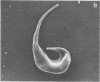
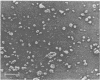
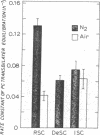
Abstract
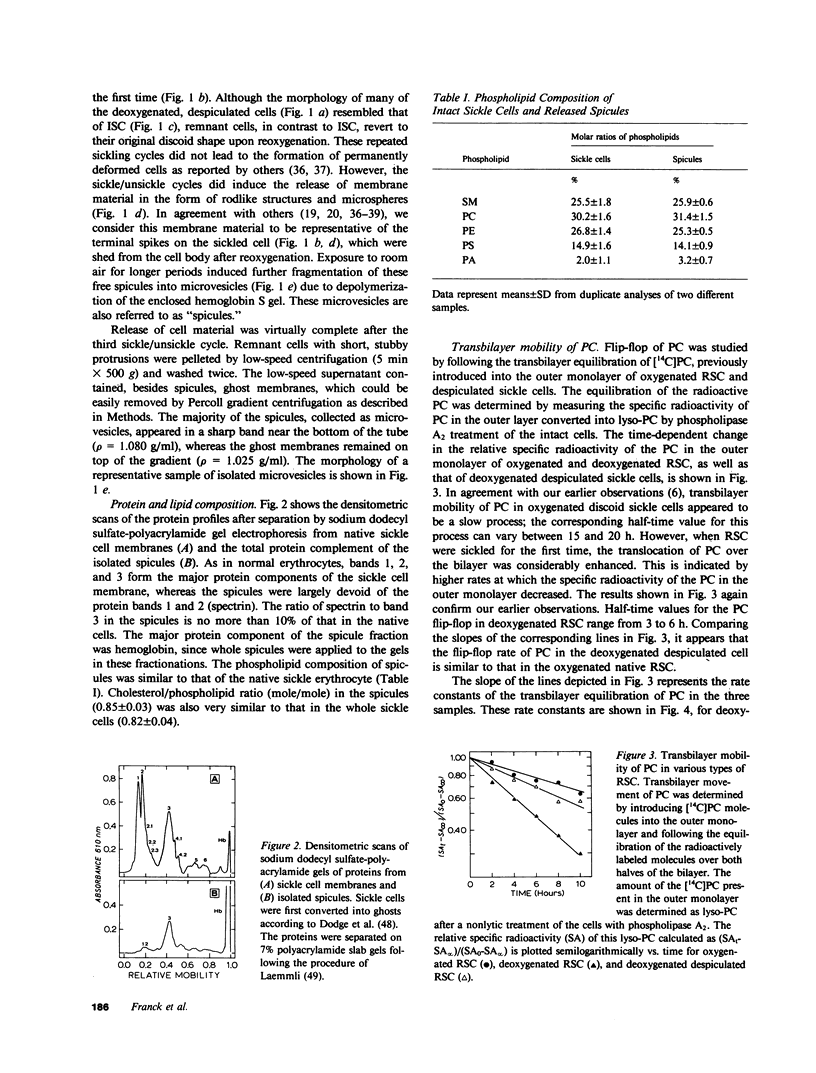
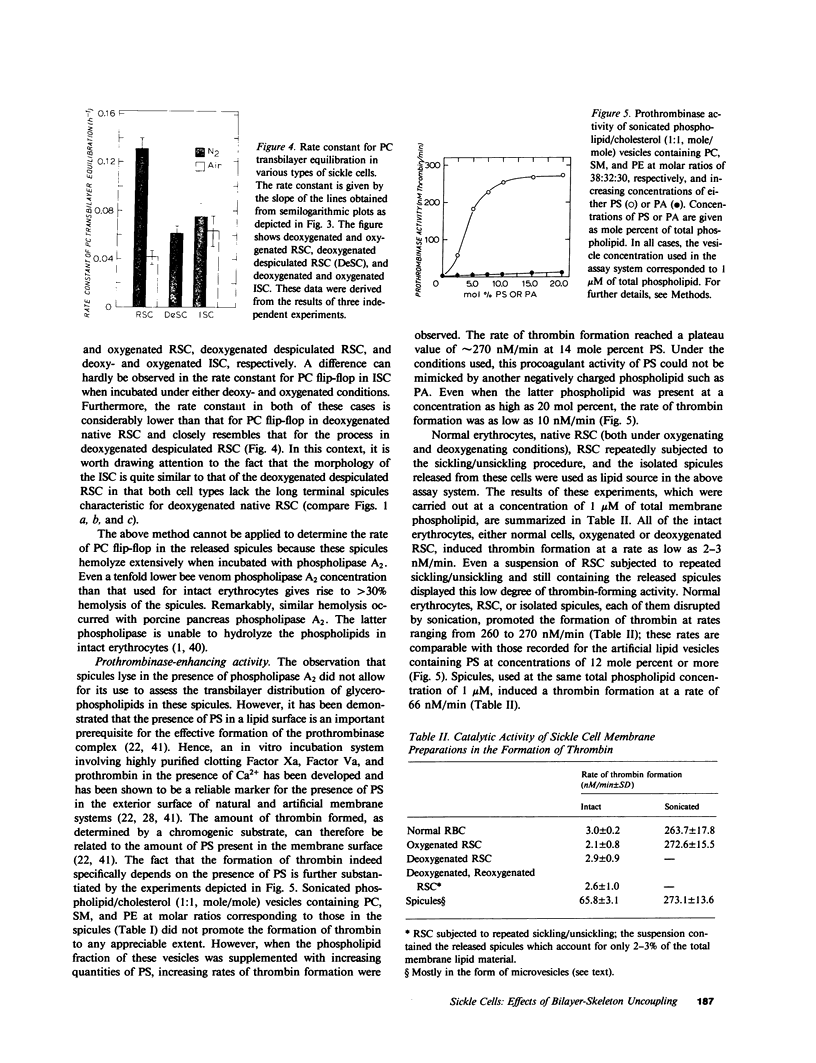
We have previously reported that the normal membrane phospholipid organization is altered in sickled erythrocytes. More recently, we presented evidence of enhanced transbilayer movement of phosphatidylcholine (PC) in deoxygenated reversibly sickled cells (RSC) and put forward the hypothesis that these abnormalities in phospholipid organization are confined to the characteristic protrusions of these cells. To test this hypothesis, we studied the free spicules released from RSC by repeated sickling and unsickling as well as the remnant despiculated cells. The rate of transbilayer movement of PC in the membrane of deoxygenated remnant despiculated cells was determined by following the fate of 14C-labelled PC, previously introduced into the outer monolayer under fully oxygenated conditions using a PC-specific phospholipid exchange protein from beef liver. The rate of transbilayer movement of PC in the remnant despiculated cells was significantly slower than in deoxygenated native RSC and was not very much different from that in oxygenated native RSC or irreversibly sickled cells. The free spicules had the same lipid composition as the native cells, but were deficient in spectrin. These spicules markedly enhanced the rate of thrombin formation in the presence of purified prothrombinase (Factor Xa, Factor Va, and Ca2+) and prothrombin, indicating the exposure of a significant fraction of phosphatidylserine (PS) in the outer monolayer. This effect was not observed when the spicules in this assay were replaced by normal erythrocytes, deoxygenated native RSC, or a deoxygenated sample of RSC after repetitive sickling/unsickling. The results are interpreted to indicate that the destabilization of the lipid bilayer in sickled cells, expressed by the enhanced flip-flop of PC and the exposure of PS in the outer monolayer, occurs predominantly in those parts of the membrane that are in spicular form.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan D., Limbrick A. R., Thomas P., Westerman M. P. Release of spectrin-free spicules on reoxygenation of sickled erythrocytes. Nature. 1982 Feb 18;295(5850):612–613. doi: 10.1038/295612a0. [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E. Bacterial infection and sickle cell anemia. An analysis of 250 infections in 166 patients and a review of the literature. Medicine (Baltimore) 1971 Mar;50(2):97–112. [PubMed] [Google Scholar]
- Bergmann W. L., Dressler V., Haest C. W., Deuticke B. Cross-linking of SH-groups in the erythrocyte membrane enhances transbilayer reorientation of phospholipids. Evidence for a limited access of phospholipids to the reorientation sites. Biochim Biophys Acta. 1984 Jan 25;769(2):390–398. doi: 10.1016/0005-2736(84)90322-5. [DOI] [PubMed] [Google Scholar]
- Bevers E. M., Comfurius P., Zwaal R. F. The nature of the binding for prothrombinase at the platelet surface as revealed by lipolytic enzymes. Eur J Biochem. 1982 Feb;122(1):81–85. doi: 10.1111/j.1432-1033.1982.tb05850.x. [DOI] [PubMed] [Google Scholar]
- Bevers E. M., Comfurius P., van Rijn J. L., Hemker H. C., Zwaal R. F. Generation of prothrombin-converting activity and the exposure of phosphatidylserine at the outer surface of platelets. Eur J Biochem. 1982 Feb;122(2):429–436. doi: 10.1111/j.1432-1033.1982.tb05898.x. [DOI] [PubMed] [Google Scholar]
- Broekhuyse R. M. Quantitative two-dimensional thin-layer chromatography of blood phospholipids. Clin Chim Acta. 1969 Mar;23(3):457–461. doi: 10.1016/0009-8981(69)90349-0. [DOI] [PubMed] [Google Scholar]
- Chiu D., Lubin B., Shohet S. B. Erythrocyte membrane lipid reorganization during the sickling process. Br J Haematol. 1979 Feb;41(2):223–234. doi: 10.1111/j.1365-2141.1979.tb05851.x. [DOI] [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Demel R. A., Geurts van Kessel W. S., Zwaal R. F., Roelofsen B., van Deenen L. L. Relation between various phospholipase actions on human red cell membranes and the interfacial phospholipid pressure in monolayers. Biochim Biophys Acta. 1975 Sep 16;406(1):97–107. doi: 10.1016/0005-2736(75)90045-0. [DOI] [PubMed] [Google Scholar]
- Eaton J. W., Jacob H. S., White J. G. Membrane abnormalities of irreversibly sickled cells. Semin Hematol. 1979 Jan;16(1):52–64. [PubMed] [Google Scholar]
- Franck P. F., Chiu D. T., Op den Kamp J. A., Lubin B., van Deenen L. L., Roelofsen B. Accelerated transbilayer movement of phosphatidylcholine in sickled erythrocytes. A reversible process. J Biol Chem. 1983 Jul 10;258(13):8436–8442. [PubMed] [Google Scholar]
- Franck P. F., Roelofsen B., Op den Kamp J. A. Complete exchange of phosphatidylcholine from intact erythrocytes after protein crosslinking. Biochim Biophys Acta. 1982 Apr 23;687(1):105–108. doi: 10.1016/0005-2736(82)90176-6. [DOI] [PubMed] [Google Scholar]
- Haest C. W. Interactions between membrane skeleton proteins and the intrinsic domain of the erythrocyte membrane. Biochim Biophys Acta. 1982 Dec;694(4):331–352. doi: 10.1016/0304-4157(82)90001-6. [DOI] [PubMed] [Google Scholar]
- Haest C. W., Plasa G., Kamp D., Deuticke B. Spectrin as a stabilizer of the phospholipid asymmetry in the human erythrocyte membrane. Biochim Biophys Acta. 1978 May 4;509(1):21–32. doi: 10.1016/0005-2736(78)90004-4. [DOI] [PubMed] [Google Scholar]
- Jackson C. M., Nemerson Y. Blood coagulation. Annu Rev Biochem. 1980;49:765–811. doi: 10.1146/annurev.bi.49.070180.004001. [DOI] [PubMed] [Google Scholar]
- Jensen M., Shohet S. B., Nathan D. G. The role of red cell energy metabolism in the generation of irreversibly sickled cells in vitro. Blood. 1973 Dec;42(6):835–842. [PubMed] [Google Scholar]
- Kamp H. H., Wirtz K. W. Phosphatidylcholine exchange protein from beef liver. Methods Enzymol. 1974;32:140–146. doi: 10.1016/0076-6879(74)32017-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lubin B., Chiu D., Bastacky J., Roelofsen B., Van Deenen L. L. Abnormalities in membrane phospholipid organization in sickled erythrocytes. J Clin Invest. 1981 Jun;67(6):1643–1649. doi: 10.1172/JCI110200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz H. U., Liu S. C., Palek J. Release of spectrin-free vesicles from human erythrocytes during ATP depletion. I. Characterization of spectrin-free vesicles. J Cell Biol. 1977 Jun;73(3):548–560. doi: 10.1083/jcb.73.3.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux S. E. Spectrin-actin membrane skeleton of normal and abnormal red blood cells. Semin Hematol. 1979 Jan;16(1):21–51. [PubMed] [Google Scholar]
- Mohandas N., Wyatt J., Mel S. F., Rossi M. E., Shohet S. B. Lipid translocation across the human erythrocyte membrane. Regulatory factors. J Biol Chem. 1982 Jun 10;257(11):6537–6543. [PubMed] [Google Scholar]
- Mombers C., Verkleij A. J., de Gier J., van Deenen L. L. The interaction of spectrin-actin and synthetic phospholipids. II. The interaction with phosphatidylserine. Biochim Biophys Acta. 1979 Mar 8;551(2):271–281. doi: 10.1016/0005-2736(89)90005-9. [DOI] [PubMed] [Google Scholar]
- Murphy J. R. Influence of temperature and method of centrifugation on the separation of erythrocytes. J Lab Clin Med. 1973 Aug;82(2):334–341. [PubMed] [Google Scholar]
- Nieuwenhuizen W., Kunze H., de Haas G. H. Phospholipase A2 (phosphatide acylhydrolase, EC 3.1.1.4) from porcine pancreas. Methods Enzymol. 1974;32:147–154. doi: 10.1016/0076-6879(74)32018-6. [DOI] [PubMed] [Google Scholar]
- Op den Kamp J. A. Lipid asymmetry in membranes. Annu Rev Biochem. 1979;48:47–71. doi: 10.1146/annurev.bi.48.070179.000403. [DOI] [PubMed] [Google Scholar]
- Padilla F., Bromberg P. A., Jensen W. N. The sickle-unsickle cycle: a cause of cell fragmentation leading to permanently deformed cells. Blood. 1973 May;41(5):653–660. [PubMed] [Google Scholar]
- Palek J., Lux S. E. Red cell membrane skeletal defects in hereditary and acquired hemolytic anemias. Semin Hematol. 1983 Jul;20(3):189–224. [PubMed] [Google Scholar]
- ROSE H. G., OKLANDER M. IMPROVED PROCEDURE FOR THE EXTRACTION OF LIPIDS FROM HUMAN ERYTHROCYTES. J Lipid Res. 1965 Jul;6:428–431. [PubMed] [Google Scholar]
- Richardson S. G., Matthews K. B., Stuart J., Geddes A. M., Wilcox R. M. Serial changes in coagulation and viscosity during sickle-cell crisis. Br J Haematol. 1979 Jan;41(1):95–103. doi: 10.1111/j.1365-2141.1979.tb03685.x. [DOI] [PubMed] [Google Scholar]
- Rouser G., Fkeischer S., Yamamoto A. Two dimensional then layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids. 1970 May;5(5):494–496. doi: 10.1007/BF02531316. [DOI] [PubMed] [Google Scholar]
- Sato S. B., Ohnishi S. Interaction of a peripheral protein of the erythrocyte membrane, band 4.1, with phosphatidylserine-containing liposomes and erythrocyte inside-out vesicles. Eur J Biochem. 1983 Jan 17;130(1):19–25. doi: 10.1111/j.1432-1033.1983.tb07111.x. [DOI] [PubMed] [Google Scholar]
- Shipolini R. A., Callewaert G. L., Cottrell R. C., Doonan S., Vernon C. A., Banks B. E. Phospholipase A from bee venom. Eur J Biochem. 1971 Jun 29;20(4):459–468. doi: 10.1111/j.1432-1033.1971.tb01414.x. [DOI] [PubMed] [Google Scholar]
- Stoffel W. Chemical synthesis of choline-labeled lecithins and sphingomyelins. Methods Enzymol. 1975;35:533–541. doi: 10.1016/0076-6879(75)35181-1. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Schroit A. J. Insertion of fluorescent phosphatidylserine into the plasma membrane of red blood cells. Recognition by autologous macrophages. J Biol Chem. 1983 Sep 25;258(18):11335–11343. [PubMed] [Google Scholar]
- Weitz M., Bjerrum O. J., Ott P., Brodbeck U. Quantitative composition and characterization of the proteins in membrane vesicles released from erythrocytes by dimyristoylphosphatidylcholine. A membrane system without cytoskeleton. J Cell Biochem. 1982;19(2):179–191. doi: 10.1002/jcb.240190208. [DOI] [PubMed] [Google Scholar]
- Westerman M. P., Allan D. Effects of valinomycin, A23187 and repetitive sickling on irreversible sickle cell formation. Br J Haematol. 1983 Mar;53(3):399–409. doi: 10.1111/j.1365-2141.1983.tb02040.x. [DOI] [PubMed] [Google Scholar]
- Williamson P., Bateman J., Kozarsky K., Mattocks K., Hermanowicz N., Choe H. R., Schlegel R. A. Involvement of spectrin in the maintenance of phase-state asymmetry in the erythrocyte membrane. Cell. 1982 Oct;30(3):725–733. doi: 10.1016/0092-8674(82)90277-x. [DOI] [PubMed] [Google Scholar]
- ZLATKIS A., ZAK B., BOYLE A. J. A new method for the direct determination of serum cholesterol. J Lab Clin Med. 1953 Mar;41(3):486–492. [PubMed] [Google Scholar]
- Zwaal R. F., Comfurius P., van Deenen L. L. Membrane asymmetry and blood coagulation. Nature. 1977 Jul 28;268(5618):358–360. doi: 10.1038/268358a0. [DOI] [PubMed] [Google Scholar]
- Zwaal R. F., Roelofsen B., Comfurius P., van Deenen L. L. Organization of phospholipids in human red cell membranes as detected by the action of various purified phospholipases. Biochim Biophys Acta. 1975 Sep 16;406(1):83–96. doi: 10.1016/0005-2736(75)90044-9. [DOI] [PubMed] [Google Scholar]
- van Meer G., Op den Kamp J. A. Transbilayer movement of various phosphatidylcholine species in intact human erythrocytes. J Cell Biochem. 1982;19(2):193–204. doi: 10.1002/jcb.240190209. [DOI] [PubMed] [Google Scholar]
- van Meer G., Poorthuis B. J., Wirtz K. W., Op den Kamp J. A., van Deenen L. L. Transbilayer distribution and mobility of phosphatidylcholine in intact erythrocyte membranes. A study with phosphatidylcholine exchange protein. Eur J Biochem. 1980 Jan;103(2):283–288. doi: 10.1111/j.1432-1033.1980.tb04313.x. [DOI] [PubMed] [Google Scholar]