Abstract
Stem cell-based therapies are emerging as a promising strategy to tackle cancer. Multiple stem cell types have been shown to exhibit inherent tropism towards tumours. Moreover, when engineered to express therapeutic agents, these pathotropic delivery vehicles can effectively target sites of malignancy. This perspective considers the current status of stem cell-based treatments for cancer and provides a rationale for translating the most promising preclinical studies into the clinic.
The ultimate goal of cancer therapy is to develop antitumour agents that robustly target cancerous cells while sparing normal cells. Currently, the major drawback of using conventional treatments is their lack of selectivity, often resulting in considerable loss of healthy tissue. Many adult stem cells (SCs) show intrinsic tumour-tropic properties, making them attractive candidates for the targeted delivery of anticancer biological agents (TABLE 1). The strategy is twofold: SCs can disseminate solid tumours and migrate towards micrometastatic lesions, enabling site-specific delivery. Furthermore, SCs can be modified to stably express or release various anticancer agents, thereby circumventing the short half-lives that many chemotherapeutic agents exhibit.
Table 1.
Stem cell sources
| Name | Sources | Advantages | Disadvantages |
|---|---|---|---|
| Embryonic SCs | Inner cell mass of the mammalian blastocyst | Pluripotent — enabling them to form derivatives of all three germ layers |
|
| Haematopoietic SCs |
|
|
Limited differentiation potential |
| Mesenchymal SCs |
|
|
|
| Neural SCs |
|
|
|
| Endothelial SCs |
|
|
Limited differentiation potential |
| Induced SCs | Derived from somatic cells using reprogramming technologies |
|
|
SC, stem cell.
Whilst the development of preclinical SC therapies remains voracious, translating the most promising into the clinic necessitates much technical and regulatory traversal. Owing to the regenerative and therapeutic potential that SCs offer medicine, research into them has garnered much interest. However, scientific concerns over the use of SC therapies to treat disease need to be resolved (BOX 1). Clinical application of SCs in the absence of solid knowledge and expertise can jeopardize progress, as exemplified by the Stamina Foundation, Italy1. These are clearly exciting and uncertain times for SC research. There is a need to separate the hope from the hype; to distinguish the therapeutic potential that SCs bring to the clinical table from the inflated promises and flimsy claims that pervade the media and scientific literature.
Box 1 | The safety of stem cells.
Stem cells (SCs) have two important properties: the ability to self-renew and differentiate into specialized cell types and the capacity to home towards sites of pathology and malignant lesions. Although these features are very important from a regenerative standpoint, they present potential safety concerns when introduced into a recipient. Of particular concern is whether SCs promote the growth of certain tumours119–121 or indeed form tumours themselves122,123. Non-immortalized adult SCs (such as mesenchymal SCs, neural SCs, haematopoietic SCs and endothelial SCs) provide fewer safety concerns than their immortalized counterparts (such as embryonic SCs and induced pluripotent SCs), especially when they are autologous and delivered into a similar niche from which they were derived. SCs that are engineered to express antitumour agents need rigorous testing to ensure that the SC has not been rendered tumorigenic. The incorporation of suicide genes into therapeutic SCs enables their controlled eradication, thereby providing a safety mechanism to alleviate this concern. Furthermore, many SC based therapies, including oncolytic virus and delivery of nanoparticles, result in the death of the SC upon release of the therapy, thereby effectively abolishing the possibility of any tumour formation or errant differentiation.
The advent of reprogramming technologies — the ability to convert a somatic cell into a pluripotent or multipotent SC — provides additional avenues for creating therapeutic patient-derived cells. Despite their huge potential, these cells tend to form teratomas in mice, which is a considerable clinical hurdle that must be overcome before these cells could be transplanted into patients, and overcoming this challenge is an area of intense research124. This has already been achieved in the case of induced neural SCs that were created from human fetal fibroblasts and shown not to aberrantly proliferate when implanted into mice125. Clearly, these safety concerns need to be addressed to limit undue harm to the patient and to prevent a situation whereby the therapeutic SC exacerbates cancer progression. Careful selection of the SC type, the purity of the SC population and the effect of any modifications on the function of the SCs should be established in preclinical studies and facilitated by a thorough understanding of SC biology.
This perspective aims to highlight the most recent advances in SC-based treatments for cancer. It initially focuses on how state of the art technologies have been applied to manipulate and deploy SCs in preclinical studies to target cancerous cells. To conclude, the successes and failures of the most recent clinical trials are discussed, allowing us to address how their outcomes can refine future trials and facilitate the transition from bench to bedside.
Stem cells for therapeutic delivery
To maximize the effectiveness of anticancer agents, it is necessary to circumvent the problems that are associated with them: namely, their often unfavourable pharmacokinetics (short half-life and biotransformation) and difficulty in producing sustained and efficacious concentrations in the vicinity of the tumour. In the case of brain tumours, failure of compounds to cross the blood–brain barrier is an additional confounding factor. The use of SCs as cellular delivery agents has been posed as a novel means to tackle these challenges, permitting a targeted and more prolonged therapeutic response than might otherwise be possible by conventional delivery methods. To maximize the therapeutic impact of SC therapy, SCs should evade the host immune system and home towards malignant foci. Although these are highly desirable characteristics, whether and to what extent these attributes exist are still being established. It is widely accepted that SCs have immunosuppressive properties by virtue of their expression of cytokines and growth factors that can modulate the cellular and innate immune pathways of the host2–4. However, several in vivo studies have found that transplantation of various adult and induced allogeneic donor SCs elicits an immune response, thereby resulting in their rejection5–9. For example, although allogeneic mesenchymal SCs (MSCs) seem to be less immunogenic than allogeneic non-SC donor cells, such as fibroblasts (as determined by their relatively long persistence in immunocompetent hosts8), they should not be considered to be immune privileged but rather to have the ability to transiently escape host rejection10.
The migratory capacity of neural SCs (NSCs) and neural progenitors was initially shown in xenograft mouse models by their ability to home to intracranial brain tumours and non-neural tumours in other regions of the body11–13. Moreover, NSCs not only integrate into the primary tumour bed but also track towards small intracranial microsatellite deposits that typify malignant brain tumours such as glioblastoma11. These tumour-tropic characteristics have been reported in numerous types of human SCs14–16. The cellular and molecular mechanisms that underlie the tumour tropism of SCs are far from being completely understood. Various chemokine–chemokine receptor pairs have been associated with tumour tropism, and perhaps the best studied is stromal cell-derived factor 1 (SDF1; also known as CXCL12) and its receptor CXC-chemokine receptor 4 (CXCR4). To date, the SDF1–CXCR4 signalling axis has been shown to have a major role in the migration of multiple SC types, including adult SCs17–20, embryonic SCs (ESCs)21 and induced pluripotent SCs (iPSCs)22. Other influential signalling pathways have been elucidated and include PI3K signalling23, urokinase-type plasminogen activator (uPA)–uPA receptor (uPAR)24,25, vascular endothelial growth factor receptor 2 (VEGFR2)26 and matrix metalloproteinase 1 (MMP1)–proteinase-activated receptor 1 (PAR1)27. The degree of SC migration towards a tumour in vivo is influenced by diverse factors, including the nature of the SC (the heterogeneity of the population, culture conditions and the expression of migratory factors) and the tumour microenvironment (the degree of hypoxia, the extent of vascularization, and inflammation). A better understanding of the factors influencing the migratory potential of SCs will allow a greater ability to tailor SC migration and ultimately increase the therapeutic potential of these SCs.
Creating anticancer stem cells
Unmodified SCs can have intrinsic antitumour effects attributed to factors that are secreted by SCs and physical interactions that are established between the SC and tumour cells28–30. In addition, SCs have been modified in various ways to treat cancer, and some of the most promising are discussed below.
Genetic modification of stem cells to secrete anticancer proteins
SC secretion of therapeutic proteins can be divided into two broad categories depending on whether they act directly on malignant cells or on supporting cells of the tumour, such as blood vessels and stroma (FIG. 1a). SCs are typically modified by viral transduction to express transgenes encoding secretable effector proteins, although non-viral methods have been reported that offer certain advantages, such as lower host immunogenicity31,32. Direct effectors include the pro-apoptotic protein tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) that binds to death receptor 4 (DR4; also known as TRAILR1) and DR5 (also known as TRAILR2) (which are preferentially expressed on cancerous cells) and induces apoptosis33,34. Using proteins that can outcompete or sterically block the binding of endogenous ligands to their cognate receptor is another strategy that results in inhibition of proliferation pathways in the cancer and associated cells. For example, SC-expression of biological agents that bind to epidermal growth factor receptor (EGFR) or its tumour-specific variant EGFRvIII (REFS 35,36), and cytokines such as interferon-β (IFNβ)37–40 and IFNα41, have all been shown to negatively regulate tumour growth in various preclinical cancer models.
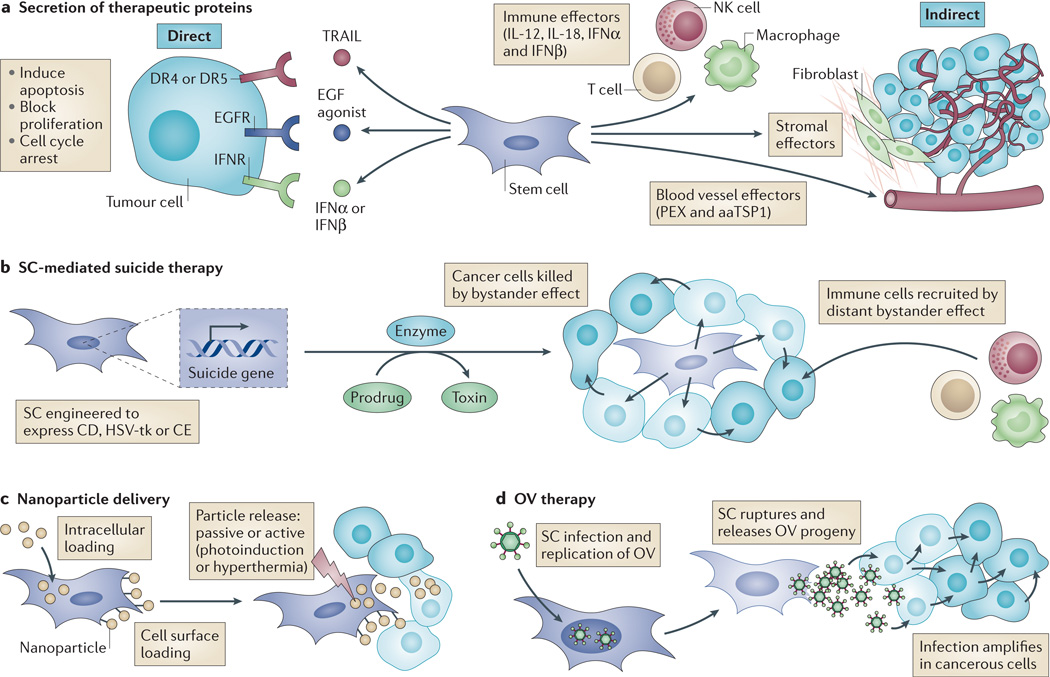
Figure 1. Using stem cells (SCs) to promote tumour cell death.
SCs can be modified in various ways to generate antitumour capabilities. a | SCs can be engineered to secrete therapeutic proteins that function directly on tumour cells or indirectly on cells of the tumour microenvironment. For example, tumour necrosis factor-related apoptosis-inducing ligand (TRAIL), epidermal growth factor (EGF) agonists or interferons (IFNα or IFNβ) can be secreted to function on death receptor 4 (DR4) and DR5, EGF receptor (EGFR) or IFN receptors (IFNRs), respectively. Alternatively, SCs can secrete stromal, immune or blood vessel effectors. b | SCs can be engineered to express a suicide gene encoding an enzyme (such as cytosine deaminase (CD), carboxylesterase (CE) or herpes simplex virus thymidine kinase (HSV-tk)) that converts a prodrug into a cytotoxin. This induces suicide of the SC, and cancer cells are killed by the bystander effect, a phenomenon that describes the movement of cytotoxin from the SC to adjacent cancer cells via a paracrine mechanism or gap junctions. The distant bystander effect describes the recruitment of host immune cells in response to death or inflammatory signals released from dying cells. c | SCs can be loaded with nanoparticles containing chemotherapy or imaging agents that are released in the vicinity of the tumour, either passively or in response to external stimuli. d | SCs can be infected with oncolytic viruses (OVs). OVs replicate within the SCs, which then rupture and release OV progeny that can infect cancerous cells and amplify infection. aaTSP1, anti-angiogenic thrombospondin 1; IL, interleukin; NK, natural killer; PEX, a fragment of matrix metalloproteinase 2.
Indirect effectors include agents that inhibit the formation of tumour-associated vasculature, such as anti-angiogenic thrombospondin 1 (TSP1; also known as THBS1)42 and PEX (a fragment of MMP2)43, effectively limiting the growth of the tumour mass by creating a hostile, non-permissive microenvironment. Secretion of immunomodulatory molecules such as interleukins (ILs) has also been tested. The rationale is that the immunosuppressive tumour milieu can be shifted into one that directs an immune response against the cancer44. Human MSCs have been engineered to secrete IL-12 or IL-18 and have been tested in mice bearing renal cell carcinoma45, cervical tumours46 and glioblastoma47–49. These studies correlated the sustained presence of SC-delivered ILs with a shift in the immune profile, including increased levels of IFNγ, activation of natural killer cells and recruitment of tumour-specific T cells, often resulting in protracted survival and thus underscoring the potential of this approach.
Genetic modification of stem cells to induce cancer cell death
SC-mediated suicide gene therapy describes a strategy whereby SCs are engineered to express an enzyme that converts a separately administered non-toxic prodrug into a cytotoxic drug that can efficiently kill surrounding cells by the bystander effect (FIG. 1b). An added benefit of SC-mediated suicide therapy is that the SC is eliminated after its therapeutic effect, thereby abolishing any concern over its long-term fate. Three major suicide gene systems are currently used. Cytosine deaminase (CD) converts 5-fluorocytosine (5-FC) to the toxic antimetabolite 5-fluorouracil. The herpes simplex virus thymidine kinase (HSV-tk) converts ganciclovir (GCV) to GCV-monophosphate, which is further phosphorylated to GCV-triphosphate, which potently blocks DNA synthesis. In addition, carboxylesterase (CE) converts the pro-drug irinotecan to the potent topoisomerase inhibitor SN-38. The CD–5-FC system has been used in modified MSCs and NSCs and applied in mouse models to treat tumours of the brain, such as glioblastoma50–53 and medulloblastoma54, often resulting in regression of the tumour mass and prolonged survival. The HSV-tk system, which relies on the formation of gap junctions between the SC and surrounding target cells for an efficient bystander effect, has shown efficacy in several animal models of cancer, including glioblastoma55,56, breast14 and prostate57. Human NSCs harnessing the CE–irinotecan system have proved to be effective in preclinical models of ovarian and lung cancer, as well as medulloblastoma58–60.
Stem cells as nanoparticle carriers
Nanoparticle delivery systems are able to contain high concentrations of often insoluble chemotherapeutic reagents, while protecting them from degradation by the harsh biological environment. Furthermore, the surface of nanoparticles can be modified to alter properties such as stability, solubility and targeting61. Although this technology offers considerable delivery benefits, using nanoparticles in vivo has been challenging owing to their efficient clearance, inefficient dissemination in solid tumours and failure to target micrometastatic lesions61. One approach to overcome these barriers is to use SCs as nanoparticle delivery agents62. SCs can be loaded with nanoparticles and administered in vivo, where they can migrate towards malignancies and deposit the loaded nanoparticles close to the tumour63. Although this ‘Trojan horse’ principle is conceptually straightforward, its successful implementation requires much technical consideration, including establishing an efficient means to load SCs without affecting their intrinsic properties, and controlling the release of nanoparticles from the SC to ensure sustained and targeted therapy (FIG. 1c). One novel approach is to load the cell membrane of MSCs with porous silica ‘nanorattles’ containing doxorubicin64. These loaded MSCs were shown to migrate towards and induce apoptosis in intracranial tumours more efficiently than injection of doxorubicin alone64. More frequently, strategies rely on the passive and/or calveolin- or clathrin-assisted uptake of nanoparticles into SCs63. Once at the tumour site, nanoparticles are released from the SC, either as a result of membrane rupture caused by cytosolic accumulation, or by facilitating death using an external source such as photoinduction65,66 or inducing hyperthermia67. Combining nanoparticle-based therapeutics with SC delivery is a nascent partnership that warrants further investigation for the treatment of cancer.
Stem cells loaded with oncolytic virus
Oncolytic viruses have the ability to selectively replicate in and kill tumour cells while sparing healthy cells68. However, their therapeutic efficacy is confounded by clearance of the virus by host defence mechanisms following systemic administration and insufficient viral spread following intratumoural administration. In an effort to improve delivery of oncolytic virus therapeutics and circumvent antiviral immunity, several studies have explored the possibility of using SCs as delivery vehicles69–72 (FIG. 1d). Different SC types have been used as host cells for the transportation and local release of intact replicating oncolytic adenoviruses administered systemically or intratumourally into murine models of ovarian cancer70, metastatic breast cancer73 and glioblastoma74–76. MSCs have recently been shown to function as effective carriers to deliver attenuated oncolytic measles virus to ovarian tumours77 and human hepatocellular carcinoma78, which would otherwise be quickly neutralized by pre-existing antiviral antibodies. Many of these studies have shown selective localization of oncolytic virus-loaded SCs to tumour xenografts and subsequent infection of tumour cells, resulting in reduced tumour volumes and a significant increase in the median survival of treated animals70,73–78.
Oncolytic HSV (oHSV) has been modified for tumour-selective replication and is inherently neurotropic, which makes it a promising candidate for brain tumour therapy68,79. Although Phase I and Ib clinical trials using oHSV for treating malignant glioblastoma post-tumour resection have shown antitumour activity, clinical response rates have been sub-optimal80–82. This could be attributed to the secondary bleeding caused by surgical intervention, causing an influx of cerebrospinal fluid into the resection cavity and a ‘washing-out’ of the oHSV. In a recent study, we loaded human MSCs with oHSV and showed that they effectively produced oHSV progeny, increased antitumour efficacy in a clinically relevant glioblastoma model and prolonged median survival in mice, compared to direct injection of purified oHSV83. SC delivery of oncolytic virus has proved to be effective in several preclinical cancer models, and the challenge is to ensure absolute safety of the viral system to avoid complications in its clinical translation.
Potentiating stem cell efficacy
Various strategies have been applied to SCs to enhance their therapeutic potential (FIG. 2). These approaches have been categorized with respect to whether they augment intrinsic SC properties, function in combination with secreted factors to enhance antitumour efficacy or improve the delivery of SCs.
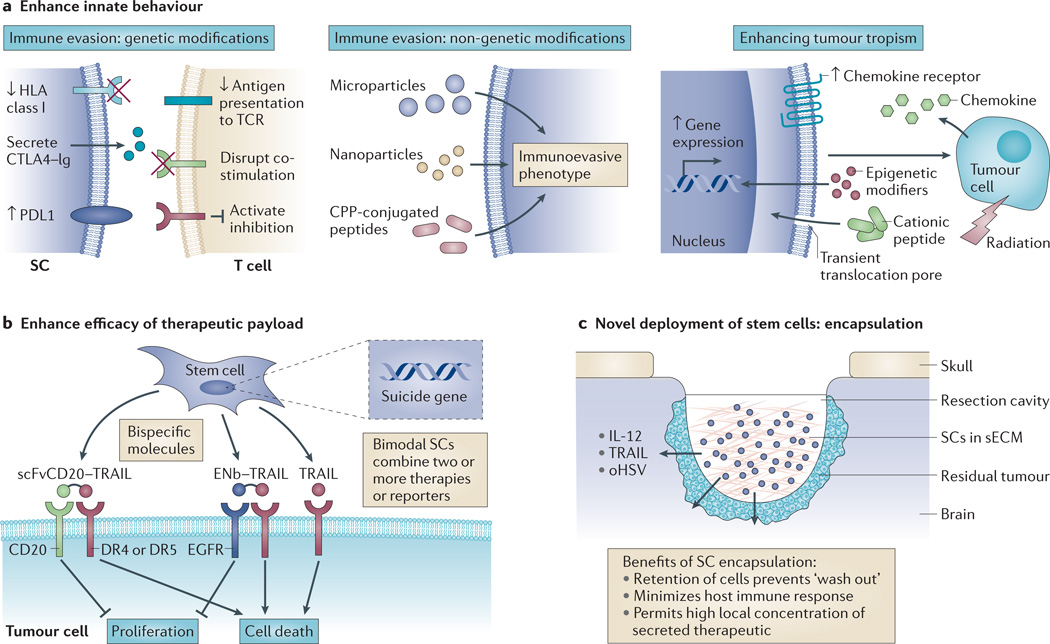
Figure 2. Potentiating stem cell (SC) efficacy.
Multiple strategies have been used to enhance the therapeutic potential of SCs. a | Immunoevasive and migratory properties can be enhanced using genetic and non-genetic approaches. For example, SCs can be genetically modified to decrease the expression of human leukocyte antigen (HLA) class I to reduce antigen presentation to T cell receptors (TCRs), cytotoxic T lymphocyte antigen 4 (CTLA4)–immunoglobulin (Ig) can be secreted to block T cell co-stimulation by CTLA4, and upregulation of programmed cell death 1 ligand 1 (PDL1) can also inhibit T cell stimulation. Non-genetic approaches to deliver immune-cell modifying proteins include conjugation of peptides to a cell-penetrating peptide (CPP) and delivery in microparticles or nanoparticles. Enhancing tumour tropism of SCs can be achieved by overexpressing chemokine receptors, upregulating gene expression using epigenetic modifiers, altering SC characteristics using cationic peptides and irradiating tumours. b | The efficacy of SC-delivered therapeutic payloads can be enhanced by engineering bispecific molecules that simultaneously target multiple receptors; for example, epidermal growth factor receptor (EGFR)-specific nanobody (ENb) bound to tumour necrosis factor-related apoptosis-inducing ligand (TRAIL), or a CD20 specific single chain Fv antibody fragment (scFvCD20) bound to TRAIL. Alternatively, bimodal SCs can be created that express a combination of two or more secreted therapeutics, suicide and/or reporter genes. c | SCs that secrete therapeutic agents (for example, interleukin 12 (IL 12), TRAIL or oncolytic herpes simplex virus (oHSV)) can be encapsulated in synthetic extracellular matrix (sECM) to increase their therapeutic effectiveness. DR, death receptor.
Enhancing innate stem cell behaviour
To maximize the success of SC-based therapies, it is crucial that they survive in vivo until the entire cancer has been eliminated and that they successfully migrate towards malignancies. Much effort is being spent in attempting to enhance these characteristics (FIG. 2a). As discussed earlier, allogeneic SCs are somewhat immune-evasive but are still rejected in an immunocompetent recipient, thereby limiting their therapeutic potential10. Torikai and colleagues84 used zinc finger nucleases to genetically edit human leukocyte antigen (HLA) class I in ESCs and demonstrated that these cells could escape lysis from HLA-restricted cytotoxic T lymphocytes. Indeed, the rapidly expanding use of genome editing technologies could be systematically applied to create genetically engineered allogeneic SCs with improved immune-evasive capabilities85,86. To prevent immune rejection of ESC-derived allografts, cytotoxic T lymphocyte-associated protein 4 (CTLA4)–immunoglobulin and programmed cell death 1 ligand 1 (PDL1) knock-in human ESCs (hESCs) were created to simultaneously disrupt T cell co-stimulatory pathways and activate T cell inhibitory pathways87. Interestingly, neither modification on its own conferred immune protection to hESC derivatives in vivo, and this highlights the necessity of a multifaceted approach to immune evasion.
Complementing the genetic manipulation of SCs is an abundance of non-genetic approaches that rely on the surface binding or intracellular loading of SCs with effector moieties. These are either directly immunosuppressive or modify the behaviour of the SC such that it adopts immunosuppressive qualities. For example, human MSCs can be loaded with microparticles containing chemicals that impart particular cellular characteristics to the targeted cells — modifying attributes such as secretion rate, differentiation potential and proliferative properties. Furthermore, surrounding cells can be influenced via a paracrine mechanism, opening up the possibility of driving an entire SC or stromal cell population towards a desired immunosuppressive phenotype88,89. Proteins that are conjugated to cell-penetrating peptides have recently been used to efficiently introduce genes of interest into SCs to manipulate their behaviour, thereby offering an additional tool to the SC-engineering repertoire32.
Enhancing the tumour-tropic potential of SCs has been achieved in various ways, many of which are built on augmenting migratory signalling pathways that already exist in the cell. Overexpressing chemokine receptors in SCs has proved to be successful in increasing chemokine-directed migration towards intracranial gliomas20,90,91. Recently, haematopoietic stem and progenitor cells were primed with cationic peptides to enhance their mobilization and homing, even in the presence of low SDF1 gradients92. The use of epigenetic modifiers was shown to promote tumour tropism of MSCs towards cancer cells in vitro93. In this study, histone deacetylase (HDAC) inhibitors were applied to MSCs, resulting in the upregulation of uPA expression and enhanced tumour-tropic behaviour93. It will be interesting to see whether these results, and promising in vitro experiments in general, can be translated to animal models. If so, the ‘priming’ of SCs with epigenetic modifiers or other modifying agents to enhance their homing abilities might have substantial clinical potential. Additionally, several groups have shown that SC tropism can be boosted by locally irradiating tumours, directed by inflammation within injured tissues94,95. This has clinical implications; as solid tumours in humans are often treated using radiotherapy, the tumour-targeting response of SC therapies following radiotherapy might also be improved as a convenient side-effect.
Enhancing stem cell efficacy using combination therapies: to sensitize or synergize?
Cancers comprise a heterogeneous population of cells that are genetically and epigenetically unstable96. In addition, cancerous cells can acquire resistance to chemotherapeutic agents, either intrinsically or during their evolution (acquired resistance), making it unlikely that a monotherapy would eradicate the disease97. Using a combination approach can provide a more effective means to treat malignancies (FIG. 2b). One strategy is to create SCs that simultaneously express and secrete different therapeutic agents that target multiple cancer pathways. Although the appropriate selection of tumour-specific targets and subsequent SC manipulation can be technically challenging, there are a growing number of studies using bimodal SCs or SCs that secrete bifunctional molecules. Examples of bimodal SCs include combining HSV-tk therapy with TRAIL in glioblastoma models55 and CD with IFNβ in glioblastoma37 and breast cancer98 models in mice. Bifunctional proteins that are able to target multiple antitumour pathways include a fusion of the pro-apoptotic TRAIL protein to an EGFR-specific nanobody (ENb; a small antibody fragment) to create an immunoconjugate (ENb–TRAIL) that can concurrently inhibit tumour growth and elicit apoptosis35. We have shown that delivery of ENb–TRAIL by NSCs significantly decreased tumour burden and prolonged survival in mouse models of highly malignant glioblastoma35. A similar strategy was used by engineering human umbilical cord MSCs to secrete a CD20-specific single chain Fv antibody fragment fused to TRAIL (scFvCD20–TRAIL)99. MSC delivery of scFvCD20–TRAIL was more efficacious than MSC delivery of TRAIL alone in a mouse model of non-Hodgkin’s lymphoma because it concomitantly inhibited proliferation and induced apoptosis specifically in cancer cells99.
To increase the effectiveness of SCs delivering a single therapy, many studies have combined an additional agent that synergizes with the SC therapy or sensitizes an otherwise resistant population to the locally delivered biological agent. For example, various classes of drug have been shown to synergize with SC-delivered TRAIL to potentiate its p53-independent pro-apoptotic mode of action. These include proteasome inhibitors such as bortezomib, HDAC inhibitors, genotoxic drugs such as cisplatin, the PI3K–mTOR inhibitor PI-103, short hairpin RNA (shRNA) and microRNA inhibitors33,100. Understanding the molecular mechanisms that underlie sensitization to TRAIL is complex and dependent on the exact mode of action of each agent. However, upregulation of death receptors, inhibition of apoptotic inhibitor proteins and activation of p53-dependent apoptosis can all contribute to this combination effect33. In another study, combining human bone marrow-derived MSCs expressing HSV-tk with valproic acid was shown to enhance the bystander effect by increasing the expression of gap junction proteins connexin 43 (also known as gap junction α1) and connexin 26 (also known as gap junction β2), thereby allowing enhanced cellular transmission of GCV-monophosphate in culture56. This response was translated in vivo; mice treated with valproic acid and GCV had significantly longer survival compared with mice treated with GCV alone56.
For SC therapies to be translated into the clinic, they must initially be incorporated into the current standard of care. Patients with glioblastoma are often treated by maximal surgical resection followed by ionizing radiation and temozolomide. Kim et al.101 demonstrated that MSCs expressing TRAIL were actually more effective at killing malignant glioblastoma in the presence of temozolomide. In another study, NSCs carrying an oncolytic virus were applied to a glioblastoma model in combination with radiation and temozolomide102. This combination was shown to prolong the survival of mice bearing patient-derived glioblastoma xenografts. Interestingly, when NSCs were administered prior to (instead of after) radiotherapy and temozolomide treatment, median mouse survival was increased by 30% (REF. 102). This result was attributed to the sensitizing effect of the oncolytic virus on glioblastoma cells towards radiotherapy, and it shows the importance of testing SC therapies within the context of the current standard of care for a given cancer.
Novel deployment of stem cells: encapsulation
The route of delivery can have a profound effect on the survival and antitumour efficacy of therapeutic SCs in vivo. For example, intranasal delivery of SCs is emerging as a novel non-invasive means for the treatment of brain tumours103,104. Another innovative strategy is the encapsulation of SCs that are implanted in vivo in tumour-bearing mice (FIG. 2c). Biodegradable hydrogels and synthetic extracellular matrix (sECM) materials composed of hyaluronic acid, alginate, agarose and other polymers allow encapsulation of cells within biocompatible and semipermeable scaffolds105. Owing to their ability to provide effective in vivo cell retention and a physiological environment that promotes cell survival and prevents immune responses, biodegradable hydrogels have been used to encapsulate SCs in various rodent cancer models106–108. Reagan et al.109 demonstrated the utility of encapsulating MSCs that were engineered to express TRAIL in porous, biocompatible silk scaffolds. Encapsulated MSCs expressing TRAIL were shown to reduce primary breast tumour growth and were also effective in mouse models of bone, lung and liver metastases109. Matrigel-encapsulated MSCs that were engineered to express modified IL-12 showed significant antitumour effects compared to non-encapsulated MSCs expressing modified IL-12 when they were administered intratumourally in mouse models of melanoma46. As the blood–brain barrier and vascular dysfunction in the tumour microenvironment are major impediments for the efficient delivery of therapeutic molecules to brain tumour cells, SC encapsulation is an important prospect for harnessing on-site delivery of tumour-specific agents. We have investigated a new approach to the treatment of glioblastoma by encapsulating therapeutic SCs in glioblastoma resection xenograft mouse models that recapitulate the clinical scenario of surgical debulking of glioblastoma110. Encapsulation of mouse and human MSCs and NSCs in sECM was shown to increase their retention in the resection cavity and permitted tumour-selective migration from the gel. Furthermore, when sECM-encapsulated SCs expressing pro-apoptotic TRAIL protein, or infected with oHSV–TRAIL, were placed in the glioblastoma tumour resection cavity, significantly increased survival of the mice was observed compared to resected mice implanted with encapsulated non-therapeutic SCs83,110. Collectively, these studies show the potential of SC encapsulation in preclinical studies and provide a platform for clinical translation in a broad spectrum of resected cancers.
Imaging stem cell fate and efficacy
To aid the clinical translation of SC-based therapies, it is necessary to determine their survival, fate and therapeutic efficacy once delivered to the patient. Several imaging modalities can be used to image SC behaviour in vivo. Optical imaging approaches have been used to assess various SC characteristics when implanted within small-animal cancer models. SCs that are engineered to stably co-express fluorescent and bioluminescent reporter constructs can be visualized using dual bioluminescence and intravital fluorescence microscopy34,110. When tumours are additionally labelled, it is possible to longitudinally image the whole process of tumour formation, SC migration and tumour killing. Subsequent histological analysis permits SC localization at a cellular level, making this a powerful approach15,34,110.
When moving to larger animal models or humans, optical imaging techniques are limited by the depth of tissue penetration, necessitating the need to explore other non-invasive imaging modalities. The bulk of these studies are currently being tested in mouse xenograft models. For example, magnetic resonance imaging (MRI) has been used to detect SCs that have been loaded with superparamagnetic particles to determine tumour tropism and therapeutic impact111–114. MRI detection of ferumoxytol-labelled human NSCs demonstrated their safety, feasibility and usefulness in mice, and contributed to the US Food and Drug Administration (FDA) allowing the investigation of this method in a first in human Phase I clinical trial (ClinicalTrials.gov identifier: NCT01172964)114. Positron emission tomography (PET) has also been applied to measure SC fate and systemic distribution115. Interestingly, HSV-tk can be used as a marker for PET imaging in combination with various radioactive substrates, and SCs expressing this gene have been non-invasively imaged at high resolution55,116. As new contrast agents are developed and imaging techniques are refined, the ability to record SC behaviour in vivo will continue to improve. This should result in a better biological understanding, which is necessary to improve the clinical success of SC-based therapies in the future.
Therapeutic stem cells in clinical trials
Despite the large number and diversity of preclinical studies using engineered SCs as therapeutic agents, their current use in clinical trials is rare. Of the existing trials, the majority involve treatment of recurrent glioblastoma, possibly reflecting the dire prognosis of this disease and the need to test novel approaches. A handful of trials are currently or will shortly be testing MSCs or NSCs that are modified to have active antitumour activity. Genexine, Inc. are sponsoring NCT02079324, which intends to establish the safety and efficacy of MSCs expressing IL-12 (GX-051) that are administered intratumourally in patients with advanced head and neck cancer. Garcia-Castro and colleagues69 explored the safety and efficacy of infusing several doses of autologous MSCs loaded with oncolytic adenovirus (ICOVIR-5) in four children with metastatic neuroblastoma who were resistant to front-line therapies. A complete clinical response was documented in one case, with the child in remission for three years post-therapy. In another trial, allogeneic immortalized NSCs that are modified to express CD are being tested in recurrent glioblastoma (ClinicalTrials.gov identifier: NCT01172964). Following surgical resection of the tumour mass, modified NSCs are injected into the resection margins and patients are treated with the oral prodrug 5-FC. Although this trial is ongoing, two more complementary trials have been approved that combine therapy with NSCs expressing CD with either leucovorin calcium (ClinicalTrials.gov identifier: NCT02015819) or irinotecan hydrochloride (ClinicalTrials.gov identifier: NCT02055196) to further sensitize glioblastoma cells. These trials are not yet recruiting patients and are probably awaiting results from the initial trial regarding the safety and efficacy of this approach.
Perspectives
Considering the extensive success of anticancer SCs in preclinical studies, why is there such a paucity of clinical trials using these cells? As witnessed by the protracted integration of other cutting-edge treatments into clinical practice, nascent therapeutic technologies require considerable understanding of fundamental mechanisms before they can be delivered with confidence. Although the ability to phenotypically tailor and image SCs has entered an exciting phase in the laboratory, their clinical translation is lagging. Even reaching a consensus on precisely defining the characteristics of a given type of SC has hindered progress117. As safety is of paramount importance, concerns should be resolved and clinical trials should proceed with due caution TABLE 2).
Table 2.
Hurdles on the way to the clinic
| Consideration | Reason | Finding a solution |
|---|---|---|
| Optimal SC type | SCs vary greatly, even within the same class of SC | Thorough molecular and functional characterization is required |
| Quality control | Minimize variability to ensure homogeneity of SC population | Good manufacturing practice-compliant isolation and culture |
| Route of administration or cell dosing | Maximize in vivo effectiveness | Rigorous preclinical characterization and appropriate scaling from animal to human required |
| Tumorigenicity or cell fate | Avoid the occurrence of therapeutic SCs forming tumours or aberrantly differentiating in host |
|
| Poor engraftment or survival | Efficacy of therapy will be impaired if SCs do not survive or are poorly engrafted in recipient |
|
| Funding | Stopping a clinical trial midway could jeopardize patient health and hinder medical benefit | Make sure costs are carefully considered and the solvency of funding sources are verified |
| Ensure modifications to SCs maintain their characteristics | Therapeutic modifications might adversely affect performance and/or safety of SCs | Test modified SCs for inappropriate genomic modifications and verify functionality in a preclinical context |
| Therapeutic SCs or secreted proteins breaking host tolerance to self-antigens | Evoking an immune response might impair effectiveness of therapy and provoke additional complications in the patient |
|
SC, stem cell.
A greater biological understanding of patient-derived tumours will improve the chances of therapeutic success by yielding new therapeutic targets and enabling mechanism-based SC therapies to be developed in response to a specific cancer profile118. Instead of stratifying patients with respect to a given therapy, the therapeutic SC itself could be engineered for optimal efficacy. The implementation of clinically relevant animal models would aid in the systematic testing and refining of SC-based therapies. Currently, models typically rely on the orthotopic introduction of established human cancer cell lines in an immunocompromised host. This situation poorly reflects the clinical scenario, especially if the therapy has an immunomodulatory component. Although no perfect animal models exist, caveats should be minimized. Using genetically engineered mouse models in which tumours arise in situ, or incorporating patient-derived tumours and/or introducing surgical resection into solid tumour studies in mice are approaches that might hold more validity and should be explored.
To conclude, SC-based antitumour therapies offer tremendous promise for the treatment of cancer. Although patients that are refractory to the current standard of care may well benefit from this novel approach, eagerness to rush through clinical trials might jeopardize their health and the integrity of the SC field. Preclinical fervour should be tempered with caution during this precarious phase, and clinical trials should be carefully considered and have rigorous scientific backing.
Acknowledgements
The authors apologize to all colleagues whose work could not be cited owing to space limitations. This work was supported by grants R01CA138922, R01CA173077, and the James S. McDonnell Foundation.
Footnotes
Competing interests statement
The authors declare no competing interests.
DATABASES
ClinicalTrials.gov: https://clinicaltrials.gov/ NCT01172964 | NCT02079324 | NCT01172964 | NCT02015819 | NCT02055196
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
Contributor Information
Daniel W. Stuckey, Molecular Neurotherapy and Imaging Laboratory and the Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts 02114, USA.
Khalid Shah, Molecular Neurotherapy and Imaging Laboratory and the Departments of Radiology and Neurology, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts 02114, USA; and the Harvard Stem Cell Institute, Harvard University, Cambridge, Massachusetts 02138, USA..
References
- 1.Abbott A. Leaked files slam stem-cell therapy. Nature. 2014;505:139–140. doi: 10.1038/505139a. [DOI] [PubMed] [Google Scholar]
- 2.Jones BJ, McTaggart SJ. Immunosuppression by mesenchymal stromal cells: from culture to clinic. Exp. Hematol. 2008;36:733–741. doi: 10.1016/j.exphem.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 3.Bernardo ME, Fibbe WE. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell. 2013;13:392–402. doi: 10.1016/j.stem.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Milwid JM, et al. Enriched protein screening of human bone marrow mesenchymal stromal cell secretions reveals MFAP5 and PENK as novel IL-10 modulators. Mol. Ther. 2014;22:999–1007. doi: 10.1038/mt.2014.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muraoka K, et al. The high integration and differentiation potential of autologous neural stem cell transplantation compared with allogeneic transplantation in adult rat hippocampus. Exp. Neurol. 2006;199:311–327. doi: 10.1016/j.expneurol.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Zhao T, Zhang ZN, Rong Z, Xu Y. Immunogenicity of induced pluripotent stem cells. Nature. 2011;474:212–215. doi: 10.1038/nature10135. [DOI] [PubMed] [Google Scholar]
- 7.Eliopoulos N, Stagg J, Lejeune L, Pommey S, Galipeau J. Allogeneic marrow stromal cells are immune rejected by MHC class I- and class II mismatched recipient mice. Blood. 2005;106:4057–4065. doi: 10.1182/blood-2005-03-1004. [DOI] [PubMed] [Google Scholar]
- 8.Zangi L, et al. Direct imaging of immune rejection and memory induction by allogeneic mesenchymal stromal cells. Stem Cells. 2009;27:2865–2874. doi: 10.1002/stem.217. [DOI] [PubMed] [Google Scholar]
- 9.Schu S, et al. Immunogenicity of allogeneic mesenchymal stem cells. J. Cell. Mol. Med. 2012;16:2094–2103. doi: 10.1111/j.1582-4934.2011.01509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: immune evasive, not immune privileged. Nature Biotech. 2014;32:252–260. doi: 10.1038/nbt.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aboody KS, et al. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc. Natl Acad. Sci. USA. 2000;97:12846–12851. doi: 10.1073/pnas.97.23.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown AB, et al. Intravascular delivery of neural stem cell lines to target intracranial and extracranial tumors of neural and non-neural origin. Hum. Gene Ther. 2003;14:1777–1785. doi: 10.1089/104303403322611782. [DOI] [PubMed] [Google Scholar]
- 13.Tang Y, et al. In vivo tracking of neural progenitor cell migration to glioblastomas. Hum. Gene Ther. 2003;14:1247–1254. doi: 10.1089/104303403767740786. [DOI] [PubMed] [Google Scholar]
- 14.Yang J, et al. Tumor tropism of intravenously injected human-induced pluripotent stem cell-derived neural stem cells and their gene therapy application in a metastatic breast cancer model. Stem Cells. 2012;30:1021–1029. doi: 10.1002/stem.1051. [DOI] [PubMed] [Google Scholar]
- 15.Shah K, et al. Glioma therapy and real-time imaging of neural precursor cell migration and tumor regression. Ann. Neurol. 2005;57:34–41. doi: 10.1002/ana.20306. [DOI] [PubMed] [Google Scholar]
- 16.Gondi CS, et al. Human umbilical cord blood stem cells show PDGF-D-dependent glioma cell tropismin vitro andin vivo. Neuro Oncol. 2010;12:453–465. doi: 10.1093/neuonc/nop049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suarez-Alvarez B, Lopez-Vazquez A, Lopez- Larrea C. Mobilization and homing of hematopoietic stem cells. Adv. Exp. Med. Biol. 2012;741:152–170. doi: 10.1007/978-1-4614-2098-9_11. [DOI] [PubMed] [Google Scholar]
- 18.Shi M, et al. Regulation of CXCR4 expression in human mesenchymal stem cells by cytokine treatment: role in homing efficiency in NOD/SCID mice. Haematologica. 2007;92:897–904. doi: 10.3324/haematol.10669. [DOI] [PubMed] [Google Scholar]
- 19.Wynn RF, et al. A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood. 2004;104:2643–2645. doi: 10.1182/blood-2004-02-0526. [DOI] [PubMed] [Google Scholar]
- 20.Park SA, et al. CXCR4-transfected human umbilical cord blood-derived mesenchymal stem cells exhibit enhanced migratory capacity toward gliomas. Int. J. Oncol. 2011;38:97–103. [PubMed] [Google Scholar]
- 21.Guo Y, Hangoc G, Bian H, Pelus LM, Broxmeyer HE. SDF-1/CXCL12 enhances survival and chemotaxis of murine embryonic stem cells and production of primitive and definitive hematopoietic progenitor cells. Stem Cells. 2005;23:1324–1332. doi: 10.1634/stemcells.2005-0085. [DOI] [PubMed] [Google Scholar]
- 22.Koizumi S, et al. Migration of mouse-induced pluripotent stem cells to glioma-conditioned medium is mediated by tumor-associated specific growth factors. Oncol. Lett. 2011;2:283–288. doi: 10.3892/ol.2011.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kendall SE, et al. Neural stem cell targeting of glioma is dependent on phosphoinositide 3-kinase signaling. Stem Cells. 2008;26:1575–1586. doi: 10.1634/stemcells.2007-0887. [DOI] [PubMed] [Google Scholar]
- 24.Vallabhaneni KC, et al. Urokinase receptor mediates mobilization, migration, and differentiation of mesenchymal stem cells. Cardiovasc. Res. 2011;90:113–121. doi: 10.1093/cvr/cvq362. [DOI] [PubMed] [Google Scholar]
- 25.Gutova M, et al. Urokinase plasminogen activator and urokinase plasminogen activator receptor mediate human stem cell tropism to malignant solid tumors. Stem Cells. 2008;26:1406–1413. doi: 10.1634/stemcells.2008-0141. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt NO, et al. Brain tumor tropism of transplanted human neural stem cells is induced by vascular endothelial growth factor. Neoplasia. 2005;7:623–629. doi: 10.1593/neo.04781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ho IA, et al. Matrix metalloproteinase 1 is necessary for the migration of human bone marrow-derived mesenchymal stem cells toward human glioma. Stem Cells. 2009;27:1366–1375. doi: 10.1002/stem.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Motaln H, et al. Human mesenchymal stem cells exploit the immune response mediating chemokines to impact the phenotype of glioblastoma. Cell Transplant. 2012;21:1529–1545. doi: 10.3727/096368912X640547. [DOI] [PubMed] [Google Scholar]
- 29.Schichor C, et al. Mesenchymal stem cells and glioma cells form a structural as well as a functional syncytium. in vitro. Exp. Neurol. 2012;234:208–219. doi: 10.1016/j.expneurol.2011.12.033. [DOI] [PubMed] [Google Scholar]
- 30.Qiao L, et al. Suppression of tumorigenesis by human mesenchymal stem cells in a hepatoma model. Cell Res. 2008;18:500–507. doi: 10.1038/cr.2008.40. [DOI] [PubMed] [Google Scholar]
- 31.Hu YL, et al. Mesenchymal stem cells as a novel carrier for targeted delivery of gene in cancer therapy based on nonviral transfection. Mol. Pharm. 2012;9:2698–2709. doi: 10.1021/mp300254s. [DOI] [PubMed] [Google Scholar]
- 32.Jo J, Hong S, Choi WY, Lee DR. Cell-penetrating peptide (CPP)-conjugated proteins is an efficient tool for manipulation of human mesenchymal stromal cells. Sci. Rep. 2014;4:4378. doi: 10.1038/srep04378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stuckey DW, Shah K. TRAIL on trial: preclinical advances in cancer therapy. Trends Mol. Med. 2013;19:685–694. doi: 10.1016/j.molmed.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sasportas LS, et al. Assessment of therapeutic efficacy and fate of engineered human mesenchymal stem cells for cancer therapy. Proc. Natl Acad. Sci. USA. 2009;106:4822–4827. doi: 10.1073/pnas.0806647106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van de Water JA, et al. Therapeutic stem cells expressing variants of EGFR-specific nanobodies have antitumor effects. Proc. Natl Acad. Sci. USA. 2012;109:16642–16647. doi: 10.1073/pnas.1202832109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Balyasnikova IV, Ferguson SD, Sengupta S, Han Y, Lesniak MS. Mesenchymal stem cells modified with a single-chain antibody against EGFRvIII successfully inhibit the growth of human xenograft malignant glioma. PLoS ONE. 2010;5:e9750. doi: 10.1371/journal.pone.0009750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ito S, et al. Human neural stem cells transduced with IFN-β and cytosine deaminase genes intensify bystander effect in experimental glioma. Cancer Gene Ther. 2010;17:299–306. doi: 10.1038/cgt.2009.80. [DOI] [PubMed] [Google Scholar]
- 38.Studeny M, et al. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-β delivery into tumors. Cancer Res. 2002;62:3603–3608. [PubMed] [Google Scholar]
- 39.Ren C, et al. Cancer gene therapy using mesenchymal stem cells expressing interferon-β in a mouse prostate cancer lung metastasis model. Gene Ther. 2008;15:1446–1453. doi: 10.1038/gt.2008.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dembinski JL, et al. Tumor stroma engraftment of gene-modified mesenchymal stem cells as anti-tumor therapy against ovarian cancer. Cytotherapy. 2013;15:20–32. doi: 10.1016/j.jcyt.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ren C, et al. Therapeutic potential of mesenchymal stem cells producing interferon-α in a mouse melanoma lung metastasis model. Stem Cells. 2008;26:2332–2338. doi: 10.1634/stemcells.2008-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Eekelen M, et al. Human stem cells expressing novel TSP-1 variant have anti-angiogenic effect on brain tumors. Oncogene. 2010;29:3185–3195. doi: 10.1038/onc.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim SK, et al. PEX-producing human neural stem cells inhibit tumor growth in a mouse glioma model. Clin. Cancer Res. 2005;11:5965–5970. doi: 10.1158/1078-0432.CCR-05-0371. [DOI] [PubMed] [Google Scholar]
- 44.Gajewski TF, et al. Cancer immunotherapy strategies based on overcoming barriers within the tumor microenvironment. Curr. Opin. Immunol. 2013;25:268–276. doi: 10.1016/j.coi.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 45.Gao P, Ding Q, Wu Z, Jiang H, Fang Z. Therapeutic potential of human mesenchymal stem cells producing IL-12 in a mouse xenograft model of renal cell carcinoma. Cancer Lett. 2010;290:157–166. doi: 10.1016/j.canlet.2009.08.031. [DOI] [PubMed] [Google Scholar]
- 46.Seo SH, et al. The effects of mesenchymal stem cells injected via different routes on modified IL-12 mediated antitumor activity. Gene Ther. 2011;18:488–495. doi: 10.1038/gt.2010.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ryu CH, et al. Gene therapy of intracranial glioma using interleukin 12 secreting human umbilical cord blood-derived mesenchymal stem cells. Hum. Gene Ther. 2011;22:733–743. doi: 10.1089/hum.2010.187. [DOI] [PubMed] [Google Scholar]
- 48.Hong X, Miller C, Savant-Bhonsale S, Kalkanis SN. Antitumor treatment using interleukin-12 secreting marrow stromal cells in an invasive glioma model. Neurosurgery. 2009;64:1139–1146. doi: 10.1227/01.NEU.0000345646.85472.EA. discussion 1146–1147. [DOI] [PubMed] [Google Scholar]
- 49.Xu G, et al. Adenoviral-mediated interleukin-18 expression in mesenchymal stem cells effectively suppresses the growth of glioma in rats. Cell Biol. Int. 2009;33:466–474. doi: 10.1016/j.cellbi.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 50.Kosaka H, et al. Therapeutic effect of suicide gene-transferred mesenchymal stem cells in a rat model of glioma. Cancer Gene Ther. 2012;19:572–578. doi: 10.1038/cgt.2012.35. [DOI] [PubMed] [Google Scholar]
- 51.Zhao Y, et al. Targeted suicide gene therapy for glioma using human embryonic stem cell-derived neural stem cells genetically modified by baculoviral vectors. Gene Ther. 2012;19:189–200. doi: 10.1038/gt.2011.82. [DOI] [PubMed] [Google Scholar]
- 52.Aboody KS, et al. Neural stem cell-mediated enzyme/prodrug therapy for glioma: preclinical studies. Sci. Transl. Med. 2013;5:184ra59. doi: 10.1126/scitranslmed.3005365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Altaner C, et al. Complete regression of glioblastoma by mesenchymal stem cells mediated prodrug gene therapy simulating clinical therapeutic scenario. Int. J. Cancer. 2014;134:1458–1465. doi: 10.1002/ijc.28455. [DOI] [PubMed] [Google Scholar]
- 54.Kim SK, et al. Human neural stem cells target experimental intracranial medulloblastoma and deliver a therapeutic gene leading to tumor regression. Clin. Cancer Res. 2006;12:5550–5556. doi: 10.1158/1078-0432.CCR-05-2508. [DOI] [PubMed] [Google Scholar]
- 55.Martinez-Quintanilla J, et al. Therapeutic efficacy and fate of bimodal engineered stem cells in malignant brain tumors. Stem Cells. 2013;31:1706–1714. doi: 10.1002/stem.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ryu CH, et al. Valproic acid enhances anti-tumor effect of mesenchymal stem cell mediated HSV-TK gene therapy in intracranial glioma. Biochem. Biophys. Res. Commun. 2012;421:585–590. doi: 10.1016/j.bbrc.2012.04.050. [DOI] [PubMed] [Google Scholar]
- 57.Lee WY, et al. Immortalized human fetal bone marrow-derived mesenchymal stromal cell expressing suicide gene for anti-tumor therapy in vitro and in vivo. Cytotherapy. 2013;15:1484–1497. doi: 10.1016/j.jcyt.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 58.Kim KY, Kim SU, Leung PC, Jeung EB, Choi KC. Influence of the prodrugs 5-fluorocytosine and CPT-11 on ovarian cancer cells using genetically engineered stem cells: tumor-tropic potential and inhibition of ovarian cancer cell growth. Cancer Sci. 2010;101:955–962. doi: 10.1111/j.1349-7006.2009.01485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hong SH, et al. Human neural stem cells expressing carboxyl esterase target and inhibit tumor growth of lung cancer brain metastases. Cancer Gene Ther. 2013;20:678–682. doi: 10.1038/cgt.2013.69. [DOI] [PubMed] [Google Scholar]
- 60.Gutova M, et al. Neural stem cell-mediated CE/CPT-11 enzyme/prodrug therapy in transgenic mouse model of intracerebellar medulloblastoma. Gene Ther. 2013;20:143–150. doi: 10.1038/gt.2012.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bertrand N, Wu J, Xu X, Kamaly N, Farokhzad OC. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv. Drug. Deliv. Rev. 2014;66:2–25. doi: 10.1016/j.addr.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roger M, et al. Mesenchymal stem cells as cellular vehicles for delivery of nanoparticles to brain tumors. Biomaterials. 2010;31:8393–8401. doi: 10.1016/j.biomaterials.2010.07.048. [DOI] [PubMed] [Google Scholar]
- 63.Auffinger B, et al. Drug-loaded nanoparticle systems and adult stem cells: a potential marriage for the treatment of malignant glioma? Oncotarget. 2013;4:378–396. doi: 10.18632/oncotarget.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li L, et al. Silica nanorattle-doxorubicin-anchored mesenchymal stem cells for tumor-tropic therapy. ACS Nano. 2011;5:7462–7470. doi: 10.1021/nn202399w. [DOI] [PubMed] [Google Scholar]
- 65.Duchi S, et al. Mesenchymal stem cells as delivery vehicle of porphyrin loaded nanoparticles: effective photoinduced in vitro killing of osteosarcoma. J. Control Release. 2013;168:225–237. doi: 10.1016/j.jconrel.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 66.Schnarr K, et al. Gold nanoparticle-loaded neural stem cells for photothermal ablation of cancer. Adv. Healthc. Mater. 2013;2:976–982. doi: 10.1002/adhm.201300003. [DOI] [PubMed] [Google Scholar]
- 67.Rachakatla RS, et al. Attenuation of mouse melanoma by A/C magnetic field after delivery of bi magnetic nanoparticles by neural progenitor cells. ACS Nano. 2010;4:7093–7104. doi: 10.1021/nn100870z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aghi M, Martuza RL. Oncolytic viral therapies - the clinical experience. Oncogene. 2005;24:7802–7816. doi: 10.1038/sj.onc.1209037. [DOI] [PubMed] [Google Scholar]
- 69.Garcia-Castro J, et al. Treatment of metastatic neuroblastoma with systemic oncolytic virotherapy delivered by autologous mesenchymal stem cells: an exploratory study. Cancer Gene Ther. 2010;17:476–483. doi: 10.1038/cgt.2010.4. [DOI] [PubMed] [Google Scholar]
- 70.Komarova S, Kawakami Y, Stoff-Khalili MA, Curiel DT, Pereboeva L. Mesenchymal progenitor cells as cellular vehicles for delivery of oncolytic adenoviruses. Mol. Cancer Ther. 2006;5:755–766. doi: 10.1158/1535-7163.MCT-05-0334. [DOI] [PubMed] [Google Scholar]
- 71.Kranzler J, Tyler MA, Sonabend AM, Ulasov IV, Lesniak MS. Stem cells as delivery vehicles for oncolytic adenoviral virotherapy. Curr. Gene Ther. 2009;9:389–395. doi: 10.2174/156652309789753347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ahmed AU, et al. Bone marrow mesenchymal stem cells loaded with an oncolytic adenovirus suppress the anti-adenoviral immune response in the cotton rat model. Mol. Ther. 2010;18:1846–1856. doi: 10.1038/mt.2010.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stoff-Khalili MA, et al. Mesenchymal stem cells as a vehicle for targeted delivery of CRAds to lung metastases of breast carcinoma. Breast Cancer Res. Treat. 2007;105:157–167. doi: 10.1007/s10549-006-9449-8. [DOI] [PubMed] [Google Scholar]
- 74.Sonabend AM, et al. Mesenchymal stem cells effectively deliver an oncolytic adenovirus to intracranial glioma. Stem Cells. 2008;26:831–841. doi: 10.1634/stemcells.2007-0758. [DOI] [PubMed] [Google Scholar]
- 75.Yong RL, et al. Human bone marrow-derived mesenchymal stem cells for intravascular delivery of oncolytic adenovirus Delta24-RGD to human gliomas. Cancer Res. 2009;69:8932–8940. doi: 10.1158/0008-5472.CAN-08-3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ahmed AU, et al. A comparative study of neural and mesenchymal stem cell-based carriers for oncolytic adenovirus in a model of malignant glioma. Mol. Pharm. 2011;8:1559–1572. doi: 10.1021/mp200161f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mader EK, et al. Optimizing patient derived mesenchymal stem cells as virus carriers for a phase clinical trial in ovarian cancer. J. Transl. Med. 2013;11:20. doi: 10.1186/1479-5876-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ong HT, et al. Systemically delivered measles virus-infected mesenchymal stem cells can evade host immunity to inhibit liver cancer growth. J. Hepatol. 2013;59:999–1006. doi: 10.1016/j.jhep.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hoffmann D, Wildner O. Comparison of herpes simplex virus- and conditionally replicative adenovirus-based vectors for glioblastoma treatment. Cancer Gene Ther. 2007;14:627–639. doi: 10.1038/sj.cgt.7701055. [DOI] [PubMed] [Google Scholar]
- 80.Markert JM, et al. Phase Ib trial of mutant herpes simplex virus G207 inoculated pre-and post-tumor resection for recurrent GBM. Mol. Ther. 2009;17:199–207. doi: 10.1038/mt.2008.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Harrow S, et al. HSV1716 injection into the brain adjacent to tumour following surgical resection of high-grade glioma: safety data and long-term survival. Gene Ther. 2004;11:1648–1658. doi: 10.1038/sj.gt.3302289. [DOI] [PubMed] [Google Scholar]
- 82.Papanastassiou V, et al. The potential for efficacy of the modified (ICP 34.5(-)) herpes simplex virus HSV1716 following intratumoural injection into human malignant glioma: a proof of principle study. Gene Ther. 2002;9:398–406. doi: 10.1038/sj.gt.3301664. [DOI] [PubMed] [Google Scholar]
- 83.Duebgen M, et al. Stem cells loaded with multimechanistic oncolytic herpes simplex virus variants for brain tumor therapy. J. Natl Cancer Inst. 2014;106:dju090. doi: 10.1093/jnci/dju090. [DOI] [PubMed] [Google Scholar]
- 84.Torikai H, et al. Toward eliminating HLA class I expression to generate universal cells from allogeneic donors. Blood. 2013;122:1341–1349. doi: 10.1182/blood-2013-03-478255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sander JD, Joung JK. CRISPR-Cas systems for editing, regulating and targeting genomes. Nature Biotech. 2014;32:347–355. doi: 10.1038/nbt.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mussolino C, Cathomen T. TALE nucleases: tailored genome engineering made easy. Curr. Opin. Biotechnol. 2012;23:644–650. doi: 10.1016/j.copbio.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 87.Rong Z, et al. An effective approach to prevent immune rejection of human ESC-derived allografts. Cell Stem Cell. 2014;14:121–130. doi: 10.1016/j.stem.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ankrum JA, et al. Engineering cells with intracellular agent-loaded microparticles to control cell phenotype. Nature Protoc. 2014;9:233–245. doi: 10.1038/nprot.2014.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sarkar D, Ankrum JA, Teo GS, Carman CV, Karp JM. Cellular and extracellular programming of cell fate through engineered intracrine-, paracrine-, and endocrine-like mechanisms. Biomaterials. 2011;32:3053–3061. doi: 10.1016/j.biomaterials.2010.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Honeth G, Staflin K, Kalliomaki S, Lindvall M, Kjellman C. Chemokine-directed migration of tumor-inhibitory neural progenitor cells towards an intracranially growing glioma. Exp. Cell Res. 2006;312:1265–1276. doi: 10.1016/j.yexcr.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 91.Kim DS, et al. Overexpression of CXC chemokine receptors is required for the superior glioma-tracking property of umbilical cord blood-derived mesenchymal stem cells. Stem Cells Dev. 2009;18:511–519. doi: 10.1089/scd.2008.0050. [DOI] [PubMed] [Google Scholar]
- 92.Ratajczak MZ, et al. A novel perspective on stem cell homing and mobilization: review on bioactive lipids as potent chemoattractants and cationic peptides as underappreciated modulators of responsiveness to SDF-1 gradients. Leukemia. 2012;26:63–72. doi: 10.1038/leu.2011.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pulukuri SM, Gorantla B, Dasari VR, Gondi CS, Rao JS. Epigenetic upregulation of urokinase plasminogen activator promotes the tropism of mesenchymal stem cells for tumor cells. Mol. Cancer Res. 2010;8:1074–1083. doi: 10.1158/1541-7786.MCR-09-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zielske SP, Livant DL, Lawrence TS. Radiation increases invasion of gene-modified mesenchymal stem cells into tumors. Int. J. Radiat. Oncol. Biol. Phys. 2009;75:843–853. doi: 10.1016/j.ijrobp.2008.06.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Klopp AH, et al. Tumor irradiation increases the recruitment of circulating mesenchymal stem cells into the tumor microenvironment. Cancer Res. 2007;67:11687–11695. doi: 10.1158/0008-5472.CAN-07-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Meacham CE, Morrison SJ. Tumour heterogeneity and cancer cell plasticity. Nature. 2013;501:328–337. doi: 10.1038/nature12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hanahan D. Rethinking the war on cancer. Lancet. 2014;383:558–563. doi: 10.1016/S0140-6736(13)62226-6. [DOI] [PubMed] [Google Scholar]
- 98.Yi BR, et al. Selective antitumor effect of neural stem cells expressing cytosine deaminase and interferon-β against ductal breast cancer cells in cellular and xenograft models. Stem Cell Res. 2014;12:36–48. doi: 10.1016/j.scr.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 99.Yan C, et al. Human umbilical cord mesenchymal stem cells as vehicles of CD20-specific TRAIL fusion protein delivery: a double-target therapy against non-Hodgkin’s lymphoma. Mol. Pharm. 2013;10:142–151. doi: 10.1021/mp300261e. [DOI] [PubMed] [Google Scholar]
- 100.Du W, Uslar L, Sevala S, Shah K. Targeting c-Met receptor overcomes TRAIL-resistance in brain tumors. PLoS ONE. 2014;9:e95490. doi: 10.1371/journal.pone.0095490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim SM, et al. Potential application of temozolomide in mesenchymal stem cell-based TRAIL gene therapy against malignant glioma. Stem Cells Transl. Med. 2014;3:172–182. doi: 10.5966/sctm.2013-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tobias AL, et al. The timing of neural stem cell-based virotherapy is critical for optimal therapeutic efficacy when applied with radiation and chemotherapy for the treatment of glioblastoma. Stem Cells Transl. Med. 2013;2:655–666. doi: 10.5966/sctm.2013-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Reitz M, et al. Intranasal delivery of neural stem/ progenitor cells: a noninvasive passage to target intracerebral glioma. Stem Cells Transl. Med. 2012;1:866–873. doi: 10.5966/sctm.2012-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Balyasnikova IV, et al. Intranasal delivery of mesenchymal stem cells significantly extends survival of irradiated mice with experimental brain tumors. Mol. Ther. 2014;22:140–148. doi: 10.1038/mt.2013.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Burdick JA, Prestwich GD. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 2011;23:H41–H56. doi: 10.1002/adma.201003963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hansen K, et al. A 3 dimensional extracellular matrix as a delivery system for the transplantation of glioma-targeting neural stem/progenitor cells. Neuro Oncol. 2010;12:645–654. doi: 10.1093/neuonc/noq002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Goren A, Dahan N, Goren E, Baruch L, Machluf M. Encapsulated human mesenchymal stem cells: a unique hypoimmunogenic platform for long-term cellular therapy. FASEB J. 2010;24:22–31. doi: 10.1096/fj.09-131888. [DOI] [PubMed] [Google Scholar]
- 108.Rihova B. Immunocompatibility and biocompatibility of cell delivery systems. Adv. Drug Deliv. Rev. 2000;42:65–80. doi: 10.1016/s0169-409x(00)00054-5. [DOI] [PubMed] [Google Scholar]
- 109.Reagan MR, et al. Stem cell implants for cancer therapy: TRAIL-expressing mesenchymal stem cells target cancer cells in situ. J. Breast Cancer. 2012;15:273–282. doi: 10.4048/jbc.2012.15.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kauer TM, Figueiredo JL, Hingtgen S, Shah K. Encapsulated therapeutic stem cells implanted in the tumor resection cavity induce cell death in gliomas. Nature Neurosci. 2012;15:197–204. doi: 10.1038/nn.3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Menon LG, et al. Imaging of human mesenchymal stromal cells: homing to human brain tumors. J. Neurooncol. 2012;107:257–267. doi: 10.1007/s11060-011-0754-7. [DOI] [PubMed] [Google Scholar]
- 112.Thu MS, et al. Iron labeling and pre-clinical MRI visualization of therapeutic human neural stem cells in a murine glioma model. PLoS ONE. 2009;4:e7218. doi: 10.1371/journal.pone.0007218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chien LY, et al. In vivo magnetic resonance imaging of cell tropism, trafficking mechanism, and therapeutic impact of human mesenchymal stem cells in a murine glioma model. Biomaterials. 2011;32:3275–3284. doi: 10.1016/j.biomaterials.2011.01.042. [DOI] [PubMed] [Google Scholar]
- 114.Gutova M, et al. Magnetic resonance imaging tracking of ferumoxytol-labeled human neural stem cells: studies leading to clinical use. Stem Cells Transl. Med. 2013;2:766–775. doi: 10.5966/sctm.2013-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hasenbach K, et al. Monitoring the glioma tropism of bone marrow-derived progenitor cells by 2-photon laser scanning microscopy and positron emission tomography. Neuro Oncol. 2012;14:471–481. doi: 10.1093/neuonc/nor228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sweeney TJ, et al. Visualizing the kinetics of tumor-cell clearance in living animals. Proc. Natl Acad. Sci. USA. 1999;96:12044–12049. doi: 10.1073/pnas.96.21.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bianco P, et al. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nature Med. 2013;19:35–42. doi: 10.1038/nm.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Garraway LA, Lander ES. Lessons from the cancer genome. Cell. 2013;153:17–37. doi: 10.1016/j.cell.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 119.Karnoub AE, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 120.Yang T, et al. Activation of mesenchymal stem cells by macrophages prompts human gastric cancer growth through NF-κB pathway. PLoS ONE. 2014;9:e97569. doi: 10.1371/journal.pone.0097569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rowan BG, et al. Human adipose tissue-derived stromal/stem cells promote migration and early metastasis of triple negative breast cancer xenografts. PLoS ONE. 2014;9:e89595. doi: 10.1371/journal.pone.0089595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Amariglio N, et al. Donor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS Med. 2009;6:e1000029. doi: 10.1371/journal.pmed.1000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rosland GV, et al. Long-term cultures of bone marrow-derived human mesenchymal stem cells frequently undergo spontaneous malignant transformation. Cancer Res. 2009;69:5331–5339. doi: 10.1158/0008-5472.CAN-08-4630. [DOI] [PubMed] [Google Scholar]
- 124.Lee AS, Tang C, Rao MS, Weissman IL, Wu JC. Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. Nature Med. 2013;19:998–1004. doi: 10.1038/nm.3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ring KL, et al. Direct reprogramming of mouse and human fibroblasts into multipotent neural stem cells with a single factor. Cell Stem Cell. 2012;11:100–109. doi: 10.1016/j.stem.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]