Cancer comprises hundreds of distinct molecular diseases; a revelation that has emerged from decades of innovations in genomic medicine and cancer biology. This broad disease profile has prompted the tailoring of cancer therapy to individual patients, with some clinical success1. The personalized approach relies on the incorporation of appropriate molecular species to target particular cancer cells – more specifically, molecularly-targeted therapy. So far, dozens of small-molecule receptor inhibitors and monoclonal antibodies are available for targeted cancer therapy2.
Nanoparticle-based imaging within the biomedical sciences has arisen from the need to probe the success of molecularly-targeted therapies. The field is currently advancing along two parallel paths. Firstly, nanomaterials can be used to monitor molecular and microenvironment changes associated with cancer – an approach known as molecular imaging. Secondly, the integration of imaging and therapeutic capabilities into single nanoparticle systems has been realized, permitting confirmation of drug delivery to tumour sites, image-guided surgery or image-guided selective tumour ablation – an approach termed diagnostic therapy or theranostics. In this Commentary, advances in both approaches made over the past three years are discussed in the context of personalized medicine. Despite these advances, further improvements in sensitivity and safety profiles are needed. Substantial physical, biological, economic, and regulatory-approval barriers must be overcome for both approaches to realize clinical translation.
Molecular Imaging
Molecular imaging consists of noninvasive mapping of molecular and cellular processes associated with disease progression in living systems3,4. The main advantage of in vivo molecular imaging is its ability to interrogate diseased tissues without biopsies or surgical procedures, and with information in hand, a more personalized treatment regimen can be applied5. Modalities that have been used for molecular imaging include positron emission tomography (PET), single photon emission computed tomography (SPECT), ultrasonography (US), optical imaging, and magnetic resonance imaging (MRI). These modalities differ in spatial resolution, depth penetration and detection sensitivity.
Molecular imaging of cancer must be highly sensitive because concentrations of biological molecules abnormally expressed in tumour tissues are, in general, very low (in the picomolar to nanomolar range). Nanoparticles are the ideal agent to address this requirement, as they have several properties that enhance imaging detection of biological targets: the ability to amplify contrast signal by incorporating tens of thousands of reporting elements (for example, radionuclides, fluorophores or gadolinium ions), unique physicochemical properties (for example, surface plasmon resonance, magnetic property, thermal- or pH-responsive phase change), the ability to modulate pharmacokinetics through surface chemistry and to integrate multiple functions in a single scaffold.
Imaging lymph nodes
The most established use of nanoparticle-based molecular imaging in cancer is the identification of lymph nodes involved with the draining of a primary tumour, namely, sentinel lymph nodes. For many years, Technetium 99m (99mTc)-labeled sulfur colloidal nanoparticles have been used in the clinic for mapping sentinel lymph nodes following interstitial administration. An important principle in the development of nanoparticles for molecular imaging, however, is the use of multivalent interactions to increase receptor binding avidity6. For example, attempts to develop a more sensitive and selective lymph node-seeking radiotracer have culminated in the recent approval by the United States Food and Drug Administration (FDA) of mannosylated dextran ([99mTc]tilmanocept, molecular weight ≈ 19,000 Da, diameter 7.1 nm)7. Tight binding to the target, owning to multiple mannose molecules in each dextran chain, ensures that this radiotracer remains in the first-echelon lymph nodes with minimal pass-through to second-echelon (non-sentinel) lymph nodes8. In general, multivalency effect is operational in targeted delivery of nanoparticles, regardless of the injection route. However, the density of homing ligands on the nanoparticle surface must be carefully titrated to achieve optimal results.
Imaging of angiogenesis
At present, most research on nanoparticle-based molecular imaging of tumours is focused on targeting angiogenic biomarkers. There is a clear clinical need for imaging angiogenic activity because angiogenesis is recognized as a distinct hallmark of cancer9 and targeting tumour vasculature is an important strategy for anticancer therapy. Whereas for therapeutic agents, high tissue concentration is paramount, for molecular imaging agents, the dominant prerequisite is high signal-to-background ratio (SBR). Because extravasation and extravascular transport of nanoparticles are slow processes, nanoparticles must have a reasonable half-life in blood circulation to reach their targets. This inevitably, however, increases background signal and decreases SBR. Therefore, it is not surprising that significant efforts have been devoted to developing imaging agents that target angiogenic blood vessels, for which prolonged circulation is neither a prerequisite nor a benefit. An additional benefit of nanoparticles for imaging of tumour blood vessels is that unlike small-molecule imaging probes, they have limited access to tumour cells, which may reduce unwanted binding to tumour cells and macrophages that also express such angiogenesis biomarker as αvβ3 integrin, thereby increasing specificity in the identification of angiogenic blood vessels.
Since the approval of the first antiangiogenic agent, bevacizumab, for the treatment of metastatic colorectal cancer in 200410, the FDA has approved several antiangiogenic drugs, including sorafenib (Nexavar), sunitinib (Sutent), pazopanib (Votrient), and everolimus (Afinitor). Hundreds of clinical trials are under way to evaluate the benefits of these drugs in treating cancer. The high cost of antiangiogenic treatment and the fact that only a fraction of patients respond have inspired the development of nanoparticle-based angiogenesis imaging agents for various imaging modalities, including nuclear imaging11–13, US14–16, photoacoustic imaging17, and MRI18–20 (Fig. 1a). The goals for such imaging agents are to identify the best patients for antiangiogenic drugs and to differentiate non-responders from responders who would continue to benefit from treatment.
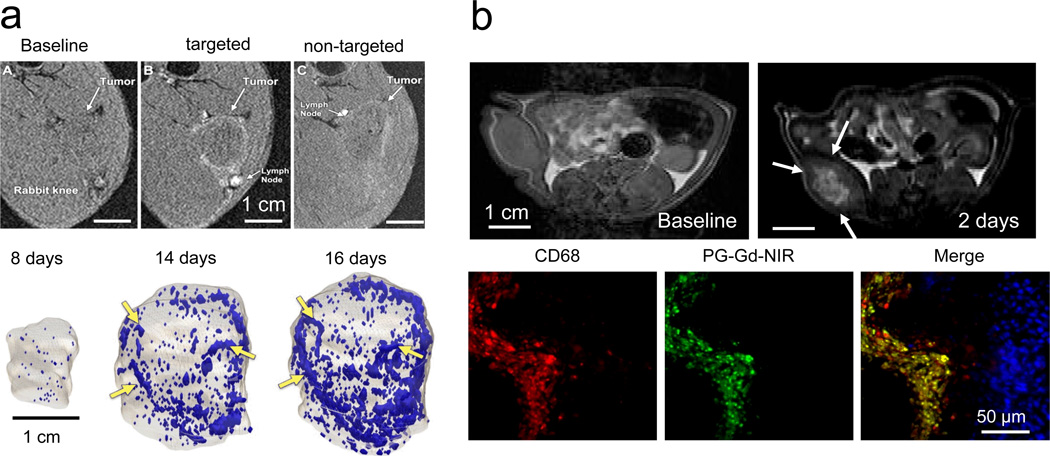
Figure 1. Examples of nanoparticles in molecular-cellular imaging.
a, In vivo T1-weighted MRI of angiogenic activity in VX2 tumours injected with αvβ3 integrin-targeted, paramagnetic perfluorocarbon nanoparticles in a rabbit model. Top: Outline of tumour periphery is clearly seen with targeted imaging agent, but not with non-targeted nanoparticles. Bottom: Neovascular maps show contrast-enhanced voxels over time. Angiogenic blood vessels markedly increased between days 8 and 14, with continued progression noted on day 16. Contrast-enhanced pixels are shown in blue. Arrows indicate examples of consistent enhancement patterns over time. Adapted from Schmieder et al.18 with permission. b, In vivo T1-weighted MRI of tumour-associated macrophages in C6 glioma in rats with poly(L-glutamic acid)-conjugated with gadolinium (Gd)-diethylenetriaminepentaacetic acid and a near infrared dye (PG-Gd-NIR813). Top: MR images acquired before and 2 days after intravenous injection of PG-Gd-NIR813. Contrast enhancement in the central rather than peripheral area of the tumour is cleared seen. Arrows indicate the tumour. Bottom: Fluorescent micrographs of tumour peri-necrotic area depicted the co-localization of PG-Gd-NIR813 (pseudo-coloured green) with tumour-associated macrophages. Tumour-associated macrophages were stained with CD68 (pseudo-coloured red). Cell nuclei were counterstained with DAPI (blue). Figure adapted from Melancon et al.24 with permission.
Nanoparticles targeting angiogenesis are probably more effective with MRI and US, than with PET and SPECT. Normally, nuclear probes (i.e. for PET and SPECT) are small molecules which are able to penetrate tissues and bind to multiple cell types. Angiogenesis biomarkers, such as the αvβ3-integrin, are highly expressed on many cell-types within an inflammatory pathology, particularly activated macrophages in the case of cancer. Thus, although detectability is very high as a consequence of multifactorial accumulation of small-molecular-weight radiotracers, the ability to diagnose malignant risk from tumours with extensive angiogenesis from lesions predominated with leukocytes participating in cancer rejection is difficult, or even, impossible. For PET or SPECT, the gain in detection sensitivity and specificity when using nanoparticles is more than offset by the increase in background noise from nanoparticles that persist in blood circulation. This means that considerable time must pass before the blood activity is low enough for adequate images to be obtained, which is clinically undesirable, particularly if a delay of many hours is needed between treatment and imaging. Also, it is difficult to match the physical half-life of the radioisotope used for nanoparticle labeling to the biological half-life of the molecular process under investigation and the half-life of nanoparticles in circulation.
MRI is particularly well suited to imaging of angiogenic status. It has excellent temporal and spatial resolution and can provide both anatomic and functional information (for example, tumour volume and information from diffusion-weighted MRI and dynamic contrast-enhanced MRI). Hak et al.21 have recently shown that it is possible to obtain quantitative information on receptor binding, internalization, and recycling dynamics using dynamic contrast-enhanced MRI and αVβ3-integrin-targeting nanoparticles containing gadolinium ions. The study combined intravital microscopy, dynamic contrast-enhanced MRI, and compartment modeling to analyze in vivo targeting kinetics of nanoparticles in the rim of a tumour. Such an approach provides insights into in vivo nanoparticle targeting that are difficult to achieve with other imaging modalities.
Imaging of tumour microenvironment
While tumour cell targeting has dominated research on small-molecular-weight-based molecular imaging, this research focus may change in the future as emerging data suggest that the cellular and extracellular matrix microenvironments, both in the primary tumour and in metastatic sites, are crucial for tumour growth22. One area where early clinical translation may be fruitful is the development of nanoparticles for imaging of macrophages, cancer-associated fibroblasts, and other components of the tumour microenvironment23. Unlike nanoparticles for imaging tumour cell targets, nanoparticles for imaging the tumour microenvironment might not need to be completely penetrant. MRI-visible nanoparticles could be taken up by circulating macrophages which in turn carry them to the tumour, making it possible to visualize tumour-associated macrophages in the peri-necrotic zone (Fig.1b)24. Clearly, a deeper understanding of the nanoparticle-microenvironment interaction is needed to design better imaging agents for this important target.
Clinical translation
While nanoparticles for sentinel lymph node imaging are well established, clinical translation of other forms of nanoparticle-based molecular imaging will be extremely challenging25. To be fully clinically translatable, the targeted imaging agents should be detectable with the imaging modality that best matches the clinical needs and related drug discovery program, demonstrate high sensitivity and high specificity compared to the current ‘gold’ standard, have an acceptable safety profile and finally, positively impact patient care.
Clinical translation and commercialization of nanoparticle-based imaging agents has been hindered, in some cases, by poor clinical performance. The history of marketing efforts for dextran-coated SPIO nanoparticles provides an illustration: This class of macrophage-avid MRI agents has been extensively studied in both preclinical and clinical settings for the assessment of lymph node metastasis23,26–28. SPIO-based products for lymph node imaging, however, were recently withdrawn from the US and European markets after several years of commercialization29. The reasons cited for the pharmaceutical companyies decision were the limited use of SPIOs by radiologists and poor reproducibility20—more specifically, the variation in physicochemical properties of the SPIOs, such as particle size, surface charge and coating material, led to a large variation in lymph node uptake of the particles. Clearly, there is a need to better understand uptake mechanisms into the target organs after intravenous administration and a need to better control the manufacturing process to ensure uniform nanoparticles with high batch-to-batch consistency.
At present, the biggest barrier to commercialization of nanoparticle-based imaging agents is their development cost. Nanoparticles for the sole use of imaging agents have a much lower return on investment and higher safety requirements than drug delivery systems, which raise billions of dollars in sales. Industry embraces nanotechnology when nanomaterials meet medical needs and the development hurdles are manageable. For nanotechnology-based imaging agents, demonstrating not only imaging of molecular targets but, more importantly, improved patient outcomes, is extremely difficult and can be costly. Therefore, investment by corporations in the development of nanoparticle-based molecular imaging agents is cautious.
Research challenges also stand in the way of broader clinical translation of nanoparticle-based molecular imaging agents. The physicochemical properties of nanoparticles need to be better understood so that circulation lifetime, biodistribution, and penetration of biological tissues can be optimized30,31. For effective imaging agents, sensitivity, which depends on target-to-background ratio, should be a top consideration. Along this line of thinking, nanoparticles smaller than 5 nm with enhanced renal clearance have been devised and tested32–34. Also, the faster nanoparticles are cleared from the body after imaging, the lower the potential for long-term toxic effects, so development of biodegradable nanoparticles is another avenue of pursuit35.
Progress on nanomaterial design often requires optimizing the balance between sensitivity and particle characteristics. Smaller nanoparticles penetrate deeper into extravascular fluid space to permit imaging of tumour-cell-specific targets, but an imaging modality such as US, sensitivity decreases with particle size. US is sensitive enough to detect a single gaseous microbubble (diameter ~1–4 µm), but its sensitivity decreases linearly with the cross-sectional area of particles36. In an attempt to optimize both tumour uptake and sensitivity, Sheeran et al. have created US contrast agents based on liquid perfluorocarbon nanodroplets37,38. Upon exposure to US pulses, these nanodroplets undergo phase transition to generate microbubbles in situ. In another approach, von Maltzahn et al. used a two-phase targeting and in vivo communication scheme involving ‘signaling’ modules and ‘receiving’ nanoparticles to amplify nanoparticle delivery39. It may be possible to extend this approach to the detection of extravascular molecular targets using smaller nanoparticles if the ‘modules’ anchored to the tumour cells can be made to transmit information to circulating nanoparticles.
Other examples of signal amplification in nanoparticles design abound. For example, nanoparticles in which chelated organic gadolinium complexes are encapsulated within fatty acid have been used to visualize enzymatic activity. After activation by lipase cleavage, the insoluble chelate becomes soluble and generates an increase in relaxivity, r1. In a rat model of acute pancreatitis, the lipase-activatable probe was successfully used for detection of early acute pancreatitis40. Another exciting development in highsensitivity detection is the design of hyperpolarized silica nanoparticles, which have been shown to be feasible for in vivo MRI41.
Theranostics
Theranostic nanomaterials, in which both imaging and therapeutic capabilities are integrated into a single platform, have shown potential for targeted drug delivery, image-guided surgery, and minimally invasive interventions. The topic of cost versus benefit of combining targeted drug delivery and imaging in nanoparticle design has been discussed by Cheng et al. recently42. The dual theranostic functionality may ensure that government and payer resources are optimally allocated and that expensive treatments are prescribed only to responding patients, and thus may have a favorable risk-benefit profile.
Image-guided surgery
Considerable efforts are being devoted to the development of targeted near-infrared fluorescent (NIRF) and surface-enhanced Raman scattering (SERS) imaging probes for evaluation of surgical margins and identification of residual disease during surgical procedures43. Recently, the impact of NIRF imaging-guided surgery on patient care was demonstrated using 5-aminolevulinic acid, a small-molecular-weight compound that is a non-fluorescent prodrug metabolically converted to fluorescent porphyrins in malignant gliomas. Tumour fluorescence derived from 5-aminolevulinic acid enabled more complete resection of contrast-enhancing tumour, leading to improved progression-free survival in patients with malignant glioma44. It is anticipated that nanoparticles for NIRF imaging with high sensitivity and specificity for various solid tumours will be developed and translated into the clinic for intraoperative guidance.
One area in which we may see early clinical translation is optical imaging to guide endoscopic surgery. Both NIRF and SERS imaging have great potential for early detection of epithelial cancers in body cavities and for ensuring clear surgical margins for minimally invasive endoscopic surgery. At present, white-light endoscopy is the standard for patient monitoring. Novel endoscope-compatible instruments and molecular imaging agents, however, are being developed for point-of-care diagnosis and treatment. For example, because colorectal cancer initially develops in the mucous membrane of the large intestine, non-absorbable, orally administered NIRF nanoparticles have been developed for endoscopic optical imaging45. Silver- and gold-based nanoparticles generate significant Raman enhancement (up to 1014 – 1015 fold over free Raman molecules) and high signal-to-noise ratio46,47. These noble metal nanoparticles may be applied topically to the inner hollow organs, such as the colon, in which situation they do not normally enter the systemic circulation, avoiding the concerns over potential toxicity associated with intravenous injection.
Nanoparticle-based dual-modality imaging probes are normally designed to be detected first by MRI, US, or nuclear imaging for presurgical assessment of deep tissue lesions, and then to be detected by optical imaging to guide intraoperative resection. Examples include PET-NIRF, SPECT-NIRF imaging probes48,49, or MRI-NIRF imaging probes50,51. The principle of this approach is that a preoperative PET, SPECT, or MRI study would direct the surgeon to the tumour site and intraoperative fluorescence visualization would then guide the surgical exposure and visual identification of the tumour.
More recently, triple-modality nanoparticle imaging probes for PET, NIRF imaging, and MRI have been synthesized and characterized. Of note is a new triple-modality MRI-photoacoustic-Raman nanoparticle described by Kircher et al.52. The agent is composed of a 60-nm gold core covered with a Raman molecular tag, a protective 30-nm silica coating, and an MRI T1 agent, 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA)-Gd. These multifunctional nanoparticles were detected by MRI, photoacoustic imaging, and Raman imaging with at least a picomolar sensitivity. Application of this probe to glioblastoma-bearing mice in vivo permitted MRI-based whole-brain preoperative macroscopic delineation of tumour, high-spatial-resolution three-dimensional photoacoustic imaging, and high-sensitivity, high-specificity, high-resolution surface imaging of tumour margins using Raman imaging. This type of triple-modality–nanoparticle approach has promise for enabling more accurate brain tumour imaging and resection52.
Selective tumour ablation
Nanoparticles with unique physicochemical properties have been created to interact with external energy sources, such as ionizing radiation, radiofrequency, light, and ultrasound, to mediate chemical, thermal, or mechanical effects for selective tumour ablation. The addition of heat to activate release of drug payload from nanocarriers can further improve antitumour efficacy. Numerous examples of this approach exist, including ultrasound-triggered drug release from microbubbles and temperature-sensitive liposomes for enhanced drug delivery to tumours53,54, NIR laser-activated drug release and thermal ablation55,56, and radiofrequency-activated drug release from temperature-sensitive liposomes57. These minimally invasive techniques offer the important advantage of low systemic toxicity. In these applications, it is beneficial to be able to assess whether nanoparticles are selectively delivered to the tumour sites before external energy is deposited. If treatment volume and exposure time are carefully controlled, this could reduce the diffusion of heat to the surrounding normal tissues. By analogy with the use of imaging in planning and delivery of radiation therapy, it is imperative that nanoparticle-mediated thermal ablation be conducted under image guidance to monitor tumour delivery of both nanoparticles and applicators. Nanoparticles can be visualized by introducing various reporter elements such as SPIO or radiotracers55,58; a novel approach to visualizing nanoparticles is to use the intrinsic properties of nanoparticles, such as NIR surface plasmon absorption for photoacoustic imaging (Fig. 2). This approach, reported by Lu et al., shows how highly functional nanoparticles can integrate tumour specificity, drug delivery, thermal ablation, and in vivo imaging into a single entity for future personalized medicine17.
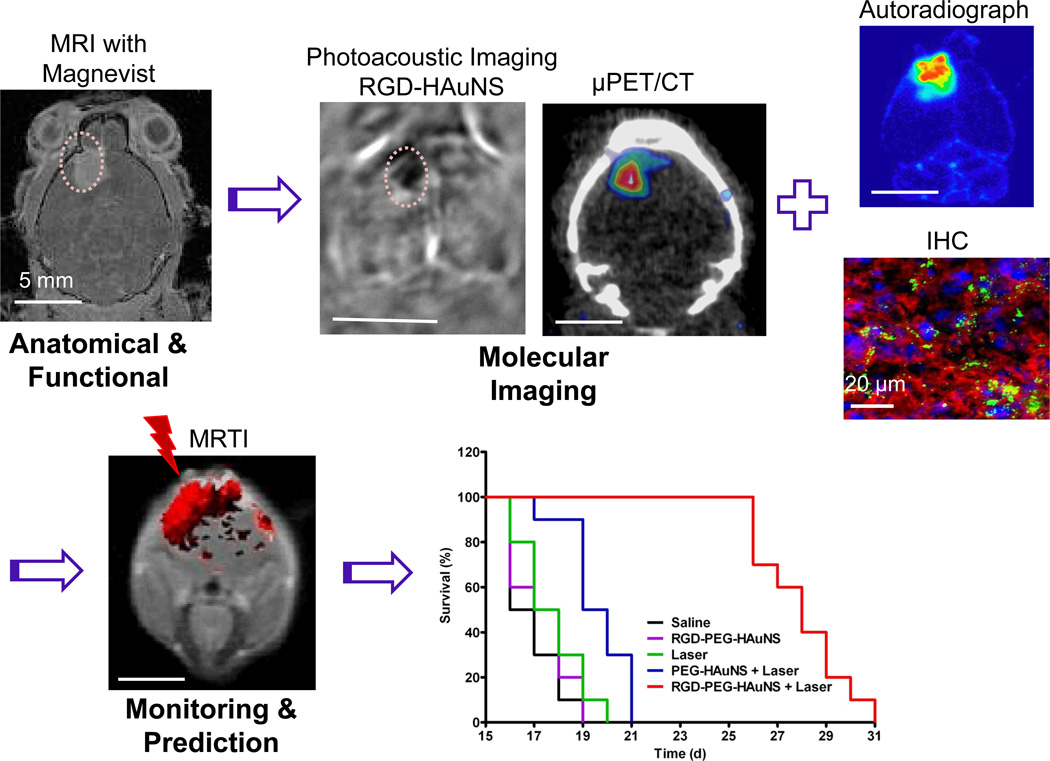
Figure 2. Image-guided minimally-invasive tumour ablation therapy mediated by molecularly targeted multifunctional nanoparticles.
In this preclinical study, photoacoustic tomography and microPET/CT with cyclic RGD peptide-conjugated hollow gold nanospheres (RGD-PEG-HAuNS) revealed the presence of integrin αvβ3-expressing U87 glioma in the brain of a mouse, which was confirmed by ex vivo analysis with autoradiography and immunohistochemical staining. Anatomical imaging with conventional MRI was also performed to confirm the presence of the tumour. Verification of tumour uptake of targeted RGD-PEG-HAuNS ensured efficient and localized heating of the tumour when it was irradiated with a near infrared laser, during which MR thermal imaging (MRTI) was used to monitor the change in temperature. Tumour-bearing mice in the group treated with intravenous injection of RGD-PEG-HAuNS plus laser lived significantly longer than mice in the groups treated with saline, RGD-PEG-HAuNS alone, laser alone, or non-targeted PEG-HAuNS plus laser. PET imaging and MRTI data may also be used for therapy planning and to predict treatment outcome using computational modeling. Adapted from Lu et al.17 with permission.
Clinical translation
A goal of targeted therapy for cancer is to give patients the right drug at the right dose at the right time. To achieve this goal, it is important to be able to report not only nanocarrier but also drug distribution to the tumour. At present, image-guided drug delivery is primarily used for preclinical optimization of the pharmacokinetics and biodistribution profiles of nanoparticles rather than the drugs they carry. In principle, it is possible to use dual-isotope SPECT to quantify and compare the biodistribution of nanocarriers and their drug payload59. Drug release and nanoparticle stability may also be assessed by optical imaging based on the quenching-dequenching phenomenon60,61. In an interesting design, liposomes loaded with a chemical shift agent and a highly fluorinated compound were used as model drugs to generate a 1H chemical exchange saturation transfer (CEST) signal. The CEST signal disappeared when the agent was released from the liposomes, while a 19F MRI signal appeared at the same time because the fluorinated compound was freed from the influence of the CEST agent in the liposomal compartment62. However, these approaches, although serving as excellent tools for preclinical studies, are very difficult if not impossible to implement under clinical settings.
Another consideration in designing theranostic nanoparticles is how to non-invasively assess treatment effect in real time and use the feed-back information to predict treatment outcome. For laser-induced photothermal ablation therapy, several FDA-cleared MR temperature imaging (MRTI) systems are commercially available. MRTI is a non-invasive method of quantifying temperature change in tissues63. Tissue damage depends on the magnitude and duration of temperature elevation and can be predicted with Arrhenius analysis64,65 or the Sapareto-Dewey isoeffect thermal dose relationship66. The implementation of these models and the development of computational tools for planning hyperthermia and ablation therapies as well as predicting heat-activated drug release deserve considerably more attention67,68. With predictive modeling and quantitative imaging to provide tumour uptake of nanoparticles as input data, nanoparticle-mediated thermal ablation therapy and drug delivery may be planned in a virtual environment to improve the likelihood of successful treatment.
Finally, increase in the level of sophistication of multifunctional nanoparticles poses significant obstacles to clinical translation. One challenge is to ensure uniform formulation of nanoparticles and batch-to-batch reproducibility. Although the benefit of increasing complexity in terms of enabling multiple functions is clear, the cost and regulatory barriers can be prohibitive42. Choi and Frangioni34 analyzed design considerations in the context of human physiology and regulatory environment and proposed three criteria to guide clinical translation of nanoparticles: degradability (complete clearance), surface charge (minimal non-specific tissue uptake) and size/shape (renal clearance). While these criteria are intended for general guidance, in the case of cancer, the first and utmost criteria should be antitumour efficacy and the therapeutic window. Innovative multifunctional theranostic nanoparticles that demonstrate significant antitumour activities in a variety of preclinical animal cancer models (ectopic, orthotopic, metastatic, syngeneic, and genetically-modified-animal models) with increased therapeutic window compared to the existing drugs may offer opportunities for success. For patients with late-stage cancer who have exhausted all existing therapies, these treatment options may prolong and improve the quality of life and deserve efforts for clinical translation.
Outlook
Multifunctional nanoparticles are attractive for combining various imaging modalities, for image-guided drug delivery and release, image-guided surgery and minimally invasive therapy. While many nanoparticle systems have been tested successfully in preclinical studies and a few have been tested in clinical trials, a myriad of obstacles must be overcome to fulfill the promise of nanoparticles in bioimaging25,69. For nanoparticle-based imaging agents, if economic and regulatory considerations prove not to be barriers, further advances in sensitivity and safety will eventually lead to improvements in clinical care.
Nanoparticle-based molecular imaging could provide spatial and temporal information regarding the presence of molecular target and the dynamics of target modulation by therapeutic intervention. Near-term developments in diagnostic nanomedicine will likely be limited to niche markets, such as characterizing tumour angiogenesis and monitoring anti-angiogenic therapy. For nanoparticle-based theranostics, imaging capabilities will help measure the dose and duration of nanoparticles delivered to the target tissues. I In addition, imaging will also make it possible to monitor response to therapy and predict treatment outcome. With photothermal conducting theranostic nanoparticles, one can envision the possibility of completely eradicating cancer cells through integration of multiple treatment modalities—e.g., photothermal ablation therapy, chemotherapy, radiotherapy, and RNA interference—into a single nanomaterial.
Research on nanoparticle-based molecular imaging and theranostics is steadily progressing. These new and complex materials require a deeper understanding of how biomaterials interact with the body on a cellular and molecular level, which in turn will lead to creation of better nanomaterials and products. Ultimately, new nanomaterials that demonstrate clear clinical benefits with a better-defined regulatory and approval process should lead to successful clinical translation. Therefore, the question is not whether nanoparticle-based biomedical imaging has a role in personalized medicine, but how and when that role will become a reality.
Acknowledgements
I would like to thank Dr. David Piwnica-Worms for helpful discussions and Stephanie Deming for editing the article. This work was supported in part by the National Cancer Institute (U54CA151668 and R01CA119387), the Viragh Family Foundation and the John S. Dunn Foundation.
References
- 1.Hamburg MA, Collins FS. N. Engl. J. Med. 2010;363:301–304. doi: 10.1056/NEJMp1006304. [DOI] [PubMed] [Google Scholar]
- 2.Zámečníkova A. Expert Opin. Drug Discov. 2014;9:77–92. doi: 10.1517/17460441.2014.865012. [DOI] [PubMed] [Google Scholar]
- 3.Mankoff DA. J. Nucl. Med. 2007;48:18N–21N. [PubMed] [Google Scholar]
- 4.Blasberg R, Piwnica-Worms D. Clin. Cancer. Res. 2012;18:631–637. doi: 10.1158/1078-0432.CCR-11-2020. [DOI] [PubMed] [Google Scholar]
- 5.Pysz MA, Gambhir SS, Willmann JK. Clin. Radiol. 2010;65:500–516. doi: 10.1016/j.crad.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weissleder R, Kelly K, Sun EY, Shtatland T, Josephson L. Nat. Biotech. 2005;23:1418–1423. doi: 10.1038/nbt1159. [DOI] [PubMed] [Google Scholar]
- 7.Vera DR, Wallace AM, Hoh CK, Mattrey RF. J. Nucl. Med. 2001;42:951–959. [PubMed] [Google Scholar]
- 8.Sondak V, et al. Ann. Surg. Oncol. 2013;20:680–688. doi: 10.1245/s10434-012-2612-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanahan D, Weinberg RA. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Hurwitz H, et al. N. Engl. J. Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 11.Morales-Avila E, et al. Bioconjug. Chem. 2011;22:913–922. doi: 10.1021/bc100551s. [DOI] [PubMed] [Google Scholar]
- 12.Yang X, et al. Biomaterials. 2011;32:4151–4160. doi: 10.1016/j.biomaterials.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen F, et al. ACS Nano. 2013;7:9027–9039. doi: 10.1021/nn403617j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pochon S, et al. Invest. Radiol. 2010;45:89–95. doi: 10.1097/RLI.0b013e3181c5927c. [DOI] [PubMed] [Google Scholar]
- 15.Deshpande N, Ren Y, Foygel K, Rosenberg J, Willmann JK. Radiology. 2011;258:804–811. doi: 10.1148/radiol.10101079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kiessling F, Fokong S, Koczera P, Lederle W, Lammers T. J. Nucl. Med. 2012;53:345–348. doi: 10.2967/jnumed.111.099754. [DOI] [PubMed] [Google Scholar]
- 17.Lu W, et al. Cancer. Res. 2011;71:6116–6121. doi: 10.1158/0008-5472.CAN-10-4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmieder AH, et al. Radiology. 2013;268:470–480. doi: 10.1148/radiol.13120789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bolley J, et al. Nanoscale. 2013;5:11478–11489. doi: 10.1039/c3nr03763k. [DOI] [PubMed] [Google Scholar]
- 20.Corot C, Warlin D. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2013;5:411–422. doi: 10.1002/wnan.1225. [DOI] [PubMed] [Google Scholar]
- 21.Hak S, et al. Angiogenesis. 2013:1–15. [Google Scholar]
- 22.Brabletz T, Lyden D, Steeg PS, Werb Z. Nat. Med. 2013;19:1104–1109. doi: 10.1038/nm.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weissleder R, Nahrendorf M, Pittet M. Nat. Mater. doi: 10.1038/nmat3780. in press. [DOI] [PubMed] [Google Scholar]
- 24.Melancon MP, et al. Biomaterials. 2010;31:6567–6573. doi: 10.1016/j.biomaterials.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lanza GM, et al. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2013;6:1–14. doi: 10.1002/wnan.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosen JE, Chan L, Shieh D-B, Gu FX. Nanomed.: Nanotechnol., Biol. 2012;8:275–290. doi: 10.1016/j.nano.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 27.Wu L, Cao Y, Liao C, Huang J, Gao F. Eur. J. Radiol. 2011;80:582–589. doi: 10.1016/j.ejrad.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 28.Harisinghani MG, et al. N. Engl. J. Med. 2003;348:2491–2499. doi: 10.1056/NEJMoa022749. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y-XJ. Quant. Imaging. Med. Surg. 2011;1:35–40. doi: 10.3978/j.issn.2223-4292.2011.08.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferrari M. Nat. Rev. Cancer. 2005;5:161–171. doi: 10.1038/nrc1566. [DOI] [PubMed] [Google Scholar]
- 31.Chauhan V, Jain RK. Nat. Mater. 2013;12:958–962. doi: 10.1038/nmat3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi HS, et al. Nat. Biotechnol. 2007;25:1165–1170. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim BH, et al. J. Am. Chem. Soc. 2011;133:12624–12631. doi: 10.1021/ja203340u. [DOI] [PubMed] [Google Scholar]
- 34.Choi HS, Frangioni JV. Mol. Imaging. 2010;9:291–310. [PMC free article] [PubMed] [Google Scholar]
- 35.Lee C-M, et al. J. Nucl. Med. 2013;54:1974–1980. doi: 10.2967/jnumed.113.122267. [DOI] [PubMed] [Google Scholar]
- 36.Unnikrishnan S, Klibanov AL. Am. J. Roentgenol. 2012;199:292–299. doi: 10.2214/AJR.12.8826. [DOI] [PubMed] [Google Scholar]
- 37.Sheeran PS, Dayton PA. Curr. Pharm. Des. 2012;18:2152–2165. doi: 10.2174/138161212800099883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sheeran PS, Luois SH, Mullin LB, Matsunaga TO, Dayton PA. Biomaterials. 2012;33:3262–3269. doi: 10.1016/j.biomaterials.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.von Maltzahn G, et al. Nat. Mater. 2011;10:545–552. doi: 10.1038/nmat3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang H-W, et al. Biomaterials. 2014;35:356–367. doi: 10.1016/j.biomaterials.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 41.Cassidy MC, Chan HR, Ross BD, Bhattacharya PK, Marcus CM. Nat. Nanotechnol. 2013;8:363–368. doi: 10.1038/nnano.2013.65. [DOI] [PubMed] [Google Scholar]
- 42.Cheng Z, Al Zaki A, Hui JZ, Muzykantov VR, Tsourkas A. Science. 2012;338:903–910. doi: 10.1126/science.1226338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Orosco RK, Tsien RY, Nguyen QT. IEEE Rev. Biomed. Eng. 2013;6:178–187. doi: 10.1109/RBME.2013.2240294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stummer W, et al. Lancet Oncol. 2006;7:392–401. doi: 10.1016/S1470-2045(06)70665-9. [DOI] [PubMed] [Google Scholar]
- 45.Sakuma S, et al. Eur. J. Pharm. Biopharm. 2011;79:537–543. doi: 10.1016/j.ejpb.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 46.Garai E, et al. J. Biomed. Optics. 2013;18:096008. doi: 10.1117/1.JBO.18.9.096008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jokerst JV, Cole AJ, Van de Sompel D, Gambhir SS. ACS Nano. 2012;6:10366–10377. doi: 10.1021/nn304347g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang R, et al. J. Nucl. Med. 2011;52:958–964. doi: 10.2967/jnumed.110.083220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ali Z, et al. Anal. Chem. 2011;83:2877–2882. doi: 10.1021/ac103261y. [DOI] [PubMed] [Google Scholar]
- 50.Cha E-J, et al. J. Controlled Release. 2011;155:152–158. doi: 10.1016/j.jconrel.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 51.Olson ES, et al. Proc. Natl. Acad. Sci. USA. 2010;107:4311–4316. doi: 10.1073/pnas.0910283107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kircher MF, et al. Nat. Med. 2012;18:829–834. doi: 10.1038/nm.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ibsen S, Schutt CE, Esener S. Drug Des. Devel. Ther. 2013;7:375–388. doi: 10.2147/DDDT.S31564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen K-J, et al. ACS Nano. 2012;7:438–446. doi: 10.1021/nn304474j. [DOI] [PubMed] [Google Scholar]
- 55.You J, et al. Cancer. Res. 2012;72:4777–4786. doi: 10.1158/0008-5472.CAN-12-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.You J, et al. J. Control Release. 2012;158:319–328. doi: 10.1016/j.jconrel.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hong CW, Libutti SK, Wood BJ. Curr. Oncol. 2013;20:e274–e277. doi: 10.3747/co.20.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Melancon MP, et al. Invest. Radiol. 2011;46:132–140. doi: 10.1097/RLI.0b013e3181f8e7d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hijnen NM, de Vries A, Nicolay K, Grüll H. Contrast Media Mol. Imaging. 2012;7:214–222. doi: 10.1002/cmmi.485. [DOI] [PubMed] [Google Scholar]
- 60.Lee HJ, et al. J. Controlled Release. 2013;172:152–158. doi: 10.1016/j.jconrel.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zou P, Chen H, Paholak HJ, Sun D. Mol. Pharm. doi: 10.1021/mp4002393. (Epub 2013/09/17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Langereis S, et al. J. Am. Chem. Soc. 2009;131:1380–1381. doi: 10.1021/ja8087532. [DOI] [PubMed] [Google Scholar]
- 63.Rieke V, Butts Pauly K. J. Magn. Reson. Imaging. 2008;27:376–390. doi: 10.1002/jmri.21265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roizin-Towle L, Pirro JP. Int. J. Radiat. Oncol. Biol. Phys. 1991;20:751–756. doi: 10.1016/0360-3016(91)90018-y. [DOI] [PubMed] [Google Scholar]
- 65.Dewhirst MW, Viglianti BL, Lora-Michiels M, Hanson M, Hoopes PJ. Int. J. Hyperthermia. 2003;19:267–294. doi: 10.1080/0265673031000119006. [DOI] [PubMed] [Google Scholar]
- 66.Sapareto SA, Dewey WC. Int. J. Radiat. Oncol. Biol. Phys. 1984;10:787–800. doi: 10.1016/0360-3016(84)90379-1. [DOI] [PubMed] [Google Scholar]
- 67.Fahrenholtz SJ, Stafford RJ, Maier F, Hazle JD, Fuentes D. Int. J. Hyperthermia. 2013;29:324–335. doi: 10.3109/02656736.2013.798036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gasselhuber A, et al. Int. J. Hyperthermia. 2010;26:499–513. doi: 10.3109/02656731003623590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dothager RS, Piwnica-Worms D. Cancer. Res. 2011;71:5611–5615. doi: 10.1158/0008-5472.CAN-11-0817. [DOI] [PubMed] [Google Scholar]