Abstract
Aims/Introduction
Diabetes has profound consequences on the cardiovascular system leading to cardiovascular morbidity and mortality in diabetic patients. Blood pressure (BP) has a characteristic and reproducible circadian pattern, with high values during the day and low values at night. A 7-day timed analysis of BP through ambulatory blood pressure monitoring has been used not only to diagnose day and night dipping patterns of blood pressure, but also to measure day-to-day variability and the circadian hyper-amplitude-tension, a condition in which excessive circadian BP amplitude precedes the chronic established hypertension. Our objective was to assess the 7-day/24-h circadian pattern of BP and heart rate in diabetic patients, as it could be helpful in the diagnosis and prevention of cardiovascular morbidity.
Materials and Methods
A total of 50 diabetic patients with type 2 diabetes and 50 non-diabetic participants were recruited for the study. General health records were individually maintained, and 7-day/24-h ambulatory blood pressure monitoring using an ambulatory blood pressure monitor was carried out.
Results
The rhythmic parameters of systolic and diastolic BP, heart rate, double amplitude, acrophase and 3-h fractionated hyperbaric index were found to be significantly high in diabetic patients. A total of 12 participants were diagnosed with circadian hyper-amplitude-tension. These data suggest that diabetic patients have certain variations in the circadian pattern of blood pressure and heart rate, which can result in disturbed vascular events, and thus are at greater risk of cardiovascular morbidity.
Conclusion
Seven-day/24-h monitoring might be useful as an early predictive tool in assessing future cardiovascular risk, guiding treatment and management of these patients.
Keywords: Circadian hyper-amplitude-tension, Diabetes, Midline-estimating statistic of rhythm
Introduction
Diabetes and hypertension are interrelated diseases, and can be classified as high-risk factors for cardiovascular disease (CVD). Hypertension occurs in approximately 30% of patients with type 1 diabetes, and in 50–80% of patients with type 2 diabetes1,2. Blood pressure (BP) is recognized as a risk factor for the development of diabetic nephropathy, retinopathy3. In patients with type 2 diabetes, hypertension usually clusters with the other components of the cardiometabolic syndrome, such as microalbuminuria, central obesity, insulin resistance, dyslipidemia, hypercoagulation, increased inflammation and left ventricular hypertrophy (LVH). In type 1 diabetes, hypertension often occurs subsequent to the development of diabetic nephropathy4. Hypertension in people with diabetes is characterized by volume expansion, increased salt sensitivity, isolated systolic blood pressure (SBP) elevation, loss of the nocturnal dipping of BP and pulse, and increased propensity toward orthostatic hypotension and albuminuria5. Patients with type 1 and type 2 diabetes frequently have circadian changes in blood pressure that correlate to nephropathy risk6. Early detection of nocturnal hypertension and early intervention with angiotensin blockade might delay progression of diabetic nephropathy. Therefore, strict control of blood pressure is very important in these patients. In most individuals, BP has a characteristic and reproducible daily pattern, as regulated by the circadian timing system7. Based on the daily pattern of BP, two broad categories of people have been recognized: dippers and non-dippers8. In patients with cardiovascular risk, the night-time dipping pattern is disturbed or absent9. A 7-day timed analysis of the records of BP through ambulatory blood pressure monitoring has been used to diagnose day-to-day variability10 and the circadian hyper-amplitude-tension (CHAT), a condition in which excessive circadian BP amplitude precedes the chronic established hypertension11. In clinical practice, it is sometimes very hard to identify the true blood pressure level when the BP variability is very high. In such cases, 24-h to 7 days of ambulatory BP monitoring (ABPM) is useful for the assessment of the actual BP level and the prediction of cardiovascular prognosis. We used a 7-day timed analysis of the records of BP through ABPM to study day-to-day variability in patients with type 2 diabetes.
Material and Methods
Registration of Volunteer Diabetic Patients
A total of 50 male participants with type 2 diabetes and 50 non-diabetic controls (age 30–70 years) with casual BP ≤140/90 mmHg were registered from the Diabetes Clinic OPD at King George Medical University, Lucknow, India. No participant was taking any antihypertensive medicine during ABPM. The protocol of the present study was approved by the Committee of Medical Ethics, Research Cell KGMU (3118/R.Cell-08), Lucknow, India, in compliance with the Declaration of Helsinki principles of medical ethics. All the participants were informed of the purpose and protocols of the study before they gave written informed consent in English and Hindi. General health records were maintained for each participant. Participants with any type of chronic complications of diabetes, pregnancy, end-stage renal diseases or significant albuminuria were excluded from the study.
ABPM
Participants underwent 7-day/24-h ABPM, which was carried out with an automated ABPM device, the A&D TM-2430 model (A&D Company, Tokyo, Japan). Participants were told to carry out all their routine activities during recording periods. The ABPM device was programmed to record BP and heart rate (HR) every 30 min during the day, and at 1-h intervals during the night. Measurements from the ABPM device were transferred and stored in a computer for further analysis. For each individual, the data were summarized in a sphygmochron (a computer comparison of patients' profile with the specified reference limit). The results were analyzed using Halberg COSINOR analysis. Each BP and HR profile was analyzed by a sphygmochron, utilizing both a parametric and non-parametric approach. ABPM records were sent to Halberg Chronobiology Center, University of Minnesota, Minnesota, Minneapolis, USA, for further interpretation. The following estimates were obtained: (i) midline-estimating statistic of rhythm (MESOR), a time structure or chronome-adjusted mean; (ii) double amplitude or predictable change (2A), which is the total change within a day or the circadian amplitude of reproducible variability within a day; (iii) acrophase, which is a measure of timing of overall high values recurring in each cycle; and (iv) CHAT, which was diagnosed in those who were having larger than usual changes in BP and overswinging BP patterns in the double amplitude. The average 7-day values of the aforementioned parameters were determined for each participant.
Statistical Analysis
Statistical significance between experimental values of ABPM of non-diabetic and diabetic participants were calculated by Halberg COSINOR analysis and Student's t-test12–14.
Results
Clinical Observations
The mean SBP/diastolic BP (DBP) of the participants recorded by mercury sphygmomanometer were 127.66/78.19 and 130.21/82.62 for non-diabetic and diabetic participants, respectively. The mean HR was 70.77 ± 6.44 b.p.m. for non-diabetic and 70.21 ± 10.20 b.p.m. for diabetic participants (Table1).
Table 1.
Basal clinical characteristics and observation of participants
| Clinical characteristics | Non-diabetic | Diabetic |
|---|---|---|
| No. participants | 50 | 50 |
| Age (years) | 52.5 ± 9.42 | 58.2 ± 13.42 |
| Height (cm) | 158.2 ± 7.26 | 159.26 ± 5.22 |
| Weight (kg) | 62.5 ± 9.66 | 67.82 ± 6.87 |
| Casual systolic blood pressure (mmHg) | 127.66 ± 7.41 | 130.21 ± 10.22 |
| Casual diastolic blood pressure (mmHg) | 78.19 ± 6.05 | 82.62 ± 5.1 |
| Casual heart rate (b.p.m.) | 70.77 ± 6.44 | 70.21 ± 10.20 |
Values represent the mean ± standard error of 50 diabetic and 50 non-diabetic participants.
ABPM
MESOR
There was a significant difference (P < 0.01) between the MESOR of SBP, DBP and HR of diabetic participants as compared with non-diabetic controls. There was a highly significant increase in MESOR; that is, 16.2% in SBP, 12.5% in DBP and 9.38% of diabetic participants (P < 0.01; Table2).
Table 2.
Seven-day/24-h midline-estimating statistic of rhythm and double amplitude of diabetic and non-diabetic participants
| MESOR | Double amplitude (predictable change) | |||
|---|---|---|---|---|
| Non-diabetic | Diabetic | Non-diabetic | Diabetic | |
| SBP (mmHg) | 110.26 ± 8.62 | 132.2 ± 10.24* | 8.23 ± 2.66 | 24.12 ± 3.82* |
| DBP (mmHg) | 75.62 ± 6.22 | 86.5 ± 12.40* | 4.26 ± 2.06 | 17.07 ± 6.33* |
| HR (b.p.m.) | 70.23 ± 10.22 | 77.86 ± 6.55* | 4.323 ± 2.44 | 14.81 ± 6.33* |
Values represent the mean ± standard error of 35 diabetic and 35 non-diabetic participants.
Significant at the level of P < 0.01. HR, heart rate; DBP, diastolic blood pressure; MESOR, midline-estimating statistic of rhythm; SBP, systolic blood pressure.
Double Amplitude (Predictable Change)
There was a significant difference between the circadian amplitude of reproducible variability of diabetic and non-diabetic participants. In diabetic participants, SBP/DBP was 24.12 ± 3.82/17.07 ± 6.33 (P < 0.01) and HR 14.81 ± 6.33 (P < 0.05), respectively (Table2).
Acrophase
Figure1 shows the circadian pattern of acrophase in diabetic and non-diabetic participants. There was a significant difference between diabetic and non-diabetic participants. Acrophase in the SBP/DBP of diabetes participants was found mostly during the early morning hours, whereas in non-diabetic participants it was found during 14.00–16.00 h.
Figure 1.
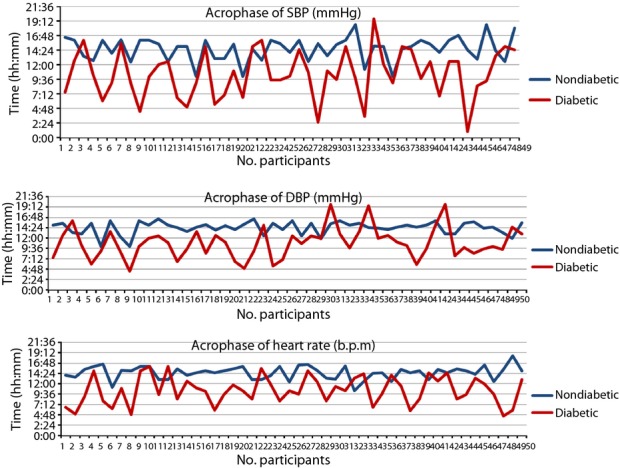
Acrophase systolic blood pressure (SBP), diastolic blood pressure (DBP) and heart rate of 7-day/24-h ambulatory blood pressure monitoring.
CHAT
CHAT was diagnosed in 12 of 50 diabetic participants. However, no CHAT was observed in non-diabetic participants. (P < 0.05). There were 38 non-dippers diagonosed in the diabetic group, whereas 42 dippers was diagonosed in the non-diabetic control group (Figure2).
Figure 2.
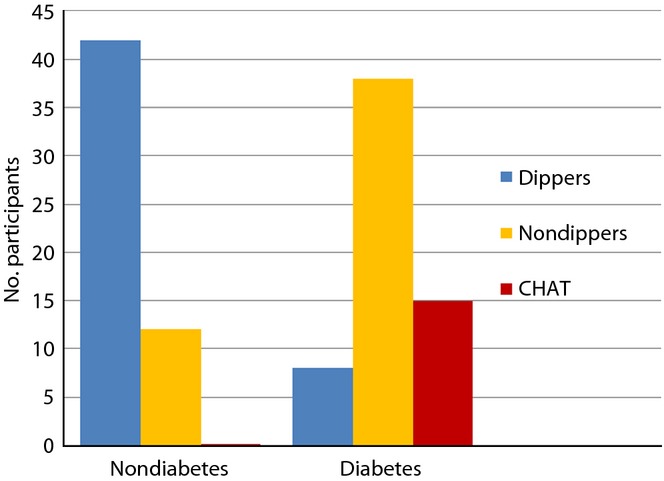
Dipping, non-dipping patterns and circadian hyper-amplitude-tension (CHAT) diagnosed in diabetes patients.
Three-Hour Hyperbaric Index
A maximum excess of SBP (12.33 ± 1.069) was observed at 12.00–15.00 h (P < 0.01). Excess values of DBP were found during 06.00–09.00 h (9.32 ± 3.21) and 15.00–18.00 h (12.01 ± 4.22, P < 0.01), which was highly significant in comparison with controls. Similarly, a significant difference between hyperbaric index values of HR in diabetic and non-diabetic participants was noted during 03.00–6.00 h (7.66 ± 2.66) and 18.00–21.00 h (8.22 ± 1.02, P < 0.01; Figure3).
Figure 3.
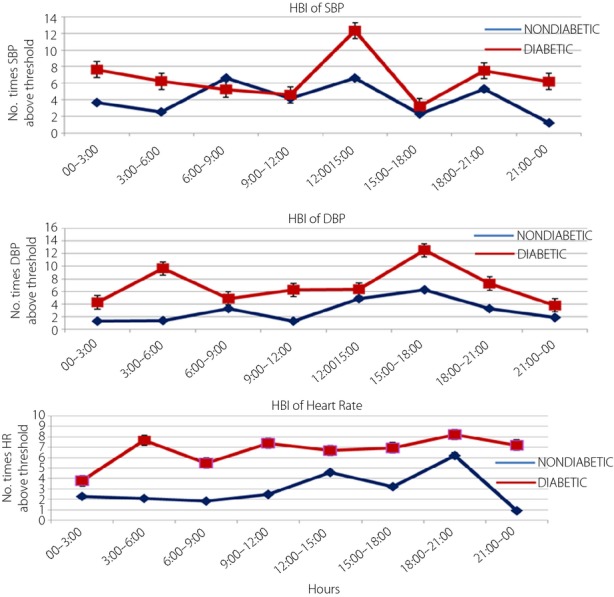
Hyperbaric indexes (HBI) of systolic blood pressure (SBP; mmHg), diastolic blood pressure (DBP; mmHg) and heart rate (HR; b.p.m.) during ambulatory blood pressure monitoring.
Discussion
ABPM has emerged as a strong predictor of variability of BP and HR, not only for the extent of hour-to-hour, but also for day-to-day variability. Seven-day/24-h monitoring is an important tool for prognostication of hypertension in diabetes15. The present study showed that diabetic participants had a disturbed circadian pattern of 7-day/24-h ambulatory BP. The MESOR in diabetic participants was significantly higher (132.2 ± 10.24/86.5 ± 12.40) than non-diabetic participants. Similarly, the MESOR of HR was significantly high (P < 0.01) compared with non-diabetic participants. Also, high BP amplitude (0.001) was observed in SBP, DBP and the HR of diabetic participants. A SBP and/or DBP that overswings (higher amplitude) along the 7-day/24-h scale is a predictor of CHAT16, which is the cause of the above-threshold variability of BP17 associated with the risk of catastrophic events, such as myocardial infarction, cerebral ischemia and nephropathy18,12. In the present study, CHAT was observed in 15 diabetic participants. The results of study therefore show that circadian (24 h) and circasemidian (7 day) patterns of ambulatory BP were disturbed in diabetic participants. In day and night difference of ambulatory BP, we found that a non-dipping pattern was significantly high in diabetic participants compared with controls (Figure2). There were 38 diabetic participants who were diagnosed as non-dippers, whereas 42 non-diabetic participants were diagnosed as dippers during the 7-day/24-h ABPM recordings. Non-dipping pattern in diabetes patients was also reported by Lurbe et al.19, Nakano et al.20, Cuspidi21 and Sierra22. In agreement with these reports, we can say that a non-dipping pattern is associated with a 150% increase in the risk of CVD in diabetes and cardiovascular prognosis. Therefore, for the prevention of CVD in diabetic patients, physicians should recognize that patients are at high risk when an abnormal circadian rhythm of BP is observed. There was a significant difference between the diabetic group and non-diabetic group in the acrophase, which is the time of excess BP. In diabetic patients, the maximum acrophase was noted during 09.00–12.00 h; whereas in the non-diabetic participants it was during 13.00–18.00 h, which is considered a clinically and chronobiologically normal pattern (Figure1). Chronobiological research shows that clinically healthy persons have a circadian rhythm of HR. As a rule, circadian rhythm acrophases (high values) are allocated in an interval of 12.00–20.00 h, and batyphases (lowest values) in an interval of 02.00–09.00 h23,24. Changes in the time structures of BP in diabetes are associated with autonomic nervous dysfunction, which can also produce cardiac autonomic neuropathy25. The hyperbaric index was calculated by numerical integration as the total area (within 1 cycle) of any given patient's BP above the threshold26,27,13. The maximum values of 3-h fractionated hyperbaric index of SBP and DBP was observed at 12.00–15.00 h and 15.00–18.00 h, which was significant in comparison with non-diabetic participants (P < 0.01; Figure3). A maximum excess of SBP was observed at 12.00–15.00 h, and DBP was found during 06.00–09.00 and 15.00–18.00 h (P < 0.01), which was highly significant in comparison with non-diabetes. Similarly, a significant difference between hyperbaric index values of HR in diabetic and non-diabetic participants was noted during 03.00–06.00 h (P < 0.01; Figure3). Our findings show that there are disturbed circadian patterns of all the chronobiological parameters of SBP, DBP and HR in diabetic patients. These data suggest that diabetic patients have certain variations in the 7-day/24-h circadian pattern of BP, and are at greater risk of cardiovascular morbidity. BP control helps to avoid cardiovascular complications in patients with type 2 diabetes28, therefore 7-day/24-h ABPM could be a powerful tool for assessing future cardiovascular risk, detecting alterations, such as absence of nocturnal BP fall, postprandial hypotension, reduced heart and increased BP variability, and guiding treatment and medicine dose in these patients.
Acknowledgments
The authors are grateful to the Halberg Chronobiology Center, University of Minnesota, for ABPM statistics help. A financial grant for the study was supported by the Indian Council of Medical research (ICMR) New Delhi. The authors declare that there are no conflicts of interest.
References
- Eguchi Kazuo. Ambulatory blood pressure monitoring in diabetes and obesity—a review. Int J Hypertens. 2011;2011:954757. doi: 10.4061/2011/954757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsberg L, Molitch M. Diabetes and hypertension: pathogenesis, prevention and treatment. Clin Exp Hypertens. 2004;26:621–628. doi: 10.1081/ceh-200031945. [DOI] [PubMed] [Google Scholar]
- James R. Sowers, murray epstein diabetes mellitus and associated hypertension, vascular disease, and nephropathy an update. Hypertension. 1995;26:869–879. doi: 10.1161/01.hyp.26.6.869. [DOI] [PubMed] [Google Scholar]
- Felício JS, de Souza ACCB, Kohlmann N, et al. Nocturnal blood pressure fall as predictor of diabetic nephropathy in hypertensive patientsv with type 2 diabetes. Cardiovasc Diabetol. 2010;9:36. doi: 10.1186/1475-2840-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Atat F, McFarlane SI, Sowers JR. Diabetes, hypertension, and cardiovascular derangements: pathophysiology and management. Curr Hypertens Rep. 2004;6:215–223. doi: 10.1007/s11906-004-0072-y. [DOI] [PubMed] [Google Scholar]
- Schutta MH. Diabetes and hypertension: epidemiology of the relationship and pathophysiology of factors associated with these comorbid conditions. J Cardiometab Syndr. 2007;2:124–130. doi: 10.1111/j.1559-4564.2007.06368.x. [DOI] [PubMed] [Google Scholar]
- Miller-Craig MW, Bishop CN, Raftery EB. Circadian variation of blood pressure. Lancet. 1978;1:795–797. doi: 10.1016/s0140-6736(78)92998-7. [DOI] [PubMed] [Google Scholar]
- Drayer JI, Weber MA. Reproducibility of blood pressure values in normotensive subjects. Clin Exp Hypertens. 1985;A7:417–422. doi: 10.3109/10641968509073567. [DOI] [PubMed] [Google Scholar]
- Pickering TG. Blood pressure measurement and detection of hypertension. Lancet. 1994;344:31–35. doi: 10.1016/s0140-6736(94)91053-7. [DOI] [PubMed] [Google Scholar]
- Kurpesia M, Trzos E, Drozdz J, et al. Myocardial ischemia and autonomic activity in dippers and non-dippers with coronary artery disease: assessment of normotensive and hypertensive patients. Int J Cardiol. 2002;83:133–142. doi: 10.1016/s0167-5273(02)00031-1. [DOI] [PubMed] [Google Scholar]
- Siegelova J, Fisher B, Havelkin A, et al. Circadian blood pressure variation analyzed from 7-day ambulatory blood pressure monitoring in patients with ischaemic heart disease. Scr Med. 2010;83:41–48. [Google Scholar]
- Cornelissen G, Halberg F. Chronomedicine. In: Armitage P, Colton T, editors. Encyclopedia of Biostatistics. Chichester, UK: Wiley; 1998. pp. 642–649. Vol 1: [Google Scholar]
- Hermida RC, Mojón A, Fernández JR, et al. The tolerance hyperbaric test: a chronobiologic approach for improved diagnosis of hypertension. Chronobiol Int. 2002;19:1183–1211. doi: 10.1081/cbi-120015960. [DOI] [PubMed] [Google Scholar]
- Fisher RA. Statistical Methods for Research Workers. 11th edn. Edinburgh, UK: Oliver and 345 Boyd Ltd; 1950. [Google Scholar]
- Kalaycioglu E, Gokdeniz T, Aykan AC, et al. The relationship between dipper/nondipper pattern and cardioankle vascular index in hypertensive diabetic patients. Blood Press Monit. 2013;18:188–194. doi: 10.1097/MBP.0b013e328362df70. http://journals.lww.com/bpmonitoring/toc/2013/08000. [DOI] [PubMed] [Google Scholar]
- Katinas G, Halberg F, Cornélissen G, et al. Transient circadian hyper-amplitude-tension (CHAT) may be intermittent: case reports illustrating gliding spectral windows. Biomed Pharmacother. 2003;57(Suppl 1):104s–109s. doi: 10.1016/j.biopha.2003.08.020. [DOI] [PubMed] [Google Scholar]
- Halberg F, Cornélissen G, Wall D, et al. Engineering and governmental challenge: 7-day/24-hour chronobiologic blood pressure and heart rate screening: part II. Biomed Instrum Technol. 2002;36:183–197. doi: 10.2345/0899-8205(2002)36[183:EAGCHC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Frattola A, Parati G, Cuspidi C, et al. Prognostic value of 24-hour blood pressurevariability. J Hypertension. 1993;11:1133–1137. doi: 10.1097/00004872-199310000-00019. Oct; [DOI] [PubMed] [Google Scholar]
- Lurbe E, Redon J, Kesani A, et al. Increase in nocturnal blood pressure and progression to microalbuminuria in type 1 diabetes. N Engl J Med. 2002;347:797–805. doi: 10.1056/NEJMoa013410. [DOI] [PubMed] [Google Scholar]
- Nakano S, Konishi K, Furuya K, et al. A prognostic role of mean 24-h pulse pressure level for cardiovascular events in type 2 diabetic subjects under 60 years of age. Diabetes Care. 2005;28:95–100. doi: 10.2337/diacare.28.1.95. [DOI] [PubMed] [Google Scholar]
- Cuspidi C, Meani S, Lonati L, et al. Short-term reproducibility of a non-dipping pattern in type 2 diabetic hypertensive patients. J Hypertens. 2006;24:647–653. doi: 10.1097/01.hjh.0000217846.65089.19. [DOI] [PubMed] [Google Scholar]
- De la Sierra A, Redon J, Banegas JR, et al. Prevalence and factors associated with circadian blood pressure patterns in hypertensive patients. Hypertension. 2009;53:466–472. doi: 10.1161/HYPERTENSIONAHA.108.124008. [DOI] [PubMed] [Google Scholar]
- Koehler F, Okano FK, Elveback LR, et al. Periodograms for the study of physiologic daily periodicity in mice and in man. Exp Med Surg. 1956;14:5–30. [PubMed] [Google Scholar]
- Radysh IV. Temporary organization of womans physiological system during adaptation to different factors of environment. Autoreferat. 1998:46. [Google Scholar]
- Matteucci E, Consani C, Masoni MC, Giampietro O. Circadian blood pressure variability in type 1 diabetes subjects and their nondiabetic siblings influence of erythrocyte electron transfer. Cardiovasc Diabetol. 2010;9:61. doi: 10.1186/1475-2840-9-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberg F, Ahlgren A, Haus E. Circadian systolic and diastolic hyperbaric indices of high school and college students. Chronobiologia. 1984;11:299–309. [PubMed] [Google Scholar]
- Hermida RC, Mojón A, Fernández JR, et al. Computer-based medical system for the computation of blood pressure excess in the diagnosis of hypertension. Biomed Instrum Technol. 1996;30:267–283. [PubMed] [Google Scholar]
- Lago RM, Singh PP, Nesto RW. Diabetes and hypertension. Nat Clin Pract Endocrinol Metab. 2007;3:667. doi: 10.1038/ncpendmet0638. [DOI] [PubMed] [Google Scholar]


