Abstract
Background
Men with biochemical recurrence (BCR) of prostate cancer are typically observed or treated with androgen deprivation therapy. Non-hormonal, non-toxic treatments to slow the rise of PSA are desirable. We studied a combination herbal supplement, Prostate Health Cocktail (PHC), in prostate cancer cell lines and in a population of men with BCR.
Methods
PC3, LAPC3, and LNCaP cells were incubated with increasing concentrations of PHC suspension. Men previously treated for prostate cancer with surgery, radiation, or both with rising PSA but no radiographic metastases were treated with 3 capsules of PHC daily; the primary endpoint was 50% PSA decline. Circulating tumor cells (CTCs) were identified using parylene membrane filters.
Results
PHC showed a strong dose-dependent anti-proliferative effect in androgen-sensitive and independent cell lines in vitro and suppression of androgen receptor expression. 40 eligible patients were enrolled in the clinical trial. Median baseline PSA was 2.8 ng/mL (1.1-84.1) and 15 men (38%) had a PSA decline on study (1%-55% reduction) ; 25 (62%) had rising PSA on study. The median duration of PSA stability was 6.4 months. Two patients had grade 2/3 transaminitis; the only other grade 2 toxicities were hyperglycemia, hypercalcemia and flatulence. There were no significant changes in testosterone or dihydrotestosterone. CTCs were identified in 19 men (47%).
Conclusion
Although the primary endpoint was not met, Prostate Health Cocktail was well tolerated and was associated with PSA declines and stabilization in a significant number of patients. This is the first report of detecting CTCs in men with BCR prostate cancer. Randomized studies are needed to better define the effect of PHC in men with BCR.
Background
While most men who develop a rising PSA after curative therapy will not die from prostate cancer, these men with biochemical recurrence (BCR) face uncertainty about when they will develop metastases and when they should start on androgen deprivation therapy (ADT). To date, no study has shown a survival advantage for initiation of ADT at BCR rather than at the time radiographic metastases are detected. Furthermore, there is increasing evidence of significant harms caused by ADT including weight gain, loss of lean muscle, development of diabetes and osteopenia1,2. Thus, aside from salvage radiation, there is no standard of care, but options include observation or intermittent ADT3. However, anxiety is an important driver of patients receiving ADT for BCR4 despite known decrements to quality of life related to treatment5. Non-hormonal, non-toxic treatments which could lower or slow the rise of PSA are highly desirable. Multiple natural remedies have been studied in this setting, such as pomegranate juice6 and fenretinide7. In the fenretinide study, zero of 23 men experienced a PSA decline, which indicates that observation or treatment with an inactive agent would be expected to result in continued PSA rise for all patients.
Prostate Health Cocktail (PHC) was formulated to include ingredients which had shown varied mechanisms of action influencing prostate cancer growth in preclinical studies in the following concentrations: vitamin D3 (cholecalciferol) 400 IU, vitamin E (alpha tocopherol) 400 IU, selenium (L-selenomethionine) 200 mcg, green tea extract (epigallocatechin) 400 mg, saw palmetto berry (permixon) 320 mg, soy isoflavones (genistein and daidzein) 20 mg each, lycopene 10 mg. For instance, vitamin D receptors are present on prostate cancer cell lines PC3, LNCaP, and DU1458 and treatment with vitamin D3 in culture resulted in decreased proliferation and increased differentiation9,10. In men with BCR, daily use of oral calcitriol, a high potency vitamin D analog, was associated with >50% PSA reductions, but this benefit was offset by clinically significant hypercalcemia11. Vitamin E has also been identified as a nutrient of interest, since the Finnish ATBC study found an incidental reduction in prostate cancer mortality in men taking vitamin E (α-tocopherol) compared to placebo12. One potential mechanism of action is inhibition of androgen receptor (AR) signaling as a transcriptional repressor, with resultant reduction in expression of PSA13. Additional studies have confirmed that vitamin E, and lycopene as well, can induce tumor necrosis in xenograft models, while downregulating androgen target genes such as IL6, and IGF114. Selenium was also unexpectedly found to be associated with a lower risk of prostate cancer in an unrelated prevention study15, though subsequent prospective study failed to confirm this effect16. In established cancer, selenium has been shown to exert a multitude of effects on cells, inducing cell cycle block and apoptosis via superoxide and caspase-9, as well as down-regulating the angiogenic switch17 and works synergistically with vitamin E to inhibit cancer cell growth18. Green tea polyphenols such as epigallocatechin (EGC) diminish AR signaling and lower circulating hepatocyte and vascular endothelial growth factor19,20. Saw palmetto inhibits growth of LNCaP cells with decreased expression of COX2 21. Soy isoflavones genistein and daidziein concentrate in prostate tissue22, where they interfere with DNA synthesis and methylation, increase expression of cell cycle regulators and decrease VEGF expression23-26. Many of the components of PHC have been found to be synergistic in preclinical study. Selenium has been shown to potentiate the antioxidant effect of vitamin E in prostate cancer cell lines. Vitamin E upregulates expression of vitamin D receptors, potentiating the anti-tumor effect of calcitriol without increasing toxicity27. Synergism has also been identified between lycopene and vitamin D3, with an additive effect on cell differentiation and inhibition of proliferation28.
We undertook preclinical and clinical study of PHC with the hypothesis that combination herbal therapy would be effective in lowering PSA in men with BCR of prostate cancer. Because better stratification tools are needed for BCR, we also studied the detection of circulating tumor cells (CTCs) in this population. While traditional methods have failed to reliably detect CTCs in BCR prostate cancer patients, we incorporated use of a novel parylene membrane filter technology which to see whether a non-EpCAM enrichment based technique would identify CTCs in this population.
Methods
In addition to obtaining an IND from the FDA (#79,284) the content of PHC was independently confirmed by NHK Laboratories, Inc (COA issued 8/2/2012).
Cell Culture and Proliferation Assay
Human prostate cancer LNCaP, PC3 and LAPC3 cells (American Type Culture Collection, Manassas, VA) were cultured and maintained at 37°C in a 5% CO2 atmosphere in RPMI-1640 medium supplemented with 5% fetal bovine serum, 100 units/mL penicillin and 100 μg/mL streptomycin. Prostate cancer cells were seeded into 96-well plates (3000 cells/well) to grow to 80% confluency prior to treatment.
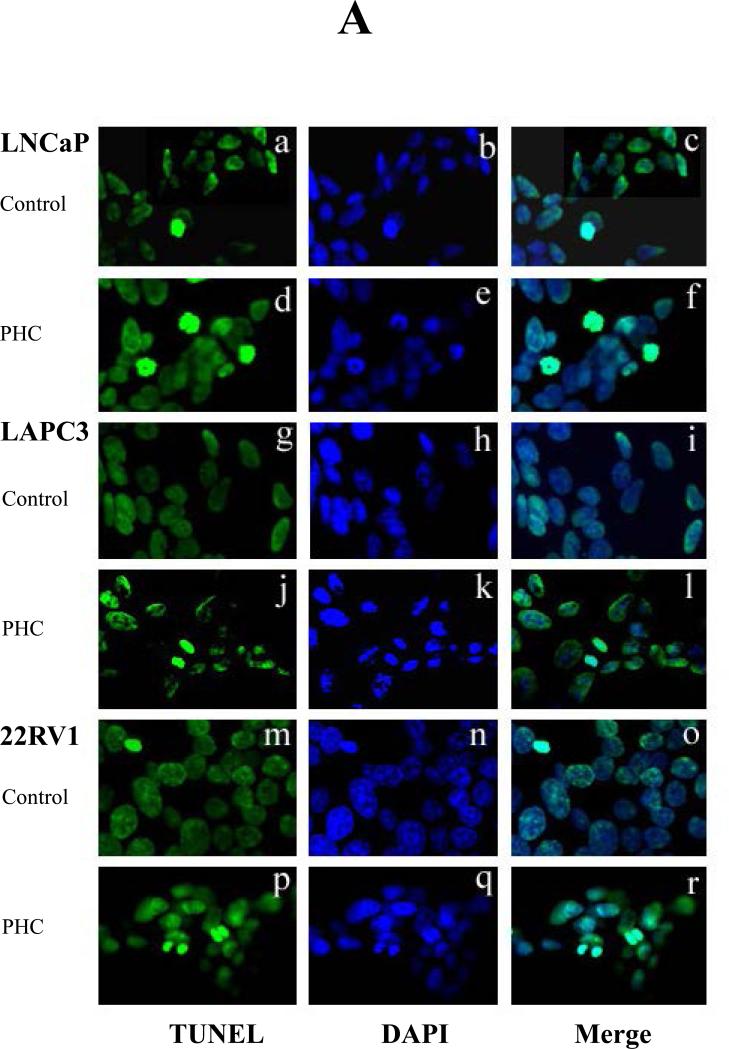
The dry PHC powder was dissolved in serum-free RPMI 1640 (SF-RPMI, 100mg/ml) by shaking for 2 hours at room temperature. After spinning (13,000 rpm), the supernatant was filtered (0.2 μm), then diluted with SF-RPMI and added to the cell cultures. The cells were treated with PHC at final concentrations of 0 (SF-RPMI as the vehicle control), 0.01, 0.1 and 1 mg/ml. Following 72 hours of incubation at 37°C in a 5% CO2 atmosphere, the cells were washed and subjected to the MTS assay. The CellTiter 96® AQueous One Solution Cell Proliferation Assay kit (Promega, Madison, WI, USA) was used following the manufacturer's instructions. The absorbance was detected at 490 nm with a Microplate Reader (VersaMAx, Molecular Devices). All analyses were performed in a quadruplicate set of wells and repeated on separate occasions. AR expression was examined by Western Blot. The PHC-induced apoptosis was examined by identifying DNA fragmentation with fluorescent TUNEL assay and counter-staining with DAPI. All apoptotic (TUNEL-positive) cell nuclei had bright green appearance under a fluorescence microscope. The result was expressed as an apoptotic index defined as the mean of TUNEL-positive cells counted per 400x field.
Clinical Trial
The protocol was IRB approved and registered on clinicaltrials.gov website (NCT00669656). Men were eligible for the trial if they had BCR after treatment for prostate cancer with curative intent using prostatectomy, radiation therapy, or both. At least two rising PSA values > 2 weeks apart were required, with a minimum absolute PSA of 1 ng/mL for prostatectomy patients and 2 ng/mL for radiation patients; radiation patients had to meet the “nadir plus two” definition of recurrence. PSA doubling time had to fall between 3 and 36 months. There had to be no radiographic evidence of metastases on a bone scan < 12 weeks and a CT scan < 8 weeks prior to enrollment. Men who had received ADT were eligible, provided the following criteria were met: for neoadjuvant and adjuvant ADT a total of 24 months were allowed provided the last injection was >12 months prior to enrollment. For men treated with ADT for biochemical recurrence, they could not have demonstrated castration resistance (2 or more rising PSA values with testosterone <50), they had to be off ADT for at least 3 months with testosterone recovery >150 ng/dL) and there had to be a documented discussion with the treating physician about the decision not to use ADT again. Normal organ function was required: calculated creatinine clearance > 50 mL/min; AST, ALT and bilirubin all had to be < 1.5 x upper limit of normal. Men taking full dose anticoagulation or antiplatelet therapy were excluded, however the use of 81 mg aspirin daily was allowed. Significant cardiac disease, including coronary disease with prior bypass surgery or stent placement, resulted in exclusion.
Subjects were provided with PHC by OncoNatural Solutions, and were instructed to consume 3 capsules daily on an empty stomach; 4 weeks constituted a treatment cycle. Toxicity assessment (Common Terminology Criteria for Adverse Events, CTC AE v 3.0) and PSA testing occurred after one cycle and then every 2 cycles for up to one year. Imaging was to be done as clinically indicated, with a recommended reassessment at the time of removal from study. Subjects were removed from study for PSA progression, which had to be confirmed by a second PSA value 4 weeks after initially meeting criteria and in the absence of documented urinary tract infection or prostatitis. For patients whose PSA levels never decreased on study, PSA progression was defined as a 25% increase over baseline and an absolute increase of 5 ng/mL. In patients whose PSA initially decreased on study, PSA progression was defined as a 25% increase and an absolute increase of 5 ng/mL above nadir OR crossing above the baseline PSA level. Subjects who were still felt to be benefitting from treatment after completing all protocol specified treatment (13 cycles) were eligible to continue receiving PHC at no cost from the manufacturer off protocol, while being followed by their regular physician. The trial was funded by the Whittier Foundation.
The primary endpoint was PSA response, initially defined as a 50% reduction in PSA29. Subjects whose PSA did not meet progression criteria (as defined above) by 12 weeks nor response were labeled as PSA stable disease for their best response. PSA DT post-treatment was calculated using all available values > 1 month after starting study therapy; only subjects with repeat PSA measurements available after starting study therapy were evaluable.
The study was originally designed with the assumption that, left untreated, >95% of men meeting inclusion criteria would continue to have rising PSA, and that a15% rate of PSA declines would represent activity worthy of additional study. A Simon Optimum two-stage design was employed31, with interim analysis after 20 subjects. With no responders, study closure would be considered. If enrollment continued, response in ≥4 of 45 subjects would indicate sufficient PHC activity to warrant further study. With this design, there was 0.9 probability (power) to conclude that PHC was worth of further study when the true response rate was 15% or greater, and 0.1 probability (alpha) of incorrectly considering PHC worthy of further study with a true response rate <4%. At the time of the interim analysis with 20 patients enrolled, none of the 19 evaluable patients had a PSA response. At that time also, based on the recently published Prostate Cancer Clinical Trials Working Group (PCCTWG) guidelines30, best PSA declines and PSA status at 12 weeks were also examined. Given the tolerability of PHC, and the fact that 6 of the 19 evaluable patients experienced some PSA decline, a decision was made to continue the trial to obtain more data on the tolerability and PSA changes associated with this supplement. The study was stopped in December 2012 after enrollment of the 43rd subject.
Testosterone, dihydrotestosterone (DHT), vitamin D, and selenium levels were drawn at cycles 1, 4, and 13. Blood samples were tested for enumeration of circulating tumor cells (CTC) using parylene membrane filters31 at cycles 1, 4, and 13.
Results
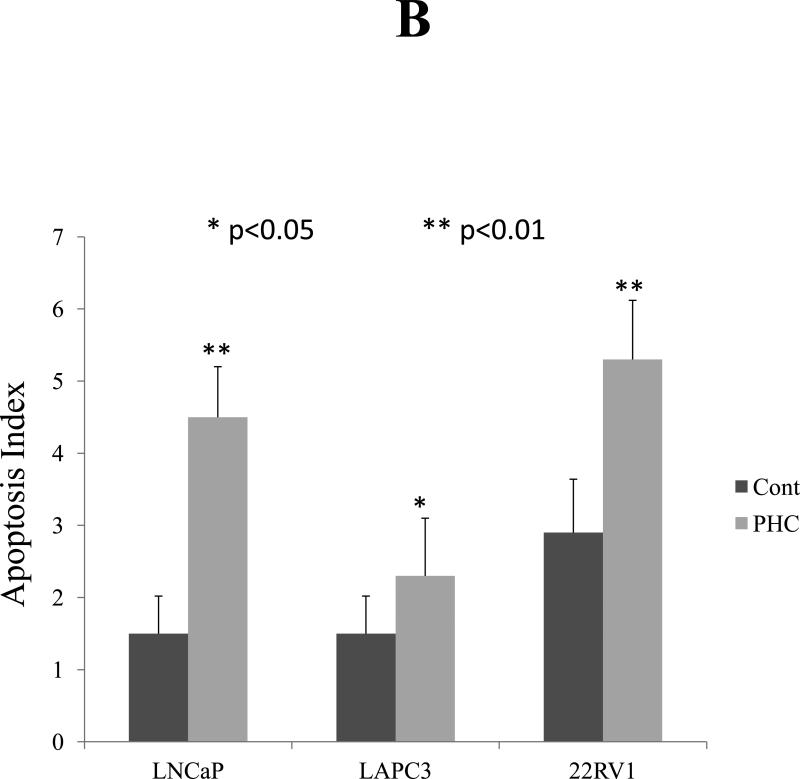
Experiments using LAPC3, PC3, and LNCaP cell cultures identified a strong dose-dependent anti-proliferative effect (Figure 1) as well as suppression of AR expression by Western blot in LNCaP and LAPC3 cells. AR was not detectable in PC3 cells (data not shown). Apoptosis was induced by exposure to PHC, as detected by the TUNEL assay (Figure 3), in all three cell lines.
Figure 1. Prostate Health Cocktail Suppresses the Proliferation of Prostate Cancer Cell Lines Dose-dependently and suppresses AR expression.
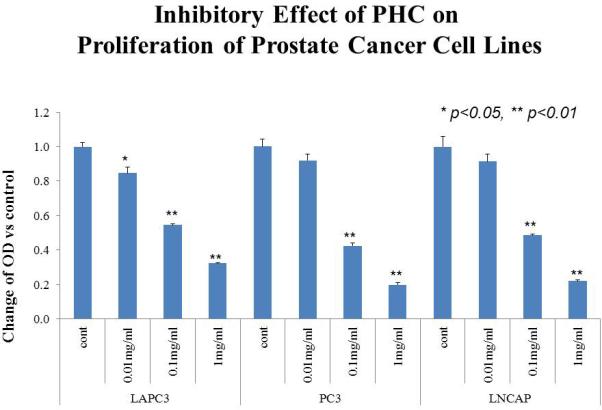
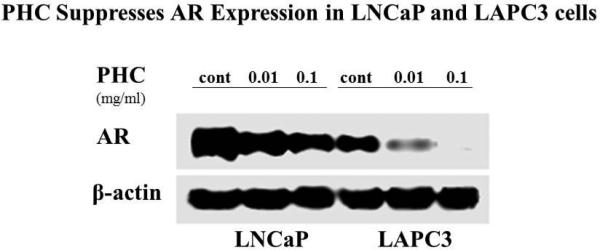
Human prostate cancer LNCaP, PC3 and LAPC3 cells were seeded into 96-well plates (3000 cells/well) to grow to 80% confluency. The dry PHC powder was dissolved in serum-free RPMI 1640 (SF-RPMI) and added to the cells at the final concentrations of 0 (SF-RPMI as the vehicle control), 0.01, 0.1 and 1 mg/ml, respectively. Following 72 hour incubation at 37°C in a 5% CO2 atmosphere, the cells were washed and subjected to the MTS assay. The absorbance was detected at 490 nm with a Microplate Reader. Optical density (OD) decreased in a dose-dependent manner, representing suppression of proliferation (A). AR expression was measured via Western Blot and also showed that PHC reduced expression in a dose-dependent pattern (B). βactin is used as control, representing protein expression of a “housekeeping” gene.
Figure 3. PHC Induces Apoptosis in Prostate Cancer Cells.
Three cells lines, LNCaP, LAPC3 and 22RV1, of prostate cancer were cultured on cover slips and treated with PHC (10 μg/ml) for 4 days. The PHC-induced apoptosis was examined by identifying DNA fragmentation with fluorescent TUNEL assay and counter-staining with DAPI. All apoptotic (TUNEL-positive) cell nuclei had bright green fluorescence under a fluorescence microscope. The result was expressed as an apoptotic index defined as the mean of TUNEL-positive cells counted per 400x field. P<0.05 indicated significant difference. Representative photomicrographs demonstrated the DNA fragmentation and dysmorphic nuclear condensation (Figure 3A), which were significantly increased in response to PHC treatment in all three cell lines (Figure 3A, d-f, j-l, p-r, Figure 3B).
Of the 43 subjects enrolled, 3 were found to be ineligible due to low baseline testosterone (1), use of excluded medication (1) or history of coronary artery disease (1). Baseline and demographic characteristics of the 40 eligible subjects are summarized in Table 1. The median age was 67 (range 54 to 84) and baseline PSA was 2.8 ng/mL (1.1-84.1). For initial local therapy, 10 men (25%) were treated with radiation, 10 men (25%) had prostatectomy only, and 20 men (50%) had received both. Of these 40, 12 men (30%) had received ADT prior to study entry; 3 for BCR and 9 as (neo)adjuvant. The median baseline PSA doubling time was 9.4 months (range 3.3 to 36.1), and was slower in those with Gleason < 7 (12 months) compared to Gleason 8-10 (7.2 months), p=0.031.
Table 1.
Baseline and Demographic Characteristics of the Study Population
| Age at On Study (Yrs) Median (Range) | 67.0 (54.8 – 84.5) | |
|---|---|---|
| Race | Number of patients | Percent |
| Caucasian | 28 | 70% |
| Hispanic | 6 | 15% |
| Black | 4 | 10% |
| Asian | 2 | 5% |
| Gleason Score at Initial Diagnosis | ||
| ≤ 7 | 31 | 78% |
| > 7 | 9 | 23% |
| Prior Treatment | ||
| Radiation Therapy Only | 10 | 25% |
| Surgery Only | 10 | 25% |
| Surgery + Radiation Therapy | 20 | 50% |
| Neo-Adjuvant/Adjuvant ADT | 9 | 22.5% |
| ADT for biochemical recurrence prior to study entry | 3 | 7.5% |
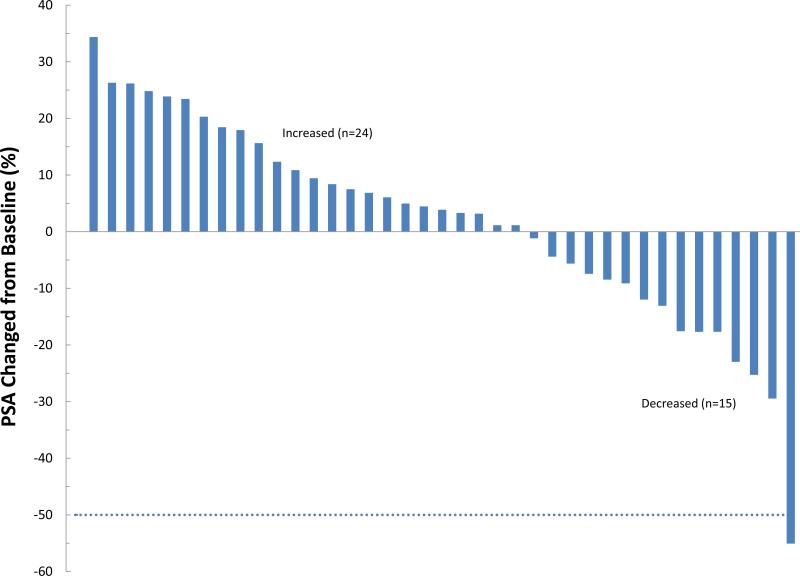
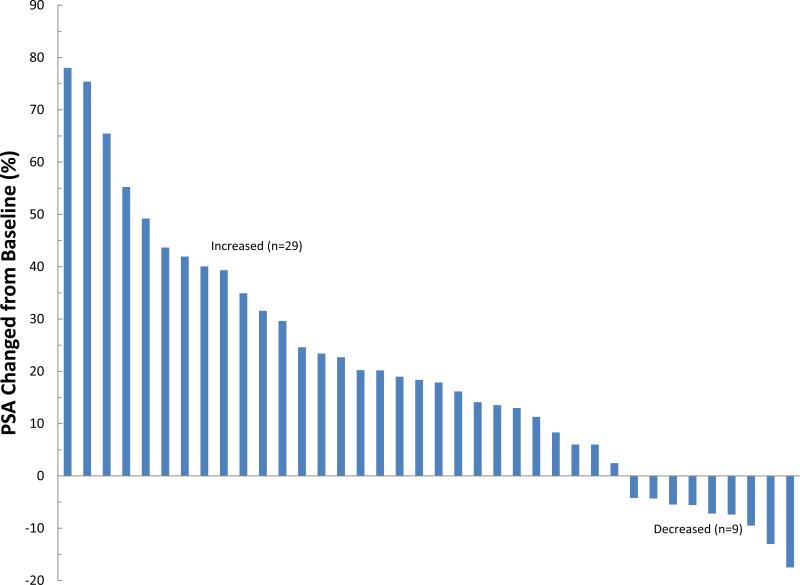
Subjects received a median of 10 cycles (1-13) of treatment; 26 subjects (65%) received treatment for at least 6 months and 8 (20%) were still on treatment at 12 months. Reasons for discontinuation included PSA or clinical progression in 19 (48%), completion of protocol therapy in 11 (28%), initiating alternate treatment for rising PSA without meeting progression criteria in 6 (15%), difficulty complying with study procedures in 2 (5%), toxicity in 1 and intercurrent illness in 1. The patient who was taken off therapy early due to intercurrent illness did not have post treatment PSA measurements; all other 39 subjects were evaluable for assessing PSA response. None of these 39 subjects experienced a complete PSA response or a confirmed PSA partial response (defined as a 50% decrease from baseline which was confirmed at least 4 weeks later). However one patient achieved a 55% decrease at cycle 13; he was taken off therapy due to completion of protocol. 15 of the 39 evaluable patients (38%) had a PSA decline with a maximum decrease ranging from -1.1% to -55%. Of the 38 men evaluable at 12 weeks, 9 (24%) had PSA decline (Figure 2). The median “best” PSA change at any time point during the study for all 39 evaluable subjects was 3.8% (range: -55% to +34.3%) and the median change in PSA at 12 weeks was 18.1% (range: -17.5% to +78.0%). The median time to PSA or clinical progression was 8.3 months (95% CI: 5.5, 11.8).. The median duration of PSA stability (from treatment start date to last assessment, without PSA progression) was 6.4 months (range: 0.6 to 12.3). PSA doubling time post-treatment was calculated for 37 men who had 2+ post-treatment PSA determinations. Median PSA DT post-treatment was 6.7 months (<0 to 27.7) compared to 9.4 months at study entry (p=0.047, sign test); 12 of the 37 evaluable men (32%) had a slower PSA DT post-treatment than at baseline. The median follow-up is 17.8 months (range 0.9 – 35.6) and 18 men have had clinical progression, with local recurrence (n=2) or distant metastatic disease (n=16) ; all but one of these progressions occurred after the patient had been taken off the PHC supplement. The median time to metastasis from study treatment start was 23 months (95% confidence interval 13.6, 33.8 months).
Figure 2. Waterfall plot of PSA changes during treatment with PHC in men with biochemically recurrent prostate cancer.
A) “best” change, which excludes a patient who came off study before completing one month due to intercurrent illness, and so was not evaluable and B) PSA change at 12 weeks. Changes are noted in percentage compared to baseline, not absolute PSA level changes.
Toxicity data are summarized in Table 2; there were no grade 4 toxicities. The only grade 3 toxicity deemed possibly related to study therapy was transaminitis, which resolved upon stopping treatment. On review, this transamnitis seemed related to alcohol intake. However, since grade 1 and 2 transaminitis were noted in 3 other patients, the possibility that this toxicity was related to PHC cannot be ruled out. One patient developed grade 3 hyponatremia and altered mental status in the setting of pneumonia, which was felt to be unrelated to PHC. There were no consistent significant changes in selenium, testosterone or DHT during study therapy (Table 3) although selenium levels increased from baseline to latest cycle (p=0.002) for the subset of 15 patients with data.
Table 2.
A summary of toxicities experienced by patients during the clinical trial.
| Toxicity Category | Toxicity | Number of Patients with Maximum Grade Experienced | |||
|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | ||
| Blood/Bone Marrow | Hemoglobin | 1 | 0 | 0 | 0 |
| Leukocytes (total WBC) | 1 | 0 | 0 | 0 | |
| Platelets | 1 | 0 | 0 | 0 | |
| Cardiac General | Hypertension | 1 | 0 | 0 | 0 |
| Constitutional Symptoms | Fatigue (asthenia, lethargy, malaise) | 2 | 0 | 0 | 0 |
| Dermatology/Skin | Rash/desquamation | 1 | 0 | 0 | 0 |
| Endocrine | Glucose, serum-high (hyperglycemia) | 3 | 1 | 0 | 0 |
| Gastrointestinal | Constipation | 2 | 0 | 0 | 0 |
| Diarrhea | 1 | 0 | 0 | 0 | |
| Distension/bloating, abdominal | 2 | 0 | 0 | 0 | |
| Flatulence | 3 | 1 | 0 | 0 | |
| Heartburn/dyspepsia | 4 | 0 | 0 | 0 | |
| Nausea | 2 | 0 | 0 | 0 | |
| Hemorrhage/Bleeding | Hemorrhage/Bleeding - Other | 1 | 0 | 0 | 0 |
| Hepatic | ALT, SGPT | 1 | 1 | 1 | 0 |
| AST, SGOT | 2 | 1 | 0 | 0 | |
| Alkaline phosphatase | 1 | 0 | 0 | 0 | |
| Bilirubin (hyperbilirubinemia) | 2 | 0 | 0 | 0 | |
| Metabolic/Laboratory | Calcium, serum-high (hypercalcemia) | 0 | 1 | 0 | 0 |
| Creatinine | 1 | 0 | 0 | 0 | |
| Potassium, serum-high (hyperkalemia) | 2 | 0 | 0 | 0 | |
| Musculoskeletal/Soft Tissue | Muscle weakness, generalized or specific area | 2 | 0 | 0 | 0 |
| Neurology | Dizzines | 1 | 0 | 0 | 0 |
| Pain | Pain (Abdomen NOS) Pain (Muscle) |
2 | 0 | 0 | 0 |
| 0 | 1 | 0 | 0 | ||
Table 3.
Changes in hormone and micronutrient levels during the study.
| Markers | Cycle 1 (Baseline) | Cycle 4 or Last Cycle after Cycle 1 | Cycle 13 or Last Cycle after Cycle 4** | p-value* | |||||
|---|---|---|---|---|---|---|---|---|---|
| N | Median (Range) | N | Median (Range) | N | Median (Range) | Cycle 4 vs. Baseline | Cycle 13 vs. Baseline | Cycle 13 vs. Cycle 4 | |
| PAP (ng/dL) | 38 | 1.2 (0.4 – 10.7) | 30 | 1.1 (0.5 – 7.8) | 16 | 1.3 (0.4 – 8.0) | 0.25 | 0.58 | 0.79 |
| Testosterone (ng/dL) | 39 | 370 (35.6 – 736) | 30 | 355 (192 – 905) | 18 | 374 (214 – 624) | 0.26 | 0.24 | 1.00 |
| DHT (ng/mL) | 36 | 29.5 (9 – 74) | 28 | 27 (9 – 91) | 16 | 31 (15 – 61) | 0.31 | 1.00 | 0.79 |
| Vit D (ng/mL) | 40 | 33 (22 – 107) | 30 | 37 (12 – 63) | 15 | 36 (23 – 75) | 0.33 | 0.58 | 0.77 |
| Selenium (mcg/L) | 37 | 149 (88 – 298) | 27 | 151 (104 – 316) | 15 | 171 (134 – 414) | 0.56 | 0.002 | 0.58 |
p-value based on sign test.
Circulating tumor cells (CTC) were collected in 33 patients at baseline, and identified in 16 (48%); 22 of the 33 patients also had specimens collected at follow up and CTCs were identified in 10 (45%) of these 22 patients.. Among patients with CTCs assessed at baseline and follow-up, the CTC count increased in 5 men and decreased in 7 men; the remaining 10 had no change. The presence of CTCs was not associated with baseline PSA DT (p=0.58), or with PSA levels at 12 weeks post treatment (p=0.44), or the development of metastases (p=0.30).
DISCUSSION
There is a substantial population of men with BCR after curative-intent local therapy for prostate cancer.. While 20% of these men will die from prostate cancer, the natural history is lengthy33. Our current risk stratification tools for prostate cancer mortality are clinical: PSA DT and Gleason Score33,34. No prospective randomized trials have yet shown that ADT improves survival when administered at BCR compared to when metastases are detected. Aside from radiation +/- ADT for BCR, the NCCN guidelines35 for this group of patients includes observation although intermittent ADT has also been utilized3. In this study, 15/39 (38.5%) of men experienced a PSA decline, and 9 of 36 (25%) had a decline at 12 weeks compared to baseline. Strengths of this study include rigorous testing of PHC for manufacturing and content. Nutritional supplements are not strictly regulated, which means that content may not be consistent, making sound clinical investigation difficult. Prior experience with PC-SPES, a supplement which induced responses, but was found to contain warfarin and estrogen,36 highlight the need for content verification. The measurement of testosterone and DHT levels during our study is another strength, since we sought to identify an active agent without the hormonal effects of ADT. Weaknesses include the lack of a placebo control, and the modest sample size.
Concerns raised by the SELECT trial about potential negative impact of vitamin E on prostate cancer incidence and selenium on diabetes risk16 could be viewed as a weakness of the composition of PHC. However it should be noted that the combination arm of the SELECT trial showed no increased risk, suggesting potential differences related to combining vitamins and micronutrients. Also, the physician's health study II did not find any difference in risk of incident prostate cancer with vitamin E supplementation37. Importantly, both of these studies were performed in the setting of primary prevention; there could be differential effects of micronutrients on normal or precancerous cells as compared to effects on established cancer cells. Epidemiologic studies of serum vitamin D levels have reported conflicting data, and ultimately both high and low vitamin D levels may be associated with higher prostate cancer risk38. The relatively low level of vitamin D contained in PHC is unlikely to push subjects into a high serum range; indeed levels did not change significantly during our study (Table 3). However, again it must be noted that these data may not applicable to a population of patients who already have prostate cancer.
We found striking in vitro activity of PHC in both androgen dependent and independent prostate cancer cell lines, and confirmed that activity in a prospective single-arm trial. One limitation is that in vivo vitamins and other components of PHC are likely metabolized, thus the direct effect of the compound may not translate to clinical benefit. The in vitro suppression of AR we identified after in vitro exposure to PHC cannot be explained by changes in circulating testosterone or DHT levels, and clinically patients were not found to have changes in circulating androgen levels, nor did they report typical side effects of hypogonadism (hot flashes, mastodynia). The AR belongs to the nuclear receptor superfamily and functions as a ligand-dependent transcription factor, binding to the androgen responsive element and recruiting co-regulatory factors. Further work will be needed to understand how PHC suppresses AR expression.
Our study is not the first to evaluate a natural supplement in men with BCR prostate cancer. Pomegranate juice6 and extract39 have both been shown to influence PSA DT as well as induce PSA declines in 13-35% of patients treated. Perifosine, studied by the California Cancer Consortium, induced PSA declines in 5 of 24 subjects, though none had a >50% PSA decline40. Cross-trial comparison is difficult, given the known prognostic influence of PSA DT and Gleason scores. In the pomegranate juice study there were no patients with Gleason >7, whereas in our study 23% of patients had Gleason 8-10 cancer; the PSA DT at baseline in the pomegranate juice study was 14 months, whereas in our population it was 9.4 months. There was no indication of how many men had received ADT in the pomegranate study; this clinical variable could also confound outcomes and of note, none of these trials utilized placebo control. The completion of multiple clinical trials utilizing non-hormonal agents for BCR prostate cancer indicates major interest in this approach.
The median time to metastasis was relatively short in our population, likely reflecting the inclusion of more aggressive populations, including those with PSA DT between 3-6 months, high Gleason scores, and previous treatment with ADT for BCR. It should also be taken in context that this figure is from study entry rather than from time of biochemical recurrence, as in published reports which found median time to metastasis of 8 years33; in order to be eligible, our patients had to have a PSA level of at least 1, and many started with higher PSA levels, representing a variable number of years since meeting the definition of BCR. In order to better determine the impact of PHC and other supplements in BCR, randomized placebo-controlled trials are needed with clinically meaningful endpoints such as time to metastasis. One meta-analysis of single arm studies of non-hormonal biologic therapies (including lenalidomide and marimostat) in men with BCR found correlation between changes in PSA DT and metastasis-free survival41. This provides rationale to conduct randomized, placebo-controlled trials of PHC or other supplements with an endpoint of metastasis-free survival.
Better prognostic markers are needed for men with BCR. CTCs have strong, independent prognostic value in the setting of metastatic, castration-resistant prostate cancer42. However, their utility in patients with BCR is unproven. One limitation for the use of CTCs in earlier stage prostate cancer is low yield; one study detected CTCs in only 3 of 36 men (8.3%) with BCR prostate cancer using the Veridex platform43. Using parylene membrane filters32,44 we identified CTCs in nearly half of our population, and noted changes in CTC counts during treatment, though our numbers were too small to allow correlation with clinical benefit. If additional studies identify an association between CTC and time to metastasis, this marker could be provide valuable stratification for men with BCR. Men predicted to have shorter time to metastasis could be enrolled on clinical trials of early aggressive interventions, creating an enriched population to enhance the likelihood of obtaining significant results. In addition, more studies are needed to discover predictive biomarkers of response to PHC, to identify patients most likely to benefit.
CONCLUSION
Although the study did not meet its primary endpoint of 50% PSA declines, we found that PHC, a combination herbal supplement, induced some PSA declines in men with BCR prostate cancer, without reducing serum androgens. There were few, transient adverse events. Additional study of this agent in a placebo-controlled trial is planned.
ACKNOWLEDGEMENTS
This study was supported by the Whittier Foundation, with additional support from the Cancer Center Core Grant Grant P30 CA014089
Footnotes
CONFLICT OF INTEREST
Dr. Pinski holds the patent for Prostate Health Cocktail, and has commercial interest in the company that distributes it, OncoNatural Solutions. The other authors declare no conflicts.
Contributor Information
Tanya B. Dorff, University of Southern California, Keck School of Medicine Norris Comprehensive Cancer Center 1441 Eastlake Ave. #3440 Los Angeles, CA 90033.
Susan Groshen, USC Keck School of Medicine, Norris Comprehensive Cancer Center Department of Preventive Medicine, Division of Biostatistics Susan.Groshen@med.usc.edu.
Denice D. Tsao-Wei, USC Keck School of Medicine, Norris Comprehensive Cancer Center Department of Preventive Medicine, Division of Biostatistics Wei_d@med.usc.edu
Shigang Xiong, USC Keck School of Medicine, Division of Medical Oncology shigangx@usc.edu.
Mitchell E. Gross, USC Keck School of Medicine, Westside Prostate Cancer Center Center for Applied Molecular Medicine mitcheeg@med.usc.edu.
Nicholas Vogelzang, Comprehensive Cancer Centers of Nevada Nicholas.Vogelzang@usoncology.com.
David I. Quinn, USC Keck School of Medicine, Norris Comprehensive Cancer Center Department of Medicine, Division of Medical Oncology diquinn@usc.edu.
Jacek K. Pinski, USC Keck School of Medicine, Norris Comprehensive Cancer Center Department of Medicine, Division of Medical Oncology Pinski@med.usc.edu.
REFERENCES
- 1.Keating NL, O'Malley J, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2008;24:4448–56. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- 2.Ross RW, Small EJ. Osteoporosis in men treated with androgen deprivation therapy for prostate cancer. J Urol. 2002;167:1952. [PubMed] [Google Scholar]
- 3.Crook JM, O'Callaghan CJ, Duncan G, Dearnaley DP, Higano CS, Horwitz EM, et al. Intermittent androgen suppression for rising PSA level after radiotherapy. NEJM. 2012;367:895–903. doi: 10.1056/NEJMoa1201546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dale W, Hemmerich J, Bylow K, Mohile S, Mullaney M, Stadler WM. Patient anxiety about prostate cancer independently predicts early initiation of androgen deprivation therapy for biochemical cancer recurrence in older men: a prospective cohort study. J Clin Oncol. 2009;27:1557–63. doi: 10.1200/JCO.2008.18.5850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fowler FJ, Jr, Collins MM, Corkery EW, Elliott DB, Barry MJ. The impact of androgen deprivation on quality of life after radical prostatectomy for prostate carcinoma. Cancer. 2002;95:287–295. doi: 10.1002/cncr.10656. [DOI] [PubMed] [Google Scholar]
- 6.Pantuck AJ, Leppert JT, Zomorodian N, Aronson W, Hong J, Barnard RJ, et al. Phase II study of pomegranate juice for men with rising prostate-specific antigen after surgery or radiation for prostate cancer. Clin Cancer Res. 2006;12:4018. doi: 10.1158/1078-0432.CCR-05-2290. [DOI] [PubMed] [Google Scholar]
- 7.Cheung E, Pinski J, Dorff T, Groshen S, Quinn DI, Reynolds CP, et al. Oral fenretinide in biochemically recurrent prostate cancer: a California Cancer Consortium phase II trial. Clin Genitourinary Cancer. 2009;7:43–50. doi: 10.3816/CGC.2009.n.008. [DOI] [PubMed] [Google Scholar]
- 8.Skowronski RJ, Peehl DM, Feldman D. Vitamin D and prostate cancer: 1,25 dihydroxyvitamin D receptors and actions in human prostate cancer cell lines. Endocrinology. 1993;132:1952–60. doi: 10.1210/endo.132.5.7682937. [DOI] [PubMed] [Google Scholar]
- 9.Moffatt KA, Johannes WU, Hedlund TE, Miller GJ. Growth inhibitory effects of 1a,25-dihydroxyvitamin D3 are mediated by increased levels of p21 in the prostatic carcinoma cell line ALVA-31. Cancer Res. 2001;61:7122–9. [PubMed] [Google Scholar]
- 10.Bauer JA, Thompson TA, Church DR, Ariazi EA, Wilding G. Growth inhibition and differentiation in human prostate carcinoma cells induced by the vitamin D analog 1a,24-dihydroxyvitamin D2. Prostate. 2003;55:159–67. doi: 10.1002/pros.10219. [DOI] [PubMed] [Google Scholar]
- 11.Beer TM, Lemmon D, Lowe BA, Henner WD. High-dose weekly oral calcitriol in patients with a rising PSA after prostatectomy or radiation for prostate carcinoma. Cancer. 2003;97:1217–24. doi: 10.1002/cncr.11179. [DOI] [PubMed] [Google Scholar]
- 12.Heinonen OP, Albanes D, Virtamo J, Taylor PR, Huttunen JK, Hartman AM, et al. Prostate cancer and supplementation with a-tocopherol and b-carotene: incidence and mortality in a controlled trial. J Natl Cancer Inst. 1998;90:440–6. doi: 10.1093/jnci/90.6.440. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Ni J, Messing EM, Chang E, Yang C-R, Yeh S. Vitamin E succinate inhibits the function of androgen receptor and the expression of prostate-specific antigen in prostate cancer cells. Proc Natl Acad Sci USA. 2002;99:7408–13. doi: 10.1073/pnas.102014399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siler U, Barella L, Spitzer V, Schnorr J, Lein M, Goralczyk R, Wertz K. Lycopene and vitamin E interfere with autocrine/paracrine loops in the Dunning prostate cancer model. The FASEB journal. 2004;18:1019–21. doi: 10.1096/fj.03-1116fje. [DOI] [PubMed] [Google Scholar]
- 15.Clark LC, Combs G, Turnbull BW, Slate EH, Chalker DK, Chow J, et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional prevention of cancer study group. JAMA. 1996;276:1957–63. [PubMed] [Google Scholar]
- 16.Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the selenium and vitamin E cancer prevention trial (SELECT). JAMA. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao R, Domann FE, Zhong W. Apoptosis induced by selenomethionine and methioninase is superoxide mediated and p53 dependent in human prostate cancer cells. Mol Cancer Ther. 2006;5:3275–84. doi: 10.1158/1535-7163.MCT-06-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zu K, Ip C. Synergy between selenium and vitamin E in apoptosis induction is associated with activation of distinctive initiator caspases in human prostate cancer cells. Cancer Res. 2003;63:6988. [PubMed] [Google Scholar]
- 19.McLarty J, Bigleow RLH, Smith M, Elmajian D, Ankem M, Cardelli JA, et al. Tea polyphenols decrease serum levels of prostate-specific antigen, hepatocyte growth factor, and vascular endothelial growth factor in prostate cancer patients and inhibit production of hepatocyte growth factor and vascular endothelial growth factor in vitro. Cancer Prev Res. 2009;2:673–82. doi: 10.1158/1940-6207.CAPR-08-0167. [DOI] [PubMed] [Google Scholar]
- 20.Tang F-Y, Nguyen N, Meydani M. Green tea catechins inhibit VEGF-induced angiogenesis in vitro through suppression of VE-cadherin phosphorylation and inactivation of Akt molecule. International Journal of Cancer. 2003;106:871–8. doi: 10.1002/ijc.11325. [DOI] [PubMed] [Google Scholar]
- 21.Goldman WH, Sharma AL, Currier SJ, Johnston PD, Rana A, Sharma CP. Saw palmetto berry extract inhibits growth and cox-2 expression in prostatic cancer cells. Cell Biol Internatl. 2001;25:1117–24. doi: 10.1006/cbir.2001.0779. [DOI] [PubMed] [Google Scholar]
- 22.Rannikko A, Petas A, Raivio T, Janne OA, Rannikko S, Adlercreutz H. Plasma and prostate phytoestrogen concentrations in prostate cancer patients after oral phytoestrogen supplementation. Prostate. 2006;66:82–7. doi: 10.1002/pros.20315. [DOI] [PubMed] [Google Scholar]
- 23.Handayani R, Rice L, Cui Y, Medrano TA, Samedi V, Baker H, et al. Soy isoflavones alter expression of genes associated with cancer progression, including interleukin-8, in androgen-independent PC-3 human prostate cancer vells. J Nutr. 2006;136:75–82. doi: 10.1093/jn/136.1.75. [DOI] [PubMed] [Google Scholar]
- 24.Fang MZ, Chen D, Sun Y, Jin Z, Christman JK, Yang CS. Reversal of hypermethylation and reactivation of p16INK4a, RARbeta, and MGMT genes by genistein and other isoflavones from soy. Clin Cancer Res. 2005;11:7033–41. doi: 10.1158/1078-0432.CCR-05-0406. [DOI] [PubMed] [Google Scholar]
- 25.Shen JC, Klein RD, Wei Q, Guan Y, Contois JH, Wang TT, et al. Low-dose genistein induces cyclin-dependent kinase inhibitors and G(1) cell cycle arrest in human prostate cancer cells. Mol Carcinog. 2000;29:92–102. doi: 10.1002/1098-2744(200010)29:2<92::aid-mc6>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 26.Guo Y, Wang S, Hoot DR, Clinton SK. Suppression of VEGF-mediated autocrine and paracrine interactions between prostate cancer cells and vascular endothelial cells by soy isoflavones. J Nutr Biochem. 2007;18:408–17. doi: 10.1016/j.jnutbio.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 27.Yin Y, Ni J, Chen M, Guo Y, Yeh S. RRR-alpha-vitamin E succinate potentiates the antitumor effect of calcitriol in prostate cancer without overt side effects. Clin Cancer Res. 2009;15:190–200. doi: 10.1158/1078-0432.CCR-08-0910. [DOI] [PubMed] [Google Scholar]
- 28.Amir H, Karas M, Giat J, Danilenko M, Levy R, Yermiahu T, et al. Lycopene and 1,25-dihydroxyvitamin D3 cooperate in the inhibition of cell cycle progression an dinduction of differentiation in HL-60 leukemic cells. Nutr Cancer. 1999;33:105–12. doi: 10.1080/01635589909514756. [DOI] [PubMed] [Google Scholar]
- 29.Scher HI, Eisenberger M, D'Amico AV, Halabi S, Small EJ, Morris M, et al. Eligibility and outcomes reporting guidelines for clinical trials for patients in the state of a rising prostate-specific antigen: recommendations from the prostate-specific antigen working group. J Clin Oncol. 2004;22:537–56. doi: 10.1200/JCO.2004.07.099. [DOI] [PubMed] [Google Scholar]
- 30.Scher HI, Halabi S, Tannock I, Morris M, Sternberg C, Carducci MA, et al. Design and endpoints of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the prostate cancer clinical trials working group. J Clin Oncol. 2008;26:1148–59. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simon R. Optimal two-stage designs for phase II clinical trials. Controlled Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 32.Lin HK, Zheng S, Williams AJ, Balic M, Groshen S, et al. Portable filter-based microdevice for detection and characterization of circulating tumor cells. Clin Cancer Res. 2010;16:5011–18. doi: 10.1158/1078-0432.CCR-10-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Freedland SJ, Humphreys EB, Mangold LA, Eisenberger M, Dorey FJ, Walsh PC, et al. Risk of prostate cancer specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005;294:433–9. doi: 10.1001/jama.294.4.433. [DOI] [PubMed] [Google Scholar]
- 34.Valicenti RK, DeSilvio M, Hanks GE, Porter A, Brereton H, Rosenthal SA, et al. Posttreatment prostate specific antigen doubling time as a surrogate endpoint for prostate cancer specific survival: an analysis of Radiation Therapy Oncology Group Protocol 92-02. Int J Radiat Oncol Biol Phys. 2006;66:1064–71. doi: 10.1016/j.ijrobp.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 35. [6/13/14]; http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf.
- 36.White J. PC-SPES: a lesson for future dietary supplement research. J Natl Cancer Inst. 2002;94:1261–2. doi: 10.1093/jnci/94.17.1261. [DOI] [PubMed] [Google Scholar]
- 37.Gaziano JM, Glynn RJ, Christen WG, Kurth T, Belanger C, MacFadyen J, et al. Vitamins E and C in the prevention of prostate and total cancer in men. JAMA. 2009;301:52–62. doi: 10.1001/jama.2008.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tuohimaa P, Tenkanen L, Ahonen M, Lumme S, Jellum E, Hallmans G, et al. Both high and low levels of blood vitamin D are associated with a higher prostate cancer risk: a longitudinal, nested case-control study in the Nordic countries. Int J Cancer. 2004;108:104–108. doi: 10.1002/ijc.11375. [DOI] [PubMed] [Google Scholar]
- 39.Paller CJ, Ye W, Wozniak PJ, Gillespie BK, Sieber PR, Greengold RH, et al. A randomized phase II study of pomegranate extract for men with rising PSA following initial therapy for localized prostate cancer. Prostate Cancer Prostat Dis. 2013;16:50–5. doi: 10.1038/pcan.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chee KG, Longmate J, Quinn DI, Chatta G, Pinski J, Twardowski P, et al. The AKT inhibitor perifosine in biochemically recurrent prostate cancer: a phase II California/Pittsburgh cancer consortium trial. Clin Genitourin Cancer. 2007;5:433–7. doi: 10.3816/CGC.2007.n.031. [DOI] [PubMed] [Google Scholar]
- 41.Antonarakis ES, Zahurak ML, Lin J, Keizman D, Carducci MA, Eisenberger MA. Changes in PSA kinetics predict metastasis-free survival in men with PSA-recurrent prostate cancer treated with nonhormonal agents. Cancer. 2012;118:1533–42. doi: 10.1002/cncr.26437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Bono JS, Scher HI, Montgomery RB, Parker C, Miller MC, Tissing H, et al. Circulating tumor cells predict survival benefit from treatment in metastatic, castration-resistant prostate cancer. Clin Cancer Res. 2008;14:6302–9. doi: 10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- 43.Aragon-Ching JB, Simmens SJ, Andrawis R, Hendricks F, Frazier H, et al. Circulating tumor cells in biochemical recurrence of prostate cancer: final results. J Clin Oncol. 2013;(suppl 6) doi: 10.1016/j.clgc.2015.04.003. abstr 179. [DOI] [PubMed] [Google Scholar]
- 44.Zheng S, Lin H, Liu JQ, Balic M, Datar R, Cote RJ, Tai YC. Membrane microfilter device for selective capture, electrolysis and genomic analysis of human circulating tumor cells. J Chromatogr A. 2007;1162:154–61. doi: 10.1016/j.chroma.2007.05.064. [DOI] [PubMed] [Google Scholar]