL
B lymphoblastoid cell line
- CTO
CellTracker™ Orange
- CTFR
CellTrace™ Far Red
- AF488
Alexa Fluor® 488
- TW
transwell
Footnotes
Disclosure
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balázs M, Martin F, Zhou T, Kearney J. Blood dendritic cells interact with splenic marginal zone B cells to initiate T-independent immune responses. Immunity. 2002;17:341–352. doi: 10.1016/s1074-7613(02)00389-8. [DOI] [PubMed] [Google Scholar]
- Bayry J, Lacroix-Desmazes S, Kazatchkine MD, Hermine O, Tough DF, Kaveri SV. Modulation of dendritic cell maturation and function by B lymphocytes. J Immunol. 2005;175:15–20. doi: 10.4049/jimmunol.175.1.15. [DOI] [PubMed] [Google Scholar]
- Bennett WE, Cohn ZA. The isolation and selected properties of blood monocytes. J Exp Med. 1966;123:145–160. doi: 10.1084/jem.123.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergtold A, Desai DD, Gavhane A, Clynes R. Cell surface recycling of internalized antigen permits dendritic cell priming of B cells. Immunity. 2005;23:503–514. doi: 10.1016/j.immuni.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Berwin B, Hart JP, Rice S, Gass C, Pizzo SV, Post SR, Nicchitta CV. Scavenger receptor-A mediates gp96/GRP94 and calreticulin internalization by antigen-presenting cells. EMBO J. 2003;22:6127–6136. doi: 10.1093/emboj/cdg572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickham K, Goodman K, Paludan C, Nikiforow S, Tsang ML, Steinman RM, Münz C. Dendritic cells initiate immune control of epstein-barr virus transformation of B lymphocytes in vitro. J Exp Med. 2003;198:1653–1663. doi: 10.1084/jem.20030646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder RJ, Anderson KM, Basu S, Srivastava PK. Heat shock protein gp96 induces maturation and migration of CD11c+ cells in vivo. J Immunol. 2000;165:6029–6035. doi: 10.4049/jimmunol.165.11.6029. [DOI] [PubMed] [Google Scholar]
- Carrasco YR, Batista FD. B cells acquire particulate antigen in a macrophage-rich area at the boundary between the follicle and the subcapsular sinus of the lymph node. Immunity. 2007;27:160–171. doi: 10.1016/j.immuni.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Chan O, Shlomchik MJ. A new role for B cells in systemic autoimmunity: B cells promote spontaneous T cell activation in MRL-lpr/lpr mice. J Immunol. 1998;160:51–59. [PubMed] [Google Scholar]
- Crowley M, Inaba K, Steinman RM. Dendritic cells are the principal cells in mouse spleen bearing immunogenic fragments of foreign proteins. J Exp Med. 1990;172:383–386. doi: 10.1084/jem.172.1.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daviet L, McGregor JL. Vascular biology of CD36: roles of this new adhesion molecule family in different disease states. Thrombosis and Haemostasis. 1997;78:65–69. [PubMed] [Google Scholar]
- Denzer K, Eijk Mv, Kleijmeer MJ, Jakobson E, Groot Cd, Geuze HJ. Follicular dendritic cells carry MHC class II-expressing microvesicles at their surface. Journal of Immunology. 2000;165:1259–1265. doi: 10.4049/jimmunol.165.3.1259. [DOI] [PubMed] [Google Scholar]
- Deola S, Panelli MC, Maric D, Selleri S, Dmitrieva NI, Voss CY, Klein H, Stroncek D, Wang E, Marincola FM. Helper B cells promote cytotoxic T cell survival and proliferation independently of antigen presentation through CD27/CD70 interactions. J Immunol. 2008;180:1362–1372. doi: 10.4049/jimmunol.180.3.1362. [DOI] [PubMed] [Google Scholar]
- Diaz-de-Durana Y, Mantchev GT, Bram RJ, Franco A. TACI-BLyS signaling via B-cell-dendritic cell cooperation is required for naive CD8+ T-cell priming in vivo. Blood. 2006;107:594–601. doi: 10.1182/blood-2004-12-4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois B, Massacrier C, Caux C. Selective attraction of naive and memory B cells by dendritic cells. Journal of Leukocyte Biology. 2001;70:633–641. [PubMed] [Google Scholar]
- Duffield JS, Tipping PG, Kipari T, Cailhier JF, Clay S, Lang R, Bonventre JV, Hughes J. Conditional ablation of macrophages halts progression of crescentic glomerulonephritis. Am J Pathol. 2005;167:1207–1219. doi: 10.1016/S0002-9440(10)61209-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunne DW, Resnick D, Greenberg J, Krieger M, Joiner KA. The type I macrophage scavenger receptor binds to gram-positive bacteria and recognizes lipoteichoic acid. Proc Natl Acad Sci USA. 1994;91:1863–1867. doi: 10.1073/pnas.91.5.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson AR, Youd ME, Corley RB. Marginal zone B cells transport and deposit IgM-containing immune complexes onto follicular dendritic cells. Int Immunol. 2004;16:1411–1422. doi: 10.1093/intimm/dxh142. [DOI] [PubMed] [Google Scholar]
- Fraser I, Hughes D, Gordon S. Divalent cation-independent macrophage adhesion inhibited by monoclonal antibody to murine scavenger receptor. Nature. 1993;364:343–346. doi: 10.1038/364343a0. [DOI] [PubMed] [Google Scholar]
- Girvan A, Aldwell FE, Buchan GS, Faulkner L, Baird MA. Transfer of macrophage-derived mycobacterial antigens to dendritic cells can induce naïve T-cell activation. Scand J Immunol. 2003;57:107–114. doi: 10.1046/j.1365-3083.2003.01191.x. [DOI] [PubMed] [Google Scholar]
- Goldstein JL, Ho YK, Basu SK, Brown MS. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc Natl Acad Sci USA. 1979;76:333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harshyne LA, Watkins SC, Gambotto A, Barratt-Boyes SM. Dendritic cells acquire antigens from live cells for cross-presentation to CTL. J Immunol. 2001;166:3717–3723. doi: 10.4049/jimmunol.166.6.3717. [DOI] [PubMed] [Google Scholar]
- Harshyne LA, Zimmer MI, Watkins SC, Barratt-Boyes SM. A role for class A scavenger receptor in dendritic cell nibbling from live cells. J Immunol. 2003;170:2302–2309. doi: 10.4049/jimmunol.170.5.2302. [DOI] [PubMed] [Google Scholar]
- Hart DN. Dendritic cells: unique leukocyte populations which control the primary immune response. Blood. 1997;90:3245–3287. [PubMed] [Google Scholar]
- Harvey BP, Gee RJ, Haberman AM, Shlomchik MJ, Mamula MJ. Antigen presentation and transfer between B cells and macrophages. Eur J Immunol. 2007;37:1739–1751. doi: 10.1002/eji.200636452. [DOI] [PubMed] [Google Scholar]
- Harvey BP, Quan TE, Rudenga BJ, Roman RM, Craft J, Mamula MJ. Editing antigen presentation: antigen transfer between human B lymphocytes and macrophages mediated by class A scavenger receptors. J Immunol. 2008;181:4043–4051. doi: 10.4049/jimmunol.181.6.4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang NN, Han SB, Hwang IY, Kehrl JH. B cells productively engage soluble antigen-pulsed dendritic cells: visualization of live-cell dynamics of B cell-dendritic cell interactions. J Immunol. 2005;175:7125–7134. doi: 10.4049/jimmunol.175.11.7125. [DOI] [PubMed] [Google Scholar]
- Inaba K, Metlay JP, Crowley MT, Steinman RM. Dendritic cells pulsed with protein antigens in vitro can prime antigen-specific, MHC-restricted T cells in situ. J Exp Med. 1990;172:631–640. doi: 10.1084/jem.172.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K, Turley S, Yamaide F, Iyoda T, Mahnke K, Inaba M, Pack M, Subklewe M, Sauter B, Sheff D, et al. Efficient presentation of phagocytosed cellular fragments on the major histocompatibility complex class II products of dendritic cells. J Exp Med. 1998;188:2163–2173. doi: 10.1084/jem.188.11.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong JMD, Schuurhuis DH, Ioan-Facsinay A, Welling MM, Camps MG, Voort EI, Huizinga Tw, Ossendorp F, Verbeek JS, Toes RE. Dendritic cells, but not macrophages or B cells, activate major histocompatibility complex class II-restricted CD4+ T cells upon immune-complex uptake in vivo. Immunology. 2006;119:499–506. doi: 10.1111/j.1365-2567.2006.02464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordo ED, Wermeling F, Chen Y, Karlsson MCI. Scavenger receptors as regulators of natural antibody responses and B cell activation in autoimmunity. Mol Immunol. 2011;48:1307–1318. doi: 10.1016/j.molimm.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Kilmon MA, Rutan JA, Clarke SH, Vilen BJ. Low-affinity, Smith antigen-specific B cells are tolerized by dendritic cells and macrophages. J Immunol. 2005;175:37–41. doi: 10.4049/jimmunol.175.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleindienst P, Brocker T. Concerted antigen presentation by dendritic cells and B cells is necessary for optimal CD4 T-cell immunity in vivo. Immunology. 2005;115:556–564. doi: 10.1111/j.1365-2567.2005.02196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzavecchia A. Receptor-mediated antigen uptake and its effect on antigen presentation to class II-restricted T lymphocytes. Annu Rev Immunol. 1990;8:773–793. doi: 10.1146/annurev.iy.08.040190.004013. [DOI] [PubMed] [Google Scholar]
- Mamula MJ. B cells: Not just making Ig anymore. Arth Rheum. 2013 doi: 10.1002/art.38208. in press. [DOI] [PubMed] [Google Scholar]
- Mannhalter JW, Zlabinger GJ, Ahmad R, Eibl MM. Human T cell proliferation in response to E. coli presented by autologous macrophages is antigen specific. Clin Exp Immunol. 1983;54:95–102. [PMC free article] [PubMed] [Google Scholar]
- Marañón C, Desoutter JF, Hoeffel G, Cohen W, Hanau D, Hosmalin A. Dendritic cells cross-present HIV antigens from live as well as apoptotic infected CD4+ T lymphocytes. Proc Natl Acad Sci USA. 2004;101:6092–6097. doi: 10.1073/pnas.0304860101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neijssen J, Herberts C, Drijfhout JW, Reits E, Janssen L, Neefjes J. Cross-presentation by intercellular peptide transfer through gap junctions. Nature. 2005;434:83–88. doi: 10.1038/nature03290. [DOI] [PubMed] [Google Scholar]
- O’Doherty U, Steinman RM, Peng M, Cameron PU, Gezelter S, Kopeloff I, Swiggard WJ, Pope M, Bhardwaj N. Dendritic cells freshly isolated from human blood express CD4 and mature into typical immunostimulatory dendritic cells after culture in monocyte-conditioned medium. J Exp Med. 1993;178:1067–1076. doi: 10.1084/jem.178.3.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape KA, Catron DM, Itano AA, Jenkins MK. The humoral immune response is initiated in lymph nodes by B cells that acquire soluble antigen directly in the follicles. Immunity. 2007;26:491–502. doi: 10.1016/j.immuni.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Phan TG, Grigorova I, Okada T, Cyster JG. Subcapsular encounter and complement-dependent transport of immune complexes by lymph node B cells. Nat Immunol. 2007;8:992–1000. doi: 10.1038/ni1494. [DOI] [PubMed] [Google Scholar]
- Probst HC, Broek M. Priming of CTLs by lymphocytic choriomeningitis virus depends on dendritic cells. J Immunol. 2005;174:3920–3924. doi: 10.4049/jimmunol.174.7.3920. [DOI] [PubMed] [Google Scholar]
- Qi H, Egen JG, Huang AYC, Germain RN. Extrafollicular activation of lymph node B cells by antigen-bearing dendritic cells. Science. 2006;312:1672–1676. doi: 10.1126/science.1125703. [DOI] [PubMed] [Google Scholar]
- Roth R, Gee RJ, Mamula MJ. B lymphocytes as autoantigen-presenting cells in the amplification of autoimmunity. Ann NY Acad Sci. 1997;815:88–104. doi: 10.1111/j.1749-6632.1997.tb52047.x. [DOI] [PubMed] [Google Scholar]
- Valdez Y, Mah W, Winslow MM, Xu L, Ling P, Townsend SE. Major histocompatibility complex class II presentation of cell-associated antigen is mediated by CD8alpha+ dendritic cells in vivo. J Exp Med. 2002;195:683–694. doi: 10.1084/jem.20010898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wykes M, Pombo A, Jenkins C, MacPherson GG. Dendritic cells interact directly with naive B lymphocytes to transfer antigen and initiate class switching in a primary T-dependent response. J Immunol. 1998;161:1313–1319. [PubMed] [Google Scholar]
- Xu H, Li X, Liu D, Li J, Zhang X, Chen X, Hou S, Peng L, Xu C, Liu W, Zhang L, Qi H. Follicular T-helper cell recruitment governed by bystander B cells and ICOS-driven motility. Nature. 2013;496:523–527. doi: 10.1038/nature12058. [DOI] [PubMed] [Google Scholar]
- Yan J, Harvey BP, Gee RJ, Shlomchik MJ, Mamula MJ. B cells drive early T cell autoimmunity in vivo prior to dendritic cell-mediated autoantigen presentation. J Immunol. 2006;177:4481–4487. doi: 10.4049/jimmunol.177.7.4481. [DOI] [PubMed] [Google Scholar]
- Yokota T, Ehlin-Henriksson B, Hansson GK. Scavenger receptors mediate adhesion of activated B lymphocytes. Exp Cell Res. 1998;239:16–22. doi: 10.1006/excr.1997.3876. [DOI] [PubMed] [Google Scholar]
- Zhao QL, Fujiwara Y, Kondo T. Mechanism of cell death induction by nitroxide and hyperthermia. Free Radic Biol Med. 2006;40:1131–1143. doi: 10.1016/j.freeradbiomed.2005.10.064. [DOI] [PubMed] [Google Scholar]