Abstract
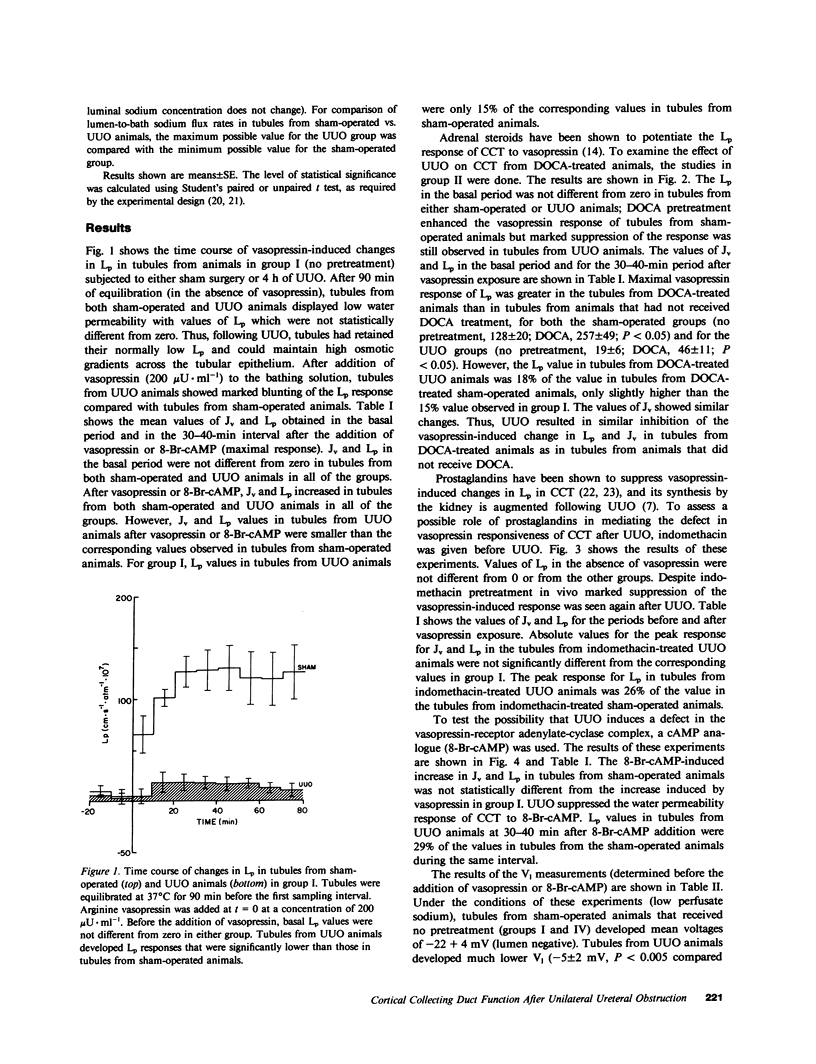
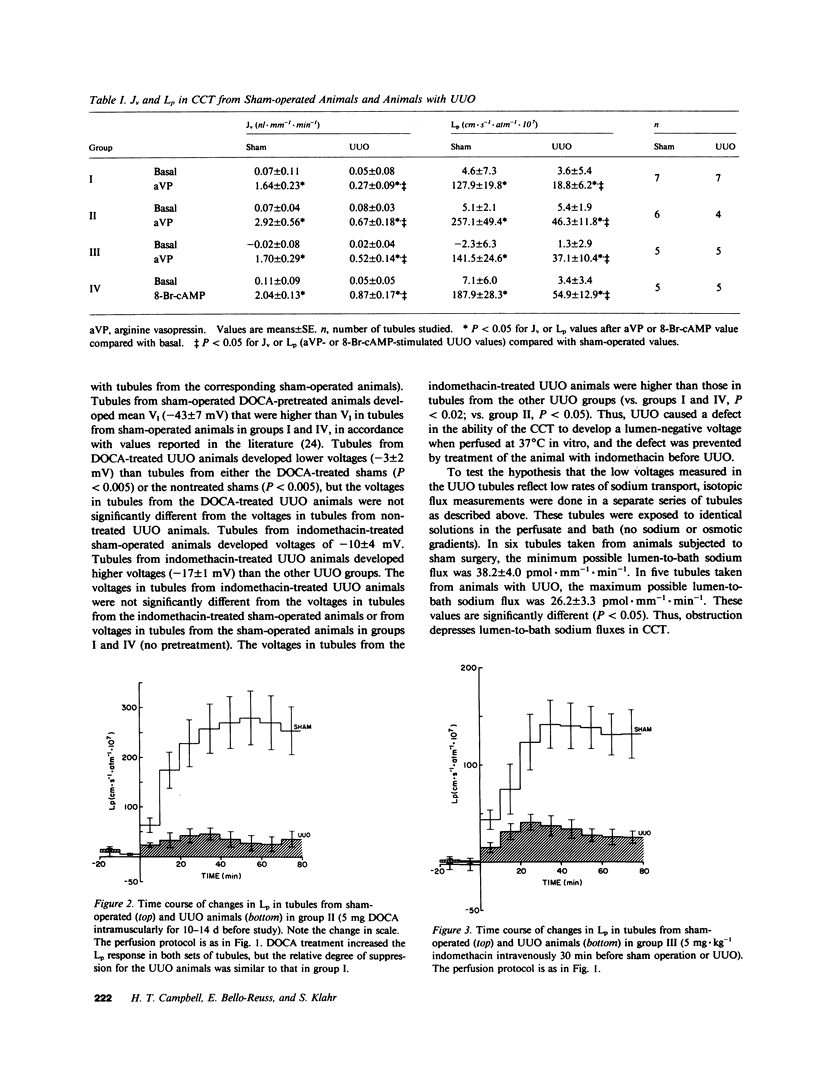
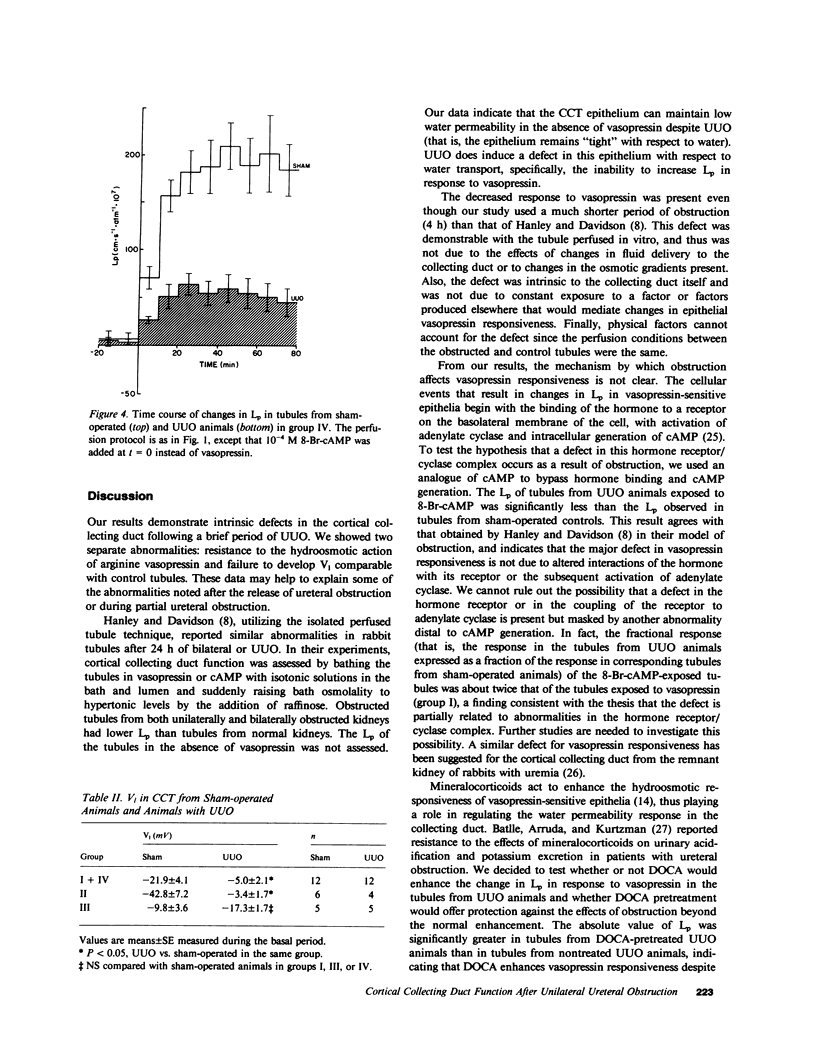
Ureteral obstruction affects the kidney's ability to conserve water and sodium. Using the isolated perfused tubule technique, we studied cortical collecting tubules (CCT) taken from rabbits subjected to a sham operation or to 4 h of unilateral ureteral obstruction (UUO). Tubules were perfused in the presence of an osmotic gradient directed to promote water movement from lumen to bath, and volume flux (Jv), hydraulic water permeability (Lp), and transepithelial voltage (V1) were determined. In tubules from sham-operated and UUO animals, basal (before exposure to vasopressin) J, and Lp were not different from zero. After addition of 200 microU . ml-1 of arginine vasopressin (aVP) to the bath, Jv and Lp increased to 1.64 +/- 0.23 nl . mm-1 . min-1 and 127.9 +/- 19.8 cm . s-1 . atm-1 x 10(7), respectively, in tubules from sham-operated animals, but not only 0.27 +/- 0.09 nl . mm-1 . min-1 an 18.8 +/- 6.2 cm . s-1 . atm-1 . 10(7) in tubules from UUO animals. Pretreatment with desoxycorticosterone acetate (DOCA) or indomethacin in vivo did not prevent the blunted vasopressin response seen in tubules taken from UUO animals. The Jv and Lp responses to the cyclic AMP (cAMP) analogue, 8-Br-cAMP, were also diminished in tubules taken from UUO animals compared with shams. V1, measured during the basal period, was diminished in tubules from UUO kidneys (-5.0 +/- 2.1 mV) compared with shams (-21.9 +/- 4.1 mV), and pretreatment with DOCA did no prevent the effects of UUO on V1. In contrast, tubules taken from animals that received indomethacin prior to UUO developed voltages not different from voltages in tubules taken from sham-operated animals (-17.3 +/- 1.7 mV). We conclude that, although CCT from UUO animals can maintain osmotic gradients, their ability to respond to vasopressin by increasing Lp is impaired by an intrinsic defect located at a step beyond the generation of cAMP, and that prostaglandin inhibition or DOCA pretreatment do not reverse the decreased responsiveness of Lp to aVP. UUO also diminished V1, and this abnormality was prevented by previous treatment with indomethacin, suggesting that prostaglandins may mediate the effect of UUO on V1.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batlle D. C., Arruda J. A., Kurtzman N. A. Hyperkalemic distal renal tubular acidosis associated with obstructive uropathy. N Engl J Med. 1981 Feb 12;304(7):373–380. doi: 10.1056/NEJM198102123040701. [DOI] [PubMed] [Google Scholar]
- Bello-Reuss E. Electrical properties of the basolateral membrane of the straight portion of the rabbit proximal renal tubule. J Physiol. 1982 May;326:49–63. doi: 10.1113/jphysiol.1982.sp014176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello-Reuss E., Higashi Y., Kaneda Y. Dopamine decreases fluid reabsorption in straight portions of rabbit proximal tubule. Am J Physiol. 1982 Jun;242(6):F634–F640. doi: 10.1152/ajprenal.1982.242.6.F634. [DOI] [PubMed] [Google Scholar]
- Burg M., Grantham J., Abramow M., Orloff J. Preparation and study of fragments of single rabbit nephrons. Am J Physiol. 1966 Jun;210(6):1293–1298. doi: 10.1152/ajplegacy.1966.210.6.1293. [DOI] [PubMed] [Google Scholar]
- Campbell W. B., Jackson E. K., Graham R. M. Saralasin-induced renin release: its blockade by prostaglandin synthesis inhibitors in the conscious rat. Hypertension. 1979 Nov-Dec;1(6):637–642. doi: 10.1161/01.hyp.1.6.637. [DOI] [PubMed] [Google Scholar]
- DiBona D. R. Cytoplasmic involvement in ADH-mediated osmosis across toad urinary bladder. Am J Physiol. 1983 Nov;245(5 Pt 1):C297–C307. doi: 10.1152/ajpcell.1983.245.5.C297. [DOI] [PubMed] [Google Scholar]
- Du Bois R., Vernoiry A., Abramow M. Computation of the osmotic water permeability of perfused tubule segments. Kidney Int. 1976 Dec;10(6):478–479. doi: 10.1038/ki.1976.135. [DOI] [PubMed] [Google Scholar]
- Fine L. G., Schlondorff D., Trizna W., Gilbert R. M., Bricker N. S. Functional profile of the isolated uremic nephron. Impaired water permeability and adenylate cyclase responsiveness of the cortical collecting tubule to vasopressin. J Clin Invest. 1978 Jun;61(6):1519–1527. doi: 10.1172/JCI109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frindt G., Windhager E. E., Taylor A. Hydroosmotic response of collecting tubules to ADH or cAMP at reduced peritubular sodium. Am J Physiol. 1982 Nov;243(5):F503–F513. doi: 10.1152/ajprenal.1982.243.5.F503. [DOI] [PubMed] [Google Scholar]
- Goldfarb S. Effects of calcium on ADH action in the cortical collecting tubule perfused in vitro. Am J Physiol. 1982 Nov;243(5):F481–F486. doi: 10.1152/ajprenal.1982.243.5.F481. [DOI] [PubMed] [Google Scholar]
- Grantham J. J., Orloff J. Effect of prostaglandin E1 on the permeability response of the isolated collecting tubule to vasopressin, adenosine 3',5'-monophosphate, and theophylline. J Clin Invest. 1968 May;47(5):1154–1161. doi: 10.1172/JCI105804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall D. A., Grantham J. J. Temperature effect on ADH response of isolated perfused rabbit collecting tubules. Am J Physiol. 1980 Dec;239(6):F595–F601. doi: 10.1152/ajprenal.1980.239.6.F595. [DOI] [PubMed] [Google Scholar]
- Hall D. A., Grantham J. J. Temperature effect on ADH response of isolated perfused rabbit collecting tubules. Am J Physiol. 1980 Dec;239(6):F595–F601. doi: 10.1152/ajprenal.1980.239.6.F595. [DOI] [PubMed] [Google Scholar]
- Hanley M. J., Davidson K. Isolated nephron segments from rabbit models of obstructive nephropathy. J Clin Invest. 1982 Jan;69(1):165–174. doi: 10.1172/JCI110427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays R. M. Alteration of luminal membrane structure by antidiuretic hormone. Am J Physiol. 1983 Nov;245(5 Pt 1):C289–C296. doi: 10.1152/ajpcell.1983.245.5.C289. [DOI] [PubMed] [Google Scholar]
- Holt W. F., Lechene C. ADH-PGE2 interactions in cortical collecting tubule. I. Depression of sodium transport. Am J Physiol. 1981 Oct;241(4):F452–F460. doi: 10.1152/ajprenal.1981.241.4.F452. [DOI] [PubMed] [Google Scholar]
- Kirschenbaum M. A., Lowe A. G., Trizna W., Fine L. G. Regulation of vasopressin action by prostaglandins. Evidence for prostaglandin synthesis in the rabbit cortical collecting tubule. J Clin Invest. 1982 Dec;70(6):1193–1204. doi: 10.1172/JCI110718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klahr S. Pathophysiology of obstructive nephropathy. Kidney Int. 1983 Feb;23(2):414–426. doi: 10.1038/ki.1983.36. [DOI] [PubMed] [Google Scholar]
- Kokko J. P. Effect of prostaglandins on renal epithelial electrolyte transport. Kidney Int. 1981 Jun;19(6):791–796. doi: 10.1038/ki.1981.82. [DOI] [PubMed] [Google Scholar]
- Lorenzen M., Taylor A., Windhager E. E. pH effect on osmotic response of collecting tubules to vasopressin and 8-CPT-cAMP. Am J Physiol. 1983 Aug;245(2):F188–F197. doi: 10.1152/ajprenal.1983.245.2.F188. [DOI] [PubMed] [Google Scholar]
- Moody T. E., Vaughn E. D., Jr, Gillenwater J. Y. Relationship between renal blood flow and ureteral pressure during 18 hours of total unilateral uretheral occlusion. Implications for changing sites of increased renal resistance. Invest Urol. 1975 Nov;13(3):246–251. [PubMed] [Google Scholar]
- Morrison A. R., Moritz H., Needleman P. Mechanism of enhanced renal prostaglandin biosynthesis in ureter obstruction. Role of de novo protein synthesis. J Biol Chem. 1978 Nov 25;253(22):8210–8212. [PubMed] [Google Scholar]
- Muldowney F. P., Duffy G. J., Kelly D. G., Duff F. A., Harrington C., Freaney R. Sodium diuresis after relief of obstructive uropathy. N Engl J Med. 1966 Jun 9;274(23):1294–1298. doi: 10.1056/NEJM196606092742304. [DOI] [PubMed] [Google Scholar]
- Schwartz M. J., Kokko J. P. Urinary concentrating defect of adrenal insufficiency. Permissive role of adrenal steroids on the hydroosmotic response across the rabbit cortical collecting tubule. J Clin Invest. 1980 Aug;66(2):234–242. doi: 10.1172/JCI109849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes J. B., Ingram M. J., Williams A. D., Ingram D. Heterogeneity of the rabbit collecting tubule: localization of mineralocorticoid hormone action to the cortical portion. Kidney Int. 1981 Sep;20(3):340–347. doi: 10.1038/ki.1981.144. [DOI] [PubMed] [Google Scholar]
- Wilson D. R., Honrath U. Cross-circulation study of natriuretic factors in postobstructive diuresis. J Clin Invest. 1976 Feb;57(2):380–389. doi: 10.1172/JCI108289. [DOI] [PMC free article] [PubMed] [Google Scholar]



