Abstract
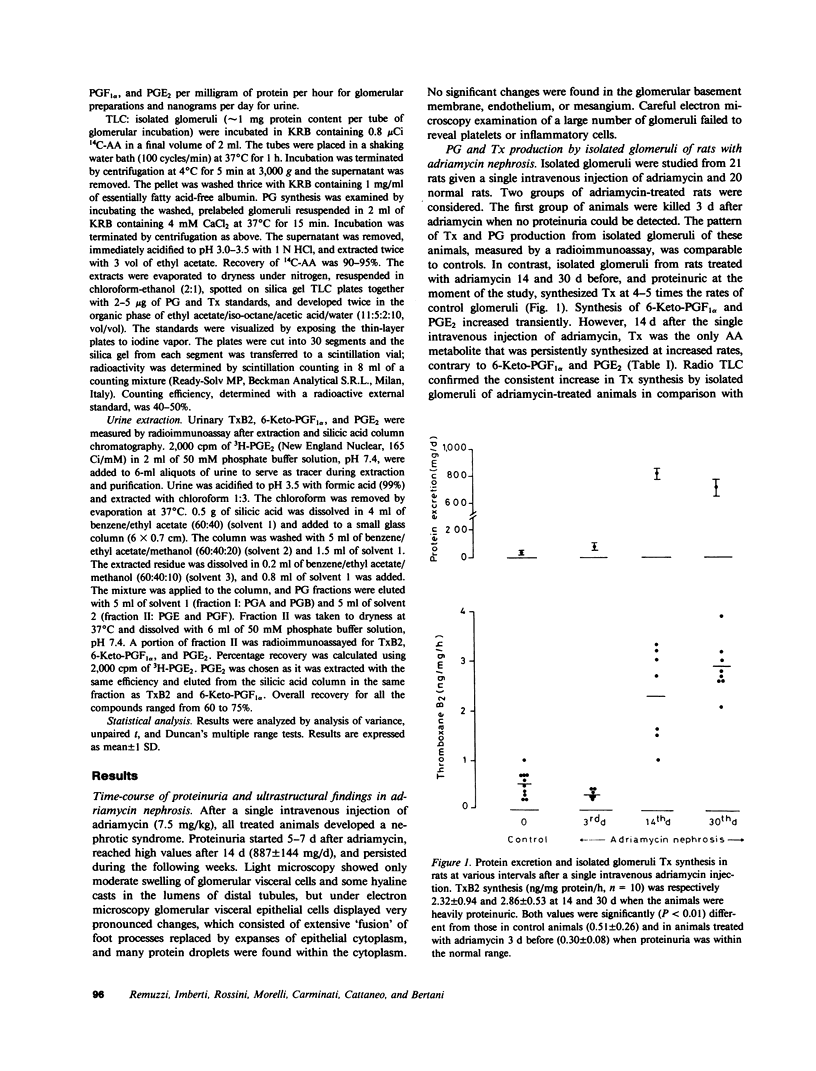
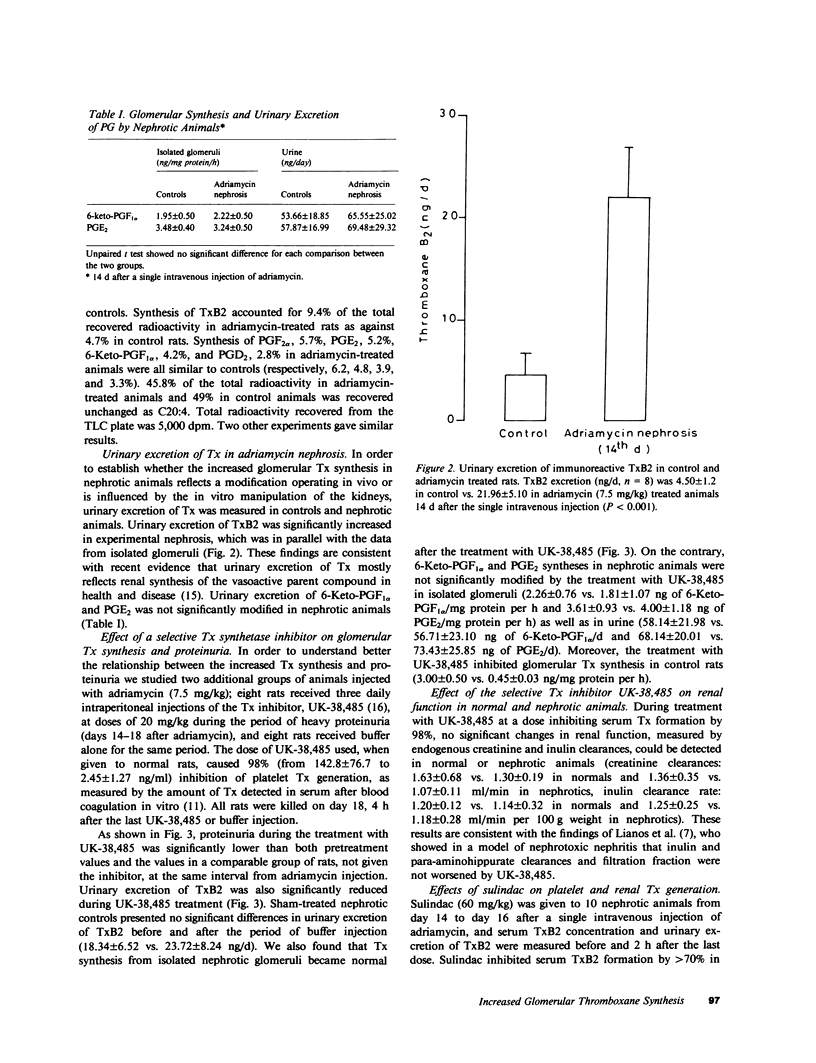
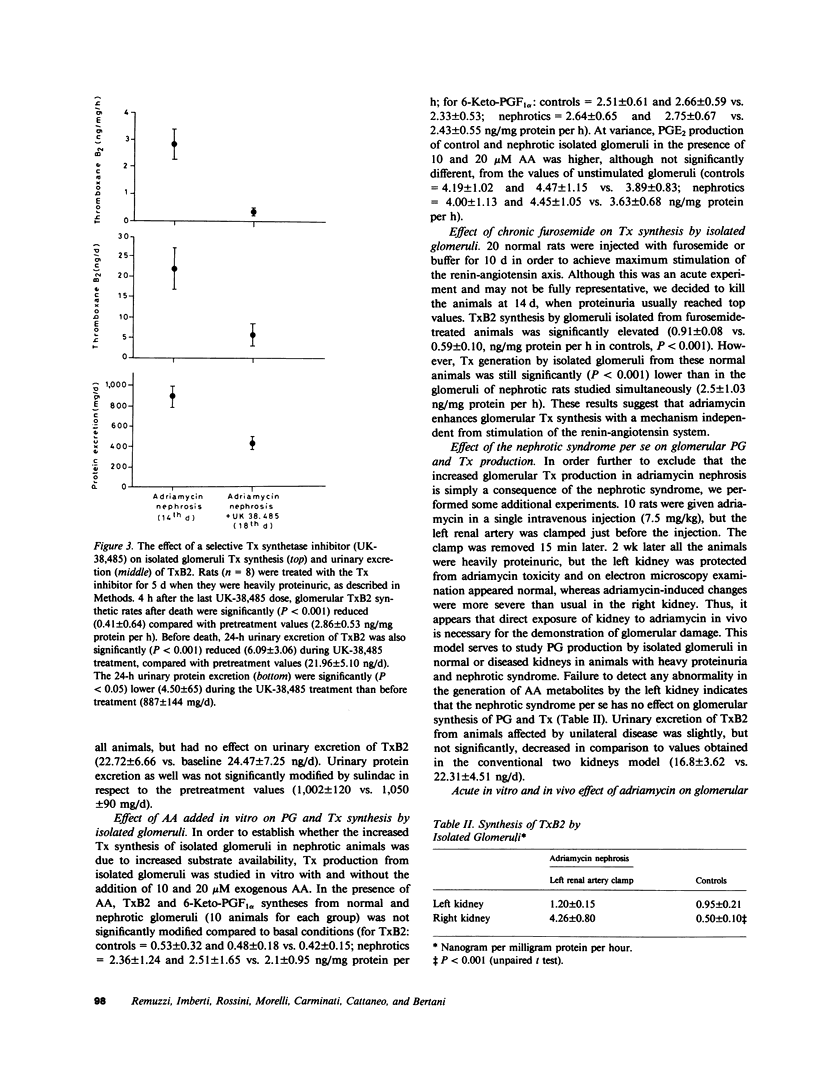
Altered glomerular metabolism of arachidonic acid (AA) has already been demonstrated in experimental nephrotoxic nephritis. The enhanced synthesis of thromboxane A2 (TxA2) in isolated glomeruli that has been found may mediate changes in renal hemodynamics. The objectives of this investigation were: to check whether glomerular AA metabolism is also altered in a model of glomerulopathy in which no leukocyte infiltration or platelet deposition could be demonstrated; to establish a correlation between the altered AA metabolism and proteinuria; and to explore whether the alteration of the prostaglandin (PG) pathway found in isolated glomeruli is an in vitro artifact or reflects a modification in vivo. We used a model of glomerular damage characterized by heavy and persistent proteinuria, which was induced in the rat by a single intravenous injection of adriamycin. At light microscopy, minimal glomerular abnormalities were found in this model. Electron microscopy showed profound alterations of glomerular epithelial cells with extensive fusion of foot processes and signs of epithelial cell activation. Electron microscopy of numerous glomeruli showed no platelet deposition or macrophage and leukocyte infiltration in this model. Isolated glomeruli from nephrotic rats studied 14 or 30 d after a single intravenous injection of adriamycin (7.5 mg/kg) when animals were heavily proteinuric generated significantly more TxB2, the stable breakdown product of TxA2, than normal glomeruli. No significant changes were found in the other major AA metabolites formed through cyclooxygenase. Urinary excretion of immunoreactive TxB2 was also significantly higher in nephrotic than in normal animals. Administration of a selective Tx synthetase inhibitor, UK-38,485, from day 14 to day 18 after adriamycin resulted in a significant reduction of proteinuria compared with pretreatment values. Glomerular synthesis and urinary excretion of TxB2 were normal during the UK-38,485 treatment. Additional experiments showed that elevated glomerular synthesis and urinary excretion of TxB2 were not a consequence of increased substrate availability. Maximal stimulation of the renin-angiotensin axis with furosemide increased glomerular TxB2 synthesis in normal rats, which was significantly lower than in nephrotic animals. Finally, experiments using a unilateral model of adriamycin nephrosis indicated that the enhancement of glomerular TxB2 synthesis is not simply a consequence of the nephrotic syndrome. We conclude that: there is an abnormality of glomerular AA metabolism in nephritic syndrome, which leads to increased TxA2 production; the increased Tx generation correlates with protein excretion and might be responsible for altering the glomerular basement membrane permeability to protein; and the alteration found in isolated glomeruli probably reflects a modification in vivo, in that urinary excretion of immunoreactive TxB2 is also consistently increased in adriamycin nephrosis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benabe J. E., Klahr S., Hoffman M. K., Morrison A. R. Production of thromboxane A2 by the kidney in glycerol-induced acute renal failure in the rabbit. Prostaglandins. 1980 Mar;19(3):333–347. doi: 10.1016/0090-6980(80)90069-6. [DOI] [PubMed] [Google Scholar]
- Bertani T., Poggi A., Pozzoni R., Delaini F., Sacchi G., Thoua Y., Mecca G., Remuzzi G., Donati M. B. Adriamycin-induced nephrotic syndrome in rats: sequence of pathologic events. Lab Invest. 1982 Jan;46(1):16–23. [PubMed] [Google Scholar]
- Dighe K. K., Smith G. W., Ungar A., Whelpdale P. H. Renal prostaglandins in renal hypertensive dogs. Clin Sci Mol Med. 1978 May;54(5):561–566. doi: 10.1042/cs0540561. [DOI] [PubMed] [Google Scholar]
- FitzGerald G. A., Pedersen A. K., Patrono C. Analysis of prostacyclin and thromboxane biosynthesis in cardiovascular disease. Circulation. 1983 Jun;67(6):1174–1177. doi: 10.1161/01.cir.67.6.1174. [DOI] [PubMed] [Google Scholar]
- Folkert V. W., Schlondorff D. Prostaglandin synthesis in isolated glomeruli. Prostaglandins. 1979 Jan;17(1):79–86. doi: 10.1016/0090-6980(79)90077-7. [DOI] [PubMed] [Google Scholar]
- Frölich J. C., Wilson T. W., Sweetman B. J., Smigel M., Nies A. S., Carr K., Watson J. T., Oates J. A. Urinary prostaglandins. Identification and origin. J Clin Invest. 1975 Apr;55(4):763–770. doi: 10.1172/JCI107987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARE R. S. Endogenous creatinine in serum and urine. Proc Soc Exp Biol Med. 1950 May;74(1):148–151. doi: 10.3181/00379727-74-17837. [DOI] [PubMed] [Google Scholar]
- Hassid A., Konieczkowski M., Dunn M. J. Prostaglandin synthesis in isolated rat kidney glomeruli. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1155–1159. doi: 10.1073/pnas.76.3.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lianos E. A., Andres G. A., Dunn M. J. Glomerular prostaglandin and thromboxane synthesis in rat nephrotoxic serum nephritis. Effects on renal hemodynamics. J Clin Invest. 1983 Oct;72(4):1439–1448. doi: 10.1172/JCI111100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michielsen P., Verberckmoes R., Desmet V., Hermerijckx W. Evolution histologique des glomérulonéphrites prolifératives diffuses traitées par l'indométhacine. J Urol Nephrol (Paris) 1969 Apr-May;75(4):315–318. [PubMed] [Google Scholar]
- Morrison A. R., Nishikawa K., Needleman P. Thromboxane A2 biosynthesis in the ureter obstructed isolated perfused kidney of the rabbit. J Pharmacol Exp Ther. 1978 Apr;205(1):1–8. [PubMed] [Google Scholar]
- Patrignani P., Filabozzi P., Catella F., Pugliese F., Patrono C. Differential effects of dazoxiben, a selective thromboxane-synthase inhibitor, on platelet and renal prostaglandin-endoperoxide metabolism. J Pharmacol Exp Ther. 1984 Feb;228(2):472–477. [PubMed] [Google Scholar]
- Poggi A., Kornblihtt L., Delaini F., Colombo T., Mussoni L., Reyers I., Donati M. B. Delayed hypercoagulability after a single dose of adriamycin to normal rats. Thromb Res. 1979;16(5-6):639–650. doi: 10.1016/0049-3848(79)90208-1. [DOI] [PubMed] [Google Scholar]
- Remuzzi G., Benigni A., Dodesini P., Schieppati A., Livio M., De Gaetano G., Day S. S., Smith W. L., Pinca E., Patrignani P. Reduced platelet thromboxane formation in uremia. Evidence for a functional cyclooxygenase defect. J Clin Invest. 1983 Mar;71(3):762–768. doi: 10.1172/JCI110824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieppati A., Dodesini P., Benigni A., Massazza M., Mecca G., Remuzzi G., Livio M., de Gaetano G., Rossi E. C. The metabolism of arachidonic acid by platelets in nephrotic syndrome. Kidney Int. 1984 Apr;25(4):671–676. doi: 10.1038/ki.1984.72. [DOI] [PubMed] [Google Scholar]
- Sraer J. D., Moulonguet-Doleris L., Delarue F., Sraer J., Ardaillou R. Prostaglandin synthesis by glomeruli isolated from rats with glycerol-induced acute renal failure. Circ Res. 1981 Sep;49(3):775–783. doi: 10.1161/01.res.49.3.775. [DOI] [PubMed] [Google Scholar]
- Sraer J., Ardaillou N., Sraer J. D., Ardaillou R. In vitro prostaglandin synthesis by human glomeruli and papillae. Prostaglandins. 1982 Jun;23(6):855–864. doi: 10.1016/0090-6980(82)90129-0. [DOI] [PubMed] [Google Scholar]
- Swartz S. L., Williams G. H., Hollenberg N. K., Levine L., Dluhy R. G., Moore T. J. Captopril-induced changes in prostaglandin production: relationship to vascular responses in normal man. J Clin Invest. 1980 Jun;65(6):1257–1264. doi: 10.1172/JCI109788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber P. C., Scherer B., Larsson C. Increase of free arachidonic acid by furosemide in man as the cause of prostaglandin and renin release. Eur J Pharmacol. 1977 Feb 7;41(3):329–332. doi: 10.1016/0014-2999(77)90326-0. [DOI] [PubMed] [Google Scholar]
- Yoshida N., Aoki N. Release of arachidonic acid from human platelets. A key role for the potentiation of platelet aggregability in normal subjects as well as in those with nephrotic syndrome. Blood. 1978 Nov;52(5):969–977. [PubMed] [Google Scholar]
- Zipser R., Myers S., Needleman P. Exaggerated prostaglandin and thromboxane synthesis in the rabbit with renal vein constriction. Circ Res. 1980 Aug;47(2):231–237. doi: 10.1161/01.res.47.2.231. [DOI] [PubMed] [Google Scholar]


