Abstract
Aims/Introduction
To compare carotid and lower limb atherosclerotic lesions, and examine if carotid atherosclerotic lesions are in line with lower limb atherosclerotic lesions, and can reflect generalized atherosclerosis in inpatients with type 2 diabetes.
Materials and Methods
This was an observational study carried out in 867 Chinese inpatients with type 2 diabetes, including 573 previously known and 294 newly diagnosed patients. Ultrasonographic assessments of intima-media thickness (IMT), plaques, and stenosis in the carotid and lower limb arteries were evaluated. Atherosclerotic lesions between the carotid and lower limb arteries were compared in both previously known and newly diagnosed diabetes, respectively.
Results
In both the known (77.3% vs 49.4%, P < 0.001) and the newly diagnosed diabetes (55.4% vs 29.9%, P < 0.001), the prevalence of atherosclerotic plaques was significantly higher in the lower limb arteries than in the carotid arteries. Likewise, the prevalence of stenosis was also significantly higher (P < 0.001) in the lower limb arteries (16.9%) than in the carotid arteries (4.2%) in the established diabetes patients. However, there was no significant difference in the mean IMT between common carotid and common femoral arteries in both the previously known (0.90 ± 0.24 mm vs 0.89 ± 0.20 mm, P = 0.675) and the newly diagnosed diabetes patients (0.86 ± 0.22 mm vs 0.85 ± 0.16 mm, P = 0.436).
Conclusions
Carotid plaques might underestimate generalized plaques in inpatients with type 2 diabetes, as shown by its significantly lower prevalence compared with that of the lower extremity arteries. A combined carotid and lower limb ultrasound examination can improve the detection of atherosclerotic lesions in inpatients with type 2 diabetes.
Keywords: Atherosclerosis, Diabetes, Epidemiology
Introduction
Atherosclerotic complications are the leading causes of morbidity and mortality among patients with type 2 diabetes mellitus1,2. In addition to coronary arteries, carotid and lower extremity arteries are two other sites where atherosclerotic lesions commonly occur2. Diabetes accelerates the progression of atherosclerosis, and increases the incidence of myocardial infarction, stroke and peripheral arterial disease2–5. Therefore, early detection of atherosclerosis in individuals with diabetes is crucial in reducing the risk of cardiovascular, cerebrovascular and amputation events.
Currently, ultrasound examination of carotid arteries is used to investigate the signs of early atherosclerotic vascular disease. Carotid atherosclerotic lesions are regarded as an indicator of generalized atherosclerosis. For example, previous studies showed that carotid atherosclerosis detected by ultrasonography could be an appropriate marker of atherosclerotic disease, and had been shown to independently reflect both cardiovascular and cerebrovascular events6–8. However, there is controversy whether carotid atherosclerosis detected by ultrasonography can be used as a surrogate marker of generalized atherosclerosis. For example, there are studies that showed that plaque formation in the carotid arteries was weakly associated with plaques in other peripheral arteries9,10. In particular, based on ultrasonography, studies comparing the difference of carotid and lower limb atherosclerotic lesions in type 2 diabetes are almost completely lacking.
In a previous study, we found that combining carotid and lower limb ultrasound examination could improve the detection of atherosclerotic plaques in patients with type 2 diabetes11. In the present study, we further compared the intima-media thickness (IMT) value and the prevalence of atherosclerotic plaques and stenosis between the carotid and lower limb arteries, and examined if carotid atherosclerotic lesions were in parallel with lower extremity atherosclerotic lesions in patients with type 2 diabetes using vascular Doppler ultrasonography. Given the fact that the duration of diabetes is an important risk factor for atherosclerosis of carotid and lower limb arteries in type 2 diabetes11, we divided the type 2 diabetics into previously known and newly diagnosed patients, and investigated if the characteristics of atherosclerotic lesions were different between the previously known and the newly diagnosed patients with type 2 diabetes.
Materials and Methods
Participants and Study Design
The current study was a cross-sectional study and partly based on data used in our previous study11. Between May 2007 and July 2008, 917 consecutive type 2 diabetes patients who were admitted to the Department of Endocrinology and Metabolism, Shanghai Jiao Tong University Affiliated Sixth People's Hospital, Shanghai, China, were observed, and valid information was available for 867 patients including 573 previously known and 294 newly diagnosed patients with type 2 diabetes in the present study. According to the World Health Organization criteria12, newly diagnosed diabetes was defined as having either fasting plasma glucose (FPG) ≥7.0 mmol/L and/or 2-h postprandial plasma glucose (2-h PPG) ≥11.1 mmol/L or a 2-h plasma glucose ≥11.1 mmol/L during a 75-g oral glucose tolerance test in patients without a history of diabetes. Previously known diabetes was defined based on self-reported physician diagnosis or use of antidiabetic agents. The definition of diabetic retinopathy, diabetic nephropathy and cardiocerebrovascular events was according to our previous study11. All participants were interviewed to obtain their history of hypertension, cardiocerebrovascular events, alcohol consumption and smoking habits, and underwent an ultrasonographic evaluation of IMT, plaque, and stenosis in both the carotid and lower extremity arteries. The study was approved by the Ethics Committee of the hospital, and all participants gave written informed consent.
Physical Examination and Laboratory Assays
A physical examination including weight, height and blood pressure was carried out. Body mass index (BMI) was calculated as weight divided by height squared. Hypertension was defined according to our previously described criteria11. Blood samples for plasma glucose; C-peptide; glycated hemoglobin A1c (HbA1c); renal function tests including blood urea nitrogen (BUN), serum creatinine (Scr) and blood uric acid (BUA); liver function tests including aspartate aminotransferase (AST), alanine aminotransferase (ALT) and γ-glutamyltransferase (γ-GT); and lipid levels including total cholesterol (TC), total triglycerides (TG), high-density lipoprotein cholesterol (HDL-C) and low density lipoprotein cholesterol (LDL-C) were obtained after an overnight fast, and 2 h after breakfast.
Ultrasonography Measurements
Color Doppler sonography was carried out with an Acuson Sequoia 512 (Siemens Medical Solutions, Mountain View, CA, USA) scanner equipped with a 5–13-MHz linear array transducer. The ultrasonographic examination was carried out by three experienced ultrasonographers according to a standardized technique. After the participants had remained in the supine position for 5 min, the transducer was placed on the neck and lower limbs to show both vessel imaging and blood flow characteristics. At each location, IMT, atherosclerotic plaques and stenosis were recorded. Carotid arteries were examined bilaterally at the levels of the common carotid arteries, the bifurcation, the external carotid arteries, and the internal carotid arteries from transverse and longitudinal orientations. Seven locations of each lower limb artery were evaluated: common femoral artery, profunda femoris artery, superficial femoral artery, popliteal artery, anterior tibial artery, posterior tibial artery and peroneal artery. The IMT was defined as the distance between the leading edge of the lumen-intima echo and the leading edge of the media-adventitia echo. The mean IMT was defined as the mean of IMT in both right and left sides of the common carotid artery or common femoral artery. Atherosclerotic plaque was defined as a focal structure encroaching into the arterial lumen of 0.5 mm or 50% of the surrounding IMT value or IMT of >1.5 mm13. Carotid atherosclerotic plaque was defined as the presence of atherosclerotic plaques in any of the aforementioned carotid arteries segments11,14. Lower limb atherosclerotic plaque was defined as the presence of atherosclerotic plaques in any of the above-mentioned lower limb artery segments11. Based on previous literature15–17, artery stenosis in the present study was classified into three categories: normal, 1–49% diameter reduction and ≥50% diameter reduction. A significant artery stenosis was defined as ≥50% stenosis. Both the intraobserver and interobserver reproducibility were determined using Spearman's correlation coefficient. The intraobserver correlation coefficients were 0.91–0.92 for carotid IMT and 0.92–0.94 for femoral IMT, 0.88–0.89 for carotid plaque and 0.89–0.94 for lower limb arterial plaque, and 0.87–0.94 for carotid stenosis and 0.94–1.00 for lower limb arterial stenosis, respectively. The interobserver correlation coefficients were 0.87–0.97 for carotid IMT and 0.95–0.97 for femoral IMT, 0.83–0.94 for carotid plaque and 0.89–0.94 for lower limb arterial plaque, and 0.82–0.89 for carotid stenosis and 0.83–0.89 for lower limb arterial stenosis, respectively.
Comparison of Carotid and Lower Limb Atherosclerotic Lesions
The comparisons of carotid and lower limb atherosclerotic lesions in the present study population were based on the comparison of atherosclerotic plaques, stenosis and IMT. The comparisons of atherosclerotic plaques and stenosis were carried out at both the patient-level and vessel-level. In the vessel-level analysis, the left carotid artery, the right carotid artery, the left lower limb artery and the right lower limb artery were considered separately. In the patient-level analysis, the prevalence of plaque and stenosis between the carotid and lower limb arteries was compared. In addition to comparing the prevalence of atherosclerotic plaques, the prevalence of artery stenosis and significant artery stenosis was also compared. The comparison of IMT was carried out between the mean common carotid IMT and the mean common femoral IMT.
Statistical Analysis
The data were analyzed using spss 11.0 for Windows (SPSS Inc., Chicago, IL, USA). For continuous variables, the Kolmogorov–Smirnov test was applied to examine normal distribution. Continuous variables were expressed as mean ± standard deviation, and compared by two-sided t-tests or, if the data were not distributed normally, variables were expressed as median with interquartile range and the Mann–Whitney U-test was used. Categorical variables were expressed as percentages and using a χ2-test or Fisher's exact test. Linear regression was used to evaluate differences of continuous variables while adjusting for age and/or sex. Binary logistic regression was used to evaluate differences of categorical variables while adjusting for age and/or sex. A two-sided alpha level of 0.05 was considered statistically significant.
Results
Clinical Characteristics of the Study Patients
The clinical characteristics of the 867 inpatients with type 2 diabetes are shown in Table1. The mean age of the previously known diabetics (62 ± 11 years) was significantly older than that of the newly diagnosed diabetics (52 ± 15 years). Interestingly, after adjustment for age and sex, only the lower limb atherosclerotic lesions in the previously known type 2 diabetics were more severe than in the newly diagnosed type 2 diabetes. The prevalence of diabetic retinopathy, diabetic nephropathy and cardiocerebrovascular events was markedly higher in the previously known diabetics than in the newly diagnosed diabetics, even after adjustment for age and sex.
Table 1.
Characteristics and comparison between previously known and newly diagnosed type 2 diabetes patients
| Variables | Previously known type 2 diabetes (n = 573) | Newly diagnosed type 2 diabetes (n = 294) | P-value | P-value* |
|---|---|---|---|---|
| Male, n (%) | 284 (49.6%) | 191 (65.0%) | <0.001 | 0.02 |
| Age (years) | 62 ± 11 | 52 ± 15 | <0.001 | <0.001 |
| Duration of diabetes (months) | 118 ± 79 | – | – | |
| Smoking, n (%) | 133 (23.2%) | 61 (20.8%) | 0.503 | 0.615 |
| Alcohol, n (%) | 162 (28.3) | 87 (29.6%) | 0.712 | 0.789 |
| Hypertension, n (%) | 308 (53.8%) | 115 (39.1%) | <0.001 | 0.242 |
| Hyperlipidemia, n (%) | 450 (78.5%) | 203 (69.0%) | 0.227 | 0.428 |
| Body mass index (kg/m2) | 24.79 ± 3.59 | 25.26 ± 3.71 | 0.079 | 0.454 |
| Waist circumference (cm)† | 89 (83–96) | 90 (84–96) | 0.508 | 0.537 |
| Systolic blood pressure (mmHg) | 133 ± 18 | 127 ± 15 | <0.001 | 0.049 |
| Diastolic blood pressure (mmHg) | 90 ± 9 | 81 ± 10 | 0.202 | 0.309 |
| Aspartate aminotransferase (U/L)† | 19 (14–29) | 26 (16.5–42.5) | <0.001 | 0.003 |
| Alanine aminotransferase (U/L)† | 19 (16–24) | 23 (17–32) | <0.001 | 0.027 |
| γ-glutamyltransferase (U/L)† | 23 (16–40) | 31 (21.5–53.5) | <0.001 | 0.479 |
| Blood urea nitrogen (μmol/L)† | 5.50 (4.53–6.70) | 4.9 (4.0–6.3) | <0.001 | 0.079 |
| Serum creatinine (μmol/L)† | 67 (56–80) | 68 (56–80) | 0.996 | 0.734 |
| Blood uric acid (μmol/L)† | 313 (255–371) | 303 (241–373) | 0.319 | 0.310 |
| Total triglycerides (mmol/L)† | 1.40 (0.98–2.12) | 1.51 (1.07–2.13) | 0.081 | 0.746 |
| Total cholesterol (mmol/L)† | 4.50 (4.00–5.30) | 4.90 (4.10–5.61) | 0.001 | 0.003 |
| High-density lipoprotein (mmol/L)† | 1.11 (0.95–1.31) | 1.05 (0.89–1.23) | 0.001 | 0.416 |
| Low-density lipoprotein (mmol/L)† | 2.91 (2.42–3.40) | 3.30 (2.57–3.99) | <0.001 | <0.001 |
| Fasting plasma glucose (mmol/L)† | 7.46 (5.84–9.30) | 8.57 (6.79–11.33) | <0.001 | <0.001 |
| 2 h plasma glucose (mmol/L) | 12.77 ± 4.44 | 15.58 ± 5.63 | <0.001 | <0.001 |
| Fasting C-peptide (ng/mL)† | 1.71 (1.07–2.59) | 1.65 (0.88–2.43) | 0.616 | 0.102 |
| 2-h Postprandial C-peptide(ng/mL)† | 3.84 (2.15–5.68) | 3.34 (1.9–5.48) | 0.105 | 0.732 |
| Glycated hemoglobin A1C (%) | 8.82 ± 2.42 | 10.86 ± 2.50 | <0.001 | <0.001 |
| Mean femoral IMT (mm) | 0.89 ± 0.20 | 0.85 ± 0.16 | 0.005 | 0.045 |
| Mean carotid IMT (mm) | 0.87 ± 0.24 | 0.79 ± 0.23 | <0.001 | 0.849 |
| Carotid plaques, n (%) | 283 (49.4%) | 88 (29.9%) | <0.001 | 0.135 |
| Lower limb plaques, n (%) | 443 (77.3%) | 163 (55.4%) | <0.001 | 0.052 |
| Carotid stenosis, n (%) | 24 (4.2%) | 9 (3.1%) | 0.412 | 0.425 |
| Lower limb stenosis, n (%) | 97 (16.9%) | 14 (4.8%) | <0.001 | 0.010 |
| Diabetic retinopathy, n (%) | 171 (29.8%) | 47 (16.0%) | <0.001 | <0.001 |
| Diabetic nephropathy, n (%) | 163 (28.4%) | 26 (8.8%) | <0.001 | <0.001 |
| Cardio-cerebrovascular events, n (%) | 132 (23%) | 23 (7.8%) | <0.001 | <0.001 |
Values are expressed as the mean ± standard deviation, or median with interquartile range, or percentages.
Non-normal distribution of continuous variables. The P-values were not adjusted for age and sex for trend.
The P values were adjusted for age and sex for trend.
Comparison of Carotid and Lower Limb Atherosclerotic Plaques
The comparison of carotid and lower limb atherosclerotic plaques is shown in Figure1. Patient-level analysis showed that in both the previously known diabetes group and in the newly diagnosed diabetes group, the prevalence of atherosclerotic plaques was significantly higher in the lower limb arteries than in the carotid arteries (all P < 0.001; Figure1a).
Figure 1.
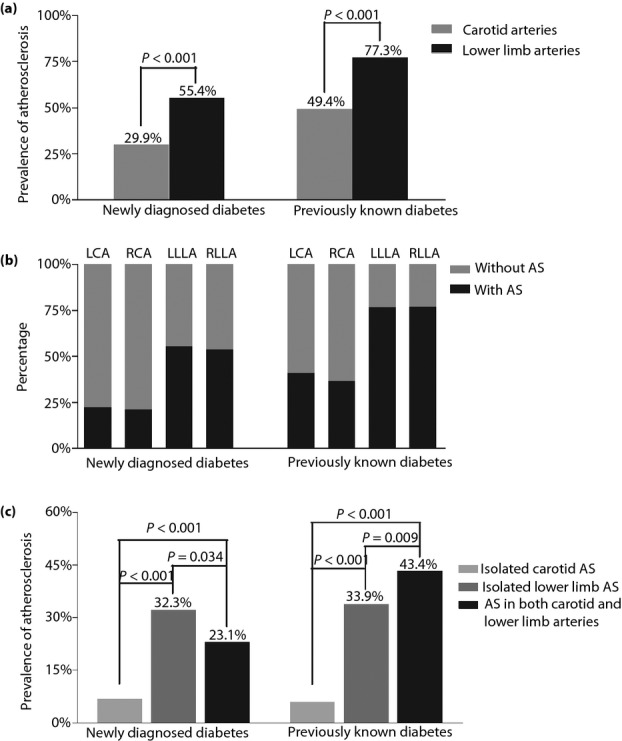
Comparison of carotid and lower limb atherosclerotic plaques in both the previously known and the newly diagnosed diabetes patients. (a) The comparison of the prevalence of atherosclerotic plaques in the carotid and lower limb arteries. (b) The comparison of the prevalence of atherosclerotic plaques in the left carotid, right carotid, left lower limb and right lower limb artery. An artery was considered normal if there was no evidence of atherosclerotic plaque present. An artery was considered “diseased” if there was evidence of any level of atherosclerotic plaque. (c) The percentage of patients with atherosclerotic plaques. The patients with plaques were divided into three groups including the patients with isolated carotid plaque, patients with isolated lower extremity plaque, and patients with plaques in both the carotid and lower extremity arteries. The P-values for three group comparisons were all <0.001 in both the newly diagnosed diabetes patients and the previously known diabetes patients. LCA, left carotid artery; LLLA, left lower limb artery; RCA, right carotid artery; RLLA, right lower limb artery.
Vessel-level analysis showed that both in the previously known group and in the newly diagnosed diabetes group, more than 50% of the left and right lower limb arteries had atherosclerotic plaques, giving them the highest prevalence of plaque in our patient population (Figure1b). Between 25 and 50% of the left and right carotid arteries had plaques in the previously known diabetes (Figure1b). Less than 25% of the left and right carotid arteries were diseased in the newly diagnosed patients with diabetes, giving them the lowest prevalence of plaque in our patient population (Figure1b).
In contrast, the prevalence of isolated carotid plaque was 6.8%, isolated lower extremity plaque 32.3%, and plaques in both the carotid and lower extremity arteries 23.1% in the newly diagnosed diabetes patients (Figure1c). However, more patients tended to have atherosclerotic plaques in both the carotid and lower limbs (43.4%) than in either the isolated carotid (5.9%) or in the isolated lower limb arteries (33.9%) in the previously known diabetes patients (Figure1c).
Comparison of Carotid and Lower Limb Atherosclerotic Stenosis
Figure2 shows the comparison of carotid and lower limb atherosclerotic stenosis. In the patient-level analysis, the prevalence of atherosclerotic stenosis was significantly higher in the lower limb arteries than in the carotid arteries in the established diabetes (Figure2a). In contrast, the prevalence of stenosis was not significantly different between the carotid and lower limb arteries in the newly diagnosed diabetes patients (Figure2a). In the newly diagnosed diabetes patients, the prevalence of isolated carotid arterial stenosis and isolated lower extremity arterial stenosis was 3.0 and 4.8%, and there was no stenosis in both the carotid and lower extremity arteries (Figure2b). In the previously known diabetes, artery stenosis tended to be more often seen just in the lower limb arteries, which was much higher than in either isolated carotid arteries or in both the carotid and lower limb arteries (Figure2b).
Figure 2.
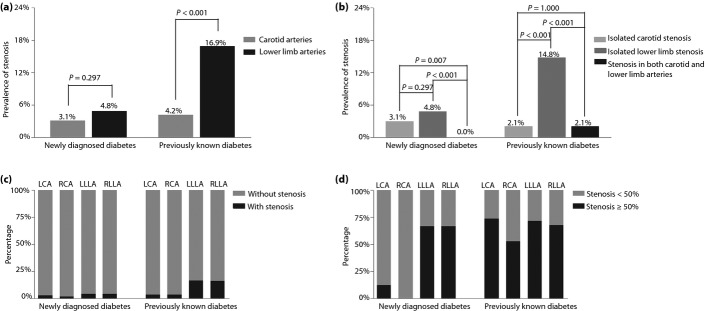
Comparison of carotid and lower limb atherosclerotic stenosis in both the previously known and the newly diagnosed diabetes patients. (a) The comparison of the prevalence of atherosclerotic stenosis in the carotid and lower limb arteries. (b) The percentage of patients with stenosis. The patients with stenosis were divided into three groups including patients with isolated carotid stenosis, patients with isolated lower extremity stenosis, and patients with stenoses in both the carotid and lower extremity arteries. The P-values for three group comparisons were 0.001 in the newly diagnosed diabetes patients and <0.001 in the previously known diabetes patients, respectively. (c) The comparison of the prevalence of atherosclerotic stenosis in the left carotid, right carotid, left lower limb and right lower limb artery. Artery stenosis including both 1–49% diameter reduction and ≥50% diameter reduction. (d) The comparison of the prevalence of significant atherosclerotic stenosis in the left carotid artery (LCA), right carotid artery (RCA), left lower limb artery (LLLA) and right lower limb artery (RCLA). Stenosis was considered significant if it caused ≥50% stenosis of a vessel.
In the vessel-level analysis, the left lower limb artery (16.6%) had the greatest prevalence in the previously known diabetes patients, followed by the right lower limb artery (16.2%), and the left and right carotid artery (all 3.3%; Figure2c). Similarly, in the newly diagnosed type 2 diabetes, the left and right lower limb artery (all 4.1%) had the greatest prevalence, followed by the left carotid artery (2.4%) and the right carotid artery (1.7%; Figure2c).
When considering the prevalence of significant artery stenosis (≥50% stenosis) in the previously known diabetes, the left carotid artery had a prevalence of 73.7%, the left lower limb artery 71.6%, the right lower limb artery 67.8% and the right carotid artery 52.6%, respectively (Figure2d). In contrast, in the newly diagnosed diabetes, the left and right lower limb arteries had the greatest prevalence of significant artery stenosis (all 66.7%), and there was a prevalence of significant artery stenosis of 12.5% in the left carotid artery (Figure2d). The right carotid artery had no significant artery stenosis in the newly diagnosed diabetes (Figure2d).
Comparison of Mean IMT Between Common Carotid and Common Femoral Arteries
The comparison of mean IMT between common carotid and common femoral arteries is presented in Figure3. In both the previously known diabetes group (0.90 ± 0.24 mm vs 0.89 ± 0.20 mm) and the newly diagnosed diabetes group (0.86 ± 0.22 mm vs 0.85 ± 0.16 mm), there was no significant difference between the mean common carotid IMT and the mean common femoral IMT (Figure3).
Figure 3.
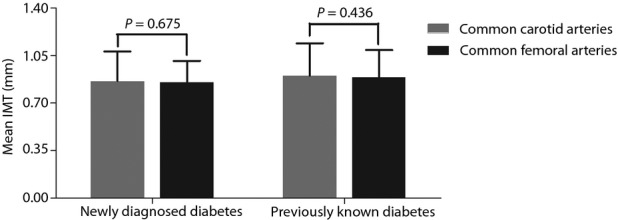
Comparison of mean intima-media thickness (IMT) between common carotid and common femoral arteries in both the previously known and the newly diagnosed diabetes patients.
Discussion
Carotid ultrasonography is presently widely used as a means of assessing atherosclerotic changes in type 2 diabetes18–20. Meanwhile, carotid atherosclerosis has been commonly recognized as an indicator of general atherosclerosis because of its relationship to coronary atherosclerosis, lower limb atherosclerosis and atherosclerosis elsewhere in the circulation21–23. For example, Hulthe et al.24 showed that the presence of atherosclerosis in the carotid arteries is known to be reflective for the extent of coronary atherosclerosis. Another previous study found that there was a significant correlation between carotid artery disease and lower extremity arterial disease in a healthy population of elderly adults with isolated systolic hypertension25. Therefore, carotid atherosclerosis appears to mark the presence of generalized atherosclerosis.
However, conflicting results have been reported for the correlation between carotid atherosclerosis and atherosclerosis elsewhere in the arterial system. Furthermore, little has been published regarding the comparison of carotid and lower limb artery atherosclerotic lesions in patients with diabetes. For example, there are studies showing that the presence of atherosclerotic plaques in the carotid arteries does not reflect atherosclerosis elsewhere in the arterial system9,10,26. This view might be further strengthened by findings from a study in which there is no correlation between the degree of lower extremity atherosclerosis and asymptomatic carotid artery stenosis >50% in male veterans with symptomatic lower limb atherosclerosis27. Furthermore, Sosnowski et al.28 believe that the presence of femoral atherosclerotic plaque more precisely than that of the carotid plaque reflects the probability and severity of coronary artery atherosclerosis.
The present findings in a hospital-based sample of type 2 diabetes also showed that the plaque and stenosis in the carotid arteries did not indicate those in the lower extremity arteries in both the known and newly diagnosed type 2 diabetes patients, although the condition was more severe in the established patients with diabetes than in the newly diagnosed patients with diabetes. We compared the prevalence of atherosclerotic plaques and stenosis between the carotid and lower extremity arteries, based on both patient-level and vessel-level analyses. Patient-level analyses showed that in both the known and the newly diagnosed patients with diabetes, the prevalence of atherosclerotic plaques was markedly higher in the lower extremity arteries than in the carotid arteries.
There was a similar trend for the prevalence of stenosis in the previously known diabetes patients. In contrast, there was no significant difference in the prevalence of stenosis between the carotid and the lower extremity arteries in the newly diagnosed diabetes patients. When considering the prevalence of significant artery stenosis in the previously known diabetes patients, there was no significant difference between the lower extremity and carotid arteries. However, for the newly diagnosed type 2 diabetes, the prevalence of the significant artery stenosis was significantly higher in the lower limb artery than in the carotid artery, which showed that significant artery stenosis occurred earlier in the limb arteries in type 2 diabetes.
A previous study showed that the prevalence of carotid plaques was 28% in type 2 diabetic patients at baseline29. After a 2-year follow up, the prevalence of carotid plaques increased to 62%29. A study by Lacroix et al.30 showed that the prevalence of carotid stenosis <60% and ≥60% was 63.6% and 4.7% in type 2 diabetic patients, respectively. The prevalence of lower extremity diseases was approximately 27% in both undiagnosed diabetes and diagnosed diabetes31. The higher prevalence of diabetic atherosclerotic lesions in the present study might be explained by the more serious condition in our studied population. Because of their more serious condition, such patients are more likely to be hospitalized, and to have a higher prevalence of atherosclerotic disease compared with the general population. In addition, it might also be explained by the different definition of atherosclerotic lesions and different measures of atherosclerosis in other studies.
Interestingly, in contrast to atherosclerotic plaques, whether in the previously known diabetes patients or in the newly diagnosed diabetes patients, there was no marked difference in the mean IMT between the common carotid and the common femoral arteries. IMT increase is an early event, and appears before plaque occurrence in the development of atherosclerosis, whereas formation of plaques represents advanced atherosclerotic lesions32. Additionally, carotid IMT is under genetic control, whereas carotid plaque is strongly influenced by environmental factors and less influenced by genetic factors32,33. Ebrahim et al.34 have provided evidence that carotid plaque predicts cardiovascular events better than carotid IMT. Furthermore, the study by Held et al.35 confirmed that carotid IMT was a weak predictor of events, and carotid plaques tended to predict the risk of cardiovascular death or myocardial infarction, whereas femoral artery IMT and plaques predicted the risk of revascularization. Therefore, based on the present results that carotid artery IMT value was similar to femoral artery IMT value, increased carotid IMT might represent a marker of general atherosclerosis and can be used as a way to detect early atherosclerosis risk populations, such as diabetic patients, in which prevention could be more efficient, but evaluations of plaques provided better prediction of cardiovascular events than assessments of IMT in patients with type 2 diabetes.
Thus, the present findings suggested that carotid plaque and stenosis might not represent atherosclerotic burden in type 2 diabetes, although it appears to do in general populations. However, carotid IMT might represent a marker of general atherosclerosis in type 2 diabetic patients. Different rheological conditions in the carotid and lower limb arteries could contribute to the differences presently observed.
The discrepancy between the present results and findings of other studies could be explained by the different definition of atherosclerosis and different populations studied by the researchers. The differences in study population and the methods used to assess atherosclerosis likely result in inconsistent results between studies. It is possible that carotid atherosclerosis is associated with lower limb atherosclerosis only in patients with a low risk of cardiovascular disease, but not in those with a high risk of cardiovascular disease, such as patients with diabetes. For example, in the Rotterdam Study, lower extremity atherosclerosis was defined as an ankle–arm index <0.90 in subjects aged ≥55 years21. However, based on ultrasound screening, the ankle-to-brachial index might underestimate the prevalence of peripheral occlusive disease in patients with diabetes36. More studies are required to determine whether carotid atherosclerosis reflects lower limb atherosclerosis in a population other than diabetes mellitus patients using ultrasound examination.
The present study has potential clinical significance and practical application. Our findings call into question the widespread use of carotid atherosclerotic plaques as an indicator of general atherosclerosis in patients with diabetes. The carotid plaque severely underestimates the burden of lower extremity atherosclerosis in both the previously known and newly diagnosed type 2 diabetes patients. The combination of carotid and lower extremity ultrasonography will be of considerable use in future clinical applications. Although screening the general population for lower limb atherosclerosis is currently of unproven value in effecting outcomes, it would be cost-effective in a population at high risk for atherosclerosis, such as diabetes, coronary artery disease and cerebrovascular disease patients.
Several potential limitations of the current study should be mentioned. First, the present sample was limited to inpatients with type 2 diabetes. Therefore, caution should be exercised in drawing conclusions regarding other populations, such as the general population and type 1 diabetic patients. Second, because our study design was cross-sectional, we cannot assess the evolution of the process of atherosclerosis. Finally, the present study was limited by the small sample size, and a further large-scale study to confirm our findings would be useful.
In conclusion, the present study provides evidence that carotid atherosclerotic plaques cannot reflect generalized atherosclerosis in type 2 dietetic patients, as shown by its significantly lower prevalence compared with that of the lower extremity arteries, which not only exists in previously known diabetics, but also in newly diagnosed diabetics. Carotid ultrasonography can provide important information of carotid arterial lesions, but cannot accurately assess the presence and severity of atherosclerosis elsewhere in type 2 diabetes. It emphasizes the value of the combination of ultrasonography of carotid and lower extremity arteries in detection of early atherosclerotic lesions in patients with type 2 diabetes.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (81170759 and 81370965), the National 973 Program (2011CB504001), Natural Science Foundation of Jiangsu Province, China (BK2009208) and the Science Foundation of Shanghai Jiao Tong University Affiliated Sixth People's Hospital (1112 and 1435). There is no financial support or relationship that might pose a conflict of interest.
References
- Haffner SM, Lehto S, Ronnemaa T, et al. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;4:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002;19:2570–2581. doi: 10.1001/jama.287.19.2570. [DOI] [PubMed] [Google Scholar]
- Aronson D, Rayfield EJ. How hyperglycemia promotes atherosclerosis: molecular mechanisms. Cardiovasc Diabetol. 2002;1:1. doi: 10.1186/1475-2840-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chait A, Bornfeldt KE. Diabetes and atherosclerosis: is there a role for hyperglycemia. J Lipid Res. 2009;50:S335–S339. doi: 10.1194/jlr.R800059-JLR200. (Suppl) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huysman E, Mathieu C. Diabetes and peripheral vascular disease. Acta Chir Belg. 2009;5:587–594. doi: 10.1080/00015458.2009.11680493. [DOI] [PubMed] [Google Scholar]
- Lorenz MW, Markus HS, Bots ML, et al. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;4:459–467. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- Salonen JT, Salonen R. Ultrasound B-mode imaging in observational studies of atherosclerotic progression. Circulation. 1993;3(Suppl):II56–II65. [PubMed] [Google Scholar]
- Peters SA, Grobbee DE, Bots ML. Carotid intima-media thickness: a suitable alternative for cardiovascular risk as outcome. Eur J Cardiovasc Prev Rehabil. 2011;2:167–174. doi: 10.1177/1741826710389400. [DOI] [PubMed] [Google Scholar]
- Pasterkamp G, Schoneveld AH, Hillen B, et al. Is plaque formation in the common carotid artery representative for plaque formation and luminal stenosis in other atherosclerotic peripheral arteries? A post mortem study. Atherosclerosis. 1998;1:205–210. doi: 10.1016/s0021-9150(97)00255-4. [DOI] [PubMed] [Google Scholar]
- Adraktas DD, Brasic N, Furtado AD, et al. Carotid atherosclerosis does not predict coronary, vertebral, or aortic atherosclerosis in patients with acute stroke symptoms. Stroke. 2010;8:1604–1609. doi: 10.1161/STROKEAHA.109.577437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Yu H, Zhu J, et al. The combination of carotid and lower extremity ultrasonography increases the detection of atherosclerosis in type 2 diabetes patients. J Diabetes Complications. 2012;1:23–28. doi: 10.1016/j.jdiacomp.2011.11.006. [DOI] [PubMed] [Google Scholar]
- Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;7:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Touboul PJ, Hennerici MG, Meairs S, et al. Mannheim intima-media thickness consensus. Cerebrovasc Dis. 2004;4:346–349. doi: 10.1159/000081812. [DOI] [PubMed] [Google Scholar]
- Li LX, Zhao CC, Ren Y, et al. Prevalence and clinical characteristics of carotid atherosclerosis in newly diagnosed patients with ketosis-onset diabetes: a cross-sectional study. Cardiovasc Diabetol. 2013;1:18. doi: 10.1186/1475-2840-12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant EG, Benson CB, Moneta GL, et al. Carotid artery stenosis: gray-scale and Doppler US diagnosis–Society of Radiologists in Ultrasound Consensus Conference. Radiology. 2003;2:340–346. doi: 10.1148/radiol.2292030516. [DOI] [PubMed] [Google Scholar]
- Koelemay MJ, den Hartog D, Prins MH, et al. Diagnosis of arterial disease of the lower extremities with duplex ultrasonography. Br J Surg. 1996;3:404–409. doi: 10.1002/bjs.1800830336. [DOI] [PubMed] [Google Scholar]
- Roederer GO, Langlois YE, Jager KA, et al. The natural history of carotid arterial disease in asymptomatic patients with cervical bruits. Stroke. 1984;4:605–613. doi: 10.1161/01.str.15.4.605. [DOI] [PubMed] [Google Scholar]
- Bonora E, Kiechl S, Oberhollenzer F, et al. Impaired glucose tolerance, Type II diabetes mellitus and carotid atherosclerosis: prospective results from the Bruneck Study. Diabetologia. 2000;2:156–164. doi: 10.1007/s001250050024. [DOI] [PubMed] [Google Scholar]
- Inukai T, Yamamoto R, Suetsugu M, et al. Small low-density lipoprotein and small low-density lipoprotein/total low-density lipoprotein are closely associated with intima-media thickness of the carotid artery in Type 2 diabetic patients. J Diabetes Complications. 2005;5:269–275. doi: 10.1016/j.jdiacomp.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Choi SW, Shin MH, Yun WJ, et al. Association between hemoglobin A1c, carotid atherosclerosis, arterial stiffness, and peripheral arterial disease in Korean type 2 diabetic patients. J Diabetes Complications. 2011;1:7–13. doi: 10.1016/j.jdiacomp.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Bots ML, Hofman A, Grobbee DE. Common carotid intima-media thickness and lower extremity arterial atherosclerosis. The Rotterdam Study. Arterioscler Thromb. 1994;12:1885–1891. doi: 10.1161/01.atv.14.12.1885. [DOI] [PubMed] [Google Scholar]
- Dropinski J, Szczeklik W, Wegrzyn W. Increased carotid artery intima-media thickness as an indicator of the onset of atherosclerosis in patients with connective tissue systemic diseases. Kardiol Pol. 2003;12:475–483. [PubMed] [Google Scholar]
- Craven TE, Ryu JE, Espeland MA, et al. Evaluation of the associations between carotid artery atherosclerosis and coronary artery stenosis. A case–control study. Circulation. 1990;4:1230–1242. doi: 10.1161/01.cir.82.4.1230. [DOI] [PubMed] [Google Scholar]
- Hulthe J, Wikstrand J, Emanuelsson H, et al. Atherosclerotic changes in the carotid artery bulb as measured by B-mode ultrasound are associated with the extent of coronary atherosclerosis. Stroke. 1997;6:1189–1194. doi: 10.1161/01.str.28.6.1189. [DOI] [PubMed] [Google Scholar]
- Sutton KC, Wolfson SK, Jr, Kuller LH. Carotid and lower extremity arterial disease in elderly adults with isolated systolic hypertension. Stroke. 1987;5:817–822. doi: 10.1161/01.str.18.5.817. [DOI] [PubMed] [Google Scholar]
- Kocak H, Gumuslu S, Sahin E, et al. Relationship between carotid artery intima-media thickness and brachial artery flow-mediated dilation in peritoneal dialysis patients. Int Urol Nephrol. 2009;2:409–416. doi: 10.1007/s11255-008-9504-y. [DOI] [PubMed] [Google Scholar]
- de Virgilio C, Toosie K, Arnell T, et al. Asymptomatic carotid artery stenosis screening in patients with lower extremity atherosclerosis: a prospective study. Ann Vasc Surg. 1997;4:374–377. doi: 10.1007/s100169900063. [DOI] [PubMed] [Google Scholar]
- Sosnowski C, Pasierski T, Janeczko-Sosnowska E, et al. Femoral rather than carotid artery ultrasound imaging predicts extent and severity of coronary artery disease. Kardiol Pol. 2007;7:760–766. discussion 767–768. [PubMed] [Google Scholar]
- Lundman P, Keech AC, Griffiths K, et al. New carotid plaque formation is very common in adult patients with Type 2 diabetes mellitus. Diabet Med. 2005;3:355–356. doi: 10.1111/j.1464-5491.2005.01411.x. [DOI] [PubMed] [Google Scholar]
- Lacroix P, Aboyans V, Criqui MH, et al. Type-2 diabetes and carotid stenosis: a proposal for a screening strategy in asymptomatic patients. Vasc Med. 2006;2:93–99. doi: 10.1191/1358863x06vm677oa. [DOI] [PubMed] [Google Scholar]
- Gregg EW, Gu Q, Williams D, et al. Prevalence of lower extremity diseases associated with normal glucose levels, impaired fasting glucose, and diabetes among U.S. adults aged 40 or older. Diabetes Res Clin Pract. 2007;3:485–488. doi: 10.1016/j.diabres.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Moskau S, Golla A, Grothe C, et al. Heritability of carotid artery atherosclerotic lesions: an ultrasound study in 154 families. Stroke. 2005;1:5–8. doi: 10.1161/01.STR.0000149936.33498.83. [DOI] [PubMed] [Google Scholar]
- Zannad F, Visvikis S, Gueguen R, et al. Genetics strongly determines the wall thickness of the left and right carotid arteries. Hum Genet. 1998;2:183–188. doi: 10.1007/s004390050804. [DOI] [PubMed] [Google Scholar]
- Ebrahim S, Papacosta O, Whincup P, et al. Carotid plaque, intima media thickness, cardiovascular risk factors, and prevalent cardiovascular disease in men and women: the British Regional Heart Study. Stroke. 1999;4:841–850. doi: 10.1161/01.str.30.4.841. [DOI] [PubMed] [Google Scholar]
- Held C, Hjemdahl P, Eriksson SV, et al. Prognostic implications of intima-media thickness and plaques in the carotid and femoral arteries in patients with stable angina pectoris. Eur Heart J. 2001;1:62–72. doi: 10.1053/euhj.1999.2006. [DOI] [PubMed] [Google Scholar]
- Potier L, Halbron M, Bouilloud F, et al. Ankle-to-brachial ratio index underestimates the prevalence of peripheral occlusive disease in diabetic patients at high risk for arterial disease. Diabetes Care. 2009;4:e44. doi: 10.2337/dc08-2015. [DOI] [PubMed] [Google Scholar]


