The negative impact of hypertension on cognitive function was already hinted at in the 1960s in a study on psychomotor speed of air traffic controllers and pilots, and has received extensive confirmation over the following decades1. Thus, hypertension has been associated with a wide variety of cognitive deficits, including reduced abstract reasoning (executive dysfunction), impaired memory, attention deficit and slowing of mental processing speed1,2. Indeed, hypertension is a leading cause of vascular cognitive impairment (VCI), a term that includes all cognitive deficits attributable to vascular factors3. The most extreme case of VCI is vascular dementia, in which multiple cognitive domains are affected, with a negative impact on the activities of daily living. Increasing evidence also suggests that hypertension is a risk factor for Alzheimer’s disease (AD), highlighting its participation in all major causes of cognitive impairment4,5. In the past three years (2011–2013), several papers published in Hypertension have provided new insight into the link between high blood pressure and dementia. These papers will be briefly discussed, highlighting their contribution to current concepts of pathobiology, prevention and treatment of the “end-organ damage” to the brain inflicted by hypertension.
Midlife hypertension and late life dementia
Although its is well established that hypertension impairs cognition, one of the key issues still unsettled concerns the temporal relationships between blood pressure elevation and cognitive decline. On the one hand, cross sectional studies indicated that individuals with dementia have lower blood pressure, challenging the involvement of hypertension6. On the other hand, longitudinal studies, in which patient were followed for decades, revealed that individuals that develop dementia have a history high blood pressure earlier in life6,7. The effect is independent of other cardiovascular risk factors or co-morbidities and is observed both in men and women. In this context, Joas et al.8 demonstrated that in women with hypertension the development of dementia is preceded by a reduction in blood pressure, an effect that correlated with a reduction in the body mass index. The causes of the reduction in blood pressure remain to be defined, but the data stress the importance of accurate blood pressure monitoring in the care of patients with hypertension at risk for cognitive dysfunction. The reduction in body mass index suggests a reduced metabolic state, which may be responsible for the blood pressure decline in the late phases of the dementia. However, there is no consensus on the association between late life dementia and low blood pressure, and there might be differences between AD and vascular dementia. For example, Ninomiya et al.9 in a population-based prospective study in Japan reported that both midlife and late-life hypertension correlates with vascular dementia, but not AD. Although this study was based on a limited number of blood pressure measurements over the years, the data suggest that dementia is not universally associated with a reduction in blood pressure. These findings challenge the potential use of blood pressure as a biomarker of incipient dementia, and stress the need for additional well-controlled longitudinal studies. Rather, the relationship between hypertension and dementia may also depend on the predominant pathology underlying the cognitive impairment.
How long does it take for newly diagnosed hypertension to induce cognitive deficits?
Kohler et al. 10 studied the cognitive trajectory of patients that developed hypertension during the study period (incident hypertension), providing a unique opportunity to examine the temporal relationships between newly diagnosed hypertension and subsequent cognitive impairment. They found that incident hypertension increases the risk of dementia later in life, but the cognitive decline develops at least one year after the hypertension making it well suited to therapeutic interventions. Successful treatment of hypertension tended to dampen the cognitive decline, with partial success, while untreated or uncontrolled hypertension had the greatest negative impact on cognition. Overall, the results support careful blood pressure monitoring and adequate treatment of hypertension. However, it has been difficult to demonstrate in randomized clinical trials that treatment of hypertension reduces dementia risk. As discussed in recent reviews4–6, assessing the efficacy of hypertension treatment has been complicated by the long interval between onset of hypertension and development of dementia, the relatively short follow-up adopted in clinical trials performed thus far, and the coexistence with AD as a cause of dementia. Nevertheless, considering its well-established beneficial effects on cardiovascular morbidity and mortality, treatment of hypertension is certainly warranted irrespective of its potential positive impact on cognitive function. Future research exploring the impact of anti-hypertensive treatment on cognition is still needed to define the best timing and target population for intervention, and to evaluate whether some antihypertensive agents are more effective than others on cognitive outcomes11.
How does hypertension cause cognitive impairment?
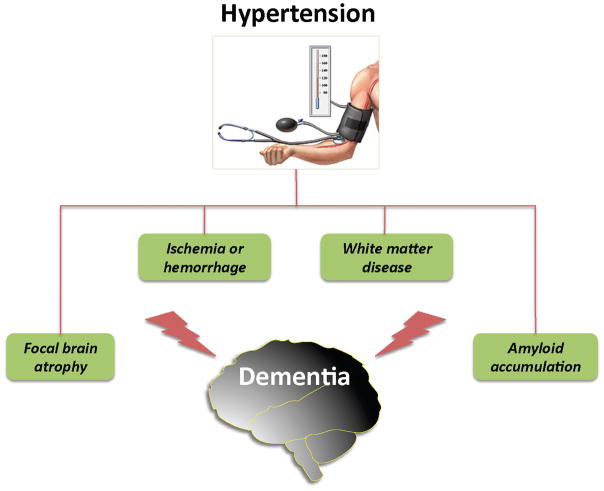
The potential factors through which hypertension contributes to dementia are illustrated in figure 1. Microvascular dysfunction and damage induced by hypertension leads to white matter disease, microinfarcts, and microhemorrhages, alterations closely correlated with the cognitive dysfunction4,5. Hypertension has major effects on the regulation of the cerebral circulation, which may impair brain structure and function by reducing vascular reserves and promoting ischemic injury4,12. One of the conditions linked to hypertension is sleep apnea, which is also a risk factor for dementia13. Capone et al.14 investigated the impact of chronic intermittent hypoxia (CIH), a model of sleep apnea, on the regulation of the cerebral circulation in mice. CIH increased blood pressure, induced cerebral endothelial dysfunction and suppressed the increases in CBF produced by neural activity, a vital homeostatic response that matches the delivery of energy substrates with the energy demands of the active brain. The cerebrovascular effects were mediated by upregulation of the potent vasoconstrictor endothelin-1, which induced the dysfunction via activation of endothelin type A receptors through NADPH oxidase-derived free radicals. Although it remains to be established whether the elevation in blood pressure induced by CIH is necessary or sufficient to induce the cerebrovascular dysfunction, the findings highlight the importance of endothelin-1-induced cerebrovascular damage in the pathophysiology of risk factors for cognitive impairment. Angiotensin-2, an octapeptide involved in the mechanisms of hypertension, has also been implicated in the vascular dysfunction underlying the effects of hypertension on the brain and leading to cognitive impairment15. Using a mouse model of cerebral hypoperfusion, which recapitulates selected features of VCI, Dong et al.16 found that inhibition of renin, the enzyme that converts angiotensinogen into angiotensin 1, protects the brain from white matter injury and associated cognitive impairment. These experimental findings are in agreement with clinical data suggesting that treatment of hypertension with inhibitors of the renin-angiotensin system may slow down cognitive decline6. However, as pointed out in the previous section, the evidence that hypertension treatment prevents dementia is not conclusive. Hypertension may also promote neocortical atrophy. Celle et al.17 demonstrated that hypertension leads to reduced gray matter volumes selectively in the left frontal lobe (supplemental motor area and superior and middle frontal gyrus), a finding linked to executive dysfunction and independent of major confounders such as age, sex, education level, and total brain volume. Although the possibility that these changes were related to local or distant microvascular pathology, e.g., microinfarcts, cannot be ruled out, the data suggest a potential mechanism for the executive dysfunction associated with hypertension. As discussed in the next section, hypertension can also influence cognition by modulating the brain levels of amyloid-β (Aβ), a peptide involved in the pathobiology of AD.
Figure 1.
Potential mechanisms of the cognitive dysfunction induced by hypertension. Hypertension-induced microvascular damage leads to subcortical and periventricular white matter damage (leukoaraiosis), microinfarcts, and microhemorrhages, and may promote amyloid accumulation in brain by impairing the vascular clearance of the Aβ peptide. Hypertension has also been associated with atrophy of the left frontal lobe, a finding that may underlies the alterations in executive function induced by hypertension.
Hypertension and Alzheimer’s disease
AD is the most common cause of dementia in the elderly, characterized pathologically by brain atrophy, accumulation of amyloid in the brain parenchyma (amyloid plaques) and blood vessels (amyloid angiopathy), as well as aggregation of the microtubule stabilizing protein tau (neurofibrillary tangles)18. Although AD has been traditionally considered a disease of neurons, recent epidemiological, pathological and experimental data implicate vascular factors in its mechanisms5,19. In particular, some studies, but not all, e.g., Ninomiya et al.9, have shown that mid life hypertension is a risk factor for AD. Although the mechanisms of the association remain unclear, there is evidence that hypertension may promote the accumulation and/or aggregation of the Aβ peptide in brain. Increases in brain amyloid have been reported in ApoE4+ hypertensive individuals, an effect reduced by antihypertensive treatment20. In this context, Shah et al.21 examined the relationship between midlife blood pressure, plasma Aβ, late life dementia and amyloid deposition at autopsy in Japanese Americans enrolled in the Honolulu Asia aging study. They found that increases in diastolic blood pressure in midlife are associated with reductions in plasma Aβ, and increased amyloid angiopathy and dementia risk. The findings raise the possibility that the cerebrovascular dysfunction and damage produced by midlife hypertension impairs the vascular clearance of brain Aβ, resulting in amyloid accumulation in cerebral blood vessels and cognitive dysfunction. These clinical-pathological observations are in agreement with experimental data demonstrating that the vascular clearance of Aβ is impaired in dysfunctional cerebral blood vessels leading to amyloid angiopathy22. Whatever the mechanisms of the interaction, the realization that cerebrovascular damage may play a role both in vascular dementia and AD supports the notion that maintaining vascular health by controlling vascular risk factors, such as hypertension, may be an important preventive strategy for late life dementia.
Acknowledgments
Sources of funding
Dr. Iadecola receives grant support form the National Institutes of Health (HL96571, NS73666) and the Alzheimer’s Association (ZEN-11-202707).
Footnotes
Disclosures
None
References
- 1.Elias MF, Goodell AL, Dore GA. Hypertension and cognitive functioning: a perspective in historical context. Hypertension. 2012;60:260–268. doi: 10.1161/HYPERTENSIONAHA.111.186429. [DOI] [PubMed] [Google Scholar]
- 2.Waldstein SR, Manuck SB, Ryan CM, Muldoon MF. Neuropsychological correlates of hypertension: review and methodologic considerations. Psychol Bull. 1991;110:451–468. doi: 10.1037/0033-2909.110.3.451. [DOI] [PubMed] [Google Scholar]
- 3.Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Faraco G, Iadecola C. Hypertension: a harbinger of stroke and dementia. Hypertension. 2013;62:810–817. doi: 10.1161/HYPERTENSIONAHA.113.01063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iadecola C. The pathobiology of vascular dementia. Neuron. 2013;80:844–866. doi: 10.1016/j.neuron.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tzourio C, Laurent S, Debette S. Is Hypertension Associated With an Accelerated Aging of the Brain? Hypertension. 2014 doi: 10.1161/HYPERTENSIONAHA.113.00147. in press. [DOI] [PubMed] [Google Scholar]
- 7.Skoog I, Lernfelt B, Landahl S, Palmertz B, Andreasson LA, Nilsson L, Persson G, Odén A, Svanborg A. 15-year longitudinal study of blood pressure and dementia. Lancet. 1996;347:1141–1145. doi: 10.1016/s0140-6736(96)90608-x. [DOI] [PubMed] [Google Scholar]
- 8.Joas E, Bäckman K, Gustafson D, Östling S, Waern M, Guo X, Skoog I. Blood pressure trajectories from midlife to late life in relation to dementia in women followed for 37 years. Hypertension. 2012;59:796–801. doi: 10.1161/HYPERTENSIONAHA.111.182204. [DOI] [PubMed] [Google Scholar]
- 9.Ninomiya T, Ohara T, Hirakawa Y, Yoshida D, Doi Y, Hata J, Kanba S, Iwaki T, Kiyohara Y. Midlife and late-life blood pressure and dementia in Japanese elderly: the Hisayama study. Hypertension. 2011;58:22–28. doi: 10.1161/HYPERTENSIONAHA.110.163055. [DOI] [PubMed] [Google Scholar]
- 10.Köhler S, Baars MAE, Spauwen P, Schievink S, Verhey FRJ, van Boxtel MJP. Temporal evolution of cognitive changes in incident hypertension: prospective cohort study across the adult age span. Hypertension. 2014;63:245–251. doi: 10.1161/HYPERTENSIONAHA.113.02096. [DOI] [PubMed] [Google Scholar]
- 11.Staessen JA, Thijs L, Richart T, Odili AN, Birkenhäger WH. Placebo-controlled trials of blood pressure-lowering therapies for primary prevention of dementia. Hypertension. 2011;57:e6–7. doi: 10.1161/HYPERTENSIONAHA.110.165142. [DOI] [PubMed] [Google Scholar]
- 12.Pires PW, Dams Ramos CM, Matin N, Dorrance AM. The effects of hypertension on the cerebral circulation. Am J Physiol Heart Circ Physiol. 2013;304:H1598–614. doi: 10.1152/ajpheart.00490.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Durgan DJ, Bryan RM. Cerebrovascular consequences of obstructive sleep apnea. J Am Heart Assoc. 2012;1:e000091. doi: 10.1161/JAHA.111.000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Capone C, Faraco G, Coleman C, Young CN, Pickel VM, Anrather J, Davisson RL, Iadecola C. Endothelin 1-dependent neurovascular dysfunction in chronic intermittent hypoxia. Hypertension. 2012;60:106–113. doi: 10.1161/HYPERTENSIONAHA.112.193672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Silva TM, Faraci FM. Effects of angiotensin II on the cerebral circulation: role of oxidative stress. Front Physiol. 2012;3:484. doi: 10.3389/fphys.2012.00484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong Y-F, Kataoka K, Toyama K, Sueta D, Koibuchi N, Yamamoto E, Yata K, Tomimoto H, Ogawa H, Kim-Mitsuyama S. Attenuation of brain damage and cognitive impairment by direct renin inhibition in mice with chronic cerebral hypoperfusion. Hypertension. 2011;58:635–642. doi: 10.1161/HYPERTENSIONAHA.111.173534. [DOI] [PubMed] [Google Scholar]
- 17.Celle S, Annweiler C, Pichot V, Bartha R, Barthélémy JC, Roche F, Beauchet O. Association between ambulatory 24-hour blood pressure levels and brain volume reduction: a cross-sectional elderly population-based study. Hypertension. 2012;60:1324–1331. doi: 10.1161/HYPERTENSIONAHA.112.193409. [DOI] [PubMed] [Google Scholar]
- 18.Huang Y, Mucke L. Alzheimer mechanisms and therapeutic strategies. Cell. 2012;148:1204–1222. doi: 10.1016/j.cell.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodrigue KM, Rieck JR, Kennedy KM, Devous MD, Diaz-Arrastia R, Park DC. Risk Factors for β-Amyloid Deposition in Healthy Aging: Vascular and Genetic Effects. JAMA Neurol. 2013:1–7. doi: 10.1001/jamaneurol.2013.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shah NS, Vidal J-S, Masaki K, Petrovitch H, Ross GW, Tilley C, DeMattos RB, Tracy RP, White LR, Launer LJ. Midlife blood pressure, plasma β-amyloid, and the risk for Alzheimer disease: the Honolulu Asia Aging Study. Hypertension. 2012;59:780–786. doi: 10.1161/HYPERTENSIONAHA.111.178962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park L, Zhou J, Zhou P, Pistick R, Jamal El S, Younkin L, Pierce J, Arreguin A, Anrather J, Younkin SG, Carlson GA, McEwen BS, Iadecola C. Innate immunity receptor CD36 promotes cerebral amyloid angiopathy. Proc Natl Acad Sci USA. 2013;110:3089–3094. doi: 10.1073/pnas.1300021110. [DOI] [PMC free article] [PubMed] [Google Scholar]