Abstract
Engineered functional organs or tissues, created with autologous somatic cells and seeded on biodegradable or hydrogel scaffolds, have been developed for use in individuals with tissue damage suffered from congenital disorders, infection, irradiation, or cancer. However, in those patients, abnormal cells obtained by biopsy from the compromised tissue could potentially contaminate the engineered tissues. Thus, an alternative cell source for construction of the neo-organ or functional recovery of the injured or diseased tissues would be useful. Recently, we have found stem cells existing in the urine. These cells are highly expandable, and have self-renewal capacity, paracrine properties, and multi-differentiation potential. As a novel cell source, urine-derived stem cells (USCs) provide advantages for cell therapy and tissue engineering applications in regeneration of various tissues, particularly in the genitourinary tract, because they originate from the urinary tract system. Importantly, USCs can be obtained via a non-invasive, simple, and low-cost approach and induced with high efficiency to differentiate into three dermal cell lineages.
Keywords: Cell therapy, Genitourinary tract, Stem cells, Tissue regeneration, Urine
Introduction
Stem cells have shown potential as a therapeutic strategy for repair of various tissues, including genitourinary organs. Stem cell-based therapy for genitourinary tissue repair is most relevant to congenital conditions or disorders such as radiation damage, chronic inflammatory diseases, and tumors. Multiple types of stem cells have been used in preclinical animal models to repair or regenerate tissue, employing either trans-differentiation or paracrine effects to stimulate endogenous cells participating in tissue regeneration. These stem cells include pluripotent stem cells such as embryonic stem cells (ESCs); induced pluripotent stem cells (iPS),1 multipotent mesenchymal stem cells (MSCs), including bone marrow-derived mesenchymal stromal cells (BMSC)2, 3, 4, 5, 6; adipose-derived stem cells (ASCs)7; hair follicle stem cells8; and amniotic fluid stem cells.9
We recently found that a subpopulation of cells isolated from urine possess biological characteristics with stem cell characteristics, i.e. clonogenicity, cell growth patterns, expansion capacity, cell surface marker expression profiles, multipotent differentiation, pro-angiogenic paracrine effects, immune-modulatory properties, and easily-induced pluripotent stem cells. Thus, we have termed these cells “urine-derived stem cells” or USCs.10, 11, 12 These stem cells can be obtained from humans and different animal species, such as monkeys, pigs, and rabbits. Although stem cells make up a small proportion of the total cell population, they play an important role in replacing aged, injured, and diseased cells and promoting tissue regeneration from organs where they originate. USCs consistently expressed MSC/pericyte markers and some key cell surface markers, but not hematopoietic stem cell markers (except for MHC-1), endothelial cell markers (CD31), or human leukocyte antigen (locus) DR (HLA-DR). Compared to other MSCs, USCs have several advantages: i) they can be obtained regardless of a person's age, gender, or health condition (except in those urinary tract infection and anuria); ii) the cells can be collected using a simple, safe, low-cost and non-invasive procedure; iii) it is easier to isolate pure stem cells, which do not require an enzyme digestion process; iv) the cells display telomerase activity so that they are able to generate more cells, but not teratomas or tumors; and v) they differentiate into podocytes, smooth muscle, and endothelial and urothelial cells with higher efficiency.10, 11, 12
Origin of USCs
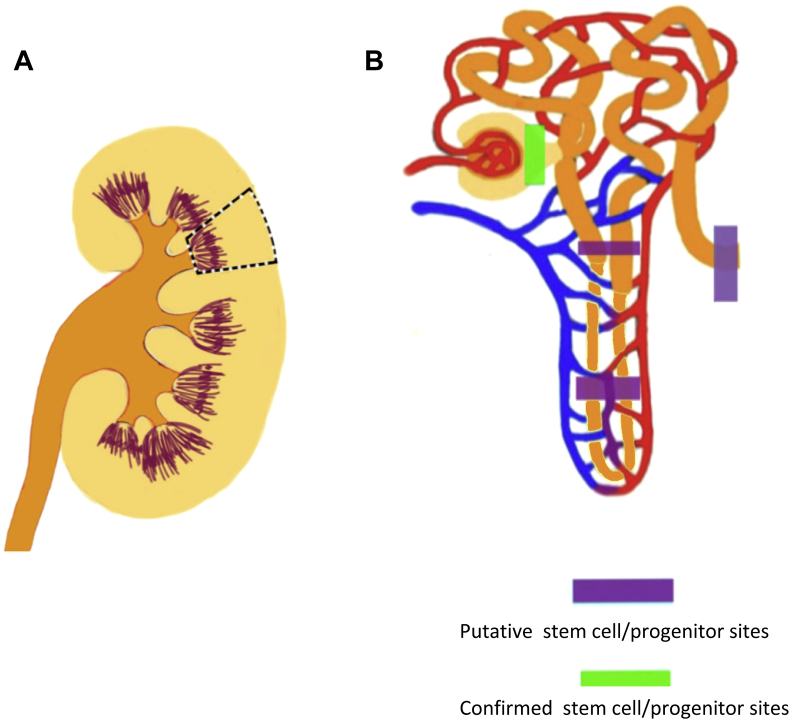
The source of endogenous stem cells in the kidney may be the renal tubules or the papilla. Glomerular parietal epithelial cells function as stem cells in the glomeruli (Fig. 1), displaying self-renewal properties and the potential to give rise to podocytes and proximal tubular cells.13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28
Figure 1.
Possible sources of endogenous renal stem cells in the kidney. (A) The dotted area depicts the potential existence of renal stem cells, which are possibly the sources of urine-derived stem cells. (B) A detailed depiction of possible locations of urine stem cells.
The epithelialmesenchymal transition (EMT) is a process by which epithelial cells lose their cell polarity to become mesenchymal stem cells; these multipotent stromal cells can differentiate into a variety of cell types in renal repair and regeneration. Parietal cells are commonly obtained from kidney tissue biopsies, but the isolation of pure parietal cells is difficult.29, 30
Identifying the origin of USCs will lead to a better understanding of the biological impact of this multipotent MSC population in the urinary tract system. There is strong evidence that USCs are most likely from glomerular parietal epithelial cells. USCs isolated from urine obtained from the upper urinary tract are similar to voided USCs in morphology, cell phenotypes, growth pattern, and differentiation capacity, suggesting that the voided USCs originate from the upper urinary tract.11, 31 In addition, urine-derived cells from women who had received transplanted kidneys from male donors contained the Y chromosome and expressed normal renal cell markers (PAX2 and PAX8), podocytes, glomerular parietal cells, and specific gene and protein markers (synaptopodin and podocin),13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 32, 33, 34, 35, 36 suggesting that USCs most likely originate from the kidney. Furthermore, USCs expressed CD146+/CD31− at a rate similar to that expressed in parietal cells and podocytes in glomerulus, while renal tubule epithelial cells, bladder and ureter urothelial and smooth muscle cells did not, implying that USCs are likely transitional cells at the parietal cell/podocyte interface originating from renal tissue10 (Fig. 2).
Figure 2.
Renal cells, including parietal cells, podocytes, and renal tubule epithelial cells, are shed off into the urine in normal physiologic conditions.
Self-renewal of USCs
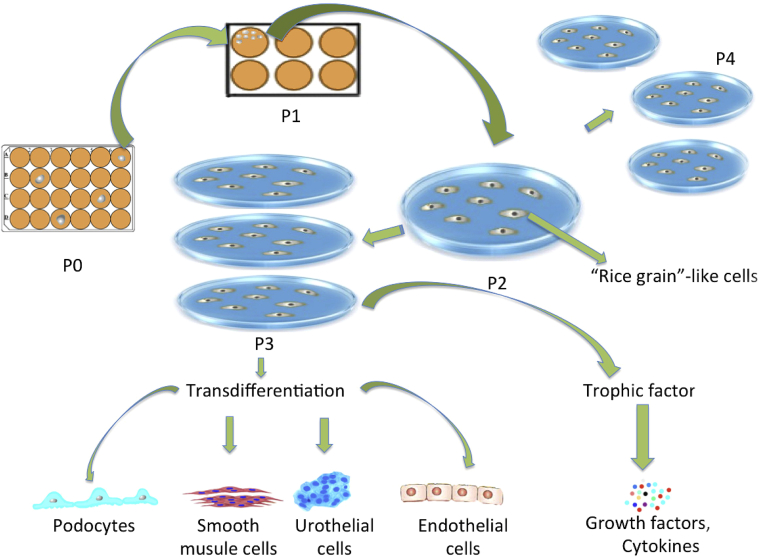
USCs can be obtained from voided urine and can generate a large number of cells from a single clone10, 37 (Fig. 3). These cells form homogenous cell types and possess highly proliferative capacity because they maintain higher telomerase activity and longer telomere length compared to other types of MSCs10 (Table 1). Upto 75% of USCs collected from middle-aged individuals expressed telomerase activity (USCs-TA+) and retained long telomere length,38 but USCs-TA+ decline to 50–60% of the USCs in people 50 years old or older. USCs-TA+ can be maintained for upto 20 passages with 67 population doublings, indicating that a single USC can generate upto 267 cells within 14 weeks. In contrast, USCs-TA− grow only for 8-10 passages with 34 population doublings. Importantly, either USCs-TA+ or USCs-TA− display normal karyotypes in culture medium even after several passages. They did not form teratomas 3 months after renal subcapsular cell implantation.38 We can now obtain 100-140 USC clones/24 h urine from each individual.39 About 1.4 × 109 cells are needed for potential use in bladder reconstruction with cell-seeded technology.40 Thus, two urine samples containing 20–30 USC clones in 400 ml can provide ample cells (1.5 × 109 USCs at p4) within 4–5 weeks to be used in cell-based therapy for genitourinary tissue or organ repair.
Figure 3.
Cell proliferation, differentiation, and trophic factor secretion by urine-derived stem cells in vitro.
Table 1.
Comparison of USCs and other types of stem cells in genitourinary tissue repair.
| Cell type/parameters | MSCs | USCs | ESC/iPS cells | Somatic cells from GU tissues |
|---|---|---|---|---|
| Original sites | Bone marrow or fat tissues | kidney | EC, SMC and UC from bladder | |
| Self-renewal and expansion capability | Limited, PD∼30, <passage 8 | High, PD 60–70 > passage 15 | Very high, PD > 200, | Limited, PD < 30, passage <8 |
| Multi-lineage differentiation capability | Multipotent, but mainly limited within mesodermal cell lineages: i.e. ostocytes, adipocytes, chondrocytes | Multipotent differentiation potential; give rise to three dermal cell lineages | Pluripotent (can form all lineages of the body) | None |
| Urothelial and endothelial differentiation capability | Low (<10%) | High (60–85%) | Low | None |
| Telomerase activity (TA)/telomere length | Cannot be detected | Upto 75% USC clones possess TA and relatively long telomeres | Possess TA and long telomeres | None |
| Harvesting methods | Invasive | Non-invasive, simple, cost-low, safe | Invasive to harvest somatic cells for iPS cells | Invasive |
| Stem cell isolation | Difficult | Very easy | Easy | None |
| Number of stem cells harvested | 1 MSC/104 bone marrow stromal cells in a newborn, 1 MSC/106 | 100–140 USCs clones/24 h urine in adult | Unknown | |
| Angiogenic trophic factors | Yes | Yes | Unknown | Moderate |
| Immnuno-modulatory properties | Yes | Yes | Unknown | Moderate |
| Rejection after implanted in vivo | No rejection reaction as allogenous or even xenogenous cells (e.g. human BMSCs, USCs) implanted in rodent, rabbit, or canine models | Likely to be rejected | No rejection as autogenous cells | |
| Teratoma formation or oncogenic potential | No | No | Yes | None |
| Preclinical Study in renal insufficiency, ED, SUI, bladder or urethral reconstruction | Effective | Effective | None, due to safety concern | Effective |
| References | 2, 3, 4, 5, 6, 53 | 11, 12, 31, 37, 39, 42, 72 | 70 | |
Abbreviation: ESC, embryonic stem cell; GU, genitourinary; PD, population doubling; BMSC, bone marrow stem cell; MSC, mesenchymal stem cell; iPS, pluripotent stem cell; TA, telomerase activity; USC, urine-derived stem cell; SMC, smooth muscle cell; UC, urothelial cell; EC, endothelial cell; ED, erectile dysfunction; SUI, stress urinary incontinence.
Multipotent differentiation of USCs
USCs can differentiate in vitro into multi-potential cells. After being induced in the appropriate culture condition, each type of differentiated USC expressed specific markers at the gene, protein, and cellular levels of osteogenic,41 chondrogenic and adipogenic myogenic,42 neurogenic43 and endothelial cell types,10 respectively. Following implantation in vivo, induced USCs can form functional bone, cartilage, fat, muscle, endothelium, and urothelium tissue.10
For ureter, bladder, or urethral tissue engineering, urothelial, endothelial, and smooth muscle cells are needed for creating urothelial mucosa, blood vessels, and muscle wall. However, a challenge in urological tissue regeneration is generating urothelial cells from BMSCs or ASCs Although BMSCs, the most commonly used MSC source, can efficiently differentiate into smooth muscle and endothelial cells,5, 6, 44, 45, 46, 47, 48, 49, 50, 51, 52 only 5–10% of BMSCs can give rise to the cells expressing urothelial gene and protein markers.5 One of the most likely reasons for this is that true stem cells in bone marrow stromal cells are very rare, depending on donor age (1/104 cells in newborns, but 1/106 in older individuals). In addition, it is very difficult to isolate pure stem cells from the large amount of somatic cells. Furthermore, it is less likely to induce stromal cells (mesodermal) to differentiate into urothelial cells (endodermal).
Using the same inductive medium as in the BMSC study,53 we found that 60%–70% of USCs differentiated into cells expressing uroepithelial cell-specific genes and protein markers (uroplakin-Ia/III), and had urothelial barrier function and tight junction ultrastructures. Urothelial differentiated USCs also expressed the genes and proteins for ZO-1, E-cadherin, and cingulin (associated with tight junctions) in a dose- and time-dependent manner. The barrier function of induced USCs reaches a maturity similar to that of urothelial cells isolated from bladder tissue 14 days after induction, significantly better than non-induced USCs, indicating that USCs possess stem cell plasticity.10
USCs can efficiently give rise to functional cells of the smooth muscle cell lineage. Myogenically-differentiated USCs expressed smoothelin, desmin, myosin, a-SM actin, and calponin at both the gene and protein levels. The mRNA and protein levels of these markers increased significantly with time in differentiation media. Functional studies demonstrated that these differentiated USCs have similar contractile properties as healthy smooth muscle cells in vitro. Myogenically-differentiated USCs formed multiple layers of smooth muscle cells beneath UC layers when subcutaneously implanted in a nude mouse model. The smooth muscle cells stained positively for a-SMA, desmin, and myosin. Scaffolds containing urothelial differentiated USCs generated stratified layers in vivo and stained positive for uroplakin-Ia and uroplakin-III (urothelial markers) and epithelial cell markers (Ck 7, Ck13, Ck20 and AE1/AE3).10, 11
We found that USCs differentiate into cells of the endothelial lineage when grown in endothelial differentiation medium containing 2 ng/ml VEGF for 12 days. Early in vitro vessel-forming was displayed 18 h after differentiated USCs (5 × 103 cells) were seeded onto Matrigel. The differentiated cells began to express the specific gene and protein markers of endothelial cells (CD31, vWF, KDR, FLT-1, FLT-1, eNOS and VE-cadherin). Induced USCs demonstrated intense immunofluorescent staining for these markers compared to non-differentiated USCs. Importantly, USCs can be efficiently differentiated into endothelial cells with barrier function. Neovessel formation occurred 4 weeks after induced USCs were subcutaneously implanted in an athymic mouse model.10
Immunoregulatory property of USCs
Regulatory T cells play an important role in induction of peripheral tolerance, inhibition of pro-inflammatory immune responses, and decreased immune reactions. USCs can impart profound immunomodulatory effects, by inhibiting proliferation of peripheral blood mononuclear cells (PBMNC) and T and B cells, and secreting interleukin (IL)-6 and IL-8.54
PBMNCs proliferated when mixed with other cells due to immune stimulation.55 However, PBMNC concentrations in USC wells were much lower than in BMSC culture wells. BrdU colorimetric ELISAs showed there was less BrdU labeled into the USC PBMNC mixed culture wells compared to BMSC culture wells. CD80 and CD86 expressed on the surface of antigen-presenting cells interact with cytotoxic T lymphocyte antigen-4 expressed on activated T cells and mediate critical T cell inhibitory signals. Flow cytometry showed that 3.35% of the BMSCs were positive for CD80 (versus 1.05% of USCs), and 1.3% of the BMSCs were positive for CD86 (versus 0.55% of USCs). Human cytokine release arrays showed that IL-6 and IL-8 concentrations were elevated after stimulation by PBMNCs in USC supernatant, which is higher than BMSC supernatant. IL-6 and IL-8 might be the main immunomodulatory cytokines to target in future studies aimed at preventing and treating diabetic bladder tissue lesions, other immune system disorders, or rejection of transplanted organs.
Trophic factors secreted by USCs and exogenous growth factors
USCs can secrete angiogenic growth factors and cytokines,56, 57 but require a favorable microenvironment to do so. We demonstrated that use of genetically modified stem cells via transfection of the VEGF gene significantly promoted myogenic differentiation of USCs and induced angiogenesis and innervation.58 However, virally delivered VEGF caused severe side effects in our animal model, including hyperemia, hemorrhage, and even death.42 Thus, a safer approach is needed for stem cell therapy to increase angiogenesis and promote muscle regeneration. Adding exogenous angiogenic factors into biodegradable polymers as delivery vehicles can be beneficial to promote regeneration and tissue healing.59 Alginate is one of the most commonly used natural hydrogels as an aqueous drug carrier for encapsulation because of its mild gelling conditions and tunable microsphere characteristics. Alginate microbeads also resist protein adsorption, making them attractive for in vivo studies.60 Alginate microbeads deliver molecules in a controlled fashion, which can stably release active FGF-1 for at least 3 weeks in vitro. This sustained release of FGF-1 promoted neovascularization in vivo without any side effects.61, 62, 63 More recently, we found that a combination of growth factors (VEGF, IGF-1, FGF-1, PDGF, HGF and NGF) released locally from alginate microbeads induced USCs to differentiate into a myogenic lineage, enhanced revascularization and innervation, and stimulated resident cell growth in vivo.42 In addition, when cultured on 3D biomaterial, stem cells had significantly enhanced cell viability, proliferation, and differentiation in vitro; and promoted tissue formation in vivo, compared to cells cultured on 2-dimensional plates.64
iPS cells reprogrammed from USCs
The ability to reprogram iPS cells from adult somatic cells could promote broad biomedical applications of stem cells, such as drug developments and tissue regeneration. Despite successful converting to iPS cells from various types of somatic cells, the process is time-consuming and inefficient. In our recent study, we created iPS cells from the urine of a child with Duchenne muscular dystrophy.65 The cells found in the patient's urine were reprogrammed to iPS more rapidly and with greater efficiency than fibroblasts or other mesenchymal cells. Following the forced expression of the four reprogramming factors oct4, sox2, klf4 and c-myc, urine cells from a healthy volunteer and a patient with Duchenne muscular dystrophy were successfully reprogrammed into iPS cells in 12 days, whereas it required more than 3 weeks to reprogram human skin fibroblasts. Cells harbored in the urine intrinsically expressed two canonical reprogramming factors, c-myc and klf4, together with highly active telomerase. The latter characteristic of urine cells contributes to improved reprogramming efficiency. Our data and other investigators' studies66, 67, 68, 69, 70 demonstrate the feasibility of rapid and efficient iPS cell generation from a sample of human urine, suggesting potential therapeutic uses of patient- derived iPS cells both for the treatment of various genetic defects via cell-based therapy and for drug screening.
Applications of USCs in animal studies
Diabetic erectile dysfunction
As with other MSCs,44, 45, 46, 47, 48, 49, 50, 51, 52, 71 we recently determined that either human USCs or USCs genetically modified with FGF2 improved erectile dysfunction in a type 2 diabetic rat model.56 USCs collected from human healthy donors and were transfected with FGF2 (USCs-FGF2). The implanted cells were injected into the cavernous tissue and tracked 1 and 4 weeks later. Implanted USCs or USCs-FGF2 had significantly higher intracavernous pressure (ICP) and a higher ratio of ICP to mean artery pressure 28 days after ICP. Although few cells were detected within the implanted sites, histological and Western blot analyses demonstrated increased expression of endothelial and smooth muscle markers within the cavernous tissue following USC or USC-FGF2 injection. This study demonstrated that paracrine effects of USCs or USCs-FGF2 induced improvement of erectile function in type 2 diabetic rats by recruiting resident cells and increasing the endothelial expression and amounts of smooth muscle.
Urinary incontinence
Impairment of sphincter muscles or their neural and vascular support leads to stress urinary incontinence.57 To determine the feasibility of using USCs, we studied the role of USCs or USCs over-expression of human VEGF165 in collagen-I gel on angiogenesis, cell survival, cell growth, myogenic phenotype differentiation of the implanted cells, and innervation following implantation in vivo. USCs were infected with adenovirus containing the VEGF and green fluorescent protein genes. A total of 5 × 106 cells, USCs alone, USCs plus endothelial cells, or human skeletal myoblasts (as control) suspended in collagen-I gel were subcutaneously implanted into nude mice. Extensive vascularization and more implanted cells was noted in VEGF-expressing USCs groups compared to the non-VEGF groups in vivo. Significantly more cells displaying endothelial markers (CD 31 and von Willebrand's factor) and myogenic markers (myf-5, MyoD, and desmin), and regenerated nerve fibers displaying neural markers (S-100, GFAP and neurofilament) were seen in grafts of VEGF-expressing USCs. Improved angiogenesis by VEGF-expressing USCs enhanced grafted cell survival, recruited resident cells, and promoted myogenic phenotype differentiation of USCs and innervation. This approach has important clinical implications for the development of cell therapies to treat stress urinary incontinence.
To provide site-specific delivery and targeted release of growth factors to implanted USCs, we prepared microbeads of alginate containing growth factors.42 The growth factors included VEGF, IGF-1, FGF-1, PDGF, HGF, and NGF. Radiolabeled growth factors were loaded separately and used to track in vitro release from the microbeads, as measured with a gamma counter over 4 weeks. In a separate experiment, in vitro endothelial differentiation of USCs via the released VEGF released from the microbeads confirmed that the released growth factors from the microbeads were bioactive. Next, USCs and microbeads were mixed with the collagen gel type 1 (2 mg/ml) and subcutaneously injected into nude mice. Four weeks after subcutaneous injection, grafted cell survival was improved and more cells expressed myogenic and endothelial cell transcripts and markers compared to controls. Compared to controls, we observed more vessel formation and innervation in USCs combined with the six growth factors and incorporated in microbeads. In conclusion, a combination of growth factors released locally from the alginate microbeads induced USCs to differentiate into a myogenic lineage, enhanced revascularization and innervation, and stimulated resident cell growth in vivo. This approach could potentially be used for cell therapy in the treatment of stress urinary incontinence. Our most recent studies demonstrated that implanted USCs restored sphincter function by increasing leak point pressure (LPP) in a rat model one week after vaginal distention injury (unpublished data).
Bladder or urethral reconstruction
The ideal stem cell sources for bladder repair would i) be able to differentiate into functional smooth muscle, urothelial, endothelial, and peripheral neurocytes with high efficiency. These promote bladder contractility and compliance, and restore histological structures with innate vasculature and innervation; ii) allow collection via a non-invasive, simple, safe, and low-cost method; iii) have universal or ‘off the shelf’ availability; and iv) generate tissue-specific or organ-specific stem cells from the urinary tract system. Currently, it is unknown whether such a ‘perfect’ stem cell exists. We do know, however, that certain cell types are more favorable than others. Although BMSCs or adipose stem cells are the most commonly used MSCs, they have some limitations in tissue engineering technology applications for lower urinary tract reconstruction, such as low differentiation capacity (<5% of urothelial cells of endodermal lineage), short lifespan in vitro (<10 passages in BMSCs), and the need for invasive collection procedures.
To be used successfully in tissue engineering approaches for lower urinary tract reconstruction, USCs must be directed to three types of bladder cells: smooth muscle, urothelial, and endothelial cells. Via trans-differentiation, USCs can give rise to all three types in vitro and in vivo.37, 72 In addition, when both myogenically-differentiated USCs and urothelially differentiated USCs were co-cultured on decellularized collagen matrix (such as SIS or bladder submucosa), the urothelium and muscle layer structure in vitro is similar to that of the bladder wall.37, 72 Further cystoplasty experiments in animal models are needed to test the feasibility and efficacy of USCs or the induced USCs.
In addition, we found that USCs differentiated into podocytes and renal tubule epithelial cells in vitro and significantly enhanced renal function after implantation of USCs by reducing collagen deposition and inhibiting fibrosis formation in a rodent model of chronic renal failure (unpublished data).
Future directions
To use stem cell therapy more efficiently for tissue repair or regeneration requires enhancing angiogenesis for survival of grafted cells, inducing innervation for functional recovery, and developing more suitable biomaterials in the future. Increasing the ratio of cell retention and improving long-term engraftment after cell implantation will lead to better genitourinary tissue regeneration. The grafted cells start to die within the first week, most probably due to ischemia, inflammation, or apoptosis due to detachment from the extracellular matrix. It is extremely important to increase viability of implanted stem cells early after cell transplantation. Controlled release of exogenous angiogenic factors, such as genetically modified stem cells, significantly increase cell retention and induce better tissue formation. Because of safety concerns, use of viral gene transfection might not be optimal.
In addition, for tubule or hollow organ tissue engineering in urology, urothelial cells seeded on the luminal side of scaffold are often lost during surgery, washed out via the urine, or mechanically ejected via the urethral catheter. To solve these problems, several methods might help: a) using biomaterials with porous micro-structure to aid cell retention within the scaffold; b) keeping the cell-seeding scaffold construct wet in the culture media, and avoid drying it out during surgery; c) inducing angiogenesis or capillary network formation early in implantation with angiogenic growth factors released from nanoparticles or microbeads, adding growth factors (such as FGF2 or IGF-1) into binding scaffolds in the site, or pretreatment of hypoxic cells; and d) promoting revascularization (artery-capillary-venous system) at the middle or late stages after the implantation using biologically safe physical stimulation, including lower-frequency electrical stimulation or low-intensity ultrasound. These methods could extend the lifespan of implanted cells in vivo to provide better tissue repair with long-term release of paracrine factors and trans-differentiation, resident cell recruitment, anti-fibroblast formation, and anti-inflammatory and anti-apoptotic effects of MSCs. In addition, innervation is critical to create a functional bladder. Stimulating peripheral nerve growth into neobladder tissue might be more efficacious than attempting to create neurogenic differentiation of MSCs.
To achieve clinically successful results, USCs need further investigation. Although USCs restore tissue or organ function in rodent models, larger animal experiments are required before cell therapy could be attempted in clinical trials. Relevant questions include: a) What is the mechanism by which USCs generate tissue repair, trans-differentiation, or trophic effects? Is it necessary to induce USCs to differentiated target cells in vitro, or can undifferentiated stem cells achieve similar or better outcomes as pre-differentiated stem cells? b) As a novel stem cell source, can USCs be used for tissue repair and regeneration in non-urinary tract sites, such as skin, vessels, heart, lung, or liver tissue?
Summary and conclusions
As a novel cell source, USCs possess an excellent feasibility and safety profile for tissue regeneration, specifically for genitourinary tissue repair. These cells express telomerase activity and are highly expandable, but do not induce teratomas or tumors in vivo. USCs not only efficiently give rise to podocytes, myocytes, and endothelial and urothelial cells, they also secrete a battery of growth factors and cytokines. Preclinical outcomes of cell therapy with USCs have been positive in models of diabetic erectile function, stress urinary incontinence, urethra and bladder reconstruction, and renal insufficiency. USCs can be obtained via a non-invasive, simple, safe and low-cost approach. Besides genitourinary tissue repair, USCs might also be a viable cell source for cell-based therapy in treatment of tissue defects or diseases in other systems.
Conflicts of interest
All authors have none to declare.
Acknowledgments
The authors acknowledge funding support from NIH grant U01CA166886 (X. Zhou); National Natural Science Foundation of China (No. 81100415) and (No. 81371704), Chongqing Natural Science Foundation of Committee of Science and Technology (No. CSTC, 2010BB5377), Doctoral Program of the Ministry of Education (No. 20115503120009).
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Moad M., Pal D., Hepburn A.C. A novel model of urinary tract differentiation, tissue regeneration, and disease: reprogramming human prostate and bladder cells into induced pluripotent stem cells. Eur Urol. Nov 2013;64(5):753–761. doi: 10.1016/j.eururo.2013.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma A.K., Bury M.I., Fuller N.J. Cotransplantation with specific populations of spina bifida bone marrow stem/progenitor cells enhances urinary bladder regeneration. Proc Natl Acad Sci U S A. Mar 5 2013;110(10):4003–4008. doi: 10.1073/pnas.1220764110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma A.K., Bury M.I., Marks A.J. A nonhuman primate model for urinary bladder regeneration using autologous sources of bone marrow-derived mesenchymal stem cells. Stem Cells. Feb 2011;29(2):241–250. doi: 10.1002/stem.568. [DOI] [PubMed] [Google Scholar]
- 4.Sharma A.K., Fuller N.J., Sullivan R.R. Defined populations of bone marrow derived mesenchymal stem and endothelial progenitor cells for bladder regeneration. J Urol. Oct 2009;182(4 suppl):1898–1905. doi: 10.1016/j.juro.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 5.Tian H., Bharadwaj S., Liu Y., Ma P.X., Atala A., Zhang Y. Differentiation of human bone marrow mesenchymal stem cells into bladder cells: potential for urological tissue engineering. Tissue Eng Part A. May 2010;16(5):1769–1779. doi: 10.1089/ten.tea.2009.0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tian H., Bharadwaj S., Liu Y. Myogenic differentiation of human bone marrow mesenchymal stem cells on a 3D nano fibrous scaffold for bladder tissue engineering. Biomaterials. Feb 2010;31(5):870–877. doi: 10.1016/j.biomaterials.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salem S.A., Hwie A.N., Saim A. Human adipose tissue derived stem cells as a source of smooth muscle cells in the regeneration of muscular layer of urinary bladder wall. Malays J Med Sci. Jul 2013;20(4):80–87. [PMC free article] [PubMed] [Google Scholar]
- 8.Drewa T., Joachimiak R., Kaznica A., Sarafian V., Pokrywczynska M. Hair stem cells for bladder regeneration in rats: preliminary results. Transplant Proc. Dec 2009;41(10):4345–4351. doi: 10.1016/j.transproceed.2009.08.059. [DOI] [PubMed] [Google Scholar]
- 9.De Coppi P., Bartsch G., Jr., Siddiqui M.M. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol. Jan 2007;25(1):100–106. doi: 10.1038/nbt1274. [DOI] [PubMed] [Google Scholar]
- 10.Bharadwaj S., Liu G., Shi Y. Multipotential differentiation of human urine-derived stem cells: potential for therapeutic applications in urology. Stem Cells. Sep 2013;31(9):1840–1856. doi: 10.1002/stem.1424. [DOI] [PubMed] [Google Scholar]
- 11.Bharadwaj S., Liu G., Shi Y. Characterization of urine-derived stem cells obtained from upper urinary tract for use in cell-based urological tissue engineering. Tissue Eng Part A. Aug 2011;17(15–16):2123–2132. doi: 10.1089/ten.tea.2010.0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y., McNeill E., Tian H. Urine derived cells are a potential source for urological tissue reconstruction. J Urol. Nov 2008;180(5):2226–2233. doi: 10.1016/j.juro.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 13.Swetha G., Chandra V., Phadnis S., Bhonde R. Glomerular parietal epithelial cells of adult murine kidney undergo EMT to generate cells with traits of renal progenitors. J Cell Mol Med. Feb 2011;15(2):396–413. doi: 10.1111/j.1582-4934.2009.00937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okamoto T., Sasaki S., Yamazaki T., Sato Y., Ito H., Ariga T. Prevalence of CD44-positive glomerular parietal epithelial cells reflects podocyte injury in adriamycin nephropathy. Nephron Exp Nephrol. 2013;124(3–4):11–18. doi: 10.1159/000357356. [DOI] [PubMed] [Google Scholar]
- 15.Moeller M.J., Kuppe C. Glomerular disease: the role of parietal epithelial cells in hyperplastic lesions. Nat Rev Nephrol. Jan 2014;10(1):5–6. doi: 10.1038/nrneph.2013.252. [DOI] [PubMed] [Google Scholar]
- 16.Pippin J.W., Sparks M.A., Glenn S.T. Cells of renin lineage are progenitors of podocytes and parietal epithelial cells in experimental glomerular disease. Am J Pathol. Aug 2013;183(2):542–557. doi: 10.1016/j.ajpath.2013.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Migliorini A., Angelotti M.L., Mulay S.R. The antiviral cytokines IFN-alpha and IFN-beta modulate parietal epithelial cells and promote podocyte loss: implications for IFN toxicity, viral glomerulonephritis, and glomerular regeneration. Am J Pathol. Aug 2013;183(2):431–440. doi: 10.1016/j.ajpath.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 18.Shankland S.J., Anders H.J., Romagnani P. Glomerular parietal epithelial cells in kidney physiology, pathology, and repair. Curr Opin Nephrol Hypertens. May 2013;22(3):302–309. doi: 10.1097/MNH.0b013e32835fefd4. [DOI] [PubMed] [Google Scholar]
- 19.Ueno T., Kobayashi N., Nakayama M. Aberrant Notch1-dependent effects on glomerular parietal epithelial cells promotes collapsing focal segmental glomerulosclerosis with progressive podocyte loss. Kidney Int. Jun 2013;83(6):1065–1075. doi: 10.1038/ki.2013.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang J., Pippin J.W., Vaughan M.R. Retinoids augment the expression of podocyte proteins by glomerular parietal epithelial cells in experimental glomerular disease. Nephron Exp Nephrol. 2012;121(1–2):e23–37. doi: 10.1159/000342808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smeets B., Moeller M.J. Parietal epithelial cells and podocytes in glomerular diseases. Seminars Nephrol. Jul 2012;32(4):357–367. doi: 10.1016/j.semnephrol.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 22.Ryu M., Migliorini A., Miosge N. Plasma leakage through glomerular basement membrane ruptures triggers the proliferation of parietal epithelial cells and crescent formation in non-inflammatory glomerular injury. J Pathol. 2012;228:482–494. doi: 10.1002/path.4046. [DOI] [PubMed] [Google Scholar]
- 23.Kabgani N., Grigoleit T., Schulte K. Primary cultures of glomerular parietal epithelial cells or podocytes with proven origin. PLoS One. 2012;7(4):e34907. doi: 10.1371/journal.pone.0034907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smeets B., Uhlig S., Fuss A. Tracing the origin of glomerular extracapillary lesions from parietal epithelial cells. J Am Soc Nephrol. Dec 2009;20(12):2604–2615. doi: 10.1681/ASN.2009010122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohse T., Chang A.M., Pippin J.W. A new function for parietal epithelial cells: a second glomerular barrier. Am J Physiol Renal Physiol. Dec 2009;297(6):F1566–F1574. doi: 10.1152/ajprenal.00214.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Appel D., Kershaw D.B., Smeets B. Recruitment of podocytes from glomerular parietal epithelial cells. J Am Soc Nephrol. Feb 2009;20(2):333–343. doi: 10.1681/ASN.2008070795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohse T., Pippin J.W., Vaughan M.R., Brinkkoetter P.T., Krofft R.D., Shankland S.J. Establishment of conditionally immortalized mouse glomerular parietal epithelial cells in culture. J Am Soc Nephrol. Oct 2008;19(10):1879–1890. doi: 10.1681/ASN.2007101087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wiggins J.E., Goyal M., Wharram B.L., Wiggins R.C. Antioxidant ceruloplasmin is expressed by glomerular parietal epithelial cells and secreted into urine in association with glomerular aging and high-calorie diet. J Am Soc Nephrol. May 2006;17(5):1382–1387. doi: 10.1681/ASN.2005111239. [DOI] [PubMed] [Google Scholar]
- 29.Yaoita E., Yoshida Y. Polygonal epithelial cells in glomerular cell culture: podocyte or parietal epithelial origin? Microsc Res Tech. May 15 2002;57(4):212–216. doi: 10.1002/jemt.10075. [DOI] [PubMed] [Google Scholar]
- 30.Norgaard J.O. Rat glomerular epithelial cells in culture. Parietal or visceral epithelial origin? Lab Invest. Sep 1987;57(3):277–290. [PubMed] [Google Scholar]
- 31.Chun S.Y., Kim H.T., Lee J.S. Characterization of urine-derived cells from upper urinary tract in patients with bladder cancer. Urology. 2012;79(5) doi: 10.1016/j.urology.2011.12.034. 1186. e1–7. [DOI] [PubMed] [Google Scholar]
- 32.Burnworth B., Pippin J., Karna P. SSeCKS sequesters cyclin D1 in glomerular parietal epithelial cells and influences proliferative injury in the glomerulus. Lab Invest. Apr 2012;92(4):499–510. doi: 10.1038/labinvest.2011.199. [DOI] [PubMed] [Google Scholar]
- 33.Chang A.M., Ohse T., Krofft R.D. Albumin-induced apoptosis of glomerular parietal epithelial cells is modulated by extracellular signal-regulated kinase 1/2. Nephrol Dial Transplant. 2012;27(4):1330–1343. doi: 10.1093/ndt/gfr483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ohse T., Vaughan M.R., Kopp J.B. De novo expression of podocyte proteins in parietal epithelial cells during experimental glomerular disease. Am J Physiol Renal Physiol. Mar 2010;298(3):F702–F711. doi: 10.1152/ajprenal.00428.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takeda Y., Shirato I., Hayashi K., He J.S., Tomino Y. Immunohistochemical analysis of protein gene product 9.5, a new marker for parietal epithelial cells of Bowman's capsules, in anti-glomerular basement membrane(GBM) antibody induced glomerulonephritis of WKY rats. Nihon Jinzo Gakkai shi. 2001;43(1):20–27. [PubMed] [Google Scholar]
- 36.Weinstein T., Cameron R., Katz A., Silverman M. Rat glomerular epithelial cells in culture express characteristics of parietal, not visceral, epithelium. J Am Soc Nephrol. Dec 1992;3(6):1279–1287. doi: 10.1681/ASN.V361279. [DOI] [PubMed] [Google Scholar]
- 37.Bodin A., Bharadwaj S., Wu S., Gatenholm P., Atala A., Zhang Y. Tissue-engineered conduit using urine-derived stem cells seeded bacterial cellulose polymer in urinary reconstruction and diversion. Biomaterials. Dec 2010;31(34):8889–8901. doi: 10.1016/j.biomaterials.2010.07.108. [DOI] [PubMed] [Google Scholar]
- 38.Shi Y.A., Liu G.H., Bharadwaj S., Atala A., Zhang Y. Urine derived stem cells with high telomerase activity for cell based therapy in urology. J Urol. 2012;187(4):e302. [Google Scholar]
- 39.Lang R., Liu G., Shi Y. Self-renewal and differentiation capacity of urine-derived stem cells after urine preservation for 24 hours. PLoS One. 2013;8(1):e53980. doi: 10.1371/journal.pone.0053980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Atala A., Bauer S.B., Soker S., Yoo J.J., Retik A.B. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. Apr 15 2006;367(9518):1241–1246. doi: 10.1016/S0140-6736(06)68438-9. [DOI] [PubMed] [Google Scholar]
- 41.Qin H., Zhu C., An Z. Silver nanoparticles promote osteogenic differentiation of human urine-derived stem cells at noncytotoxic concentrations. Int J Nanomedicine. 2014;9:2469–2478. doi: 10.2147/IJN.S59753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu G., Pareta R.A., Wu R. Skeletal myogenic differentiation of urine-derived stem cells and angiogenesis using microbeads loaded with growth factors. Biomaterials. Jan 2013;34(4):1311–1326. doi: 10.1016/j.biomaterials.2012.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guan J.J., Niu X., Gong F.X. Biological characteristics of human-urine-derived stem cells: potential for cell-based therapy in neurology. Tissue Eng Part A. 2014;20(13–14):1794–1806. doi: 10.1089/ten.tea.2013.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kovanecz I., Rivera S., Nolazco G. Separate or combined treatments with daily sildenafil, molsidomine, or muscle-derived stem cells prevent erectile dysfunction in a rat model of cavernosal nerve damage. J Sex Med. Nov 2012;9(11):2814–2826. doi: 10.1111/j.1743-6109.2012.02913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qiu X., Villalta J., Ferretti L. Effects of intravenous injection of adipose-derived stem cells in a rat model of radiation therapy-induced erectile dysfunction. J Sex Med. Jul 2012;9(7):1834–1841. doi: 10.1111/j.1743-6109.2012.02753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun C., Lin H., Yu W. Neurotrophic effect of bone marrow mesenchymal stem cells for erectile dysfunction in diabetic rats. Int J Androl. Aug 2012;35(4):601–607. doi: 10.1111/j.1365-2605.2012.01250.x. [DOI] [PubMed] [Google Scholar]
- 47.Ma L., Hellstrom W.J. Words of wisdom. Re: treatment of erectile dysfunction in the obese type 2 diabetic ZDF rat with adipose tissue-derived stem cells. Eur Urol. Jan 2011;59(1):168–169. doi: 10.1016/j.eururo.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 48.Huang Y.C., Ning H., Shindel A.W. The effect of intracavernous injection of adipose tissue-derived stem cells on hyperlipidemia-associated erectile dysfunction in a rat model. J Sex Med. Apr 2010;7(4 Pt 1):1391–1400. doi: 10.1111/j.1743-6109.2009.01697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garcia M.M., Fandel T.M., Lin G. Treatment of erectile dysfunction in the obese type 2 diabetic ZDF rat with adipose tissue-derived stem cells. J Sex Med. Jan 2010;7(1 Pt 1):89–98. doi: 10.1111/j.1743-6109.2009.01541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin G., Banie L., Ning H., Bella A.J., Lin C.S., Lue T.F. Potential of adipose-derived stem cells for treatment of erectile dysfunction. J Sex Med. Mar 2009;6(Suppl 3):320–327. doi: 10.1111/j.1743-6109.2008.01190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Song Y.S., Lee H.J., Park I.H., Lim I.S., Ku J.H., Kim S.U. Human neural crest stem cells transplanted in rat penile corpus cavernosum to repair erectile dysfunction. BJU Int. Jul 2008;102(2):220–224. doi: 10.1111/j.1464-410X.2008.07469.x. discussion 224. [DOI] [PubMed] [Google Scholar]
- 52.Bivalacqua T.J., Deng W., Kendirci M. Mesenchymal stem cells alone or ex vivo gene modified with endothelial nitric oxide synthase reverse age-associated erectile dysfunction. Am J Physiol Heart Circ Physiol. Mar 2007;292(3):H1278–H1290. doi: 10.1152/ajpheart.00685.2006. [DOI] [PubMed] [Google Scholar]
- 53.Zhang Y., Lin H.K., Frimberger D., Epstein R.B., Kropp B.P. Growth of bone marrow stromal cells on small intestinal submucosa: an alternative cell source for tissue engineered bladder. BJU Int. Nov 2005;96(7):1120–1125. doi: 10.1111/j.1464-410X.2005.05741.x. [DOI] [PubMed] [Google Scholar]
- 54.Wu R.P., Soland M., Liu G. The 3rd Annual Regenerative edicine Foundation Conference 2012 Abstract Book. Oct. 18–19, 2012. Immunomodulatory properties of urine derived stem cells. Charlotte, NC, USA. [Google Scholar]
- 55.Nawa Y., Teshima T., Sunami K. Responses of granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cells to alloantigen stimulation. Blood. Aug 15 1997;90(4):1716–1718. [PubMed] [Google Scholar]
- 56.Ouyang B., Sun X., Han D. Human urine-derived stem cells alone or genetically-modified with FGF2 improve type 2 diabetic erectile dysfunction in a rat model. PLoS One. 2014;9(3):e92825. doi: 10.1371/journal.pone.0092825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu G., Wang X., Sun X., Deng C., Atala A., Zhang Y. The effect of urine-derived stem cells expressing VEGF loaded in collagen hydrogels on myogenesis and innervation following after subcutaneous implantation in nude mice. Biomaterials. Nov 2013;34(34):8617–8629. doi: 10.1016/j.biomaterials.2013.07.077. [DOI] [PubMed] [Google Scholar]
- 58.Albersen M., Fandel T.M., Lin G. Injections of adipose tissue-derived stem cells and stem cell lysate improve recovery of erectile function in a rat model of cavernous nerve injury. J Sex Med. Oct 2010;7(10):3331–3340. doi: 10.1111/j.1743-6109.2010.01875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Camarata P.J., Suryanarayanan R., Turner D.A., Parker R.G., Ebner T.J. Sustained release of nerve growth factor from biodegradable polymer microspheres. Neurosurgery. Mar 1992;30(3):313–319. doi: 10.1227/00006123-199203000-00001. [DOI] [PubMed] [Google Scholar]
- 60.Rajesh Pareta J.P.M., Farney Alan C., Emmanuel C. Opara bioartificial pancreas: evaluation of crucial barriers to clinical application. In: Randhawa G., editor. Organ Donation Transplant – Public Policy Clin Perspectives. InTech; 2012. [Google Scholar]
- 61.Moya M.L., Lucas S., Francis-Sedlak M. Sustained delivery of FGF-1 increases vascular density in comparison to bolus administration. Microvasc Res. Sep 2009;78(2):142–147. doi: 10.1016/j.mvr.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 62.Moya M.L., Garfinkel M.R., Liu X. Fibroblast growth factor-1 (FGF-1) loaded microbeads enhance local capillary neovascularization. J Surg Res. May 15 2010;160(2):208–212. doi: 10.1016/j.jss.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moya M.L., Cheng M.H., Huang J.J. The effect of FGF-1 loaded alginate microbeads on neovascularization and adipogenesis in a vascular pedicle model of adipose tissue engineering. Biomaterials. Apr 2010;31(10):2816–2826. doi: 10.1016/j.biomaterials.2009.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kang S.W., Seo S.W., Choi C.Y., Kim B.S. Porous poly(lactic-co-glycolic acid) microsphere as cell culture substrate and cell transplantation vehicle for adipose tissue engineering. Tissue Eng Part C Methods. Mar 2008;14(1):25–34. doi: 10.1089/tec.2007.0290. [DOI] [PubMed] [Google Scholar]
- 65.Guan X., Mack D.L., Moreno C.M. Dystrophin-deficient cardiomyocytes derived from human urine: new biologic reagents for drug discovery. Stem Cell Res. Mar 2014;12(2):467–480. doi: 10.1016/j.scr.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou T., Benda C., Dunzinger S. Generation of human induced pluripotent stem cells from urine samples. Nat Protoc. Dec 2012;7(12):2080–2089. doi: 10.1038/nprot.2012.115. [DOI] [PubMed] [Google Scholar]
- 67.Zhou J., Wang X., Zhang S. Generation and characterization of human cryptorchid-specific induced pluripotent stem cells from urine. Stem Cells Dev. Mar 1 2013;22(5):717–725. doi: 10.1089/scd.2012.0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou T., Benda C., Duzinger S. Generation of induced pluripotent stem cells from urine. J Am Soc Nephrol. Jul 2011;22(7):1221–1228. doi: 10.1681/ASN.2011010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jia B., Chen S., Zhao Z. Modeling of hemophilia A using patient-specific induced pluripotent stem cells derived from urine cells. Life Sci. 2014;108(1):22–29. doi: 10.1016/j.lfs.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 70.Xue Y., Cai X., Wang L. Generating a non-integrating human induced pluripotent stem cell bank from urine-derived cells. PLoS One. 2013;8(8) doi: 10.1371/journal.pone.0070573. e70573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lin G., Wang G., Banie L. Treatment of stress urinary incontinence with adipose tissue-derived stem cells. Cytotherapy. 2010;12(1):88–95. doi: 10.3109/14653240903350265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu S., Liu Y., Bharadwaj S., Atala A., Zhang Y. Human urine-derived stem cells seeded in a modified 3D porous small intestinal submucosa scaffold for urethral tissue engineering. Biomaterials. Feb 2011;32(5):1317–1326. doi: 10.1016/j.biomaterials.2010.10.006. [DOI] [PubMed] [Google Scholar]