Abstract
Aims/Introduction
The present study applied the Wilson–Cleary model of health-related quality of life (HRQOL) by using the structural equation modeling (SEM) approach to understand the interrelationships among clinical, sociodemographic and psychological characteristics in older people with diabetes.
Materials and Methods
This was a cross-sectional study with 452 Chinese older people with diabetes recruited from three primary care clinics. A series of assessments were made, including four instruments: the Chinese version of the Short Form 36 Health Survey, Older American Resources and Services Multidimensional Functional Assessment Questionnaire, Rand Mental Health Inventory and Medical Outcomes Study Social Support Survey; and clinical outcomes (diabetes-related characteristics and physiological data).
Results
In the present study, we identified six patient individual and environmental characteristics, namely, age, sex, physical activity, psychological distress, social support and adequacy of income, that significantly influence HRQOL directly or by way of physical functional status and general health perception.
Conclusions
Improving social and financial support as well as providing interventions to promote physical activity and to cope with psychological distress in this patient population might be effective to eventually enhance their HRQOL. The present findings add to the literature the underlying complex biological and psychological processes of HRQOL, and take the body of knowledge in HRQOL of older people with diabetes to a theoretical level, and provide insights for development of appropriate strategies to optimize their HRQOL.
Keywords: Diabetes, Quality of life, Structural equation modeling
Introduction
Diabetes, a chronic degenerative disease that demands life-long lifestyle modifications and adherence to a treatment regime by the people affected1, has a negative impact on the individual's health-related quality of life (HRQOL)2–5. The importance of optimizing HRQOL has been increasingly reiterated not only because of the associations between poor HRQOL and adverse outcomes in people with diabetes2,3, but also because of a paradigm shift from the biomedical paradigm that focuses mainly on physiological outcomes to the social science paradigm that encompasses the functioning and overall well-being as the focus of diabetes care delivery2,3,6. Key drivers contributing to the paradigm shift include the increasing penetration of patient empowerment7 and the adoption of the chronic care model8 to collaboratively plan the care with the patients who face the consequences of the impact of such care on their daily lives7. In such a shift, HRQOL has become a frequent outcome measure in diabetes care2,3.
To date, developing interventions that can improve both physiological outcomes and HRQOL in diabetic patients remains a constant challenge1. Research evidence is required to guide the planning of appropriate approaches to diabetes care. However, research has mainly focused on association analysis between HRQOL and variables logically identified from a clinical perspective4,9–13. Whereas psychosocial variables are increasingly included in the analysis (for example, depression5,9, psychological well-being10, treatment satisfaction10,11 and quality of care provision12), there has been a lack of a theoretical framework to systematically select variables for investigation. This has resulted in a gap in the diabetes research literature about an empirically tested theoretical framework to understand the relationship between HRQOL, and physiological and psychosocial variables as well as the determinants of HRQOL. This gap in the literature might hamper the development of appropriate means to optimize both physiological outcomes and the HRQOL of people with diabetes.
This gap in the literature is obvious in Chinese populations with diabetes. China is one of the top two global epicenters of the diabetes epidemic, and, given its large population, might bear a higher diabetes-related burden14. Whereas a handful of HRQOL studies have been published on Chinese people who resided in Taiwan15–17, Shanghai18 and Hong Kong19,20, they mainly focused on scale validation or investigating the impact of living with diabetes. The inclusion of variables for the investigation in these studies might be considered as atheoretical.
Furthermore, diabetes prevalence in Chinese older people is high, with one-quarter and one-fifth of older adults being affected therewith in Hong Kong21 and mainland China22, respectively. Care planning with older adults with diabetes is considered to be more complicated than with younger adults, and it requires empirical support9,23. An understanding of the relationship between HRQOL and key health outcomes guided by a theoretical framework is required for Chinese older adults with diabetes.
In 1995, Wilson and Cleary24 published their seminal conceptual model of HRQOL, which provides a causal pathway linking traditional clinical variables to HRQOL. Conceptually, Wilson and Cleary24 linked five health concepts on a continuum (Figure1), they are: (i) physiological factors; (ii) symptoms; (iii) functional health; (iv) general health perceptions; and (v) HRQOL. Beginning at the clinical level (biological, objective) at one end of the continuum and moving outward to the individuals' interaction with the environment to perceive a level of quality of life (psychological, subjective) at the other end, this model integrates both biomedical and social science paradigms. In terms of application, the model provides a theoretical basis for the selection of variables according to the series of health concepts on the causal pathway for clinical attention and research. The model has been empirically tested, and is widely regarded as facilitating the understanding of associations among objective clinical outcomes and subjective patient experiences in chronic diseases, including generalized anxiety disorder25, HIV/AIDS26,27 and heart failure28. However, suchlike published work in diabetes is scarce29.
Figure 1.
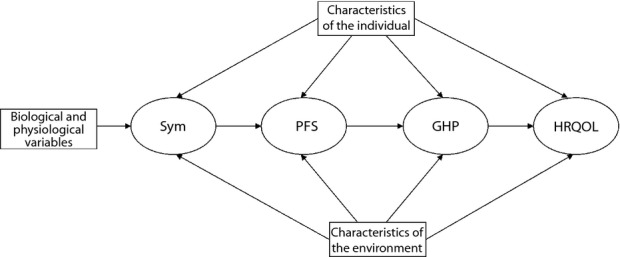
The Wilson–Cleary conceptual path model of health-related quality of life. GHP, general health perception; HRQOL, health-related quality of life; PFS, physical functional status; Sym, symptom status.
The present study therefore applied the Wilson–Cleary model of HRQOL24 by using structural equation modeling (SEM) to understand the relationships among clinical and psychological outcomes in community-dwelling older Hong Kong Chinese people with diabetes.
Materials and Methods
Setting and Participants
The present cross-sectional study, carried out in 2010, used a quota sampling method to select three primary care clinics as the setting for recruiting community-dwelling patients from each of the three geographical regions of Hong Kong, namely, Hong Kong Island, Kowloon and New Territories. It was estimated that 50% of diabetic patients attended primary care clinics for diabetes care. The quota sampling method was used to recruit 150 patients from each of the three clinics. All diabetic patients aged ≥60 years who attended the clinics were eligible. Those with language problems, such as stroke patients who exhibited speech difficulties, were excluded from the study. The final sample (n = 452) consisted of 152, 151 and 149 patients from each of the three clinics. All patients were informed about the study and asked to sign a consent form before data collection. Patient anonymity was preserved throughout the study.
Assessments Used in the Wilson–Cleary Model
A questionnaire was developed, guided by the conceptual model (Figure1), in order to collect the variables (Table1) of interest for testing. The questionnaire consisted of a sociodemographic sheet, a health assessment form and four instruments. After obtaining approval from the regional ethical committees and written consent from the patients, the data were collected by a trained research nurse by face-to-face interview. The latest physiological data were retrieved from the patients' records. In the SEM methodology, latent variables (represented by ovals in Figures1 and 2), as opposed to observed variables (represented by rectangles in Figures1 and 2), cannot be observed directly, and they are inferred indirectly by means of observed variables. In the present study, for example, symptom status was regarded as a latent variable that could be inferred from observed variables, such as the number of comorbidities, and the number of somatic symptoms and the self-rated severity of fatigue.
Table 1.
Characteristics of the study sample (n = 452)
| Mean (SD)/median (IRQ)/n (%) | |
|---|---|
| Sociodemographic characteristics | |
| Age (years)* | 71.8 (7.3) |
| 60 – 64 | 84 (18.6%) |
| 67 – 69 | 95 (21.0%) |
| 70 – 74 | 109 (24.1%) |
| ≥75 | 164 (36.3%) |
| Sex | |
| Male | 185 (40.9%) |
| Female | 267 (59.1%) |
| Marital status | |
| Married | 318 (70.5%) |
| Single/divorced/widowed/separated | 133 (29.5%) |
| No. children | |
| 0 | 25 (5.5%) |
| 1 – 2 | 155 (34.3%) |
| 3 – 4 | 175 (38.7%) |
| ≥5 | 97 (21.5%) |
| Living alone | |
| No | 405 (89.6%) |
| Yes | 47 (10.4%) |
| Education | |
| No formal education | 118 (26.5%) |
| Primary | 171 (38.3%) |
| Secondary | 71 (15.9%) |
| Tertiary or above | 86 (19.3%) |
| Adequacy of income | |
| Not enough | 54 (12.0%) |
| Just enough | 180 (40.1%) |
| More than enough | 215 (47.9%) |
| Working status | |
| Retired/unemployed | 337 (74.6%) |
| Full-time/part-time working/housekeeper | 115 (25.4%) |
| Lifestyle characteristics | |
| Smoking status | |
| Never smoker | 350 (77.4%) |
| Ex-smoker | 82 (18.1%) |
| Current smoker | 20 (4.4%) |
| Drinking status | |
| Never drinker | 397 (87.8%) |
| Former drinker | 35 (7.7%) |
| Current drinker | 20 (4.4%) |
| Doing exercise regularly | |
| No | 86 (19.0%) |
| Yes | 366 (81.0%) |
| Frequency of exercise per week | |
| 0 | 86 (19.6%) |
| 1 – 3 | 85 (19.4%) |
| 4 – 6 | 41 (9.3%) |
| 7 | 227 (51.7%) |
| Average duration per each time of exercise | |
| Does not exercise | 86 (19.5%) |
| ≤30 min | 138 (31.3%) |
| 31–60 min | 149 (33.8%) |
| >60 min | 68 (15.4%) |
| Average time spent on exercise per day (h)* | 0.6 (0.6) |
| Diabetes-related characteristics | |
| Time since diagnosed with diabetes (years) | |
| <5 | 128 (28.8%) |
| 5 to <10 | 92 (20.7%) |
| 10 to <20 | 149 (33.6%) |
| ≥20 | 75 (16.9%) |
| Onset age (years) | |
| <60 | 182 (41.3%) |
| 60–64 | 89 (20.2%) |
| 65–69 | 84 (19.0%) |
| 70–74 | 39 (8.8%) |
| ≥75 | 47 (10.7%) |
| Type of treatment | |
| Dietary control only | 44 (9.9%) |
| OHA | 363 (81.2%) |
| Insulin therapy | 5 (1.1%) |
| OHA and insulin therapy | 35 (7.8%) |
| Presence of diabetes complications | |
| No/unknown | 395 (88.8%) |
| Yes | 50 (11.2%) |
| Participated in diabetes education | |
| No | 159 (35.8%) |
| Yes | 285 (64.2%) |
| Physiological data | |
| Bodyweight (kg)* | 60.7 (10.8) |
| Body height (cm)* | 157.2 (8.4) |
| Body mass index (kg/m2)* | 24.4 (3.9) |
| Systolic blood pressure (mmHg)* | 137.3 (16.6) |
| Diastolic blood pressure (mmHg)* | 73.4 (10.8) |
| Urine protein (g/L)† | 0.11 (0.07–0.44) |
| Urine albumin-to-creatinine ratio (mmol/L)† | 1.70 (0.80–3.80) |
| Triglycerides (mmol/L)† | 1.31 (0.94–1.82) |
| Total cholesterol (mmol/L)* | 4.72 (0.87) |
| LDL cholesterol (mmol/L)* | 2.87 (0.75) |
| HDL cholesterol (mmol/L)* | 1.22 (0.53) |
| HbA1c (%)* | 7.16 (1.06) |
| Comorbidity | |
| Hypertension | 362 (80.1%) |
| Cardiovascular disease | 22 (4.9%) |
| Cerebrovascular disease | 29 (6.4%) |
| Chronic lung disease | 5 (1.1%) |
| Dementia | 5 (1.1%) |
| Parkinsonism | 3 (0.7%) |
| Arthritis | 117 (25.9%) |
| Eyesight impairment | 130 (28.8%) |
| Hearing impairment | 35 (7.7%) |
| Hyperlipidemia | 74 (16.5%) |
| Prostate disease | 11 (2.5%) |
| Cancer | 6 (1.3%) |
| Others | 112 (24.8%) |
| Total number of comorbidities | |
| 0 | 42 (9.3%) |
| 1 | 159 (35.2%) |
| 2 | 146 (32.3%) |
| ≥3 | 105 (23.2%) |
| Somatic symptoms | |
| Loss of appetite | 67 (14.8%) |
| Fatigue | 186 (41.2%) |
| Chest pain | 70 (15.5%) |
| Breathing difficulty | 54 (11.9%) |
| Stomachache | 71 (15.7%) |
| Headache | 81 (17.9%) |
| Dizziness | 105 (23.3%) |
| Joint swelling and stiffness | 144 (31.9%) |
| Constipation | 121 (26.8%) |
| Insomnia | 188 (41.7%) |
| Others | 4 (0.9%) |
| Total number of the above somatic symptoms | |
| 0 | 90 (19.9%) |
| 1 | 113 (25.0%) |
| 2 | 73 (16.2%) |
| 3 | 61 (13.5%) |
| 4 | 37 (8.2%) |
| ≥5 | 78 (17.3%) |
| Self-rated measures | |
| Self-rated severity of chronic pain (range 0–10)* | 2.4 (2.8) |
| Self-rated severity of fatigue (range 0–10)* | 2.9 (2.6) |
| Self-rated general health status (range 0–10)* | 4.3 (2.0) |
| OMFAQ (Chinese version)‡ | |
| Physical self-maintenance score* | 13.6 (1.1) |
| Instrumental activities of daily living score* | 13.5 (1.7) |
| SF-36 (Chinese version)‡ | |
| Physical functioning* | 77.9 (21.0) |
| Role physical* | 68.6 (42.6) |
| Bodily pain* | 75.0 (27.3) |
| General health* | 50.9 (18.1) |
| Vitality* | 67.4 (17.6) |
| Social functioning* | 86.3 (20.4) |
| Role emotional* | 87.4 (32.3) |
| Mental health* | 79.0 (14.8) |
| RMHI (Chinese version)‡ | |
| Distress scale | |
| Anxiety* | 17.4 (6.5) |
| Depression* | 7.9 (3.6) |
| Loss of behavioral/emotional control* | 15.8 (4.7) |
| Total distress score | 41.1 (13.5) |
| Wellbeing scale | |
| Positive affect* | 44.3 (9.1) |
| Emotional tie* | 13.8 (2.7) |
| Total well-being score* | 58.0 (11.1) |
| MOS-SSS (Chinese version)‡ | |
| Tangible* | 68.7 (26.1) |
| Affectionate* | 66.5 (21.4) |
| Positive social interaction* | 61.2 (22.4) |
| Emotional–informational* | 58.9 (23.4) |
| Total score* | 63.8 (20.5) |
HbA1c, glycosylated hemoglobin; HDL, high-density lipoprotein; IRQ, interquartile range; LDL, low-density lipoprotein; MOS-SSS, Medical Outcomes Study Social Support Survey; OHA, oral hypoglycemic agents; OMFAQ, Older American Resources and Services Multidimensional Functional Assessment Questionnaire; RMHI, Rand Mental Health Inventory; SF-36; Short Form 36 Health Survey. n = 452. Data marked
are presented as mean (standard deviation [SD]) and
are presented as median (inter-quartile range [IQR]), all others are presented as frequency (percentage).
All the scales were scored so that a high score indicates a better underlying trait being measured except for the distress scales of the Rand Mental Health Inventory, where a higher score corresponds to greater extent of distress.
Figure 2.
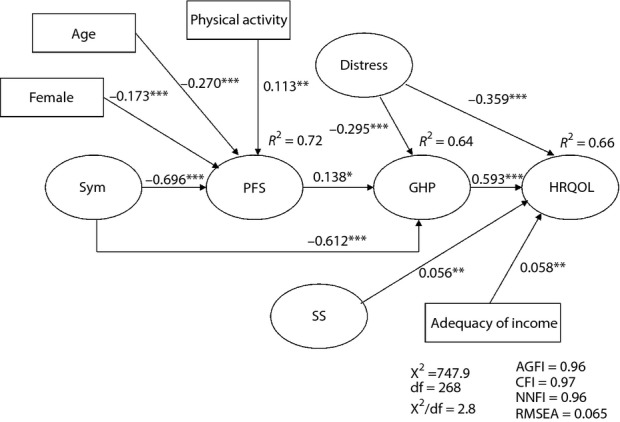
A path model of health-related quality of life for Hong Kong community-dwelling older Chinese people with diabetes. Latent and observed variables are represented by ellipses and rectangles, respectively. Directional effects are shown using single-headed arrows. *P < 0.05; **P < 0.01; ***P < 0.001. Distress, psychological distress; GHP, general health perception; HRQOL, health-related quality of life; PFS, physical functional status; SS, social support; Sym, symptom status.
HRQOL
We used the Short Form 36 Health Survey (SF-36) for the measurement of HRQOL because of its popularity as a generic instrument for HRQOL in diabetic populations and its sensitivity to changes in health status – including the presence of diabetes complications and other comorbidities2,30. In addition, research supports the usefulness of all the subscales for research purposes in older people31.
The Chinese version of SF-36 (SF-36-C)20 has been validated and used with Chinese people in Hong Kong. The HRQOL in the present study was assessed by six of the eight subscales of the SF-36-C20, namely, role functional physical, role functioning emotional, mental health, social functioning, bodily pain and vitality.
General Health Perception
Variables reflecting general health perception were obtained from three self-rated one-item measures, namely, self-rated general health status, and self-rated severity of chronic pain and fatigue; and one of eight subscales of SF-36-C20, general health perceptions. The self-rated measures were self-developed, anchoring on an 11-point scale (0 = extremely poor, or not at all severe; 10 = extremely well, or extremely severe).
Physical Functional Status
Variables reflecting physical functional status were obtained from two instruments: the Chinese version of the Older American Resources and Services Multidimensional Functional Assessment Questionnaire (OMFAO-C); and one of the eight subscales of SF-36-C20, physical functioning. The OMFAQ-C32 has been used with Chinese people in Hong Kong, and it has shown adequate psychometric property. The OMFAQ-C has two-seven-item subscales: the physical self-maintenance scale and the instrumental activities of daily living scale.
Symptom Status
Variables reflecting symptom status were obtained from the self-reported comorbidity characteristics, including the presence of comorbidities, such as hypertension, and the number of such comorbidities; somatic symptom characteristics, including the presence of symptoms such as loss of appetite, and the number of such symptoms; and self-rated measures, including self-rated severity of chronic pain and fatigue. Somatic symptom was measured by a self-developed list of 10 symptoms, including insomnia and constipation.
Clinical Variables
Clinical variables were obtained from diabetes-related characteristics, including the time since diagnosis, the age of onset and the type of diabetes treatment; and physiological data, including the glycosylated hemoglobin (HbA1c) level, blood pressure and the lipid profile. These three physiological data are the major physiological markers of diabetes control33. Blood pressure was taken on every clinic visit. A sample of blood was taken half-yearly for HbA1c and yearly for the lipid profile.
Characteristics of the Individual
Variables reflecting the characteristics of the individual were obtained from: (i) sociodemographic characteristics, including age, sex and working status; (ii) lifestyle characteristics, including smoking and drinking status, and the time spent on exercise per day; and (iii) psychological distress. The latter was measured by the Chinese version of the Rand Mental Health Inventory (RMHI-C), which assesses the mental health of Chinese people with adequate psychometric property34. The 38-item RMHI-C has two higher-order subscales: psychological well-being and psychological distress. Psychological well-being is further divided into two-second-order subscales: emotional ties and general positive affect. Psychological distress is divided into three subscales: anxiety, depression, and loss of behavioral and emotional control.
Characteristics of the Environment
Variables reflecting the characteristics of the environment were obtained from sociodemographic characteristics, including marital status and adequacy of income; and social support. The latter was measured by the Chinese version of the Medical Outcomes Study Social Support Survey (MOS-SSS-C), which assesses the self-perceived adequacy of functional social support with adequate psychometric property35. It has four subscales measuring the perceived adequacy of tangible support, informational and emotional support, positive social interaction, and affectionate support.
Statistical Analysis
Data were summarized and presented using appropriate descriptive statistics. We followed the two-step approach recommended by Anderson and Gerbing36 to SEM. First, confirmatory factor analysis (CFA) was carried out to justify the measurement models (i.e., the grouping of observed variables) for the latent variables. Second, an initial structural path model was built on the basis of the Wilson–Cleary HRQOL model (Figure1). The initial path model was then modified by adding plausible paths with the use of modification indices and trimmed subsequently to obtain the final model (Figure2) by deleting insignificant paths.
The SEM was carried out by using LISREL 8.8 (Scientific Software International, Inc., Skokie, IL, USA), and the parameters were estimated by the robust maximum likelihood method, which allows violation of multivariate normality assumption of the data. As the chi square-test is sensitive to sample size and violation of the multivariate normality assumption37, consequently, several goodness-of-fit indices were used to assess the overall fit of the path models. Guided by Schermelleh–Engel et al.37, the following fit indices were chosen: (i) the chi square-statistic to degree of freedom ratio (χ2/df); (ii) the root mean square error of approximation (RMSEA); (iii) adjusted goodness-of-fit index (AGFI); (iv) the comparative fit index (CFI); and (v) the non-normed fit index (NNFI). A value of 2 <χ2/df ≤3 indicating an acceptable fit to the data, with a value of χ2/df ≤2 indicating a good fit37. The AGFI, CFI and NNFI usually range from 0 to 1, with AGFI ≥0.85, CFI ≥0.95 and NNFI ≥0.95 indicating an acceptable fit to the data37, whereas AGFI ≥0.9, CFI ≥0.97 and NNFI ≥0.97 indicating a good fit37. The smaller the value of RMSEA indicates a better fit, with values ≤0.08 indicating an acceptable fit and with values ≤0.05 indicating a good fit37. All other statistical analyses were carried out using SPSS 18.0 (SPSS Inc., Chicago, IL, USA). All statistical tests were two-sided, and a P-value <0.05 was considered statistically significant.
Results
Patient Characteristics
A total of 586 eligible subjects had been approached in the three primary clinics, among them 452 subjects agreed to participate in the study (77% response rate). Table1 shows the descriptive information of the 452 participants included in the study.
Structural Equation Modeling
Applying the first step of the two-step approach36 to SEM, a CFA was carried out to justify the measurement models for the latent variables. Initially, both the observed variables of self-rated fatigue and self-rated chronic pain were allowed to measure both the latent variables of symptom status and general health perception. By CFA, it was found that self-rated severity of chronic pain and fatigue did not reflect the latent variables of general health perception and symptom status, respectively. A first-order CFA was then carried out to assess the adequacy of the measurement model of the latent variables, including clinical variables, symptom status, physical functional status, general health perception, HRQOL, psychological distress and social support. The latent variables were allowed to correlate freely with each other. The results of the CFA showed an adequate fit to the data (RMSEA = 0.079, AGFI = 0.95, CFI = 0.95 and NNFI = 0.94). Table2 gives the final observed variables chosen to measure the latent variables involved in the present study.
Table 2.
Observed variables for measuring the latent variables included in the final path model
| Latent factors | Observed variables/Indicators | Descriptions |
|---|---|---|
| Age | ||
| Sex | Binary variable: 0 = Male, 1 = Female | |
| Physical activity | Average time spent on exercise per week (h) | |
| Adequacy of income | Ranged from 0 = extremely not enough to 4 = more than enough | |
| Symptom status | No. comorbidities | The lower the number the better |
| No. somatic symptoms | The lower the number the better | |
| Self-rated severity of chronic pain | Ranged 0 – 10, the lower the score the better | |
| Physical functional status | OMFAQ-C physical self-maintenance score | Ranged 0 – 14, the higher the score the better |
| OMFAQ-C instrumental activities of daily living score | Ranged 0 – 14, the higher the score the better | |
| SF36-C physical functioning subscale score | Ranged 0 – 100, the higher the score the better | |
| General health perception | Self-rated general health status | Ranged 0 – 10, the higher the score the better |
| Self-rated severity of fatigue | Ranged 0 – 10, the higher the score the worse | |
| SF-36-C general health subscale score | Ranged 0 – 100, the higher the score the better | |
| Health-related quality of life | SF-36-C bodily pain subscale score | Ranged 0 – 100, the higher the score the better |
| SF-36-C role physical subscale score | Ranged 0 – 100, the higher the score the better | |
| SF-36-C vitality subscale score | Ranged 0 – 100, the higher the score the better | |
| SF-36-C social functioning subscale score | Ranged 0 – 100, the higher the score the better | |
| SF-36-C role emotional subscale score | Ranged 0 – 100, the higher the score the better | |
| SF-36-C mental health subscale score | Ranged 0 – 100, the higher the score the better | |
| Psychological distress | RMHI-C anxiety subscale score | Ranged 10 – 60, the higher the score the greater extent of anxiety |
| RMHI-C depression subscale score | Ranged 5 – 29, the higher the score the greater extent of depression | |
| RMHI-C loss of hehavioral/emotional control subscale score | Ranged 9 – 53, the higher the score the poorer behavioral/emotional control | |
| Social support | MOS-SSS-C tangible subscale score | Ranged 0 – 100, the higher the score the better |
| MOS-SSS-C affecctionate subscale score | Ranged 0 – 100, the higher the score the better | |
| MOS-SSS-C positive social interaction subscale score | Ranged 0 – 100, the higher the score the better | |
| MOS-SSS-C emotional–informational subscale score | Ranged 0 – 100, the higher the score the better |
MOS-SSS-C, Chinese version of the Medical Outcomes Study Social Support Survey; OMFAQ-C, Chinese version of the Older American Resources and Services Multidimensional Functional Assessment Questionnaire; RMHI-C, Chinese version of the Rand Mental Health Inventory; SF-36-C; Chinese version of the Short Form 36 Health Survey.
Applying the second step of the two-step approach36 to SEM, plausible pathways on the Wilson–Cleary model24 linking sociodemographic, lifestyle, diabetes-related, and clinical variables to the latent constructs were added and examined. The path model was refined subsequently with the use of modification indices for adding plausible paths and trimmed by deleting insignificant paths subsequently.
Figure2 shows the final path model obtained, as well as the standardized path coefficients and proportions of variance of endogenous variables explained. The chi square of the final model was 747.9 with a degree of freedom (df) of 268, P < 0.001. The goodness-of-fit indices (χ2/df = 2.8, RMSEA = 0.065, AGFI = 0.96, CFI = 0.97 and NNFI = 0.96) showed that the final model was a reasonably good fit to the data37. All path coefficients were significant (P < 0.05). The model explained for 66, 64, and 72% of the variance of HRQOL, general health perception, and physical functional status, respectively.
Our final model (Figure2) shows that no considered clinical variables, namely, diabetes-related characteristics (e.g., type of treatment) and physiological data (e.g., HbA1c), had any significant direct or indirect influence on HRQOL. Among the considered variables reflecting the characteristics of the individual, age (standardized path coefficient β = −0.27), female sex (β = −0.17) and physical activity (β = 0.11) had a significant effect on physical functional status, which in turn affected HRQOL through general health perception (β = 0.59), whereas psychological distress had both a direct (β = −0.36) and indirect (β = −0.30) effect through general health perception on HRQOL. Among the considered variables reflecting characteristics of the environment, sociodemographic characteristics, such as marital status, had no significant direct or indirect influence on HRQOL, whereas adequacy of income (β = 0.06) and social support (β = 0.06) both had a significant direct influence on HRQOL. Of note, symptom status had a significant direct path (β = −0.61) to general health perception in addition to the indirect pathway (β = −0.70) from physical functional status to general health perception (β = 0.14).
In summary, we identified nine determinants with both a direct and indirect effect on the HRQOL of the studied population (Figure2). The four determinants with a direct effect on HRQOL (66% of variance explained) were: general health perception, psychological distress, adequacy of income and social support. The three determinants with an indirect effect on HRQOL through general health perception (64% of variance explained) were: symptom status, physical functional status and psychological status. A further four determinants that exerted an indirect effect on HRQOL through physical functional status (72% of variance explained) were: symptom status, age, sex and physical activity. The determinants of sex, age, physical activity and psychological distress are categorized as characteristics of the individual (Figure2), whereas social support and adequacy of income are categorized as socioeconomic characteristics of the environment.
Discussion
To our knowledge, this is the first study to examine the determinants of HRQOL based on the Wilson–Cleary model24 in community-dwelling older Hong Kong Chinese people with diabetes. Our findings delineate an overall path model linking the patients' individual and environmental characteristics, symptom status, physical functional status, general health perception, and HRQOL with directional pathways. This model can enhance our understanding of the underlying complex biological and psychological processes impacting on the HRQOL of older people with diabetes.
The present study not only adds to the growing body of literature on the Wilson–Cleary model25–28, and supports its usefulness as a theoretical framework to understand the relationship among the clinical and psychological outcomes of patients with chronic illness, but also provides insights to enhance HRQOL among diabetic patients. In the present study, we identified six patient individual and environmental characteristics, namely, age, sex, physical activity, psychological distress, social support and adequacy of income, that significantly influenced HRQOL directly or by way of physical functional status and general health perception. In particular, improving social and financial support, as well as providing interventions to promote physical activity and to cope with psychological distress in this patient population might be effective to eventually enhance their HRQOL.
We have identified four previous applications of the Wilson–Cleary model24 in populations with chronic illness25–28. The study25 of patients with generalized anxiety disorder used the measure of clinician-rated severity of the illness as the clinical outcome, and it was influential on HRQOL. One of the two studies of patients with HIV/AIDS26 showed that the physiological outcome is one of the determinants. The other study in patients with HIV/AIDS27 again showed that the clinical variables including physiological outcomes are influential on HRQOL. The last study partially applied the Wilson–Cleary model in Hong Kong Chinese patients with heart failure28, in which the role of clinical outcomes on HRQOL was not examined. SEM was used in the first three studies, and adequate fit to the Wilson–Cleary model was found in all of them. Although the present study results are largely consistent with the Wilson–Cleary model, diabetes-related characteristics and physiological outcomes, including time since diagnosis, age of onset, type of diabetes treatment, HbA1c level, blood pressure and lipid profile are all not associated with symptom status as suggested by the theoretical HRQOL model. This can probably be related to the fact that our sample was drawn from community-dwelling older adults with stable diabetes control. Given that diabetes is an insidious disease, most diabetic patients perceive a stable health status until they suffer from diabetes complications, such as retinopathy, neuropathy and nephropathy. Therefore, the diabetes-related clinical and physiological variables might not be perceived as factors influencing the studied population's HRQOL through symptom status. A cohort study with community-dwelling UK older adults38 showed that functional well-being and emotional experience are more instrumental to daily living, and in turn exert more influence on HRQOL. A national study with USA older adults39 showed that emotional well-being and activities of daily living impact on HRQOL. Nevertheless, a recent Taiwanese study40 reported that high HbA1c level could adversely affect HRQOL in diabetic patients with <10-year disease duration. Further research is therefore warranted to confirm the role of diabetes-related characteristics and physiological outcomes in influencing HRQOL of diabetic patients, particularly those who have stable diabetes control.
While using a theoretical framework to guide the study was our strength, attention should be drawn to the limitations of the study, including a non-probability sample of patients who were community-dwelling older adults with type 2 diabetes attending primary care. The results showed that our sample generally had stable diabetes control, as shown by the physiological data, which reflected the diabetic patient profile expected at the primary care setting31. The present findings might not be generalized to patients of other age groups, or with unstable conditions or with advanced diabetes complications and who attend tertiary care. Our sample generally engaged in a healthy lifestyle, and had low levels of severity of fatigue and chronic pain. However, these findings were only self-reported. Another limitation lies with the cross-sectional design of the present study; our results cannot establish any cause–effect relationships. Finally, some caution is required in interpreting the study results. Two of the eight subscales of the SF-36, the physical functional and general health subscales, were used to measure physical functional status and general health perception in the Wilson–Cleary model, and the remaining six subscales were used to measure HRQOL. Although the internal consistency of the eight SF-36 subscale scores of our sample was reasonably high (Cronbach's alpha 0.82), it is possible that our measurement model of HRQOL might not fully reflect the usual concept of HRQOL. Furthermore, the generic measurement of the symptom status in our path model might not appropriately reflect the symptoms of diabetic patients, such as, hypoglycemia and hyperglycemia. This might explain why diabetes-related characteristics and physiological outcomes were not associated with the symptom status of the model. Nevertheless, the present findings provide important implications for knowledge development in enhancing HRQOL in older people with diabetes.
For healthcare providers of primary care setting, our findings provide an important insight into optimizing both the physiological and psychological outcomes of older adults with diabetes. Healthcare providers should focus not only on diabetes-related treatment and physiological outcomes, but on caring for the person as a holistic being who faces challenges arising from living with diabetes as well as other challenges arising from the total life context – the biopsychosocial environment – reflecting the philosophy of integration both of biomedical and social science paradigms in healthcare of the Wilson–Cleary model24. Furthermore, as shown in the present findings, patients’ experiences, such as the severity of fatigue, the number of somatic symptoms, the number of comorbidities, the ability to take care of activities of daily living, the severity of chronic pain and psychological distress, are influential on HRQOL. These experiences resemble some features of ‘geriatric syndrome’, which ‘is related to the impairment of multiple systems due to aging as well as age-related disease’41. Taking the present findings and the literature together, we recommend that healthcare providers should adopt an empowerment approach7 and a chronic care model8 in order to develop an equal partnership with Chinese older adults so as to plan appropriate care that can optimize both their physiological and psychological well-being. Healthcare providers should mobilize resources to create a supportive environment, such as social support and financial adequacy, for older adults. In addition, a heightened awareness of the opportunistic screening of geriatric syndrome among such older adults should be developed9,41.
Acknowledgments
We thank all the participating clinics, the staff of the clinics who facilitated the recruitment of participants, and especially all of the participants of the study. This study received no funding from any agency in the public, commercial or not-for-profit sectors.
References
- Harkness E, MacDonald W, Valderas J, et al. Identifying psychosocial interventions that improve both physical and mental health in patients with diabetes: a systematic review and meta-analysis. Diabetes Care. 2010;33:926–930. doi: 10.2337/dc09-1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Norris SL, Chowdhury FM, et al. The effects of interventions on health-related quality of life among persons with diabetes: a systematic review. Med Care. 2007;45:820–834. doi: 10.1097/MLR.0b013e3180618b55. [DOI] [PubMed] [Google Scholar]
- Ali S, Stone M, Skinner TC, et al. The association between depression and health-related quality of life in people with type 2 diabetes: a systematic literature review. Diabetes Metab Res Rev. 2010;26:75–89. doi: 10.1002/dmrr.1065. [DOI] [PubMed] [Google Scholar]
- Landman GWD, van Hateren KJJ, Kleefstra N, et al. Health-related quality of life and mortality in a general and elderly population of patients with type 2 diabetes (ZODIAC-18) Diabetes Care. 2010;33:2378–2382. doi: 10.2337/dc10-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldney RD, Philips PJ, Fisher LJ, et al. Diabetes, depression, and quality of life: a population study. Diabetes Care. 2004;27:1066–1070. doi: 10.2337/diacare.27.5.1066. [DOI] [PubMed] [Google Scholar]
- Glasgow RE, Peeples M, Skovlund SE. Where is the patient in diabetes performance measures? The case for including patient-centered and self-management measures. Diabetes Care. 2008;31:1046–1050. doi: 10.2337/dc07-1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RM, Funnell MM. Patient empowerment: myths and misconceptions. Patient Educ Couns. 2010;79:277–282. doi: 10.1016/j.pec.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland PAO, Hudson SV, Piasecki A, et al. Features of the chronic care model (CCM) associated with behavioral counselling and diabetes care in community primary care. J Am Board Fam Med. 2010;23:295–305. doi: 10.3122/jabfm.2010.03.090141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laiteerapong N, Karter AJ, Liu JY, et al. Correlates of quality-of-life in older adults with diabetes: the diabetes and aging study. Diabetes Care. 2011;34:1749–1753. doi: 10.2337/dc10-2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best JH, Rubin RR, Peyrot M, et al. Weight-related quality of life, health utility, psychological well-being, and satisfaction with exenatide once weekly compared with sitagliptin or pioglitazone after 26 weeks of treatment. Diabetes Care. 2011;34:314–319. doi: 10.2337/dc10-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logtenberg SJ, Kleefstra N, Houweling ST, et al. Health-related quality of life, treatment satisfaction and costs associated with intraperitoneal versus subcutaneous insulin administration in type 1 diabetes. A randomized controlled trial. Diabetes Care. 2010;33:1169–1172. doi: 10.2337/dc09-1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins MM, O'Sullivan T, Harkins V, et al. Quality of life and quality of care in patients with diabetes experiencing different models of care. Diabetes Care. 2009;32:603–605. doi: 10.2337/dc08-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ose D, Wensing M, Szecsenyi J, et al. Impact of primary care-based disease management on the health-related quality of life in patients with type 2 diabetes and comorbidity. Diabetes Care. 2009;32:1594–1596. doi: 10.2337/dc08-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu BF. Globalization of diabetes: the role of diet, lifestyle, and genes. Diabetes Care. 2011;34:1249–1257. doi: 10.2337/dc11-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CY, Perng SJ, Chen HF, et al. The impact of learned resourcefulness on quality of life in type 2 diabetic patients: a cross-sectional correlational study. J Nurs Res. 2008;16:264–274. doi: 10.1097/01.jnr.0000387314.97515.8c. [DOI] [PubMed] [Google Scholar]
- Huang IC, Hwang CC, Wu MY, et al. Diabetes-specific or generic measures for health-related quality of life? Evidence from psychometric validation of the D-39 and SF-36. Value Health. 2008;11:450–461. doi: 10.1111/j.1524-4733.2007.00261.x. [DOI] [PubMed] [Google Scholar]
- Li TC, Lin CC, Liu CS, et al. Validation of the Chinese version of the diabetes impact measurement scales amongst people suffering from diabetes. Qual Life Res. 2006;15:1613–1619. doi: 10.1007/s11136-006-0024-x. [DOI] [PubMed] [Google Scholar]
- Tang WL, Wang YM, Du WM, et al. Assessment of quality of life and relevant factors in elderly diabetic patients in the Shanghai community. Pharmacoepidemiol Drug Saf. 2006;15:123–130. doi: 10.1002/pds.1166. [DOI] [PubMed] [Google Scholar]
- Shiu ATY, Thompson DR, Wong RYM. Quality of life and its predictors among Hong Kong Chinese patients with diabetes. J Clin Nurs. 2008;17:125–132. doi: 10.1111/j.1365-2702.2007.02036.x. [DOI] [PubMed] [Google Scholar]
- Lam CKL, Tse EYY, Gandek B, et al. The SF-36 summary scales were valid, reliable, and equivalent in a Chinese population. J Clin Epidemiol. 2005;58:815–822. doi: 10.1016/j.jclinepi.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Janus ED. Epidemiology of cardiovascular risk factors in Hong Kong. Clin Exp Pharmacol Physiol. 1997;24:987–988. doi: 10.1111/j.1440-1681.1997.tb02736.x. [DOI] [PubMed] [Google Scholar]
- Yang W, Lu J, Weng J, et al. Prevalence of diabetes among men and women in China. N Engl J Med. 2010;362:1090–1101. doi: 10.1056/NEJMoa0908292. [DOI] [PubMed] [Google Scholar]
- Schuz B, Wurm S, Schollgen I, et al. What do people include when they self-rate their health? Differential associations according to health status in community-dwelling older adults. Qual Life Res. 2011;20:1573–1580. doi: 10.1007/s11136-011-9909-4. [DOI] [PubMed] [Google Scholar]
- Wilson IB, Cleary PD. Linking clinical variables with health-related quality of life: a conceptual model of patient outcomes. JAMA. 1995;273:59–65. [PubMed] [Google Scholar]
- Wyrwich KW, Harnam N, Locklear JC, et al. Understanding the relationships between health outcomes in generalized anxiety disorder clinical trials. Qual Life Res. 2011;20:255–262. doi: 10.1007/s11136-010-9734-1. [DOI] [PubMed] [Google Scholar]
- Sousa KH, Kwok OM. Putting Wilson and Cleary to the test: analysis of a HRQOL conceptual model using structural equation modeling. Qual Life Res. 2006;15:725–737. doi: 10.1007/s11136-005-3975-4. [DOI] [PubMed] [Google Scholar]
- Nokes KM, Holzemer WL, Corless IB, et al. Health-related quality of life in persons younger and older than 50 who are living with HIV/AIDS. Res Aging. 2000;22:290–310. [Google Scholar]
- Yu DSF, Lee DTF, Woo J. Health-related quality of life in elderly Chinese patients with heart failure. Res Nurs Health. 2004;27:332–344. doi: 10.1002/nur.20030. [DOI] [PubMed] [Google Scholar]
- Chia L. 2007. The characteristics that associate with health-related quality of life in patients with type-2 diabetes. PhD thesis, University of Pittsburgh, Pittsburgh, PA, USA. http://etd.library.pitt.edu/ETD/available/etd-06072007-092944/unrestricted/Chia_Lichun_04_Apr_2007.pdf accessed on 12 March 2013)
- Speight J, Reaney MD, Barnard KD. Not all roads lead to Rome – a review of quality of life measurement in adults with diabetes. Diabet Med. 2009;26:315–327. doi: 10.1111/j.1464-5491.2009.02682.x. [DOI] [PubMed] [Google Scholar]
- Mishra GD, Gale CR, Sayer AA, et al. How useful are the SF-36 sub-subscales in older people? Mokken scaling of data from the HALCyon programme. Qual Life Res. 2011;20:1005–1010. doi: 10.1007/s11136-010-9838-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu HC, Chen YC, Mau LW, et al. An evaluation of the reliability and validity of the Chinese-version OARS Multidimensional Functional Assessment Questionnaire. Chinese J Public Health. 1997;16:119–132. [Google Scholar]
- American Diabetes Association. Executive summary: standards of medical care in diabetes – 2010. Diabetes Care. 2010;33(Supp. 1):S4–S10. doi: 10.2337/dc12-s004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Wu SC, Krause NM, et al. The structure of the Mental Health Inventory among Chinese in Taiwan. Med Care. 1992;30:659–676. doi: 10.1097/00005650-199208000-00001. [DOI] [PubMed] [Google Scholar]
- Yu DSF, Lee DTF, Woo J. Psychometric properties of the Chinese version of the Medical Outcomes Study Social Support Survey (MOS-SSS-C) Res Nurs Health. 2004;27:135–143. doi: 10.1002/nur.20008. [DOI] [PubMed] [Google Scholar]
- Anderson JC, Gerbing DW. Structural equation modeling in practice: a review and recommended two-step approach. Psychol Bull. 1988;103:411–423. [Google Scholar]
- Schermelleh-Engel K, Moosbrugger H, Müller H. Evaluating the fit of structural equation models: tests of significance and descriptive goodness-of-fit measures. Methods Psychol Res Online. 2003;8:23–74. [Google Scholar]
- Brett CE, Gow AJ, Corley J, et al. Psychosocial factors and health as determinants of quality of life in community- dwelling older adults. Qual Life Res. 2012;21:505–516. doi: 10.1007/s11136-011-9951-2. [DOI] [PubMed] [Google Scholar]
- Baernholdt M, Hinton I, Yan G, et al. Factors associated with quality of life in older adults in the United States. Qual Life Res. 2012;21:527–534. doi: 10.1007/s11136-011-9954-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RH, Wu LC, Hsu HY. A path model of health-related quality of life in type 2 diabetic patients: a cross-sectional study in Taiwan. J Adv Nurs. 2011;67:2658–2667. doi: 10.1111/j.1365-2648.2011.05701.x. [DOI] [PubMed] [Google Scholar]
- Araki A, Ito H. Diabetes mellitus and geriatric syndromes. Geriatr Gerontol Int. 2009;9:105–114. doi: 10.1111/j.1447-0594.2008.00495.x. [DOI] [PubMed] [Google Scholar]


