Thrombocytopenia is a highly prevalent complication in patients with advanced liver disease and carries a heightened risk for bleeding, which, in turn, is associated with increases in ambulatory visits, hospital stays and platelet transfusions. This review begins with a discussion of several aspects of thrombocytopenia including its etiology, immunological mechanisms and the role of thrombopoietin. The article concludes with an overview of management strategies.
Keywords: Advanced liver disease, Cirrhosis, Splenectomy, Splenic artery embolization, Thrombocytopenia, Thrombopoietin stmulators
Abstract
Thrombocytopenia (defined as a platelet count <150×109/L) is a well-known complication in patients with liver cirrhosis and has been observed in 76% to 85% of patients. Significant thrombocytopenia (platelet count <50×109/L to 75×109/L) occurs in approximately 13% of patients with cirrhosis. Thrombocytopenia can negatively impact the care of patients with severe liver disease by potentially interfering with diagnostic and therapeutic procedures. Multiple factors can contribute to the development of thrombocytopenia including splenic platelet sequestration, immunological processes, bone marrow suppression by chronic viral infection, and reduced levels or activity of the hematopoietic growth factor thrombopoietin. The present review focuses on the etiologies and management options for severe thrombocytopenia in the setting of advanced liver disease.
Abstract
La thrombocytopénie (définie comme une numération plaquettaire inférieure à 150×109/L) est une complication bien connue chez les patients atteints de cirrhose du foie, qui s’observe chez 76 % à 85 % des patients. Une thrombocytopénie importante (numération plaquettaire de moins de à 50×109/L à 75×109/L) se déclare chez environ 13 % des patients atteints de cirrhose. La thrombocytopénie peut nuire aux soins des patients atteints d’une maladie hépatique grave et compromettre les interventions diagnostiques et thérapeutiques. De nombreux facteurs peuvent contribuer à l’apparition d’une thrombocytopénie, y compris la séquestration des plaquettes spléniques, les processus immunologiques, la suppression de la moelle épinière par une infection virale chronique et le ralentissement de la thrombopoïétine, un facteur de croissance hématopoïétique. La présente analyse porte sur les étiologies et les options de prise en charge de la thrombocytopénie grave en cas de maladie hépatique avancée.
Thrombocytopenia is a well-known complication in advanced liver disease, with an incidence of 77% to 85% in patients with cirrhosis (1). Patients with chronic liver disease and thrombocytopenia are at increased risk for bleeding, requiring recurrent platelet transfusions, increased ambulatory visits and inpatient hospital stays compared with individuals without thrombocytopenia (2). It has been estimated that the annual health care costs of a patient with hepatitis C virus (HCV) infection with and without thrombocytopenia is $37,924 and $12,174, respectively (2). Thrombocytopenia can also interfere with diagnostic and therapeutic procedures in patients with advanced liver disease. For example, patients with chronic HCV-related cirrhosis, who are not candidates for liver transplantation, often cannot be treated with inter-feron (IFN) therapy because of low platelet counts. This is clinically important because successful therapy for HCV infection may reduce the progression to hepatocellular carcinoma (3). Liver transplantation can be safely avoided if timely IFN therapy is provided to HCV patients. Repetitive platelet transfusions are not a practical solution to thrombocytopenia because of the short half-life of platelets and the associated alloimmunization that ultimately develops. The risk of transfusion-associated complications also significantly increases with repeated transfusions. The characterization of thrombocytopenia in these patient populations in the literature is sparse; accordingly, the present review concentrates on the etiology and management of thrombocytopenia in ‘advanced liver disease’ as a whole and HCV infection when mentioned.
ETIOLOGY
Thrombocytopenia in patients with advanced liver disease is secondary to hypersplenism, possible immune-mediated mechanisms, direct viral suppression of platelet production and decreased thrombopoietin (TPO) production from the diseased liver. The general approach to the diagnosis and major mechanisms of thrombocytopenia is highlighted in Figure 1.
Figure 1).
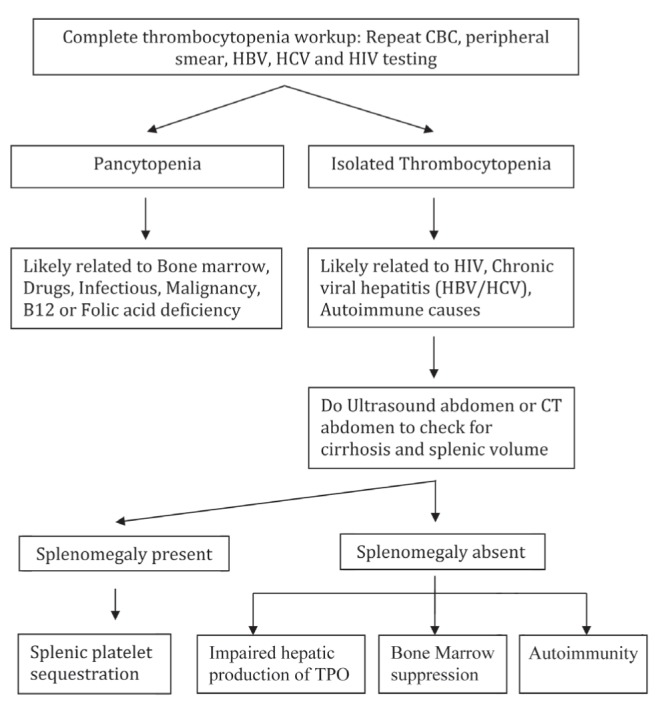
General approach and mechanism to the diagnosis of thrombocytopenia in liver disease. CBC Complete blood count; CT Computed tomography; HBV Hepatitis B virus; HCV Hepatitis C virus; TPO Thrombopoietin
Hypersplenism
Most cases of thrombocytopenia in the setting of liver disease are associated with splenomegaly (4). Splenomegaly is defined as enlargement of the spleen, diagnosed either on physical examination or imaging studies. In general, splenic enlargement to approximately 285 g is palpable (5). Normal splenic size on ultrasound is <13 cm in length and ≤15 cm in thickness and, on computed tomography scan is <10 cm (6). Hepatic diseases account for 36% of cases of splenomegaly; 35% are attributed to hematological conditions, 16% to infectious diseases (50% in AIDS/HIV), 4% to primary spleen disorders, 5% to inflammatory conditions and 4% to other causes (7). Splenomegaly is a common finding in patients with cirrhosis and portal hypertension. Conversely, not all patients with cirrhosis and portal hypertension have splenomegaly (8). To date, the reasons behind this discordance are unclear. It has also been noted that portal venous pressure only increases to a certain level, after which portal venous pressure decreases with an increase in total portal systemic shunt and splenic shunt (9). This may partially explain the occasional lack of correlation between portal venous pressure and splenic size. The etiology of splenomegaly in patients with liver cirrhosis is multifactorial and may be a congestive or hyperplastic phenomenon (10). In most cases, increases in portal venous pressure cause splenic congestion, thereby leading to the development of splenomegaly with resulting increased platelet sequestration and subsequent thrombocytopenia.
Immunological mechanisms of thrombocytopenia in cirrhosis and HCV patients
Noting a variable response in thrombocytopenia after portal vein decompression in cirrhotic patients, Jabbour et al (11) questioned whether other mechanisms of thrombocytopenia may be occurring in specific patient populations. It has been long observed that HCV patients are prone to several autoimmune disorders, suggesting the possibility of an autoimmune etiology for thrombocytopenia in this patient population (12–14). A recent study by Olariu et al (15) investigating thrombocytopenia in HCV patients found that both decreased platelet production by bone marrow suppression (the ‘central’ mechanism) and increased destruction by autoimmune processes (the ‘peripheral’ mechanism) was present in approximately 93.3% of patients with severe thrombocytopenia and 64% of patients with moderate thrombocytopenia. However, these conclusions should be viewed with caution because there were several limitations to the study, such as excluding patients with cirrhosis and, thereby, potentially excluding the majority of patients with splenomegaly. Moreover, there was no mention of the bone marrow biopsy or megakaryocyte quantification methods used. It was also noted that only dual-colour immunofloresence and flow cytometry could identify both mature and immature megakaryocytes as opposed to light microscopy, which may exclude immature megakaryocytes (16).
The role of the spleen in the autoimmune process of immune thrombocytopenic purpura (ITP) has been extensively studied (17). The etiology of thrombocytopenia in liver cirrhosis may have some similarities with ITP. Autoimmune antibodies possibly contribute to the immunological destruction of platelets in both; however, the severity of thrombocytopenia in liver cirrhosis is typically less than that found in ITP. This may be explained by impaired functioning of the reticuloendothelial system, thereby decreasing platelet opsonizing capacity acting via Fc receptors in cirrhotic patients (18). Other explanations may be differences in the subclass activation of immunoglobulin (Ig) G isotypes in cirrhotic patients. Both platelet-associated antibodies (PAIgG and anti-GP antibodies) and serum circulating antiplatelet antibodies are found in patients with cirrhosis (19,20). Increased PAIgG autoimmune antibodies in cirrhosis patients were first noted by Landolfi et al (21). Subsequent studies have also shown an inverse correlation between platelet counts and PAIgG levels (22–24). PAIgG is also increased in many nonimmune causes of thrombocytopenia and hypergammaglobulinemia. The PAIgG assay is not regarded to be an appropriate test for the diagnosis of ITP by the American Society of Hematology; hence, its diagnostic role in HCV or cirrhotic patients is also questionable (25).
The role of other antiplatelet antibodies (anti-GP antibodies) in cirrhosis patients was first studied by Pereira et al (26) in 1995. Anti-GPIIb-IIIa antibody-producing B cells are present in approximately 99% of patients with liver cirrhosis irrespective of the etiology of cirrhosis (19,26). They are found to be the strongest independent factor associated with thrombocytopenia and inversely correlated with platelet count (19). The glycocalicin index, which measures platelet turnover rate, is abnormally high in liver cirrhosis patients. Reticulated platelets, which reflect the rate of bone marrow platelet production, is increased in all patients with alcoholic cirrhosis, hepatitis B virus cirrhosis and ITP patients (20,27–29). This, in addition to the presence of anti-GPIIb-IIIa autoantibodies, suggests at least some immuno-logical component of platelet destruction and thrombocytopenia in some advanced liver disease patients (20). The low reticulated platelet count in the HCV subgroups may be explained by the central effect of HCV in bone marrow suppression (20).
The role of TPO
Immunological processes may partially explain thrombocytopenia in cirrhotic patients in whom portal vein congestion has not been demonstrated; however, in some cases, immune complexes cannot be implicated. There is mounting evidence that impaired hepatic production of TPO may be a major cause of thrombocytopenia in liver disease. TPO is synthesized in the liver and is the principle physiological regulator of platelet production (30). Thrombocytopenic patients with advanced liver disease have inappropriately low levels of TPO (31–33). Patients with splenomegaly and normal platelet counts have significantly higher TPO levels than those with thrombocytopenia, which suggests that higher TPO levels result in a compensatory increase in platelet production (34). Serum TPO levels correlate inversely with the severity of liver disease as reflected by the degree of fibrosis, Child-Pugh class and other synthetic measures of liver function (35,36). TPO levels and platelet counts increase after orthotopic liver transplantation, strongly supporting impaired TPO production as a primary cause of thrombocytopenia in at least some patients (37,38).
MANAGEMENT: OVERVIEW
The management of thrombocytopenia in cirrhotic patients has been a challenging problem for many years. Splenorenal shunts were developed in the 1960s but later abandoned due to a high mortality rate from liver failure (39,40). Splenectomy and, later, partial splenectomy (PS), gained popularity because of fewer complications associated with the procedure. With refined laparoscopic techniques, splenectomies have been performed since the late 1990s with minimal complications and better resolution of thrombocytopenia compared with PS. Splenic embolization has been successfully performed since the 1970s. Now, partial splenic embolization (PSE) is gaining in popularity. Newer techniques, such as radiofrequency ablation (RFA) and TPO stimulators, are currently being investigated to treat the thrombocytopenia associated with liver disease. Here, we discuss the merits and drawbacks of each intervention, with a special emphasis on recent advances in the management of thrombocytopenia in chronic liver disease patients (Table 1). Figures 2 and 3 summarize the general approach to management.
TABLE 1.
Summary of management of thrombocytopenia in liver disease
| Procedure | Advantages | Disadvantages | Comments |
|---|---|---|---|
| Laparoscopic splenectomy |
|
|
|
| Partial splenic artery embolization |
|
|
|
| Radiofrequency splenic ablation |
|
|
|
| Shunt procedures |
|
|
|
| Antiviral therapy |
|
|
|
| Immune suppression |
|
|
|
| Thrombopoietin stimulators |
|
|
|
AASLD American Association for the Study of Liver Diseases; HCV Hepatitis C virus; ITP Immune thrombocytopenic purpura; PSE Partial splenic embolization; RFA Radiofrequency ablation; TIPS Transjugular intrahepatic portosystemic shunts
Figure 2).
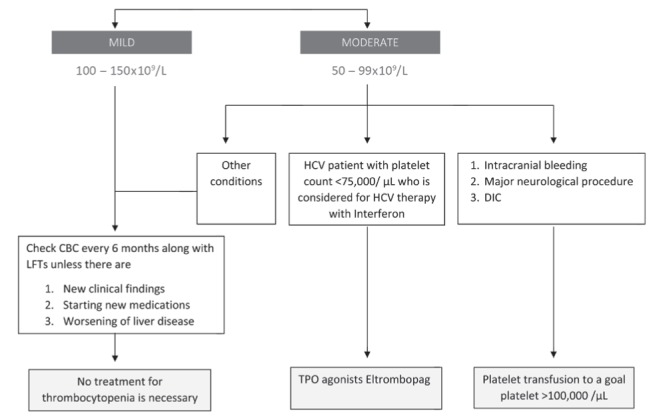
Cirrhosis and thrombocytopenia. CBC Complete blood count; HCV Hepatitis C virus; DIC Disseminated intravascular coagulation; LFTs Liver function tests; TPO Thrombopoietin
Figure 3).
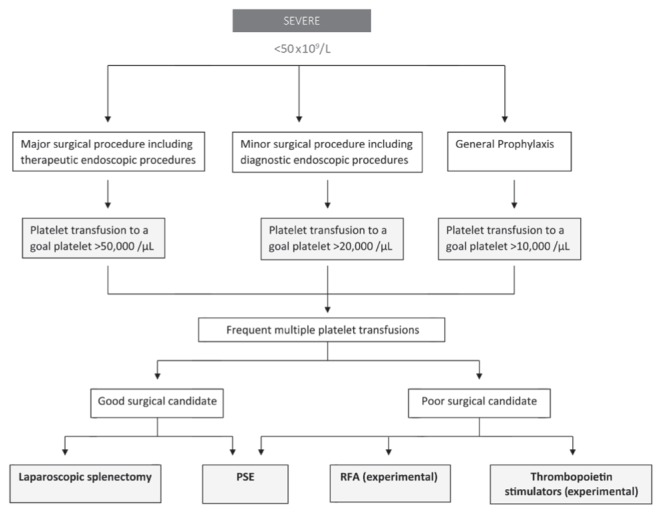
Cirrhosis and thrombocytopenia. PSE Partial splenic embolization; RFA Radiofrequency ablation
Splenectomy
Splenomegaly is a common complication in patients with cirrhosis, with portal hypertension being the primary mechanism of pathogenesis. Splenectomy is a common surgical strategy for correcting thrombocytopenia due to hypersplenism. Open splenectomy and laparoscopic splenectomy are the two most widely available procedures commonly performed for severe thrombocytopenia.
Since the 1950s, open splenectomies were primarily performed in cirrhotic patients with splenomegaly to relieve portal hypertension and reduce variceal bleeding (41,42). Due to the high risk for bleeding, some studies preferred shunt procedures to open splenectomy (43,44). Additional complications reported after open splenectomy include portal vein thrombosis (8% to 10%) and wound pain (45,46). Due to advances in laparoscopic surgical techniques, laparoscopic splenectomies have been performed since the early 1990s on patients who were initially not considered for this procedure (47,48). Comparative studies investigating open versus laproscopic splenectomy in cirrhosis patients to improve platelet counts were largely missing in the literature until recently, when Hayashi et al (49) first published a case series involving HCV patients. In this study, the mean hospital stay, significant complication rate and transfusion rate were nine days, 14% and 71% for open splenectomy compared with 2.6 days, 0% and 0%, respectively, for laparoscopic splenectomy. Other recent studies have favoured laparoscopic approaches to decrease complication rates. Compared with open splenectomy, laparoscopic splenectomy has resulted in less blood loss and shorter hospital stays (50). Currently, due to lack of strong supportive evidence of efficacy and a high associated complication rate, open splenectomy to improve thrombocytopenia is not commonly practiced or recommended.
Portal and splenic vein thrombosis are the main complications of laparoscopic splenectomy (19% to 55%) compared with open splenectomy (8% to 10%) (45,46,51–54). Risk factors for thrombosis include larger spleen size and large splenic vein diameter (55). The most significant complication following laparoscopic splenectomy is bleeding. The amount of bleeding in laparoscopic splenectomy groups is significantly less compared with open splenectomy groups (51,56–58). The conversion rate from laparoscopic to open splenectomy due to bleeding ranges from 0% to 9.6% (56,58). Overall complication rates in cirrhotic patients range from 2.5% to 17% (57,59). However, in patients with massive splenomegaly, the complication rate can be as high as 56% (60). The indication of laparoscopic splenectomy in the management of thrombocytopenia is largely limited because of the associated surgical complications with the procedure, as mentioned above.
Splenic artery embolization
The use of splenic artery embolization (SAE) in the management of thrombocytopenia was first reported by Maddison (61) in 1973 as a proposed alternative to splenectomy in surgically unfit patients. The two types of SAE techniques widely published in the literature include total embolization and partial embolization. Due to an increased risk for splenic abscess from total splenic embolization, partial SAE (PSE) is now the preferred option in patients who are candidates (62,63). Embolization occludes the arterial supply of the spleen peripherally with ischemic necrosis of functional splenic tissue followed by a decrease in spleen size. Partial embolization allows the preservation of some normal splenic tissue and thereby avoids the theoretical risk of overwhelming postembolization sepsis. The role of preoperative splenic volume in the management of thrombocytopenia in patients with cirrhosis is a matter of significant debate. Unfortunately, there were no clear guidelines to direct splenectomy or PSE based on splenic volume. The recommended splenic infarct volume to effectively increase the platelet counts (at one year) and decrease the post-PSE complication is between 388 mL and 540 mL (64). One institution’s approach is shown in Table 2; however, this approach needs to be further validated (65). In patients with cirrhosis and splenic volume <400 mL, splenectomy is mainly preferred because PSE can cause significant inflammatory reactions and preclude further splenectomy if needed. In patients with splenic volume <700 mL, the infarcted splenic volume is shown to correlate (r=0.53; P≤0.001) with the long-term (one year) increase in platelet counts; however, in patients with splenic volume ≥700 mL, noninfarcted splenic volume (r=−0.71; P=0.003) and splenic infarction ratio (r=0.72; P=0.002) were shown to correlate (65).
TABLE 2.
Management of thrombocytopenia due to cirrhosis at a single centre
| Preoperative splenic volume | Procedure | Target |
|---|---|---|
| <400 mL | Left splenectomy | |
| 400 mL to 700 mL | Single partial splenic embolization or laparoscopic splenectomy (in selective cases) | Infarcted splenic area (Infarcted splenic volume <540 mL) |
| >700 mL | Repeated partial splenic embolization (two-month interval) or laparoscopic splenectomy (in selective cases) | Noninfarcted splenic area (Noninfarcted splenic ratio <20% and noninfarcted splenic volume <170 mL) |
Data adapted from reference 64
The extent and sustainability of improvements in platelet counts appears to be dependent on the extent of SAE. It has been reported that embolization of <30% of splenic mass results in an unsustained improvement in platelet counts, but embolization of >50% is associated with an increased risk for complications (66,67). Complication rates are reported to be approximately 28% with <50% embolization, 56% with 50% to 70% embolization, and 95% with >70% embolization (68). The complications reported with PSE are pneumonia, peritonitis, splenic abscess and portal vein thrombosis (67). Laparoscopic splenectomy has a higher morbidity rate compared with PSE (16% to 36% versus 0% to 16%, respectively) (69,70). Splenic abscess is a severe complication of PSE, with a reported incidence of up to 16%; death may occur in 6% of cases (69,70). Retrospective series suggest that PSE can control cytopenias in nearly all patients (70,71). In a randomized trial, PSE was comparable with splenectomy with regard to increasing platelet counts (211×109/L versus 240×109/L at two weeks, and 146×109/L versus 322×109/L at six months). The patients in the PSE group also experienced fewer episodes of portal vein thrombosis (72).
It should be noted that in other studies, patients with HCV infection had a poorer response rate to PSE, which was believed to be secondary to possible immunological mechanisms contributing to the thrombocytopenia, which PSE could not correct (73,74).
RFA of the spleen
RFA of the spleen is a minimally invasive, relatively new procedure that has shown promising results in patients with cirrhosis and splenomegaly (75). In a randomized controlled trial comparing RFA with laparoscopic splenectomy, platelet counts improved after RFA but were not sustained after 12 months and 24 months of follow-up (76). Suboptimal ablation was likely the reason for the unsustained platelet counts. Patients with >70% ablation showed sustained platelet counts similar to splenectomized patients. The other benefits of RFA were improved liver function secondary to improved hepatic artery blood flow to the liver, increased oxygenation and decreased liver sinusoidal congestion (77). The main advantages of RFA over PSE were lower complication rates, cost effectiveness and convenience. The major complications in this group of patients included hemorrhagic shock and intra-abdominal bleeding. Complications such as hyperpyrexia, splenic rupture or abscess, which are commonly observed in patients with PS, were absent in patients treated with RFA (76). Further clinical trials with longer follow-up studies are needed to definitively elucidate its effectiveness.
Shunt procedures
Splenic congestion is believed to be the primary mechanism of thrombocytopenia in patients with cirrhosis. After the success of surgical splenectomy in improving thrombocytopenia, the focus shifted to shunt procedures. Shunt procedures decompress the spleen and decrease portal pressures, thereby creating a ‘physiological splenectomy’. The types of shunts widely published in the literature for these purposes are portocaval shunts, splenorenal shunts and transjugular intrahepatic portosystemic shunts (TIPS). When compared with a control group, patients with portocaval shunts and distal splenorenal shunts have experienced more complications, such as hepatic encephalopathy and hepatic failure, and minimal improvements in hypersplenism and thrombocytopenia (78,79). These approaches have now been largely abandoned. TIPS has been in use for >20 years to treat the complications of portal hypertension. Several case series suggested an improvement in the platelet count within a few days of TIPS placement (80–82). However, prospective series do not support this because the mean platelet counts appear to remain unchanged after TIPS (83,84). Complications include encephalopathy (up to 45%) and portal vein thrombosis (15%) (85). Recent practice guidelines update the use of TIPS in cirrhosis. TIPS for the treatment of hypersplenism are currently discouraged by the American Association for the Study of Liver Diseases (85).
Antiviral therapy to improve thrombocytopenia
The role of antiviral therapy in the management of thrombocytopenia is a much-debated topic. Direct viral suppression of megakaryocytes and, therefore, TPO deficiency causing thrombocytopenia, has been proposed. New questions have arisen as to whether the eradication of HCV would itself improve thrombocytopenia.
In one of the earlier case series investigasting the treatment of HCV to improve thrombocytopenia with or without cirrhosis, Rajan and Liebman (86) showed that IFN therapy can improve platelet counts in severely thrombocytopenic patients (range 16×109/L to 48×109/L) (86). In a study involving eight patients, 63% (five of eight) of patients experienced improvement in their platelet counts to >50×109/L. Two patients had neither an improvement in platelet count nor an antiviral response to IFN. The most significant response (one of one) was observed in a patient who achieved a sustained virological response (SVR). In patients with cirrhosis (three of eight), platelet counts improved in two. Patients who had improved platelet counts also had decreased rheumatoid factor antibodies and cryoglobulins, as well as improved transaminase levels. In another study (74), the authors showed that all of the patients (n=5) with thrombocytopenia (<150×109/L) who achieved an SVR with IFN improved their platelet counts at least by 5% (two patients between 5% and 15%, one between 15% and 25%, and two >25%). The limitations of this study were small sample size, the majority of the patients treated with IFN in the thrombocytopenia group were mildly thrombocytopenic (80% of the patient’s platelets counts >100×109/L) (74). Further randomized control studies and clinical trials are needed to evaluate the role of anti-viral therapy in the management of thrombocytopenia.
Immune suppression for the treatment of thrombocytopenia
The role of autoantibodies in thrombocytopenia, especially in HCV, is well known. Steroids and other immunosuppressive agents have been used for the treatment of ITP. The treatment of HCV in patients with liver transplantation shows that immunosuppression increases the viral load and recurrence of HCV infection even after achieving an SVR (87–90). Presently, immunosuppression cannot be recommended to improve thrombocytopenia in this patient population.
TPO stimulators
Clinical studies investigating thrombopoietic growth factors showed that they were effective in increasing the platelet count in several clinical settings including myelodysplastic syndromes, nonmyeloablative chemotherapy and ITP. The development of first-generation thrombopoietic growth factors (recombinant human TPO and pegylated recombinant human megakaryocyte growth and development factor [PEG-rHuMGDF]) was stopped due to development of antibodies to PEG-rHuMGDF (91). Of the second-generation thrombopoietic growth factors, only romiplostim and eltrombopag have been extensively tested in human diseases. Both increased platelet counts in patients with ITP and could do so for a prolonged time without apparent untoward effects. Both drugs are now United States Food & Drug Administration-approved for the treatment of patients with chronic ITP. Studies are ongoing in patients with thrombocytopenia related to chemotherapy, hepatitis C and myelodysplastic syndromes. McHutchinson et al (92) conducted a phase II randomized controlled trial involving 74 patients with HCV-related cirrhosis. Only 6% of patients in the placebo group completed the 12-week antiviral course. In contrast, 36%, 53% and 65% of patients receiving 30 mg, 50 mg and 75 mg of eltrombopag completed the same antiviral course, respectively. Moreover, 75% to 95% of patients in the eltrombopag group achieved the primary end point (platelet count 100×109/L at week 4) in a dose-dependent manner (92). The Eltrombopag Evaluated for Its Ability to Overcome Thrombocytopenia and Enable Procedures (ELEVATE) study is a randomized, double-blind, placebo-controlled, multinational follow-up study in patients with thrombocytopenia and chronic liver disease (93). It was terminated following the identification of an imbalance of thrombosis of the portal venous system in the patients treated with eltrombopag versus matching placebo. Six patients (4%) in the eltrombopag group and one (1%) in the placebo group experienced a thrombotic event of the portal venous system. Of note, five of the six patients treated with eltrombopag experienced the portal venous thrombosis at platelet counts >200×109/L (93). Eltrombopag was recently United States Food & Drug Administration-approved for thrombocytopenia in patients with HCV who are eligable for antiviral therapy with IFN and ribavirin (94). Its effects on newer antiviral medications, such as sofosbuvir and semepivir, is yet to be determined. Phase III studies of romiplostim treatment in HCV-associated thrombocytopenia are currently ongoing. Some of the risk for thrombosis in these patients is dose related, although portal vein thrombosis has also been reported with this agent (95). TPO stimulators may play an important role in the management of thrombocytopenia in patients with advanced liver disease, primarily in patients who are poor surgical candidates.
CONCLUSIONS
Hypersplenism in patients with advanced liver disease remains a challenging issue and thrombocytopenia is a multifactorial problem. Currently available therapeutic solutions are not without significant complications. Intuitively, it would appear that correcting the underlying liver disease with immune therapy, use of immunosuppressives or liver transplantation would provide the best solution with fewest complications. Less is known about the impact of platelet autoantibodies and direct viral suppression of megakaryocytes. Management of thrombocytopenia in patients with cirrhosis should be a goal-oriented and team approach. In HCV patients with high viral loads and mild thrombocytopenia without accompanying cirrhosis, IFN therapy can be safely considered in the hope of improving platelet counts. Combination therapy consisting of pegylated IFN and TPO stimulators, such as eltrombopag, can also be considered in these patients. In patients with splenomegaly, PSE and laparoscopic splenectomy can be considered as therapeutic options. The role of immune suppression and TPO stimulators in patients with massive splenomegaly in whom these interventions are considered unsafe is an area for ongoing investigation.
Footnotes
DISCLOSURES: The authors have no financial disclosures or conflicts of interest to declare.
REFERENCES
- 1.Qamar AA, Grace ND, Groszmann RJ, et al. Portal Hypertension Collaborative Group Incidence, prevalence, and clinical significance of abnormal hematologic indices in compensated cirrhosis. Clin Gastroenterol Hepatol. 2009;7:689–95. doi: 10.1016/j.cgh.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poordad F, Theodore D, Sullivan J, Grotzinger K. Medical resource utilisation and healthcare costs in patients with chronic hepatitis C viral infection and thrombocytopenia. J Med Econ. 2011;14:194–206. doi: 10.3111/13696998.2011.562266. [DOI] [PubMed] [Google Scholar]
- 3.Morihara D, Kobayashi M, Ikeda K, et al. Effectiveness of combination therapy of splenectomy and long-term interferon in patients with hepatitis C virus-related cirrhosis and thrombocytopenia. Hepatol Res. 2009;39:439–47. doi: 10.1111/j.1872-034X.2008.00481.x. [DOI] [PubMed] [Google Scholar]
- 4.Sakai K, Iwao T, Oho K, Toyonaga A, Sata M. Propranolol ameliorates thrombocytopenia in patients with cirrhosis. J Gastroenterol. 2002;37:112–8. doi: 10.1007/s005350200005. [DOI] [PubMed] [Google Scholar]
- 5.Aito H. The estimation of the size of the spleen by radiological methods. A comparative radiographic, gamma imaging and ultrasonic study. Ann Clin Res. 1974;6(Suppl):1–54. [PubMed] [Google Scholar]
- 6.Tamayo SG, Rickman LS, Mathews WC, et al. Examiner dependence on physical diagnostic tests for the detection of splenomegaly: A prospective study with multiple observers. J Gen Intern Med. 1993;8:69–75. doi: 10.1007/BF02599986. [DOI] [PubMed] [Google Scholar]
- 7.O’Reilly RA. Splenomegaly in 2,505 patients at a large university medical center from 1913 to 1995. 1963 to 1995: 449 patients. West J Med. 1998;169:88–97. [PMC free article] [PubMed] [Google Scholar]
- 8.Gibson PR, Gibson RN, Ditchfield MR, Donlan JD. Splenomegaly – an insensitive sign of portal hypertension. Aust N Z J Med. 1990;20:771–4. doi: 10.1111/j.1445-5994.1990.tb00421.x. [DOI] [PubMed] [Google Scholar]
- 9.Ohnishi K, Nakayama T, Saito M, et al. Effects of propranolol on portal hemodynamics in patients with chronic liver disease. Am J Gastroenterol. 1985;80:132–5. [PubMed] [Google Scholar]
- 10.Bolognesi M, Merkel C, Sacerdoti D, Nava V, Gatta A. Role of spleen enlargement in cirrhosis with portal hypertension. Dig Liver Dis. 2002;34:144–50. doi: 10.1016/s1590-8658(02)80246-8. [DOI] [PubMed] [Google Scholar]
- 11.Jabbour N, Zajko A, Orons P, Irish W, Fung JJ, Selby RR. Does transjugular intrahepatic portosystemic shunt (TIPS) resolve thrombocytopenia associated with cirrhosis? Dig Dis Sci. 1998;43:2459–62. doi: 10.1023/a:1026634215918. [DOI] [PubMed] [Google Scholar]
- 12.Clifford BD, Donahue D, Smith L, et al. High prevalence of serological markers of autoimmunity in patients with chronic hepatitis C. Hepatology. 1995;2:613–9. [PubMed] [Google Scholar]
- 13.Hadziyannis SJ. Nonhepatic manifestations and combined diseases in HCV infection. Dig Dis Sci. 1996;41(12 Suppl):63S–74S. doi: 10.1007/BF02087878. [DOI] [PubMed] [Google Scholar]
- 14.Tran A, Benzaken S, Yang G, et al. Chronic hepatitis C and autoimmunity: Good response to immunosuppressive treatment. Dig Dis Sci. 1997;42:778–80. doi: 10.1023/a:1018812113732. [DOI] [PubMed] [Google Scholar]
- 15.Olariu M, Olariu C, Olteanu D. Thrombocytopenia in chronic hepatitis C. J Gastrointest Liver Dis. 2010;19:381–5. [PubMed] [Google Scholar]
- 16.Law HK, Bol SJ, Palatsides M, Williams NT. Analysis of human megakaryocytic cells using dual-color immunofluorescence labeling. Cytometry. 2000;41:308–15. [PubMed] [Google Scholar]
- 17.Kuwana M, Okazaki Y, Kaburaki J, Kawakami Y, Ikeda Y. Spleen is a primary site for activation of platelet-reactive T and B cells in patients with immune thrombocytopenic purpura. J Immunol. 2002;168:3675–82. doi: 10.4049/jimmunol.168.7.3675. [DOI] [PubMed] [Google Scholar]
- 18.Rimola A, Soto R, Bory F, Arroyo V, Piera C, Rodes J. Reticuloendothelial system phagocytic activity in cirrhosis and its relation to bacterial infections and prognosis. Hepatology. 1984;4:53–8. doi: 10.1002/hep.1840040109. [DOI] [PubMed] [Google Scholar]
- 19.Kajihara M, Kato S, Okazaki Y, et al. A role of autoantibody-mediated platelet destruction in thrombocytopenia in patients with cirrhosis. Hepatology. 2003;37:1267–76. doi: 10.1053/jhep.2003.50209. [DOI] [PubMed] [Google Scholar]
- 20.Pradella P, Bonetto S, Turchetto S, et al. Platelet production and destruction in liver cirrhosis. J Hepatol. 2011;54:894–900. doi: 10.1016/j.jhep.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 21.Landolfi R, Leone G, Fedeli G, Storti S, Laghi F, Bizzi B. Platelet-associated IgG in acute and chronic hepatic diseases. Scand J Haematol. 1980;25:417–22. doi: 10.1111/j.1600-0609.1981.tb01423.x. [DOI] [PubMed] [Google Scholar]
- 22.Barrison IG, Knight ID, Viola L, Boots MA, Murray-Lion IM, Mitchell TR. Platelet associated immunoglobulins on chronic liver disease. Br J Haematol. 1981;48:347–50. [PubMed] [Google Scholar]
- 23.Graber D, Giuliani D, Leevy CM, Morse BS. Platelet-associated IgG in hepatitis and cirrhosis. J Clin Immunol. 1984;4:108–11. doi: 10.1007/BF00915043. [DOI] [PubMed] [Google Scholar]
- 24.de Noronha R, Taylor BA, Wild G, Triger DR, Greaves M. Inter-relationships between platelet count, platelet IgG, serum IgG, immune complexes and severity of liver disease. Clin Lab Haematol. 1991;13:127–35. doi: 10.1111/j.1365-2257.1991.tb00261.x. [DOI] [PubMed] [Google Scholar]
- 25.George JN, Woolf SH, Raskob GE, et al. Idiopathic thrombocytopenic purpura: A practice guideline developed by explicit methods for the American Society of Hematology. Blood. 1996;88:3–40. [PubMed] [Google Scholar]
- 26.Pereira J, Accatino L, Alfaro J, Brahm J, Hidalgo P, Mezzano D. Platelet autoantibodies in patients with chronic liver disease. Am J Hematol. 1995;50:173–8. doi: 10.1002/ajh.2830500305. [DOI] [PubMed] [Google Scholar]
- 27.Michur H, Maślanka K, Szczepiński A, Mariańska B. Reticulated platelets as a marker of platelet recovery after allogeneic stem cell transplantation. Int J Lab Hematol. 2008;30:519–25. doi: 10.1111/j.1751-553X.2007.00993.x. [DOI] [PubMed] [Google Scholar]
- 28.Thomas-Kaskel AK, Mattern D, Köhler G, Finke J, Behringer D. Reticulated platelet counts correlate with treatment response in patients with idiopathic thrombocytopenic purpura and help identify the complex causes of thrombocytopenia in patients after allogeneic hematopoietic stem cell transplantation. Cytometry B Clin Cytom. 2007;72:241–8. doi: 10.1002/cyto.b.20163. [DOI] [PubMed] [Google Scholar]
- 29.Harrison P, Robinson MS, Mackie IJ, Machin SJ. Reticulated platelets. Platelets. 1997;8:379–83. doi: 10.1080/09537109777050. [DOI] [PubMed] [Google Scholar]
- 30.Kuter DJ, Begley CG. Recombinant human thrombopoietin: Basic biology and evaluation of clinical studies. Blood. 2002;100:3457–69. doi: 10.1182/blood.V100.10.3457. [DOI] [PubMed] [Google Scholar]
- 31.Martin TG, III, Somberg KA, Meng YG, et al. Thrombopoietin levels in patients with cirrhosis before and after orthotopic liver transplantation. Ann Intern Med. 1997;127:285–8. doi: 10.7326/0003-4819-127-4-199708150-00005. [DOI] [PubMed] [Google Scholar]
- 32.Ishikawa T, Ichida T, Matsuda Y, et al. Reduced expression of thrombopoietin is involved in thrombocytopenia in human and rat liver cirrhosis. J Gastroenterol Hepatol. 1998;13:907–13. doi: 10.1111/j.1440-1746.1998.tb00760.x. [DOI] [PubMed] [Google Scholar]
- 33.Peck-Radosavljevic M, Zacherl J, Meng YG, et al. Is inadequate thrombopoietin production a major cause of thrombocytopenia in cirrhosis of the liver? J Hepatol. 1997;27:127–31. doi: 10.1016/s0168-8278(97)80291-7. [DOI] [PubMed] [Google Scholar]
- 34.Giannini E, Botta F, Borro P, et al. Relationship between thrombopoietin serum levels and liver function in patients with chronic liver disease related to hepatitis C virus infection. Am J Gastroenterol. 2003;98:2516–20. doi: 10.1111/j.1572-0241.2003.08665.x. [DOI] [PubMed] [Google Scholar]
- 35.Kawasaki T, Takeshita A, Souda K, et al. Serum thrombopoietin levels in patients with chronic hepatitis and liver cirrhosis. Am J Gastroenterol. 1999;94:1918–22. doi: 10.1111/j.1572-0241.1999.01231.x. [DOI] [PubMed] [Google Scholar]
- 36.Giannini E, Borro P, Botta F, et al. Serum thrombopoietin levels are linked to liver function in untreated patients with hepatitis C virus-related chronic hepatitis. J Hepatol. 2002;37:572–7. doi: 10.1016/s0168-8278(02)00274-x. [DOI] [PubMed] [Google Scholar]
- 37.Peck-Radosavljevic M, Wichlas M, Zacherl J, et al. Thrombopoietin induces rapid resolution of thrombocytopenia after orthotopic liver transplantation through increased platelet production. Blood. 2000;95:795–801. [PubMed] [Google Scholar]
- 38.Yanaga K, Tzakis AG, Shimada M, et al. Reversal of hypersplenism following orthotopic liver transplantation. Ann Surg. 1989;210:180–3. doi: 10.1097/00000658-198908000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mutchnick MG, Lerner E, Conn HO. Portal-systemic encephalopathy and portacaval anastomosis: A prospective, controlled investigation. Gastroenterology. 1974;66:1005–19. [PubMed] [Google Scholar]
- 40.Vang J, Simert G, Hansson JA, Thylen U, Bengmark TS. Results of a modified distal spleno-renal shunt for portal hypertension. Ann Surg. 1977;185:224–8. doi: 10.1097/00000658-197702000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lord JW., Jr The surgical management of secondary hypersplenism. Surgery. 1951;29:407–18. [PubMed] [Google Scholar]
- 42.Linton RR, Ellis DS, Geary JE. Critical comparative analysis of early and late results of splenorenal and direct portacaval shunts performed in 169 patients with portal cirrhosis. Ann Surg. 1961;154:446–59. doi: 10.1097/00000658-196109000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamamoto S, Hidemura R. Surgical treatment of portal hypertension with special reference to the feature of intrahepatic circulatory disturbances. Jpn Circ J. 1964;28:178–80. doi: 10.1253/jcj.28.178. [DOI] [PubMed] [Google Scholar]
- 44.El-Khishen MA, Henderson JM, Millikan WJ, Jr, Kutner MH, Warren WD. Splenectomy is contraindicated for thrombocytopenia secondary to portal hypertension. Surg Gynecol Obstet. 1985;160:233–8. [PubMed] [Google Scholar]
- 45.Hassn AM, Al-Fallouji MA, Ouf TI, Saad R. Portal vein thrombosis following splenectomy. Br J Surg. 2000;87:362–73. doi: 10.1046/j.1365-2168.2000.01383-16.x. [DOI] [PubMed] [Google Scholar]
- 46.Winslow ER, Brunt LM, Drebin JA, Soper NJ, Klingensmith ME. Portal vein thrombosis after splenectomy. Am J Surg. 2002;184:631–5. doi: 10.1016/s0002-9610(02)01095-4. [DOI] [PubMed] [Google Scholar]
- 47.Carroll BJ, Phillips EH, Semel CJ, Fallas M, Morgenstern L. Laparoscopic splenectomy. Surg Endosc. 1992;6:183–5. doi: 10.1007/BF02210877. [DOI] [PubMed] [Google Scholar]
- 48.Delaitre B, Maignien B, Icard P. Laparoscopic splenectomy. Br J Surg. 1992;79:1334. doi: 10.1002/bjs.1800791230. [DOI] [PubMed] [Google Scholar]
- 49.Hayashi PH, Mehia C, Joachim Reimers H, Solomon HS, Bacon BR. Splenectomy for thrombocytopenia in patients with hepatitis C cirrhosis. J Clin Gastroenterol. 2006;40:740–4. doi: 10.1097/00004836-200609000-00015. [DOI] [PubMed] [Google Scholar]
- 50.Shigekawa Y, Uchiyama K, Takifuji K, et al. A laparoscopic splenectomy allows the induction of antiviral therapy for patients with cirrhosis associated with hepatitis C virus. Am Surg. 2011;77:174–9. [PubMed] [Google Scholar]
- 51.Watanabe Y, Horiuchi A, Yoshida M, et al. Significance of laparoscopic splenectomy in patients with hypersplenism. World J Surg. 2007;31:549–55. doi: 10.1007/s00268-006-0504-8. [DOI] [PubMed] [Google Scholar]
- 52.Ushitora Y, Tashiro H, Takahashi S, et al. Splenectomy in chronic hepatic disorders: Portal vein thrombosis and improvement of liver function. Dig Surg. 2011;28:9–14. doi: 10.1159/000321886. [DOI] [PubMed] [Google Scholar]
- 53.Ikeda M, Sekimoto M, Takiguchi S, et al. High incidence of thrombosis of the portal venous system after laparoscopic splenectomy: A prospective study with contrast-enhanced CT scan. Ann Surg. 2005;241:208–16. doi: 10.1097/01.sla.0000151794.28392.a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harris W, Marcaccio M. Incidence of portal vein thrombosis after laparoscopic splenectomy. Can J Surg. 2005;48:352–4. [PMC free article] [PubMed] [Google Scholar]
- 55.Kinjo N, Kawanaka H, Akahoshi T, et al. Risk factors for portal venous thrombosis after splenectomy in patients with cirrhosis and portal hypertension. Br J Surg. 2010;97:910–6. doi: 10.1002/bjs.7002. [DOI] [PubMed] [Google Scholar]
- 56.Hashizume M, Tomikawa M, Akahoshi T, et al. Laparoscopic splenectomy for portal hypertension. Hepatogastroenterology. 2002;49:847–52. [PubMed] [Google Scholar]
- 57.Zhu JH, Wang YD, Ye ZY, et al. Laparoscopic versus open splenectomy for hypersplenism secondary to liver cirrhosis. Surg Laparosc Endosc Percutan Tech. 2009;19:258–62. doi: 10.1097/SLE.0b013e3181a6ec7c. [DOI] [PubMed] [Google Scholar]
- 58.Kercher KW, Carbonell AM, Heniford BT, Matthews BD, Cunningham DM, Reindollar RW. Laparoscopic splenectomy reverses thrombocytopenia in patients with hepatitis C cirrhosis and portal hypertension. J Gastrointest Surg. 2004;8:120–6. doi: 10.1016/j.gassur.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 59.Wang Y, Zhan X, Zhu Y, Xie Z, Zhu J, Ye Z. Laparoscopic splenectomy in portal hypertension: A single-surgeon 13-year experience. Surg Endosc. 2010;24:1164–9. doi: 10.1007/s00464-009-0744-4. [DOI] [PubMed] [Google Scholar]
- 60.Patel AG, Parker JE, Wallwork B, et al. Massive splenomegaly is associated with significant morbidity after laparoscopic splenectomy. Ann Surg. 2003;238:235–40. doi: 10.1097/01.sla.0000080826.97026.d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maddison EF. Embolic therapy of hypersplenism. Invest Radiol. 1973;8:280–1. [Google Scholar]
- 62.Witte CL, Ovitt TW, Van Wyck DB, Witte MH, O’Mara RE, Woolfenden JM. Ischemic therapy in thrombocytopenia from hypersplenism. Arch Surg. 1976;111:1115–21. doi: 10.1001/archsurg.1976.01360280073012. [DOI] [PubMed] [Google Scholar]
- 63.Wholey MH, Chamorro HA, Rao G, Chapman W. Splenic infarction and spontaneous rupture of the spleen after therapeutic embolization. Cardiovasc Radiol. 1978;1:249–53. doi: 10.1007/BF02552051. [DOI] [PubMed] [Google Scholar]
- 64.Hayashi H1, Beppu T, Okabe K, et al. Therapeutic factors considered according to the preoperative splenic volume for a prolonged increase in platelet count after partial splenic embolization for liver cirrhosis. J Gastroenterol. 2010;45:554–9. doi: 10.1007/s00535-009-0185-9. [DOI] [PubMed] [Google Scholar]
- 65.Hayashi H, Beppu T, Okabe K, Masuda T, Okabe H, Baba H. Risk factors for complications after partial splenic embolization for liver cirrhosis. Br J Surg. 2008;95:744–50. doi: 10.1002/bjs.6081. [DOI] [PubMed] [Google Scholar]
- 66.Lee CM, Leung TK, Wang HJ, et al. Evaluation of the effect of partial splenic embolization on platelet values for liver cirrhosis patients with thrombocytopenia. World J Gastroenterol. 2007;28:619–22. doi: 10.3748/wjg.v13.i4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhu K, Meng X, Qian J, et al. Partial splenic embolization for hypersplenism in cirrhosis: A long-term outcome in 62 patients. Dig Liver Dis. 2009;41:411–6. doi: 10.1016/j.dld.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 68.Mukaiya M, Hirata K, Yamashiro K, Katsuramaki T, Kimura H, Denno R. Changes in portal hemodynamics and hepatic function after partial splenic embolization (PSE) and percutaneous transhepatic obliteration (PTO) Cancer Chemother Pharmacol. 1994;(33 Suppl):S37–S41. doi: 10.1007/BF00686666. [DOI] [PubMed] [Google Scholar]
- 69.Sakai T, Shiraki K, Inoue H, et al. Complications of partial splenic embolization in cirrhotic patients. Dig Dis Sci. 2002;47:388–91. doi: 10.1023/a:1013786509418. [DOI] [PubMed] [Google Scholar]
- 70.N’Kontchou G, Seror O, Bourcier V, et al. Partial splenic embolization in patients with cirrhosis: Efficacy, tolerance and long-term outcome in 32 patients. Eur J Gastroenterol Hepatol. 2005;17:179–84. doi: 10.1097/00042737-200502000-00008. [DOI] [PubMed] [Google Scholar]
- 71.Romano M, Giojelli A, Capuano G, Pomponi D, Salvatore M. Partial splenic embolization in patients with idiopathic portal hypertension. Eur J Radiol. 2004;49:268–73. doi: 10.1016/S0720-048X(03)00134-7. [DOI] [PubMed] [Google Scholar]
- 72.Amin MA, el-Gendy MM, Dawoud IE, Shoma A, Negm AM, Amer TA. Partial splenic embolization versus splenectomy for the management of hypersplenism in cirrhotic patients. World J Surg. 2009;33:1702–10. doi: 10.1007/s00268-009-0095-2. [DOI] [PubMed] [Google Scholar]
- 73.Hernandez F, Blanquer A, Linares M, Lopez A, Tarin F, Cervero A. Autoimmune thrombocytopenia associated with hepatitis C virus infection. Acta Haematol. 1998;99:217–20. doi: 10.1159/000040842. [DOI] [PubMed] [Google Scholar]
- 74.Pockros PJ, Duchini A, McMillan R, Nyberg LM, McHutchison J, Viernes E. Immune thrombocytopenic purpura in patients with chronic hepatitis C virus infection. Am J Gastroenterol. 2002;97:2040–5. doi: 10.1111/j.1572-0241.2002.05845.x. [DOI] [PubMed] [Google Scholar]
- 75.Liu Q, Ma K, He Z, et al. Radiofrequency ablation for hypersplenism in patients with liver cirrhosis: A pilot study. J Gastrointest Surg. 2005;9:648–57. doi: 10.1016/j.gassur.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 76.Feng K, Ma K, Liu Q, Wu Q, Dong J, Bie P. Randomized clinical trial of splenic radiofrequency ablation versus splenectomy for severe hypersplenism. Br J Surg. 2011;98:354–61. doi: 10.1002/bjs.7367. [DOI] [PubMed] [Google Scholar]
- 77.Liu Q, Ma K, Song Y, Zhou N, He Z. Two-year follow-up of splenic radiofrequency ablation in patients with cirrhotic hypersplenism: Does increased hepatic arterial flow induce liver regeneration? Surgery. 2008;143:509–18. doi: 10.1016/j.surg.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 78.Mutchnick MG, Lerner E, Conn HO. Portal-systemic encephalopathy and portacaval anastomosis: A prospective, controlled investigation. Gastroenterology. 1974;66:1005–19. [PubMed] [Google Scholar]
- 79.Vang J, Simert G, Hansson JA, Thylen U, Bengmark TS. Results of a modified distal spleno-renal shunt for portal hypertension. Ann Surg. 1977;185:224–8. doi: 10.1097/00000658-197702000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Alvarez OA, Lopera GA, Patel V, Encarnacion CE, Palmaz JC, Lee M. Improvement of thrombocytopenia due to hypersplenism after transjugular intrahepatic portosystemic shunt placement in cirrhotic patients. Am J Gastroenterol. 1996;91:134. [PubMed] [Google Scholar]
- 81.Lawrence SP, Lezotte DC, Durham JD, et al. Course of thrombocytopenia of chronic liver disease after transjugular intrahepatic portosystemic shunts (TIPS). A retrospective analysis. Dig Dis Sci. 1995;40:1575. doi: 10.1007/BF02285211. [DOI] [PubMed] [Google Scholar]
- 82.Pursnani KG, Sillin LF, Kaplan DS. Effect of transjugular intrahepatic portosystemic shunt on secondary hypersplenism. Am J Surg. 1997;173:169. doi: 10.1016/s0002-9610(97)00006-8. [DOI] [PubMed] [Google Scholar]
- 83.Sanyal AJ, Freedman AM, Purdum PP, et al. The hematologic consequences of transjugular intrahepatic portosystemic shunts. Hepatology. 1996;23:32. doi: 10.1002/hep.510230105. [DOI] [PubMed] [Google Scholar]
- 84.Jabbour N, Zajko A, Orons P, Irish W, Fung JJ, Selby RR. Does transjugular intrahepatic portosystemic shunt (TIPS) resolve thrombocytopenia associated with cirrhosis? Dig Dis Sci. 1998;43:2459–62. doi: 10.1023/a:1026634215918. [DOI] [PubMed] [Google Scholar]
- 85.Boyer TD, Haskal Z. AASLD Practice Guidelines: The role of transjugular intrahepatic portosystemic shunt in the management of portal hypertension. Hepatology. 2010;51:1–16. doi: 10.1002/hep.23383. [DOI] [PubMed] [Google Scholar]
- 86.Rajan S, Liebman HA. Treatment of hepatitis C related thrombocytopenia with interferon alpha. Am J Hematol. 2001;68:202–9. doi: 10.1002/ajh.1180. [DOI] [PubMed] [Google Scholar]
- 87.Iga D, Tomimatsu M, Endo H, Ohkawa S, Yamada O. Improvement of thrombocytopenia with disappearance of HCV RNA in patients treated by interferon-alpha therapy: Possible etiology of HCV-associated immune thrombocytopenia. Eur J Haematol. 2005;75:417–23. doi: 10.1111/j.1600-0609.2005.00524.x. [DOI] [PubMed] [Google Scholar]
- 88.Lin A, Thadareddy A, Goldstein MJ, Lake-Bakaar G. Immune suppression leading to hepatitis C virus re-emergence after sustained virological response. J Med Virol. 2008;80:1720–2. doi: 10.1002/jmv.21257. [DOI] [PubMed] [Google Scholar]
- 89.Lladó L, Fabregat J, Castellote J, et al. THOSIN Study Group Impact of immunosuppression without steroids on rejection and hepatitis C virus evolution after liver transplantation: Results of a prospective randomized study. Liver Transpl. 2008;14:1752–60. doi: 10.1002/lt.21629. [DOI] [PubMed] [Google Scholar]
- 90.Lake JR. The role of immunosuppression in recurrence of hepatitis C. Liver Transpl. 2003;9:S63–6. doi: 10.1053/jlts.2003.50264. [DOI] [PubMed] [Google Scholar]
- 91.Li J, Yang C, Xia Y, et al. Thrombocytopenia caused by the development of antibodies to thrombopoietin. Blood. 2001;98:3241–8. doi: 10.1182/blood.v98.12.3241. [DOI] [PubMed] [Google Scholar]
- 92.McHutchison JG, Dusheiko G, Shiffman ML, et al. TPL102357 Study Group Eltrombopag for thrombocytopenia in patients with cirrhosis associated with hepatitis C. N Engl J Med. 2007;29:2227–36. doi: 10.1056/NEJMoa073255. [DOI] [PubMed] [Google Scholar]
- 93.The ELEVATE study. < www.clinicaltrials.gov> TPL104054. NCT00678587 (Accesed March 1, 2013)
- 94.Afdhal NH, Dusheiko GM, Theodore D, et al. Eltrombopag increases platelet numbers in thrombocytopenic patients with HCV infection andcirrhosis, allowing for effective antiviral therapy. Gastroenterology. 2014;146:442–52. doi: 10.1053/j.gastro.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 95.Dultz G, Kronenberger B, Azizi A, et al. Portal vein thrombosis as complication of romiplostim treatment in a cirrhotic patient with hepatitis C-associated immune thrombocytopenic purpura. J Hepatol. 2011;55:229–32. doi: 10.1016/j.jhep.2011.01.020. [DOI] [PubMed] [Google Scholar]


