Abstract
Spiroacetals can be formed through a one-pot sequence of a hetero Diels-Alder reaction, an oxidative carbon–hydrogen bond cleavage, and an acid treatment. This convergent approach expedites access to a complex molecular subunit that is present in numerous biologically active structures. The utility of the protocol is demonstrated through its application to a brief synthesis of the actin-binding cytotoxin bistramide A.
Keywords: cycloaddition, C–H activation, spiro compound, natural products, stereoselectivity
Transformations that generate multiple product-relevant bonds facilitate complex molecule synthesis.[1] Intermolecular cycloaddition reactions such as the hetero Diels-Alder reaction[2] are ideally suited for this objective. Oxidative carbon–hydrogen bond functionalization[3] also introduces product-relevant bonds from structurally simple precursors. This manuscript describes a telescoped sequence comprised of an asymmetric hetero Diels-Alder reaction and oxidative carbon–hydrogen bond functionalization to access spiroacetals. These units are components of numerous biologically active structures[4] and have inspired multiple synthetic approaches.[5] The mild and convergent protocol described herein provides a step economical approach to the construction of these structures. The applicability of the sequence to complex molecule synthesis is demonstrated through the total synthesis of the cytotoxin bistramide A.
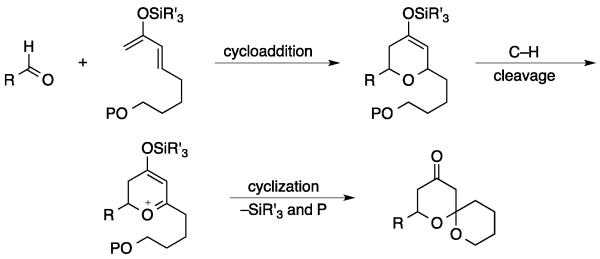
The strategy is illustrated in Scheme 1. The fragment coupling phase of this transformation can be achieved through the union of a silyloxy diene with an aldehyde to yield a 4-silyloxy dihydropyran. Related structures have been used as precursors for spiroacetals through multistep sequences.[6], [7] However the electron-rich alkene and cation-stabilizing oxygen make the dihydropyran an ideal substrate for in situ oxidative carbon–hydrogen bond cleavage by 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) in accord with our studies on related transformations.[8] The resulting dihydropyrone can undergo intramolecular nucleophilic addition[9] to yield the target product in which two rings and three bonds are created from readily-available precursors.
Scheme 1.
Convergent approach to spiroacetal synthesis.
Scheme 2 details the initial demonstration of the process. Condensing aldehyde 1 with diene 2 (neat) in the presence of Jacobsen’s catalyst (3a)[10] provided adduct 4. Diluting the crude mixture with CH2Cl2 followed by adding DDQ yielded 5 nearly instantaneously through carbon–hydrogen bond cleavage and silyl group loss from the intermediate oxocarbenium ion. The sequence was completed by protodesilylation with p-TsOH•H2O to deliver spiroacetal 6 in 78% isolated yield and 91% ee. The oxygen in the ring appears to be essential for promoting rapid oxidation in consideration of Mayr’s studies[11] showing that carbocyclic enolsilanes add to DDQ rather than form enones.
Scheme 2.
One pot enantioselective cycloaddition/oxidation spiroacetal construction. TBDPS = tert-Butyl diphenylsilyl.
A brief study on the scope of the process is shown in Table 1. Tetrahydrofuran-containing products can be prepared (entry 1) by shortening the tether between the diene and silyl ether units. Non-functionalized aliphatic aldehydes are suitable substrates (entry 2), providing the products in excellent enantiomeric purity. Aromatic aldehydes yield aryl-substituted spiroacetals (entry 3). Chiral, dienes can be employed in the process (entries 4 and 5). These reactions deliver products as single enantiomers in accord with the Horeau principle.[12] The molecular complexity of the targets in these examples suggests that this strategy is viable for late stage fragment coupling syntheses of spiroacetal containing natural products.
Table 1.
Scope exploration.[a]
| entry | diene | aldehyde | product | yield[b] (ee) |
|---|---|---|---|---|
| 1 |

|
1 |

|
72% (85%) |
| 2 | 2 |

|

|
50 (>99%) |
| 3 | 2 |

|

|
53 (84%) |
| 4 |

|
1 |

|
71 (>99%)[c] |
| 5 |

|
1 |

|
75 (>99%)[c] |
See the Supporting Information for detailed procedures, substrate syntheses, and stereochemical determination.
Yield of isolated, purified product.
Diastereomeric products were removed by chromatography.
The total synthesis of the sponge-derived cytotoxin bistramide A (17, Scheme 3)[13] was initiated to illustrate the merits of this protocol. The structure of bistramide A was confirmed through total synthesis by the Kozmin group.[14] Several total and partial syntheses of 17 and related structures subsequently appeared.[15] Crystallographic studies[16] showed that interactions between actin (the biological target)[17] and 17 are dominated by the spiroacetal subunit, leading to rational analog designs.[18]
Scheme 3.
Retrosynthetic analysis of bistramide A.
We envisioned bistramide A to arise from fragments 18 and 19 through amide bond formation. The core spiroacetal unit of 18 will be formed from the cycloaddition/oxidative cyclization between aldehyde 20 and diene 21. The righthand fragment can result from the union of amine 23 and a tetrahydropyran-contaning carboxylic acid derived from alcohol 24.
Our approach to the spiroacetal subunit (Scheme 4) commenced with the oxidation, Brown crotylation,[19] and silylation of 4-choloro-1-butanol to yield 25. Suzuki coupling[20] with iodo enone 26[21] yielded a ketone that was converted to silyloxy diene 27 under standard conditions. The spiroacetal was constructed by coupling 27 with aldehyde 28, which can be accessed in one step from (+)-β-citronellene,[22] in the presence of ent-3b (the enantiomer of 3b). DDQ treatment and acid-mediated ring closure yielded spirocycle 29 in 58% yield as a single stereoisomer. Ketone deoxygenation through a mild variant of the Wolff-Kishner reduction[23] and cross metathesis with methacrolein mediated by the Grela-Grubbs catalyst (30)[24] provided aldehyde 31. A diastereoselective addition of Me2Zn in the presence of (−)-MIB (32)[25] and the conversion of the chloride to an azido group completed the synthesis of spiroacetal subunit 33 as a single stereoisomer within the limits of NMR detection.
Scheme 4.
Synthesis of the spiroacetal subunit. Reagents and conditions: a) (COCl)2, DMSO, Et3N, CH2Cl2, −78 °C, 83%; b) trans-2-Butene, nBuLi, KOtBu, (−)-(Ipc)2BOMe, THF, then BF3•OEt2, then aldehyde, −78 °C to rt, 67%, 90% ee; c) TESCl, imidazole, CH2Cl2, quantitative; d) (9-BBN)2, THF, 0 °C to rt, then 26, Pd(dppf)Cl2, K3PO4, H2O, CH2Cl2, 73%; e) TESOTf, Et3N, CH2Cl2, 0 °C, 96%; f) 28, 3b, 4 Å MS, then DDQ, CH2Cl2, then p-TsOH•H2O, 58%; g) p-TsNHNH2, MeOH; h) NaBH3CN, THF, MeOH, pH > 4, 0 °C; i) NaOAc, EtOH, 75 °C, 54% (three steps); j) Methacrolein, 30, CH2Cl2, 40 °C, 68%; k) Me2Zn, 32, hexane, 86%; l) NaN3, DMF, 60 °C, quantitative. TES = triethylsilyl, Mes = 2,4,6-trimethylphenyl, Ipc = isopinocamphenyl, BBN = borabicyclononane, dppf = diphenylphosphino-ferrocene, OTf = trifluoromethanesulfonate, Ts = toluenesulfonyl.
We prepared the 2,6-trans-tetrahydropyran in the right fragment through homoallylic alcohol hydroformylation, oxocarbenium ion formation, and nucleophilic addition.[26] The route is shown in Scheme 5. Conversion of 1,3-propanediol to homoallylic alcohol 34 proceeded through selective monosilylation, oxidation, and asymmetric crotylation. Hydroformylation was achieved through rhodium catalysis in the presence of Breit’s exceptional DPPon ligand (35).[27] The resultant lactol was converted to an acetate group in situ to yield 36 in 91% overall yield. The addition of (E)-3-penten-2-one to 36 in the presence of TMSOTf[15b,d] provided 37, with concomitant cleavage of the silyl ether through an acidic work-up. Oxidizing the primary alcohol to a carboxylic acid was achieved through CrO3 and H5IO6. The crude acid was converted to activated ester 38 with N-hydroxysuccinimide and DCC. Coupling 38 with known β-amino acid 39[14] provided an amide that was transformed to activated ester 40 without purification.
Scheme 5.
Synthesis of the right-hand fragment. Reagents and conditions: a) TBSCl, imidazole, THF, 95%; b) SO3•Py, DMSO, Et3N, CH2Cl2, 88%; c) cis-2-Butene, nBuLi, KOtBu, (+)-(Ipc)2BOMe, THF, then BF3•OEt2, then aldehyde, −78 °C to rt, 67%, 90% ee; d) Rh(CO)2acac, 35, H2/CO (1:1, 8 atm), then Ac2O Et3N, DMAP, 91%; e) (E)-3-Penten-2-one, TMSOTf, Et3N, CH2Cl2, −78 °C, 57%; f) H5IO6, CrO3, CH3CN, H2O, 0 °C; g) N-Hydroxysuccinimide, DCC, CH3CN, 81% (two steps); h) 39, iPr2NEt, DMF; i) N-Hydroxysuccinimide, DCC, CH3CN, 50% (two steps). Py = pyridine, acac = acetylacetonate, DMAP = 4-dimethylaminopyridine, DCC = dicyclohexyl carbodiimide.
The synthesis was completed (Scheme 6) by reducing azide 33 with PMe3 in aqueous THF. The crude amine was mixed with 40 to provide bistramide A in 69% yield. The longest linear sequence in this route is 14 steps from commercially available starting materials, making this the shortest reported synthesis of this natural product (the shortest previous synthesis took 17 steps from commercially available materials). Significantly, this strategy results in a substantial reduction in the overall step count in comparison to published sequences to this family of molecules.
Scheme 6.

Completion of the synthesis. Reagents and conditions: a) PMe3, THF, H2O; b) 40, DMF, 69% (two steps).
We have demonstrated that the benefits of fragment-coupling asymmetric cycloaddition reactions can be merged with the complexity-increasing capabilities of oxidative carbon–hydrogen bond cleavage for a convergent synthesis of spiroacetals. The substrates are easily prepared, functional group tolerance is high, and stereocontrol is excellent, indicating that this protocol will be applicable to natural product synthesis. The rapid complexity that this sequence provides was exploited in the shortest reported synthesis of the actin-binding cytotoxin bistramide A.
Supplementary Material
Footnotes
This work was supported by a grant from the National Institutes of Health, Institute of General Medicine (P50-GM067082).
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/anie.201xxxxxx.
References
- [1].a) Hendrickson JB. J. Am. Chem. Soc. 1975;97:5784. [Google Scholar]; b) Wender PA, Verma VA, Paxton TJ, Pillow TH. Acc. Chem. Res. 2008;41:40. doi: 10.1021/ar700155p. [DOI] [PubMed] [Google Scholar]; c) Newhouse T, Baran PS, Hoffmann RW. Chem. Soc. Rev. 2009;38:3010. doi: 10.1039/b821200g. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Gaich T, Baran PS. J. Org. Chem. 2010;75:4657. doi: 10.1021/jo1006812. [DOI] [PubMed] [Google Scholar]
- [2].For reviews, see: Pellissier H. Tetrahedron. 2009;65:2839. Danishefsky SJ. Aldrichimica Acta. 1986;19:59.
- [3].a) Gutekunst WR, Baran PS. Chem. Soc. Rev. 2011;40:1976. doi: 10.1039/c0cs00182a. [DOI] [PubMed] [Google Scholar]; b) Brückl T, Baxter RD, Ishihara Y, Baran PS. Acc. Chem. Res. 2012;45:826. doi: 10.1021/ar200194b. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Yamaguchi J, Yamaguchi AD, Itami K. Angew. Chem. 2012;124:9092. doi: 10.1002/anie.201201666. [DOI] [PubMed] [Google Scholar]; Angew. Chem., Int. Ed. 2012;51:8960. [Google Scholar]; d) Chen DY-K, Youn SW. Chem. Eur. J. 2012;18:9452. doi: 10.1002/chem.201201329. [DOI] [PubMed] [Google Scholar]
- [4].a) Francke W, Kitching W. Curr. Org. Chem. 2001;5:233. [Google Scholar]; b) Jacobs MF, Kitching W. Curr. Org. Chem. 1998;2:395. [Google Scholar]
- [5].a) Palmes JA, Aponick A. Synthesis. 2012;44:3699. [Google Scholar]; b) Raju BR, Saikia AK. Molecules. 2008;13:1942. doi: 10.3390/molecules13081942. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Aho JE, Pihko PM, Rissa TK. Chem. Rev. 2005;105:4406. doi: 10.1021/cr050559n. [DOI] [PubMed] [Google Scholar]; d) Mead KT, Brewer BN. Curr. Org. Chem. 2003;7:227. [Google Scholar]
- [6].a) Danishefsky SJ, Pearson WH. J. Org. Chem. 1983;48:3865. [Google Scholar]; b) Danishefsky SJ, Armistead DM, Wincott FE, Selnick HG, Hungate R. J. Am. Chem. Soc. 1989;111:2976. [Google Scholar]
- [7].For reviews of oxidative and cycloaddition-based approaches to spiroacetals, see: Sperry J, Liu Y-C, Brimble MA. Org. Biomol. Chem. 2010;8:29. doi: 10.1039/b916041h. Rizzacasa MA, Pollex A. Org. Biomol. Chem. 2009;7:1053. doi: 10.1039/b819966n.
- [8].a) Peh GR, Floreancig PE. Org. Lett. 2012;14:5614. doi: 10.1021/ol302744t. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Han X, Floreancig PE. Org. Lett. 2012;14:3808. doi: 10.1021/ol301720u. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Cui Y, Floreancig PE. Org. Lett. 2012;14:1720. doi: 10.1021/ol3002877. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Brizgys GJ, Jung HH, Floreancig PE. Chem. Sci. 2012;3:438. [Google Scholar]; e) Jung HH, Floreancig PE. Tetrahedron. 2009;65:10830. doi: 10.1016/j.tet.2009.10.088. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Liu L, Floreancig PE. Org. Lett. 2009;11:3152. doi: 10.1021/ol901188q. [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Liu L, Floreancig PE. Angew. Chem. 2010;122:6030. [Google Scholar]; Angew. Chem. Int. Ed. 2010;49:5894. [Google Scholar]; h) Liu L, Floreancig PE. Angew. Chem. 2010;122:3133. [Google Scholar]; Angew. Chem Int. Ed. 2010;49:3069. [Google Scholar]; i) Tu W, Floreancig PE. Angew. Chem. 2009;121:4627. doi: 10.1002/anie.200901489. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2009;48:4567. [Google Scholar]; j) Tu W, Liu L, Floreancig PE. Angew. Chem. 2008;120:4252. doi: 10.1002/anie.200706002. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2008;47:4184. [Google Scholar]
- [9].For another example of spiroacetal formation through a formal hydride loss from an oxacycle, see: Donohoe TJ, Lipiński RM. Angew. Chem. 2013;125:2551. doi: 10.1002/anie.201208919. Angew. Chem. Int. Ed. 2013;52:2491.
- [10].Dossetter AG, Jamison TF, Jacobsen EN. Angew. Chem. 1999;111:2549. doi: 10.1002/(sici)1521-3773(19990816)38:16<2398::aid-anie2398>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]; Angew Chem. Int. Ed. 1999;38:2398. [Google Scholar]
- [11].Guo X, Mayr H. J. Am. Chem. Soc. 2013;135:12377. doi: 10.1021/ja405890d. for related earlier studies, see: Ryu I, Murai S, Hatayama Y, Sonoda N. Tetrahedron Lett. 1978;19:3455. Bhattacharya A, DiMichele LM, Dolling U-H, Grabowski EJJ, Grenda VJ. J. Org. Chem. 1989;54:6118. Hodgson DM, Moreno-Clavijo E, Day SE, Man S. Org. Biomol. Chem. 2013;11:5362. doi: 10.1039/c3ob41251b.
- [12].The Horeau principle describes the statistical enantiomeric excess enhancement when two enantiomerically enriched species are coupled due to diastereomer formation. For a review see: Rautenstrauch V. Bull. Chem. Soc. Fr. 1994;131:515.
- [13].a) Gouiffès D, Moreau S, Helbecque N, Berrier JL, Hénichart JP, Barbin Y, Laurent D, Verbist JF. Tetrahedron. 1988;44:451. [Google Scholar]; b) Griffiths G, Garrone B, Deacon E, Owen P, Pongracz J, Mead G, Bradwell A, Watters D, Lord J. Biochem. Biophys. Res. Commun. 1996;222:802. doi: 10.1006/bbrc.1996.0830. [DOI] [PubMed] [Google Scholar]; c) Foster MP, Mayne CL, Dunkel R, Pugmire RJ, Grant DM, Kornprobst JM, Verbist JF, Biard JF, Ireland CM. J. Am. Chem. Soc. 1992;114:1110. [Google Scholar]
- [14].Statsuk AV, Liu D, Kozmin SA. J. Am. Chem. Soc. 2004;126:9546. doi: 10.1021/ja046588h. [DOI] [PubMed] [Google Scholar]
- [15].a) Wipf P, Hopkins TD. Chem. Commun. 2005:3421. doi: 10.1039/b505100b. [DOI] [PubMed] [Google Scholar]; b) Crimmins MT, DeBaille AC. J. Am. Chem. Soc. 2006;128:4936. doi: 10.1021/ja057686l. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Lowe JT, Wrona IE, Panek JS. Org. Lett. 2007;9:327. doi: 10.1021/ol062957y. [DOI] [PubMed] [Google Scholar]; d) Yadav JS, Chetia L. Org. Lett. 2007;9:4587. doi: 10.1021/ol702095n. [DOI] [PubMed] [Google Scholar]; e) Tomas L, af Gennäs GB, Hiebel MA, Hampson P, Gueyrard D, Pelotier B, Yli-Kauhaluoma J, Piva O, Lord JM, Goekjian PG. Chem. Eur. J. 2012;18:7452. doi: 10.1002/chem.201102462. [DOI] [PubMed] [Google Scholar]; f) Commandeur M, Commandeur C, Cossy J. Org. Lett. 2011;13:6018. doi: 10.1021/ol202483u. [DOI] [PubMed] [Google Scholar]
- [16].Rizvi SA, Tereshko V, Kossiakoff AA, Kozmin SA. J. Am. Chem. Soc. 2006;128:3882. doi: 10.1021/ja058319c. [DOI] [PubMed] [Google Scholar]
- [17].a) Statsuk AV, Bai R, Baryza JL, Verma VA, Hamel E, Wender PA, Kozmin SA. Nature Chem. Biol. 2005;1:383. doi: 10.1038/nchembio748. [DOI] [PubMed] [Google Scholar]; b) Rizvi SA, Courson DS, Keller VA, Rock RS, Kozmin SA. Proc. Natl. Acad. Sci. USA. 2008;105:4088. doi: 10.1073/pnas.0710727105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Rizvi SA, Liu S, Chen Z, Skau C, Pytynia M, Kovar DR, Chmura SJ, Kozmin SA. J. Am. Chem. Soc. 2010;132:7288. doi: 10.1021/ja101811x. [DOI] [PubMed] [Google Scholar]
- [19].Brown HC, Bhat KS. J. Am. Chem. Soc. 1986;108:293. [Google Scholar]
- [20].Miyaura N, Ishiyama T, Sasaki H, Ishikawa M, Satoh M, Suzuki A. J. Am. Chem. Soc. 1989;111:314. [Google Scholar]
- [21].Rivero MR, Buchwald SL. Org. Lett. 2007;9:973–976. doi: 10.1021/ol062978s. [DOI] [PubMed] [Google Scholar]
- [22].Fürstner A, Feyen F, Prinz H, Waldmann H. Angew. Chem. 2003;115:5519. doi: 10.1002/anie.200352268. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2003;42:5361. [Google Scholar]
- [23].Nair V, Sinhababu A. J. Org. Chem. 1978;43:5013. [Google Scholar]
- [24].Grela K, Harutyunyan S, Michrowska A. Angew. Chem. 2002;114:4210. doi: 10.1002/1521-3773(20021104)41:21<4038::AID-ANIE4038>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2002;41:4038. [Google Scholar]
- [25].Nugent WA. Chem. Commun. 1999:1369. [Google Scholar]
- [26].Jung HH, Seiders JR, II, Floreancig PE. Angew. Chem. 2007;119:8616. doi: 10.1002/anie.200702999. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2007;46:8464. [Google Scholar]
- [27].a) Breit B, Seiche W. J. Am. Chem. Soc. 2003;125:6608. doi: 10.1021/ja0348997. [DOI] [PubMed] [Google Scholar]; b) Gellrich U, Seiche W, Keller M, Breit B. Angew. Chem. 2012;124:11195. doi: 10.1002/anie.201203768. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2012;51:11033. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.