Abstract
Background
Parental smoking and exposure of the mother or the child to environmental tobacco smoke (ETS) as risk factors for Acute non-Lymphocytic Leukemia (AnLL) were investigated.
Methods
Incident cases of childhood AnLL were enrolled in 14 Italian Regions during 1998–2001. We estimated odds ratios (OR) and 95% confidence intervals (95%CI) conducting logistic regression models including 82 cases of AnLL and 1,044 controls. Inverse probability weighting was applied adjusting for: age; sex; provenience; birth order; birth weight; breastfeeding; parental educational level age, birth year, and occupational exposure to benzene.
Results
Paternal smoke in the conception period was associated with AnLL (OR for ≥11 cigarettes/day = 1.79, 95% CI 1.01–3.15; P trend 0.05). An apparent effect modification by maternal age was identified: only children of mothers aged below 30 presented increased risks. We found weak statistical evidence of an association of AnLL with maternal exposure to ETS (OR for exposure>3 hours/day = 1.85, 95%CI 0.97–3.52; P trend 0.07). No association was observed between AnLL and either maternal smoking during pregnancy or child exposure to ETS.
Conclusions
This study is consistent with the hypothesis that paternal smoke is associated with AnLL. We observed statistical evidence of an association between maternal exposure to ETS and AnLL, but believe bias might have inflated our estimates.
Introduction
Acute leukemia is the most common childhood cancer; acute lymphoblastic leukemia (ALL) accounts for 75–80% of total cases of childhood leukemia, acute non-lymphocytic leukemia (AnLL) for about 20%. [1] Established risk factors, such as exposure to ionizing radiations and genetic syndromes, explain no more than 10% of cases; [2] Suggested risk factors include: car exhaust fumes, pesticides, non-ionizing radiation, pets, antiepileptic drugs, maternal alcohol consumption, maternal illicit drug use (cannabis sativa), maternal age, paternal age, breast feeding, birth order, chemical contamination in drinking water, both viral and bacterial infections, and parental cigarette smoking. [3]–[5] Alongside occupational exposure to benzene, [6] active tobacco smoking is an established risk for adult myeloid leukemia. [7] According to the International Agency for Research on Cancer (IARC), the available body of evidence suggests a consistent association of childhood leukemia with preconception and with combined paternal and maternal smoking. [7] Conversely, studies on maternal tobacco smoking often showed modest increases in risk, or null or inverse associations. [7] Only one study was included on second hand smoke and leukemia (namely chronic lymphocytic leukemia) reporting a positive association. [7] Most of the evidence on the relationship between cigarette smoking and childhood leukemia regards ALL, [8], while there is scant evidence for AnLL. [7], [9] As shown in supplemental Table S1, several studies highlighted that paternal smoking around the time of conception is a risk factor for childhood ALL. A meta-analysis of heavy paternal smoking (20+ cigarettes/day) highlighted a substantial increase in the risk of childhood leukemia (OR 1.44, 95%CI 1.24–1.68) [8].
Our aim was to investigate parental cigarette consumption and second-hand smoke exposure as risk factors for childhood AnLL, using data collected in a large case-control study primarily designed to evaluate the role of physical agents (including electromagnetic fields), parental occupation and environmental exposure in childhood hematopoietic malignancies. [10]–[11]
Methods
Study population
SETIL (Studio sulla Eziologia dei Tumori Infantili Linfoemopoietici, study on the etiology of childhood lympho-hematopoietic malignancies) is a population-based case-control study conducted in Italy between 1998 and 2003. Details of the study have been given elsewhere. [10]–[11] Thanks to the support of the Italian Association of Pediatric Hematology and Oncology almost all incident cases of childhood acute leukemia (aged between 0 and 10) in 14 Italian Regions were collected; [12] second primary neoplasms were excluded. Cases were individually matched for date of birth, sex and residence area with 2 population controls randomly drawn from Local Health Authority registries. Parents of eligible cases were contacted through the pediatric oncologist, parents of controls through their general practitioner; eligible subjects were asked to participate in a direct interview (non responders were 8% among cases and 29% among controls). During the study period 82 cases of AnLL, 601 cases of ALL and 1,044 controls (128 matched to AnLL cases and 916 matched to ALL cases) were enrolled.
Information was collected from parents of cases and controls in a direct interview using a standardized questionnaire that was constructed to collect data on many putative causes of childhood leukemia, including personal characteristics and exposure to physical, chemical and biological agents. For practical reasons, interviewers were not blinded to the case or control status of the child.
In the present analysis of AnLL, we broke the individual matching, and included the 82 cases of AnLL and all 1,044 sampled controls (irrespectively of individual matching with AnLL or ALL cases). Matching was retained in additional sensitivity analyses.
The SETIL study was conceived to investigate the etiology of hematopoietic malignancies. Findings on the association between tobacco smoke and risk of childhood acute lymphoblastic leukemia have been recently reported. [13] Queries about collaborations and access to the data can be addressed to the principal investigator of the SETIL Study (Prof. Corrado Magnani; email: magnani@med.unipmn.it). The SETIL study participated in the Childhood Leukemia International Consortium (CLIC, https://clic.berkeley.edu/about). [14]
The SETIL study was authorized by the ethics committee for the Piedmont Region (authorization n.2886, on 15/2/1999; letter n. 1852/28.3 on 17/2/1999) and later by the corresponding board of each participating research unit. Written informed consent was obtained from all participating subjects. The ethics committee approved the consent procedure.
Exposure variables and covariates
An English language translation of the smoking sections of the SETIL questionnaire is presented in appendix S1. Available information on paternal smoking status in the period of conception enabled us to classify fathers in four categories: never a smoker; former smoker; smoker, 1 to 10 cigarettes per day; and smoker, 11 or more cigarettes per day. Based on preliminary analyses, never smokers and former smokers were merged, creating the category of non-smokers with reference to the period of conception. Information on the smoking status of fathers (smoker or non-smoker) was also available for the pregnancy and the period between birth and diagnosis. As expected, an excellent agreement (Cohen's kappa = 0.96) was found between paternal smoking status in the conception period and smoking status after the child's birth.
For maternal smoking, information was available separately for each trimester of pregnancy. Since the consumption of cigarettes tended to be stable across the pregnancy (Cohen's kappa between first and third trimester = 0.92), smoking status was classified according to the first trimester of pregnancy. After a preliminary analysis and considering the small numbers of active smokers — only three mothers of cases declared they had smoked more than 10 cigarettes/day — a dichotomous variable was created: non-smoker (never a smoker or former smoker); smoker. Mothers were asked to declare how many hours per day they had been exposed to Environmental Tobacco Smoking (ETS) during pregnancy. A three-level variable was created using the collected information: never exposed to passive smoking, and two levels of exposure based on the median of exposure to passive smoking among controls' mothers.
Exposure of children to ETS, measured in cigarettes per day, was collected for every year of life; Hence, we created a cumulative exposure index equal to the number of cigarettes to which the children had been exposed (ETS). Again, a three-level variable was created: never exposed to ETS, and two levels of exposure based on the median of exposure to passive smoking among controls.
Possible confounders were selected a-priori and included: sex, age group (less than two years; between two and four years; between four and six years; more than six years), residence area (part of Italy: North, except Lombardy; Lombardy; center; South and islands), birth order; birth weight; duration of breastfeeding; maternal and paternal age at child's birth; maternal and paternal education level; and parental occupational exposure to benzene. Exposure to benzene was assessed by industrial hygienists on the basis of information gathered with a job specific questionnaire. Detailed methods for the evaluation of exposure to benzene were presented in Miligi et al. [10]
Statistical Analysis
Unmatched analyses were performed in order to avoid the loss of cases (9 cases were in matching strata without controls). To increase statistical power, considering that the sampling procedure and collection of information were the same for controls matched to AnLL and to ALL cases, we included all the 1,044 enrolled controls in the analysis and not only the 128 individually matched with AnLL cases. Unmatched analyses models always included age, gender and residence area. Matching was retained in additional sensitivity analyses.
In contingency tables, statistical independence of variables was tested using χ2 test or Fisher exact test, according to Cochran rule. [15] We examined associations between AnLL and each of the aforementioned sources of exposure to tobacco smoke. Odds Ratios (OR) and relative 95% Confidence Intervals (95% CI) were obtained with unconditional logistic regression models. Linear trends for ordinal exposure variables were evaluated using the Wald test, treating the variable as a continuous variable (introduced in the model with 1 degree of freedom). To test for possible interactions on a multiplicative scale, product terms for the interaction between the exposure variable and the proposed effect modifier were created and likelihood ratio tests were used to compare models with and without the interaction terms.
The limited number of cases (n = 82) did not allow the direct inclusion of all covariates in multivariate logistic regression models. To deal with the small number of events per parameter, we performed two separate sets of analyses. Firstly, we adjusted for putative confounders (parameterized as presented in Table 1) via inverse probability weighting (IPW). [16] Then the conditional probability of being exposed given the individual covariates were estimated by fitting probit (for dichotomous exposure) or multinomial probit (for categorical exposure) regression models and we calculated robust standard error for the inference. [16]–[18]. A second set of regression models including covariates selected based on the change-in-estimates methods were fitted, using a threshold for inclusion of a 10% change in the odds ratios of interest [19]. All analyses were performed using Stata 12.1 SE (Stata corporation, Texas, TX) and all tests were 2 sided. A p-value of 0.05 or less was considered statistically significant.
Table 1. Characteristics of Acute Non-Lymphocytic Leukemia Cases and Controls in the SETIL Case-Control Study, Italy, 1998-2003.
| AnLL cases | Controls matched to AnLL cases | All sampled controls | P valuea | ||||
| No. | % | No. | % | No. | % | ||
| Genderd | |||||||
| Female | 39 | 47.6 | 62 | 48.4 | 482 | 46.2 | |
| Male | 43 | 52.4 | 66 | 51.6 | 562 | 52.8 | Na |
| Age at study reference date (years)d | |||||||
| ≤1 | 21 | 25.6 | 35 | 27.3 | 156 | 14.9 | |
| 2–3 | 13 | 15.9 | 18 | 14.1 | 351 | 33.6 | |
| 4–5 | 13 | 15.9 | 15 | 11.7 | 233 | 22.3 | |
| ≥6 | 35 | 42.7 | 60 | 46.9 | 304 | 29.1 | Na |
| Residence area (part of Italy)d | |||||||
| North (except Lombardy) | 22 | 26.8 | 33 | 25.8 | 250 | 24.0 | |
| Lombardy | 16 | 19.5 | 32 | 25.0 | 260 | 24.9 | |
| Center | 17 | 20.7 | 23 | 18.0 | 257 | 24.6 | |
| South and islands | 27 | 32.9 | 40 | 31.2 | 277 | 26.5 | Na |
| Birth order | |||||||
| First born | 39 | 47.6 | 68 | 53.1 | 551 | 52.8 | |
| Second born | 31 | 37.8 | 39 | 30.5 | 379 | 36.3 | |
| Third born and others | 12 | 14.6 | 21 | 16.4 | 113 | 10.8 | 0.49b |
| Birth weight (g) | |||||||
| <3,000 | 19 | 23.2 | 31 | 24.2 | 239 | 22.9 | |
| 3,000–3,299 | 18 | 22.0 | 28 | 21.9 | 246 | 23.6 | |
| 3,300–3,599 | 23 | 28.0 | 29 | 22.7 | 254 | 24.4 | |
| ≥3,600 | 22 | 26.8 | 40 | 31.2 | 304 | 29.2 | 0.89b |
| Duration of breastfeeding (months) | |||||||
| 0 | 12 | 14.6 | 26 | 20.5 | 232 | 22.3 | |
| 1–3 | 32 | 39.0 | 32 | 25.2 | 267 | 25.7 | |
| 4–6 | 19 | 23.2 | 37 | 29.1 | 233 | 22.4 | |
| >6 | 19 | 23.2 | 32 | 25.2 | 308 | 29.6 | 0.04b |
| Maternal age at child's birth (years) | |||||||
| ≤24 | 14 | 17.1 | 23 | 18.1 | 140 | 13.4 | |
| 25–29 | 25 | 30.5 | 41 | 32.3 | 382 | 36.7 | |
| 30–34 | 30 | 36.6 | 44 | 34.6 | 359 | 34.5 | |
| ≥35 | 13 | 15.8 | 19 | 15.0 | 160 | 15.4 | 0.65b |
| Birth year of the mother | |||||||
| <1960 | 15 | 18.3 | 22 | 17.3 | 145 | 13.9 | |
| 1960–1964 | 27 | 32.9 | 32 | 25.2 | 328 | 31.5 | |
| 1965–1969 | 22 | 26.8 | 53 | 31.7 | 373 | 35.8 | |
| ≥1970 | 18 | 22.0 | 20 | 15.8 | 195 | 18.7 | 0.36b |
| Maternal educational level | |||||||
| Less than high school | 46 | 56.1 | 45 | 35.2 | 400 | 38.4 | |
| High school | 26 | 31.7 | 64 | 50.0 | 503 | 48.3 | |
| University | 10 | 12.2 | 19 | 14.8 | 139 | 13.3 | <0.01b |
| Paternal age at child's birth (years) | |||||||
| ≤24 | 3 | 3.7 | 6 | 4.7 | 33 | 3.3 | |
| 25–29 | 18 | 22.2 | 29 | 22.8 | 241 | 23.8 | |
| 30–34 | 3 | 40.7 | 45 | 35.4 | 385 | 38.0 | |
| ≥35 | 27 | 33.3 | 47 | 37.0 | 353 | 34.9 | 0.96b |
| Paternal educational level | |||||||
| Less than high school | 47 | 58.0 | 54 | 42.2 | 463 | 44.6 | |
| High school | 24 | 29.6 | 59 | 46.1 | 424 | 40.9 | |
| University | 10 | 12.4 | 15 | 11.7 | 151 | 14.6 | 0.06b |
| Parental occupational exposure to benzene | |||||||
| Absent | 80 | 97.6 | 126 | 98.4 | 1,009 | 96.7 | |
| Present | 2 | 2.4 | 2 | 1.6 | 35 | 3.3 | 0.65c |
Abbreviations: AnLL, acute non-lymphocytic leukemia; NA, not appropriate.
Comparison between cases and all controls sampled in the SETIL study (AnLL controls + ALL controls).
P values from χ2 test.
P values from Fisher exact test.
Matching variables.
Results
Characteristics of study participants by case-control status are reported in Table 1. The entire sample of controls, mainly consisting of subjects matched to ALL cases, has a different age distribution compared to AnLL cases and their matched controls. The duration of breastfeeding was comparable in AnLL cases and their matched controls; conversely, long breastfeeding periods were more frequent in the control sample. Parents of cases usually had a lower educational level than controls' parents. All other considered characteristics seemed to have comparable distribution among cases and controls.
The ORs for the association between exposures to tobacco smoke and risk of AnLL are presented in Table 2. Estimates for both the matched and unmatched analyses are reported. In the unmatched analysis, ORs were estimated with reference to the subpopulation with complete data on putative confounders. Depending on the studied exposure, this restriction determined the exclusion of 33–39 controls and, only for paternal smoking in the conception period, of one case. Estimates based on the entire sample were consistent with those presented in Table 2.
Table 2. Association Between Acute non-Lymphocytic Leukemia and Sources of Exposure to Tobacco Smoke. The SETIL Study, Italy, 1998–2003.
| Unmatched analysis | ||||||||||||||
| Matched analysisa | Crude estimates | Models adjusted by sex, age and residence area | Models selected through change-in-estimates strategy | Models weighted by the inverse probability of exposuref | ||||||||||
| Exposure | Ca | Co | OR | 95%CI | Ca | Co | OR | 95%CI | OR | 95%CI | OR | 95%CI | OR | 95%CI |
| Paternal smoking in the conception period | ||||||||||||||
| Non smoker | 38 | 80 | 1.00 | Ref. | 38 | 612 | 1.00 | Ref. | 1.00 | Ref. | 1.00b | Ref.b | 1.00 | Ref. |
| Smoker, 1–10 cigs/day | 12 | 15 | 1.95 | 0.76–5.04 | 12 | 123 | 1.57 | 0.80–3.09 | 1.74 | 0.87–3.48 | 1.59b | 0.80–3.18b | 1.34 | 0.65–2.76 |
| Smoker, ≥11 cigs/day | 30 | 33 | 1.76 | 0.91–3.41 | 30 | 264 | 1.83 | 1.11–3.02 | 1.90 | 1.14–3.17 | 1.79b | 1.07–3.00b | 1.79 | 1.01–3.15 |
| P trend | 0.09 | 0.02 | 0.01 | 0.02 | 0.05 | |||||||||
| Maternal smoking in the 1st trimester of pregnancy | ||||||||||||||
| Non smoker | 71 | 115 | 1.00 | Ref. | 70 | 893 | 1.00 | Ref. | 1.00 | Ref. | 1.00c | Ref.c | 1.00 | Ref. |
| Smoker | 11 | 14 | 1.22 | 0.47–3.12 | 11 | 111 | 1.26 | 0.65–2.46 | 1.35 | 0.68–2.66 | 1.30c | 0.66–2.56c | 0.83 | 0.38–1.81 |
| Maternal exposure to ETS during the pregnancy | ||||||||||||||
| Not exposed | 49 | 84 | 1.00 | Ref. | 48 | 692 | 1.00 | Ref. | 1.00 | Ref. | 1.00d | Ref.d | 1.00 | Ref. |
| ≤3 hours/day | 15 | 22 | 0.99 | 0.43–2.29 | 15 | 188 | 1.15 | 0.63–2.10 | 1.03 | 0.55–1.92 | 1.04d | 0.56–1.92d | 0.89 | 0.46–1.72 |
| >3 hours/day | 17 | 19 | 1.69 | 0.78–3.64 | 17 | 115 | 2.13 | 1.18–3.83 | 1.94 | 1.06–3.54 | 2.12d | 1.16–3.86d | 1.85 | 0.97–3.52 |
| P trend | 0.23 | 0.02 | 0.06 | 0.03 | 0.07 | |||||||||
| Cumulative exposure of child to ETS | ||||||||||||||
| Not exposed | 52 | 89 | 1.00 | Ref. | 52 | 718 | 1.00 | Ref. | 1.00 | Ref. | 1.00e | Ref.e | 1.00 | Ref. |
| <4000 cigs | 15 | 20 | 1.33 | 0.57–3.07 | 15 | 151 | 1.37 | 0.75–2.50 | 1.27 | 0.69–2.36 | 1.18e | 0.64–2.18e | 1.25 | 0.63–2.48 |
| ≥4000 cigs | 15 | 20 | 1.59 | 0.65–3.87 | 14 | 130 | 1.49 | 0.80–2.76 | 1.51 | 0.78–2.92 | 1.33e | 0.69–2.57e | 1.15 | 0.45–2.95 |
| P trend | 0.29 | 0.15 | 0.18 | 0.39 | 0.77 | |||||||||
Abbreviations: 95%CI, 95% confidence interval; cigs, cigarettes; ETS, environmental tobacco smoke; OR, odds ratio; Ref, reference category.
Logistic regression models conditioned on matching variables (date of birth, sex, residence area of the child).
Logistic regression model adjusted by age class and maternal educational level.
Logistic regression model adjusted by age class and maternal educational level.
Logistic regression model adjusted by duration of breastfeeding and paternal educational level.
Logistic regression model adjusted by age class, maternal and paternal educational level.
Logistic regression model adjusted sex, age class, residence area, birth order, birth weight, duration of breastfeeding, maternal and paternal age at child birth, maternal and paternal educational level, birth year of the mother, and parental occupational exposure to benzene (inverse probability weighting).
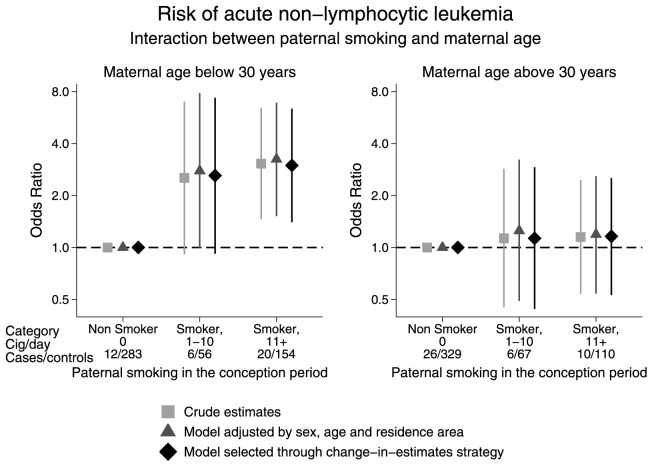
As shown in table 2, in matched analysis, paternal smoking in the conception period presented signs of association with the risk of AnLL (OR of smokers, 1–10 cigarettes/day = 1.95, 95%CI 0.76–5.04; OR of smokers, 11 or more cigarettes/day = 1.76, 95%CI 0.91, 3.41; P for trend 0.09). Unmatched analysis of paternal smoking produced similar estimates (adjusted OR of smokers, 1–10 cigarettes/day = 1.34, 95%CI 0.65–2.76; OR of smokers, 11 or more cigarettes/day = 1.79, 95%CI 1.01, 3.15; P for trend 0.05). Although supported by very weak statistical evidence (P = 0.18), the study of the interaction between paternal smoking and maternal age at child's birth showed interesting estimates (Figure 1). Apparently, paternal smoking affected the risk of childhood AnLL only among children born from mothers aged below 30 years, a cut-off selected a priori based on median maternal age. In the multivariable model selected with the change-in-estimate method and including age at diagnosis and maternal educational level, the adjusted OR for moderate smokers (1–10 cigarettes/day) was 2.61 (95%CI0.92–7.36), while the OR for heavy-smoker fathers (11 or more cigarettes/day) was 2.99 (95%CI1.40–6.37). Estimates for children born from mothers aged above 30 years were close to the unit (adjusted OR of smokers, 1–10 cigarettes/day = 1.13, 95%CI0.44–2.92, OR of smokers, 11 or more cigarettes/day = 1.16, 95%CI0.53–2.53). Of note, almost no evidence was found of an interaction between paternal age and paternal smoking during the conception period (at multivariate analysis p interaction = 0.40, data not shown).
Figure 1. Association Between Paternal Smoking Status During the Period of Conception and Risk of Childhood Acute Non-Lymphocytic Leukemia, According to Maternal Age at Delivery.
The SETIL Study, Italy, 1998–2003.
Maternal smoking during the first trimester of pregnancy did not show clear signs of association with the risk of childhood AnLL (Table 2). However, in unmatched analysis, marginal evidence of an association of AnLL with high levels of maternal exposure to ETS during the pregnancy (adjusted OR of mothers exposed more than 3 hours/day = 1.85, 95%CI 0.97–3.52; P for trend = 0.07) were observed. However, the exclusion of active-smoker mothers (n = 117) from the analysis determined a decrease of the estimates (adjusted OR of mothers exposed more than 3 hours/day = 1.42, 95%CI 0.69–2.95). The further adjustment by paternal smoking (an exposure that is likely to be associated with maternal exposure to ETS) caused a modest increase of the estimates (adjusted OR of mothers exposed more than 3 hours/day = 1.61, 95%CI 0.73–3.53).
As shown in Table 2, no evidence supported an association between the exposure of the child to ETS and the risk of AnLL (for children exposed to 4,000 or more cigarettes, OR adjusted through IPW = 1.15, 95%CI 0.45–2.95; P for trend = 0.77).
Discussion
In this analysis of data from a population-based case-control study moderate evidence supporting the hypothesis that children of fathers who smoked in the period of conception have an increased risk of AnLL was found. Interestingly, an apparent effect modification by maternal age was also identified. Indeed, only children of mothers aged below 30 years at the delivery presented an increased risk. We also found weak signs of an association between maternal exposure to second-hand smoke and risk of childhood AnLL. No sign of association was found for maternal smoking during pregnancy. Finally, we did not find any evidence supporting an association between child exposure to second-hand smoke and risk of AnLL.
Plausibility of the results and evidence from previous studies
An association between paternal smoking before the pregnancy and risk of childhood leukemia has already been reported. [7]–[9], [20] However, most of the positive findings regarded ALL, while only limited evidence supports the association between AnLL and paternal smoking. [7] It should be considered that studies on AnLL and paternal smoking are all case-control studies and they are often underpowered, due to the rarity of the disease. Since tobacco smoke is an established leukemogenic in adults, [7] the biological plausibility of an association with childhood AnLL is high. Furthermore, the possible effect of exposure to tobacco smoke of the gametes or the embryo/fetus in utero on the risk of childhood AnLL is in line with the “two hits” model proposed by Greaves. [21] Moderate/weak evidence of a possible interaction between paternal smoking and maternal age at delivery was observed. Possible explanations of the observed interaction could be chance or a strong pattern of confounders differentially acting in the two maternal age strata. However, further investigations should be carried out before excluding causality, since during pre-implantation embryogenesis complex interactions exist between paternal and maternal factors and the biochemical environment. [22]
Our analysis did not produce evidence supporting an association between maternal tobacco smoking and risk of childhood AnLL. Results were broadly in line with those of previous studies. [7] However, one should consider our sample only included 19 women (3 cases and 16 controls) who declared having smoked more than 10 cigarettes/day during the first trimester of pregnancy.
Results for maternal exposure to second-hand smoke suggest a possible association with AnLL: to the best of our knowledge, this finding is the first supporting this association [23], [24] which makes us cautious in interpreting this apparent association as causal since we consider the self-assessment of second-hand smoke to be a measure prone to misclassification and recall bias. In fact, the presence of a raised risk only for maternal exposure to ETS and not for maternal active smoking is difficult to explain from a biological point of view. Furthermore, evidence suggesting a strong recall bias for maternal exposure to ETS emerged from a former study of ALL performed data from the SETIL study [13].
In most past studies on exposure of children to second-hand smoke and risk of AnLL authors used parental smoking status after pregnancy as a proxy of exposure, and most findings were negative. [25] In the SETIL study, a quantification of child exposure was attempted with direct questions in the questionnaire, but we failed to find any sign of an association between second-hand smoke and AnLL risk.
Strengths and limitations
One strength of this study is the population based design: the identification of incident cases in participating Regions proved to be very accurate [12] and information on exposures was collected by trained interviewers.
Conversely, several limitations should be considered: the response rate of controls was 0.71 and we cannot exclude a selection bias. Recall bias is always a concern when investigating self-reported exposures. Nevertheless, a Swedish study highlighted that retrospective recall of pregnancy smoking is fairly stable over time. [26] Also, interviewed subjects and interviewers were unaware of the hypothesis investigated in the present report since studying the association between smoking and childhood ALL was not one of the main purposes of the SETIL study; furthermore, the sections aimed at collecting information on smoking were only a small part of the entire questionnaire. On the balance, we do not believe that recall bias is a serious limitation for the study of parental active smoking; on the contrary, recall bias could affect the study of ETS. As the SETIL study was not primarily designed to study the effect of tobacco smoking, misclassification of exposure could be an issue, particularly for ETS exposure.
In the present analysis we were unable to consider the effect of residential and domestic exposure to benzene, possible confounders of the relationship between exposure to cigarette smoke and risk of childhood AnLL.
We decided to break the matching in order to avoid loss of cases and expand the control group. Therefore, we should consider a possible bias due to the use of unconditional logistic regression in analysis that involved both matched and unmatched controls, with respect to AnLL cases. Of note, estimates from conditional logistic regression models (matched analysis) were consistent with the results from unmatched analysis.
The use of a propensity score or inverse probability weighting in case-control studies has been reported to be more problematic than in cohort studies, since estimates might be affected by an artefactual effect modification and residual confounding [27]. To assess whether this sort of bias might influence our estimates a supplemental set of analyses where covariates were selected based on the change-in-estimates method was performed. It is noteworthy to observe that figures from the two sets of analyses were consistent.
Conclusions
Our study supports the hypothesis that paternal smoking is associated with the risk of childhood AnLL; we also found signs of a possible effect modification due to maternal age at delivery that should be considered in future investigations. We found weak evidence of a possible effect of maternal exposure to second-hand smoke on the risk of childhood AnLL. This finding has to be consider with a degree of caution since recall bias is likely. No evidence at all emerged in our analysis for maternal smoking and exposure of the child to second-hand smoke; these results are broadly in line with knowledge from previous researches, but we should underline that the power of our study to detect an association for these exposures was low.
Supporting Information
Studies on Paternal Tobacco Smoking and Risk of Childhood Acute non-Lymphocytic Leukemia.
(DOCX)
Smoking questionnaire.
(DOCX)
Acknowledgments
The SETIL study was financially supported by research grants received by AIRC (Italian Association on Research on Cancer), MIUR (Ministry for Instruction, University and Research, PRIN Program), Ministry of Health (Ricerca Sanitaria Finalizzata Program), Ministry of Labour and Welfare, Associazione Neuroblastoma, Piemonte Region (Ricerca Sanitaria Finalizzata Regione Piemonte Program), Liguria Region, ONLUS Comitato per la vita "Daniele Chianelli"- Associazione per la Ricerca e la Cura delle Leucemie, Linfomi e Tumori di Adulti e Bambini, (Perugia).
Contributors
The members of the SETIL Study Group (Principal Investigator: Prof. Corrado Magnani, email: magnani@med.unipmn.it) are:
Aricò Maurizio, AOU Meyer, Firenze, Italia;
Assennato Giorgio, ARPA Puglia, Bari, Italy;
Bernini Gabriella, Dipartimento di Oncoematologia, Azienda Ospedaliera Universitaria Meyer, Firenze, Italy (retired);
Biddau Pierfranco, Ospedale Microcitemico, Cagliari, Italia;
Bisanti Luigi, ASL di Milano, Milano, Italia;
Bochicchio Francesco, Istituto Superiore di Sanità, Roma, Italia;
Bocchini Vittorio, Istituto Nazionale per la Ricerca sul Cancro, Genova, Italia;
Cannizzaro Santina, Lega Italiana per la Lotta contro i Tumori Onlus, Ragusa, Italia;
Casotto Veronica, IRCCS Burlo Garofolo, Trieste, Italia;
Celentano Egidio, ARSAN - Agenzia Regionale Sanitaria della Campania, Napoli, Italia;
Chiavarini Manuela, Dipartimento di Specialità Medico Chirurgiche e Sanità Pubblica – Sezione di Sanità Pubblica, Facoltà di Medicina e Chirurgia, Università degli Studi di Perugia, Perugia, Italia;
Cocco Pierluigi, Università di Cagliari, Cagliari, Italia;
Cuttini Marina, Ospedale Pediatrico Bambino Gesù, Roma, Italia;
de Nichilo Gigliola, SPESAL, Barletta, Italia;
De Salvo Gian Luigi, Istituto Oncologico Veneto IRCCS, Padova, Italia;
Forastiere Francesco, Department of Epidemiology, Regional Health Authority - Lazio Region, Rome, Italy;
Gafà Lorenzo, Lega Italiana per la Lotta contro i Tumori Onlus, Ragusa Ibla, Italia;
Galassi Claudia, AOU S.Giovanni Battista e CPO Piemonte, Torino, Italia;
Greco Alessandra, Istituto Oncologico Veneto—IRCCS Padova, Padova, Italia;
Guarino Erni, Istituto Nazionale Tumori, Napoli, Italia
Haupt Riccardo, Istituto Giannina Gaslini, Genova, Italia;
Lagorio Susanna, National Institute of Health, Rome, Italia;
Locatelli Franco, Università di Pavia, Pavia, Italia;
Luzzatto Lia Lidia, ASL 1, Torino, Italy;
Kirchmayer Ursula, Dipartimento Epidemiologia Regione Lazio, Roma, Italia;
Masera Giuseppe, Università Milano Bicocca, Monza, Italia;
Massaglia Pia, Neuropsichiatria Infantile, Torino, Italia;
Merlo Domenico Franco, IRCCS Azienda Ospedaliera Universitaria San Martino- IST Istituto Nazionale per Minelli Liliana, Università degli Studi di Perugia, Perugia, Italia;
Monetti Daniele, Istituto Oncologico Veneto IRCCS, Padova, Italia;
Mosciatti Paola, Università di Camerino, Camerino, Italia;
la Ricerca sul Cancro, Genova, Italia;
Michelozzi Paola, Department of Epidemiology, Regional Health Authority – Lazio – Region, Rome, Italy;
Nuccetelli Cristina, Istituto Superiore di Sanità, Roma, Italia;
Pannelli Franco, Università di Camerino, Dipartimento di Medicina Sperimentale e di Sanità Pubblica, Camerino, Italy;
Pession Andrea, University of Bologna, Bologna, Italia;
Polichetti Alessandro, National Institute of Health, Roma, Italia;
Poggi Vincenzo, A.O.R.N. Santobono – Pausilipon, Napoli, Italia;
Pulsoni Alessandro, La Sapienza, Università di Roma, Roma, Italia;
Sampietro Giuseppe, ASL Città di Milano, Milano, Italia;
Schilirò Gino, Università di Catania, Catania, Italia;
Risica Serena, Istituto Superiore di Sanità, Roma, Italia;
Rizzari Carmelo, A.O. San Gerardo, Fondazione MBBM, Monza, Italia;
Targhetta Roberto, Servizio di Oncoematologia Pediatrica, Ospedale Microcitemico, Cagliari, Italia;
Torregrossa Maria Valeria, Università degli Studi di Palermo; Palermo, Italia;
Valenti Rosaria Maria, Università degli Studi di Palermo, Palermo, Italia;
Varotto Stefania, Dipartimento di Pediatria, Università di Padova, Italia;
Zambon Paola, Registro Tumori del Veneto, Università di Padova, Padova, Italia;
Andrea Farioli's work on this paper was supported by the Master's Degree in Epidemiology, University of Turin.
A special thank to Ms. Victoria Franzinetti for her careful revision of the text.
Funding Statement
The SETIL study was financially supported by research grants received by AIRC (Italian Association on Research on Cancer), MIUR (Ministry for Instruction, University and Research, PRIN Program), Ministry of Health (Ricerca Sanitaria Finalizzata Program), Ministry of Labour and Welfare, Associazione Neuroblastoma, Piemonte Region (Ricerca Sanitaria Finalizzata Regione Piemonte Program), Liguria Region, Comitato per la vita "Daniele Chianelli"- Associazione per la Ricerca e la Cura delle Leucemie, Linfomi e Tumori di Adulti e Bambini, (Perugia). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Pui CH (2006) Childhood Leukemia. 2nd ed. Cambridge University Press: New York. [Google Scholar]
- 2. Greaves MF, Alexander FE (1993) An infectious etiology for common acute lymphoblastic leukemia in childhood? Leukemia 7: 349–360. [PubMed] [Google Scholar]
- 3. Belson M, Kingsley B, Holmes A (2007) Risk factors for acute leukemia in children: a review. Environ Health Perspect 115: 138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Eden T (2010) Aetiology of childhood leukaemia. Cancer Treat Rev 36: 286–297 10.1016/j.ctrv.2010.02.004 [DOI] [PubMed] [Google Scholar]
- 5. Greaves M (2006) Infection, immune responses and the aetiology of childhood leukaemia. Nat Rev Cancer 6: 193–203 10.1038/nrc1816 [DOI] [PubMed] [Google Scholar]
- 6. Schnatter AR, Rosamilia K, Wojcik NC (2005) Review of the literature on benzene exposure and leukemia subtypes. Chem Biol Interact 153–154: 9–21 10.1016/j.cbi.2005.03.039 [DOI] [PubMed] [Google Scholar]
- 7.WHO-IARC (2009) IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 100. A Review of Human Carcinogens. Part E: Personal Habits and Indoor Combustions. Lyon: WHO Press. [PMC free article] [PubMed] [Google Scholar]
- 8. Milne E, Greenop KR, Scott RJ, Bailey HD, Attia J, et al. (2012) Parental prenatal smoking and risk of childhood acute lymphoblastic leukemia. Am J Epidemiol 175: 43–53 10.1093/aje/kwr275 [DOI] [PubMed] [Google Scholar]
- 9. Chang JS (2009) Parental smoking and childhood leukemia. Methods Mol Biol 472: 10–37 10.1007/978-1-60327-492-05 [DOI] [PubMed] [Google Scholar]
- 10. Miligi L, Benvenuti A, Mattioli S, Salvan A, Tozzi GA, et al. (2013) Risk of childhood leukaemia and non-Hodgkin's lymphoma after parental occupational exposure to solvents and other agents: the SETIL Study. Occup Environ Med 70: 648–655 10.1136/oemed-2012-100951 [DOI] [PubMed] [Google Scholar]
- 11. Badaloni C, Ranucci A, Cesaroni G, Zanini G, Vienneau D, et al. (2013) Air pollution and childhood leukaemia: a nationwide case-control study in Italy. Occup Environ Med 70: 876–883 10.1136/oemed-2013-101604 [DOI] [PubMed] [Google Scholar]
- 12. AIRTUM Working Group (2008) Italian cancer figures-report 2008. 1. Childhood cancer. (In Italian) Epidemiol Prev 32 1, 13–35 – [PubMed] [Google Scholar]
- 13. Farioli A, Legittimo P, Mattioli S, Miligi L, Benvenuti A et al. (2014) Tobacco smoke and risk of childhood acute lymphoblastic leukemia: findings from the SETIL case-control study. Cancer Causes Control, doi: 10.1007/s10552-014-0371-9 [DOI] [PubMed] [Google Scholar]
- 14. Metayer C, Milne E, Clavel J, Infante-Rivard C, Petridou E, et al. (2013) The Childhood Leukemia International Consortium. Cancer Epidemiol 37: 336–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cochran WG (1954) Some methods for strengthening the common χ2 tests. Biometrics 10: 417–451. [Google Scholar]
- 16. Robins JM, Hernán MA, Brumback B (2000) Marginal structural models and causal inference in epidemiology. Epidemiology 11: 550–560. [DOI] [PubMed] [Google Scholar]
- 17. Tchernis R, Horvitz-Lennon M, Normand SL (2005) On the use of discrete choice models for causal inference. Stat Med 24: 2197–2212 10.1002/sim.2095 [DOI] [PubMed] [Google Scholar]
- 18. Hernan MA, Brumback BA, Robins JM (2000) Marginal Structural Models to Estimate the Causal Effect of Zidovudine on the Survival of HIV-Positive Men. Epidemiology 11: 561–570. [DOI] [PubMed] [Google Scholar]
- 19. Maldonado G, Greenland S (1993) Simulation study of confounder-selection strategies. Am J Epidemiol 138: 923–36. [DOI] [PubMed] [Google Scholar]
- 20. Lee KM, Ward MH, Han S, Ahn HS, Kang HJ, et al. (2009) Paternal smoking, genetic polymorphisms in CYP1A1 and childhood leukemia risk. Leuk Res 33: 250–258 10.1016/j.leukres.2008.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Greaves M (2005) In utero origins of childhood leukaemia. Early Hum Dev 81: 123–129 10.1016/j.earlhumdev.2004.10.004 [DOI] [PubMed] [Google Scholar]
- 22. Ménézo YJ (2006) Paternal and maternal factors in preimplantation embryogenesis: interaction with the biochemical environment. Reprod Biomed Online 12: 616–621 10.1016/S1472-6483(10)61188-1 [DOI] [PubMed] [Google Scholar]
- 23. Trédaniel J, Boffetta P, Little J, Saracci R, Hirsch A (1994) Exposure to passive smoking during pregnancy and childhood, and cancer risk: the epidemiological evidence. Paediatr Perinat Epidemiol 8: 233–255 10.1111/j.1365-3016.1994.tb00455.x [DOI] [PubMed] [Google Scholar]
- 24. Sasco AJ, Vainio H (1999) From in utero and childhood exposure to parental smoking to childhood cancer: a possible link and the need for action. Hum Exp Toxicol 18: 192–201 10.1191/096032799678839905 [DOI] [PubMed] [Google Scholar]
- 25. Boffetta P, Trédaniel J, Greco A (2000) Risk of childhood cancer and adult lung cancer after childhood exposure to passive smoke: A meta-analysis. Environ Health Perspect 108: 73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Post A, Gilljam H, Bremberg S, Galanti MR (2008) Maternal smoking during pregnancy: a comparison between concurrent and retrospective self-reports. Paediatr Perinat Epidemiol 22: 155–161 10.1111/j.1365-3016.2007.00917.x [DOI] [PubMed] [Google Scholar]
- 27. Månsson R, Joffe MM, Sun W, Hennessy S (2007) On the estimation and use of propensity scores in case-control and case-cohort studies. Am J Epidemiol 166: 332–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Studies on Paternal Tobacco Smoking and Risk of Childhood Acute non-Lymphocytic Leukemia.
(DOCX)
Smoking questionnaire.
(DOCX)