Multiple myeloma (MM) is characterized by the accumulation of malignant plasma cells in the bone marrow (BM). The introduction of novel agents that include thalidomide, bortezomib (bz) and lenalidomide into the treatment of MM has significantly improved response, progression and survival rates.1 However, in spite of these improvements, MM is still regarded as incurable as the vast majority of patients will eventually relapse and die of therapy-refractory disease. One possible explanation for disease relapse is the persistence of distinct tumor populations resistant to current therapies. According to current models, cancer stem cells or tumor-initiating cells represent minor subpopulations of tumor cells that possess the capacity to recapitulate tumors in vivo upon transplantation as well as increased drug resistance.2 In previous studies, MM has been found to consist of a heterogeneous mixture of cells. CD138-positive (CD138+) plasma cells form the tumor bulk, but studies tracking tumor-specific immunoglobulin heavy chain rearrangements have also identified relatively rare CD138-negative (CD138−) clonotypic idiotype-positive (Id+) memory B cells.3 In human MM this minor population of CD138− has been found to be highly tumorigenic in immunodeficient NOD/Scid mice and relatively resistant to many drugs compared with CD138+ plasma cells. However, other studies have demonstrated that tumor cell engraftment and growth is restricted to CD138+ plasma cells in the SCID-Hu mouse model.4 Therefore, the expression of CD138 by tumorigenic MM cells remains controversial. It is possible that these disparate findings are because of the xenografting models used to study human MM. Therefore, we investigated the functional properties, including clonogenic growth, engraftment potential and drug resistance of CD138− and CD138+ tumor populations in a more physiological context by studying the syngeneic immunocompetent 5T33 MM mouse model of MM.
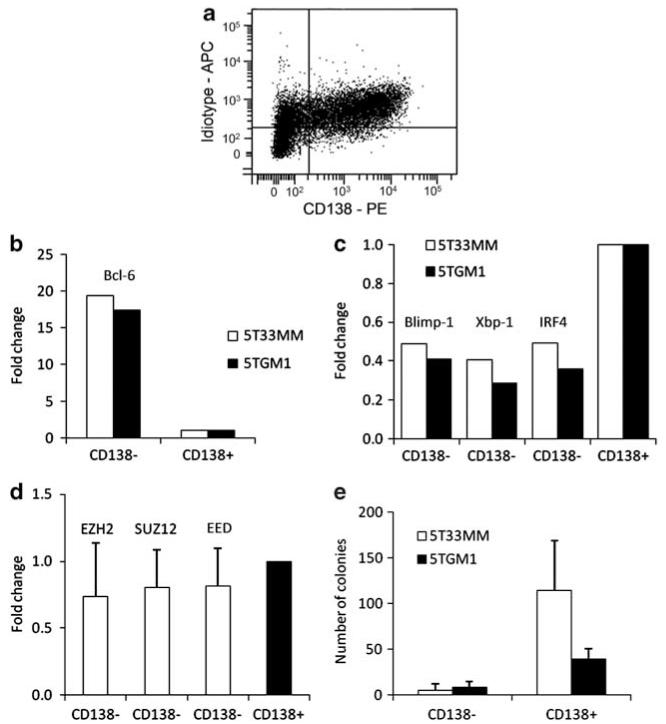
We have previously characterized the 5TMM model and found that it recapitulates the critical interactions between MM tumor cells and the BM microenvironment.5 5T33 MM cells were originally obtained from elderly C57Bl/KaLwRij mice that spontaneously developed MM and have been continuously propagated by serial syngeneic transplantation into young unaffected and immunocompetent mice. We isolated 5T33 MM cells from the BM of affected mice by flushing hind limb long bones and crushing vertebrae followed by the depletion of CD11b+ myeloid cells using magnetic-activated cell sorting. CD138 expression of purified 5T33 MM and 5TGM1 cells, an in vitro growing cell line derived from 5T33 MM cells,6 was analyzed by flow cytometry. Similar to previous reports of human MM, Id+ 5T33 MM cells heterogeneously express CD138 (Figure 1a). The majority of the cells in each line are CD138+, but small populations of 5T33 MM (6–15%, Figure 1a) and 5TGM1 (5–7%; data not shown) are CD138−. We sorted 5T33 MM cells by FACS into Id+ CD138+ and Id+ CD138− populations and found that both fractions express identical VH transcripts by RT-PCR confirming their clonal relationship (data not shown). The absence of CD138 may either reflect a less-differentiated state or shedding of CD138 from cell surface during apoptosis, therefore, we analyzed the differentiation status of CD138− and CD138+ cells by real-time Q-PCR for transcription factors involved in the differentiation of normal B cells to plasma cells. In both 5T33 MM and 5TGM1 cells the expression of B-cell lymphoma 6 characteristic of B cells was greater in CD138− cells than CD138+ cells whereas B-lymphocyte-induced maturation protein 1, X-box binding protein-1 and Interferon regulatory factor 4 characteristics of plasma cells were greater in CD138+ than in CD138− cells (Figure 1b and c). These findings are similar to the identification of a less-differentiated CD138− population in the human MM cell line RPMI8226 cell line after coculture with stromal cells.7 We previously identified a novel gene profile in 5T33 MM cells that overlaps with self-renewing embryonic stem cells and is epigenetically silenced by the Polycomb repressive complex 2 (PRC2).8 Activating mutations or overexpression of PRC2 components have been reported in hematopoietic tumors including CD138+ MM plasma cells.8,9 We examined the expression of active components of the PRC2 complex EZH2, SUZ12 and EED in CD138+ and CD138− 5T33 MM cells and detected similar expression of the PRC2 complex in each cell population (Figure 1d). Therefore, CD138− and CD138+ cells within 5T33 MM and 5TGM1 MM may express both distinct differentiation and similar self-renewal programs.
Figure 1.
(a) CD138 expression on 5T33 MM cells analyzed by flow cytometry. Purified 5T33 MM cells were analyzed by a membranic staining with PE labeled anti-CD138 and simultaneous staining with anti-5T33 MM idiotype antibodies, which were detected with rat anti-mouse IgG1-APC. Irrelevant isotype controls were used as control. Dot plot represents anti-idiotype and anti-CD138 staining of events gated on 7AAD- living cells. (b, c) B-cell lymphoma 6 (Bcl-6), B-lymphocyte-induced maturation protein 1 (Blimp-1), X-box binding protein-1 (Xbp-1) and Interferon regulatory factor 4 (IRF4) expression in 5T33 MM and 5TGM1 sorted CD138− cells compared with CD138+ cells. Real-time Q-PCR showing fold increase/decrease of mRNA levels in CD138− samples compared with CD138+ samples (equal to 1). To standardize the amount of sample RNA, we used an endogenous reference gene. Results of one independent sample of three are shown. Primer sequences are available upon request. (d) Expression of EZH2, SUZ12 and EED in the 5T33 MM CD138− cells compared with CD138+ cells. Real-time Q-PCR showing fold increase/decrease of mRNA levels in CD138− samples compared with CD138+ samples (equal to 1). To standardize the amount of sample RNA, we used an endogenous reference gene. Results shown are combined from two independent samples repeated twice. Primer sequences are available upon request. (e) Clonogenicity of CD138− and CD138+ populations in 5T33 MM model. Sorted populations were plated in methylcellulose medium at a concentration of 5×104/ml. After 10 days incubation at 37 °C and 5% CO2, the number of colonies was counted. The mean value±s.d. of two (5T33 MM) and three (5TGM1) independent experiments is shown.
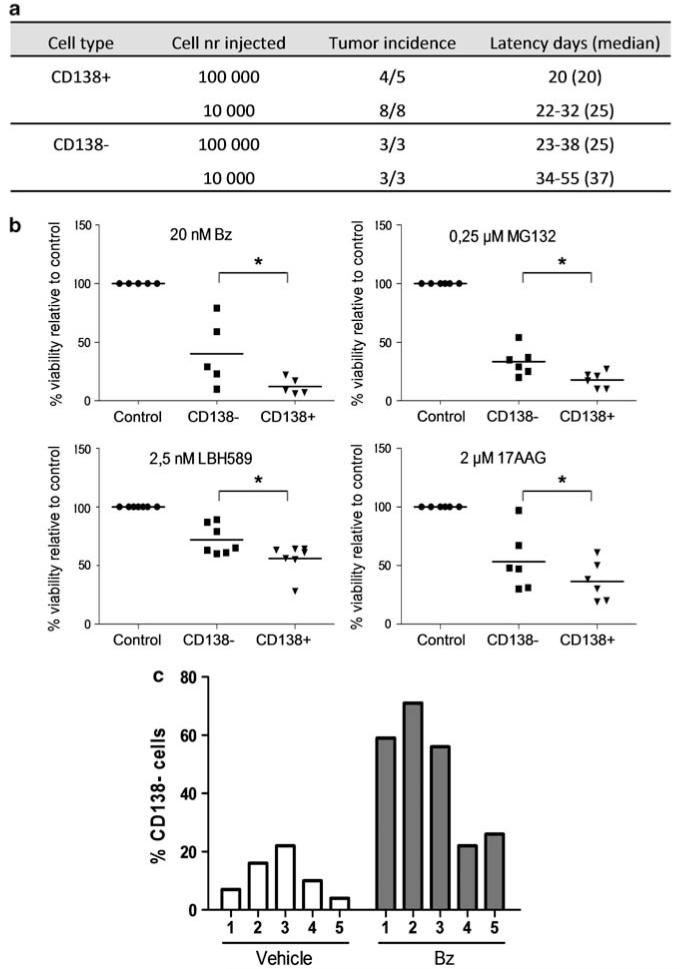
We next investigated the clonogenic growth capacity of CD138− and CD138+ cells in vitro and in vivo. Sorted 5T33 MM and 5TGM1 cells (>90% purity) were evaluated for colony forming potential by plating in methylcellulose (5 × 104 cells per ml). CD138− cells were found to generate less tumor colonies compared with CD138+ cells in each cell line (Figure 1e). We also injected sorted 5T33 MM cells into C57BlKaLwRij mice at different cell concentrations to examine tumorigenic potential. Recipient mice were killed following the development of symptoms and MM engraftment was confirmed by the detection of paraprotein in the serum and by the identification of malignant plasma cells within the BM on May-Grünwald-Giemsa stained cytospins (results not shown). Figure 2a demonstrates that both populations were capable of initiating MM growth in vivo, although with some delay in the engraftment of the CD138− population compared with CD138+ cells. Therefore, both CD138− and CD138+ cells are clonogenic in vitro and in vivo with the CD138+ population exhibiting a higher frequency of tumorigenic cells. These results are similar to previous studies demonstrating that both CD138− and CD138+ cells within human MM cell lines are clonogenic.4 In contrast, the engraftment of primary clinical specimens is restricted to CD138− cells in NOD/Scid mice and CD138+ cells in SCID-hu mice.4,10,11 Therefore, the tumor-initiating potential of MM cells is likely to be dependent upon the specific in vivo model utilized, and both CD138− and CD138+ MM cells are important for MM growth in a syngeneic immunocompetent model dependent on the BM microenvironment.
Figure 2.
(a) Tumor engraftment studies with CD138− and CD138+ populations of 5T33 MM. (b) Drug sensitivity of CD138− and CD138+ 5T33 MM cells. Sorted populations were incubated with bz, MG132, LBH589 and 17AAG at the indicated concentrations. After 20 h, the viability was measured by the CellTiter-Glo Luminescent Viability Assay. Results are given as the percentage viability relative to control (100%). Each dot represents the result of an independent experiment. *Indicates P-value<0.05 (Mann–Whitney test). (c) Percentage CD138− cells after bz treatment in vivo measured with flow cytometry. Results shown are from five mice in each group. Differences between vehicle and bz-treated group are significant (P<0.008, Mann–Whitney test).
These findings suggest that both CD138− and CD138+ cells contribute to disease propagation and should be considered when developing novel targeted therapeutical strategies for MM. We investigated the sensitivity of 5T33 MM CD138− and CD138+ cells to several drugs currently undergoing clinical testing in MM. We plated sorted cells in 96-well plates (1 × 103 – 10 × 103 cells per well), treated with the proteasome inhibitors bz and MG132, the HDAC inhibitor LBH589, and the HSP90 inhibitor 17AAG, then quantified cell viability (Figure 2b) and caspase 3/7 activity (data not shown) after 20 h. We found that 5T33 MM CD138− cells were less sensitive than CD138+ cells to all of the drugs studied (Figure 2b). Similarly, CD138− 5TGM1 cells were more viable after bz treatment than CD138+ 5TGM1 cells (57±19 vs 11±5%; data not shown). Furthermore, sensitivity to melphalan and the immunomodulatory drug lenalidomide was investigated on 5TGM1 subsets. In accordance with bz, CD138− 5TGM1 cells were more viable than CD138+ 5TGM1 cells after treatment with melphalan (52±8.5% vs 9±5%) and lenalidomide (60±14% vs 14±8.7%). We also examined the phenotype of 5T33 MM cells after in vivo treatment with bz (0.6 mg/kg, subcutaneous, 2× per week, starting 1 week after injection). Compared with a vehicle control, a tumor reduction of ~47% was observed in bz treated mice, and the percentage of viable (7AAD-) CD138− 5T33 MM cells was significantly increased (Figure 2c). Taken together these results indicate that CD138− cells are less sensitive to treatment both in vitro and in vivo. In human MM, increased STAT3/ERK1/2 signaling in CD138− cells has been suggested to be involved in the relative drug resistance of this population.4,7
Our results suggest that the tumor-initiating potential of MM cells is not dependent on the expression of CD138 alone, but that both CD138− and CD138+ cells are important in MM pathogenesis. This notion is supported by a recent study suggesting that CD138− plasma cells in MM clinical samples are more immature and proliferative.13 From our study, it seems that the CD138+ population is enriched for clonogenic MM cells, as it displayed increased clonogenic and tumor-initiating capacity than the CD138− population. This hypothesis is supported by the fact that populations with stem cell characteristics, identified by side population and aldehyde dehydrogenase assays, are predominantly (>97%) CD138+ (data not shown). It is possible that CD138− cells are increased in self-renewal and long-term proliferative potential as previously described, but we did not detect significantly enhanced ability to form colonies during serial plating in methylcellulose (data not shown). In addition, CD138− and CD138+ myeloma cells display differential sensitivities to drugs with CD138− cells relatively resistant compared with CD138+ cells. Therefore, future studies in MM examining potential therapies and drug resistance should include not only purified CD138+ cells but also CD138− cells.
ACKNOWLEDGEMENTS
We would like to thank Angelo Willems and Carine Seynaeve for their expert technical assistance and Jean-Marc Lazou for the sorting of the cells. This work was financially supported by the Fonds voor Wetenschappelijk Onderzoek Vlaanderen (FWO-Vl), Swedish Cancer Society, Swedish Research Council, the European Stem cell network (EUFP6 MSCNET), the Onderzoeksraad Vrije Universiteit Brussel and the ‘Vlaamse Liga tegen Kanker’. E Menu, E Van Valckenborgh and E De Bruyne are postdoctoral fellows of FWO-Vl and S Lub is a research fellow of FWO-Vl.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Palumbo A, Rajkumar SV. Treatment of newly diagnosed myeloma. Leukemia. 2009;23:449–456. doi: 10.1038/leu.2008.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 3.Rasmussen T, Lodahl M, Hancke S, Johnsen HE. In multiple myeloma clonotypic CD38− /CD19+ / CD27+ memory B cells recirculate through bone marrow, peripheral blood and lymph nodes. Leuk Lymphoma. 2004;45:1413–1417. doi: 10.1080/10428190410001655157. [DOI] [PubMed] [Google Scholar]
- 4.Huff CA, Matsui W. Multiple myeloma cancer stem cells. J Clin Oncol. 2008;26:2895–2900. doi: 10.1200/JCO.2007.15.8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vanderkerken K, Asosingh K, Croucher P, Van Camp B. Multiple myeloma biology: lessons from the 5TMM models. Immunol Rev. 2003;194:196–206. doi: 10.1034/j.1600-065x.2003.00035.x. [DOI] [PubMed] [Google Scholar]
- 6.Oyajobi BO, Franchin G, Williams PJ, Pulkrabek D, Gupta A, Munoz S, et al. Dual effects of macrophage inflammatory protein-1alpha on osteolysis and tumor burden in the murine 5TGM1 model of myeloma bone disease. Blood. 2003;102:311–319. doi: 10.1182/blood-2002-12-3905. [DOI] [PubMed] [Google Scholar]
- 7.Fuhler GM, Baanstra M, Chesik D, Somasundaram R, Seckinger A, Hose D, et al. Bone marrow stromal cell interaction reduces syndecan-1 expression and induces kinomic changes in myeloma cells. Exp Cell Res. 2010;316:1816–1828. doi: 10.1016/j.yexcr.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 8.Kalushkova A, Fryknas M, Lemaire M, Fristedt C, Agarwal P, Eriksson M, et al. Polycomb target genes are silenced in multiple myeloma. PLoS One. 2010;5:e11483. doi: 10.1371/journal.pone.0011483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin-Perez D, Piris MA, Sanchez-Beato M. Polycomb proteins in hematologic malignancies. Blood. 2010;116:5465–5475. doi: 10.1182/blood-2010-05-267096. [DOI] [PubMed] [Google Scholar]
- 10.Yaccoby S, Epstein J. The proliferative potential of myeloma plasma cells manifest in the SCID-hu host. Blood. 1999;94:3576–3582. [PubMed] [Google Scholar]
- 11.Dongkyoon K, Weissman I. Enrichment of xenotransplantable clonal cells in CD38high/CD138+ cells of multiple myeloma patients; AACR 101st Annual Meeting; 2010; Abstract 4315. [Google Scholar]
- 12.Kelly PN, Dakic A, Adams JM, Nutt SL, Strasser A. Tumor growth need not be driven by rare cancer stem cells. Science. 2007;317:337. doi: 10.1126/science.1142596. [DOI] [PubMed] [Google Scholar]
- 13.Reid S, Yang S, Brown R, Kabani K, Aklilu E, Ho PJ, et al. Characterisation and relevance of CD138-negative plasma cells in plasma cell myeloma. Int J Lab Hematol. 2010;32(6 Part 1):e190–e196. doi: 10.1111/j.1751-553X.2010.01222.x. [DOI] [PubMed] [Google Scholar]