Abstract
Older patients with Burkitt lymphoma/leukemia (BL) have inferior outcomes. Because cyclophosphamide is highly active in BL and can be dose-escalated without stem-cell rescue, we designed a short, cyclophosphamide-intensive regimen without anthracyclines for patients aged ≥30 with untreated, non-HIV-associated BL/atypical BL. Two cycles involving cyclophosphamide 1500 mg/m2, vincristine, rituximab, prednisone, methotrexate 3 g/m2, and intrathecal cytarabine were delivered 2 weeks apart, followed by intensification with high-dose cyclophosphamide (50 mg/kg/day for 4 days) and rituximab. Of 21 patients, median age 53 (range, 34–75), 71% had stage IV, 95% were high-risk and 29% had performance status 3–4. Response occurred in all evaluable patients post-cycle 2 and in 76% post-intensification. Five non-relapse deaths occurred (four before intensification). The estimated 1-year and 3-year event-free survival was 52%; 1-year and 3-year overall survival was 57%. Seventeen (81%) received intensification (median 30 days to intensification). Brief, anthracycline-sparing, intensive cyclophosphamide (BASIC) therapy yields durable remissions in poorer-risk BL/atypical BL.
Keywords: Atypical Burkitt lymphoma, Burkitt lymphoma, cyclophosphamide, unclassifiable B-cell lymphoma
Introduction
Burkitt lymphoma/leukemia (BL) and B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and BL (atypical BL) are rare, highly aggressive neoplasms that are potentially curable with intensive, multiagent systemic and central nervous system (CNS) directed therapies. Regimens for sporadic or atypical BL cure most pediatric and young adult patients, but older individuals are underrepresented in this literature [1–4]. Moreover, older individuals tend to have inferior outcomes attributed in part to toxicities and resulting delays in scheduled therapy [3–6]. Age-related variations in disease biology, while incompletely defined, also likely contribute to the outcome differences seen in children and very young adults, as compared to older individuals, treated with the same or similarly intensive regimens [1,4]. In an LMB (Mature B-cell Lymphoma) study for adult BL, even age ≥ 33 was associated with shorter survival [5]. Thus, more effective and tolerable regimens are needed for older individuals with these diseases.
Cyclophosphamide is among the most active agents in BL where, remarkably, it can produce durable remissions as a single agent [7,8]. In transplant and in a variety of other settings, it has been repeatedly demonstrated that cyclophosphamide can be safely dose-escalated, and that even at the highest tolerable doses, hematopoiesis rapidly and predictably reconstitutes [9–13]. In contrast to cyclophosphamide, the importance of anthracyclines in BL has never been clearly established, and particularly in children, excellent results have been reported with intensive regimens without anthracyclines [14,15]. We hypothesized that cyclophosphamide intensification in BL or atypical BL might reduce the need for other cytotoxic agents and shorten treatment duration, thus potentially improving safety without compromising cure rates in older patients. We report the results of a pilot trial of a new approach to BL or atypical BL in older adults that involves brief, anthracycline-sparing, intensive cyclophosphamide (BASIC) therapy.
Materials and methods
Patients
An investigator-initiated, single-arm, pilot phase 2 trial was conducted at Johns Hopkins University (21 patients) and Hahnemann University (two patients) from 2005 to 2010 (http://www.clinicaltrials.gov, NCT00133991). The individual institutional review boards approved the study, and all participants gave written informed consent.
Entry requirements consisted of patients aged ≥ 30 years with newly diagnosed classic BL or unclassifiable B-cell lymphoma/leukemia with features intermediate between diffuse large B-cell lymphoma and BL per World Health Organization criteria (previously termed atypical Burkitt or Burkitt-like lymphoma) [16]. Patients with human immunodeficiency virus (HIV) infection, known significant cardiac dysfunction or irreversible renal dysfunction were excluded; acute renal failure, even if necessitating dialysis, was permitted. Treatment could begin while awaiting HIV testing. Prior treatment was limited to steroids, two doses of intrathecal (i.t.) chemotherapy and surgical resection. No restrictions were placed on performance status or disease extent.
BASIC treatment plan
Cycles 1 and 2 each consisted of cyclophosphamide, vincristine, prednisone and rituximab with CNS prophylaxis or treatment (Table I). The cycle length was 14 days, contingent upon absolute neutrophil count (ANC) recovery to ≥ 500/μL and completion of leucovorin rescue. The specified regimen was cyclophosphamide 1500 mg/m2 intravenously (i.v.) day 1; vincristine 1.4 mg/m2 (2 mg cap) i.v. day 1; prednisone 100 mg by mouth days 1–5; rituximab 375 mg/m2 i.v. days 1 and 8; methotrexate 3 g/m2 i.v. day 8 with leucovorin rescue; cytarabine 100 mg intrathecally (i.t.) on days 1, 4, 8 and 11; and filgrastim. To prevent delay in cytotoxic chemotherapy, cycle 1, day 1 rituximab was given after completion of cyclophosphamide and vincristine. To reduce acute toxicity, the protocol was amended to permit delay or omission of the first rituximab. In cases of significant lymphocytosis, rituximab could also be administered in divided doses. Tumor lysis syndrome prophylaxis followed standard practice, including aggressive hydration, serial laboratory monitoring, allopurinol and/or rasburicase and phosphate binders.
Table I.
BASIC therapy schema.
| Drug* | Dose | Time |
|---|---|---|
| Cycles 1 and 2 | ||
| Cyclophosphamide | 1500 mg/m2/day i.v. over 1 h | Day 1 |
| Mesna† | 900 mg/m2/day i.v. in divided doses | Day 1 |
| Vincristine‡ | 1.4 mg/m2/day i.v. push (2 mg cap) | Day 1 |
| Prednisone§ | 100 mg/day p.o. | Days 1–5 |
| Rituximab¶ | 375 mg/m2/day i.v. | Days 1 and 8 |
| Filgrastim | 5 µg/kg/day s.c. | Day 3, until post-nadir ANC ≥ 500/μL |
| Methotrexate** | 3 g/m2 i.v. over 2 h | Day 8 |
| Leucovorin†† | 25 mg/m2 i.v. every 6 h | 24 h after start of MTX, until clearance |
| Cytarabine‡‡§§ | 100 mg i.t. ± hydrocortisone 50 mg i.t. | Days 1, 4, 11; day 8 if i.v. MTX omitted |
| Cyclophosphamide intensification | ||
| Rituximab | 375 mg/m2/day i.v. | Day 1 |
| Cyclophosphamide¶¶ | 50 mg/kg/day i.v. over 1 – 2 h | Days 2–5 |
| Mesna¶¶ | 40 mg/kg/day i.v. in divided doses | Days 2–5 |
| Filgrastim*** | 5 μg/kg/day s.c. | Day 11, until post-nadir ANC ≥ 1000/μL |
| Rituximab | 375 mg/m2/day i.v. | Weekly for 4 doses, when ANC ≥ 1000/μL |
| Cytarabine††† | 100 mg i.t. ± hydrocortisone 50 mg i.t. | Maintenance regimen, if prior CNS disease |
ANC, absolute neutrophil count; BASIC, brief, anthracycline-sparing, intensive cyclophosphamide; CNS, central nervous system; i.t., intrathecal; i.v., intravenous; MTX, methotrexate; p.o., by mouth; s.c, subcutaneous.
Commence cycle 2 on day 15 and commence cyclophosphamide intensification on cycle 2, day 15, provided ANC ≥ 500/μL, leucovorin rescue completed, and filgrastim last given ≥ 24 h prior.
Or hydration with forced diuresis.
Vincristine omitted if direct bilirubin ≥5.0 mg/dL; 50% reduction (1 mg cap) suggested if direct bilirubin 3.0 – 4.9 mg/dL.
Or dexamethasone i.v. equivalent.
Cycle 1, day 1 rituximab given after cyclophosphamide, vincristine and prednisone. Subsequent doses of rituximab preceded cytotoxic chemotherapy. First rituximab could be given in divided doses if the circulating lymphocyte count was high, delayed until day 4, 5 or 6 of cycle 1, or omitted.
MTX omitted if estimated creatinine clearance < 50 mL/min. Dose reduction or omission for other reasons (e.g. hyperbilirubinemia, effusion) permitted.
Leucovorin dose titrated according to MTX level. Leucovorin could be given orally when MTX level < 0.2 μM/L and discontinued when <0.05μM/L.
Omission of cycle 1, day 1 i.t. cytarabine permitted if no apparent CNS disease. Time points for i.t. treatment are ± 1 day, separated by ≥ 48 h.
In patients with CNS involvement, twice-weekly i.t. cytarabine was to continue after day 11 if there were treatment delays.
High-dose cyclophosphamide with mesna dosed according to lesser of ideal and actual body weight, and administered with aggressive hydration; other chemotherapy dosed according to actual weight.
Or pegfilgrastim 6 mg s.c. once.
Suggested maintenance after CNS clearance: once weekly for four doses, then every other week for four doses.
Intensification with cyclophosphamide was due on day 15 of cycle 2. This consisted of rituximab day 1; high-dose cyclophosphamide (50 mg/kg i.v. daily, days 2–5) without stem-cell rescue; filgrastim; and, when the ANC was ≥ 1000/μL, once-weekly rituximab for 4 weeks (Table I). High-dose cyclophosphamide could be given in a specialized outpatient setting.
Eligibility criteria for cyclophosphamide intensification consisted of absence of disease progression; Eastern Cooperative Oncology Group (ECOG) performance status < 4; ANC≥500/μL; last filgrastim ≥ 24 h beforehand; completion of leucovorin rescue; cardiac ejection fraction ≥ 40%; not on dialysis; and transaminases ≤ 5 times the upper limit of normal.
The same regimen of intrathecal chemotherapy and high-dose methotrexate was utilized for prophylaxis and treatment of CNS disease. In addition, patients with CNS involvement were to continue twice-weekly i.t. cytarabine during treatment delays and to receive maintenance i.t. therapy after CNS clearance (per Table I). Radiation therapy was permitted.
Antibiotic prophylaxis during cycles 1 and 2 included ciprofloxacin from day 8 and Pneumocystis jiroveci prophylaxis. Prophylaxis during intensification included fluconazole, norfloxacin or ciprofloxacin, and Pneumocystis jiroveci and herpesvirus prophylaxis.
Evaluations
Diagnostic tissue was centrally reviewed by a Johns Hopkins hematopathologist in all cases. The presence of a MYC-immunoglobulin gene (MYC-IG) translocation was determined by conventional cytogenetics or interphase fluorescence in situ hybridization (FISH). FISH analysis for t(8;14) in most cases included probes for a MYC breakaway (8q24.1;var) and t(8;22). BCL2 expression was assessed by immunohistochemistry. FISH for t(14;18) was not routinely performed. Per the Magrath criteria, low-risk disease was defined as a single extraabdominal mass or completely resected abdominal disease and a normal lactate dehydrogenase; all other presentations were considered high-risk [2].
Toxicities were graded by National Cancer Institute Common Terminology Criteria, version 3.0 [17], with censoring upon relapse. To identify patients with relapsed or progressive disease, disease status was assessed before cyclophosphamide intensification by computed tomography (CT) and by bone marrow biopsy and lumbar puncture if previously abnormal. Formal response was assessed following high-dose cyclophosphamide using the 2007 International Working Group (IWG) criteria [18], with 18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET)/CT performed in 16/17 cases. Imaging was reviewed by one nuclear medicine specialist for reporting purposes.
Statistics
The primary objectives were to estimate response rate and 1-year event-free and overall survival, and to describe feasibility and safety. The study aimed to accrue ≥ 20 evaluable patients, based upon 82% power (two-sided alpha, 0.05) to detect an absolute 20% improvement in 1-year overall survival compared to an estimated historical rate from the literature of 50% [6,19]. A composite endpoint of death or failure to reach complete remission (CR) was utilized for early monitoring.
Event-free survival (EFS) was defined as time to progression or relapse, myelodysplasia or acute leukemia, death or last evaluation. Survival was estimated using the Kaplan-Meier method and compared by the log-rank test. Hazard ratios (HRs) were estimated using Cox proportional hazards models. Cumulative incidences of relapse and non-relapse mortality were estimated by competing-risk analysis using Gray's method [20], wherein non-relapse mortality was a competing risk for relapse and relapse was a competing risk for non-relapse mortality. A description of predictive factors was prespecified. Outcomes until 19 January 2012 are reported.
Results
Patient characteristics
Twenty-one evaluable patients, median age 53 (range, 34–75), were accrued (Table II). Two additional participants were withdrawn because HIV testing returned positive. This was a poor-risk cohort, with 52% being over age 50, 81% having extranodal disease (including 14% with known CNS disease), 95% being high-risk by the Magrath criteria, and 29% presenting with a performance status of 3 or 4. Two patients presented with spinal cord compression, three had early acute renal failure managed with hemodialysis, one had preexisting sepsis and hypoxemia, one had a recent acute coronary syndrome, and one had a diverting colostomy for bowel obstruction due to lymphoma.
Table II.
Patient characteristics.
| Variable | Result |
|---|---|
| Number evaluable | 21 |
| Patient age, years | |
| Median (range) | 53 (34–75) |
| 30–40 | 5 (24%) |
| 41–50 | 5 (24%) |
| 51–60 | 5 (24%) |
| >60 | 6 (29%) |
| Male sex | 14 (67%) |
| Ann Arbor stage, n | |
| I | 1 |
| IE | 2 |
| II | 2 |
| IIE | 1 |
| III | 0 |
| IV | 15 |
| B symptoms present | 6 (29%) |
| Histology | |
| Classic BL | 11 (52%) |
| Documented t(8;14) or t(8;22) | 6 |
| Other MYC translocation | 1 |
| MYC translocation absent | 4 |
| Atypical BL* | 10 (48%) |
| Documented t(8;14) or t(8;22) | 4 |
| Other MYC translocation | 1 |
| MYC translocation absent | 4 |
| BCL2 expression† | |
| Documented double-hit, n‡ | |
| Age-unadjusted IPI risk category | |
| Low | 5 (24%) |
| Low-intermediate | 2 (10%) |
| High-intermediate | 5 (24%) |
| High | 9 (43%) |
| High-risk by Magrath criteria | 20 (95%) |
| ECOG performance status, n | |
| 0 or 1 | 11 |
| 2 | 4 |
| 3 | 4 |
| 4 | 2 |
| Elevated LDH | 18 (86%) |
| Ratio, LDH:ULN | |
| Mean | 16 |
| Median (range) | 6.6 (0.6–148) |
| Extranodal sites | |
| 0 | 3 (14%) |
| 1 | 8 (38%) |
| 2 or more | 10 (48%) |
| Bone marrow biopsy positive | |
| Yes | 10 (48%)§ |
| < 20% involved | 1 |
| 20–90% | 2 |
| > 90% and/or nearly completely ef aced | 6 |
| Involved, degree not estimated | 1 |
| No | 11 (52%) |
| Circulating blasts or atypical lymphocytes | 8 (38%) |
| CNS involvement at presentation, n | |
| Yes | 3¶ |
| No or not identified | 16 |
| Unknown | 2 |
| Concurrent RT for CNS disease ** | 3 (14%) |
| RT to other sites** | 2 (10%) |
| Tumor-related renal insufficiency or failure | 5 (24%) |
| Epidural cord compression | 2 (10%) |
BL, Burkitt lymphoma/leukemia; CNS, central nervous system; ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; RT, radiation therapy; ULN, upper limit of normal.
Includes two cases in which classic BL could not be excluded, and which were therefore excluded from comparison of outcomes by histology.
Assessed by immunohistochemistry. MYC translocation testing and BCL2 immunohistochemistry not performed in one case. One case without detected MYC translocation had three MYC signals on FISH.
Defined by a MYC/8q24 translocation combined with another recurrent breakpoint.
Five other patients had stage IV disease based on extranodal involvement other than bone marrow.
All leptomeningeal.
Excluding RT for relapsed or progressive disease
Efficacy
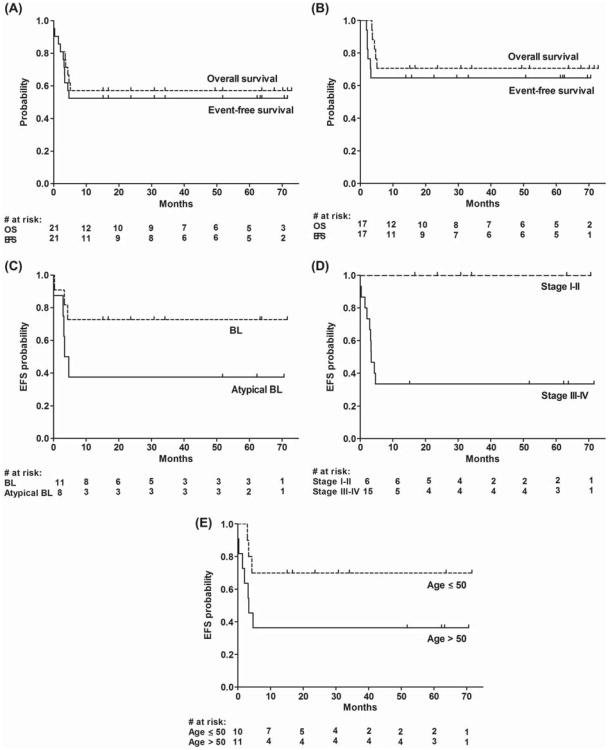
With a median follow-up of 52 months (range, 15–72 months) in patients without events, 12/21 patients are alive, 11 without relapse. For all eligible patients, the actuarial 1-year and 3-year EFS was 52% (95% confidence interval [CI], 35–79%) after treatment initiation [Figure 1(A)]. The estimated 1-year and 3-year overall survival was 57% (95% CI, 40–83%) [Figure 1(A)]. At 6 months and beyond, the cumulative incidences of relapse and non-relapse mortality were each 24%, with all events occurring < 5 months after treatment initiation.
Figure 1.
Survival outcomes. (A) Event-free survival (EFS) and overall survival of all evaluable patients after treatment initiation, and (B) of patients after high-dose cyclophosphamide. (C) EFS after treatment initiation according to diagnosis, (D) stage and (E) age.
After two cycles, 17 patients were evaluable for early response. Of these, eight (47%) had CR, eight (47%) had CR unconfirmed (CRu), one (6%) had partial remission (PR), and none had stable, relapsed or progressive disease by CT-based criteria [21]. The other patients were inevaluable because of death (n = 3) or insufficient imaging (n = 1).
Four patients (age range, 59–75) did not receive cyclophosphamide intensification due to early non-relapse death or comorbidities. Seventeen of 21 patients (81%) received and completed cyclophosphamide intensification (median age 49, range 38–65), of whom 11 (65%) had CR, two (12%) had PR and four (24%) had relapsed or progressive disease on post-treatment assessment by the 2007 IWG criteria [18]. Following high-dose cyclophosphamide, the estimated 1-year and 3-year EFS was 65% (95% CI, 46–92%) and the estimated 1-year and 3-year overall survival was 71% (95% CI, 52–96%) [Figure 1(B)].
Of the 11 patients with CR after cyclophosphamide intensification, 10 remain alive in continued remission 14–71 months (median, 56 months) after high-dose cyclophosphamide. Of the two patients in PR after cyclophosphamide intensification, one remains in remission after consolidative radiation therapy and the other, whose disease rapidly progressed, is in CR 4 years after myeloablative, matched sibling donor transplant. The remaining four patients with relapsed or progressive disease after high-dose cyclophosphamide (all of whom had achieved CR or CRu by CT-based criteria prior to intensification) died from lymphoma, one despite myeloablative, haploidentical transplant.
Descriptive analyses
For descriptive purposes, outcomes according to presenting features are shown in Figures 1(C)–1(E). On unadjusted analysis, patients with classic BL had a tendency toward better EFS than those with atypical BL [estimated EFS at 1 year and beyond, 73% vs. 38%, p = 0.13; Figure 1(C)] and tended to be younger (median age 42 vs. 56; p = 0.009 by Wilcoxon's test). The estimated 1-year and longer-term EFS of the six patients with stage I–II disease, all having an International Prognostic Index of ≤ 2, was 100%, vs. 33% in advanced-stage patients [p = 0.01; Figure 1(D)]. Compared to patients aged ≤ 50, patients aged > 50 had a tendency toward an inferior EFS (36% vs. 70% at 1 year and beyond for age > 50 and ≤ 50, respectively, p = 0.10; HR 3.0, 95% CI, 0.8–11.5) and overall survival (36% vs. 80%, p = 0.04; HR 4.4, 95% CI, 0.9–21.3) on unadjusted analysis [Figure 1(E)]. Of the six patients aged > 60, four had non-relapse deaths and two are alive with durable CRs.
Of the five patients who relapsed or progressed, all had stage IV disease, including three who presented with known CNS involvement (Table III). Of the two with a documented “double-hit” lymphoma [2,22], one (with MYC and BCL6 translocations) had primary progressive disease after cyclophosphamide intensification, and one (with MYC and BCL2 translocations) remains in CR at 52 months. An additional patient with atypical BL having t(8;14) and BCL2 expression, but in whom t(14;18) was inevaluable by FISH, remains in CR at 62 months.
Table III.
Treatment failures.
| Age | Type of failure | TTF | CNS + at diagnosis | MTX given | RT for CNS disease |
|---|---|---|---|---|---|
| 50 | Relapse (systemic, CNS*) | 2.9 months | NE | 0 | 0 |
| 54 | Relapse (systemic, CNS*) | 3.2 months | 1† | 0 | 1 |
| 42 | Relapse (systemic) | 3.3 months | 1† | 1 | 1 |
| 65 | Relapse (systemic) | 3.4 months | 1† | 0 | 1 |
| 49 | Relapse (systemic) | 4.3 months | 0 | 1 | 0 |
| 59 | NRM (tumor lysis, cycle 1) | 1 day | NE | — | 0 |
| 75 | NRM (sepsis, cycle 1) | 8 days | 0 | — | 0 |
| 69 | NRM (multiorgan failure, cycle 1) | 1.4 months | 0 | 1 | 0 |
| 63 | NRM (comorbidities, cycle 2) | 2.1 months | 0 | 0 | 0 |
| 65 | NRM (late sepsis after high-dose Cy) | 4.7 months | 0 | 1 | 0 |
1, yes, 0, no; BL, Burkitt lymphoma/leukemia; CNS, central nervous system; Cy, cyclophosphamide; MTX, high-dose methotrexate; NE, not evaluable; NRM, non-relapse mortality; RT, radiation therapy (prior to relapse); TTF, time to treatment failure, measured after treatment initiation.
Leptomeningeal and parenchymal.
Leptomeningeal.
Toxicity
The median time between start of therapy and cyclophosphamide intensification was 30 days (range, 28–40).
There were five treatment-related deaths, of which four occurred in cycle 1 or 2 (Table III). Three patients died during cycle 1: a 59-year-old from acute tumor lysis syndrome on day 1, in the face of overwhelming disease volume; a 75-year-old from neutropenic sepsis on day 8 (having sepsis and pneumonia on initial presentation); and a 69-year-old from multiorgan failure with fungemia on day 46, preceded by respiratory arrest of unclear etiology on day 20 and protracted methotrexate toxicity. One additional patient, age 63, did not receive high-dose cyclophosphamide because of comorbidities during cycles 1–2, including delirium and pneumonia preceded by grade 4 neutropenic sepsis, and died in hospice.
Six patients (29%) either presented with or developed acute tumor lysis syndrome upon treatment initiation. Three of these received hemodialysis. Effusions, renal failure, hyperbilirubinemia and concurrent brain radiation led to omission of high-dose methotrexate in 9/37 (24%) cycles. The first rituximab dose was omitted in one case and given in divided doses in four cases.
The median time between cycles 1 and 2 was 14 days (range, 14–21). Likewise, the median time between cycle 2 and cyclophosphamide intensification was 14 days (range, 14 – 22). Grade 4 neutropenia (ANC < 500/μL) occurred in 17/20 evaluable patients (85%) during cycle 1 and in 14/18 (78%) during cycle 2. In cycles 1 and 2, a total of nine documented episodes of grade 3 infection (seven neutropenic) and two episodes of grade 4 infection (both neutropenic) occurred, all of which resolved.
Hematopoietic recovery after completion of high-dose cyclophosphamide was brisk and universal. The median time to a sustained ANC ≥ 500/μL was 16 days (range, 10–21). The median time to a sustained platelet count ≥ 20 000/μL, without transfusion in the preceding week, was 22 days (range, 0–31). Most patients required red blood cell and platelet transfusions.
During or immediately following cyclophosphamide intensification, there were no life-threatening infections save for one associated with late-onset neutropenia as described below. All nine episodes of grade 3 infection (five neutropenic) resolved, including a case of varicella zoster virus meningitis 3.6 months after high-dose cyclophosphamide. Of the 11 patients who received outpatient high-dose cyclophosphamide, six had hospitalizations, most commonly for infection.
Mucositis was less than grade 3. Other toxicities included grade ≥ 3 sensory and/or motor neurotoxicity in five patients: four with grade 3 neurotoxicity, and one with grade 4 ischemic stroke (having a history of stroke) preceded by a grade 3 axonal and demyelinating polyneuropathy. Additionally, there was a case of self-limited grade 3 pericarditis attributable to cyclophosphamide and a case of grade 3 anasarca with contrast nephropathy.
Following neutrophil recovery, seven episodes of grade ≥ 3 neutropenia (ANC < 1000/μL) without apparent cause (such as infection) were documented in 6/17 patients (35%) at a median of 7.2 months (range, 2.6–11.8) after high-dose cyclophosphamide completion. This includes a case of fatal, late neutropenic sepsis (2.6 months after cyclophosphamide completion) in a 65-year-old debilitated by a stroke. The other episodes carried no major clinical consequences. The frequency of late-onset neutropenia (attributable in part to rituximab) [23] may be underestimated, as this often occurred beyond the period of intensive laboratory monitoring. No treatment-related myelodysplastic syndrome or acute leukemia has been diagnosed.
Discussion
High CR rates have been achieved in older adults with BL or atypical BL with a number of intensive approaches [1–3,24]. However, relapse and toxicity remain as significant problems in older patients [1,3,6]. Modifications of standard intensive BL regimens have been suggested to overcome often prohibitive toxicities [1,24,25]. In this older patient cohort (30% were over age 60), including patients with poor performance status (29% had a performance status of 3 or 4) and substantial comorbidities, this very intensive but brief regimen was associated with a 57% overall survival at 1 year. The outcomes are encouraging given the poor-risk nature of this cohort. The entry criteria were broad, with very few restrictions on organ dysfunction and no restrictions placed on performance status, maximum age, disease extent including CNS disease, or concurrent infection. These patients therefore seem representative of the patients actually encountered in practice. Nevertheless, the planned dose intensity was achievable, and 81% of eligible patients were able to complete treatment.
While there is a general consensus that older patients with BL have worse outcomes, there is no universally agreed-upon definition of an “older” patient with BL [4]. In a study of hyperCVAD (hyperfractionated cyclophosphamide, vincristine, cytarabine, doxorubicin), the estimated 3-year overall survival was 77% for patients aged < 60 and 17% for those aged ≥ 60 [19]. In a Cancer and Leukemia Group B trial, patients aged ≥ 50 were more than twice as likely to not complete therapy and had greater incidences of disease progression, toxicity and death [6]. The tendency toward worse outcomes with greater age may reflect tumor biology, reduced ability to tolerate toxicities and/or altered drug metabolism. The patients in our study with atypical BL tended to have worse outcomes than those with classic BL, potentially due to their significantly older age, worse biology, or the relative importance of anthracyclines in atypical as compared with classic BL.
Cyclophosphamide spares the early hematopoietic stem cells responsible for bone marrow reconstitution, and thus at high doses (50 mg/kg/day for 4 days) is lymphoablative but not fully myeloablative [12,13]. Phase 2 studies have demonstrated that transplant doses of cyclophosphamide can be administered without the need for stem-cell rescue, eliminating the problem of tumor reseeding from a contaminated autograft [9–11]. Moreover, when used as a single agent in lymphomas and autoimmune disorders, high-dose cyclophosphamide was associated with few serious side effects other than brief, transient cytopenias and a very low mortality [9–11]. In the present study, four out of five treatment-related deaths occurred during the conventional-dose therapy, prior to high-dose cyclophosphamide, and the one death afterward was associated with late-onset neutropenia and thus possibly related to rituximab [23]. Thus, the duration of aplasia (similar to autologous transplant) and low treatment-related mortality after high-dose cyclophos phamide was in keeping with previous experiences. Because of its toxicity profile, treatment intensification with high-dose cyclophosphamide can be pursued safely in older patients and importantly does not preclude salvage autologous or allogeneic transplant.
Few prospective studies of rituximab-containing regimens for BL have been published [3,26–30]. These studies suggest that it is feasible to integrate rituximab safely both in the non-HIV and HIV settings, including in the CODOX-M/IVAC (cyclophosphamide, vincristine, doxorubicin, methotrexate, ifosfamide, etoposide, cytarabine) regimen [26,28]. Rituximab has single-agent activity in BL [29], in which some [3,26] but not all [31] retrospective comparisons suggest it reduces relapse. However, rituximab may also complicate the initial management of high-grade disease. Given the risk of first-dose reactions in patients who may be clinically and metabolically unstable, this protocol mandated that the first rituximab dose be given after cyclophosphamide and vincristine.
In this study, five of 11 patients aged > 50 had a non-relapse death, and all five non-relapse deaths occurred in patients aged ≥ 59. Because the entry criteria were very liberal, such that a number of participants might not have ordinarily been considered clinical trial candidates, differentiating toxicities of this investigational regimen from general chemotherapeutic toxicities can be difficult. At least two of the four deaths that occurred during conventional-dose therapy (acute tumor lysis syndrome; neutropenic sepsis) may have occurred regardless of the specific regimen. Nevertheless, the toxicity of this regimen may be problematic in older patients with a marginal performance status or serious comorbidities, and continued efforts to reduce toxicity in such patients would be important. For example, some regimens for high-grade malignancies incorporate a “prephase,” such as fractionated cyclophosphamide and steroids, with the intent of reducing the severity of tumor lysis [6,7]. It is possible that a prephase or divided dosing of the cycle 1 cyclophosphamide could reduce early morbidity in patients with high disease burden or marginal clinical status.
In BL and atypical BL, neurotoxicity concerns have prompted modifications such as reductions in vincristine and in the intensity of CNS prophylaxis [1,24,25]. High-dose methotrexate dosed at 3 g/m2, as done in the BASIC regimen, appears to be less toxic than more aggressive dosing though similarly effective [25]. Additional approaches to target sanctuary sites of disease (e.g. other systemic agents that penetrate the blood–brain barrier, radiation therapy) may enhance efficacy, but may also cause neurotoxicity.
How the BASIC regimen compares to commonly used regimens for BL or atypical BL [2,3,24] is unknown. The study did not detect a 20% improvement in 1-year overall survival compared to the estimated historical rate, with its 95% CI ranging from 40 to 83%. Such comparisons are limited in part by sample size, coupled with differences in published studies with respect to organ dysfunction, comorbidities, performance status and disease extent.
In conclusion, we report encouraging outcomes from this pilot initiative for poorer-risk patients with non-HIV-associated BL or atypical BL. The BASIC platform, relying heavily on cyclophosphamide and including CNS prophylaxis, produces durable remissions with acceptable toxicities, while shortening the treatment duration. Continued efforts to improve outcomes, including treatment toxicities, are needed especially in poorer-risk patients. Given the efficacy and short duration of the BASIC regimen, comparative studies and building upon this platform would be of interest.
Acknowledgments
This work was supported by the National Institutes of Health (K23 CA124465 to Y.L.K., P01 CA015396 to R.J.J.); the National Cancer Institute Lymphoma SPORE (P50 CA09688 to R.F.A.); and philanthropic support of research (to L.J.S.).
Footnotes
Presented in part at the 2009 American Society of Hematology annual meeting.
Potential conflict of interest: Disclosure forms provided by the authors are available with the full text of this article at http://www.informahealthcare.com/lal.
References
- 1.Mead GM, Sydes MR, Walewski J, et al. An international evaluation of CODOX-M and CODOX-M alternating with IVAC in adult Burkitt's lymphoma: results of United Kingdom Lymphoma Group LY06 study. Ann Oncol. 2002;13:1264–1274. doi: 10.1093/annonc/mdf253. [DOI] [PubMed] [Google Scholar]
- 2.Magrath I, Adde M, Shad A, et al. Adults and children with small non-cleaved-cell lymphoma have a similar excellent outcome when treated with the same chemotherapy regimen. J Clin Oncol. 1996;14:925–934. doi: 10.1200/JCO.1996.14.3.925. [DOI] [PubMed] [Google Scholar]
- 3.Thomas DA, Faderl S, O'Brien S, et al. Chemoimmunotherapy with hyper-CVAD plus rituximab for the treatment of adult Burkitt and Burkitt-type lymphoma or acute lymphoblastic leukemia. Cancer. 2006;106:1569–1580. doi: 10.1002/cncr.21776. [DOI] [PubMed] [Google Scholar]
- 4.Kelly JL, Toothier SR, Ciminello L, et al. Outcomes of patients with Burkitt lymphoma older than age 40 treated with intensive chemotherapeutic regimens. Clin Lymphoma Myeloma. 2009;9:307–310. doi: 10.3816/CLM.2009.n.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Divine M, Casassus P, Koscielny S, et al. Burkitt lymphoma in adults: a prospective study of 72 patients treated with an adapted pediatric LMB protocol. Ann Oncol. 2005;16:1928–1935. doi: 10.1093/annonc/mdi403. [DOI] [PubMed] [Google Scholar]
- 6.Lee EJ, Petroni GR, Schiffer CA, et al. Brief-duration high-intensity chemotherapy for patients with small noncleaved-cell lymphoma or FAB L3 acute lymphocytic leukemia: results of Cancer and Leukemia Group B study 9251. J Clin Oncol. 2001;19:4014–4022. doi: 10.1200/JCO.2001.19.20.4014. [DOI] [PubMed] [Google Scholar]
- 7.Burkitt D. Long-term remissions following one and two-dose chemotherapy for African lymphoma. Cancer. 1967;20:756–759. doi: 10.1002/1097-0142(1967)20:5<756::aid-cncr2820200530>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 8.Traore F, Coze C, Atteby JJ, et al. Cyclophosphamide monotherapy in children with Burkitt lymphoma: a study from the French-African Pediatric Oncology Group (GFAOP) Pediatr Blood Cancer. 2011;56:70–76. doi: 10.1002/pbc.22746. [DOI] [PubMed] [Google Scholar]
- 9.Gladstone DE, Bolaños-Meade J, Huff CA, et al. High-dose cyclophosphamide and rituximab without stem cell transplant: a feasibility study for low grade B-cell, transformed and mantle cell lymphomas. Leuk Lymphoma. 2011;52:2076–2081. doi: 10.3109/10428194.2011.594191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brodsky RA. High-dose cyclophosphamide for autoimmunity and alloimmunity. Immunol Res. 2010;47:179–184. doi: 10.1007/s12026-009-8149-y. [DOI] [PubMed] [Google Scholar]
- 11.DeZern AE, Petri M, Drachman DB, et al. High-dose cyclophosphamide without stem cell rescue in 207 patients with aplastic anemia and other autoimmune diseases. Medicine (Baltimore) 2011;90:89–98. doi: 10.1097/MD.0b013e318210e685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brodsky RA, Jones RJ. Aplastic anaemia. Lancet. 2005;365:1647–1656. doi: 10.1016/S0140-6736(05)66515-4. [DOI] [PubMed] [Google Scholar]
- 13.Emadi A, Jones RJ, Brodsky RA. Cyclophosphamide and cancer: golden anniversary. Nat Rev Clin Oncol. 2009;6:638–647. doi: 10.1038/nrclinonc.2009.146. [DOI] [PubMed] [Google Scholar]
- 14.Anderson JR, Wilson JF, Jenkin DT, et al. Childhood non-Hodgkin's lymphoma. The results of a randomized therapeutic trial comparing a 4-drug regimen (COMP) with a 10-drug regimen (LSA2-L2) N Engl J Med. 1983;308:559–565. doi: 10.1056/NEJM198303103081003. [DOI] [PubMed] [Google Scholar]
- 15.Mazza JJ, Hines JD, Andersen JW, et al. Aggressive chemotherapy in the treatment of Burkitt's and non-Burkitt's undifferentiated lymphoma. Leuk Lymphoma. 1995;18:289–296. doi: 10.3109/10428199509059620. [DOI] [PubMed] [Google Scholar]
- 16.Swerdlow SH, Campo E, Harris NL, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. Vol. 2008 Lyon: IARC; [Google Scholar]
- 17.National Cancer Institute Common Terminology Criteria, version 3.0.2006. Available from: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf.
- 18.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 19.Tomas DA, Cortes J, O'Brien S, et al. Hyper-CVAD program in Burkitt's-type adult acute lymphoblastic leukemia. J Clin Oncol. 1999;17:2461–2470. doi: 10.1200/JCO.1999.17.8.2461. [DOI] [PubMed] [Google Scholar]
- 20.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 21.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244–1253. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 22.Li S, Lin P, Fayad LE, et al. B-cell lymphomas with MYC/8q24 rearrangements and IGH@BCL2/t(14;18)(q32;q21): an aggressive disease with heterogeneous histology, germinal center B-cell immunophenotype and poor outcome. Mod Pathol. 2012;25:145–156. doi: 10.1038/modpathol.2011.147. [DOI] [PubMed] [Google Scholar]
- 23.Nitta E, Izutsu K, Sato T, et al. A high incidence of late-onset neutropenia following rituximab-containing chemotherapy as a primary treatment of CD20-positive B-cell lymphoma: a single-institution study. Ann Oncol. 2007;18:364–369. doi: 10.1093/annonc/mdl393. [DOI] [PubMed] [Google Scholar]
- 24.Rizzieri DA, Johnson JL, Niedzwiecki D, et al. Intensive chemotherapy with and without cranial radiation for Burkitt leukemia and lymphoma: final results of Cancer and Leukemia Group B Study 9251. Cancer. 2004;100:1438–1448. doi: 10.1002/cncr.20143. [DOI] [PubMed] [Google Scholar]
- 25.LaCasce A, Howard O, Li S, et al. Modified Magrath regimens for adults with Burkitt and Burkitt-like lymphomas: preserved efficacy with decreased toxicity. Leuk Lymphoma. 2004;45:761–767. doi: 10.1080/1042819031000141301. [DOI] [PubMed] [Google Scholar]
- 26.Corazzelli G, Frigeri F, Russo F, et al. RD-CODOX-M/IVAC with rituximab and intrathecal liposomal cytarabine in adult Burkitt lymphoma and “unclassifiable” highly aggressive B-cell lymphoma. Br J Haematol. 2012;156:234–244. doi: 10.1111/j.1365-2141.2011.08947.x. [DOI] [PubMed] [Google Scholar]
- 27.Barta SK, Lee JY, Kaplan LD, et al. Pooled analysis of AIDS malignancy consortium trials evaluating rituximab plus CHOP or infusional EPOCH chemotherapy in HIV-associated non-Hodgkin lymphoma. Cancer. 2011 Dec 16; doi: 10.1002/cncr.26723. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noy A, Kaplan L, Lee J. Feasibility and toxicity of a modified dose intensive R-CODOX-M/IVAC for HIV-associated Burkitt and atypical Burkitt lymphoma (BL): preliminary results of a prospective multicenter phase II trial of the AIDS Malignancy Consortium (AMC) Blood. 2009;114(Suppl. 1) Abstract 3673. [Google Scholar]
- 29.Meinhardt A, Burkhardt B, Zimmermann M, et al. Phase II window study on rituximab in newly diagnosed pediatric mature B-cell non-Hodgkin's lymphoma and Burkitt leukemia. J Clin Oncol. 2010;28:3115–3121. doi: 10.1200/JCO.2009.26.6791. [DOI] [PubMed] [Google Scholar]
- 30.Rizzieri DA, Johnson JL, Byrd JC, et al. Efficacy and toxicity of rituximab and brief duration, high intensity chemotherapy with filgrastim support for Burkitt or Burkitt-like leukemia/lymphoma: Cancer and Leukemia Group B (CALGB) Study 10002. Blood. 2010;116(Suppl. 1) doi: 10.1111/bjh.12736. Abstract 858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barnes BA, LaCasce AS, Feng Y, et al. Rituximab added to CODOX-M/IVAC has no clear benefit compared to CODOX-M/IVAC alone in adult patients with Burkitt lymphoma. Blood. 2009;114(Suppl. 1) Abstract 1667. [Google Scholar]