Acute upper gastrointestinal bleeding accounts for a significant number of hospital admissions per year, and is a major contributor to mortality and morbidity. Despite advances in endoscopic hemostatic techniques and pharmacological management, previous studies showed that a subset of patients tend to rebleed within three days of initial hemostatic treatment. However, many of these earlier studies excluded individuals who did not necessarily reflect contemporary patient populations, nor have more recent randomized clinical trials clarified current rebleeding rates. Accordingly, this comprehensive review aimed to shed light on the timing of peptic ulcer rebleeding in patients with high-risk stigmata.
Keywords: Endoscopic therapy, High-risk stigmata, Peptic ulcer bleeding, Rebleeding, Timing
Abstract
BACKGROUND:
Peptic ulcer rebleeding (PUR) usually occurs within three days following endoscopic hemostasis. However, recent data have increasingly suggested delayed rebleeding.
OBJECTIVE:
To better characterize the timing of PUR (Forrest Ia to IIb) following initially successful endoscopic hemostasis.
METHODS:
An exhaustive literature search (1989 to 2013), with cross-referencing, was performed to identify pertinent randomized controlled trial (RCT) arms. Patients receiving high-dose proton pump inhibitor (PPI) infusion following successful modern-day endoscopic hemostasis were included. A sensitivity analysis included any patients receiving PPI doses >40 mg daily. The main outcome measure was 30-day rebleeding, while weighted mean averages at t = three, seven, 14 and 28 to 30 days are also reported.
RESULTS:
Of 756 citations, six RCTs were included (561 patients; 58.5% to 89.5% male; 55.3 to 67.5 years of age). Among patients receiving high-dose PPI (five RCTs [393 patients]), 11.5% (95% CI 8.4% to 14.7%) experienced rebleeding, 55.6% (95% CI 41.1% to 70.1%) rebled within three days, 20% (95% CI 8.3% to 31.7%) between four and seven days, 17.8% (95% CI 6.6% to 28.9%) at eight to 14 days, and 6.7% (95% CI 0% to 14%) at 15 to 28 to 30 days. Using the relaxed lower PPI dosing threshold, similar respective rates were 14.4% (95% CI 11.5% to 17.3%) overall, with interval rates of 39.5% (95% CI 28.9% to 50.15%), 34.6% (95% CI 24.2% to 44.9%), 19.7% (95% CI 11% to 28.4%) and 6.2% (95% CI 0.95% to 11.5%). Qualitative review of patient characteristics, limited by small sample size, possible bias and study heterogeneity, suggested increased patient comorbidity and postendoscopic use of lower PPI dosing may predict delayed rebleeding.
CONCLUSION:
In patients with high-risk PUR undergoing successful endoscopic hemostasis, most rebled within three days, with many experiencing later rebleeding. Additional research is needed to better predict such an outcome.
Abstract
HISTORIQUE :
La reprise du saignement d’un ulcère gastroduodénal (RSUG) se produit généralement dans les trois jours suivant l’hémostase endoscopique. Cependant, des données récentes indiquent de plus en plus une reprise tardive du saignement.
OBJECTIF :
Mieux caractériser le moment de la RSUG (Forrest Ia à IIb) après une hémostase endoscopique d’abord réussie.
MÉTHODOLOGIE :
Les chercheurs ont procédé à une analyse bibliographique complète (de 1989 à 2013) avec référencement pour extraire les essais aléatoires et contrôlés pertinents (EAC). Étaient inclus les patients qui recevaient une infusion d’inhibiteur de la pompe à protons (IPP) à forte dose après une hémostase endoscopique moderne réussie. L’analyse de sensibilité incluait tous les patients ayant reçu une dose quotidienne d’IPP supérieure à 40 mg. La principale mesure d’issue était une reprise du saignement dans les 30 jours, tandis que les moyennes pondérées à t=trois, sept, 14 et 28 à 30 jours étaient également précisées.
RÉSULTATS :
Six EAC faisaient partie des 756 articles (561 patients; 58,5 % à 89,5 % d’hommes; 55,3 à 67,5 ans). Chez les patients qui avaient reçu un IPP à forte dose (cinq EAC [393 patients]), 11,5 % (95 % IC 8,4 % à 14,7 %) ont subi une reprise des saignements, 55,6 % (95 % IC 41,1 % à 70,1 %) se sont remis à saigner dans les trois jours, 20 % (95 % IC 8,3 % à 31,7 %) au bout de quatre à sept jours, 17,8 % (95 % IC 6,6 % à 28,9 %) au bout de huit à 14 jours, et 6,7 % (95 % IC 0 % à 14 %) au bout de 15 à entre 28 et 30 jours. Selon un seuil posologique rabaissé d’IPP, les taux similaires respectifs étaient de 14,4 % (95 % IC 11,5 % à 17,3 %) dans l’ensemble, selon des taux d’intervalle de 39,5 % (95 % IC 28,9 % à 50,15 %), 34,6 % (95 % IC 24,2 % à 44,9 %), 19,7 % (95 % IC 11 % à 28,4 %) et 6,2 % (95 % IC 0,95 % à 11,5 %). Une analyse qualitative des caractéristiques des patients, limitée par la petite taille de l’échantillon, les biais possibles et l’hétérogénéité des études, a laissé supposer qu’une comorbodité accrue des patients et une utilisation postendoscopique de posologies d’IPP plus faibles peuvent être prédictives d’une reprise tardive du saignement.
CONCLUSION :
La plupart des patients très vulnérables à une RSUG qui subissent une hémostase endoscopique réussie se remettent à saigner dans les trois jours, et bon nombre présentent une reprise tardive du saignement. D’autres recherches s’imposent pour mieux prédire un tel résultat.
Acute upper gastrointestinal bleeding accounts for >400,000 hospitalizations per year in the United States, with the majority of cases being nonvariceal in origin. Peptic ulcer bleeding accounts for most cases of nonvariceal upper gastrointestinal bleeding and is a major cause of morbidity and mortality (1,2). Recent advances in endoscopic hemostatic techniques and the use of high-dose intravenous (IV) proton pump inhibitors (PPIs) have been shown to improve outcomes in peptic ulcer bleeding (3). However, approximately 11% to 16% of patients with ulcers with high-risk stigmata (Forrest Ia to IIb) rebleed after initial endoscopic hemostasis, with most rebleeding reported to occur in the first 72 h (4–6).
The finding that most rebleeding occurs within the first three days is supported by several studies in which endoscopic follow-up of Forrest Ia to IIb ulcers showed healing with a clean base by day 3 to 4 (7–11); however, most of these older studies excluded patients with comorbidities, or patients on anticoagulants or nonsteroidal anti-inflammatory drugs (NSAIDs), which is not reflective of a contemporary patient population (7,9,11). In addition to these landmark studies, most of the current data on peptic ulcer rebleeding are derived from trials using endoscopic hemostatic techniques or pharmacological therapies that are not consistent with current recommendations, with use of epinephrine injection alone, low PPI doses or no PPI pharmacotherapy. In fact, more recent data from randomized trials suggest higher rates of rebleeding after three days (12–18).
The timing of rebleeding in the era of high-dose PPI and modern-day endoscopic therapy thus remains unclear. In the context of increasing health care costs and pressure to discharge patients sooner, with many centres not tightly adhering to guidelines (19,20), it is particularly important to clarify the timing of rebleeding.
The aim of the present systematic review was, accordingly, to examine the timing of peptic ulcer rebleeding in patients who exhibited high-risk stigmata having received recommended contemporary endoscopic and pharmacological therapies.
METHODS
Search strategy
A comprehensive computerized medical literature search was performed using the MEDLINE, EMBASE, Cochrane library and ISI Web of Knowledge databases from 1989 to September 2013. A highly sensitive search strategy was used to identify randomized controlled trials with a combination of controlled vocabulary and text words related to “upper gastrointestinal bleeding”, “endoscopic therapy” and “PPIs”. In addition, recursive searches and cross-referencing were performed; manual searches of articles identified after the initial search were also conducted.
Study selection
All human, adult studies published in French or English were considered. All randomized controlled trials were included if the study population fulfilled the following criteria: patients with peptic ulcer bleeding exhibiting high-risk stigmata (Forrest Ia to IIb); patients in whom successful initial hemostasis was achieved using contemporary endoscopic hemostatic methods (excluding epinephrine monotherapy), followed by PPIs at a dose >40 mg once daily. In addition, recorded outcomes needed to include rebleeding at different time points, up to 30 days. Any treatment arms of any of the studies not fulfilling these criteria were excluded, as were patients who underwent second-look endoscopy.
Validity assessment and data abstraction
Two reviewers independently identified and examined the relevant studies. A third independent reviewer resolved disagreements on specific studies. The quality of each study was assessed using modified Jadad criteria, in which an additional point was attributed for the description of allocation concealment, a priori sample size estimation, description and number of drop-outs, adequate description of the population selection and characteristics, for a total of 10 points from the initial five-point score (21). The Cochrane risk-of-bias tool (22) was also used.
Assessment of heterogeneity
Comparative qualitative analyses evaluated the homogeneity of study characteristics, such as patient populations, interventions and outcomes across studies, guiding possible sensitivity analyses.
Statistical heterogeneity was not sought because no inferential calculations were performed as part of the present analysis.
Principal outcome, data synthesis and analysis
Among all trials selected, only the arms satisfying the aforementioned criteria were considered.
Only descriptive data were generated from the present analysis, including patient characteristics and the main outcome of time to rebleeding.
Rebleeding at different time points – three days, seven days, 14 days, and 28 to 30 days – was examined. Weighted averages of rebleeding rates among all included studies were calculated for each time point; 95% CIs were also reported. Additional data pertaining to different patient characteristics and previously recognized predictors of rebleeding were extracted and analyzed. The primary analysis included the assessment of study arms having administered only high-dose IV PPI (80 mg bolus followed by 8 mg/h for three days); a planned sensitivity analysis examined studies including any dose of PPI >40 mg daily.
All statistical analyses were performed using SAS version 9.2 (SAS Institute Inc, USA).
RESULTS
Study identification
A total of 756 citations were initially identified; reasons for excluding studies are listed in Figure 1. Randomized controlled trials were excluded if epinephrine monotherapy was used in part of the patient population, such as in the study by Sung et al (15). Overall, eight arms from six full-text randomized controlled trials (13,16,18,23–25) were included, yielding a total of 561 patients.
Figure 1).
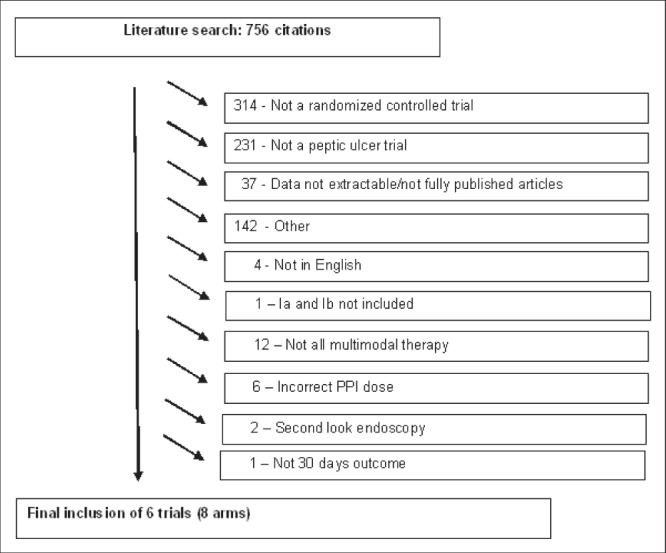
STROBE (STrengthening the Reporting of OBservational studies in Epidemiology) diagram. PPI Proton pump inhibitor
High-dose PPI (80 mg IV bolus followed by 8 mg/h for 72 h) was used in five study arms (13,16,18,23,24), which comprised the primary analysis, yielding a total of 393 patients. The sensitivity analysis included any studies using PPI doses >40 mg (total of the above eight arms).
Study characteristics
Characteristics of the included studies are shown in Table 1 and summarized below.
TABLE 1.
Study characteristics
| Characteristic | Author (reference), year | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Chen et al (23), 2012 | Chiu et al (25), 2003 (low-dose PPI)* | Lau et al (24), 2000 | Cheng et al (16), 2005 | Cheng et al (16), 2005 (low-dose PPI) | Zargar et al (13), 2006 | Choi et al (18), 2009 | Choi et al (18), 2009 (low-dose PPI) | |
| Forrest classification | Ia to IIa | Ia to IIb | Ia to IIa | SRH, Major | SRH, Major | Ia to IIa | Ia to IIb | Ia to IIb |
| Study period | January 2008 to August 2010 | August 1999 to January 2001 | May 1998 to July 1999 | January 2001 to April 2003 | January 2001 to April 2003 | January 2001 to August 2003 | May 2004 to April 2008 | May 2004 to April 2008 |
| n in arm | 100 | 100 | 120 | 52 | 53 | 102 | 19 | 21 |
| ASA score (1 to 5) | 1 or 2: 65% | 1: 43% | 1 or 2: 58.3% | 2: 7% | 2: 4% | N/A | N/A | N/A |
| 3: 26% | 2: 37% | 3: 26.7% | 3: 26% | 3: 29% | ||||
| 4: 8% | 3: 15% | 4: 15% | 4: 19% | 4: 19% | ||||
| 4: 1% | 5: 1% | 5: 0% | ||||||
| Hemodynamic Instability, % | 28.00 | 46.80 | 13.30 | N/A | N/A | 27.50 | N/A | N/A |
| Comorbid illness, % | N/A | 69.10 | 25 | 100 | 100 | 24.5 | 42.1 | 47.60 |
| Active bleeding, % | 42.00 | 49 | 53.3 | N/A | N/A | 36.3 | 31.6 | 14.3 |
| Ulcer size, cm, mean ± SD | 1.09±0.54 | 0.9±0.5 | 1.2±1.1 | 1.2±0.8 | 1.2±1 | 1.2±0.8 | 1.4±1.2 | 1.4±0.8 |
| Duodenal ulcer, % | 47.00 | 57.00 | 54.20 | 36.50 | 37.70 | 82.40 | 57.90 | 38.10 |
| Gastric ulcer, % | 53.00 | 43.00 | 44.00 | 53.80 | 54.70 | 17.60 | 42.10 | 61.90 |
| Endoscopic treatment | Epinephrine injection + heater probe | Epinephrine injection + heater probe | Epinephrine injection + heater probe | Epinephrine injection + heater probe | Epinephrine injection + heater probe | Epinephrine injection + heater probe | Epinephrine injection + APC or hemoclips | Epinephrine injection + APC or hemoclips |
| Pharmacological treatment | Pantoprazole 80 mg IV bolus then 8 mg/h × 72 h | Omeprazole 40 mg IV BID × 3 days | Omeprazole 80 mg IV bolus then 8 mg/h × 72 h | Omeprazole 80 mg IV bolus then 200 mg/day × 72 h | Omeprazole 80 mg IV bolus then 80 mg/day × 72 h | Pantoprazole 80 mg IV bolus then 8 mg/h × 72 h | Pantoprazole 80 mg IV bolus then 8 mg/h × 72 h | Pantoprazole 40 mg IV bolus then 4 mg/h × 72 h |
| NSAID use, % | 39 | 6.4† | 32.5 | 25 | 22.60 | 18 | 57.90 | 57.10 |
| Age, years, mean ± SD | 65.5 (15) | 67.5 (12.6) | 64 (17.2) | 62.5 (12.5) | 65.8 (13.8) | 55.3 (9.2) | 61 (16.5) | 57.4 (14.5) |
| Sex, male/female, %/% | 79/21 | 66/34 | 66.7/33.3 | 69.2/30.8 | 58.5/41.5 | 68.6/31.4 | 89.5/10.5 | 85.7/14.3 |
| Modified Jadad score | 9 | 6 | 8 | 6 | 6 | 10 | 6 | 6 |
No second-look endoscopy arm;
Only acetylsalicylic acid use reported. APC Argon plasma coagulation; ASA American Association of Anesthesiologists; BID Twice per day; IV Intravenous; N/A Not applicable; NSAID Nonsteroidal anti-inflammatory drug; PPI Proton pump inhibitor; SRH Stigmata of recent hemorrhage
Study populations:
All patients exhibited bleeding gastric or duodenal ulcers demonstrating high-risk stigmata. Most studies used the Forrest classification and included Forrest type Ia to IIb ulcers (Ia: spurting; Ib: oozing; IIa: visible vessel; and IIb: adherent clot). One study classified ulcers according to stigmata of recent hemorrhage (16), either major or minor in type, as described in the study by Yang et al (10). The patients ranged in age from 55.3 to 67.5 years, with 58.5% to 89.5% being male. Active bleeding (Forrest Ia and Ib) occurred in 14.3% patients (18) to up to 53.3% (24). The presence of ≥1 comorbidities ranged from 24.5% of patients (13) to 100% of patients in the study by Cheng et al (16). Hemodynamic instability or shock was recorded in four of six studies. It ranged from 13.3% (24) to up to 46.8% of patients in the study by Chiu et al (25). The proportion of patients categorized according to American Society of Anesthesiologists (ASA) scores varied (Table 1). The use of NSAIDs was recorded in almost all studies and ranged from 18% (13) to up to 57.9% (18). Mean (± SD) ulcer size ranged from to 0.9±0.5 cm (25) to 1.4±1.2 cm (18).
Outcome definition
The definition of rebleeding was similar among all studies. Rebleeding was defined clinically according to different parameters, including the presence of melena, hematemesis or fresh blood in the nasogastric tube, a change in vital signs (systolic blood pressure <90 mmHg, heart rate >100 beats/min to 110 beats/min), drop in hemoglobin level, or sudden increase in transfusion requirements. Following clinical suspicion, rebleeding was then confirmed on endoscopy.
Performance of endoscopy and adjuvant therapy
Initial endoscopy was performed within 24 h of admission in all studies. In five of the six trials, endoscopic therapy involved epinephrine injection combined with thermocoagulation. In the study by Choi et al (18), epinephrine was used in addition to argon plasma coagulation and/or application of hemoclips.
Different PPI regimens were used among the studies with lower PPI dosing. One arm of the trial by Choi et al (18) used pantoprazole 40 mg IV bolus followed by 4 mg/h for 72 h (18). One arm of the study by Cheng et al (25) used omeprazole 80 mg IV bolus followed by 80 mg infusion per day for three days, and Chiu et al (16) used omeprazole 40 mg IV twice daily for three days (25).
One-half of the trials used pantoprazole (13,18,23), while the other one-half used omeprazole (16,24,25).
Study quality
The quality of studies on the modified Jadad score varied from 6 to 10 points (Table 1), with a mean of 7.5±1.8. The Cochrane risk-of-bias tool revealed overall low bias with a potential of bias for Choi et al (18) and Chen et al (23), in which the treatment allocation was not blinded (Figure 2).
Figure 2).
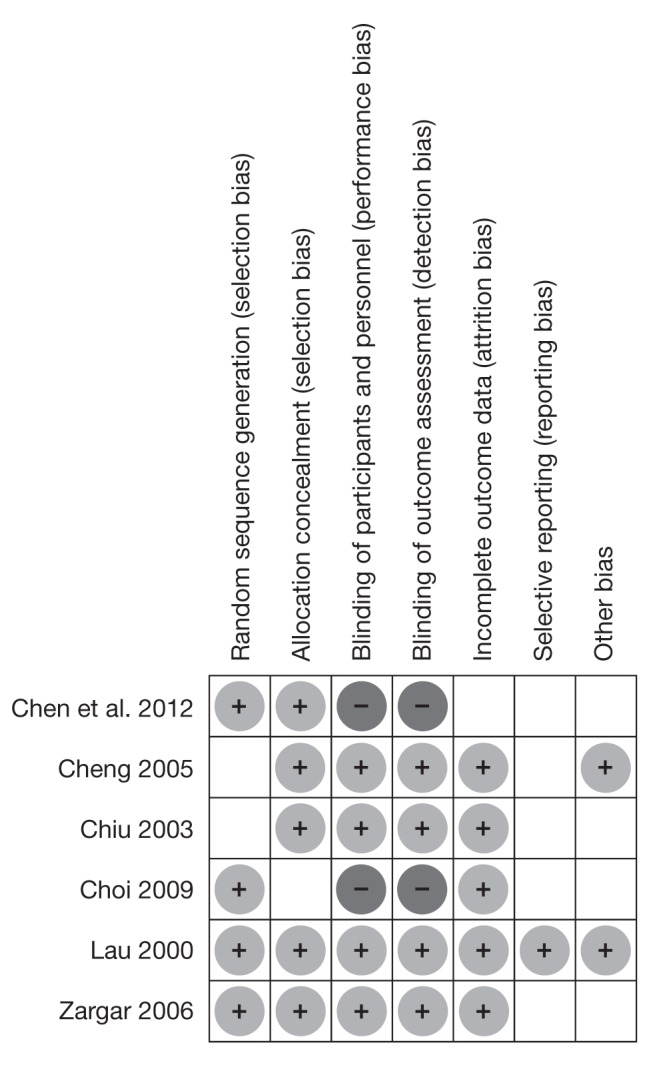
Risk of bias summary
Primary analysis: rebleeding in patients receiving high-dose PPIs
Among the 393 patients receiving high-dose PPI, 45 rebled within the first 30 days, corresponding an overall rate of rebleeding of 11.5% (95% CI 8.4% to14.7%). The rebleeding rate, broken down according to time following initial endoscopic hemostasis, is shown in Figure 3, with 55.6% rebleeding occurring within the first three days.
Figure 3).
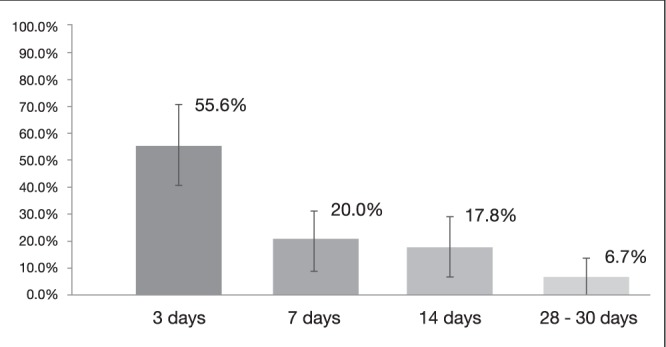
Rebleeding rates at different time points in patients receiving high-dose proton pump inhibitors (95% CIs represented by vertical lines)
Sensitivity analysis: rebleeding in patients receiving PPI doses >40 mg once daily
When including all patients receiving PPI at a dose >40 mg once daily, 81 of 561 patients rebled at 30 days, corresponding to an overall rebleeding rate of 14.4% (95% CI 11.5% to 17.3%). In this broader patient population, among those who rebled, 39.5% (95% CI 28.9% to 50.15%) did so within three days.
Of note, in the study by Cheng at al (16), rebleeding rates showed a different trend. In the high-dose PPI arm, the overall rebleeding rate was 40.4% at 30 days. Among the patients who rebled, 38.1% did so by day 3, 14.3% between days 4 and 7, 38.1% between seven and 14 days, and 9.5% between day 14 and 28 to 30. An exploratory a posteriori sensitivity analysis was subsequently performed. When excluding this study, the rebleeding rate among studies using high-dose PPI at day 3 was 70.8%, 25% between days 4 and 7, and 4.2% between day 15 and 28 to 30. Among studies using all PPI doses, rebleeding was 48.6%, 48.6% and 2.7% at day 3, between days 4 to 7, and between day 14 and 28 to 30, respectively.
DISCUSSION
The timing of rebleeding after endoscopic hemostasis in peptic ulcer bleeding remains unclear in the era of high-dose PPI and contemporary endoscopic therapy. Most of the current data regarding the timing of peptic ulcer rebleeding are obtained from studies using endoscopic hemostatic techniques and pharmacological therapies that are not consistent with current recommendations, with no PPI pharmacotherapy or low PPI doses, as well as epinephrine injection alone.
The finding that most rebleeding occurs within the first three days is also supported by several landmark studies in which endoscopic follow-up of ulcers showed healing with a clean base by day 3 to 4; however, most of these older studies excluded patients on NSAIDs or anticoagulants, and patients with any comorbidities, which is not reflective of a contemporary patient population (7,9,11).
The aim of the present systematic review was, thus, to better examine the timing of peptic ulcer rebleeding in patients exhibiting high-risk stigmata who received recommended contemporary endoscopic and pharmacological therapies.
Our primary analysis evaluated studies using high-dose PPI infusion. Among patients who rebled, 55.6% did so within three days. This is higher than what has been reported in the literature, with rebleeding rates of up to 95% in the first three days (7,8,11,26).
When analyzing rebleeding rates among the included studies, the study by Cheng et al (16) appears to stand out, demonstrating different trends in rebleeding with an overall high rate of rebleeding of 40.4%. Furthermore, among patients who rebled, only 38.1% did so by day 3. When excluding this outlier study, 70.8% of patients rebled by day 3, which is more consistent with rates reported in the literature.
We closely examined the study by Cheng et al (16) to better understand the difference in rebleeding trends reported in this trial. In this study, patients had to have at least one comorbidity to be included. In other words, 100% of their patients had at least one other coexisting illness, which is higher than all other included studies that enrolled patient populations without comorbidities in proportions of up to 75%. Furthermore, in the study by Cheng et al (16), up to 48.1% had ≥2 comorbidities. Although they did not report the rates of hemodynamic instability or shock, 48% of their patients had an ASA score ≥3, demonstrating a sicker patient profile than all other included studies. When including all studies using PPI doses >40 mg daily, only 39.5% of patients who rebled did so within the first 72 h, with the remainder rebleeding mostly between days 4 and 7. When excluding the ‘outlier’ study by Cheng et al (16), the trend toward increased delayed rebleeding persisted, with 51.3% of patients rebleeding after the three-day period, including 48.6% bleeding by day 7.
To determine why most patients rebled after the three-day period in the lower-dose PPI analysis, we performed an explorative qualitative review, comparing patient characteristics among the included studies. Unfortunately, no patient-level data were available. However, on qualitatively reviewing the data, several factors appeared to be possibly associated with delayed rebleeding.
First, higher rates of delayed rebleeding were found when including studies using PPIs at lower doses, which suggests that PPI dosing may not only affect rebleeding rates, as it is already established, but may also impact the timing of rebleeding. Interestingly, a recently published RCT demonstrated decreased rates of delayed peptic ulcer rebleeding among high-risk patients receiving a prolonged 11-day course of twice daily PPI dosing (27).
In addition to lower PPI doses, the presence of coexisting illness may also impact the timing of rebleeding. Among the included studies, trials enrolling patients with higher rates of comorbidities appear to demonstrate delayed rebleeding. Cheng et al (16), as described above, included all patients with at least one comorbidity and had higher rates of delayed rebleeding. Another included study, by Chiu et al (25), had patients with high rates of comorbidities (up to 69.1%). Rebleeding was also delayed in this trial, with most patients rebleeding between days 4 and 7. Choi et al (18) included up to 47.6% of patients with comorbidities (up to 42.1% in the high-dose PPI arm). In these arms, the only patient who rebled did so on day 6 – after the 72 h period.
The presence of comorbidities as a factor leading to increased rebleeding and mortality has been demonstrated in the literature (3,28–30); however, its association with delayed rebleeding had not yet been shown. In the present systematic review, no data could be extracted regarding the different types of comorbidities because most studies reported these without further categorization according to system. Cheng et al (16) identified the presence of hypoalbuminemia (albumin <30 g/L) as well as having ≥2 comorbidities as associated with a higher trend of rebleeding between days 4 and 14 in univariable analysis. End-stage renal disease (ESRD) was also associated, but with more delayed rebleeding. Only ESRD was significantly increased among patients who rebled between days 15 to 28 in multivariable analysis.
Patients with renal disease and ESRD are not only at increased risk for upper gastrointestinal bleeding (31), but also appear to have worse outcomes related to peptic ulcer bleeding. Patients with ESRD exhibit higher rates of rebleeding (up to 40.6% in study by Cheung et al [32]), as well as increased delayed rebleeding (beyond seven days) (16,33). ESRD has also been associated with increased long-term peptic ulcer rebleeding (34).
Patients with ESRD may be at higher risk for peptic ulcer rebleeding due to multiple factors, mainly bleeding diathesis. This is believed to be due to platelet dysfunction in the context of uremia, as well as impaired platelet-vessel wall interaction (35). Dialysis may also contribute to increased bleeding through exposure to heparin as well as through continuous platelet activation, due to interaction of blood with artificial surfaces (35,36).
In addition, ESRD is associated with lower albumin levels and poor nutrition, thereby possibly leading to slower ulcer healing and, perhaps, a greater risk of rebleeding (33). The impact of gastric acid secretion remains unclear because it has been shown to be increased in some studies, and decreased or normal in others (33,37).
Hemodynamic instability and higher ASA scores have also been shown in the literature to be associated with higher rates of rebleeding and overall poorer prognosis (3,38–40).
Interestingly, in the present analysis, studies demonstrating higher rates of delayed rebleeding had higher rates of unstable patients as well as a higher number of patients with an ASA score >3 (16,25). However, hemodynamic instability and ASA score were not systematically recorded in all included studies.
CONCLUSION
The results of the present systematic review appear to demonstrate higher rates of delayed rebleeding when including studies using modern-day endoscopic hemostatic technique as well as intravenous PPI. A qualitative review of patient characteristics from studies exhibiting delayed rebleeding suggests that possible associations may include increased patient comorbidity and use of lower doses of PPI following endoscopic hemostasis, as well as increased hemodynamic instability and higher ASA scores. However, such associations are limited by lack of patient-level data and the limited number of studies available. In addition, there existed significant clinical heterogeneity. Timing of rebleeding among patients receiving modern-day therapy and determinants of delayed rebleeding should be further explored in future research, as this could greatly impact on the outcomes of patients with peptic ulcer bleeding (and perhaps other etiologies) by adapting care to patients at increased risk for delayed rebleeding, through the use of second-look endos-copy, or better adapted follow-up oral PPI dosing.
Figure 4).
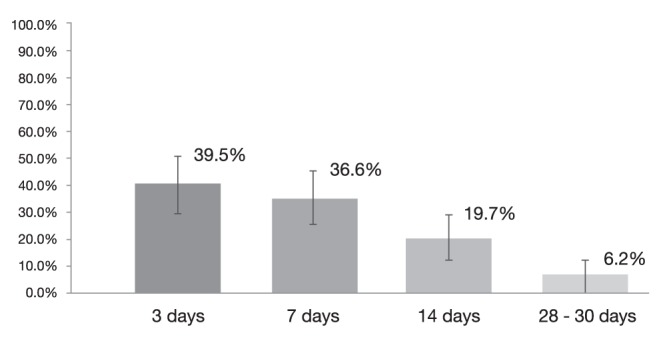
Rebleeding rates at different time points in patients receiving lower doses of proton pump inhibitors
Footnotes
DISCLOSURES: Dr Alan Barkun is a consultant for Olympus and Takeda Canada; is a speaker for Takeda Canada and AstraZeneca; is on the advisory board for Pendopharm Canada; and received research funding from Boston Scientific and Cook. Dr Sara El Ouali, Ms Myriam Martel and Dr Davide Maggio have no financial disclosures or conflicts of interest to declare.
FUNDING: None.
AUTHOR’S CONTRIBUTIONS: Conception and design: Sara El Ouali, Alan N Barkun, Myriam Martel and Davide Maggio; analysis and interpretation of data: Sara El Ouali, Alan N Barkun, Myriam Martel and Davide Maggio. Drafting of the manuscript: Sara El Ouali, Alan N Barkun, Myriam Martel and Davide Maggio. Critical revision of the article for important intellectual content: Sara El Ouali, Alan N Barkun, Myriam Martel and Davide Maggio. Final approval of the article: Sara El Ouali, Alan N Barkun, Myriam Martel and Davide Maggio.
REFERENCES
- 1.Garcia-Iglesias P, Villoria A, Suarez D, et al. Meta-analysis: Predictors of rebleeding after endoscopic treatment for bleeding peptic ulcer. Aliment Pharmacol Ther. 2011;34:888–900. doi: 10.1111/j.1365-2036.2011.04830.x. [DOI] [PubMed] [Google Scholar]
- 2.Gralnek IM, Barkun AN, Bardou M. Management of acute bleeding from a peptic ulcer. N Engl J Med. 2008;359:928–37. doi: 10.1056/NEJMra0706113. [DOI] [PubMed] [Google Scholar]
- 3.Barkun AN, Bardou M, Kuipers EJ, et al. International consensus recommendations on the management of patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med. 2010;152:101–13. doi: 10.7326/0003-4819-152-2-201001190-00009. [DOI] [PubMed] [Google Scholar]
- 4.Barkun A, Sabbah S, Enns R, et al. The Canadian Registry on Nonvariceal Upper Gastrointestinal Bleeding and Endoscopy (RUGBE): Endoscopic hemostasis and proton pump inhibition are associated with improved outcomes in a real-life setting. Am J Gastroenterol. 2004;99:1238–46. doi: 10.1111/j.1572-0241.2004.30272.x. [DOI] [PubMed] [Google Scholar]
- 5.Peura DA, Lanza FL, Gostout CJ, Foutch PG. The American College of Gastroenterology Bleeding Registry: Preliminary findings. Am J Gastroenterol. 1997;92:924–8. [PubMed] [Google Scholar]
- 6.Vreeburg EM, Snel P, de Bruijne JW, Bartelsman JF, Rauws EA, Tytgat GN. Acute upper gastrointestinal bleeding in the Amsterdam area: Incidence, diagnosis, and clinical outcome. Am J Gastroenterol. 1997;92:236–43. [PubMed] [Google Scholar]
- 7.Hsu PI, Lai KH, Lin XZ, et al. When to discharge patients with bleeding peptic ulcers: A prospective study of residual risk of rebleeding. Gastrointest Endosc. 1996;44:382–7. doi: 10.1016/s0016-5107(96)70085-8. [DOI] [PubMed] [Google Scholar]
- 8.Hsu PI, Lin XZ, Chan SH, et al. Bleeding peptic ulcer – risk factors for rebleeding and sequential changes in endoscopic findings. Gut. 1994;35:746–9. doi: 10.1136/gut.35.6.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lau JY, Chung SC, Leung JW, Lo KK, Yung MY, Li AK. The evolution of stigmata of hemorrhage in bleeding peptic ulcers: A sequential endoscopic study. Endoscopy. 1998;30:513–8. doi: 10.1055/s-2007-1001336. [DOI] [PubMed] [Google Scholar]
- 10.Yang CC, Shin JS, Lin XZ, Hsu PI, Chen KW, Lin CY. The natural history (fading time) of stigmata of recent hemorrhage in peptic ulcer disease. Gastrointest Endosc. 1994;40:562–6. doi: 10.1016/s0016-5107(94)70253-5. [DOI] [PubMed] [Google Scholar]
- 11.Lin HJ, Perng CL, Lee FY, Lee CH, Lee SD. Clinical courses and predictors for rebleeding in patients with peptic ulcers and non-bleeding visible vessels: A prospective study. Gut. 1994;35:1389–93. doi: 10.1136/gut.35.10.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laine L, Jensen DM. Management of patients with ulcer bleeding. Am J Gastroenterol. 2012;107:345–60. doi: 10.1038/ajg.2011.480. [DOI] [PubMed] [Google Scholar]
- 13.Zargar SA, Javid G, Khan BA, et al. Pantoprazole infusion as adjuvant therapy to endoscopic treatment in patients with peptic ulcer bleeding: Prospective randomized controlled trial. J Gastroenterol Hepatol. 2006;21:716–21. doi: 10.1111/j.1440-1746.2006.04292.x. [DOI] [PubMed] [Google Scholar]
- 14.Jensen DM, Pace SC, Soffer E, Comer GM, Study G. Continuous infusion of pantoprazole versus ranitidine for prevention of ulcer rebleeding: A U.S. multicenter randomized, double-blind study. Am J Gastroenterol. 2006;101:1991–9. doi: 10.1111/j.1572-0241.2006.00773.x. [DOI] [PubMed] [Google Scholar]
- 15.Sung JJ, Barkun A, Kuipers EJ, et al. Intravenous esomeprazole for prevention of recurrent peptic ulcer bleeding: A randomized trial. Ann Intern Med. 2009;150:455–64. doi: 10.7326/0003-4819-150-7-200904070-00105. [DOI] [PubMed] [Google Scholar]
- 16.Cheng HC, Kao AW, Chuang CH, Sheu BS. The efficacy of high- and low-dose intravenous omeprazole in preventing rebleeding for patients with bleeding peptic ulcers and comorbid illnesses. Dig Dis Sci. 2005;50:1194–201. doi: 10.1007/s10620-005-2759-6. [DOI] [PubMed] [Google Scholar]
- 17.Chiu PW, Lam CY, Lee SW, et al. Effect of scheduled second therapeutic endoscopy on peptic ulcer rebleeding: A prospective randomised trial. Gut. 2003;52:1403–7. doi: 10.1136/gut.52.10.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi KD, Kim N, Jang IJ, et al. Optimal dose of intravenous pantoprazole in patients with peptic ulcer bleeding requiring endoscopic hemostasis in Korea. J Gastroenterol Hepatol. 2009;24:1617–24. doi: 10.1111/j.1440-1746.2009.05939.x. [DOI] [PubMed] [Google Scholar]
- 19.Barkun AN, Bhat M, Armstrong D, et al. Effectiveness of disseminating consensus management recommendations for ulcer bleeding: A cluster randomized trial. CMAJ. 2013;185:E156–66. doi: 10.1503/cmaj.120095. 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayes SM, Murray S, Dupuis M, Dawes M, Hawes IA, Barkun AN. Barriers to the implementation of practice guidelines in managing patients with nonvariceal upper gastrointestinal bleeding: A qualitative approach. Can J Gastroenterol. 2010;24:289–96. doi: 10.1155/2010/878135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 22.Higgins JP, Green S, Cochrane Collaboration . Cochrane handbook for systematic reviews of interventions. Chichester: Hoboken: Wiley-Blackwell; 2008. [Google Scholar]
- 23.Chen CC, Lee JY, Fang YJ, et al. Randomised clinical trial: High-dose vs. standard-dose proton pump inhibitors for the prevention of recurrent haemorrhage after combined endoscopic haemostasis of bleeding peptic ulcers. Aliment Pharmacol Ther. 2012;35:894–903. doi: 10.1111/j.1365-2036.2012.05047.x. [DOI] [PubMed] [Google Scholar]
- 24.Lau JY, Sung JJ, Lee KK, et al. Effect of intravenous omeprazole on recurrent bleeding after endoscopic treatment of bleeding peptic ulcers. N Engl J Med. 2000;343:310–6. doi: 10.1056/NEJM200008033430501. [DOI] [PubMed] [Google Scholar]
- 25.Chiu PW, Lam CY, Lee SW, et al. Effect of scheduled second therapeutic endoscopy on peptic ulcer rebleeding: A prospective randomised trial. Gut. 2003;52:1403–7. doi: 10.1136/gut.52.10.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laine L, Jensen DM. Management of patients with ulcer bleeding. Am J Gastroenterol. 2012;107:345–60. doi: 10.1038/ajg.2011.480. [DOI] [PubMed] [Google Scholar]
- 27.Cheng HC, Wu CT, Chang WL, Cheng WC, Chen WY, Sheu BS. Double oral esomeprazole after a 3-day intravenous esomeprazole infusion reduces recurrent peptic ulcer bleeding in high-risk patients: A randomised controlled study. Gut. 2014 Mar 21; doi: 10.1136/gutjnl-2013-306531. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 28.Cappell MS, Nadler SC. Increased mortality of acute upper gastrointestinal bleeding in patients with chronic obstructive pulmonary disease. A case controlled, multiyear study of 53 consecutive patients. Dig Dis Sci. 1995;40:256–62. doi: 10.1007/BF02065406. [DOI] [PubMed] [Google Scholar]
- 29.Lin HJ, Wang K, Perng CL, Lee FY, Lee CH, Lee SD. Natural history of bleeding peptic ulcers with a tightly adherent blood clot: A prospective observation. Gastrointest Endosc. 1996;43:470–3. doi: 10.1016/s0016-5107(96)70288-2. [DOI] [PubMed] [Google Scholar]
- 30.Cheng HC, Chuang SA, Kao YH, Kao AW, Chuang CH, Sheu BS. Increased risk of rebleeding of peptic ulcer bleeding in patients with comorbid illness receiving omeprazole infusion. Hepatogastroenterology. 2003;50:2270–3. [PubMed] [Google Scholar]
- 31.Wasse H, Gillen DL, Ball AM, et al. Risk factors for upper gastrointestinal bleeding among end-stage renal disease patients. Kidney Int. 2003;64:1455–61. doi: 10.1046/j.1523-1755.2003.00225.x. [DOI] [PubMed] [Google Scholar]
- 32.Cheung J, Yu A, LaBossiere J, Zhu Q, Fedorak RN. Peptic ulcer bleeding outcomes adversely affected by end-stage renal disease. Gastrointest Endosc. 2010;71:44–9. doi: 10.1016/j.gie.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 33.Tseng GY, Fang CT, Lin HJ, et al. Efficacy of an intravenous proton pump inhibitor after endoscopic therapy with epinephrine injection for peptic ulcer bleeding in patients with uraemia: A case-control study. Aliment Pharmacol Ther. 2009;30:406–13. doi: 10.1111/j.1365-2036.2009.04049.x. [DOI] [PubMed] [Google Scholar]
- 34.Wu CY, Wu MS, Kuo KN, Wang CB, Chen YJ, Lin JT. Long-term peptic ulcer rebleeding risk estimation in patients undergoing haemodialysis: A 10-year nationwide cohort study. Gut. 2011;60:1038–42. doi: 10.1136/gut.2010.224329. [DOI] [PubMed] [Google Scholar]
- 35.Boccardo P, Remuzzi G, Galbusera M. Platelet dysfunction in renal failure. Semin Thromb Hemost. 2004;30:579–89. doi: 10.1055/s-2004-835678. [DOI] [PubMed] [Google Scholar]
- 36.Kaw D, Malhotra D. Platelet dysfunction and end-stage renal disease. Semin Dial. 2006;19:317–22. doi: 10.1111/j.1525-139X.2006.00179.x. [DOI] [PubMed] [Google Scholar]
- 37.Ponticelli C, Passerini P. Gastrointestinal complications in renal transplant recipients. Transpl Int. 2005;18:643–50. doi: 10.1111/j.1432-2277.2005.00134.x. [DOI] [PubMed] [Google Scholar]
- 38.Garcia-Iglesias P, Villoria A, Suarez D, et al. Meta-analysis: Predictors of rebleeding after endoscopic treatment for bleeding peptic ulcer. Aliment Pharmacol Ther. 2011;34:888–900. doi: 10.1111/j.1365-2036.2011.04830.x. [DOI] [PubMed] [Google Scholar]
- 39.Chiu PW, Joeng HK, Choi CL, Kwong KH, Ng EK, Lam SH. Predictors of peptic ulcer rebleeding after scheduled second endoscopy: Clinical or endoscopic factors? Endoscopy. 2006;38:726–9. doi: 10.1055/s-2006-925179. [DOI] [PubMed] [Google Scholar]
- 40.Corley DA, Stefan AM, Wolf M, Cook EF, Lee TH. Early indicators of prognosis in upper gastrointestinal hemorrhage. Am J Gastroenterol. 1998;93:336–40. doi: 10.1111/j.1572-0241.1998.00336.x. [DOI] [PubMed] [Google Scholar]


