Abstract
The advent of imatinib has dramatically improved outcomes in patients with chronic myelogenous leukemia (CML). It has become the standard of care for all patients with newly diagnosed chronic-phase CML based on its successful induction of durable responses in most patients. However, its use is complicated by the development of resistance in some patients. Dose escalation might overcome this resistance if detected early. The second-generation tyrosine kinase inhibitors (TKIs) dasatinib and nilotinib provide effective therapeutic options for managing patients resistant or intolerant to imatinib. Recent studies have shown that dasatinib and nilotinib provide quicker and potentially better responses than standard-dose imatinib when used as a first-line treatment. The goal of therapy for patients with CML is the achievement of a complete cytogenetic response, and eventually a major molecular response, to prevent disease progression to accelerated or blast phase. Selecting the appropriate TKI depends on many factors, including disease phase, primary or secondary resistance to TKI, the agent’s side effect profile and its relative effectiveness against BCR-ABL mutations, and the patient’s tolerance to therapy. In October 2010, NCCN organized a task force consisting of a panel of experts from NCCN Member Institutions with expertise in the management of patients with CML to discuss these issues. This report provides recommendations regarding the selection of TKI therapy for the management of patients with CML based on the evaluation of available published clinical data and expert opinion among the task force members.
Keywords: Chronic myelogenous leukemia, CML, BCR-ABL, tyrosine kinase inhibitor, TKI, imatinib, high-dose imatinib, dasatinib, nilotinib, mutations, suboptimal response, disease progression, accelerated phase, blast phase, allogeneic HSCT
Chronic myelogenous leukemia (CML) is a stem cell malignancy that is characterized by a reciprocal translocation between chromosomes 9 and 22 [t(9;22)], resulting in the formation of the Philadelphia chromosome. This translocation results in the head to tail fusion of the breakpoint cluster region (BCR) gene on chromosome 22 at band q11 and the Abelson murine leukemia (ABL) gene located on chromosome 9 at band q34.1 The fusion gene, BCR-ABL, encodes a protein (p210BCR-ABL) with deregulated tyrosine kinase activity that plays a central role in the initial development of CML. CML has 3 phases, chronic, accelerated, and blast, identified by clinical and pathologic characteristics, although most of the genetic changes in progression occur in the transition from chronic to accelerated phase.2 Most cases of CML in the United States are diagnosed in the chronic phase. Untreated CML typically progresses from chronic phase through an accelerated phase at a median of approximately 4 years, and eventually leads to terminal blast-phase disease, with death occurring from bleeding and infectious complications.3
First-Line Treatment: Imatinib Versus Second-Generation Tyrosine Kinase Inhibitors
Imatinib
Imatinib is a selective inhibitor of the BCR-ABL tyrosine kinase. The results of the IRIS (International Randomized Study of Interferon and STI571) trial established the safety and efficacy of imatinib in patients with newly diagnosed chronic-phase CML (CP-CML).4,5 This trial randomized 1106 patients to undergo initial therapy with either imatinib, 400 mg daily, or interferon-α plus low-dose cytarabine. At 8-year follow-up, 55% of patients remained on imatinib, and the estimated rates of event-free survival, freedom from progression, and overall survival were 81%, 92%, and 85%, respectively.6 Major molecular response (MMR) increased from 24% at 6 months and 39% at 12 months to the best observed MMR rate of 86%. None of the patients with documented MMR at 12 months progressed to accelerated or blast phase. Among the 359 patients who crossed over from interferon-α plus cytarabine to imatinib, 93% experienced a complete hematologic response (CHR), 86% a major cytogenetic response (MCyR), and 81% a complete cytogenetic response (CCyR) after a medial follow-up of 54 months.7 Estimated rates of freedom from progression and overall survival were 91% and 89%, respectively, after 48 months.
Imatinib is well tolerated. The most commonly reported nonhematologic adverse events are edema, muscle cramps, diarrhea, nausea, musculoskeletal pain, rash, abdominal pain, fatigue, and headache, but none of these led to discontinuation of treatment. In the IRIS trial, the most frequently reported grade 3 or 4 hematologic toxicities were neutropenia (17%), thrombocytopenia (9%), and anemia (4%).5 Hypophosphatemia, with associated changes in bone and mineral metabolism, has been noted in a small group of patients.8 Congestive heart failure and cardiotoxicity associated with long-term imatinib treatment was reported.9 However, this seems to be rare, as shown by the analysis of 1276 patients treated with imatinib at MD Anderson Cancer Center.10 After a median follow-up of 47 months, 22 (1.7%) patients were found to have congestive heart failure during imatinib therapy.10 Of these patients, 13 had received prior treatment with cardiotoxic drugs and likely had preexisting conditions predisposing them to congestive heart failure.
High-Dose Imatinib
Most patients retain variable levels of residual molecular disease at the 400-mg dose of imatinib, and therefore several studies have evaluated the efficacy of high-dose imatinib in newly diagnosed patients.
The single-arm trial Rationale and Insight for Gleevec High-Dose Therapy (RIGHT) showed that among 115 patients with newly diagnosed CML, the CCyR and MMR rates were higher with the 400-mg twice-daily dosage than those with the 400-mg daily dosage reported in the IRIS trial.11,12 At 18 months, MCyR and CCyR rates were 90% and 85%, respectively, compared with the estimated 85% and 69% rates for standard-dose imatinib previously published in the IRIS trial. At 12 months the MMR rate was 54% compared with an estimated 39% for standard-dose imatinib seen in the IRIS study. Grade 1 to 2 toxicities and grade 3 to 4 adverse events were higher among patients receiving high-dose imatinib compared with those treated with standard-dose imatinib in the IRIS trial. Although high-dose imatinib resulted in rapid and deep responses, most of the patients (70%) enrolled in this study were low risk based on Sokal score.
The investigators of the TIDEL (Therapeutic Intensification in De Novo Leukemia) trial also reported superior responses (MMR at 12 and 24 months was 55% and 77%, respectively) in patients receiving 600 mg of imatinib as the initial dose compared with those receiving less than 600 mg (MMR at 12 and 24 months was 32% and 53%, respectively).13
In a phase II trial conducted by the GIMEMA CML working party, high-dose imatinib also induced rapid cytogenetic and molecular responses in patients with intermediate Sokal risk.14 The response rates at 12 months were better than those documented in the IRIS study for patients with intermediate risk treated with 400 mg of imatinib. A phase III study conducted by European LeukemiaNet (ELN), which randomized patients with high Sokal risk to 400 or 800 mg of imatinib, showed no significant difference between the groups in terms of CCyR and MMR rates at 12 months (Table 1).15
Table 1.
Selected Response and PFS Rates for Front-Line Standard- or High-Dose Imatinib in Clinical Trials
| ELN Study (12-Month Response Rates)15 |
TOPS Study (12-Month Response Rates)16 |
German CML IV Study (12-Month Response Rates)17 |
||||
|---|---|---|---|---|---|---|
| Response | Imatinib 400 mg/d |
Imatinib 800 mg/d |
Imatinib 400 mg/d |
Imatinib 800 mg/d |
Imatinib 400 mg/d |
Imatinib 800 mg/d |
| CCyR* | 58% | 64% | 66% | 70% | 50% | 63% |
| MCyR | 74% | 68% | NR | NR | NR | NR |
| MMR‡ | 41% | 49% | 40% | 46% | 31% | 56% |
| PFS† | 84% | 91% | 95% | 97% | 88% | 88% |
Abbreviations: CCyR, complete cytogenetic response; CML, chronic myelogenous leukemia; ELN, European LeukemiaNet; MCyR, major cytogenetic response; MMR, major molecular response; NR, not reported; PFS, progression-free survival; TOPS, Tyrosine Kinase Inhibitor Optimization and Selectivity trial.
Primary end point of ELN study.
Primary end point of the TOPS study
PFS rate at 36 months (ELN study), at 18 months (TOPS study), and 5 years (German CML IV study).
The prospective randomized phase III TOPS (Tyrosine Kinase Inhibitor Optimization and Selectivity) trial compared the efficacy of high-dose and standard-dose imatinib in 476 patients with newly diagnosed CP-CML.16 Results showed that high-dose imatinib induced more rapid responses than standard-dose. At 6 months, MMR and CCyR rates were significantly higher among patients assigned to 800 mg of imatinib than among those on 400 mg. However, at 12 months, MMR (46% vs. 40%) and CCyR (70% vs. 66%) rates were comparable between the arms (Table 1). Grade 3 or 4 hematologic adverse events were higher in patients assigned to 800 mg, and half of the patients required dose reduction to less than 600 mg. The MMR rate correlated with average dose intensity in this arm. At 12 months, MMR was observed in 62% patients with an average dose intensity of 600 to 799 mg/d, and in 38% of patients with an average dose intensity of 400 to 599 mg/d.16 These data suggest that patients who can tolerate high-dose imatinib can experience superior cytogenetic and molecular responses compared with those who need dose reductions. The estimated progression-free survival rates at 18 months for 800 and 400 mg were 97% and 95%, respectively. No significant difference in CCyR and MMR rates were seen between the arms according to Sokal risk scores. The progression-free survival rate was slightly higher for patients with high Sokal risk receiving 800 mg than for those assigned to 400 mg (96% vs. 88%, respectively).
The German CML IV study also reported significantly faster response rates with 800 mg of imatinib compared with 400 mg of imatinib and 400 mg of imatinib plus interferon.17 More rapid achievement of MMR with 800 mg of imatinib was observed in patients with low and intermediate risk, but not in those with high risk. MMR rates at 12 months were 56%, 31%, and 33% for low-, intermediate-, and high-risk groups, respectively. The overall survival (92%) and progression-free survival (88%) rates at 5 years were not different between treatment arms (Table 1).
Does High-Dose Imatinib Have a Role?
Efficacy
In newly diagnosed patients, high-dose imatinib induces higher and faster incidences of CCyR and MMR compared with standard-dose imatinib early on, but no difference in response rates were seen at 12 months.11–17 None of the studies showed that 800 mg of imatinib had lower rates of disease progression than standard-dose imatinib, despite improved early responses.
Toxicity
High-dose imatinib is associated with higher rates of dose interruption, reduction, and discontinuation in a substantial number of patients because of grade 3 or 4 adverse events.
Conclusions
When data are presented in the form of intent-to-treat analysis, high-dose imatinib does not appear superior to standard-dose imatinib. This is confounded by the fact that more patients in the high-dose arm require dose reduction for toxicity. However, patients who can actually tolerate the higher dose of imatinib clearly experience better response rates than those receiving the standard dose. Currently, high-dose imatinib is not recommended as initial therapy for patients with newly diagnosed CML.
Dasatinib
Dasatinib is a potent, orally available ABL kinase inhibitor. It binds to both the active and inactive conformation of the ABL kinase domain and has in vitro activity against nearly all imatinib-resistant BCR-ABL mutations except T315I.18 It also inhibits the Src family of kinases, PDGFR, and KIT. In a 2-arm phase II trial, dasatinib induced rapid responses in patients with early CP-CML.19 In historical comparison, the CCyR rates at 3, 6, and 12 months were comparable to those achieved with high-dose imatinib and better than those achieved with standard-dose imatinib. No significant differences were seen in response rate and toxicity between the arms (100 mg once daily or 50 mg twice daily).
The DASISION study (Dasatinib versus Imatinib Study in Treatment-Naive CML Patients) compared dasatinib and imatinib in patients with newly diagnosed CP-CML.20 In this study, 519 patients were randomized to receive dasatinib (100 mg once daily; 259 patients) or imatinib (400 mg once daily; 260 patients). CCyR rate at 12 months was the primary end point. After a minimum follow-up of 12 months, the MMR and confirmed CCyR rates were higher with dasatinib than imatinib (46% vs. 28%, and 77% vs. 66%, respectively). The rates of CCyR at 3, 6, and 9 months after initiation of therapy were 54%, 73%, and 78%, respectively, for dasatinib compared with 31%, 59%, and 67%, respectively, for imatinib. The rates of MMR at 3, 6, and 9 months after dasatinib treatment were 8%, 27%, and 39%, respectively, compared with 0.4%, 8%, and 18%, respectively, for imatinib. Although a trend was seen in favor of dasatinib, progression to the accelerated or blast phase was not statistically different between the groups, with 5 patients on dasatinib (2%) and 9 patients on imatinib (3.5%) meeting the definition of progression. At 18 months, the overall survival rate was 98% and 96% for dasatinib and imatinib, respectively; the rate of confirmed CCyR at 18 months continued to be higher for dasatinib than for imatinib (78% vs. 70%, respectively); the rates of progression-free survival at 18 months were 95% for dasatinib and 94% for imatinib.21 The rate of MMR at any time was also higher for dasatinib than for imatinib (57% and 41%, respectively). The response rates were also higher across all risk groups for dasatinib. The CCyR and MMR rates were 73% and 51%, respectively, for dasatinib among high-risk patients. The corresponding rates were 64% and 30%, respectively, for imatinib in the same patient population.
In the Intergroup phase II randomized trial (S0325), 100 mg of dasatinib induced deeper molecular responses (3-log reductions in BCR-ABL transcript level) at 12 months compared with 400 mg of imatinib (59% vs. 43%) in patients with newly diagnosed CP-CML.22 Follow-up is ongoing to evaluate whether the short-term deeper molecular response will translate into improved long-term outcomes.
Nilotinib
Nilotinib is an orally available, highly selective inhibitor of BCR-ABL tyrosine kinase that is more potent than imatinib. As with dasatinib, nilotinib is also active against nearly all imatinib-resistant BCR-ABL mutations except T315I.23 In phase II studies, 400 mg of nilotinib given twice daily as first-line therapy induced high rates of CCyR and MMR in patients with early CP-CML, with most patients reaching these responses early during their therapy.24,25
In the ENESTnd (Evaluating Nilotinib Efficacy and Safety in Clinical Trials Newly Diagnosed Patients) study, the efficacy and safety of nilotinib (300 mg twice daily, n = 282; or 400 mg twice daily, n = 281) were compared with those of imatinib (400 mg once daily, n = 283) in patients with newly diagnosed CP-CML.26 MMR rate at 12 months was the primary end point. At 12 months, the rates of MMR for nilotinib were 44% for the 300-mg dose and 43% for the 400-mg dose versus 22% for imatinib. The CCyR rates by 12 months were also higher for nilotinib than for imatinib (80% for the 300-mg dose and 78% for the 400-mg dose vs. 65% for imatinib). Patients receiving nilotinib at either of the 2 dose levels had a significant improvement in the time to progression to the accelerated or blast phase compared with those receiving imatinib. The rate of progression to accelerated or blast phase was 4% with imatinib and less than 1% with nilotinib (P = .01 for 300 mg and P = .004 for 400 mg). With a median follow-up of 18 months, the overall best MMR rate was superior for nilotinib at either dose level (66% for 300 mg and 62% for 400 mg) compared with imatinib (40%), and the corresponding CCyR rates were 85%, 82%, and 74%, respectively. The estimated overall survival rates at 18 months were 98.5%, 99%, and 97%, respectively, for 300 mg of nilotinib, 400 mg of nilotinib, and imatinib.27 Superior rates of CCyR and MMR were observed in both nilotinib arms compared with the imatinib arm across all Sokal risk groups (see Table 2 for the calculation of relative risk). Among patients with a high Sokal risk, CCyR rates by 12 months were 74%, 63%, and 49%, for patients receiving 300 mg of nilotinib, 400 mg of nilotinib, and imatinib, respectively. MMR rates at 18 months in these patients were 59%, 51%, and 28%, respectively, for the 3 treatment arms. Among the 3 study groups, nilotinib, 300 mg daily, had the lowest rates of discontinuation from adverse events or laboratory abnormalities.
Table 2.
Calculation of Relative Risk*
| Study | Calculation | Risk Definition by Calculation | |
|---|---|---|---|
| Sokal et al.146 | Exp 0.0116 × (age in years − 43.4) + 0.0345 × (spleen − 7.51) + 0.188 × [(platelet count ÷ 700)2 − 0.563] + 0.0887 × (blast cells – 2.10) | Low | < 0.8 |
| Intermediate | 0.8–1.2 | ||
| High | > 1.2 | ||
| Hasford et al.147 | 0.666 when age ≥ 50 years + (0.042 × spleen) + 1.0956 when platelet count > 1500 × 109L + (0.0584 × blast cells) + 0.20399 when basophils > 3% + (0.0413 × eosinophils) × 100 | Low | < 780 |
| Intermediate | 781–1480 | ||
| High | > 1480 | ||
Calculation of relative risk found at http://www.icsg.unibo.it/rrcalc.asp. Age is in years. Spleen is in centimeter below the costal margin (maximum distance). Blast cells, eosinophils, and basophils are in percents of peripheral blood differential. All factors must be collected before any treatment.
From Baccarani M, Cortes J, Pane F, et al. Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol 2009;27:6041–6051. Reprinted with permission. © 2008 American Society of Clinical Oncology. All Rights Reserved.
Are Early Short-Term End Points Good Enough to Recommend Second-Generation TKIs at Diagnosis?
Although most patients experience response to imatinib, some may experience a loss of response or discontinue treatment from drug toxicity, also referred to as intolerance. Data from the recent randomized, phase III, first-line therapy studies have shown that patients with CP-CML randomized to either dasatinib or nilotinib met their study-specific end points more frequently than those treated with imatinib. Nilotinib, 300 mg twice daily, and dasatinib, 100 mg daily, are now approved by the FDA for the treatment of adults with newly diagnosed Ph-positive CP-CML. However, the efficacy evidence supporting the use of dasatinib and nilotinib as first-line therapy are based on early, short-term end points.
Efficacy
Quicker and better CCyR and MMR rates observed in the 2 studies favor the second-generation TKIs over imatinib (Table 3). Earlier studies have suggested that achievement of CCyR on imatinib is a major prognostic factor for overall and progression-free survival,5,6,28 and that early molecular response is predictive of long-term disease stability with lack of progression and durability of CCyR.29–31 Interestingly, CCyR was rare in patients treated with interferon (and MMR was not even established during the interferon era); however, in rare patients with a sustained CCyR, not only was discontinuation of interferon possible but also many patients have remained recurrence-free several years off therapy.32–34 Improved long-term outcomes using the response criteria of CCyR at 12 months have not yet been established with TKI therapy. Failure of response to imatinib (based on follow-up from the IRIS trial) is characterized by the lack of CCyR at 18 months.
Table 3.
Selected Response and Progression-Free Survival Rates for Frontline Tyrosine Kinase Inhibitors in Phase III Randomized Studies
| Phase III DASISION Study20(%) |
Phase III ENESTnd Study26 (%) |
||||
|---|---|---|---|---|---|
| Response Criteria | Dasatinib 100 mg daily |
Imatinib 400 mg daily |
Nilotinib 300 mg twice daily |
Nilotinib 400 mg twice daily |
Imatinib 400 mg daily |
| CCyR* at 12 months | 83 | 72 | 80 | 78 | 65 |
| MMR† at 12 months | 46 | 28 | 44 | 43 | 22 |
| Progression to AP/BP | 1.9 | 3.5 | < 1 | < 1 | 4 |
Abbreviations: AP, accelerated phase; BP, blast phase; CCyR, complete cytogenetic response; DASISION, Dasatinib versus Imatinib Study in Treatment-Naïve CML Patients; ENESTnd, Evaluating Nilotinib Efficacy and Safety in Clinical Trials Newly Diagnosed Patients; MMR, major molecular response.
Primary end point for the DASISION study (CCyR is defined as the absence of Ph+ metaphases, was determined on the basis of G-banding in ≥ 20 cells in metaphase per bone marrow sample).
Primary end point for the ENESTnd study (MMR is defined as BCR-ABL transcript level ≤ 0.1 % in peripheral blood on real-time quantitative polymerase chain reaction assay, as expressed on the International Scale).
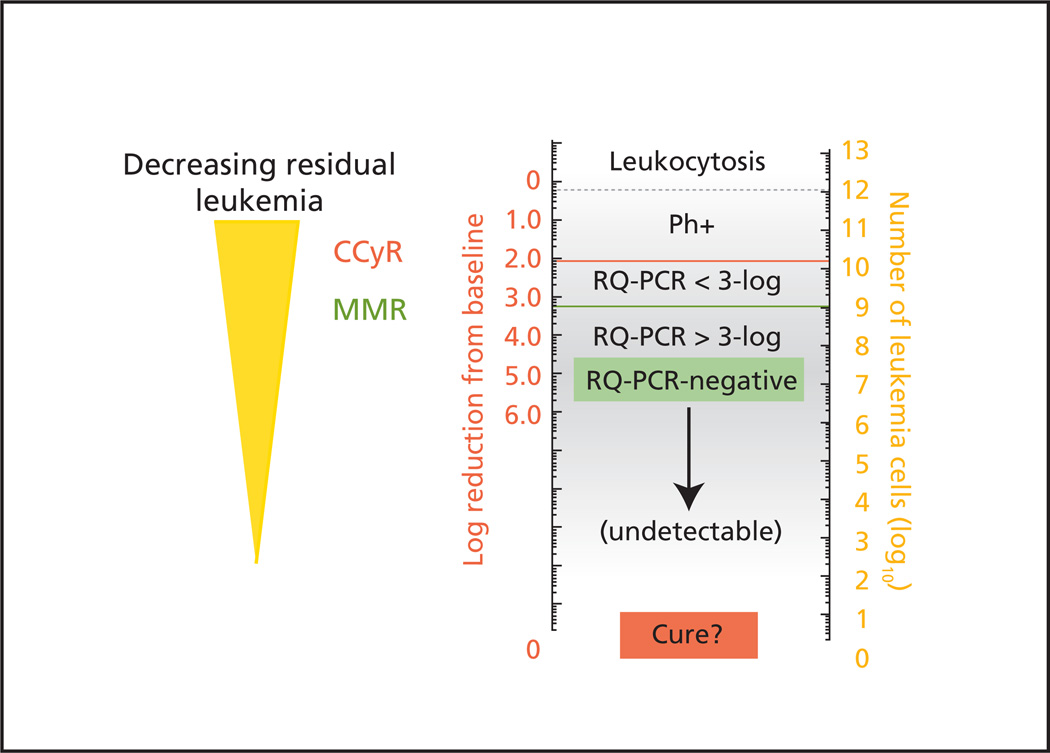
Most patients who have clinically undetectable levels of BCR-ABL transcripts according to the most sensitive polymerase chain reaction (PCR) measures have residual disease (that may eventually lead to disease relapse), supporting the notion that the currently approved TKIs do not completely eradicate CML in most patients (Figure 1). Currently no reliable predictive assays exist to assess the effect of therapy on minimal residual disease or to identify the patient population who may be at high risk for disease progression. In the absence of these biologic assays, lack of disease progression is an important end point and, if quantified, may be the best indicator that TKI therapy is really impacting the biology of the disease. Disease transformation is associated with a poor outcome. The risk of transformation is highest during the first 3 years of TKI therapy. The rate of disease progression to accelerated or blast phase was significantly lower for nilotinib (1% vs. 4% for imatinib) and no patients with low Sokal risk have progressed at 18 months.26 A trend was seen for a similar difference with dasatinib (Table 3).
Figure 1.
Schematic representation of decreasing residual disease related to numbers of BCR-ABL transcripts in the peripheral blood (left scale) and estimated number of residual leukemia cells in a patient’s body (right scale).
Abbreviations: CCyR, complete cytogenetic response; MMR, major molecular response; RQ-PCR, real-time quantitative polymerase chain reaction.
From Goldman JM. How I treat chronic myeloid leukemia in the imatinib era. Blood 2007;110:2831. Reproduced with permission of the American Society of Hematology.
Safety
Both phase III studies support the finding that each of the 3 TKIs is fairly well tolerated. Drug-related adverse events were primarily grade 1 or 2, and grade 3 or 4 nonhematologic adverse events were uncommon in both studies.
In the DASISION study, grade 3 or 4 neutropenia and anemia occurred with similar frequency among patients receiving dasatinib and imatinib, whereas thrombocytopenia was more frequent with dasatinib (Table 4).20 Nonhematologic adverse events, except pleural effusions, were more frequent with imatinib. Pleural effusions, although infrequent, were reported only with dasatinib. Overall, the rates of therapy discontinuation due to drug-related side effects were similar among patients receiving dasatinib and imatinib (5% and 4%, respectively).
Table 4.
Selected Toxicities of Dasatinib and Imatinib in the DASISION Study20
| Toxicity | All Grades (%) |
Grade 3 or 4 (%) |
||
|---|---|---|---|---|
| Dasatinib 100 mg daily |
Imatinib 400 mg daily |
Dasatinib 100 mg daily |
Imatinib 400 mg daily |
|
| Hematologic Toxicities | ||||
| Neutropenia | 65 | 58 | 21 | 20 |
| Thrombocytopenia | 70 | 62 | 19 | 10 |
| Anemia | 90 | 84 | 10 | 7 |
| Nonhematologic Toxicities | ||||
| Fluid retention | 19 | 42 | 1 | 1 |
| Superficial edema | 9 | 36 | 0 | < 1 |
| Pleural effusion | 10 | 0 | 0 | 0 |
| Diarrhea | 17 | 17 | < 1 | 1 |
| Nausea | 8 | 20 | 0 | 0 |
| Vomiting | 5 | 10 | 0 | 0 |
| Musculoskeletal pain | 11 | 14 | 0 | < 1 |
| Rash | 11 | 17 | 0 | 1 |
| Headache | 12 | 10 | 0 | 0 |
| Fatigue | 8 | 10 | < 1 | 0 |
In ENESTnd study, the nonhematologic adverse events of nausea, diarrhea, vomiting, muscle spasm, and edema of any grade were higher for patients receiving imatinib. Conversely, rates of rash, headache, and alopecia were higher with nilotinib at both dose levels. Grade 3 or 4 neutropenia was more frequent in the imatinib group, whereas thrombocytopenia and anemia were similar in all groups (Table 5).26 Electrolyte abnormalities were more frequent with nilotinib at both dose levels than with imatinib. The rates of discontinuations because of adverse events were slightly higher for those receiving 400 mg of nilotinib and imatinib (9% and 7%, respectively) compared with 5% among those receiving 300 mg of nilotinib.
Table 5.
Selected Toxicities of Nilotinib and Imatinib in the ENESTnd Study26
| Toxicity | All Grades (%) |
Grade 3 or 4 (%) |
||||
|---|---|---|---|---|---|---|
| Nilotinib 300 mg twice daily |
Nilotinib 400 mg twice daily |
Imatinib 400 mg daily |
Nilotinib 300 mg twice daily |
Nilotinib 400 mg twice daily |
Imatinib 400 mg daily |
|
| Hematologic Toxicities | ||||||
| Neutropenia | 43 | 38 | 68 | 12 | 10 | 20 |
| Thrombocytopenia | 48 | 49 | 56 | 10 | 12 | 9 |
| Anemia | 38 | 38 | 47 | 3 | 3 | 5 |
| Nonhematologic Toxicities | ||||||
| Peripheral edema | 5 | 5 | 14 | 0 | 0 | 0 |
| Diarrhea | 8 | 6 | 21 | 1 | 0 | 1 |
| Nausea | 11 | 19 | 31 | < 1 | 1 | 0 |
| Vomiting | 5 | 9 | 14 | 0 | 1 | 0 |
| Muscle spasm | 7 | 6 | 24 | 0 | 1 | 1 |
| Rash | 31 | 36 | 11 | < 1 | 3 | 1 |
| Headache | 14 | 21 | 8 | 1 | 1 | 0 |
| Fatigue | 11 | 9 | 8 | 0 | 1 | < 1 |
All TKIs have effects on QTc interval prolongation, but only the nilotinib labeling contains a black box warning regarding the risk of QTc interval prolongation, because sudden cardiac death has been reported in patients receiving nilotinib. Although no patient in the ENESTnd study had a QTc interval corrected for heart rate of more than 500 ms, electrocardiograms are recommended at baseline, 7 days after initiation, and periodically thereafter during nilotinib treatment.
Knowing that both second-generation TKIs have very good efficacy in the up-front setting, differences in their potential toxicity profiles may be helpful when choosing a second-generation TKI over imatinib as first-line therapy. In general, the choice of first-line therapy in a given patient may depend on physician’s experience, the patient’s age and ability to tolerate therapy, and the presence of comorbid conditions. For example, based on the toxicity profile, nilotinib may be preferred for patients deemed to be at risk for developing pleural effusions. Alternatively, dasatinib may be preferred in patients with a history of pancreatitis or hyperglycemia.
Practical Issues
The recommended starting dose of imatinib is 400 mg daily and the starting dose of dasatinib from the DASISION study is 100 mg daily, whereas the recommended dose of nilotinib is 300 mg twice daily in the ENESTnd study. The twice-daily dosing schedule of nilotinib may have more issues with patient compliance than the once-daily dosing of imatinib and dasatinib. Another factor that may impact compliance is the recommendation that food should not be consumed from 2 hours before and 1 hour after nilotinib dosing because of increased bioavailability after food consumption. The concern is that the increased bioavailability may have an adverse effect on QTc prolongation. Electrocardiogram monitoring at regular intervals is recommended for those receiving nilotinib, and careful attention to correcting electrolyte abnormalities, such as hypokalemia and hypomagnesemia, is also recommended.
NCCN Recommendations
Imatinib (400 mg daily) is still considered a reasonable first-line therapy choice for patients with newly diagnosed CP-CML. Given the recent data showing superior efficacy of nilotinib and dasatinib in these patients, high-dose imatinib currently has only a limited role in first-line therapy.
Second-generation TKIs should be strongly considered as first-line therapy for intermediate- to high-risk patients based on Sokal or Hasford score (Table 2). This recommendation is based on limited data indicating that these agents are associated with lower risk of disease progression in this patient population. Long-term follow-up is needed to determine whether second-generation TKIs should be implemented as standard first-line therapy in such a risk-adapted fashion.
Switching to dasatinib (100 mg daily) or nilotinib (300 mg twice daily) is recommended in select patients who are not able to tolerate imatinib because of grade 3 or 4 acute nonhematologic toxicities, or a change should be considered for evidence of chronic and persistent grade 1 or 2 nonhematologic toxicities.
Because no data exist regarding the time points for monitoring response to second-generation TKIs, the panel currently believes that physicians should monitor patients for response and toxicities when using second-generation TKIs, following the guidelines recommended for imatinib (Table 6). Further follow-up of the ENESTnd and DASISION trials will likely lead to clearer definitions of response failure to second-generation TKIs used in frontline therapy.
Table 6.
Recommendations for Monitoring Response to TkIs
| Test | Indication |
|---|---|
| Bone marrow cytogenetics | At diagnosis. If collection of bone marrow is not feasible, FISH on a peripheral blood specimen using dual probes for the BCR and ABL genes is an acceptable method of confirming CML diagnosis. |
| At 6, 12, and 18 months from initiation of therapy to assess response to TKI therapy. If a CCyR is achieved at either of the earlier time points, then cytogenetics do not need to be repeated in stable patients. | |
| Rising levels of BCR-ABL transcript (1-log increase) without an MMR. | |
| Quantitative real-time polymerase chain reaction (qPCR) | At diagnosis to establish baseline BCR-ABL transcript level. |
| Every 3 months when a patient is experiencing response to treatment and every 3–6 months after CCyR. | |
| If a rising level of BCR-ABL transcript (1-log increase) is present with an MMR, qPCR analysis should be repeated in 1–3 months. |
Abbreviations: CCyR, complete cytogenetic response; CML, chronic myelogenous leukemia; FISH, fluorescence in situ hybridization; MMR, major molecular response; TKI, tyrosine kinase inhibitor.
Second-Line Treatment: Second-Generation TKIs vs. High-Dose Imatinib
Dasatinib
The START (SRC/ABL Tyrosine kinase inhibition Activity: Research Trials of dasatinib) program trials evaluated the safety and efficacy of dasatinib, 70 mg twice daily, in patients who did not respond to prior imatinib therapy because of resistance or intolerance across all phases of CML.
Chronic Phase
Dasatinib showed significant efficacy in the second-line setting in patients with CP-CML in the START-C trial (n = 387).35,36 The primary end point was MCyR rate. Two-year follow-up data showed overall CHR, MCyR, CCyR, and MMR rates of 91%, 62%, 53%, and 47%, respectively. These responses were also durable, with 88% of patients maintaining MCyR at 24 months. The overall and progression-free survival rates were 94% and 80%, respectively.37,38 At the time of switching, patients with a prior CCyR on imatinib had the highest rates of CCyR, MMR, and 2-year progression-free survival (84%, 75.5%, and 97%, respectively) compared with those with no CCyR on imatinib (50%, 35.5%, and 75.5%, respectively).39
In a recent dose-optimization phase III randomized study (CA180-034), patients with CP-CML who were resistant or intolerant to imatinib who received dasatinib, 100 mg once daily, experienced comparable CCyR (50% vs. 54%), MCyR (63% vs.61%), progression-free survival (80% vs. 76%), and overall survival (91% and 88%) rates at 24 months to those of patients who received dasatinib, 70 mg twice daily.40 Dasatinib, 100 mg daily, was also associated with a lower incidence of any-grade pleural effusion and grade 3 or 4 thrombocytopenia; fewer patients required dose interruption, dose reduction, and toxicity-related discontinuation. The recommended starting dose is 100 mg once daily for patients with CP-CML who are resistant or intolerant to imatinib.
Accelerated and Blast Phases
Dasatinib was also clinically active and well tolerated in patients with accelerated-phase CML (AP-CML) and blast-phase CML (BP-CML).
In the START-A trial, with a median follow-up of 14 months, among patients with AP-CML, dasatinib induced major hematologic response (MaHR), CHR, MCyR, and CCyR in 64%, 45%, 39%, and 32% of patients, respectively.41 At 24 months, MCyR was maintained in 61% of patients experiencing response. The 24-month progression-free and overall survival rates were 46% and 72%, respectively.42
In patients with BP-CML (START-B and START-L), the response rates depended on whether the patients were in myeloid blast phase (MBP) or lymphoid blast phase (LBP).43 After a minimum follow-up of 12 months, MaHR, MCyR, and CCyR rates were 34%, 33%, and 26%, respectively, for patients with MBP. The corresponding response rates were 35%, 52%, and 46%, respectively, for those with LBP. However, patients with MBP had better median progression-free and overall survival (6.7 and 11.8 months, respectively) than patients with LBP (3.0 and 5.3 months, respectively).43
Recently, the results of phase III randomized studies showed that once-daily dosing of dasatinib at 140 mg has similar efficacy to the 70-mg twice-daily dosing with an improved safety profile in patients with AP-CML and BP-CML resistant or intolerant to imatinib.44,45 Among patients with AP-CML, rates were comparable for MaHR (66% vs. 68%, respectively), MCyR (39% vs. 43%, respectively), 24-month progression-free survival (51% vs. 55%, respectively), and overall survival (63% vs. 72%, respectively) for patients receiving dasatinib, 140 mg once daily or 70 mg twice daily. Once-daily treatment was associated with an improved safety profile.44 In patients with MBP-CML, the MaHR rate was 28% for both 140 mg once-daily and 70 mg twice-daily dasatinib. In patients with LBP-CML, the MaHR rates were 42% and 32%, respectively, for dasatinib at 140 mg once daily and 70 mg twice daily.45 The MCyR rates were 25% and 28%, respectively, for patients in MBP and 50% and 40%, respectively, for those with LBP. The progression-free and overall survival rates at 24 months were 24% and 28% for patients with MBP-CML, and the respective values in patients with LBP-CML were 21% and 16%.45 The recommended starting dose of dasatinib is 140 mg once daily for patients with disease progression to accelerated or blast phase.
Nilotinib
Nilotinib for imatinib-resistant or -intolerant disease was evaluated at 400 mg twice daily in all phases of CML.
Chronic Phase
A phase II multicenter study (nilotinib 2101) evaluated the efficacy of nilotinib in 321 patients with CP-CML.46,47 The rate of MCyR was the primary end point. Two-year follow-up data confirmed that nilotinib resulted in rapid and durable hematologic and cytogenetic responses.48 Overall, MMR, MCyR, and CCyR occurred in 28%, 59%, and 44% of patients, respectively. The responses were durable, with 84% maintaining CCyR and 77% maintaining MCyR at 24 months. The estimated overall progression-free and overall survival rates at 24 months were 64% and 87%, respectively. At study entry, MCyR, MMR, and progression-free survival rates were higher in patients with CHR (73%, 38%, and 77%, respectively) compared with 52%, 22%, and 56%, respectively, among patients without CHR.
Accelerated and Blast Phases
After 12-months of follow-up, hematologic response was observed in 47% and MCyR in 29% of patients with AP-CML.49 Long-term follow-up data confirmed that responses are durable, with 54% maintaining the hematologic response and 70% maintaining MCyR at 24 months.50 Estimated overall survival at 24 months was 67%.
Nilotinib has also shown activity in patients with BP-CML. As with dasatinib, the response rates depend on the type of BP-CML. In a phase II study of 136 patients with BP-CML, CHR was seen at 24-month follow-up in 13% of patients with MBP and LBP.51 MCyR was seen in 38% of patients with MBP and 52% of patients with LBP. CCyR was seen in 30% of patients with MBP and 32% of patients with LBP, respectively. The overall survival rates were 42% and 27% at 12 and 24 months, respectively. However, the responses were not durable. The duration of MCyR was 11 months for patients with MBP and 3 months for those with LBP.
High-Dose Imatinib
Dose escalation of imatinib up to 800 mg daily has been shown to overcome some of the primary resistance, but the duration of responses has typically been short.52–54 The results of a recent study with a median follow-up of 61 months indicated that dose escalation induced CCyR in 52% of patients with cytogenetic failure and only in 5% of patients with hematologic failure on standard-dose imatinib.55 The estimated 2- and 3-year event-free and overall survival rates were 57% and 47%, and 84% and 76%, respectively. Responses were also durable; 88% of patients with MCyR sustained their response beyond 2 years. Dose escalation was particularly effective in patients with cytogenetic relapse on standard-dose imatinib. In this group of patients, CCyR and MCyR rates were 73% and 87%, respectively, compared with 52% and 60% for the group of patients with cytogenetic failure. In a retrospective analysis of 106 patients from the IRIS trial who received imatinib at a dose of 400 mg daily and subsequently underwent dose escalation to either 600 or 800 mg daily, the estimated progression-free survival rates at 12 and 36 months after the dose escalation were 94% and 89%, respectively.56 These results support that dose escalation of imatinib is an appropriate option for patients in chronic phase experiencing suboptimal cytogenetic response or cytogenetic relapse.
The START-R trial evaluated the efficacy of imatinib (800 mg daily) and dasatinib (70 mg twice daily) in 150 patients with imatinib-resistant CP-CML.57,58 At a minimum follow-up of 2 years, dasatinib showed higher rates of CHR (93% vs. 82%), MCyR (53% vs. 33%), CCyR (44% vs. 18%), and MMR (29% vs. 12%) relative to high-dose imatinib. Responses with dasatinib were also durable, with 90% of patients maintaining a MCyR at 18 months compared with 74% of patients treated with high-dose imatinib.58 The estimated progression-free survival at 2 years was higher with dasatinib (86%) relative to high-dose imatinib (65%), indicating that dasatinib is an effective treatment for patients with CP-CML resistant to conventional imatinib doses.
Selection of Second-Line Therapy
High-Dose Imatinib
In the initial report from the START-R trial, dasatinib was clearly superior to imatinib, 800 mg, in patients for whom treatment with 600 mg of imatinib failed, whereas response rates were equivalent for both treatment arms in patients for whom treatment with 400 mg of imatinib failed.57 However, the 2-year follow-up data suggest that dasatinib is clearly superior to imatinib, 800 mg, in patients resistant to imatinib at doses of 400 or 600 mg daily.58 Dose escalation of imatinib is also unlikely to benefit those with hematologic resistance or those who never had a cytogenetic response with standard-dose imatinib. Dose escalation of imatinib might be beneficial for patients with suboptimal response to imatinib, 400 mg daily (see Suboptimal Response, page S-14).
Second’Generation TKIs
Dasatinib and nilotinib have been found to be effective and well-tolerated in patients who develop resistance or intolerance to imatinib. Dasatinib is approved for treatment of these patients in all phases of CML, whereas nilotinib is approved for treatment of these patients with CP- or AP-CML. Substantial long-term efficacy and safety data are available for dasatinib and nilotinib in patients for whom treatment with imatinib fails, but no randomized comparison has been performed between the agents. Therefore, the panel believes that differences in safety profiles and activity against specific mutations should be considered when choosing between dasatinib and nilotinib.
Efficacy
Among patients with CP-CML, the overall MCyR rates for dasatinib and nilotinib at the currently recommended doses were 63% and 59%, respectively, at 24-month follow-up.37,40,48 MCyR rates were similar in imatinib-resistant patients, whereas the corresponding response rates were higher with dasatinib in the imatinib-intolerant patients in all phases of CML (Table 7). The lower response rates with nilotinib seen in the imatinib-intolerant patients may be because imatinib-intolerant patients with prior MCyR were excluded from nilotinib trials. The overall progression-free survival rates for dasatinib and nilotinib are 80% and 64%, respectively, at the currently recommended doses. The rate of progression to accelerated or blast phase was less than 3% for dasatinib at 36 months follow-up. Most patients who experienced progression while receiving dasatinib remained in chronic phase.40
Table 7.
Response and PFS Rates for Dasatinib37,40 and Nilotinib48 in Imatinib-Resistant or -Intolerant Patients With Chronic-Phase Chronic Myelogenous Leukemia at 24-Month Follow-Up
| Response | Overall Population (%) |
Imatinib-Resistant Patients (%) |
Imatinib-Intolerant Patients (%) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Dasatinib 70 mg twice daily |
Dasatinib 100 mg daily |
Nilotinib 400 mg twice daily |
Dasatinib 70 mg twice daily |
Dasatinib 100 mg daily |
Nilotinib 400 mg twice daily |
Dasatinib 70 mg twice daily |
Dasatinib 100 mg daily |
Nilotinib 400 mg twice daily |
|
| CHR | 88 | 92 | NR | 89 | 89 | NR | 86 | 100 | NR |
| MCyR | 61 | 63 | 59 | 57 | 59 | 56 | 74 | 77 | 66 |
| CCyR | 54 | 50 | 44 | 48 | 44 | 41 | 69 | 67 | 51 |
| MMR | 38 | 37 | 28 | 34 | 35 | NR | 51 | 43 | NR |
| PFS | 76 | 80 | 64 | 75 | NR | NR | 94 | NR | NR |
Abbreviations: CHR, complete hematologic response; CCyR, complete cytogenetic response; MCyR, major cytogenetic response; MMR, major molecular response; NR, not reported; PFS, progression-free survival.
Among patients with AP-CML, the overall 24-month MCyR rates were 39% and 32%, and the overall survival rates were 72% and 67% at the currently recommended doses of dasatinib and nilotinib, respectively. MCyR and CCyR rates were higher for dasatinib in both imatinib-resistant and imatinib-intolerant patients with AP-CML (Table 8).
Table 8.
Response and Overall Survival Rates for Dasatinib44 and Nilotinib50 in Imatinib-Resistant or -Intolerant Patients with Accelerated-Phase Chronic Myelogenous Leukemia at 24-Month Follow-Up
| Response | Overall (%) |
Imatinib-Resistant Patients (%) |
Imatinib-Intolerant Patients (%) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Dasatinib 70 mg twice daily |
Dasatinib 140 mg daily |
Nilotinib 400 mg twice daily |
Dasatinib 70 mg twice daily |
Dasatinib 140 mg daily |
Nilotinib 400 mg twice daily |
Dasatinib 70 mg twice daily |
Dasatinib 140 mg daily |
Nilotinib 400 mg twice daily |
|
| CHR | 52 | 47 | 31 | 51 | 50 | 30 | 53 | 39 | 37 |
| MCyR | 43 | 39 | 32 | 42 | 36 | 30 | 44 | 46 | 41 |
| CCyR | 33 | 32 | 20 | 33 | 29 | 18 | 33 | 41 | 30 |
| OS | 63 | 72 | 67 | NR | NR | NR | NR | NR | NR |
Abbreviations: CHR, complete hematologic response; CCyR, complete cytogenetic response; MCyR, major cytogenetic response; MMR, major molecular response; NR, not reported; OS, overall survival.
Toxicity
Most of the adverse events were grade 1 or 2 and were manageable with dose reduction or interruption and appropriate supportive care (Table 9). Cross-intolerance profile of dasatinib and nilotinib in imatinib-intolerant patients also must be considered. Among imatinib-intolerant patients with CP-CML, those who switched to dasatinib because of nonhematologic intolerance had the highest CCyR, MMR, and 2-year progression-free survival rates (78.9%, 60.1%, and 94.4%, respectively) compared with those with hematologic intolerance (43.5%, 28.3% and 68% respectively).39 Nilotinib has minimal cross-intolerance in imatinib-intolerant patients in chronic and accelerated phase. Thrombocytopenia was the only hematologic toxicity leading to imatinib intolerance that recurred with any significant frequency during nilotinib therapy.59
Table 9.
Selected Grade 3 or 4 Toxicities for Dasatinib40,44 and Nilotinib48,50 in Imatinib-Resistant or -Intolerant Patients at 24-Month Follow-Up
| Grade 3 or 4 | Chronic Phase (%) |
Accelerated Phase (%) |
||||
|---|---|---|---|---|---|---|
| Dasatinib 70 mg twice daily |
Dasatinib 100 mg daily |
Nilotinib 400 mg twice daily |
Dasatinib 70 mg twice daily |
Dasatinib 140 mg daily |
Nilotinib 400 mg twice daily |
|
| Hematologic Toxicities | ||||||
| Neutropenia | 45 | 35 | 31 | 69 | 59 | 42 |
| Thrombocytopenia | 38 | 23 | 30 | 67 | 64 | 41 |
| Anemia | 18 | 13 | 11 | 43 | 48 | 25 |
| Nonhematologic Toxicities | ||||||
| Rash | 2 | 2 | 2 | 1 | 0 | NR |
| Headache | 3 | 1 | 2 | 1 | 1 | NR |
| Diarrhea | 4 | 1 | 2 | 3 | 3 | < 1 |
| Nausea | 1 | 1 | < 1 | 2 | 1 | < 1 |
| Vomiting | 0 | 1 | < 1 | 1 | 1 | NR |
| Dyspnea | 5 | 3 | NR | 7 | 3 | NR |
| Bleeding | 2 | 1 | NR | 7 | 8 | NR |
| Fatigue | 4 | 2 | 1 | 3 | 2 | < 1 |
| Musculoskeletal pain | 4 | 2 | NR | 2 | 0 | NR |
| Superficial edema | 1 | 0 | 0 | 0 | 1 | NR |
| Pleural effusion | 5 | 2 | < 1 | 6 | 7 | NR |
Abbreviation: NR, not reported.
Dasatinib at 70 mg twice daily was associated with significantly higher rates of myelosuppression, pleural effusion, and bleeding. The incidences of myelosuppression and pleural effusion were significantly lower with dasatinib, 100 mg daily.60 Pleural effusions during dasatinib therapy increased in patients with advanced disease. A history of cardiac disease, hypertension, and use of a twice-daily schedule were identified as factors associated with development of pleural effusions.61 Close monitoring and timely intervention are necessary for patients at risk for developing pleural effusions. Lymphocytosis from the clonal expansion of natural killer/T cells has been reported during dasatinib treatment in patients in all stages of CML who are resistant or intolerant to imatinib, and lymphocytosis has been associated with increased incidence of pleural effusion.62 Cytogenetic response rates to dasatinib were shown to be higher in this group of patients.60,63 Similar effects were observed among patients treated with dasatinib as first-line therapy in the DASISION study.64 Further studies are needed to confirm these preliminary findings.
Nilotinib has been known to cause QTc interval prolongation. In patients with CP-CML, QTc interval prolongation exceeding 500 ms occurred in 1% of patients.47 Among patients with AP-CML, none experienced a prolongation exceeding 500 ms; increases in the QTc interval from baseline of more than 60 ms were observed in 4% of patients.49 The ENACT trial, a phase III multicenter open label trial in imatinib-resistant or -intolerant patients in all CML phases, also reported very low incidences of QTc prolongation exceeding 500 ms.65 Nevertheless, the prescribing information for nilotinib carries a black box warning for QTc prolongation. Food should not be consumed from 2 hours before to 1 hour after nilotinib dosing because of increased bioavailability after food consumption and this may have an adverse effect on QTc prolongation. Therefore, monitoring of electrocardiograms at baseline, 7 days after initiation, and periodically thereafter is recommended for all patients receiving nilotinib. In addition, careful monitoring and correction of electrolyte abnormalities is required.
Activity Against BCR-ABL Mutations
Point mutations in the ABL kinase domain are emerging to be the major mechanism of resistance to imatinib.66,67 The presence of the T315I mutation confers the highest resistance to all of the currently approved TKIs, and is associated with disease progression and poor survival depending on the phase of CML.68–70 In addition to T315I, available evidence (although limited) supports the emergence of other BCR-ABL mutations resistant to dasatinib and nilotinib.71,72 F317L, V299L, and T315A mutations are highly resistant to dasatinib, whereas the mutations Y253H/F, E255K/V, and F359 confer a high degree of resistance to nilotinib. Inhibitory concentration (IC50) values of TKIs against kinase domain mutations in vitro have been reportedly useful for predicting response to therapy and long-term outcome, especially in patients with CP-CML receiving second-generation TKIs after imatinib failure.73 Among 169 patients in whom mutations were detectable after imatinib failure, response and event-free survival rates were higher in those with low IC50 mutations than in those with intermediate or high IC50 mutations. Overall survival was not dependent on the IC50 values. Among patients with advanced phases, the in vitro sensitivity of mutations had minimal or no impact on survival.73
In the START-C trial, among patients in whom baseline data on mutations were available, T315I, Y253H/F, E255K/V, F317L, and F359 mutations were identified in 2%, 9%, 4%, 3%, and 5% of patients, respectively. V299L or T315A mutations were not detected at baseline.74 The rates of CCyR were 69%, 40%, and 50%, respectively, for patients with mutations Y253H/F, E255K/V, and F359. No patients with F317 and T315I mutation experienced a CCyR. These findings were also confirmed in an analysis of 1043 imatinib-resistant patients.75 After 2 years of follow-up, dasatinib treatment (at all doses) of imatinib-resistant patients with or without a mutation induced comparable response (CCyR, 43% vs. 47%, respectively; MCyR, 55% vs. 58%, respectively) and progression-free survival rates (70% vs. 80%).75 High response rates were observed in patients with the highly imatinib-resistant mutations, including L248, Y253, E255, F359, and H396, in addition to other common mutations in G250 and M351 (Table 10). The response rates for patients with nilotinib-resistant mutations Y253H, E255K/V, and F359V/C were 61%, 38%/36%, and 60%/52%, respectively. The presence of T315I and F317L mutations at baseline was associated with less favorable responses. Other studies have also reported similar findings in patients with F317 mutations at baseline; the outcome is dependent on the CML phase, and the F317 mutation was sensitive to other TKIs.76,77
Table 10.
Efficacy of Dasatinib75 and Nilotinib78 Based on BCR-ABL Mutation Status in Patients with Chronic-Phase Chronic Myelogenous Leukemia Resistant to Imatinib
| Mutation | Rates of CCyR, = n/N (%) |
|
|---|---|---|
| Dasatinib | Nilotinib | |
| No mutation | 196/421 (43%) | 35/87 (40%) |
| Any mutation | 164/384 (47%) | 32/100 (32%) |
| T315I | 0/21 (0%) | NR |
| F317L | 1/14 (7%) | NR |
| F359C | 3/5 (60%) | 0/11 (0%) |
| F359V | 14/27 (52%) | 0/11 (0%) |
| P-Loop Mutations | ||
| L248V | 6/15 (40%) | NR |
| G250E | 20/60 (33%) | 3/5 (60%) |
| Y253H | 14/23 (61%) | 0/8 (0%) |
| E255K | 6/16 (38%) | 0/7 (0%) |
| E255V | 4/11 (36%) | 0/7 (0%) |
Abbreviations: CCyR, complete cytogenetic response; NR, not reported.
In an analysis of patients enrolled in the pivotal phase II trials of nilotinib, significant responses were achieved in patients without baseline mutations and in those who harbored mutations with high in vitro sensitivity to nilotinib.78 After 12 months of therapy, MCyR, CCyR, and MMR were achieved in 60%, 40%, and 29% of patients without baseline mutations, versus 49%, 32%, and 22% of patients with mutations, respectively. E255K/V, T315I, F359C/V, G250E, and Y253H mutations were identified in 7%, 6%, 4%, 4%, and 3% of imatinib-resistant patients.78 T315I, Y253H, E255K/V, and F359V/C mutations were associated with lower response rates and high risk of disease progression (Table 10). None of the patients experienced a CCyR within 12 months of therapy.78 E255V/K and F359V/C were associated with a high rate of disease progression (86% and 92%, respectively). These mutations were also associated with less durable responses and shorter event-free survival in patients with AP-CML.
NCCN Recommendations
Dasatinib or nilotinib are equally effective for treating patients in whom first-line imatinib therapy failed because of resistance or intolerance. The panel believes that not enough data are currently available to recommend one over the other as the preferred second-line therapy based on efficacy data.
Dasatinib and nilotinib are recommended as options for patients with CP-CML for whom standard-dose imatinib fails (less than CHR at 3 months, no cytogenetic response at 6 months, minor or no cytogenetic response at 12 months, or less than CCyR at 18 months) or for those with disease progression to accelerated or blast phase.
Dose escalation of imatinib may be an appropriate option for a subset of patients with cytogenetic failure and a previous cytogenetic response to standard-dose imatinib.
Dasatinib potentially inhibits Src family of kinases, the activation of which has been implicated as a mechanism of BCR-ABL–independent imatinib resistance, suggesting the presence of a trend toward better clinical outcome with dasatinib in an imatinib-resistant population.
Mutational analysis at the time of imatinib failure provides additional guidance in the selection of optimal second-line TKI therapy only in patients with identifiable mutations. Mutation testing is recommended before switching to second-line TKI. However, selection of second-line TKI therapy based on mutational analysis should be individualized.
In patients with no identifiable mutations, the panel recommends that the following issues be considered in choosing a second-line therapy: prior response to imatinib therapy, TKI therapy used in the first-line setting, the safety profile of each agent, patient’s comorbid conditions, patient compliance, and physician’s experience.
Suboptimal Response
The ELN guidelines published in 2006 defined suboptimal responses to imatinib as less than a CHR at 3 months, less than an MCyR at 6 months, less than a CCyR at 12 months, or less than an MMR at 18 months.79 In the updated guidelines published in 2009, suboptimal response is defined as no cytogenetic response at 3 months, less than a partial cytogenetic response (PCyR) at 6 months, a PCyR at 12 months, and less than an MMR at 18 months (Table 11).80 Patients with suboptimal response are described as those who “may still have a substantial benefit from continuing imatinib but that the long-term outcome is not likely to be optimal, so the patient becomes eligible for other treatments.” However, the ELN does not recommend automatically changing therapy for a suboptimal response.
Table 11.
NCCN and European LeukemiaNet Response Criteria for Patients With Chronic-Phase Chronic Myelogenous Leukemia
| NCCN |
European LeukemiaNet80 |
||||
|---|---|---|---|---|---|
| Evaluation Time (mo) |
Optimal Response |
Failure | Optimal Response |
Suboptimal Response |
Failure |
| 3 | CHR | Less than CHR | CHR and at least an mCyR | No CCyR | Less than CHR |
| 6 | CCyR or PCyR | No CyR | At least a PCyR | Less than PCyR | No CyR |
| 12 | CCyR | Less than PCyR | CCyR | PCyR | Less than PCyR |
| 18 | CCyR | Less than CCyR | MMR | Less than MMR | Less than CCyR |
Abbreviations: CHR, complete hematologic response (white blood cell count < 10 × 109/L, platelet count < 450 × 109/L, no immature myelocytes, promyelocytes, or blasts in peripheral blood and disappearance of palpable splenomegaly); CCyR, complete cytogenetic response (no Ph+ metaphases); CyR, cytogenetic response; mCyR, minor cytogenetic response (> 35% Ph+ metaphases); MMR, major molecular response (reduction in BCR-ABL/control gene ratio; ≥ 3-log reduction from a standardized based or ≤ 0.1 % on the international scale); PCyR, partial cytogenetic response (1%–35% Ph+ metaphases).
Suboptimal response to imatinib could result from many factors, including poor compliance to imatinib therapy, individual variation in drug metabolism, aberrant expression of drug transporters, and differences in the intrinsic biology of disease, which might result in competition between clones highly sensitive to imatinib and those that are resistant. These possibilities are not mutually exclusive.
Compliance to Therapy
In the ADAGIO (Adherence Assessment with Glivec: Indicators and Outcomes) study, which evaluated the outcomes of nonadherence to imatinib therapy in patients with CML, nonadherence was associated with poorer response to imatinib.81 Patients with suboptimal response had significantly higher mean percentages of imatinib not taken (23%) than did those with optimal response (7%). The data from Hammersmith hospital showed a strong correlation between adherence rate and the 6-year probability of achieving a MMR (94.5% for patients with a greater than 90% adherence rate compared with 28% for those with an adherence rate of 90% or less).82 Adherence was identified as the only independent predictor for achieving a complete molecular response (CMR) on standard-dose imatinib and MMR on high-dose imatinib.
Poor adherence to imatinib can also have an impact on imatinib plasma levels, which in some studies have been shown to correlate with response to therapy in patients taking a standard dose of imatinib.83 In the IRIS study, maintaining an imatinib trough level at or higher than 1000 ng/mL was important for achieving CCyR.84 Initial results of the TIDEL II trial showed that selective dose escalation may be able to overcome the adverse prognostic impact of low plasma concentrations of standard-dose imatinib and result in reduction in BCR-ABL transcript levels.85 The long-term follow-up is ongoing.
Aberrant Expression of Drug Transporters
The expression of human organic cation transporter-1 (hOCT-1) has also been correlated with response to imatinib. Imatinib uptake into a CML cell line with high hOCT-1 expression was greater than into cell lines with modest or low expression.86 Higher activity of hOCT-1 is associated with excellent molecular response irrespective of dose, whereas response was highly dose-dependent in patients with low hOCT-1 activity.87,88 Most patients with suboptimal response to imatinib have been reported to have low hOCT-1 activity. In the updated analysis of patients enrolled in the TIDEL II trial, MMR rate at 60 months was higher for patients with high hOCT-1 activity compared with those with low hOCT-1 activity (89% vs. 55%, respectively).85 These differences were highly significant in patients who averaged less than 600 mg/d of imatinib.
Notably, cellular uptake of dasatinib or nilotinib seems to be independent of hOCT-1 expression.89–91 Thus, preliminary findings suggest that patients with low hOCT-1 expression might have better outcomes with imatinib dose escalation, dasatinib, or nilotinib.
Genetic Heterogeneity and Clonal Competition
Suboptimal response could arise from the intrinsic progression program characteristic to CML. Uninhibited BCR-ABL drives the genetic changes that cause progression to advanced-phase disease. Therefore, some patients at diagnosis will already have substantial changes in their genome, while appearing to be in chronic phase. The major change in expression has been shown to be produced by the transition from chronic to accelerated phase, and that in resistant patients in chronic phase, gene expression signatures are very similar to those in patients in accelerated phase.2,92 Thus, suboptimal response could be derived from clones that have genetic changes associated with progression, and thus a relatively resistant phenotype, despite the gross pathologic characteristics of chronic phase.
In addition, the development of the BCR-ABL kinase domain mutations may contribute to suboptimal response. A rising BCR-ABL level has been associated with an increased risk of BCR-ABL mutations, a high risk of progression, and a risk for molecular relapse.93–98 Imagine that at diagnosis a patient has 2 clonal populations, with the dominant clone sensitive to imatinib and the minor clone resistant. With the selective pressure of imatinib, and the dominant clone declines but the selective clone increases; the result is that the total number of CML cells decline, but slowly, as the sensitive clonal population is replaced by the resistant population.
Dasatinib and nilotinib have been effective against many of the imatinib-resistant BCR-ABL mutations.75,78 However, no strong data support that treating suboptimal response or molecular relapse with a second-generation TKI will have any impact on the long-term clinical outcome.
Conclusions
Assessment of adherence to therapy and implementation of interventions in patients with poor adherence may improve clinical outcomes.” Evaluation of hOCT-1 expression may be reasonable in patients receiving imatinib. Mutation analyses in patients with suboptimal response may be helpful in the selection of alternate therapy.
The prognostic implications of suboptimal response may also be different depending on the time point. Thus, the outcomes of patients with suboptimal response at 6 months are more similar to those of patients who met the criteria for failure, whereas the outcomes of patients with a suboptimal response at 18 months are very similar to those of patients with an optimal response. Some studies suggest that patients with a suboptimal response at 12 months have a poor prognosis more similar to that of patients who meet criteria for failure, and others have shown that these patients have an outcome closer to that of patients with an optimal response, with a similar transformation-free survival but worse event-free survival.100–102
Available data suggest that patients with suboptimal response represent a subgroup that requires careful monitoring and may benefit from alternate treatment options. A few early reports have suggested that dose escalation of imatinib to 800 mg as tolerated100,103–105 or switching to dasatinib40,106 or nilotinib105,107,108 are effective strategies in patients with suboptimal response to standard-dose imatinib.
NCCN Recommendations
The NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) for Chronic Myelogenous Leukemia have incorporated patients who meet the ELN criteria for suboptimal response: minor cytogenetic response at 6 months and partial cytogenetic response at 12 months (to view the most recent version of these guidelines, visit the NCCN Web site at www.NCCN.org). Continuation of the current TKI therapy at the same dose or dose escalation of imatinib to 800 mg as tolerated are included as options for this group of patients. The panel currently feels that no strong evidence exists showing that a change of therapy would improve response rate among patients with suboptimal response. NCCN supports trials that study whether intervention for suboptimal response or molecular relapse affects short- and long-term outcomes.
Can TKI Therapy Ever be Discontinued?
TKI therapy has become the standard therapy for patients with CML. Extended follow-up data from the IRIS trial showed that the BCR-ABL levels continued to decline in many of the patients, and that the number of patients attaining undetectable BCR-ABL levels increased between 3 and 6 years of imatinib treatment.109 However, the disease usually relapses if imatinib is stopped even in patients who experience CMR,110 although there have been anecdotal reports of no subsequent relapse after discontinuation of imatinib therapy.111,112
Results from 2 ongoing studies have shown that that imatinib can be safely discontinued in some patients in stable CMR for 2 years or more with close molecular monitoring.113,114 Ross et al.114 reported that the rate of sustained CMR after 12 months off treatment was 67% for the entire cohort (n = 18; 5 patients had received only imatinib and 13 patients had received interferon followed by imatinib). However, the sample size was small and the median follow-up was only 7 months for patients who had received only imatinib. In the Stop Imatinib (STIM) study, among 69 patients who had a follow-up of more than 12 months, 39% remained in CMR and 58% experienced relapse within 6 months after discontinuation of imatinib. The molecular relapse-free survival was 41% and 38%, respectively, at 1 and 2 years.113 All patients who had a molecular relapse responded to reintroduction of imatinib, suggesting that discontinuation did not lead to acquired resistance.
Low Sokal risk score, male sex, and longer duration of imatinib treatment were identified as prognostic factors for the maintenance of CMR after withdrawal of imatinib.113 The results of the STIM study suggest that discontinuation of imatinib may be possible only in patients with a sustained CMR (greater than 5-log reduction in BCR-ABL transcript levels and undetectable transcripts by reverse transcriptase-PCR) for at least 2 consecutive years on imatinib. Additional prospective studies are needed to confirm these initial findings.
NCCN Recommendations
Currently, discontinuation of imatinib is not recommended outside the context of a clinical trial for patients experiencing response.
In the absence of data from studies evaluating the possibility of discontinuing dasatinib and nilotinib in responding patients, the findings from the studies involving patients treated with imatinib should be extrapolated to patients undergoing second-generation TKI therapy.
The panel believes evidence is insufficient to favor the continuation of TKI therapy during pregnancy. Potential benefit of TKI therapy for the mother or its potential risk to the fetus must be carefully evaluated on an individual basis.
Allogeneic Hematopoietic Stem Cell Transplant
Allogeneic hematopoietic stem cell transplant (HSCT) is a potentially curative treatment for patients with CML, but the excellent results with imatinib and other second-generation TKIs have challenged its role. The widespread application of allogeneic HSCT has been limited by donor availability and the high toxicity of the procedure in older patients. Ongoing advances in human leukocyte antigen (HLA) typing and the use of less-toxic regimens are broadening the use of HSCT. The advent of molecular DNA assessment of HLA typing has enabled a rigorous and stringent selection of unrelated matched donors, which has translated into greatly improved transplant outcomes, so that results with unrelated, fully matched donors are comparable to those of matched related donors.115–117 Investigational approaches using nonmyeloablative (reduced-intensity conditioning) “mini transplants” are promising and show that molecular remissions may be achieved in patients with CML.118–120
Although TKI therapy has dramatically improved the outcomes of patients with CP-CML, its use in first-line treatment will fail in some patients because of intolerance or resistance, leading to disease progression. Patients with T3151 mutation do not experience response to any of the currently approved TKI therapies. Second-generation TKIs are not very effective for managing patients with AP-CML or BP-CML.121 Thus, allogeneic HSCT has a definite, though more limited, role in the management of patients with CML.122
Prognostic Factors
The outcome of allogeneic HSCT is influenced by several pretransplant variables, such as the disease phase, HLA matching, age, sex, and time from diagnosis to transplant.123 Low HSCT comorbidity index (HCT-CI) and low C-reactive protein were recently identified as prognostic indicators for lower nonrelapsed mortality rate and a somewhat improved survival rate.124 The disease phase at the time of transplant remains an important prognostic factor. Outcomes after transplant are clearly superior for patients in chronic phase than in those in advanced phase.125
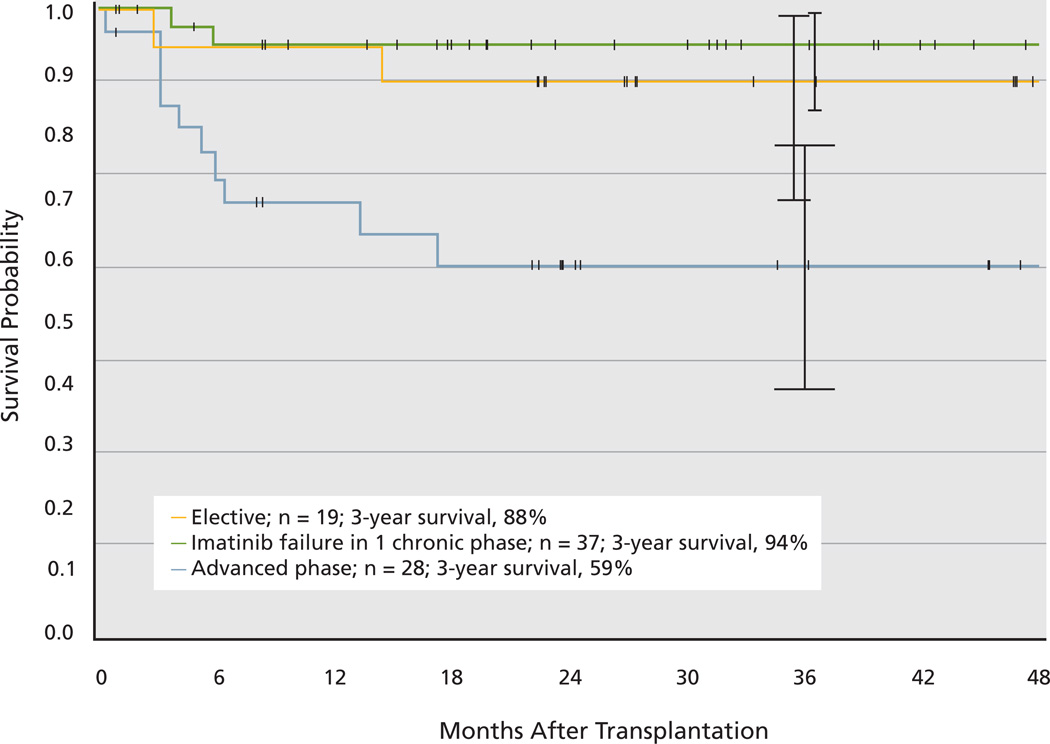
Data from the International Bone Marrow Transplant Registry (IBMTR) between 1998 and 2008 show a probability of 3-year survival of 70% to 72% for patients who underwent a transplant in chronic phase and 45% to 56% for those who underwent a transplant in accelerated phase.126 In the imatinib era, a significant improvement in survival occurred compared with previous time periods.127 Survival has improved across all European Group for Blood & Marrow Transplantation (EBMT) risk groups because of significant reduction in treatment-related mortality and incidences of relapse.128 However, survival is still poor for patients undergoing a transplant in accelerated or blast phase (40%–47% and 16%, respectively) compared with 70% for those who underwent a transplant in chronic phase. Results from the German CML Study IV confirmed these findings.129 In a subgroup analysis among 84 patients who underwent allogeneic HSCT because of either a high risk score at diagnosis, imatinib failure, or disease progression, the 3-year survival rates were 94% for patients with chronic phase and 59% for those with advanced phase (Figure 2).129 Most patients in chronic phase underwent a transplant with an EBMT score equal to or higher than 3. Treatment-related mortality was 8%. Complete molecular remissions were observed in 88% of patients who underwent a transplant. A more recent report from the Center for International Blood and Marrow Transplant Research showed that patients who undergo allogeneic HSCT for CML in first chronic phase and experience remission for at least 5 years have favorable subsequent long-term survival.130
Figure 2.
Survival probability after allogeneic stem cell transplant. Tick marks indicate last observation of living patients.
From Saussele S, Lauseker M, Gratwohl A, et al. Allogeneic hematopoietic stem cell transplantation (allo SCT) for chronic myeloid leukemia in the imatinib era: evaluation of its impact within a subgroup of the randomized German CML Study IV. Blood 2010;115:1883; reproduced with permission of the American Society of Hematology. (C)2011.
Effect of Prior TKI Therapy
Concern exists that previous imatinib therapy might have a detrimental effect on subsequent transplant outcomes, as was shown with busulfan and interferon.131–133 However, results from several studies have confirmed that the use of imatinib before transplant was not associated with a significant increase in death, relapse rate, or nonrelapse mortality compared with no prior use.134–138 In fact, the IBMTR data on 409 patients who received imatinib before transplantation and 900 who did not showed that prior use of imatinib was associated with improved survival for patients undergoing transplantation in chronic phase, although this was limited to patients who underwent a transplant because of intolerance to rather than failure of imatinib treatment.136 This survival benefit was not seen in patients who underwent a transplant in advanced phase.
Some studies have also shown that the use of second-generation TKIs before allogeneic HSCT does not affect the outcome of transplant nor increase transplantrelated toxicity.139–141
When to Transplant in the TKI Era?
Given the successful induction of durable responses with imatinib in most patients, and the recent results showing superior early efficacy of nilotinib and dasatinib in newly diagnosed patients, allogeneic HSCT is no longer recommended as a first-line treatment option for patients with CP-CML. In a randomized study comparing primary HSCT and drug treatment in 621 newly diagnosed patients,142 only 354 were eligible for HSCT based on the availability of a related donor; 123 underwent an HSCT, and 219 received the best possible drug treatment (interferon until imatinib became available later in the trial). Imatinib was offered to patients for whom treatment with interferon failed. Survival with drug therapy was clearly superior for the first 5 years. Survival differences were significant in low-risk patients and no survival difference was observed in intermediate- or high-risk patients.142
Allogeneic HSCT is the preferred option for first-line therapy in rare patients presenting with accelerated or blast phase disease at diagnosis, those who have the T315I mutation, and rare patients who are intolerant to all of the TKIs. A recent report from MD Anderson Cancer Center indicated that allogeneic HSCT is an effective strategy for patients with CML who have the T315I mutation, particularly those in earlier stages. Patients who underwent a transplant in chronic phase had the best outcome.143
Allogeneic HSCT should be considered for patients with CP-CML for whom second-line TKI therapy has failed and for those who experience disease progression to accelerated or blast phase on TKI therapy. Treatment with an alternate TKI (not received before) may be beneficial as a bridge to transplantation.
Early cytogenetic response to second-line TKIs has been shown to predict survival and may help guide the timing of transplant in eligible candidates.144,145 Among patients receiving dasatinib or nilotinib, those experiencing MCyR after 12 months of treatment had a significant survival advantage and significantly lower rates of progression than those experiencing minor cytogenetic response or only CHR.144 Patients with a minimal cytogenetic response at 3 months, PCyR at 6 months, and CCyR at 12 months had significantly better outcomes than those with lesser degrees of cytogenetic response.145
Currently, no definite recommendations exist for specific time points at which to switch patients to allogeneic HSCT based on response to second-line TKI therapy. Available data suggest that evaluating cytogenetic responses at 3, 6, and 12 months may be useful when considering switching a patient with a suitable donor to allogeneic HSCT.
NCCN Recommendations
Chronic Phase
- Patients for whom first-line TKI therapy failed should be evaluated for allogeneic HSCT at 3, 6, 12, and 18 months depending on response to second-line TKI therapy:
-
➤Less than CHR at 3 months
-
➤No cytogenetic response at 6 months
-
➤Minor or no cytogenetic response at 12 months
-
➤Partial cytogenetic response at 18 months
-
➤Cytogenetic relapse
-
➤
Disease Progression
Consider allogeneic HSCT depending on response to second-line TKI therapy for patients experiencing disease progression to accelerated phase.
TKI therapy (alone or in combination with induction chemotherapy) followed by HSCT for patients with disease progression to blast phase.
Individual Disclosures for the NCCN Task Force: Tyrosine Kinase Inhibitor Therapy Selection in the Management of Patients With Chronic Myelogenous Leukemia Panel Members
| Panel Member | Clinical Research Support | Advisory Boards, Speakers Bureau, Expert Witness, or Consultant |
Patent, Equity, or Royalty |
Other | Date Completed |
|---|---|---|---|---|---|
| Susan O’Brien, MD | None | None | None | None | 12/23/10 |
| Ellin Berman, MD | None | None | None | None | 1/14/11 |
| Joseph O. Moore, MD |
ARIAD Pharmaceuticals, Inc. |
Amgen Inc.; Genentech, Inc.; and Novartis Pharmaceuticals Corporation |
None | None | 1/14/11 |
| Javier Pinilla-Ibarz, MD, PhD |
Bristol-Myers Squibb Company; Exelixis Inc.; Novartis Pharmaceuticals Corporation; and Innovive Pharmaceuticals, Inc. |
Novartis Pharmaceuticals Corporation; and Innovive Pharmaceuticals, Inc. |
None | None | 12/15/09 |
| Jerald P. Radich, MD | Novartis Pharmaceuticals Corporation |
Novartis Pharmaceuticals Corporation; and Bristol-Myers Squibb Company |
None | None | 1/17/11 |
| Paul J. Shami, MD | Genzyme Corporation | Genzyme Corporation; and Novartis Pharmaceuticals Corporation |
None | None | 10/7/10 |
| B. Douglas Smith, MD |
Bristol-Myers Squibb Company; Novartis Pharmaceuticals Corporation; Calistoga Pharmaceuticals, Inc.; Infinity Pharmaceuticals, Inc.; and Merck Serono |
Bristol-Myers Squibb Company; and Novartis Pharmaceuticals Corporation |
None | None | 1/18/11 |
| David S. Snyder, MD | Novartis Pharmaceuticals Corporation; Bristol- Myers Squibb Company; and Merck & Co., Inc. |
Novartis Pharmaceuticals Corporation; and Bristol-Myers Squibb Company |
None | None | 1/14/11 |
| Hema M. Sundar, PhD |
None | None | None | Cephalon, Inc. |
7/19/10 |
| Moshe Talpaz, MD | ARIAD Pharmaceuticals, Inc. |
Novartis Pharmaceuticals Corporation; and Bristol-Myers Squibb Company |
None | None | 1/18/11 |
| Meir Wetzler, MD | Bristol-Myers Squibb Company; and Cheme Genex Pharmaceuticals |
Bristol-Myers Squibb Company; and Novartis Pharmaceuticals Corporation |
None | None | 12/6/09 |
Footnotes
Disclosure of Affiliations and Significant Relationships
Dr. O’Brien has disclosed that she has no financial interests, arrangements, or affiliations with the manufacturer of products and devices discussed in this report or who may financially support the educational activity.
Dr. Berman has disclosed that she has no financial interests, arrangements, or affiliations with the manufacturer of products and devices discussed in this report or who may financially support the educational activity.
Dr. Moore has disclosed that he has financial interests, arrangements, or affiliations with the manufacturer of products and devices discussed in this report or who may financially support the educational activity. He receives research support from ARIAD Pharmaceuticals, Inc.; is on the advisory board for Amgen Inc.; is a member of the speakers’ bureau for Genentech, Inc.; and a consultant for Novartis Pharmaceuticals Corporation.
Dr. Pinilla-Ibarz has disclosed that he has financial interests, arrangements, or affiliations with the manufacturer of products and devices discussed in this report or who may financially support the educational activity. He receives research support from Bristol-Myers Squibb Company, Exelixis Inc., Novartis Pharmaceuticals Corporation, and Innovive Pharmaceuticals, Inc. He is also on the advisory board and a member of the speakers’ bureau for Novartis Pharmaceuticals Corporation, and a consultant for Innovive Pharmaceuticals, Inc.
Dr. Radich has disclosed that he has financial interests, arrangements, or affiliations with the manufacturer of products and devices discussed in this report or who may financially support the educational activity. He receives grant support related to research from Novartis Pharmaceuticals Corporation, and is on the advisory board for Novartis Pharmaceuticals Corporation and Bristol-Myers Squibb Company.
Dr. Shami has disclosed that he has financial interests, arrangements, or affiliations with the manufacturer of products and devices discussed in this report or who may financially support the educational activity. He receives research support from Genzyme Corporation and is an advisory board member for Genzyme Corporation and Novartis Pharmaceuticals Corporation.
Dr. Smith has disclosed that he has financial interests, arrangements, or affiliations with the manufacturer of products and devices discussed in this report or who may financially support the educational activity. He receives grant or research support from Bristol-Myers Squibb Company; Novartis Pharmaceuticals Corporation; Calistoga Pharmaceuticals, Inc.; Infinity Pharmaceuticals, Inc.; and Merck Serono. He is also a consultant for Bristol-Myers Squibb Company and is an advisory board member for Novartis Pharmaceuticals Corporation.
Dr. Snyder has disclosed that he has financial interests, arrangements, or affiliations with the manufacturer of products and devices discussed in this report or who may financially support the educational activity. He receives grant or research support from Novartis Pharmaceuticals Corporation, Bristol-Myers Squibb Company, and Merck & Co., Inc. He is also on the advisory board and a member of the speakers’ bureau for Novartis Pharmaceuticals Corporation and Bristol-Myers Squibb Company.
Dr. Sundar has disclosed that she has financial interests, arrangements, or affiliations with the manufacturer of products and devices discussed in this report or who may financially support the educational activity. Her spouse is employed by Cephalon, Inc. She is an employee of the National Comprehensive Cancer Network.
Dr. Talpaz has disclosed that he has financial interests, arrangements, or affiliations with the manufacturer of products and devices discussed in this report or who may financially support the educational activity. He receives research support from ARIAD Pharmaceuticals, Inc.; is on the advisory board for Novartis Pharmaceuticals Corporation and Bristol-Myers Squibb Company; and is on the speakers’ bureau for Novartis Pharmaceuticals Corporation.
Dr. Wetzler has disclosed that he has financial interests, arrangements, or affiliations with the manufacturer of products and devices discussed in this report or who may financially support the educational activity. He receives research support from Bristol-Myers Squibb Company and ChemGenex Pharmaceuticals, and is a member of the speakers’ bureau for Bristol-Myers Squibb Company and Novartis Pharmaceuticals Corporation.
Contributor Information
Susan O’Brien, The University of Texas MD Anderson Cancer Center.
Ellin Berman, Memorial Sloan-Kettering Cancer Center.
Joseph O. Moore, Duke University Medical Center and Duke Cancer Institute.
Javier Pinilla-Ibarz, H. Lee Moffitt Cancer Center & Research Institute.
Jerald P. Radich, Fred Hutchinson Cancer Research Center.
Paul J. Shami, Huntsman Cancer Institute at the University of Utah.
B. Douglas Smith, The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins.
David S. Snyder, City of Hope Comprehensive Cancer Center.
Hema M. Sundar, National Comprehensive Cancer Network.
Moshe Talpaz, University of Michigan Comprehensive Cancer Center.
Meir Wetzler, Roswell Park Cancer Institute.
References
- 1.Faderl S, Talpaz M, Estrov Z, et al. The biology of chronic myeloid leukemia. N Engl J Med. 1999;341:164–172. doi: 10.1056/NEJM199907153410306. [DOI] [PubMed] [Google Scholar]
- 2.Radich JP, Dai H, Mao M, et al. Gene expression changes associated with progression and response in chronic myeloid leukemia. Proc Natl Acad Sci U S A. 2006;103:2794–2799. doi: 10.1073/pnas.0510423103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sawyers CL. Chronic myeloid leukemia. N Engl J Med. 1999;340:1330–1340. doi: 10.1056/NEJM199904293401706. [DOI] [PubMed] [Google Scholar]
- 4.O’Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarahine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 5.Druker BJ, Guilhot F, O’Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 6.Deininger M, O’Brien SG, Guilhot F, et al. International randomized study of interferon vs STI571 (IRIS) 8-year follow up: sustained survival and low risk for progression or events in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) treated with imatinib [abstract] Blood. 2009;114 Abstract 1126. [Google Scholar]
- 7.Guilhot F, Druker B, Larson RA, et al. High rates of durable response are achieved with imatinib after treatment with interferon alpha plus cytarabine: results from the International Randomized Study of Interferon and STI571 (IRIS) trial. Haematologica. 2009;94:1669–1675. doi: 10.3324/haematol.2009.010629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berman E, Nicolaides M, Maki RG, et al. Altered bone and mineral metabolism in patients receiving imatinib mesylate. N Engl J Med. 2006;354:2006–2013. doi: 10.1056/NEJMoa051140. [DOI] [PubMed] [Google Scholar]
- 9.Kerkela R, Grazette L, Yacobi R, et al. Cardiotoxicity of the cancer therapeutic agent imatinib mesylate. Nat Med. 2006;12:908–916. doi: 10.1038/nm1446. [DOI] [PubMed] [Google Scholar]
- 10.Atallah E, Durand JB, Kantarjian H, Cortes J. Congestive heart failure is a rare event in patients receiving imatinib therapy. Blood. 2007;110:1233–1237. doi: 10.1182/blood-2007-01-070144. [DOI] [PubMed] [Google Scholar]
- 11.Kantarjian H, Talpaz M, O’Brien S, et al. High-dose imatinib mesylate therapy in newly diagnosed Philadelphia chromosome-positive chronic phase chronic myeloid leukemia. Blood. 2004;103:2873–2878. doi: 10.1182/blood-2003-11-3800. [DOI] [PubMed] [Google Scholar]
- 12.Cortes JE, Kantarjian HM, Goldberg SL, et al. High-dose imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: high rates of rapid cytogenetic and molecular responses. J Clin Oncol. 2009;27:4754–4759. doi: 10.1200/JCO.2008.20.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hughes TP, Branford S, White DL, et al. Impact of early dose intensity on cytogenetic and molecular responses in chronic-phase CML patients receiving 600 mg/day of imatinib as initial therapy. Blood. 2008;112:3965–3973. doi: 10.1182/blood-2008-06-161737. [DOI] [PubMed] [Google Scholar]
- 14.Castagnetti F, Palandri F, Amabile M, et al. Results of high-dose imatinib mesylate in intermediate Sokal risk chronic myeloid leukemia patients in early chronic phase: a phase 2 trial of the GIMEMA CML Working Party. Blood. 2009;113:3428–3434. doi: 10.1182/blood-2007-08-103499. [DOI] [PubMed] [Google Scholar]
- 15.Baccarani M, Rosti G, Castagnetti F, et al. Comparison of imatinib 400 mg and 800 mg daily in the front-line treatment of high-risk, Philadelphia-positive chronic myeloid leukemia: a European LeukemiaNet Study. Blood. 2009;113:4497–4504. doi: 10.1182/blood-2008-12-191254. [DOI] [PubMed] [Google Scholar]
- 16.Cortes JE, Baccarani M, Guilhot F, et al. Phase III, randomized, open-label study of daily imatinib mesylate 400 mg versus 800 mg in patients with newly diagnosed, previously untreated chronic myeloid leukemia in chronic phase using molecular end points: tyrosine kinase inhibitor optimization and selectivity study. J Clin Oncol. 2010;28:424–430. doi: 10.1200/JCO.2009.25.3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hehlmann R, Lauseker M, Jung-Munkwitz S, et al. Treatment optimization by high-dose imatinib: randomized comparison of imatinib 800 mg versus imatinib 400 mg {+/−} IFN in newly diagnosed BCR-ABL positive chronic phase (CP) CML: the German CML-study IV [abstract] J Clin Oncol. 2010;28(Suppl l) Abstract 6517. [Google Scholar]
- 18.Shah NP, Tran C, Lee FY, et al. Overriding imatinib resistance with a novel ABL kinase inhibitor. Science. 2004;305:399–401. doi: 10.1126/science.1099480. [DOI] [PubMed] [Google Scholar]
- 19.Cortes JE, Jones D, O’Brien S, et al. Results of dasatinib therapy in patients with early chronic-phase chronic myeloid leukemia. J Clin Oncol. 2010;28:398–404. doi: 10.1200/JCO.2009.25.4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kantarjian H, Shah NP, Hochhaus A, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2010;362:2260–2270. doi: 10.1056/NEJMoa1002315. [DOI] [PubMed] [Google Scholar]
- 21.Shah N, Kantarjian H, Hochhaus A, et al. Dasatinib versus imatinib in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) in the DASISION trial: 18-month follow-up [abstract] Blood. 2010;116 Abstract 206. [Google Scholar]
- 22.Radich JP, Kopecky KJ, Kamel-Reid S, et al. A randomized phase II trial of dasatinib 100 mg vs imatinib 400 mg in newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP): the S0325 Intergroup trial [abstract] Blood. 2010;116 doi: 10.1182/blood-2012-02-410688. Abstract LBA-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weisberg E, Manley PW, Breitenstein W, et al. Characterization of AMN107, a selective inhibitor of native and mutant Bcr-Abl. Cancer Cell. 2005;7:129–141. doi: 10.1016/j.ccr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 24.Cortes JE, Jones D, O’Brien S, et al. Nilotinib as front-line treatment for patients with chronic myeloid leukemia in early chronic phase. J Clin Oncol. 2010;28:392–397. doi: 10.1200/JCO.2009.25.4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosti G, Palandri F, Castagnetti F, et al. Nilotinib for the frontline treatment of Ph+ chronic myeloid leukemia. Blood. 2009;114:4933–4938. doi: 10.1182/blood-2009-07-232595. [DOI] [PubMed] [Google Scholar]
- 26.Saglio G, Kim DW, Issaragrisil S, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010;362:2251–2259. doi: 10.1056/NEJMoa0912614. [DOI] [PubMed] [Google Scholar]
- 27.Hughes TP, Hochhaus A, Saglio G, et al. ENESTnd update: continued superiority of nilotinib versus imatinib in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) [abstract] Blood. 2010;116 Abstract 207. [Google Scholar]
- 28.de Lavallade H, Apperley JF, Khorashad JS, et al. Imatinib for newly diagnosed patients with chronic myeloid leukemia: incidence of sustained responses in an intention-to-treat analysis. J Clin Oncol. 2008;26:3358–3363. doi: 10.1200/JCO.2007.15.8154. [DOI] [PubMed] [Google Scholar]
- 29.Cortes J, Talpaz M, O’Brien S, et al. Molecular responses in patients with chronic myelogenous leukemia in chronic phase treated with imatinib mesylate. Clin Cancer Res. 2005;11:3425–3432. doi: 10.1158/1078-0432.CCR-04-2139. [DOI] [PubMed] [Google Scholar]
- 30.Iacobucci I, Saglio G, Rosti G, et al. Achieving a major molecular response at the time of a complete cytogenetic response (CCgR) predicts a better duration of CCgR in imatinib-treated chronic myeloid leukemia patients. Clin Cancer Res. 2006;12:3037–3042. doi: 10.1158/1078-0432.CCR-05-2574. [DOI] [PubMed] [Google Scholar]
- 31.Hughes TP, Hochhaus A, Branford S, et al. Long-term prognostic significance of early molecular response to imatinib in newly diagnosed chronic myeloid leukemia: an analysis from the International Randomized Study of Interferon and STI571 (IRIS) Blood. 2010;116:3758–3765. doi: 10.1182/blood-2010-03-273979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonifazi F, de Vivo A, Rosti G, et al. Chronic myeloid leukemia and interferon-alpha: a study of complete cytogenetic responders. Blood. 2001;98:3074–3081. doi: 10.1182/blood.v98.10.3074. [DOI] [PubMed] [Google Scholar]
- 33.Kantarjian HM, O’Brien S, Cortes JE, et al. Complete cytogenetic and molecular responses to interferon-alpha-based therapy for chronic myelogenous leukemia are associated with excellent long-term prognosis. Cancer. 2003;97:1033–1041. doi: 10.1002/cncr.11223. [DOI] [PubMed] [Google Scholar]
- 34.Mahon FX, Delbrel X, Cony-Makhoul P, et al. Follow-up of complete cytogenetic remission in patients with chronic myeloid leukemia after cessation of interferon alfa. J Clin Oncol. 2002;20:214–220. doi: 10.1200/JCO.2002.20.1.214. [DOI] [PubMed] [Google Scholar]
- 35.Talpaz M, Shah NP, Kantarjian H, et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med. 2006;354:2531–2541. doi: 10.1056/NEJMoa055229. [DOI] [PubMed] [Google Scholar]
- 36.Hochhaus A, Kantarjian HM, Baccarani M, et al. Dasatinib induces notable hematologic and cytogenetic responses in chronic-phase chronic myeloid leukemia after failure of imatinib therapy. Blood. 2007;109:2303–2309. doi: 10.1182/blood-2006-09-047266. [DOI] [PubMed] [Google Scholar]
- 37.Mauro MJ, Baccarani M, Cervantes F, et al. Dasatinib 2-year efficacy in patients with chronic-phase chronic myelogenous leukemia (CML-CP) with resistance or intolerance to imatinib (START-C) [abstract] J Clin Oncol. 2008;26 Abstract 7009. [Google Scholar]
- 38.Baccarani M, Rosti G, Saglio G, et al. Dasatinib time to and durability of major and complete cytogenetic response (MCyR and CCyR) in patients with chronic myeloid leukemia in chronic phase (CML-CP) [abstract] Blood. 2008;112 Abstract 450. [Google Scholar]
- 39.Khoury HJ, Mauro MJ, Matloub Y, et al. Dasatinib is well-tolerated and efficacious in imatinib-intolerant patients with chronic-phase chronic myeloid leukemia (CP-CML) [abstract] Blood. 2009;114 Abstract 1128. [Google Scholar]
- 40.Shah NP, Kim DW, Kantarjian H, et al. Potent, transient inhibition of BCR-ABL with dasatinib 100 mg daily achieves rapid and durable cytogenetic responses and high transformation-free survival rates in chronic phase chronic myeloid leukemia patients with resistance, suboptimal response or intolerance to imatinib. Haematologica. 2010;95:232–240. doi: 10.3324/haematol.2009.011452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Apperley JF, Cortes JE, Kim DW, et al. Dasatinib in the treatment of chronic myeloid leukemia in accelerated phase after imatinib failure: the START A trial. J Clin Oncol. 2009;27:3472–3479. doi: 10.1200/JCO.2007.14.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rea D, Dombret H, Kim DW, et al. Dasatinib efficacy in patients with imatinib-resistant/-intolerant chronic myeloid leukemia in accelerated phase: 24-month data from START-A [abstract] Haematologica. 2008;93(Suppl l) Abstract 0982. [Google Scholar]
- 43.Cortes J, Kim DW, Raffoux E, et al. Efficacy and safety of dasatinib in imatinib-resistant or -intolerant patients with chronic myeloid leukemia in blast phase. Leukemia. 2008;22:2176–2183. doi: 10.1038/leu.2008.221. [DOI] [PubMed] [Google Scholar]
- 44.Kantarjian H, Cortes J, Kim DW, et al. Phase 3 study of dasatinib 140 mg once daily versus 70 mg twice daily in patients with chronic myeloid leukemia in accelerated phase resistant or intolerant to imatinib: 15-month median follow-up. Blood. 2009;113:6322–6329. doi: 10.1182/blood-2008-11-186817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saglio G, Hochhaus A, Goh YT, et al. Dasatinib in imatinib-resistant or imatinib-intolerant chronic myeloid leukemia in blast phase after 2 years of follow-up in a phase 3 study: efficacy and tolerability of 140 milligrams once daily and 70 milligrams twice daily. Cancer. 2010;116:3852–3861. doi: 10.1002/cncr.25123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kantarjian H, Giles F, Wunderle L, et al. Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. N Engl J Med. 2006;354:2542–2551. doi: 10.1056/NEJMoa055104. [DOI] [PubMed] [Google Scholar]
- 47.Kantarjian HM, Giles F, Gattermann N, et al. Nilotinib (formerly AMN107), a highly selective BCR-ABL tyrosine kinase inhibitor, is effective in patients with Philadelphia chromosome positive chronic myelogenous leukemia in chronic phase following imatinib resistance and intolerance. Blood. 2007;110:3540–3546. doi: 10.1182/blood-2007-03-080689. [DOI] [PubMed] [Google Scholar]
- 48.Kantarjian HM, Giles FJ, Bhalla KN, et al. Nilotinib is effective in patients with chronic myeloid leukemia in chronic phase after imatinib resistance or intolerance: 24-month follow-up results. Blood. 2011;117:1141–1145. doi: 10.1182/blood-2010-03-277152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.le Coutre P, Ottmann OG, Giles F, et al. Nilotinib (formerly AMN107), a highly selective BCR-ABL tyrosine kinase inhibitor, is active in patients with imatinib-resistant or -intolerant accelerated-phase chronic myelogenous leukemia. Blood. 2008;111:1834–1839. doi: 10.1182/blood-2007-04-083196. [DOI] [PubMed] [Google Scholar]
- 50.Le Coutre PD, Giles F, Hochhaus A, et al. Nilotinib in chronic myeloid leukemia patients in accelerated phase (CML-AP) with imatinib (IM) resistance or intolerance: longer follow-up results of a phase II study [abstract] J Clin Oncol. 2009;27(Suppl 15) Abstract 7057. [Google Scholar]
- 51.Giles FJ, Kantarjian H, le Coutre PD, et al. Use of nilotinib to induce responses with 24-month (mo) minimum follow-up in patients (pts) with chronic myeloid leukemia in blast crisis (CML-BC) resistant to or intolerant of imatinib [abstract] J Clin Oncol. 2010;28(Suppl 15) Abstract 6510. [Google Scholar]
- 52.Kantarjian HM, Talpaz M, O’Brien S, et al. Dose escalation of imatinib mesylate can overcome resistance to standard-dose therapy in patients with chronic myelogenous leukemia. Blood. 2003;101:473–475. doi: 10.1182/blood-2002-05-1451. [DOI] [PubMed] [Google Scholar]
- 53.Marin D, Goldman JM, Olavarria E, Apperley JF. Transient benefit only from increasing the imatinib dose in CML patients who do not achieve complete cytogenetic remissions on conventional doses. Blood. 2003;102:2702–2704. doi: 10.1182/blood-2003-06-2042. [DOI] [PubMed] [Google Scholar]
- 54.Zonder JA, Pemberton P, Brandt H, et al. The effect of dose increase of imatinib mesylate in patients with chronic or accelerated phase chronic myelogenous leukemia with inadequate hematologic or cytogenetic response to initial treatment. Clin Cancer Res. 2003;9:2092–2097. [PubMed] [Google Scholar]
- 55.Jabbour E, Kantarjian HM, Jones D, et al. Imatinib mesylate dose escalation is associated with durable responses in patients with chronic myeloid leukemia after cytogenetic failure on standard-dose imatinib therapy. Blood. 2009;113:2154–2160. doi: 10.1182/blood-2008-04-154344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kantarjian HM, Larson RA, Guilhot F, et al. Efficacy of imatinib dose escalation in patients with chronic myeloid leukemia in chronic phase. Cancer. 2009;115:551–560. doi: 10.1002/cncr.24066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kantarjian H, Pasquini R, Hamerschlak N, et al. Dasatinib or high-dose imatinib for chronic-phase chronic myeloid leukemia after failure of first-line imatinib: a randomized phase 2 trial. Blood. 2007;109:5143–5150. doi: 10.1182/blood-2006-11-056028. [DOI] [PubMed] [Google Scholar]
- 58.Kantarjian H, Pasquini R, Levy V, et al. Dasatinib or high-dose imatinib for chronic-phase chronic myeloid leukemia resistant to imatinib at a dose of 400 to 600 milligrams daily: two-year follow-up of a randomized phase 2 study (START-R) Cancer. 2009;115:4136–4147. doi: 10.1002/cncr.24504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jabbour E, Kantarjian HM, Baccarani M, et al. Minimal cross-intolerance between nilotinib and imatinib in patients with imatinib-intolerant chronic myeloid leukemia in chronic phase (CML-CP) or accelerated phase (CML-AP) [abstract] Blood. 2008;112 doi: 10.1182/blood-2010-11-318949. Abstract 3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Porkka K, Khoury HJ, Paquette RL, et al. Dasatinib 100 mg once daily minimizes the occurrence of pleural effusion in patients with chronic myeloid leukemia in chronic phase and efficacy is unaffected in patients who develop pleural effusion. Cancer. 2010;116:377–386. doi: 10.1002/cncr.24734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Quintas-Cardama A, Kantarjian H, O’Brien S, et al. Pleural effusion in patients with chronic myelogenous leukemia treated with dasatinib after imatinib failure. J Clin Oncol. 2007;25:3908–3914. doi: 10.1200/JCO.2007.12.0329. [DOI] [PubMed] [Google Scholar]
- 62.Mustjoki S, Ekblom M, Arstila TP, et al. Clonal expansion of T/NK-cells during tyrosine kinase inhibitor dasatinib therapy. Leukemia. 2009;23:1398–1405. doi: 10.1038/leu.2009.46. [DOI] [PubMed] [Google Scholar]
- 63.Schiffer CA, Cortes JE, Saglio G, et al. Association of lymphocytosis following treatment with dasatinib with response and outcome [abstract] J Clin Oncol. 2010;28 Abstract 6553. [Google Scholar]
- 64.Schiffer CA, Cortes JE, Saglio G, et al. Lymphocytosis following first-line treatment for CML in chronic phase with dasatinib is associated with improved responses: a comparison with imatinib [abstract] Blood. 2010;116 Abstract 358. [Google Scholar]
- 65.Breccia M, Alimena G. Nilotinib: a second-generation tyrosine kinase inhibitor for chronic myeloid leukemia. Leuk Res. 2010;34:129–134. doi: 10.1016/j.leukres.2009.08.031. [DOI] [PubMed] [Google Scholar]
- 66.Branford S, Rudzki Z, Walsh S, et al. Detection of BCR-ABL mutations in patients with CML treated with imatinib is virtually always accompanied by clinical resistance, and mutations in the ATP phosphate-binding loop (P-loop) are associated with a poor prognosis. Blood. 2003;102:276–283. doi: 10.1182/blood-2002-09-2896. [DOI] [PubMed] [Google Scholar]
- 67.Soverini S, Colarossi S, Gnani A, et al. Contribution of ABL kinase domain mutations to imatinib resistance in different subsets of Philadelphia-positive patients: by the GIMEMA Working Party on Chronic Myeloid Leukemia. Clin Cancer Res. 2006;12:7374–7379. doi: 10.1158/1078-0432.CCR-06-1516. [DOI] [PubMed] [Google Scholar]
- 68.Jabbour E, Kantarjian H, Jones D, et al. Characteristics and outcomes of patients with chronic myeloid leukemia and T315I mutation following failure of imatinib mesylate therapy. Blood. 2008;112:53–55. doi: 10.1182/blood-2007-11-123950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nicolini FE, Hayette S, Corm S, et al. Clinical outcome of 27 imatinib mesylate-resistant chronic myelogenous leukemia patients harboring a T315I BCR-ABL mutation. Haematologica. 2007;92:1238–1241. doi: 10.3324/haematol.11369. [DOI] [PubMed] [Google Scholar]
- 70.Soverini S, Martinelli G, Rosti G, et al. ABL mutations in late chronic phase chronic myeloid leukemia patients with up-front cytogenetic resistance to imatinib are associated with a greater likelihood of progression to blast crisis and shorter survival: a study by the GIMEMA Working Party on Chronic Myeloid Leukemia. J Clin Oncol. 2005;23:4100–4109. doi: 10.1200/JCO.2005.05.531. [DOI] [PubMed] [Google Scholar]
- 71.Branford S, Melo JV, Hughes TP. Selecting optimal second-line tyrosine kinase inhibitor therapy for chronic myeloid leukemia patients after imatinib failure: does the BCR-ABL mutation status really matter? Blood. 2009;114:5426–5435. doi: 10.1182/blood-2009-08-215939. [DOI] [PubMed] [Google Scholar]
- 72.Redaelli S, Piazza R, Rostagno R, et al. Activity of bosutinib, dasatinib, and nilotinib against 18 imatinib-resistant BCR/ABL mutants. J Clin Oncol. 2009;27:469–471. doi: 10.1200/JCO.2008.19.8853. [DOI] [PubMed] [Google Scholar]
- 73.Jabbour E, Jones D, Kantarjian HM, et al. Long-term outcome of patients with chronic myeloid leukemia treated with second-generation tyrosine kinase inhibitors after imatinib failure is predicted by the in vitro sensitivity of BCR-ABL kinase domain mutations. Blood. 2009;114:2037–2043. doi: 10.1182/blood-2009-01-197715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Deininger MWN, Mauro MJ, Matloub Y, et al. Prevalence of T315I, dasatinib-specific resistant mutations (F317L, V299L, and T315A), and nilotinib-specific resistant mutations (P-loop and F359) at the time of imatinib resistance in chronic-phase chronic myeloid leukemia (CP-CML) [abstract] Blood. 2008;112 Abstract 3236. [Google Scholar]
- 75.Muller MC, Cortes JE, Kim DW, et al. Dasatinib treatment of chronic-phase chronic myeloid leukemia: analysis of responses according to preexisting BCR-ABL mutations. Blood. 2009;114:4944–4953. doi: 10.1182/blood-2009-04-214221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jabbour E, Kantarjian HM, Jones D, et al. Characteristics and outcome of chronic myeloid leukemia patients with F317L BCR-ABL kinase domain mutation after therapy with tyrosine kinase inhibitors. Blood. 2008;112:4839–4842. doi: 10.1182/blood-2008-04-149948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Soverini S, Colarossi S, Gnani A, et al. Resistance to dasatinib in Philadelphia-positive leukemia patients and the presence or the selection of mutations at residues 315 and 317 in the BCR-ABL kinase domain. Haematologica. 2007;92:401–404. doi: 10.3324/haematol.10822. [DOI] [PubMed] [Google Scholar]
- 78.Hughes T, Saglio G, Branford S, et al. Impact of baseline BCR-ABL mutations on response to nilotinib in patients with chronic myeloid leukemia in chronic phase. J Clin Oncol. 2009;27:4204–4210. doi: 10.1200/JCO.2009.21.8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Baccarani M, Saglio G, Goldman J, et al. Evolving concepts in the management of chronic myeloid leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2006;108:1809–1820. doi: 10.1182/blood-2006-02-005686. [DOI] [PubMed] [Google Scholar]
- 80.Baccarani M, Cortes J, Pane F, et al. Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol. 2009;27:6041–6051. doi: 10.1200/JCO.2009.25.0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Noens L, van Lierde MA, De Bock R, et al. Prevalence, determinants, and outcomes of nonadherence to imatinib therapy in patients with chronic myeloid leukemia: the ADAGIO study. Blood. 2009;113:5401–5411. doi: 10.1182/blood-2008-12-196543. [DOI] [PubMed] [Google Scholar]
- 82.Marin D, Bazeos A, Mahon EX, et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol. 2010;28:2381–2388. doi: 10.1200/JCO.2009.26.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Picard S, Titier K, Etienne G, et al. Trough imatinib plasma levels are associated with both cytogenetic and molecular responses to standard-dose imatinib in chronic myeloid leukemia. Blood. 2007;109:3496–3499. doi: 10.1182/blood-2006-07-036012. [DOI] [PubMed] [Google Scholar]
- 84.Larson RA, Druker BJ, Guilhot F, et al. Imatinib pharmacokinetics and its correlation with response and safety in chronic-phase chronic myeloid leukemia: a subanalysis of the IRIS study. Blood. 2008;111:4022–4028. doi: 10.1182/blood-2007-10-116475. [DOI] [PubMed] [Google Scholar]
- 85.Osborn MP, White DL, Saunders VA, et al. Early dose-escalation in chronic myeloid leukaemia patients with low plasma imatinib levels leads to equivalent BCR-ABL values and drug levels at 6 months to those with optimal drug levels: first analysis from the TIDEL II trial of de-novo patients treated with 600mg imatinib [abstract] Blood. 2009;114 Abstract 1131. [Google Scholar]
- 86.Wang L, Giannoudis A, Lane S, et al. Expression of the uptake drug transporter hOCT1 is an important clinical determinant of the response to imatinib in chronic myeloid leukemia. Clin Pharmacol Ther. 2008;83:258–264. doi: 10.1038/sj.clpt.6100268. [DOI] [PubMed] [Google Scholar]
- 87.White DL, Saunders VA, Dang P, et al. Most CML patients who have a suboptimal response to imatinib have low OCT-1 activity: higher doses of imatinib may overcome the negative impact of low OCT-1 activity. Blood. 2007;110:4064–4072. doi: 10.1182/blood-2007-06-093617. [DOI] [PubMed] [Google Scholar]
- 88.White DL, Dang P, Engler J, et al. Functional activity of the OCT-1 protein is predictive of long-term outcome in patients with chronic-phase chronic myeloid leukemia treated with imatinib. J Clin Oncol. 2010;28:2761–2767. doi: 10.1200/JCO.2009.26.5819. [DOI] [PubMed] [Google Scholar]
- 89.Hiwase DK, Saunders V, Hewett D, et al. Dasatinib cellular uptake and efflux in chronic myeloid leukemia cells: therapeutic implications. Clin Cancer Res. 2008;14:3881–3888. doi: 10.1158/1078-0432.CCR-07-5095. [DOI] [PubMed] [Google Scholar]
- 90.White DL, Saunders VA, Dang P, et al. OCT-1-mediated influx is a key determinant of the intracellular uptake of imatinib but not nilotinib (AMN107): reduced OCT-1 activity is the cause of low in vitro sensitivity to imatinib. Blood. 2006;108:697–704. doi: 10.1182/blood-2005-11-4687. [DOI] [PubMed] [Google Scholar]
- 91.Davies A, Jordanides NE, Giannoudis A, et al. Nilotinib concentration in cell lines and primary CD34(+) chronic myeloid leukemia cells is not mediated by active uptake or efflux by major drug transporters. Leukemia. 2009;23:1999–2006. doi: 10.1038/leu.2009.166. [DOI] [PubMed] [Google Scholar]
- 92.Oehler VG, Yeung KY, Choi YE, et al. The derivation of diagnostic markers of chronic myeloid leukemia progression from microarray data. Blood. 2009;114:3292–3298. doi: 10.1182/blood-2009-03-212969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Branford S, Rudzki Z, Parkinson I, et al. Real-time quantitative PCR analysis can be used as a primary screen to identify patients with CML treated with imatinib who have BCR-ABL kinase domain mutations. Blood. 2004;104:2926–2932. doi: 10.1182/blood-2004-03-1134. [DOI] [PubMed] [Google Scholar]
- 94.Khorashad JS, de Lavallade H, Apperley JF, et al. Finding of kinase domain mutations in patients with chronic phase chronic myeloid leukemia responding to imatinib may identify those at high risk of disease progression. J Clin Oncol. 2008;26:4806–4813. doi: 10.1200/JCO.2008.16.9953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang L, Knight K, Lucas C, Clark R. The role of serial BCR-ABL transcript monitoring in predicting the emergence of BCR-ABL kinase mutations in imatinib-treated patients with chronic myeloid leukemia. Haematologica. 2006;91:235–239. [PubMed] [Google Scholar]
- 96.Press RD, Galderisi C, Yang R, et al. A half-log increase in BCR-ABL RNA predicts a higher risk of relapse in patients with chronic myeloid leukemia with an imatinib-induced complete cytogenetic response. Clin Cancer Res. 2007;13:6136–6143. doi: 10.1158/1078-0432.CCR-07-1112. [DOI] [PubMed] [Google Scholar]
- 97.Kantarjian HM, Shan J, Jones D, et al. Significance of rising levels of minimal residual disease in patients with Philadelphia chromosome-positive chronic myelogenous leukemia (Ph+ CML) in complete cytogenetic response (CGCR) [abstract] Blood. 2008;112 doi: 10.1200/JCO.2008.18.6999. Abstract 445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Press RD, Willis SG, Laudadio J, et al. Determining the rise in BCR-ABL RNA that optimally predicts a kinase domain mutation in patients with chronic myeloid leukemia on imatinib. Blood. 2009;114:2598–2605. doi: 10.1182/blood-2008-08-173674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Partridge AH, Avorn J, Wang PS, Winer EP. Adherence to therapy with oral antineoplastic agents. J Natl Cancer Inst. 2002;94:652–661. doi: 10.1093/jnci/94.9.652. [DOI] [PubMed] [Google Scholar]
- 100.Alvarado Y, Kantarjian H, O’Brien S, et al. Significance of suboptimal response to imatinib, as defined by the European LeukemiaNet, in the long-term outcome of patients with early chronic myeloid leukemia in chronic phase. Cancer. 2009;115:3709–3718. doi: 10.1002/cncr.24418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Castagnetti F, Gugliotta G, Palandri F, et al. Chronic myeloid leukemia (CML) patients with “suboptimal” response to imatinib (IM) according to european leukemianet criteria have a poorer outcome with respect to “optimal” responders: a GIMEMA CML Working Party analysis [abstract] Blood. 2009;114 Abstract 2196. [Google Scholar]
- 102.Marin D, Milojkovic D, Olavarria E, et al. European Leukemia Net criteria for failure or suboptimal response reliably identify patients with CML in early chronic phase treated with imatinib whose eventual outcome is poor. Blood. 2008;112:4437–4444. doi: 10.1182/blood-2008-06-162388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Breccia M, Stagno F, Vigneri P, et al. Imatinib dose escalation in 74 failure or suboptimal response chronic myeloid leukaemia patients at 3-year follow-up. Am J Hematol. 2010;85:375–377. doi: 10.1002/ajh.21680. [DOI] [PubMed] [Google Scholar]
- 104.Koh Y, Kim I, Yoon SS, et al. Phase IV study evaluating efficacy of escalated dose of imatinib in chronic myeloid leukemia patients showing suboptimal response to standard dose imatinib. Ann Hematol. 2010;89:725–731. doi: 10.1007/s00277-010-0910-8. [DOI] [PubMed] [Google Scholar]
- 105.Yeung DT, Osborn M, White DL, et al. Selective escalation of imatinib therapy and early switching to nilotinib in de novo chronic phase CML patients: interim results from the TIDEL-II trial [abstract] Blood. 2010;116 Abstract 209. [Google Scholar]
- 106.Hochhaus A, Muller MC, Radich J, et al. Dasatinib-associated major molecular responses are rapidly achieved in patients with chronic myeloid leukemia in chronic phase (CML-CP) following resistance, suboptimal response, or intolerance on imatinib [abstract] Blood. 2008;112 Abstract 1095. [Google Scholar]
- 107.Nicolini FE, Kim DW, Ceglarek B, et al. Impact of prior therapy and suboptimal response to imatinib on the efficacy and safety of nilotinib among 1,422 patients with imatinib-resistant or -intolerant chronic myeloid leukemia (CML) in chronic phase (CP): sub-analyses of the ENACT (Expanding Nilotinib Access In Clinical Trials) study [abstract] Blood. 2009;114 Abstract 2201. [Google Scholar]
- 108.Yang AS, Jillella A, Miller CB, et al. Nilotinib-associated molecular responses achieved in chronic myeloid leukemia in chronic phase (CML-CP) patients with a suboptimal molecular response to imatinib [abstract] Blood. 2009;114 Abstract 2206. [Google Scholar]
- 109.Branford S, Seymour JF, Grigg A, et al. BCR-ABL messenger RNA levels continue to decline in patients with chronic phase chronic myeloid leukemia treated with imatinib for more than 5 years and approximately half of all first-line treated patients have stable undetectable BCR-ABL using strict sensitivity criteria. Clin Cancer Res. 2007;13:7080–7085. doi: 10.1158/1078-0432.CCR-07-0844. [DOI] [PubMed] [Google Scholar]
- 110.Cortes J, O’Brien S, Kantarjian H. Discontinuation of imatinib therapy after achieving a molecular response. Blood. 2004;104:2204–2205. doi: 10.1182/blood-2004-04-1335. [DOI] [PubMed] [Google Scholar]
- 111.Guastafierro S, Falcone U, Celentano M, et al. Is it possible to discontinue imatinib mesylate therapy in chronic myeloid leukemia patients with undetectable BCR/ABL? A case report and a review of the literature. Leuk Res. 2009;33:1079–1081. doi: 10.1016/j.leukres.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 112.Verma D, Kantarjian H, Jain N, Cortes J. Sustained complete molecular response after imatinib discontinuation in a patient with chronic myeloid leukemia not previously exposed to interferon alpha. Leuk Lymphoma. 2008;49:1399–1402. doi: 10.1080/10428190802043903. [DOI] [PubMed] [Google Scholar]
- 113.Mahon FX, Rea D, Guilhot J, et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010:1029–1035. doi: 10.1016/S1470-2045(10)70233-3. [DOI] [PubMed] [Google Scholar]
- 114.Ross DDM, Grigg A, Schwarer A, et al. The majority of chronic myeloid leukaemia patients who cease imatinib after achieving a sustained complete molecular response (CMR) remain in cmr, and any relapses occur early [abstract] Blood. 2008;112 Abstract 1102. [Google Scholar]
- 115.Davies SM, DeFor TE, McGlave PB, et al. Equivalent outcomes in patients with chronic myelogenous leukemia after early transplantation of phenotypically matched bone marrow from related or unrelated donors. Am J Med. 2001;110:339–346. doi: 10.1016/s0002-9343(01)00629-5. [DOI] [PubMed] [Google Scholar]
- 116.Hansen JA, Gooley TA, Martin PJ, et al. Bone marrow transplants from unrelated donors for patients with chronic myeloid leukemia. N Engl J Med. 1998;338:962–968. doi: 10.1056/NEJM199804023381405. [DOI] [PubMed] [Google Scholar]
- 117.Liu QF, Xu XJ, Chen YK, et al. Long-term outcomes of HLA-matched sibling compared with mismatched related and unrelated donor hematopoietic stem cell transplantation for chronic phase chronic myelogenous leukemia: a single institution experience in China. Ann Hematol. 2010 doi: 10.1007/s00277-010-1081-3. in press. [DOI] [PubMed] [Google Scholar]
- 118.Champlin R, de Lima M, Kebriaei P, et al. Nonmyeloablative allogeneic stem cell transplantation for chronic myelogenous leukemia in the imatinib era. Clin Lymphoma Myeloma. 2009;9:S261–S265. doi: 10.3816/CLM.2009.s.021. [DOI] [PubMed] [Google Scholar]
- 119.Crawley C, Szydlo R, Lalancette M, et al. Outcomes of reduced-intensity transplantation for chronic myeloid leukemia: an analysis of prognostic factors from the Chronic Leukemia Working Party of the EBMT. Blood. 2005;106:2969–2976. doi: 10.1182/blood-2004-09-3544. [DOI] [PubMed] [Google Scholar]
- 120.Or R, Shapira MY, Resnick I, et al. Nonmyeloablative allogeneic stem cell transplantation for the treatment of chronic myeloid leukemia in first chronic phase. Blood. 2003;101:441–445. doi: 10.1182/blood-2002-02-0535. [DOI] [PubMed] [Google Scholar]
- 121.Kantarjian H, O’Brien S, Talpaz M, et al. Outcome of patients with Philadelphia chromosome-positive chronic myelogenous leukemia post-imatinib mesylate failure. Cancer. 2007;109:1556–1560. doi: 10.1002/cncr.22569. [DOI] [PubMed] [Google Scholar]
- 122.Radich J. Stem cell transplant for chronic myeloid leukemia in the imatinib era. Semin Hematol. 2010;47:354–361. doi: 10.1053/j.seminhematol.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gratwohl A, Hermans J, Goldman JM, et al. Risk assessment for patients with chronic myeloid leukaemia before allogeneic blood or marrow transplantation. Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Lancet. 1998;352:1087–1092. doi: 10.1016/s0140-6736(98)03030-x. [DOI] [PubMed] [Google Scholar]
- 124.Pavlu J, Kew AK, Taylor-Roberts B, et al. Optimizing patient selection for myeloablative allogeneic hematopoietic cell transplantation in chronic myeloid leukemia in chronic phase. Blood. 2010;115:4018–4020. doi: 10.1182/blood-2010-01-263624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Horowitz MM, Rowlings PA, Passweg JR. Allogeneic bone marrow transplantation for CML: a report from the International Bone Marrow Transplant Registry. Bone Marrow Transplant. 1996;17(Suppl 3):S5–S6. [PubMed] [Google Scholar]
- 126.Pasquini MC, Wang Z. [Accessed January 11, 2011];CIBMTR Summary Slides 2010. Available at: http://www.cibmtr.org.
- 127.Boehm A, Walcherberger B, Sperr WR, et al. Improved outcome in patients with chronic myeloid leukemia after allogeneic hematopoietic stem cell transplantation over the past 25 years: a single center experience. Biol Blood Marrow Transplant. 2011;17:133–140. doi: 10.1016/j.bbmt.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 128.Gratwohl A, Brand R, Apperley J. Allogeneic hematopoietic stem cell transplantation for chronic myeloid leukemia in Europe 2006: transplant activity, long-term data and current results. An analysis by the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT) Haematologica. 2006;91:513–521. [PubMed] [Google Scholar]
- 129.Saussele S, Lauseker M, Gratwohl A, et al. Allogeneic hematopoietic stem cell transplantation (allo SCT) for chronic myeloid leukemia in the imatinib era: evaluation of its impact within a subgroup of the randomized German CML Study IV. Blood. 2010;115:1880–1885. doi: 10.1182/blood-2009-08-237115. [DOI] [PubMed] [Google Scholar]
- 130.Goldman JM, Majhail NS, Klein JP, et al. Relapse and late mortality in 5-year survivors of myeloablative allogeneic hematopoietic cell transplantation for chronic myeloid leukemia in first chronic phase. J Clin Oncol. 2010;28:1888–1895. doi: 10.1200/JCO.2009.26.7757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Beelen DW, Graeven U, Elmaagacli AH, et al. Prolonged administration of interferon-alpha in patients with chronic-phase Philadelphia chromosome-positive chronic myelogenous leukemia before allogeneic bone marrow transplantation may adversely affect transplant outcome. Blood. 1995;85:2981–2990. [PubMed] [Google Scholar]
- 132.Goldman JM, Szydlo R, Horowitz MM, et al. Choice of pretransplant treatment and timing of transplants for chronic myelogenous leukemia in chronic phase. Blood. 1993;82:2235–2238. [PubMed] [Google Scholar]
- 133.Morton AJ, Gooley T, Hansen JA, et al. Association between pretransplant interferon-alpha and outcome after unrelated donor marrow transplantation for chronic myelogenous leukemia in chronic phase. Blood. 1998;92:394–401. [PubMed] [Google Scholar]
- 134.Anderlini P, Sheth S, Hicks K, et al. Re: imatinib mesylate administration in the first 100 days after stem cell transplantation. Biol Blood Marrow Transplant. 2004;10:883–884. doi: 10.1016/j.bbmt.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 135.Deininger M, Schleuning M, Greinix H, et al. The effect of prior exposure to imatinib on transplant-related mortality. Haematologica. 2006;91:452–459. [PubMed] [Google Scholar]
- 136.Lee SJ, Kukreja M, Wang T, et al. Impact of prior imatinib mesylate on the outcome of hematopoietic cell transplantation for chronic myeloid leukemia. Blood. 2008;112:3500–3507. doi: 10.1182/blood-2008-02-141689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Oehler VG, Gooley T, Snyder DS, et al. The effects of imatinib mesylate treatment before allogeneic transplantation for chronic myeloid leukemia. Blood. 2007;109:1782–1789. doi: 10.1182/blood-2006-06-031682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nicolini F, Modolo L, Raus N, et al. Allogeneic stem cell transplantation for blast crisis (BC) chronic myelogenous leukemia (CML) in the tyrosine kinase inhibitors (TKIs) era. On behalf of the Societe Francaise De Greffe De Moelle Et De Therapie Cellulaire and the French Group of CML [abstract] Blood. 2010;116 Abstract 2266. [Google Scholar]
- 139.Breccia M, Palandri F, Iori AP, et al. Second-generation tyrosine kinase inhibitors before allogeneic stem cell transplantation in patients with chronic myeloid leukemia resistant to imatinib. Leuk Res. 2010;34:143–147. doi: 10.1016/j.leukres.2009.04.036. [DOI] [PubMed] [Google Scholar]
- 140.Jabbour E, Cortes J, Kantarjian H, et al. Novel tyrosine kinase inhibitor therapy before allogeneic stem cell transplantation in patients with chronic myeloid leukemia: no evidence for increased transplant-related toxicity. Cancer. 2007;110:340–344. doi: 10.1002/cncr.22778. [DOI] [PubMed] [Google Scholar]
- 141.Shimoni A, Leiba M, Schleuning M, et al. Prior treatment with the tyrosine kinase inhibitors dasatinib and nilotinib allows stem cell transplantation (SCT) in a less advanced disease phase and does not increase SCT toxicity in patients with chronic myelogenous leukemia and Philadelphia positive acute lymphoblastic leukemia. Leukemia. 2009;23:190–194. doi: 10.1038/leu.2008.160. [DOI] [PubMed] [Google Scholar]
- 142.Hehlmann R, Berger U, Pfirrmann M, et al. Drug treatment is superior to allografting as first-line therapy in chronic myeloid leukemia. Blood. 2007;109:4686–4692. doi: 10.1182/blood-2006-11-055186. [DOI] [PubMed] [Google Scholar]
- 143.Velev N, Cortes J, Champlin R, et al. Stem cell transplantation for patients with chronic myeloid leukemia resistant to tyrosine kinase inhibitors with BCR-ABL kinase domain mutation T315I. Cancer. 2010;116:3631–3637. doi: 10.1002/cncr.25092. [DOI] [PubMed] [Google Scholar]
- 144.Tam CS, Kantarjian H, Garcia-Manero G, et al. Failure to achieve a major cytogenetic response by 12 months defines inadequate response in patients receiving nilotinib or dasatinib as second or subsequent line therapy for chronic myeloid leukemia. Blood. 2008;112:516–518. doi: 10.1182/blood-2008-02-141580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Milojkovic D, Nicholson E, Apperley JF, et al. Early prediction of success or failure of treatment with second-generation tyrosine kinase inhibitors in patients with chronic myeloid leukemia. Haematologica. 2010;95:224–231. doi: 10.3324/haematol.2009.012781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sokal J, Cox E, Baccarani M, et al. Prognostic discrimination in “good-risk” chronic granulocytic leukemia. Blood. 1984;63:789–799. [PubMed] [Google Scholar]
- 147.Hasford J, Pfirrmann M, Hehlmann R, et al. A new prognostic score for survival of patients with chronic myeloid leukemia treated with interferon alfa. Writing Committee for the Collaborative CML Prognostic Factors Project Group. J Natl Cancer Inst. 1998;90:850–858. doi: 10.1093/jnci/90.11.850. [DOI] [PubMed] [Google Scholar]