Abstract
Genome-wide association studies (GWASs) of major depressive disorder (MDD) have yet to identify variants that surpass the threshold for genome-wide significance. A recent study reported that runs of homozygosity (ROH) are associated with schizophrenia, reflecting a novel genetic risk factor resulting from increased parental relatedness and recessive genetic effects. Here we undertake an analysis of ROH for MDD using the 9,238 MDD cases and 9,521 controls reported in a recent mega-analysis of 9 GWAS. Since evidence for association with ROH could reflect a recessive mode of action at loci, we also conducted a genome-wide association analyses under a recessive model.
The genome-wide association analysis using a recessive model found no significant associations. Our analysis of ROH suggested that there was significant heterogeneity of effect across studies in effect (p=0.001), and it was associated with genotyping platform and country of origin. The results of the ROH analysis show that differences across studies can lead to conflicting systematic genome-wide differences between cases and controls that are unaccounted for by traditional covariates. They highlight the sensitivity of the ROH method to spurious associations, and the need to carefully control for potential confounds in such analyses. We found no strong evidence for a recessive model underlying MDD.
Introduction
Major depressive disorder (MDD) is one of the leading burdens of disease in the world, with a lifetime prevalence of ~15% (Hasin and others 2005; Kessler and others 2003). It has been found to be moderately heritable, from 31 to 42% (Sullivan and others 2000), though with greater heritability in severe, recurrent forms of the disorder (Levinson 2006; McGuffin and others 1996). A recent mega-analysis of nine genome-wide association studies found no significant associations with individual genetic variants (Psychiatric Genomics Consortium MDD Working Group 2012), compared to ~5 genome-wide significant associations in similar sized studies of other psychiatric disorders (Ripke and others 2011; Sklar and others 2011). These association studies are conducted under an additive model, but some risk variants may be recessive, for which individuals with two copies of an allele are at greater risk than would be predicted from twice a single allele’s effect. In a fully recessive model only those with 2 copies of the risk allele are at risk, though there is also the possibility of partial recessive effects. As selection acts to remove deleterious alleles with respect to overall fitness from the population, genetic risk variants that are recessive can escape selection longer. Inbreeding within families (e.g. consanguineous marriages) often exposes such recessive alleles due to an increased likelihood of alleles at each locus being identical by descent. Until recently studies of inbreeding were focused on families or communities in which inbreeding is expressed relative to the founder generation, which is assumed to be unrelated and where inbreeding information was determined from self-reports or knowledge of pedigrees (pedigree inbreeding). For example, Rudan et al. (2003) found a higher incidence of six complex genetic diseases/disorders including MDD among Croatian villages with higher levels of pedigree inbreeding (Rudan and others 2003). However, by using genome-wide genotype data it is possible to estimate an individual’s inbreeding from more distant common ancestors to provide evidence for whether a recessive genetic model is more appropriate for a disorder.
One method to analyse the effect of inbreeding from genome-wide genotype data is to identify segments of continuous homozygous SNPs, reflecting blocks of the genome that are identical by descent from a common ancestor. Runs of homozygosity (ROH) capture inbreeding effects that are due to common or rare causal variants better than a simple measure of excess number of homozygous SNPs across the genome, which tends to only capture the recessive effects of common variants (Keller and others 2011). An association between percentage of genome covered by ROH (FROH) and schizophrenia has been reported (Keller and others 2012). Due to the possibility of genetic overlap between MDD and schizophrenia (Schulze and others 2012), a similar association between FROH and MDD might be expected. However, MDD has a lower heritability (h2 ~ .37, Sullivan and others 2000) than schizophrenia (h2 ~ .81, Sullivan and others 2003), which should attenuate genetic relationships. Moreover, some authors have suggested that MDD may not be under negative selection and the casual genetic variants may be beneficial in some circumstances (Belsky and Pluess 2009; Nesse 1999; Power and others 2012; Watson and Andrews 2002). Here we look at the association between MDD and SNPs in 9,238 cases and 9,521 controls across 9 studies (Table 1) (Consortium 2012) under a recessive genetic model. We also use genome-wide estimates of inbreeding to look for a consistent difference between cases and controls across 9 studies of MDD, in order to support a recessive model of the disorder.
Table 1.
Summary statistics of each of the nine studies and results of runs of homozygosity analysis
| Study | N cases |
N controls |
Platform | Recurrence (%) |
Ascertainment | Country | Number of SNPsb |
Mean FROH (%)c |
SD of Mean FROH (%)c |
Traditional F estimate (%)d |
Mean size of ROH (kb) |
SD for Mean size of kb |
Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RADIANT UK | 1,625 | 1,588 | Illumina 610K |
97.1 | Clinical | UK | 263,728 | 0.10 | 0.41 | 0.1 | 3294 | 1373 | (Lewis and others 2010) |
| MDD2000 QIMR-610k |
433 | 751 | Illumina 610K |
57.6 | Population | Australia | 275,259 | 0.09 | 0.19 | −0.01 | 3376 | 1049 | (Wray and others 2012) |
| MDD2000 QIMR-317k |
1,017 | 960 | Illumina 317Ka |
51.4 | Population | Australia | 210,075 | 0.09 | 0.24 | 0.06 | 3330 | 937 | (Wray and others 2012) |
| Radiant + Bonn/Mannheim |
935 | 1,290 | Illumina 610K |
74.2 | Clinical | Germany | 260,561 | 0.10 | 0.30 | 0.06 | 3580 | 1565 | (Rietschel and others 2010) |
| GAIN | 1,694 | 1,767 | Perlegen 600K |
50.7 | Clinical /population |
The Netherlands |
199,749 | 0.12 | 0.36 | 0.15 | 3780 | 1840 | (Sullivan and others 2009) |
| GenRED | 1,030 | 1,253 | Affymetrix 6.0 |
100 | Volunteers | USA | 253,826 | 0.08 | 0.18 | 0.09 | 3238 | 763 | (Shi and others 2011) |
| GSK | 887 | 864 | Illumina 550K |
100 | Clinical | Germany | 281,029 | 0.16 | 0.67 | 0.14 | 3732 | 1711 | (Muglia and others 2010) |
| MPIP | 376 | 537 | Illumina 317K |
36.7 | Clinical | Germany | 215,393 | 0.11 | 0.30 | 0.04 | 3533 | 1424 | (Ising and others 2009) |
| STAR*D | 1,241 | 511 | Affymetrix 5.0 |
74.3 | Clinical | USA | 155,428 | 0.10 | 0.40 | 0.17 | 3362 | 783 | (Shyn and others 2011) |
Genotyping was on either Illumina 317K or Illumina 370K SNPs, but only SNPs from Illumina 317K were used for imputation.
After QC and pruning on linkage disequilibrium
FROH is the percentage of the genome covered by runs of homozygosity.
This measure of F reflects an individual’s observed number of homozygous loci compared to that expected under Hardy-Weinberg equilibrium.
Material and Methods
Sample
In this report, we analyzed individual data from the nine discovery samples (Ising and others 2009; Lewis and others 2010; Muglia and others 2010; Rietschel and others 2010; Shi and others 2011; Shyn and others 2011; Sullivan and others 2009; Wray and others 2012) of the PGC-MDD that together comprise 9,238 cases and 9,521 controls. Full sample details are given in the supplementary materials of the original analysis (Psychiatric Genomics Consortium MDD Working Group 2012), and are outlined in Table 1. All subjects were of European ancestry (as determined from genome-wide genotypes). Cases were required to have diagnoses of DSM-IV lifetime MDD established using structured diagnostic instruments from direct interviews by trained interviewers (two studies required recurrent MDD and one recurrent, early-onset MDD) or clinician-administered DSM-IV checklists. Studies ascertained cases mostly from clinical sources, and controls were largely randomly selected from the population and screened for lifetime history of MDD.
Method of ROH calling and analysis
Genotyping was described in the supplementary materials in the original analysis (Psychiatric Genomics Consortium MDD Working Group 2012). All samples were genotyped with single nucleotide polymorphism (SNP) arrays of greater than 200K genome-wide SNPs, with analysis restricted to polymorphic SNP probes. In the original analysis, imputation to the CEU HapMap3 reference sample (Altshuler and others 2010), 1,235,109 autosomal SNPs, was performed using Beagle 3.0.4 (Browning and Browning 2009). In order to perform an association analysis under a recessive model or call runs of homozygosity (ROH), imputed SNP dosage data was converted to discrete genotype calls, keeping those SNPs with a probability of at least 0.95. The use of imputed SNPs helped to increase similarity of genomic coverage across studies. SNPs with a missingness of >2% or minor allele frequency (MAF) <5% were removed, as were then individuals with missingness over 2%. Prior to analysis SNPs were pruned for LD within PLINK, removing any SNPs with an R2 0.90 with any other SNP in a 50 SNP window. The use of imputed data in ROH has previously been shown to give similar results to those restricting to only genotyped SNPs (Keller and others 2012). The calling of ROH and percentage of genome covered by ROH per individual (FROH) were derived within PLINK (Purcell and others 2007) following the same method found to optimally detect effects of autozygosity, as described in Howrigan et al (2011). In particular, we used a series of sliding windows across the genome to call ROH within each individual separately. The size of the windows was set to 65 consecutive SNPs, so any single SNP would be found in 65 different windows. If at least 4 (>5%) of these windows contained entirely homozygous SNPs, then the SNP in question could be included within a ROH. Within windows, one missing SNP was allowed. To avoid false positives, only ROH with a minimum of 65 consecutive SNPs covering 2.3Mb were used when calculating FROH. In addition, the required minimum density in a ROH was set at 200 kb per SNP and the maximum gap between two consecutive homozygous SNPs was set at 500 kb. The estimate of the total genome captured was 2.77 × 109 bp. The analysis was performed by study, using FROH as a predictor of case-control status in a logistic regression. Percentage of SNPs missing, a SNP-by-SNP measure of homozygosity determined by PLINK’s --het command, and the first 5 ancestry-informative principal components were used as covariates. The SNP-by-SNP measure of homozygosity was included to correct for differences in genomic-homozygosity levels unrelated to inbreeding, such as DNA quality or population ancestry. A mixed model was also examined combining all samples, using study as a random effect. This analysis was performed in STATA (StataCorp. 2011).
Genome-wide recessive model
The genome-wide recessive model analysis used the autosomal dosage data converted to genotype calls as described above in the analysis of ROH. Analyses was performed in PLINK (Purcell and others 2007), using the --recessive command. The first 5 ancestry-informative principal components were included as covariates. Analysis was restricted to autosomes. Each study was analysed separately and then a meta-analysis was performed for each SNP across studies (using fixed effect p value in PLINK). As the risk allele is set as the minor allele by default, and this may differ by study for alleles at frequencies near 0.5, we used the minor allele in the analysis of imputed data from the whole sample as a reference. A p-value <5×10−8 was considered as genome-wide significant. For this significance cut-off, we had 90% power to detect a relative risk of 1.47 for the rare recessive genotype for SNPs with a MAF from 0.3–0.5. However power decreased rapidly for those alleles with lower MAF, with 90% power to detect those with a relative risk of 1.81 and MAF of 0.2, or with a relative risk of 2.21 and MAF of 0.15. For those SNPs with lower MAF, power reduced rapidly for a recessive model. Calculations were performed using CaTS Power Calculator (Skol and others 2006).
Results
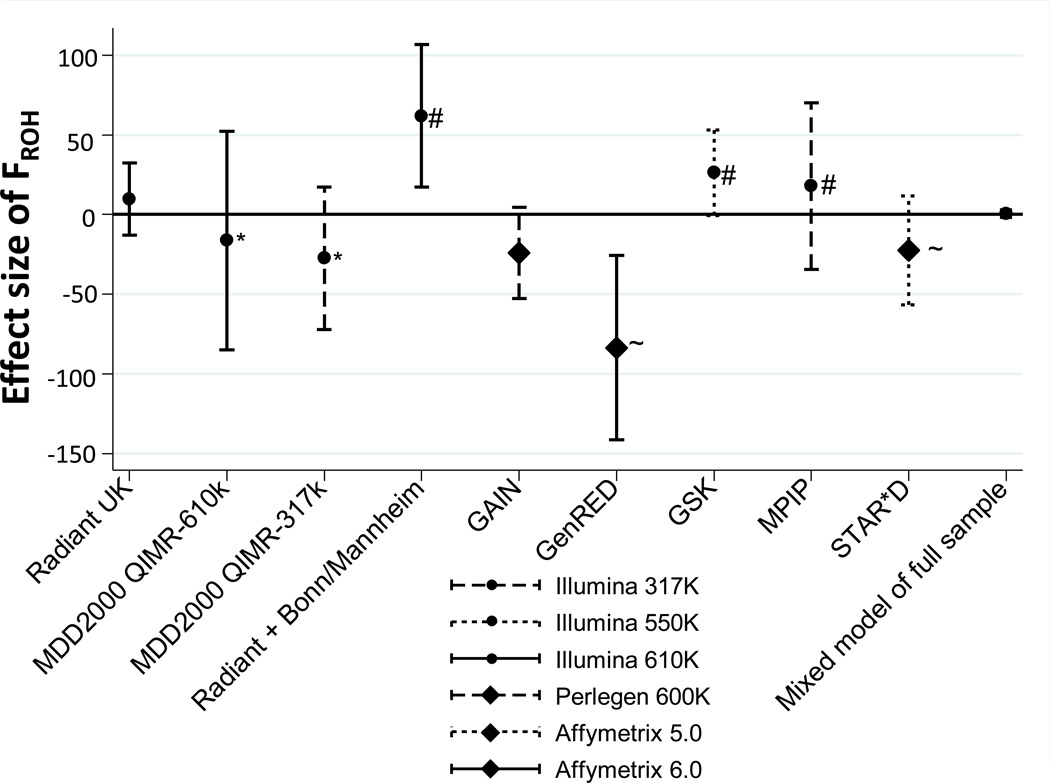
Across all samples the average percentage of the genome covered by ROH (FROH) was 0.11% (95% CI 0.102–0.112; Table 1), similar albeit slightly lower than average FROH (0.15%) reported in an earlier report using the same parameters (Keller and others 2012). In our mixed model analysis across all samples with study as a random effect, we found no significant effect of FROH on MDD status. However, there was substantial heterogeneity in direction of effect across studies (p=0.001, Figure 1). Overall, 4 studies showed increased FROH in cases (one significantly, p=0.007), while 5 studies showed increased FROH in controls (one where p=0.005). Including further principal components (up to 20) and increasing ROH size (up to 170 SNPs) made no difference to the results.
Figure 1.
Beta coefficient from logistic regression of FROH predicting MDD per study (with 95% CI, accounting for covariates). Positive effects suggest that ROHs are a risk factor for MDD. Note that though no combined effect in the mixed model of the full sample, in Illumina studies increased FROH was a risk factor (p=0.02) and in non-Illumina studies it was protective (p=0.007). Note also the consistency in studies from the same country: *Australian studies; ~American studies; # German studies.
To explore this apparent heterogeneity we examined two features of the included studies that might provide insight into the results. The first issue was potential poor matching of cases and controls which we tested within the combined RADIANT German and Bonn-Mannheim sample. Here cases were recruited from both of these two studies, whereas the controls were collected and genotyped only as part of the Bonn-Mannheim study. However, excluding the RADIANT cases and restricting to only the matched Bonn-Mannheim study’s cases and controls still resulted in a significant association with ROH (p=0.03), as both sets of cases were found to have similar mean FROH. This tentatively suggested that the heterogeneity apparent across studies was not replicated within studies. Secondly, we were interested in the effects of genomic coverage on ROH. Cases and controls from the Queensland Institute of Medical Research (QIMR) were recruited as one sample but included as two independent cohorts, based on their genotyping platform (Illumina 317k and 610k chips). When we restricted the analysis to only those SNPs directly genotyped on both platforms (202,062 SNPs), we found that the mean FROH for the QIMR-610k sample reduced from .086% to .077%, compared to .078% for the QIMR-317k sample. This implies, as expected, that genotyping platform and genome coverage were influencing estimates of mean FROH.
To better understand potential sources of heterogeneity in our findings, we used a linear meta-regression with a study’s effect size as the outcome and features of the studies individually analysed as predictors. We tested percentage of cases with recurrent MDD, mean FROH and genome-wide homozygosity, country of recruitment, presence of copy number variant probes on the platform, and genotyping platform as potential predictors of direction of effect (see Supplementary Table 1). Genotyping platform was nominally associated with effect size (p=0.05), and the direction of effect of FROH differed when studies on non-Illumina platforms were analysed separately. Within the 3 non-Illumina genotyped studies, increased inbreeding was protective against MDD (p=0.007) while within the 6 Illumina genotyped studies inbreeding was a significant risk factor for MDD (p=0.02). We noted that when more than one study was recruited from a country, the direction of effect for ROH was consistent across all studies recruited from the same country (see Figure 1). However, this was only a significant predictor in the meta-regression when distinguishing between German and non-German studies (p=0.02). This level of confounding from genotyping and country likely reduced our power to detect a true effect of ROH and MDD.
In the meta-analysis of a recessive model of association, 929,138 SNPs were analysed, though not all appeared in all 9 studies due to differences in genotyping coverage. The most significant recessive association was for rs13261582 on chromosome 8 in an intergenic region between SNTB1 and HAS2 (odds ratio of 2.0 for the minor A allele, MAF 0.22, p=2.58×10−6). However this SNP was only present in two studies (GenRED and STAR*D), and so is only supported by a subset of the sample. It did not appear among the reported top SNPs from the primary analysis of this dataset under an additive genetic model in the full sample (p=0.12). The results of the meta-analysis also showed a lower median p-value than expected by chance (λGC 0.97, see Supplementary Figures 1 and 2 for Manhattan and QQ plots). The λGC was 1.03 for SNPs with MAF > 0.2 and 0.90 for SNPs with MAF < 0.2, implying smaller differences of genotype frequencies between cases and controls than expected by chance for SNPS with low MAF. This is possibly the result of the less accurate imputation of rare alleles.
Discussion
Our analyses show systematic differences in FROH between cases and controls that differ in direction across studies. There are several explanations for these results, mostly highlighting limitations of this analysis. Firstly, we found systematic differences in mean FROH between studies. This is not unexpected and likely reflects the density of the genomic coverage and the accuracy of imputation, since SNPs were restricted to those with high quality imputation. A similar level of variation in FROH was observed in the Psychiatric GWAS Consortium’s analysis of FROH across 17 studies of schizophrenia (Keller and others 2012), though they did not report any heterogeneity of effect as a function of genotyping platform or country of recruitment. It seems unlikely that the heterogeneity of effect in the present study could be the result of differing SNP inclusion on the platforms, because such an explanation would imply systematic differences between cases and controls in the probability of homozygosity across SNPs as a function of platform. More likely in our opinion is the possibility that factors related to ascertainment of cases and controls differed across studies and influenced overall homozygosity. Such factors could include changes in homozygosity levels due to length and quality of DNA storage, or differences in ascertainment of cases and controls across populations. It is noteworthy that the two out of nine studies that genotyped controls independently of cases (GenRED and STAR*D) both showed higher FROH in controls than cases. Further, studies appear to cluster by country of origin and direction of the effect of FROH. All three German studies had increased FROH in cases for example, while the two Australian and two US studies all showed increased FROH in controls. This may reflect confounding demographic factors specific to each country. These unknown confounders, such as urban/rural status or religion, that influence both distant inbreeding (FROH) and MDD could explain the differences in effects between studies. A recent analysis of ROH and MDD in a partially overlapping sample of the GAIN study analysis here found exactly that, with religion confounding of the association because of reduced levels of depression but increased inbreeding in within the religious population of the Netherlands (Abdellaoui and others 2013). Certainly the initial hypothesis of this study, that an association with inbreeding would reflect negative selection on MDD and an excess of recessive causal mutations, seems an implausible explanation for the observed heterogeneity as the evolutionary cost of MDD status seems unlikely to have differed greatly among the ancestors of those included in the present study. Any of these explanations for the results of the FROH analysis may give some insight into why the mega-analysis of the 9 studies did not lead to any replicable genome-wide significant findings.
Our results from the genome-wide association analysis of MDD under a recessive model also produced no evidence for a recessive model, failing to produce any genome-wide significant associations. It is possible that our underlying model of recessive effect is unsuitable for an outbred population. Here we looked at a recessive effect for the minor allele, but two alternate models may also have been viable: compound heterozygote and overdominance. Compound heterozygosity is an additional risk in individuals carrying two recessive but non-identical alleles within a genetic locus, while overdominance is the increased risk of homozygosity of any allele compared to being heterozygous. However, our analysis of both would have been restricted by low power and the use of biallelic markers, and were, therefore, not performed. Both the GWAS and ROH analyses suggest though that there is no underlying recessive model of MDD, at least not of large effect. Such an association was previously reported for schizophrenia in a similarly sized sample (Keller and others 2012), showing an increase of risk for schizophrenia by 17% for every additional percentage of the genome covered by ROH and was taken as evidence for historical selection against schizophrenia risk variants. The lack of a similar association here adds molecular evidence to that from epidemiological studies suggesting MDD has little impact on reproductive fitness compared to other psychiatric disorders, and so is under substantially less negative selection (e.g. Power and others 2012).
These results also highlight that analysis of FROH appears to be sensitive to systematic differences between studies that are ostensibly unrelated to MDD status, potentially give rise to either false positive or false negative results. This suggests there are genome-wide differences in homozygosity and/or inbreeding between populations that are not corrected for by methods such as ancestry-informative principal components. We recommend the use of large combined samples in the analysis of FROH as a predictor of traits and disorders, due to the high risk of spurious associations within one study. Preferably such analyses should be done in datasets with access to data on potential social and demographic confounders. One possible further improvement might be the development of novel methods for analysing ROH, particularly in imputed genotype data where probability for homozygosity across a region is available. As similar heterogeneity across studies was not seen in other analyses of ROH within consortia (Keller and others 2012; McQuillan and others 2012), the significant heterogeneity in our results suggest that MDD is particularly sensitive to differing demographics in the ascertainment of cases and controls and this may present a problem to genome-wide polygenic approaches such as ROH. Certainly no strong evidence for a recessive model was apparent, supporting the view of MDD being under weaker negative selection than other psychiatric disorders.
Supplementary Material
Acknowledgements
Collaborators:
| Michael R | Barnes | GlaxoSmithKline | UK |
| Thomas | Bettecken | Max Planck Institute of Psychiatry | Germany |
| Elisabeth B | Binder | Max Planck Institute of Psychiatry | Germany |
| Douglas HR | Blackwood | University of Edinburgh | UK |
| Dorret I | Boomsma | VU University, Amsterdam | The Netherlands |
| Gerome | Breen | Institute of Psychiatry, King's College London | UK |
| René | Breuer | Central Inst Mental Health, University of Heidelberg | Germany |
| Enda M | Byrne | Queensland Institute of Medical Research | Australia |
| Sven | Cichon | University of Bonn | Germany |
| William H | Coryell | University of Iowa | USA |
| Nick | Craddock | Cardiff University | UK |
| Ian W | Craig | Institute of Psychiatry, King's College London | UK |
| Darina | Czamara | Max Planck Institute of Psychiatry | Germany |
| Eco J | De Geus | VU University, Amsterdam | The Netherlands |
| Franziska | Degenhardt | University of Bonn | Germany |
| Anne E | Farmer | Institute of Psychiatry, King's College London | UK |
| Josef | Frank | Central Inst Mental Health, University of Heidelberg | Germany |
| Scott D | Gordon | Queensland Institute of Medical Research | Australia |
| Magdalena | Gross | University of Bonn | Germany |
| Steven P | Hamilton | University of California, San Francisco | USA |
| Andrew C | Heath | Washington University, St Louis | USA |
| Anjali K | Henders | Queensland Institute of Medical Research | Australia |
| Stefan | Herms | University of Bonn | Germany |
| Ian B | Hickie | University of Sydney, Sydney | Australia |
| Susanne | Hoefels | University of Bonn | Germany |
| Florian | Holsboer | Max Planck Institute of Psychiatry | Germany |
| Witte J | Hoogendijk | Erasmus Medical Center | The Netherlands |
| Jouke Jan | Hottenga | VU University, Amsterdam | The Netherlands |
| Marcus | Ising | Max Planck Institute of Psychiatry | Germany |
| Ian | Jones | Cardiff University | UK |
| Lisa | Jones | University of Birmingham | UK |
| Tzeng | Jung-Ying | North Carolina State University | USA |
| James A | Knowles | University of Southern California | USA |
| Martin A | Kohli | Max Planck Institute of Psychiatry | Germany |
| Ania | Korszun | Queen Mary University of London | UK |
| William B | Lawson | Howard University | USA |
| Douglas F | Levinson | Stanford University | USA |
| Cathryn M | Lewis | Institute of Psychiatry, King's College London | UK |
| Danyu | Lin | University of North Carolina | USA |
| Susanne | Lucae | Max Planck Institute of Psychiatry | Germany |
| Donald | MacIntyre | University of Edinburgh | UK |
| Pamela AF | Madden | Washington University, St Louis | USA |
| Wolfgang | Maier | University of Bonn | Germany |
| Nicholas G | Martin | Queensland Institute of Medical Research | Australia |
| Manuel | Mattheisen | University of Bonn | Germany |
| Patrick J | McGrath | Columbia University | USA |
| Peter | McGuffin | Institute of Psychiatry, King's College London | UK |
| Andrew | McIntosh | University of Edinburgh | UK |
| Alan | McLean | University of Edinburgh | UK |
| Christel M | Middeldorp | VU University, Amsterdam | The Netherlands |
| Lefkos | Middleton | Imperial College | UK |
| Grant M | Montgomery | Queensland Institute of Medical Research | Australia |
| Pierandrea | Muglia | GlaxoSmithKline | Italy |
| Bertram | Müller-Myhsok | Max Planck Institute of Psychiatry | Germany |
| Benjamin | Neale | Harvard University/Broad Institute | USA |
| Markus M | Noethen | University of Bonn | Germany |
| Willem A | Nolen | Groningen University Medical Center | The Netherlands |
| Dale R | Nyholt | Queensland Institute of Medical Research | Australia |
| Brenda P | Penninx | VU University Medical Center, Amsterdam | The Netherlands |
| Michele L | Pergadia | Washington University, St Louis | USA |
| James B | Potash | University of Iowa | USA |
| Marcella | Rietschel | Central Inst Mental Health, University of Heidelberg | Germany |
| Stephan | Ripke | Harvard University/Broad Institute | USA |
| William A | Scheftner | Rush University Medical Center | USA |
| Thomas G | Schulze | University of Goettingen | USA |
| Jianxin | Shi | National Cancer Institute | USA |
| Stanley I | Shyn | University of Washington | USA |
| Susan L | Slager | Mayo Clinic | USA |
| Johannes H | Smit | VU University Medical Center, Amsterdam | The Netherlands |
| Michael | Steffens | University of Bonn | Germany |
| Patrick F | Sullivan | University of North Carolina | USA |
| Federica | Tozzi | GlaxoSmithKline | Italy |
| Jens | Treutlein | Central Inst Mental Health, University of Heidelberg | Germany |
| Manfred | Uhr | Max Planck Institute of Psychiatry | Germany |
| Edwin JCG | van den Oord | Virginia Commonwealth University | USA |
| Gerard | VU University Medical Center, Amsterdam | The Netherlands | |
| Myrna M | Weissman | Columbia University | USA |
| Gonneke | Willemsen | VU University, Amsterdam | The Netherlands |
| Naomi R | Wray | The University of Queensland | Australia |
| Frans G | Zitman | Leiden University Medical Center, Leiden | The Netherlands |
The PGC was funded by NIMH Grants MH085520 (lead PI PFS) and MH080403. The Bonn/Mannheim (BoMa) GWAS was supported by the German Federal Ministry of Education and Research, within the context of the National Genome Research Network 2 (NGFN-2), the National Genome Research Network plus (NGFNplus) and the Integrated Genome Research Network (IG) MooDS (Grant 01GS08144 to S Cichon and MM Nöethen, and Grant 01GS08147 to M Rietschel). The GenRED GWAS project was supported by NIMH R01 Grants MH061686 (DF Levinson), MH059542 (WH Coryell), MH075131 (WB Lawson), MH059552 (JB Potash), MH059541 (WA Scheftner) and MH060912 (MM Weissman). We acknowledge the contributions of Dr George S Zubenko and Dr Wendy N Zubenko, Department of Psychiatry, University of Pittsburgh School of Medicine, to the GenRED I project. The NIMH Cell Repository at Rutgers University and the NIMH Center for Collaborative Genetic Studies on Mental Disorders made essential contributions to this project. Genotyping was carried out by the Broad Institute Center for Genotyping and Analysis with support from Grant U54 RR020278 (which partially subsidized the genotyping of the GenRED cases). Collection and quality control analyses of the control data set were supported by grants from NIMH and the National Alliance for Research on Schizophrenia and Depression. We are grateful to Knowledge Networks (Menlo Park, CA, USA) for assistance in collecting the control data set. We express our profound appreciation to the families who participated in this project, and to the many clinicians who facilitated the referral of participants to the study. Max Planck Institute of Psychiatry MARS study was supported by the BMBF Program Molecular Diagnostics: Validation of Biomarkers for Diagnosis and Outcome in Major Depression (01ES0811). Genotyping was supported by the Bavarian Ministry of Commerce, and the Federal Ministry of Education and Research (BMBF) in the framework of the National Genome Research Network (NGFN2 and NGFN-Plus, FKZ 01GS0481 and 01GS08145). The Netherlands Study of Depression and Anxiety (NESDA) and the Netherlands Twin Register (NTR) contributed to GAIN-MDD and to MDD2000. Funding was from: the Netherlands Organization for Scientific Research (MagW/ZonMW Grants 904-61-090, 985-10- 002, 904-61-193, 480-04-004, 400-05-717, 912-100-20; Spinozapremie 56-464-14192; Geestkracht program Grant 10-000-1002); the Center for Medical Systems Biology (NWO Genomics), Biobanking and Biomolecular Resources Research Infrastructure, VU University’s Institutes for Health and Care Research and Neuroscience Campus Amsterdam, BIC/BioAssist/RK (2008.024); the European Science Foundation (EU/QLRT-2001-01254); the European Community’s Seventh Framework Program (FP7/2007–2013); ENGAGE (HEALTH-F4-2007-201413); and the European Science Council (ERC, 230374). Genotyping was funded in part by the Genetic Association Information Network (GAIN) of the Foundation for the US National Institutes of Health, and analysis was supported by grants from GAIN and the NIMH (MH081802). CM Middeldorp was supported by the Netherlands Organization for Scientific Research (NOW-VENI grant 916-76-125). Funding for the QIMR samples was provided by the Australian National Health and Medical Research Council (241944, 339462, 389927, 389875, 389891, 389892, 389938, 442915, 442981, 496675, 496739, 552485, 552498, 613602, 613608, 613674, 619667), the Australian Research Council (FT0991360, FT0991022), the FP-5 GenomEUtwin Project (QLG2-CT- 2002-01254) and the US National Institutes of Health (AA07535, AA10248, AA13320, AA13321, AA13326, AA14041, MH66206, DA12854, DA019951), and the Center for Inherited Disease Research (Baltimore, MD, USA). We thank the twins and their families registered at the Australian Twin Registry for their participation in the many studies that have contributed to this research. RADIANT was funded by: a joint grant from the UK Medical Research Council and GlaxoSmithKline (G0701420); the National Institute for Health Research (NIHR) Specialist Biomedical Research Centre for Mental Health at the South London and Maudsley NHS Foundation Trust and the Institute of Psychiatry, King’s College London; and the UK Medical Research Council (G0000647). The GENDEP study was funded by a European Commission Framework 6 grant, EC Contract Ref.: LSHB-CT-2003-503428. Genotyping of STAR*D was supported by an NIMH Grant to SP Hamilton (MH072802). STAR*D was funded by the National Institute of Mental Health (contract N01MH90003) to the University of Texas Southwestern Medical Center at Dallas (AJ Rush, principal investigator). We would like to thank the numerous researchers within the Psychiatric GWAS Consortium for their contributions. We also thank the thousands of people with MDD who donated time and effort to make this research possible.
Footnotes
Financial Disclosure:
The authors declare no conflicts of interest.
References
- Abdellaoui A, Hottenga JJ, Xiao X, Scheet P, Ehli EA, Davies GE, Hudziak JJ, Smit DJ, Bartels M, Willemsen G, Brooks A, Sullivan PF, Smit JH, de Geus EJ, Penninx BW, Boomsma DI. Association Between Autozygosity and Major Depression: Stratification Due to Religious Assortment. Behavior Genetics. 2013 doi: 10.1007/s10519-013-9610-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altshuler DM, Gibbs RA, Peltonen L, Dermitzakis E, Schaffner SF, Yu F, Bonnen PE, de Bakker PI, Deloukas P, Gabriel SB, Gwilliam R, Hunt S, Inouye M, Jia X, Palotie A, Parkin M, Whittaker P, Chang K, Hawes A, Lewis LR, Ren Y, Wheeler D, Muzny DM, Barnes C, Darvishi K, Hurles M, Korn JM, Kristiansson K, Lee C, McCarrol SA, Nemesh J, Keinan A, Montgomery SB, Pollack S, Price AL, Soranzo N, Gonzaga-Jauregui C, Anttila V, Brodeur W, Daly MJ, Leslie S, McVean G, Moutsianas L, Nguyen H, Zhang Q, Ghori MJ, McGinnis R, McLaren W, Takeuchi F, Grossman SR, Shlyakhter I, Hostetter EB, Sabeti PC, Adebamowo CA, Foster MW, Gordon DR, Licinio J, Manca MC, Marshall PA, Matsuda I, Ngare D, Wang VO, Reddy D, Rotimi CN, Royal CD, Sharp RR, Zeng C, Brooks LD, McEwen JE. Integrating common and rare genetic variation in diverse human populations. Nature. 2010;467(7311):52–58. doi: 10.1038/nature09298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Pluess M. Beyond Diathesis Stress: Differential Susceptibility to Environmental Influences. Psychological Bulletin. 2009;135(6):885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Browning BL, Browning SR. A unified approach to genotype imputation and haplotype-phase inference for large data sets of trios and unrelated individuals. American Journal of Human Genetics. 2009;84(2):210–223. doi: 10.1016/j.ajhg.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consortium MDDWGotPG. A mega-analysis of genome-wide association studies for major depressive disorder. Molecular Psychiatry. 2012 doi: 10.1038/mp.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Goodwin RD, Stinson FS, Grant BF. Epidemiology of major depressive disorder - Results from the National Epidemiologic Survey on Alcoholism and Related Conditions. Archives of General Psychiatry. 2005;62(10):1097–1106. doi: 10.1001/archpsyc.62.10.1097. [DOI] [PubMed] [Google Scholar]
- Howrigan DP, Simonson MA, Keller MC. Detecting autozygosity through runs of homozygosity: a comparison of three autozygosity detection algorithms. Bmc Genomics. 2011;12:460. doi: 10.1186/1471-2164-12-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ising M, Lucae S, Binder EB, Bettecken T, Uhr M, Ripke S, Kohli MA, Hennings JM, Horstmann S, Kloiber S, Menke A, Bondy B, Rupprecht R, Domschke K, Baune BT, Arolt V, Rush AJ, Holsboer F, Muller-Myhsok B. A Genomewide Association Study Points to Multiple Loci That Predict Antidepressant Drug Treatment Outcome in Depression. Archives of General Psychiatry. 2009;66(9):966-+. doi: 10.1001/archgenpsychiatry.2009.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller MC, Simonson MA, Ripke S, Neale BM, Gejman PV, Howrigan DP, Lee SH, Lencz T, Levinson DF, Sullivan PF. Runs of homozygosity implicate autozygosity as a schizophrenia risk factor. PLoS Genet. 2012;8(4):e1002656. doi: 10.1371/journal.pgen.1002656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller MC, Visscher PM, Goddard ME. Quantification of inbreeding due to distant ancestors and its detection using dense single nucleotide polymorphism data. Genetics. 2011;189(1):237–249. doi: 10.1534/genetics.111.130922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS. The epidemiology of major depressive disorder - Results from the National Comorbidity Survey Replication (NCS-R) Jama-Journal of the American Medical Association. 2003;289(23):3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Levinson DF. The genetics of depression: a review. Biol Psychiatry. 2006;60(2):84–92. doi: 10.1016/j.biopsych.2005.08.024. [DOI] [PubMed] [Google Scholar]
- Lewis CM, Ng MY, Butler AW, Cohen-Woods S, Uher R, Pirlo K, Weale ME, Schosser A, Paredes UM, Rivera M, Craddock N, Owen MJ, Jones L, Jones I, Korszun A, Aitchison KJ, Shi JX, Quinn JP, MacKenzie A, Vollenweider P, Waeber G, Heath S, Lathrop M, Muglia P, Barnes MR, Whittaker JC, Tozzi F, Holsboer F, Preisig M, Farmer AE, Breen G, Craig IW, McGuffin P. Genome-Wide Association Study of Major Recurrent Depression in the UK Population. American Journal of Psychiatry. 2010;167(8):949–957. doi: 10.1176/appi.ajp.2010.09091380. [DOI] [PubMed] [Google Scholar]
- McGuffin P, Katz R, Watkins S, Rutherford J. A hospital-based twin register of the heritability of DSM-IV unipolar depression. Archives of General Psychiatry. 1996;53(2):129–136. doi: 10.1001/archpsyc.1996.01830020047006. [DOI] [PubMed] [Google Scholar]
- McQuillan R, Eklund N, Pirastu N, Kuningas M, McEvoy BP, Esko T, Corre T, Davies G, Kaakinen M, Lyytikainen LP, Kristiansson K, Havulinna AS, Gogele M, Vitart V, Tenesa A, Aulchenko Y, Hayward C, Johansson A, Boban M, Ulivi S, Robino A, Boraska V, Igl W, Wild SH, Zgaga L, Amin N, Theodoratou E, Polasek O, Girotto G, Lopez LM, Sala C, Lahti J, Laatikainen T, Prokopenko I, Kals M, Viikari J, Yang J, Pouta A, Estrada K, Hofman A, Freimer N, Martin NG, Kahonen M, Milani L, Heliovaara M, Vartiainen E, Raikkonen K, Masciullo C, Starr JM, Hicks AA, Esposito L, Kolcic I, Farrington SM, Oostra B, Zemunik T, Campbell H, Kirin M, Pehlic M, Faletra F, Porteous D, Pistis G, Widen E, Salomaa V, Koskinen S, Fischer K, Lehtimaki T, Heath A, McCarthy MI, Rivadeneira F, Montgomery GW, Tiemeier H, Hartikainen AL, Madden PA, d'Adamo P, Hastie ND, Gyllensten U, Wright AF, van Duijn CM, Dunlop M, Rudan I, Gasparini P, Pramstaller PP, Deary IJ, Toniolo D, Eriksson JG, Jula A, Raitakari OT, Metspalu A, Perola M, Jarvelin MR, Uitterlinden A, Visscher PM, Wilson JF. Evidence of inbreeding depression on human height. PLoS Genet. 2012;8(7):e1002655. doi: 10.1371/journal.pgen.1002655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muglia P, Tozzi F, Galwey NW, Francks C, Upmanyu R, Kong XQ, Antoniades A, Domenici E, Perry J, Rothen S, Vandeleur CL, Mooser V, Waeber G, Vollenweider P, Preisig M, Lucae S, Muller-Myhsok B, Holsboer F, Middleton LT, Roses AD. Genome-wide association study of recurrent major depressive disorder in two European case-control cohorts. Molecular Psychiatry. 2010;15(6):589–601. doi: 10.1038/mp.2008.131. [DOI] [PubMed] [Google Scholar]
- Nesse RM. Proximate and evolutionary studies of anxiety, stress and depression: synergy at the interface. Neurosci Biobehav Rev. 1999;23(7):895–903. doi: 10.1016/s0149-7634(99)00023-8. [DOI] [PubMed] [Google Scholar]
- Power RA, Kyaga S, Uher R, Maccabe JH, Langstrom N, Landen M, McGuffin P, Lewis CM, Lichtenstein P, Svensson AC. Fecundity of Patients With Schizophrenia, Autism, Bipolar Disorder, Depression, Anorexia Nervosa, or Substance Abuse vs Their Unaffected Siblings. Arch Gen Psychiatry. 2012:1–8. doi: 10.1001/jamapsychiatry.2013.268. [DOI] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC. PLINK: a tool set for whole-genome association and population-based linkage analyses. American Journal of Human Genetics. 2007;81(3):559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietschel M, Mattheisen M, Frank J, Treutlein J, Degenhardt F, Breuer R, Steffens M, Mier D, Esslinger C, Walter H, Kirsch P, Erk S, Schnell K, Herms S, Wichmann HE, Schreiber S, Jockel KH, Strohmaier J, Roeske D, Haenisch B, Gross M, Hoefels S, Lucae S, Binder EB, Wienker TF, Schulze TG, Schmal C, Zimmer A, Juraeva D, Brors B, Bettecken T, Meyer-Lindenberg A, Muller-Myhsok B, Maier W, Nothen MM, Cichon S. Genome-wide association-, replication-, and neuroimaging study implicates HOMER1 in the etiology of major depression. Biol Psychiatry. 2010;68(6):578–585. doi: 10.1016/j.biopsych.2010.05.038. [DOI] [PubMed] [Google Scholar]
- Ripke S, Sanders AR, Kendler KS, Levinson DF, Sklar P, Holmans PA, Lin DY, Duan J, Ophoff RA, Andreassen OA, Scolnick E, Cichon S, St Clair D, Corvin A, Gurling H, Werge T, Rujescu D, Blackwood DH, Pato CN, Malhotra AK, Purcell S, Dudbridge F, Neale BM, Rossin L, Visscher PM, Posthuma D, Ruderfer DM, Fanous A, Stefansson H, Steinberg S, Mowry BJ, Golimbet V, De Hert M, Jonsson EG, Bitter I, Pietilainen OP, Collier DA, Tosato S, Agartz I, Albus M, Alexander M, Amdur RL, Amin F, Bass N, Bergen SE, Black DW, Borglum AD, Brown MA, Bruggeman R, Buccola NG, Byerley WF, Cahn W, Cantor RM, Carr VJ, Catts SV, Choudhury K, Cloninger CR, Cormican P, Craddock N, Danoy PA, Datta S, de Haan L, Demontis D, Dikeos D, Djurovic S, Donnelly P, Donohoe G, Duong L, Dwyer S, Fink-Jensen A, Freedman R, Freimer NB, Friedl M, Georgieva L, Giegling I, Gill M, Glenthoj B, Godard S, Hamshere M, Hansen M, Hansen T, Hartmann AM, Henskens FA, Hougaard DM, Hultman CM, Ingason A, Jablensky AV, Jakobsen KD, Jay M, Jurgens G, Kahn RS, Keller MC, Kenis G, Kenny E, Kim Y, Kirov GK, Konnerth H, Konte B, Krabbendam L, Krasucki R, Lasseter VK, Laurent C, Lawrence J, Lencz T, Lerer FB, Liang KY, Lichtenstein P, Lieberman JA, Linszen DH, Lonnqvist J, Loughland CM, Maclean AW, Maher BS, Maier W, Mallet J, Malloy P, Mattheisen M, Mattingsdal M, McGhee KA, McGrath JJ, McIntosh A, McLean DE, McQuillin A, Melle I, Michie PT, Milanova V, Morris DW, Mors O, Mortensen PB, Moskvina V, Muglia P, Myin-Germeys I, Nertney DA, Nestadt G, Nielsen J, Nikolov I, Nordentoft M, Norton N, Nothen MM, O'Dushlaine CT, Olincy A, Olsen L, O'Neill FA, Orntoft TF, Owen MJ, Pantelis C, Papadimitriou G, Pato MT, Peltonen L, Petursson H, Pickard B, Pimm J, Pulver AE, Puri V, Quested D, Quinn EM, Rasmussen HB, Rethelyi JM, Ribble R, Rietschel M, Riley BP, Ruggeri M, Schall U, Schulze TG, Schwab SG, Scott RJ, Shi J, Sigurdsson E, Silverman JM, Spencer CC, Stefansson K, Strange A, Strengman E, Stroup TS, Suvisaari J, Terenius L, Thirumalai S, Thygesen JH, Timm S, Toncheva D, van den Oord E, van Os J, van Winkel R, Veldink J, Walsh D, Wang AG, Wiersma D, Wildenauer DB, Williams HJ, Williams NM, Wormley B, Zammit S, Sullivan PF, O'Donovan MC, Daly MJ, Gejman PV. Genome-wide association study identifies five new schizophrenia loci. Nature Genetics. 2011;43(10):969–976. doi: 10.1038/ng.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudan I, Rudan D, Campbell H, Carothers A, Wright A, Smolej-Narancic N, Janicijevic B, Jin L, Chakraborty R, Deka R, Rudan P. Inbreeding and risk of late onset complex disease. Journal of Medical Genetics. 2003;40(12):925–932. doi: 10.1136/jmg.40.12.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze TG, Akula N, Breuer R, Steele J, Nalls MA, Singleton AB, Degenhardt FA, Nothen MM, Cichon S, Rietschel M, McMahon FJ. Molecular genetic overlap in bipolar disorder, schizophrenia, and major depressive disorder. World J Biol Psychiatry. 2012 doi: 10.3109/15622975.2012.662282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Potash JB, Knowles JA, Weissman MM, Coryell W, Scheftner WA, Lawson WB, DePaulo JR, Gejman PV, Sanders AR, Johnson JK, Adams P, Chaudhury S, Jancic D, Evgrafov O, Zvinyatskovskiy A, Ertman N, Gladis M, Neimanas K, Goodell M, Hale N, Ney N, Verma R, Mirel D, Holmans P, Levinson DF. Genome-wide association study of recurrent early-onset major depressive disorder. Molecular Psychiatry. 2011;16(2):193–201. doi: 10.1038/mp.2009.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyn SI, Shi J, Kraft JB, Potash JB, Knowles JA, Weissman MM, Garriock HA, Yokoyama JS, McGrath PJ, Peters EJ, Scheftner WA, Coryell W, Lawson WB, Jancic D, Gejman PV, Sanders AR, Holmans P, Slager SL, Levinson DF, Hamilton SP. Novel loci for major depression identified by genome-wide association study of Sequenced Treatment Alternatives to Relieve Depression and meta-analysis of three studies. Mol Psychiatry. 2011;16(2):202–215. doi: 10.1038/mp.2009.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklar P, Ripke S, Scott LJ, Andreassen OA, Cichon S, Craddock N, Edenberg HJ, Nurnberger JI, Jr, Rietschel M, Blackwood D, Corvin A, Flickinger M, Guan W, Mattingsdal M, McQuillin A, Kwan P, Wienker TF, Daly M, Dudbridge F, Holmans PA, Lin D, Burmeister M, Greenwood TA, Hamshere ML, Muglia P, Smith EN, Zandi PP, Nievergelt CM, McKinney R, Shilling PD, Schork NJ, Bloss CS, Foroud T, Koller DL, Gershon ES, Liu C, Badner JA, Scheftner WA, Lawson WB, Nwulia EA, Hipolito M, Coryell W, Rice J, Byerley W, McMahon FJ, Schulze TG, Berrettini W, Lohoff FW, Potash JB, Mahon PB, McInnis MG, Zollner S, Zhang P, Craig DW, Szelinger S, Barrett TB, Breuer R, Meier S, Strohmaier J, Witt SH, Tozzi F, Farmer A, McGuffin P, Strauss J, Xu W, Kennedy JL, Vincent JB, Matthews K, Day R, Ferreira MA, O'Dushlaine C, Perlis R, Raychaudhuri S, Ruderfer D, Hyoun PL, Smoller JW, Li J, Absher D, Thompson RC, Meng FG, Schatzberg AF, Bunney WE, Barchas JD, Jones EG, Watson SJ, Myers RM, Akil H, Boehnke M, Chambert K, Moran J, Scolnick E, Djurovic S, Melle I, Morken G, Gill M, Morris D, Quinn E, Muhleisen TW, Degenhardt FA, Mattheisen M, Schumacher J, Maier W, Steffens M, Propping P, Nothen MM, Anjorin A, Bass N, Gurling H, Kandaswamy R, Lawrence J, McGhee K, McIntosh A, McLean AW, Muir WJ, Pickard BS, Breen G, St Clair D, Caesar S, Gordon-Smith K, Jones L, Fraser C, Green EK, Grozeva D, Jones IR, Kirov G, Moskvina V, Nikolov I, O'Donovan MC, Owen MJ, Collier DA, Elkin A, Williamson R, Young AH, Ferrier IN, Stefansson K, Stefansson H, Thornorgeirsson T, Steinberg S, Gustafsson O, Bergen SE, Nimgaonkar V, Hultman C, Landen M, Lichtenstein P, Sullivan P, Schalling M, Osby U, Backlund L, Frisen L, Langstrom N, Jamain S, Leboyer M, Etain B, Bellivier F, Petursson H, Sigur Sson E, Muller-Mysok B, Lucae S, Schwarz M, Schofield PR, Martin N, Montgomery GW, Lathrop M, Oskarsson H, Bauer M, Wright A, Mitchell PB, Hautzinger M, Reif A, Kelsoe JR, Purcell SM. Large-scale genome-wide association analysis of bipolar disorder identifies a new susceptibility locus near ODZ4. Nature Genetics. 2011;43(10):977–983. doi: 10.1038/ng.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skol AD, Scott LJ, Abecasis GR, Boehnke M. Joint analysis is more efficient than replication-based analysis for two-stage genome-wide association studies. Nature Genetics. 2006;38(2):209–213. doi: 10.1038/ng1706. [DOI] [PubMed] [Google Scholar]
- StataCorp. Stata Statistical Software: Release. Vol. 12. College Station, TX: StataCorp LP; 2011. [Google Scholar]
- Sullivan PF, de Geus EJC, Willemsen G, James MR, Smit JH, Zandbelt T, Arolt V, Baune BT, Blackwood D, Cichon S, Coventry WL, Domschke K, Farmer A, Fava M, Gordon SD, He Q, Heath AC, Heutink P, Holsboer F, Hoogendijk WJ, Hottenga JJ, Hu Y, Kohli M, Lin D, Lucae S, MacIntyre DJ, Maier W, McGhee KA, McGuffin P, Montgomery GW, Muir WJ, Nolen WA, Nothen MM, Perlis RH, Pirlo K, Posthuma D, Rietschel M, Rizzu P, Schosser A, Smit AB, Smoller JW, Tzeng JY, van Dyck R, Verhage M, Zitman FG, Martin NG, Wray NR, Boomsma DI, Penninx BWJH. Genome-wide association for major depressive disorder: a possible role for the presynaptic protein piccolo. Molecular Psychiatry. 2009;14(4):359–375. doi: 10.1038/mp.2008.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF, Kendler KS, Neale MC. Schizophrenia as a complex trait - Evidence from a meta-analysis of twin studies. Archives of General Psychiatry. 2003;60(12):1187–1192. doi: 10.1001/archpsyc.60.12.1187. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: Review and meta-analysis. American Journal of Psychiatry. 2000;157(10):1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- Watson PJ, Andrews PW. Toward a revised evolutionary adaptationist analysis of depression: the social navigation hypothesis. J Affect Disord. 2002;72(1):1–14. doi: 10.1016/s0165-0327(01)00459-1. [DOI] [PubMed] [Google Scholar]
- Wray NR, Pergadia ML, Blackwood DHR, Penninx BWJH, Gordon SD, Nyholt DR, Ripke S, MacIntyre DJ, McGhee KA, Maclean AW, Smit JH, Hottenga JJ, Willemsen G, Middeldorp CM, de Geus EJC, Lewis CM, McGuffin P, Hickie IB, van den Oord EJCG, Liu JZ, Macgregor S, McEvoy BP, Byrne EM, Medland SE, Statham DJ, Henders AK, Heath AC, Montgomery GW, Martin NG, Boomsma DI, Madden PAF, Sullivan PF. Genome-wide association study of major depressive disorder: new results, meta-analysis, and lessons learned. Molecular Psychiatry. 2012;17(1):36–48. doi: 10.1038/mp.2010.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.