Abstract
Molecular sensors able to detect ions are of interest due to their potential application in areas such as pollutant sequestration. Alkynylplatinum(II) terpyridine complexes with an amide-based receptor moiety have been synthesized and characterized. Their anion binding properties based on host–guest interactions have been examined with the use of UV-vis absorption and emission spectral titration studies. Spectral changes were observed for both complexes upon the addition of spherical and nonspherical anions. Their titration profiles were shown to be in good agreement with theoretical results predicting a 1:1 binding model, and the binding constants were determined from the experimental data. Drastic color changes from yellow to orange–red were observed for one of the complexes upon titration with fluoride (F−) ion in acetone. These changes were ascribed to the deprotonation of the amide functionalities induced by F− ion, and this was confirmed by the restoration of spectral changes upon addition of trifluoroacetic acid to the F− ion–complex mixture as well as by electrospray ionization mass spectrometry (ESI-MS) data.
Keywords: host–guest systems, luminescence, molecular recognition, platinum, sensors
Molecular design for ion sensing has long been an interest of study because of its potential applications in the fields of pollutant sequestration, biomedical and environmental monitoring, as well as ion transport.1 Owing to the diverse range of geometries and basicities of different anions, complexation of anions with the receptor molecules is imposed with much more challenges and would require a delicate design of host molecules when compared with cation sensing.2 Amide-based ligands belong to the neutral-type anion receptors. Because of the highly accessible hydrogen-bond donor with directional hydrogen-bonding being involved for the host–guest interaction,2, 3 these features provide them with a differentiating power to screen anions of different geometries or hydrogen-bonding requirements,2 and they are thus commonly employed in the design of anion sensors.
Luminescent ion sensors generally consist of two components: an ion receptor site responsible for the selective recognition of the target ion, and a luminophore for signaling the binding events as an optical response due to the changes in photophysical properties upon molecular recognition. The utilization of luminescent transition metal complexes in the design of ion sensors has attracted enormous interest because of their phosphorescence nature arising from enhanced intersystem crossing as a result of the larger spin-orbit coupling favored by the presence of a heavy metal center.4 The large Stokes shifts and long excited state lifetimes render this class of luminescent ion sensors less susceptible to interference from short-lived background fluorescence signals and light scattering, which are the common problems encountered by the sensors of organic luminophores.4b Together with the high sensitivity of their photoluminescence properties towards microenvironmental changes, commonly associated with metal-to-ligand charge transfer (MLCT) excited states, the luminescent transition metal complexes could potentially be employed as the signal transduction component in the design of ion sensors.
Square-planar d8 platinum(II) polypyridine complexes are one of the well-studied classes of transition metal complexes, with intriguing spectroscopic and luminescence properties; many of these properties are found to be associated with their strong propensity to arrange themselves in oligomeric structures via Pt⋅⋅⋅Pt and ligand aromatic π–π stacking interactions.4a, 5–11 Such self-assembly behaviors have also been demonstrated for the detection of various biomacromolecules and for the monitoring of enzyme kinetics.9 In addition, our group has also demonstrated the incorporation of versatile receptor functionalities on the alkynyl ligands of the platinum(II) terpyridine complexes, with significant color changes and emission intensity enhancement upon the addition of cations.12 These studies not only suggest the strong dependence of the photophysical properties of these complexes on the microenvironmental changes upon the inclusion of ions in the receptor sites, but also illustrate that they are promising candidates for the exploration as probing sensors for ion binding events.
Herein, we report the syntheses and characterization of alkynylplatinum(II) complexes with amide-based terpyridine ligands. The host–guest interactions between the amide-based receptor moiety and various spherical and nonspherical anions have also been examined using UV-vis absorption and emission spectral titration studies to assess the anion binding properties of these metal complexes. In particular, drastic color changes have been observed for one of the complexes upon F− ion addition, which could be attributed to the F− ion-induced deprotonation of the amide functionalities.
2,2′:6′,2“-Terpyridine-3′,5′-dicarboxylic acid phenylamide and 2,2′:6′,2”-terpyridine-3′,5′-dicarboxylic acid hexylamide were prepared from 2,2′:6′,2“-terpyridine-3′,5′-dicarboxylic acid with procedures given in the Supporting Information. These newly synthesized amide-containing terpyridine ligands were used to prepare their respective chloroplatinum(II) terpyridine complexes based on the modification of literature procedures.7d,7f, 13 Complexes 1 and 2
 were synthesized by reacting the corresponding chloroplatinum(II) precursor complexes with phenylacetylene in degassed N,N-dimethylformamide in the presence of triethylamine and a catalytic amount of copper(I) iodide. These complexes with hexafluorophosphate (PF6−) as counter anions were metathesized from their triflate (OTf−) salts upon the addition of a saturated methanolic solution of ammonium hexafluorophosphate, followed by subsequent recrystallization from the acetonitrile/diethyl ether solution of the complexes. These complexes were characterized by 1H NMR spectroscopy, IR spectroscopy and positive fast atom bombardment mass spectrometry (FAB-MS) and gave satisfactory elemental analyses.
were synthesized by reacting the corresponding chloroplatinum(II) precursor complexes with phenylacetylene in degassed N,N-dimethylformamide in the presence of triethylamine and a catalytic amount of copper(I) iodide. These complexes with hexafluorophosphate (PF6−) as counter anions were metathesized from their triflate (OTf−) salts upon the addition of a saturated methanolic solution of ammonium hexafluorophosphate, followed by subsequent recrystallization from the acetonitrile/diethyl ether solution of the complexes. These complexes were characterized by 1H NMR spectroscopy, IR spectroscopy and positive fast atom bombardment mass spectrometry (FAB-MS) and gave satisfactory elemental analyses.
Dissolution of 1 and 2 in acetonitrile or acetone gave yellow solutions at room temperature. The electronic absorption spectra of these complexes at 298 K showed similar absorption pattern with intense intraligand (IL) [π→π*] transitions of the terpyridine and alkynyl ligands at about 250–347 nm, and less intense absorptions at about 445 nm, assignable as the admixtures of MLCT [dπ(Pt)→π*(tpy)] and alkynyl-to-terpyridine ligand-to-ligand charge transfer (LLCT) transitions. Upon photoexcitation at ≥400 nm, both complexes showed structureless emission bands at about 652–662 nm, which are originated from an excited state of triplet MLCT (3MLCT)/triplet LLCT (3LLCT) characters (Table S1 in the Supporting Information).
Upon addition of Cl− or Br− ions to the acetonitrile solution of 1 (0.1 m nBu4NPF6), diminution in the intensities and slight red shifts were observed for the low-energy MLCT/LLCT absorption band at about 467 nm (Figure S1 in the Supporting Information). Isosbestic points were observed for the UV-vis absorption profiles, suggesting that a clean conversion from the platinum(II) complex to the ion-bound adducts was involved. No significant spectral changes were found for the case with the addition of I− ions, while precipitations occurred upon the addition of F−, AcO− or H2PO4− ions to the solution of complex 1. Changes to the emission spectra of 1 have also been monitored with the addition of Cl− and Br− ions.14 Upon excitation at the isosbestic wavelength, a decrease in intensities was observed for the 3MLCT/3LLCT emission (Figure 1). The emission lifetime of 1 was shown to decrease upon addition of these ions (Table S2 in the Supporting Information). It could possibly be ascribed to the phosphorescence quenching brought about by photoinduced electron transfer due to the enhanced electron density on the amide functionalities upon anion inclusion. The emission titration profiles obtained for the respective spherical anions were fitted to the theoretical equation for 1:1 binding. The close agreement obtained between the experimental and the theoretical prediction indicated the involvement of a 1:1 complexation stoichiometry under the conditions investigated. The binding constant of 1 towards Cl− ions was found to be higher than that for Br− ions (Table S3 in the Supporting Information), attributed to a smaller size of the Cl− ion, which would favor a stronger extent of hydrogen-bonding interaction with the amide-based receptor sites on 1. Based on 3σ/m,15 where σ is the standard deviation of blank solution from three independent measurements and m is the slope of the linear region of titration plots based on the changes in the emission spectra, the detection limit of 1 towards Cl− and Br− ions was found to be in the micromolar range (Table S3 in the Supporting Information).
Figure 1.
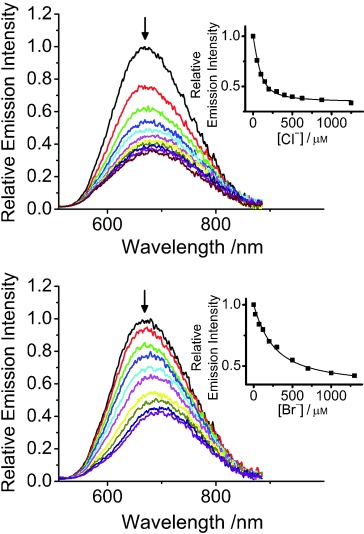
Changes in the emission spectra of 1 (100 μm) in acetonitrile (0.1 m nBu4NPF6) upon addition of nBu4NCl (top) and nBu4NBr (bottom). Insets: plot of relative emission intensity at 667 nm against various concentrations of Cl− and Br− ions and their theoretical fit for 1:1 binding stoichiometry.
With the replacement of phenylamide by hexylamide, the solubility of 2 was enhanced. The F− ion binding properties of 2 in acetonitrile (0.1 m nBu4NPF6) and acetone (0.1 m nBu4NPF6) were examined. Upon addition of the same amount of F− ions, a significant red shift was observed in the low-energy absorption band with measurements performed in acetone when compared with that performed in acetonitrile (Figure 2), suggesting that the polarity of the solvents may affect the recognition abilities of the receptor sites. Solvent polarity has been known to determine the ion-pairing association between the oppositely charged species, in which the electrostatic interaction would be enhanced with the use of a less polar solvent.16 Therefore, acetone, a comparatively less polar solvent (dielectric constant: ɛacetone=20.7 and ɛacetonitrile=37.5),16b would favor the formation of ion pairs for binding interaction, and in turn lessen the competition for solvation of the target ions.16a Because of such a solvent effect, the ion binding studies of 2 were performed in acetone.
Figure 2.
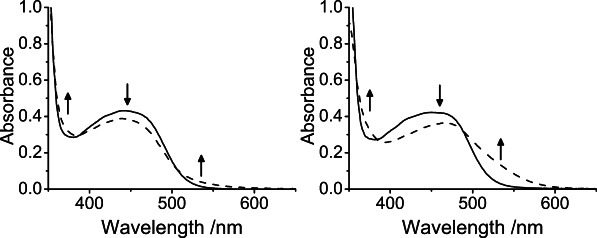
Changes to the UV-vis absorption spectra of 2 (100 μm) (solid line) in (left) acetonitrile (0.1 m nBu4NPF6) and (right) acetone (0.1 m nBu4NPF6) upon addition of nBu4NF (4 equiv) (dashed line).
Spectroscopic titration studies have been performed to investigate the host–guest interaction between 2 and F− ions. Upon addition of 0–2 equivalents of F− ions, the MLCT/LLCT absorption spectral changes were found to be similar to those observed in the case of 1 with the presence of Cl− or Br− ions except with a more pronounced extent resulted (top panel, Figure 3). Further addition of F− ions resulted in a substantial red shift with dramatic color changes from yellow to orange–red. Upon excitation at the isosbestic wavelength, the 3MLCT/3LLCT emission was gradually quenched with increasing concentration of F− ions, together with the emergence of a new lower-energy emission band at about 750 nm with lower intensity (bottom panel, Figure 3). Excitation spectra of 2 in the absence and in presence of F− ions showed different excitation bands (Figure S2 in the Supporting Information). Together with the spectral changes observed in the UV-vis titration studies, two different origins were thought to be responsible for the emission spectral changes. A nice fit to the theoretical equation of 1:1 binding mode was obtained for the emission spectral titration profile, suggestive of the formation of ion-bound adduct with 1:1 stoichiometric ratio.
Figure 3.
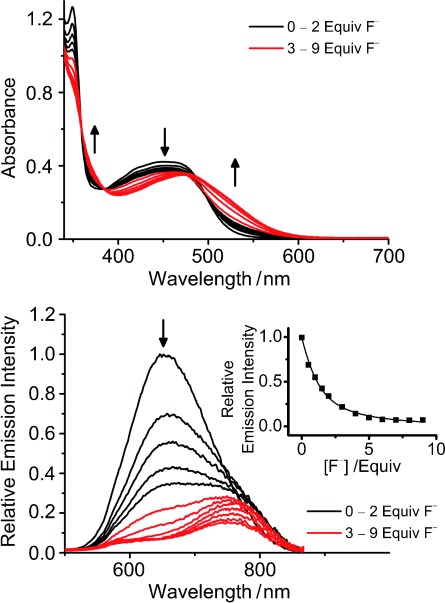
Changes to the UV-vis absorption (top) and emission (bottom) spectra of 2 (100 μm) in acetone (0.1 m nBu4NPF6) upon addition of nBu4NF. Inset: plot of relative emission intensity at 653 nm against various concentrations of F− ion and its theoretical fit for 1:1 binding stoichiometry.
F− ions have been known to induce the deprotonation of neutral amide-based receptor sites owing to their strong basicity and the formation of the highly stable HF2− anion.17 In order to confirm the possible involvement of a deprotonation process that gave rise to the dramatic spectral changes, trifluoroacetic acid, which has been shown to transfer protons to the deprotonated amide receptor sites,18 was employed in the present investigation. Upon addition of trifluoroacetic acid to the F− ion–complex mixture, the absorption and emission bands were found to be nearly restored to that of the complex alone (Figure 4), along with the color changes from orange–red to yellow. Control experiments have been performed to confirm that the addition of trifluoroacetic acid alone would only account for minor spectral changes of the metal complexes, while the absorption and emission spectra were found to show no significant changes upon the addition of sodium trifluoroacetate to the F− ion–complex mixture (Figures S3 and S4 in the Supporting Information). Moreover, the quenched emission of the Cl− ion–complex mixture, of which hydrogen-bonding interactions were involved for the binding event, was shown to remain nearly unchanged upon the addition of trifluoroacetic acid (Figure S5 in the Supporting Information). Together with the 1:1 binding stoichiometry determined, these observations suggest that the dramatic color changes of 2 upon addition of high concentrations of F− ions are likely associated with the F− ion-induced mono-deprotonation of the amide-based receptor moiety. The use of trifluoroacetic acid has allowed the regeneration of 2 by proton transfer process via Brønsted acid–base reactions, which was responsible for the restoration of the yellow color of the complex solution. Such deprotonation–protonation processes have been demonstrated to be highly reversible for at least three cycles. In the 19F NMR study, other than the observation of the characteristic doublet at a δ value of −73 ppm, which was assignable to the PF6− counter anion, two signals were observed at δ values of −115 ppm and −152 ppm upon addition of an excess of nBu4NF in the [D6]acetone solution of 2 (Figure S6 in the Supporting Information). These signals were assigned as the free F− ion19 and the HF2− ion,20 suggesting the occurrence of the F− ion-induced deprotonation. Also, negative electrospray ionization mass spectrometry (ESI-MS) spectrum with a signal at m/z 839 corresponding to [M − PF6+2 F+H2O]− was observed (Figure S7 in the Supporting Information), establishing the existence of the neutral mono-deprotonated [M − PF6 − H] and the stable HF2− anion upon addition of high concentrations of F− ions.
Figure 4.
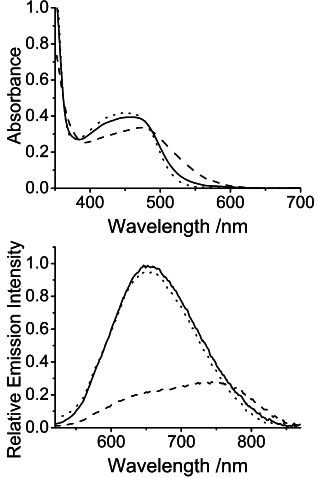
Changes to the UV-vis absorption (top) and emission (bottom) spectra of 2 (100 μm) (solid line) in acetone (0.1 m nBu4NPF6) in the presence of nBu4NF (4 equiv) (dashed line), and in the mixture in the presence of trifluoroacetic acid (4 equiv) (dotted line).
The binding affinities of 2 towards other anions have also been examined by monitoring the UV-vis and emission spectral changes upon titration (Figure 5; see also, Figures S8 and S9 in the Supporting Information), in which the resultant spectral changes were similar to that of 1 in the presence of Cl− or Br− ions.14 The emission lifetime of 2 was also found to decrease in the presence of the anions (Table S2 in the Supporting Information). The titration profiles have been fitted to the theoretical equation for 1:1 binding mode with nice agreement obtained, suggestive of the 1:1 host–guest relationship for 2 and the respective anions. The binding constants determined (Table S3 in the Supporting Information) were found to correlate with the size and basicity of the spherical anions, in the order: F− >Cl− >Br− >I−. Moreover, 2 has also been shown to exhibit strong binding constants towards the non-spherical AcO− ion, possibly ascribed to its high basicity. However, precipitations occurred upon the addition of H2PO4− ions to the complex solution of 2, preventing further study. The detection limits of the anions investigated, determined as 3σ/m15 from three individual measurements, were found to be ranging from 5.75 to 23.09 μm (Table S3 in the Supporting Information).
Figure 5.
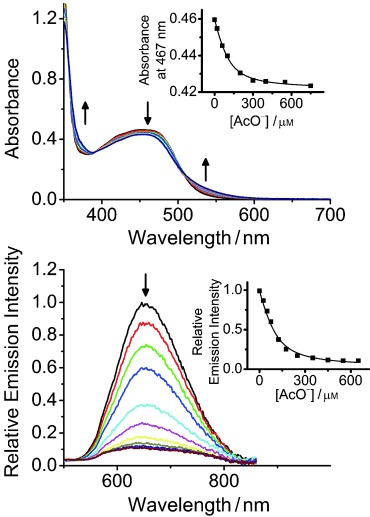
Changes to the UV-vis absorption (top) and emission (bottom) spectra of 2 (100 μm) in acetone (0.1 m nBu4NPF6) upon addition of nBu4NAcO. Insets: plot of absorbance at 467 nm and relative emission intensity at 653 nm against various concentrations of AcO− ions and their theoretical fit for 1:1 binding stoichiometry.
To conclude, functionalization of the alkynlplatinum(II) terpyridine complexes with amide-based receptor moiety allows these metal complexes to serve as potential anion sensing probes based on the host–guest interaction. These complexes could signal the anion binding events with UV-vis absorption and emission spectral changes, with their anion binding affinity found to be dependent on the size and/or the basicity of the anions investigated. The F− ion-induced deprotonation of the amide-based receptor sites on the metal complexes would result in drastic color changes, which has provided a convenient sensing strategy for the visual detection of F− ions.
Acknowledgments
V.W.-W.Y. acknowledges the support from the University Grants Committee Areas of Excellence Scheme (AoE/P-03/08) and a General Research Fund grant (HKU 7064/11P) from the Research Grants Council of Hong Kong Special Administrative Region, P. R. China. M.C.-L.Y. acknowledges the receipt of a postgraduate studentship, administered by The University of Hong Kong.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
References
- 1.Steed JW. Chem. Soc. Rev. 2009;38:506–519. doi: 10.1039/b810364j. [DOI] [PubMed] [Google Scholar]
- 2.Beer PD, Gale; PA. Angew. Chem. Int. Ed. 2001;40:486–516. [PubMed] [Google Scholar]
- Angew. Chem. 2001;113:502–532. [Google Scholar]
- 3.Caltagirone C, Gale PA. Chem. Soc. Rev. 2009;38:520–563. doi: 10.1039/b806422a. [DOI] [PubMed] [Google Scholar]
- 4a.Yam VWW, Wong KMC. Top. Curr. Chem. 2005;257:1–32. doi: 10.1007/b136069. [DOI] [PubMed] [Google Scholar]
- 4b.Zhao Q, Li F, Huang C. Chem. Soc. Rev. 2010;39:3007–3030. doi: 10.1039/b915340c. [DOI] [PubMed] [Google Scholar]
- 5a.Miller JS, editor. Extended Linear Chain Compounds, Vol. 1. New York: Plenum Press; 1982. [Google Scholar]
- 5b.Osborn RS, Rogers D. J. Chem. Soc. Dalton Trans. 1974:1002–1004. [Google Scholar]
- 5c.Miskowski VM, Houlding VH. Inorg. Chem. 1991;30:4446–4452. doi: 10.1021/ic991369w. [DOI] [PubMed] [Google Scholar]
- 5d.Houlding VH, Miskowski VM. Coord. Chem. Rev. 1991;111:145–152. [Google Scholar]
- 5e.Büchner R, Cunningham CT, Field JS, Haines RJ, McMillin DR, Summerton GC. J. Chem. Soc. Dalton Trans. 1999:711–718. [Google Scholar]
- 5f.Yam VWW, Wong KMC, Zhu N. J. Am. Chem. Soc. 2002;124:6506–6507. doi: 10.1021/ja025811c. [DOI] [PubMed] [Google Scholar]
- 6a.Miskowski VM, Houlding VH. Inorg. Chem. 1989;28:1529–1533. doi: 10.1021/ic991369w. [DOI] [PubMed] [Google Scholar]
- 6b.Herber RH, Croft M, Coyer MJ, Bilash B, Sahiner A. Inorg. Chem. 1994;33:2422–2426. [Google Scholar]
- 6c.Connick WB, Henling LM, Marsh RE, Gray HB. Inorg. Chem. 1996;35:6261–6265. [Google Scholar]
- 6d.Connick WB, Marsh RE, Schaefer WP, Gray HB. Inorg. Chem. 1997;36:913–922. [Google Scholar]
- 7a.Jennette KW, Lippard SJ, Vassiliades GA, Bauer WR. Proc. Natl. Acad. Sci. USA. 1974;71:3839–3843. doi: 10.1073/pnas.71.10.3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7b.Jennette KW, Gill JT, Sadownick JA, Lippard SJ. J. Am. Chem. Soc. 1976;98:6159–6168. [Google Scholar]
- 7c.Yip HK, Cheng LK, Cheung KK, Che CM. J. Chem. Soc. Dalton Trans. 1993:2933–2938. [Google Scholar]
- 7d.Bailey JA, Hill MG, Marsh RE, Miskowski VM, Schaefer WP, Gray HB. Inorg. Chem. 1995;34:4591–4599. [Google Scholar]
- 7e.Hill MG, Bailey JA, Miskowski VM, Gray HB. Inorg. Chem. 1996;35:4585–4590. [Google Scholar]
- 7f.Büchner R, Field JS, Haines RJ, Cunningham CT, McMillin DR. Inorg. Chem. 1997;36:3952–3956. [Google Scholar]
- 7g.Yam VWW, Tang RPL, Wong KMC, Cheung KK. Organometallics. 2001;20:4476–4482. [Google Scholar]
- 8a.Yu C, Wong KMC, Chan KHY, Yam VWW. Angew. Chem. Int. Ed. 2005;44:791–794. doi: 10.1002/anie.200461261. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. 2005;117:801–804. [Google Scholar]
- 8b.Yu C, Chan KHY, Wong KMC, Yam VWW. Chem. Eur. J. 2008;14:4577–4584. doi: 10.1002/chem.200800276. [DOI] [PubMed] [Google Scholar]
- 8c.Wong KMC, Yam VWW. Acc. Chem. Res. 2011;44:424–434. doi: 10.1021/ar100130j. [DOI] [PubMed] [Google Scholar]
- 9a.Yu C, Chan KHY, Wong KMC, Yam VWW. Proc. Natl. Acad. Sci. USA. 2006;103:19652–19657. doi: 10.1073/pnas.0604998104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9b.Yu C, Chan KHY, Wong KMC, Yam VWW. Chem. Commun. 2009:3756–3758. doi: 10.1039/b903080h. [DOI] [PubMed] [Google Scholar]
- 9c.Yeung MCL, Wong KMC, Tsang YKT, Yam VWW. Chem. Commun. 2010;46:7709–7711. doi: 10.1039/c0cc02631j. [DOI] [PubMed] [Google Scholar]
- 9d.Chung CYS, Chan KHY, Yam VWW. Chem. Commun. 2011;47:2000–2002. doi: 10.1039/c0cc04437g. [DOI] [PubMed] [Google Scholar]
- 9e.Chung CYS, Yam VWW. J. Am. Chem. Soc. 2011;133:18775–18784. doi: 10.1021/ja205996e. [DOI] [PubMed] [Google Scholar]
- 9f.Chung CYS, Yam VWW. Chem. Sci. 2013;4:377–387. [Google Scholar]
- 9g.Yeung MCL, Yam VWW. Chem. Sci. 2013;4:2928–2935. [Google Scholar]
- 10a.Yam AYY, Wong KMC, Wang G, Yam VWW. Chem. Commun. 2007:2028–2030. doi: 10.1039/b705062c. [DOI] [PubMed] [Google Scholar]
- 10b.Tam AYY, Wong KMC, Yam VWW. Chem. Eur. J. 2009;15:4775–4778. [Google Scholar]
- 10c.Po C, Tam AYY, Wong KMC, Yam VWW. J. Am. Chem. Soc. 2011;133:12136–12143. doi: 10.1021/ja203920w. [DOI] [PubMed] [Google Scholar]
- 10d.Po C, Ke Z, Tam AYY, Chow HF, Yam VWW. Chem. Eur. J. 2013;19:15735–15744. doi: 10.1002/chem.201302702. [DOI] [PubMed] [Google Scholar]
- 11a.Yam VWW, Chan KHY, Wong KMC, Chu BWK. Angew. Chem. 2006;118:6315–6319. [Google Scholar]
- Angew. Chem. Int. Ed. 2006;45:6169–6173. doi: 10.1002/anie.200600962. [DOI] [PubMed] [Google Scholar]
- 11b.Leung SYL, Tam AYY, Tao CH, Chow HS, Yam VWW. J. Am. Chem. Soc. 2012;134:1047–1056. doi: 10.1021/ja208444c. [DOI] [PubMed] [Google Scholar]
- 11c.Leung SYL, Lam WH, Yam VWW. Proc. Natl. Acad. Sci. USA. 2013;110:7986–7991. doi: 10.1073/pnas.1301252110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11d.Leung SYL, Yam VWW. Chem. Sci. 2013;4:4228–4234. [Google Scholar]
- 12a.Wong KMC, Tang WS, Lu XX, Zhu Z, Yam VWW. Inorg. Chem. 2005;44:1492–1498. doi: 10.1021/ic049079p. [DOI] [PubMed] [Google Scholar]
- 12b.Tang WS, Lu XX, Wong KMC, Yam VWW. J. Mater. Chem. 2005;15:2714–2720. [Google Scholar]
- 12c.Lo HS, Yip SK, Wong KMC, Zhu N, Yam VWW. Organometallics. 2006;25:3537–3540. [Google Scholar]
- 13a.Cummings SD, Eisenberg R. Inorg. Chem. 1995;34:2007–2014. [Google Scholar]
- 13b.Howe-Grant M, Lippard SJ. Inorg. Synth. 1980;20:101–105. [Google Scholar]
- 14. In view of the poor solubility in pure aqueous media, the anion binding properties of these two metal complexes in Tris-HCl (10 mm, pH 7.8)/acetonitrile or Tris-HCl (10 mm, pH 7.8)/acetone mixture (1:1 vv) were examined. Only a slight decrease in emission intensities (<10 %) was observed upon addition of 1000 μm of the anions investigated. Such a significant reduction in sensitivity in the aqueous buffer/acetonitrile mixture or aqueous buffer/acetone mixture can probably be ascribed to the disruption of the hydrogen-bonding interactions between the anion and the amide-based receptor due to the presence of water molecules.
- 15.Batey HD, Whitwood AC, Duhme-Klair AK. Inorg. Chem. 2007;46:6516–6528. doi: 10.1021/ic700554n. [DOI] [PubMed] [Google Scholar]
- 16a.Reynes O, Maillard F, Moutet JC, Royal G, Saint-Aman E, Stanciu G, Dutasta JP, Gosse I, Mulatier JC. J. Organomet. Chem. 2001;637–639:356–363. [Google Scholar]
- 16b.Kui SCF, Law YC, Tong GSM, Lu W, Yuen MY, Che CM. Chem. Sci. 2011;2:221–228. [Google Scholar]
- 17a.Amendola V, Esteban-Gómez D, Fabbrizzi L, Licchelli M. Acc. Chem. Res. 2006;39:343–353. doi: 10.1021/ar050195l. [DOI] [PubMed] [Google Scholar]
- 17b.Gunnlaugsson T, Glynn M, Tocci GM, Kruger PE, Pfeffer FM. Coord. Chem. Rev. 2006;250:3094–3117. [Google Scholar]
- 17c.Amendola V, Fabbrizzi L. Chem. Commun. 2009:513–531. doi: 10.1039/b808264m. [DOI] [PubMed] [Google Scholar]
- 18.Saha D, Das S, Mardanya S, Baitalik S. Dalton Trans. 2012;41:8886–8898. doi: 10.1039/c2dt30633f. [DOI] [PubMed] [Google Scholar]
- 19a.Wang DX, Zheng QY, Wang QQ, Wang MX. Angew. Chem. Int. Ed. 2008;47:7485–7488. doi: 10.1002/anie.200801705. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. 2008;120:7595–7598. [Google Scholar]
- 19b.Dey SK, Das G. Chem. Commun. 2011;47:4983–4985. doi: 10.1039/c0cc05430e. [DOI] [PubMed] [Google Scholar]
- 20a.Kang SO, Powell D, Day VW, Bowman-James K. Angew. Chem. Int. Ed. 2006;45:1921–1925. doi: 10.1002/anie.200504066. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. 2006;118:1955–1959. [Google Scholar]
- 20b.Trembleau L, Smith TAD, Abdelrahman MH. Chem. Commun. 2013;49:5850–5852. doi: 10.1039/c3cc43019g. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary


