Summary
The TP53 tumour suppressor is activated in response to distinct stimuli, including an ARF-dependent response to oncogene stress and an ATM/ATR-dependent response to DNA damage. In human T-cell acute lymphoblastic leukaemia (T-ALL), TP53-dependent tumour suppression is typically disabled via biallelic ARF deletions. In murine models, loss of Arf (Cdkn2a) or Tp53 markedly accelerates the onset of Myc-induced lymphoblastic malignancies. In zebrafish, no ARF ortholog has been identified, but the sequence of ARF is very poorly conserved evolutionarily, making it difficult to exclude the presence of a zebrafish ARF ortholog without functional studies. Here we show that tp53 mutations have no significant influence on the onset of myc-induced T-ALL in zebrafish, consistent with the lack of additional effects of Tp53 loss on lymphomagenesis in Arf-deficient mice. By contrast, irradiation leads to complete T-ALL regression in tp53 wild-type but not homozygous mutant zebrafish, indicating that the tp53-dependent DNA damage response is intact. We conclude that tp53 inactivation has no impact on the onset of myc-induced T-ALL in the zebrafish, consistent with the lack of a functional ARF ortholog linking myc-induced oncogene stress to tp53-dependent tumour suppression. Thus, the zebrafish model is well suited to the study of ARF-independent pathways in T-ALL pathobiology.
Keywords: Myc, Tp53, ARF, tumour suppression, T-cell acute lymphoblastic leukaemia
Introduction
The TP53 transcription factor (also known as P53, or TRP53 in mice) is a central tumour suppressor whose activation induces apoptosis, cell cycle arrest or senescence (Junttila and Evan 2009, Levine 1997, Lu, et al 2009). Indeed, TP53-dependent tumour suppressor pathways are inactivated in most, if not all, human tumours. The TP53 protein is activated in response to distinct stimuli, including an ARF-dependent response to aberrant oncogene activation and an ATM/ATR-dependent response to DNA damage. The ARF-dependent activation of TP53, which is known to be induced by MYC overexpression, plays a central role in tumour suppression (Eischen, et al 1999, Kamijo, et al 1997, Schmitt, et al 1999, Zindy, et al 1998). Indeed, deletions of Arf (Cdkn2a) or inactivation of Tp53 each markedly accelerate the onset of T-cell acute lymphoblastic leukaemia in murine models (Blyth, et al 1995, Treanor, et al 2011, Volanakis, et al 2009). Moreover, in the setting of murine spontaneous or radiation-induced lymphomagenesis, the tumour suppressor function of TP53 is entirely dependent on ARF (Christophorou, et al 2006, Efeyan, et al 2006). In human T-cell acute lymphoblastic leukaemia (T-ALL), TP53-dependent tumour suppression is typically disabled via ARF inactivation resulting from homozygous deletions of the CDKN2A locus (encoding both ARF and p16INK4a) (Fizzotti, et al 1995, Haidar, et al 1995, Hebert, et al 1994, Okuda, et al 1995). By contrast, TP53 mutations are very rare in T-ALL clinical samples at initial diagnosis, although they do occur more frequently in heavily treated patients at the time of relapse (Diccianni, et al 1994, Hsiao, et al 1994, Wada, et al 1993).
Evolutionarily, ARF is conserved in mammals and chickens, but this gene has not been identified in amphibians or bony fish (including zebrafish and fugu) (Gilley and Fried 2001, Kim, et al 2003, Sherr 2006). A previous attempt to identify an ARF ortholog expressed in the normal liver of fugu, using 5'RACE from the locus syntenic to mammalian CDKN2A, failed to reveal an alternative reading frame transcript expressed from this locus (Gilley and Fried 2001). However, this study suffered from the fact that ARF is not expressed in normal liver cells in the absence of oncogene stress (Gromley, et al 2009, Zindy, et al 2003). In addition, the chicken ARF locus differs from its mammalian counterpart in that it encodes only ARF, with no associated CDK inhibitor expressed from the same locus, thus making chicken ARF impossible to identify via 5'RACE from a CDK locus (Kim, et al 2003). Because sequence of ARF is extremely poorly conserved evolutionarily, even among closely related mammals (Sherr 2006), it is very difficult to exclude the presence of a zebrafish ARF ortholog based on bioinformatics approaches alone, and it remains unclear whether or not zebrafish harbour a functional ortholog of ARF.
Here, we show that tp53 mutations fail to accelerate the onset of myc-induced T-ALL in the zebrafish, consistent with the lack of a functional ARF ortholog linking myc-induced oncogene stress to tp53-dependent tumour suppression in zebrafish thymocytes. We additionally find that the tp53-dependent apoptotic response to DNA damage is retained during thymocyte transformation, indicating that the tp53 axis is intact, but fails to be activated by the myc-induced stress response that is triggered by ARF in mammalian cells.
Materials and methods
Genomic sequence analysis
Human (Homo sapiens), mouse (Mus musculus), chicken (Gallus gallus), and frog (Xenopus tropicalis) genomes were accessed using the UCSC genome browser (http://genome.ucsc.edu). The zebrafish genome was accessed using the Vega Sanger genome browser (http://vega.sanger.ac.uk). Genome builds utilized were as follows: Human, GRCh37/hg19; Mouse, NCBI37/mm9; Chicken, WUGSC 2.1/galGal3; Frog, JGI 4.2/xenTro3; Zebrafish, VEGA47. Databases were accessed on November 6, 2013.
Transgenic and mutant zebrafish lines
The tp53M214K mutant, rag2:LoxP-dsRed2-stop-LoxP-EGFP-Myc transgenic, hsp70:Cre transgenic, and rag2:MYC-ER transgenic zebrafish lines have previously been described (Berghmans, et al 2005, Feng, et al 2007a, Gutierrez, et al 2011a, Langenau, et al 2005). Genotyping for tp53, hsp70:Cre, rag2:LoxP-dsRed2-stop-LoxP-EGFP-Myc, and rag2:MYC-ER transgenes was performed via polymerase chain reaction (PCR) on genomic DNA, as previously described (Berghmans, et al 2005, Feng, et al 2007a, Feng, et al 2007b, Gutierrez, et al 2011a).
Activation of conditional myc transgenes
Activation of myc expression in rag2:LoxP-dsRed2-stop-LoxP-EGFP-Myc; hsp70:Cre double-transgenic zebrafish larvae was performed via heat shock treatment at 37°C × 45 min at 6 days post-fertilization, before returning zebrafish to their baseline 28°C ambient temperature, as previously described (Feng, et al 2007a). Post-translational activation of the MYC-ER oncoprotein in rag2:MYC-ER transgenic zebrafish was performed by raising animals off-line in 750 ml of water containing 50 μg/L (129 nM) 4-hydroxytamoxifen beginning at 5 days post-fertilization, with weekly water changes, as previously described (Gutierrez, et al 2011a).
T-ALL monitoring
Zebrafish were monitored for T-ALL development via weekly fluorescence microscopy beginning at 4 weeks of age. Tumour onset was defined as development of a fluorescent mass arising from the thymus that was greater than twice the diameter of normal thymus, together with invasion of adjacent tissues and structures. Fluorescence microscopy was performed using a Nikon SMZ1500 microscope and an EXFO X-Cite 120 Fluorescence Illumination System. Images were captured using a Nikon DS2MBWc camera and Nikon NIS-Elements F Package version 3.00 software. All zebrafish images shown represent merged fluorescence (shown in green for green fluorescence protein [GFP] fluorescence) and brightfield (shown in grayscale) images. Fluorescence and brightfield images were merged using Adobe Photoshop 7.0.
Statistical analyses
Differences in tumour-free survival were assessed by the log-rank test, and time-to-event distributions were estimated using the Kaplan-Meier method. Statistical significance was defined as P < 0.05.
Results
Evolution of the INK4a/ARF/INK4b locus
The mammalian CDKN2A locus encodes two proteins, p16INK4a and ARF (p14ARF in humans, p19ARF in mice), whose transcripts originate from distinct promoters and first exons that are then spliced into the same second and third exons (Quelle, et al 1995). However, p16INK4a and ARF are transcribed using distinct reading frames through exons 2 and 3, thus they harbour no sequence homology at the protein level. In mammals, CDKN2A is located immediately downstream of CDKN2B, a locus that encodes p15INK4b (a distinct CDK inhibitor that is related to p16INK4a) but that does not contain an alternative reading frame or encode an ARF paralog (Fig 1a and b). Chickens have an ortholog of CDKN2B and ARF is also conserved in chickens, in a genomic location syntenic to mammalian ARF immediately downstream of CDKN2B, but, in the chicken, ARF is encoded by itself and the chicken does not contain an INK4a ortholog (Fig 1c) (Kim, et al 2003). In frogs and teleosts (bony fish, such as zebrafish and fugu), this genomic locus is simpler, in that they each harbour a single CDK inhibitor gene at the location syntenic to mammalian CDKN2B, but no ARF or CDKN2A orthologs have been identified within this locus or elsewhere in their respective genomes (Fig 1d and e) (Gilley and Fried 2001). Thus, the divergence of teleosts from mammals appears to have occurred prior to the duplication of INK4a/b and the evolutionary appearance of ARF.
Fig 1.
Evolution of the INK4a/ARF/INK4b locus. (A–B) The human and mouse CDKN2A loci, which encode INK4a and ARF, are shown together with the flanking loci CDKN2B (encoding INK4b) and MTAP. (C) The syntenic locus in chickens (Gallus gallus), where the ARF locus does not encode a corresponding INK4a paralog, but is syntenic to mammalian CDKN2A, between CDKN2B and MTAP. (D–E) Frogs and zebrafish genomes lack recognized ARF orthologs, and have a single cyclin-dependent kinase inhibitor gene at this locus. This CDK inhibitor is annotated as cdkn2b, which encodes p15INK4b, but shows similar sequence similarity to mammalian p16INK4a proteins, and probably represents the ancestral gene whose duplication gave rise to mammalian INK4a and INK4b.
tp53 mutations do not accelerate onset of myc-induced T-ALL in the zebrafish
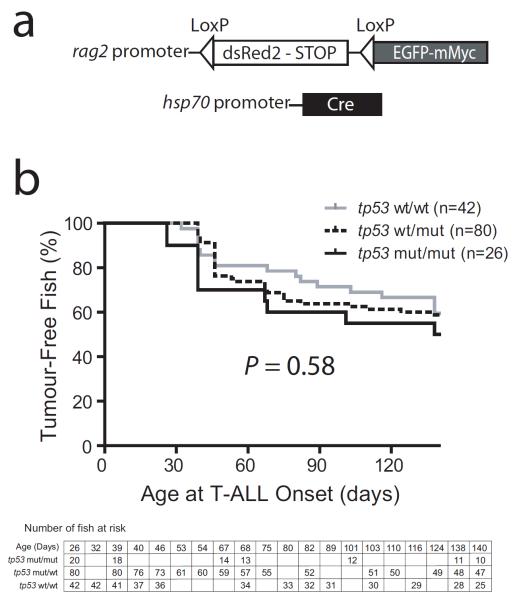
Experimental studies in mammalian systems have revealed that ARF is activated downstream of oncogene stress, which leads to induction of a robust tp53-dependent tumour suppressor response (Eischen, et al 1999, Schmitt, et al 1999, Zindy, et al 1998). To functionally test whether zebrafish harbour a functional ARF ortholog linking myc-induced oncogene stress to tp53-dependent tumour suppression, we crossed zebrafish harbouring a tp53M214K mutation, which encodes a transactivation-defective Tp53 protein (Berghmans, et al 2005), into our Cre/Lox-regulated model of myc-induced T-ALL (Fig 2a) (Feng, et al 2007a). tp53-heterozygous mutant zebrafish that also expressed rag2:LoxP-dsRed2-stop-LoxP-EGFP-Myc and hsp70:Cre transgenes were crossed to tp53-heterozygous mutant animals. Cre expression was induced in all offspring from this cross using heat shock treatment at 6 days post-fertilization (dpf), in order to excise the dsRed2-stop cassette and activate expression of EGFP-Myc. Following heat shock treatment, T-ALL onset was monitored by weekly fluorescence imaging beginning at 4 weeks of age, and tp53 genotyping was performed at the end of the experiment. We thus compared T-ALL incidence amongst tp53 wild-type (n = 42), heterozygous (n = 80), or homozygous mutant (n = 26) zebrafish that expressed both the rag2:LoxP-dsRed2-stop-LoxP-EGFP-Myc and hsp70:Cre transgenes. These numbers provided 82% power to detect a 30% difference between tp53 wild-type and homozygous mutant zebrafish, but this experiment revealed no significant influence of tp53 mutations on T-ALL onset in this line (P = 0.58; Fig 2b).
Fig 2.
Mutation of tp53 does not accelerate onset of T-ALL in the Cre-LoxP regulated conditional model. (A) Schematic depiction of the transgenes utilized in the Cre-LoxP regulated conditional model of myc-induced T-ALL. (B) Kaplan-Meier analysis of leukaemia-free survival in tp53 wild-type, heterozygous or homozygous mutant siblings. All zebrafish analysed expressed both rag2:LoxP-dsRed2-Stop-LoxP-EGFP-mMyc and hsp70:Cre transgenes, and were subjected to heat shock treatment at 6 dpf. The table indicates the number of zebrafish at risk.
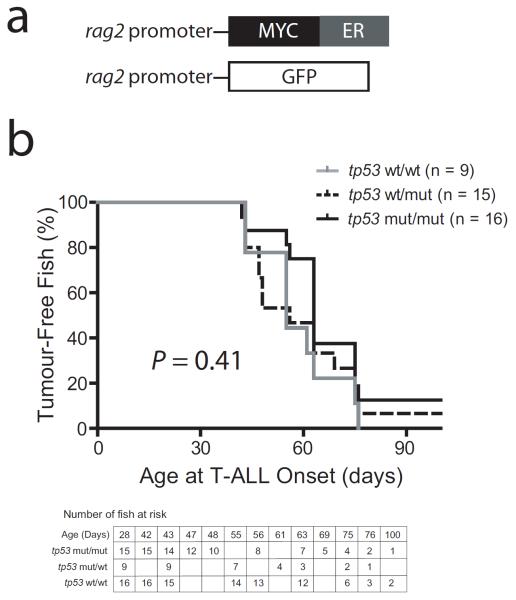
Recent work has shown that aberrant proliferation can be driven by low-level activation of Myc, below the threshold required to trigger the ARF-Tp53 tumour suppressor response (Murphy, et al 2008). Thus, we postulated that cooperation between TP53 mutations and MYC might be unmasked in a different MYC-transgenic line in which T-ALL develops with high penetrance and short latency. To address this issue, we took advantage of our rag2:MYC-ER line (Fig 3a) (Gutierrez, et al 2011a). We crossed the tp53M214K mutation into the rag2:MYC-ER line, and subsequently mated tp53-heterozygous zebrafish that also expressed rag2:MYC-ER and rag2:GFP transgenes to tp53-heterozygotes. MYC-ER activation was induced by raising offspring in 50 μg/l (129 nM) 4-hydroxytamoxifen, beginning at 5 dpf, and T-ALL onset was monitored by weekly fluorescence imaging, beginning at 4 weeks of age. We thus compared T-ALL onset amongst tp53 wild-type (n = 9), tp53 heterozygous (n = 15), or tp53 homozygous mutant (n = 16) zebrafish that each expressed both rag2:MYC-ER and rag2:GFP, numbers that provided 81% power to detect a 45% difference between the tp53 wild-type and homozygous mutant groups. This experiment revealed no detectable effect of tp53 mutations on T-ALL onset (P = 0.41; Fig 3b).
Fig 3.
Mutation of tp53 does not accelerate onset of T-ALL in the highly penetrant rag2:MYC-ER transgenic line. (A) Schematic depiction of the rag2:GFP and rag2:MYC-ER transgenes utilized. (B) Kaplan-Meier analysis of leukaemia-free survival in tp53 wild-type, heterozygous, or homozygous mutant siblings that expressed both rag2:MYC-ER and rag2:GFP transgenes. All zebrafish were raised in 50 μg/l (129 nM) 4-hydroxytamoxifen, beginning at 5 dpf, to activate the MYC-ER transgene. Table indicates the number of zebrafish at risk.
The tp53-dependent DNA damage response is retained during thymocyte transformation
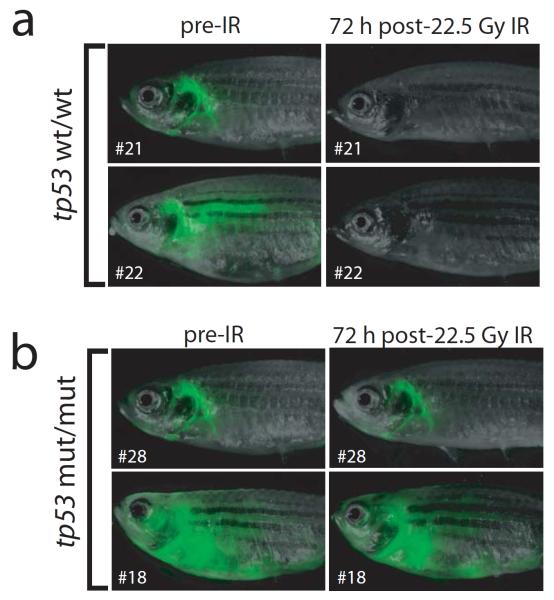
The lack of acceleration of T-ALL onset in our Myc transgenic models with mutant tp53 raises the question of whether Tp53 is functionally active at all in these tumour cells. To test whether zebrafish thymocytes are capable of inducing Tp53 function, we assessed the response of established T-ALL tumours of tp53-wild type or homozygous mutant genotypes to irradiation. Delivery of a 22.5 Gy dose of total body irradiation to tp53-wild type, rag2:MYC-ER; rag2:GFP zebrafish with T-ALL led to complete loss of all GFP-positive T-ALL cells 72 h post-irradiation in all zebrafish examined (Fig 4a; n=4). By contrast, irradiation had no discernible effect on GFP-positive T-ALL cells in any of the tp53 homozygous mutant zebrafish examined (Fig 4b; n=4). Thus, we conclude that the tp53-dependent DNA damage response is intact in myc-induced T-ALL cells of tp53 wild-type zebrafish.
Fig 4.
The tp53-dependent DNA damage response is retained during thymocyte transformation. (A) Two representative tp53-wild type rag2:MYC-ER; rag2:GFP double-transgenic zebrafish, shown at baseline and 72 h post-irradiation with 22.5 gray. (B) Two representative tp53-homozygous mutant, rag2:MYC-ER; rag2:GFP double-transgenic zebrafish, shown at baseline and 72 h post-irradiation with 22.5 Gray.
Discussion
Tumour suppressor pathways have evolved to protect long-lived organisms from malignancy. The TP53 transcription factor is a central suppressor of mammalian tumorigenesis that is activated by distinct pathways, including an ARF-dependent response to oncogene stress and ARF-independent pathways in response to DNA damage (Junttila and Evan 2009, Levine 1997, Lu, et al 2009). ARF is conserved in mammals and chickens, but ARF orthologs have not been identified in the genomes of other vertebrates including bony fish and amphibians, although the very poor sequence conservation of ARF among species makes its presence impossible to exclude via bioinformatic approaches (Gilley and Fried 2001, Kim, et al 2003, Sherr 2006). We now demonstrate that inactivation of Tp53 fails to accelerate the onset of myc-induced T-ALL in the zebrafish, consistent with findings in ARF-deficient mouse models of lymphoma (Christophorou, et al 2006, Efeyan, et al 2006). These data thus strongly suggest that zebrafish thymocytes lack a functional ARF ortholog linking myc-induced oncogenic stress to tp53-dependent tumour suppression. We have considered an alternative potential explanation for our findings, which is that the zebrafish tp53M214K mutation used in our studies may not fully abrogate tp53-dependent tumour suppression. Although we cannot rule out modest residual tumour suppressor activity in tp53M214K mutant zebrafish, this mutation has a major impact on tp53-dependent tumour suppression, as evidenced by its inability to transactivate Cdkn2a (p21) (Berghmans, et al 2005), a key mediator of tp53-dependent tumour suppression (el-Deiry, et al 1993, Martin-Caballero, et al 2001), as well as the spontaneous development of tumours in homozygous tp53M214K mutant zebrafish (Berghmans, et al 2005). Thus, the lack of any detectable interaction between Myc and Tp53 in our studies is most consistent with absence of a functional ARF ortholog in zebrafish. Moreover, we have previously shown that the zebrafish locus syntenic to mammalian INK4a/ARF/INK4b is not deleted in zebrafish myc-induced T-ALL (Langenau, et al 2005), suggesting that inactivation of ARF drives selection for biallelic INK4/ARF locus deletions in human T-ALL.
Interestingly, the evolutionary emergence of ARF correlates closely with loss of limb- and organ-regenerative capacity in adult animals, and ARF has been directly implicated in the suppression of mammalian regenerative capacity (Brockes and Kumar 2008, Pajcini, et al 2010). These findings thus suggest that emergence of the ARF-Tp53 pathway represents an evolutionary tradeoff between regenerative capacity and tumour suppression, with tumour suppression being evolutionarily favoured in long-lived organisms.
Our findings, that the zebrafish tp53M214K mutation fails to accelerate myc-induced T-ALL, contrasts with data from our laboratory and others, which demonstrate that this zebrafish tp53 mutation accelerates the onset of a range of solid tumours induced by other oncogenes, including AKT-induced liposarcoma, RAS-induced rhabdomyosarcoma and hepatocellular carcinoma, BRAF-induced melanoma, and EWSR1-FLI1-induced Ewing's sarcoma (Gutierrez, et al 2011b, Langenau, et al 2007, Leacock, et al 2012, Nguyen, et al 2011, Patton, et al 2005). Moreover, these tp53-mutant zebrafish spontaneously develop malignant peripheral nerve sheath tumours (Berghmans, et al 2005). These findings highlight the context specificity of tp53-dependent tumour suppressor pathways. Indeed, human T-ALL diagnostic specimens almost always inactivate ARF rather than TP53, whereas human rhabdomyosarcomas and osteosarcomas preferentially acquire mutations of TP53 itself. Taken together, these data thus suggest that TP53-dependent tumour suppression in MYC-induced T-ALL is largely dependent on ARF, whereas ARF-independent functions of TP53 appear to have more prominent tumour suppressor function in solid tumours.
Despite the apparent absence of a functional ARF ortholog in the zebrafish, ARF-independent tumour suppressor and oncogenic pathways are highly conserved between zebrafish and humans (White, et al 2013). Indeed, the zebrafish model has already revealed key insights into mechanisms of oncogenic transformation by canonical T-ALL oncogenes and tumour suppressors, including MYC, NOTCH, and PTEN (Blackburn, et al 2012, Gutierrez, et al 2011a, Langenau, et al 2003). Moreover, recent studies leveraging findings in the zebrafish model have revealed pathways that regulate the dissemination of localized thymic lymphomas to T-ALL, which are conserved in humans (Feng, et al 2010). Furthermore, the zebrafish model is particularly well-suited to small molecule screens to discover novel T-ALL therapeutic agents that act via ARF-independent mechanisms, as recently demonstrated (Gutierrez, et al 2014). Thus, we conclude that the zebrafish model system is ideally suited to the study of ARF-independent pathways in the molecular pathogenesis of T-ALL.
Acknowledgements
This work was supported by grants from the National Institutes of Health, USA (Grant NCI 5P01CA68484 to ATL and grants NCI 1K08CA133103 and NCI 5R21CA167124 to AG), as well as an award from the American Society of Hematology Harold Amos Medical Faculty Development Program (AG). Alejandro Gutierrez is a Research Fellow of the Gabrielle's Angel Foundation for Cancer Research. Hui Feng is supported by NIH grant NCI K99CA134743. David M. Langenau is supported by NIH grants K01AR055619, 1RO1CA154923, and 1R21CA156056, an American Cancer Society Research Scholar Grant, the Leukemia Research Foundation, the Alex Lemonade Stand Foundation, and the Harvard Stem Cell Institute.
Footnotes
The authors have no conflicts of interest to disclose.
Author Contributions AG designed and performed research and wrote the manuscript. HF, DML and YS designed and performed research and analysed data. OC performed research and analysed data. DSN analysed data. ATL supervised research and co-wrote the manuscript.
References
- Berghmans S, Murphey RD, Wienholds E, Neuberg D, Kutok JL, Fletcher CD, Morris JP, Liu TX, Schulte-Merker S, Kanki JP, Plasterk R, Zon LI, Look AT. tp53 mutant zebrafish develop malignant peripheral nerve sheath tumors. Proc Natl Acad Sci U S A. 2005;102:407–412. doi: 10.1073/pnas.0406252102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn JS, Liu S, Raiser DM, Martinez SA, Feng H, Meeker ND, Gentry J, Neuberg D, Look AT, Ramaswamy S, Bernards A, Trede NS, Langenau DM. Notch signaling expands a pre-malignant pool of T-cell acute lymphoblastic leukemia clones without affecting leukemia-propagating cell frequency. Leukemia. 2012;26:2069–2078. doi: 10.1038/leu.2012.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blyth K, Terry A, O'Hara M, Baxter EW, Campbell M, Stewart M, Donehower LA, Onions DE, Neil JC, Cameron ER. Synergy between a human c-myc transgene and p53 null genotype in murine thymic lymphomas: contrasting effects of homozygous and heterozygous p53 loss. Oncogene. 1995;10:1717–1723. [PubMed] [Google Scholar]
- Brockes JP, Kumar A. Comparative aspects of animal regeneration. Annu Rev Cell Dev Biol. 2008;24:525–549. doi: 10.1146/annurev.cellbio.24.110707.175336. [DOI] [PubMed] [Google Scholar]
- Christophorou MA, Ringshausen I, Finch AJ, Swigart LB, Evan GI. The pathological response to DNA damage does not contribute to p53-mediated tumour suppression. Nature. 2006;443:214–217. doi: 10.1038/nature05077. [DOI] [PubMed] [Google Scholar]
- Diccianni MB, Yu J, Hsiao M, Mukherjee S, Shao LE, Yu AL. Clinical significance of p53 mutations in relapsed T-cell acute lymphoblastic leukemia. Blood. 1994;84:3105–3112. [PubMed] [Google Scholar]
- Efeyan A, Garcia-Cao I, Herranz D, Velasco-Miguel S, Serrano M. Tumour biology: Policing of oncogene activity by p53. Nature. 2006;443:159. doi: 10.1038/443159a. [DOI] [PubMed] [Google Scholar]
- Eischen CM, Weber JD, Roussel MF, Sherr CJ, Cleveland JL. Disruption of the ARF-Mdm2-p53 tumor suppressor pathway in Myc-induced lymphomagenesis. Genes Dev. 1999;13:2658–2669. doi: 10.1101/gad.13.20.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- Feng H, Langenau DM, Madge JA, Quinkertz A, Gutierrez A, Neuberg DS, Kanki JP, Look AT. Heat-shock induction of T-cell lymphoma/leukaemia in conditional Cre/lox-regulated transgenic zebrafish. Br J Haematol. 2007a;138:169–175. doi: 10.1111/j.1365-2141.2007.06625.x. [DOI] [PubMed] [Google Scholar]
- Feng H, Langenau DM, Madge JA, Quinkertz A, Gutierrez A, Neuberg DS, Kanki JP, Thomas Look A. Heat-shock induction of T-cell lymphoma/leukaemia in conditional Cre/lox-regulated transgenic zebrafish. Br J Haematol. 2007b;138:169–175. doi: 10.1111/j.1365-2141.2007.06625.x. [DOI] [PubMed] [Google Scholar]
- Feng H, Stachura DL, White RM, Gutierrez A, Zhang L, Sanda T, Jette CA, Testa JR, Neuberg DS, Langenau DM, Kutok JL, Zon LI, Traver D, Fleming MD, Kanki JP, Look AT. T-lymphoblastic lymphoma cells express high levels of BCL2, S1P1, and ICAM1, leading to a blockade of tumor cell intravasation. Cancer Cell. 2010;18:353–366. doi: 10.1016/j.ccr.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fizzotti M, Cimino G, Pisegna S, Alimena G, Quartarone C, Mandelli F, Pelicci PG, Lo Coco F. Detection of homozygous deletions of the cyclin-dependent kinase 4 inhibitor (p16) gene in acute lymphoblastic leukemia and association with adverse prognostic features. Blood. 1995;85:2685–2690. [PubMed] [Google Scholar]
- Gilley J, Fried M. One INK4 gene and no ARF at the Fugu equivalent of the human INK4A/ARF/INK4B tumour suppressor locus. Oncogene. 2001;20:7447–7452. doi: 10.1038/sj.onc.1204933. [DOI] [PubMed] [Google Scholar]
- Gromley A, Churchman ML, Zindy F, Sherr CJ. Transient expression of the Arf tumor suppressor during male germ cell and eye development in Arf-Cre reporter mice. Proc Natl Acad Sci U S A. 2009;106:6285–6290. doi: 10.1073/pnas.0902310106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez A, Grebliunaite R, Feng H, Kozakewich E, Zhu S, Guo F, Payne E, Mansour M, Dahlberg SE, Neuberg DS, den Hertog J, Prochownik EV, Testa JR, Harris M, Kanki JP, Look AT. Pten mediates Myc oncogene dependence in a conditional zebrafish model of T cell acute lymphoblastic leukemia. J Exp Med. 2011a;208:1595–1603. doi: 10.1084/jem.20101691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez A, Snyder EL, Marino-Enriquez A, Zhang YX, Sioletic S, Kozakewich E, Grebliunaite R, Ou WB, Sicinska E, Raut CP, Demetri GD, Perez-Atayde AR, Wagner AJ, Fletcher JA, Fletcher CD, Look AT. Aberrant AKT activation drives well-differentiated liposarcoma. Proc Natl Acad Sci U S A. 2011b;108:16386–16391. doi: 10.1073/pnas.1106127108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez A, Pan L, Groen RW, Baleydier F, Kentsis A, Marineau J, Grebliunaite R, Kozakewich E, Reed C, Pflumio F, Poglio S, Uzan B, Clemons P, Verplank L, An F, Burbank J, Norton S, Tolliday N, Steen H, Weng AP, Yuan H, Bradner JE, Mitsiades C, Look AT, Aster JC. Phenothiazines induce PP2A-mediated apoptosis in T cell acute lymphoblastic leukemia. J Clin Invest. 2014;124:644–655. doi: 10.1172/JCI65093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haidar MA, Cao XB, Manshouri T, Chan LL, Glassman A, Kantarjian HM, Keating MJ, Beran MS, Albitar M. p16INK4A and p15INK4B gene deletions in primary leukemias. Blood. 1995;86:311–315. [PubMed] [Google Scholar]
- Hebert J, Cayuela JM, Berkeley J, Sigaux F. Candidate tumor-suppressor genes MTS1 (p16INK4A) and MTS2 (p15INK4B) display frequent homozygous deletions in primary cells from T- but not from B-cell lineage acute lymphoblastic leukemias. Blood. 1994;84:4038–4044. [PubMed] [Google Scholar]
- Hsiao MH, Yu AL, Yeargin J, Ku D, Haas M. Nonhereditary p53 mutations in T-cell acute lymphoblastic leukemia are associated with the relapse phase. Blood. 1994;83:2922–2930. [PubMed] [Google Scholar]
- Junttila MR, Evan GI. p53--a Jack of all trades but master of none. Nat Rev Cancer. 2009;9:821–829. doi: 10.1038/nrc2728. [DOI] [PubMed] [Google Scholar]
- Kamijo T, Zindy F, Roussel MF, Quelle DE, Downing JR, Ashmun RA, Grosveld G, Sherr CJ. Tumor suppression at the mouse INK4a locus mediated by the alternative reading frame product p19ARF. Cell. 1997;91:649–659. doi: 10.1016/s0092-8674(00)80452-3. [DOI] [PubMed] [Google Scholar]
- Kim SH, Mitchell M, Fujii H, Llanos S, Peters G. Absence of p16INK4a and truncation of ARF tumor suppressors in chickens. Proc Natl Acad Sci U S A. 2003;100:211–216. doi: 10.1073/pnas.0135557100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenau DM, Traver D, Ferrando AA, Kutok JL, Aster JC, Kanki JP, Lin S, Prochownik E, Trede NS, Zon LI, Look AT. Myc-induced T cell leukemia in transgenic zebrafish. Science. 2003;299:887–890. doi: 10.1126/science.1080280. [DOI] [PubMed] [Google Scholar]
- Langenau DM, Feng H, Berghmans S, Kanki JP, Kutok JL, Look AT. Cre/lox-regulated transgenic zebrafish model with conditional myc-induced T cell acute lymphoblastic leukemia. Proc Natl Acad Sci U S A. 2005;102:6068–6073. doi: 10.1073/pnas.0408708102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenau DM, Keefe MD, Storer NY, Guyon JR, Kutok JL, Le X, Goessling W, Neuberg DS, Kunkel LM, Zon LI. Effects of RAS on the genesis of embryonal rhabdomyosarcoma. Genes Dev. 2007;21:1382–1395. doi: 10.1101/gad.1545007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leacock SW, Basse AN, Chandler GL, Kirk AM, Rakheja D, Amatruda JF. A zebrafish transgenic model of Ewing's sarcoma reveals conserved mediators of EWS FLI1 tumorigenesis. Dis Model Mech. 2012;5:95–106. doi: 10.1242/dmm.007401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- Lu WJ, Amatruda JF, Abrams JM. p53 ancestry: gazing through an evolutionary lens. Nat Rev Cancer. 2009;9:758–762. doi: 10.1038/nrc2732. [DOI] [PubMed] [Google Scholar]
- Martin-Caballero J, Flores JM, Garcia-Palencia P, Serrano M. Tumor susceptibility of p21(Waf1/Cip1)-deficient mice. Cancer Res. 2001;61:6234–6238. [PubMed] [Google Scholar]
- Murphy DJ, Junttila MR, Pouyet L, Karnezis A, Shchors K, Bui DA, Brown-Swigart L, Johnson L, Evan GI. Distinct thresholds govern Myc's biological output in vivo. Cancer Cell. 2008;14:447–457. doi: 10.1016/j.ccr.2008.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen AT, Emelyanov A, Koh CH, Spitsbergen JM, Lam SH, Mathavan S, Parinov S, Gong Z. A high level of liver-specific expression of oncogenic Kras(V12) drives robust liver tumorigenesis in transgenic zebrafish. Dis Model Mech. 2011;4:801–813. doi: 10.1242/dmm.007831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda T, Shurtleff SA, Valentine MB, Raimondi SC, Head DR, Behm F, Curcio-Brint AM, Liu Q, Pui CH, Sherr CJ. Frequent deletion of p16INK4a/MTS1 and p15INK4b/MTS2 in pediatric acute lymphoblastic leukemia. Blood. 1995;85:2321–2330. [PubMed] [Google Scholar]
- Pajcini KV, Corbel SY, Sage J, Pomerantz JH, Blau HM. Transient inactivation of Rb and ARF yields regenerative cells from postmitotic mammalian muscle. Cell Stem Cell. 2010;7:198–213. doi: 10.1016/j.stem.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton EE, Widlund HR, Kutok JL, Kopani KR, Amatruda JF, Murphey RD, Berghmans S, Mayhall EA, Traver D, Fletcher CD, Aster JC, Granter SR, Look AT, Lee C, Fisher DE, Zon LI. BRAF mutations are sufficient to promote nevi formation and cooperate with p53 in the genesis of melanoma. Curr Biol. 2005;15:249–254. doi: 10.1016/j.cub.2005.01.031. [DOI] [PubMed] [Google Scholar]
- Quelle DE, Zindy F, Ashmun RA, Sherr CJ. Alternative reading frames of the INK4a tumor suppressor gene encode two unrelated proteins capable of inducing cell cycle arrest. Cell. 1995;83:993–1000. doi: 10.1016/0092-8674(95)90214-7. [DOI] [PubMed] [Google Scholar]
- Schmitt CA, McCurrach ME, de Stanchina E, Wallace-Brodeur RR, Lowe SW. INK4a/ARF mutations accelerate lymphomagenesis and promote chemoresistance by disabling p53. Genes Dev. 1999;13:2670–2677. doi: 10.1101/gad.13.20.2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr CJ. Divorcing ARF and p53: an unsettled case. Nat Rev Cancer. 2006;6:663–673. doi: 10.1038/nrc1954. [DOI] [PubMed] [Google Scholar]
- Treanor LM, Volanakis EJ, Zhou S, Lu T, Sherr CJ, Sorrentino BP. Functional interactions between Lmo2, the Arf tumor suppressor, and Notch1 in murine T-cell malignancies. Blood. 2011;117:5453–5462. doi: 10.1182/blood-2010-09-309831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volanakis EJ, Williams RT, Sherr CJ. Stage-specific Arf tumor suppression in Notch1-induced T-cell acute lymphoblastic leukemia. Blood. 2009;114:4451–4459. doi: 10.1182/blood-2009-07-233346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada M, Bartram CR, Nakamura H, Hachiya M, Chen DL, Borenstein J, Miller CW, Ludwig L, Hansen-Hagge TE, Ludwig WD, Reiter A, Mizoguchi H, Koeffler HP. Analysis of p53 mutations in a large series of lymphoid hematologic malignancies of childhood. Blood. 1993;82:3163–3169. [PubMed] [Google Scholar]
- White R, Rose K, Zon L. Zebrafish cancer: the state of the art and the path forward. Nat Rev Cancer. 2013;13:624–636. doi: 10.1038/nrc3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zindy F, Eischen CM, Randle DH, Kamijo T, Cleveland JL, Sherr CJ, Roussel MF. Myc signaling via the ARF tumor suppressor regulates p53-dependent apoptosis and immortalization. Genes Dev. 1998;12:2424–2433. doi: 10.1101/gad.12.15.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zindy F, Williams RT, Baudino TA, Rehg JE, Skapek SX, Cleveland JL, Roussel MF, Sherr CJ. Arf tumor suppressor promoter monitors latent oncogenic signals in vivo. Proc Natl Acad Sci U S A. 2003;100:15930–15935. doi: 10.1073/pnas.2536808100. [DOI] [PMC free article] [PubMed] [Google Scholar]