Abstract
Purpose:
To better understand the pathogenesis of thyroid-associated orbitopathy (TAO) through elucidating the role of thyrotropin receptor (TSHR) and CD40 in the expression of interleukin-8 (IL-8) in peripheral blood fibrocytes. Fibrocytes infiltrate the orbit of patients with TAO, where they differentiate into fibroblasts. Fibrocyte precursors occur with increased frequency in the peripheral blood expressing TSHR and CD40 in TAO patients. We hypothesize that in vitro derived fibrocytes and peripheral blood fibrocyte precursors express proinflammatory chemoattractant molecules including IL-8 initiated by TSHR and CD40 signaling. Since nearly all TAO patients express activating antibodies to TSHR, this is particularly relevant for activation of peripheral blood fibrocytes.
Methods:
TSHR and CD40 expression on peripheral blood fibrocytes was determined by flow cytometry. IL-8 RNA was quantitated by real-time polymerase chain reaction. IL-8 protein production was measured by Luminex and flow cytometry. Thyroid-stimulating hormone and CD40 ligand–stimulated phosphorylation of Akt in peripheral blood fibrocytes was studied by flow cytometry.
Results:
Both TSHR- and CD40-mediated signaling lead to IL-8 expression in mature fibrocytes. Fibrocyte precursors assayed directly from circulating peripheral blood demonstrate intracellular IL-8 expression with addition of thyroid-stimulating hormone or CD40 ligand. TSHR- and CD40-induced IL-8 production is mediated by Akt phosphorylation.
Conclusions:
Peripheral blood TSHR+ and CD40+ fibrocytes express IL-8 and may promote the recruitment of inflammatory cells, mitogenesis, and tissue remodeling in TAO. TSHR- and CD40-mediated IL-8 signaling is mediated by Akt. Delineating the molecular mechanisms of fibrocyte immune function may provide potential therapeutic targets for TAO.
INTRODUCTION
Graves disease is an autoimmune disease where circulating autoantibodies cause hyperthyroidism and lead to thyrotoxicosis. These antibodies, originally referred to as long-acting thyroid stimulators, are directed against the thyrotropin receptor (TSHR). They mimic the agonist activity of thyroid-stimulating hormone (TSH) but are not subject to the normal feedback mechanisms in the anterior pituitary.1 Graves disease is approximately 7- to 10-fold more frequent in women and typically occurs between 20 and 50 years of age.2,3 Clinical manifestations of Graves disease encompass thyroid enlargement and thyrotoxicosis, inflammation and remodeling of the orbit, and rarely transformation of the skin. Thyroid-associated orbitopathy (TAO) is a chronic inflammatory process of the orbit tissues affecting 25% to 50% of Graves disease patients.3,4 In the United States, the annual incidence rate of TAO has been estimated to be 16 cases per 100,000 population for women and 2.9 cases for men.5 While TAO more commonly affects females, men typically have more severe disease manifestations, including optic neuropathy.6 Age at onset is most common between 30 and 50 years, but severe cases occur more frequently in those older than 50 years.6,7 The prevalence of TAO is strongly associated with smoking,8 which also appears to increase the risk for developing severe ophthalmopathy.9,10
Sight-threatening disease occurs in approximately 5% of TAO patients.9,10 Conversely, 10% of those manifesting TAO fail to have endocrinologic aberration. Regardless of whether thyroid dysfunction or TAO develops first, the other becomes apparent within 18 months in 85% of patients.10 Isbister and Rundle11 were the first to divide the course of TAO into active (dynamic) and inactive (static) disease phases. Signs and symptoms of active TAO include progressive proptosis, conjunctival injection, chemosis, diplopia, corneal ulceration, and rarely loss of sight from optic nerve compression. Initial manifestations include enlargement of the extraocular muscles, expansion of orbital fat and connective tissue, and eventual progression to fibrosis and scarring of these tissues.12 The tissue expansion occurs within the relatively fixed dimensions imposed by the bony orbit and results from inflammation, accumulation of glycosaminoglycans, and increased fat volume. Inactive disease is characterized by resolution of inflammatory signs, typically occurring within 18 to 24 months of its first appearance. Long-term disease manifestations are heterogeneous but can include stable proptosis, eyelid retraction, or persistent restrictive strabismus.
Current treatment options for TAO include symptomatic treatment such as lubricating ointments and artificial tears for mild cases. In more severe cases, corticosteroids are considered the main treatment option for patients with significant inflammatory changes.13 Steroids can be administered orally or by intravenous route, but both options are associated with considerable side effects.14 Immunosuppressive agents such as cyclosporine have been investigated and may offer efficacy, particularly in combination with steroids.15 Other treatment options for severe disease include biologic agents or orbital decompression in cases associated with optic neuropathy. Thyroid-associated ophthalmopathy is unique among autoimmune processes in that the acute inflammatory phase is self-limiting. Therefore patient selection and treatment risks must be adequately addressed, since available therapies for TAO are associated with potentially serious side effects.16 This is an area where new therapies and new treatment approaches are greatly needed.
In addition to the pain and discomfort associated with the condition, patients experience considerable distress even in mild cases, due to altered appearance and a reduced quality of life.17 The long-term manifestations of TAO are considered to be inadequately managed and treated.13
IMMUNOLOGY OF GRAVES DISEASE
Adults normally exhibit tolerance to “self” antigens in a process of immune selection during fetal life known as central tolerance. However, under certain circumstances tolerance to self is lost, leading to autoimmunity. The molecular mechanisms for autoimmunity are likely multifactorial but include molecular mimicry, abnormal protein modification, release of sequestered antigens, and epitope spreading.
While Graves disease is a systemic disease, its manifestations exhibit an anatomic site-selective predilection. Thyroid dysfunction is the principal hallmark of Graves disease and occurs in greater than 90% of patients during the course of their disease. Hyperthyroidism results from activating antibodies that bind to TSHR on thyroid epithelial cells and mimic the actions of TSH. Thus TSHR appears to be at least one autoantigen important in this process. Analogous to other autoimmune diseases, several additional autoantigens and immune modifications are required to fully express disease manifestations. Genome-wide screening analyses have implicated CD40, TSHR, and major histocompatibility complex class II molecules in the process, but the mechanisms of participation are unclear.18
IMMUNOLOGY OF THYROID-ASSOCIATED ORBITOPATHY
The active phase of TAO is characterized by orbital and periorbital inflammation targeting connective tissue and fat, most likely orbital fibroblasts.19,20 Although the exact mechanisms are not yet understood, the pathophysiology of TAO appears to arise from the interactions of orbital fibroblasts, immune cells, and cytokines, creating a proinflammatory milieu for orbital tissue remodeling.21 Electron and light microscopy suggest that the muscle cells remain intact early in the disease. However, intense infiltration of T lymphocytes, mast cells, and occasional B cells often intercalate between extraocular muscle fibers and can be found in orbital fat. Among the chemokines examined, the proinflammatory and chemoattractant cytokines interleukin-622,23 and interleukin-824 have been demonstrated to be present in higher concentrations in the tears of Graves disease patients with TAO compared to Graves disease patients without TAO. IL-8 expression was increased in the orbital adipose tissue of 5 of 6 patients with Graves disease and TAO compared to control patients with Graves disease but without TAO.25 These findings suggest that interleukins 6 and 8 may be mediators of disease activity in TAO. These cytokines may be produced by infiltrating mononuclear cells and resident fibroblasts, since they are also detected in areas devoid of mononuclear infiltration.
ROLE OF ORBITAL FIBROBLASTS AND FIBROCYTES IN THE PATHOGENESIS OF THYROID-ASSOCIATED ORBITOPATHY
Several studies have demonstrated that orbital fibroblasts, especially those from patients with Graves disease, are unique with respect to how they respond to several proinflammatory cytokines. The divergent phenotype of these cells may underlie the anatomic site-selective manifestations of Graves disease. Orbital fibroblasts from Graves disease patients also exhibit enhanced production of extracellular matrix components such as hyaluronan in response to these cytokines.26 Thus, these fibroblasts produce proinflammatory molecules and components of connective tissue that promote site-specific tissue remodeling occurring in TAO.
Orbital fibroblasts in patients with TAO express elevated levels of the signaling and activation receptor CD40 compared to orbital fibroblasts in patients without inflammatory orbital disease. Furthermore, ligation of CD40 caused up-regulation of IL-6 and IL-8 in orbital fibroblasts of TAO patients compared to controls.22 Similarly, other studies have shown that orbital fibroblasts express low basal levels of IL-6 and IL-8 with increased expression of both in response to CD40 ligation.27 Exposure to lipopolysaccharide, which induces CD40 expression, also triggers an increase in the expression of IL-8 by orbital fibroblasts.28 The activation of CD40 and consequent cytokine expression represent a potential mechanism contributing to development of TAO.22,27
T cells may also play an important role in orbital fibroblast activation mediated by CD40 interactions. Orbital fibroblasts from TAO patients display increased CD40 expression, which provides T-cell costimulation and enhanced proinflammatory cytokine production, including interleukin-1 (IL-1), IL-6, and IL-8.22,29 These cytokines are potent inducers of prostaglandin H synthase-2, hyaluronan synthase, and uridine diphosphate glucose dehydrogenase genes, leading to inflammation and hyaluronan production.30–33 Thus, disruption of fibroblast–T cell interactions mediated by CD40 could represent an important therapeutic target in TAO. Administration of therapeutic blocking antibodies against CD40 ligand has already proven effective in preclinical mouse models of diabetes and inflammatory bowel disease.34–36
Fibrocytes mediate pathologic, site-specific inflammatory processes. They arise from a small subset of leukocytes (circulating fibrocytes); infiltrate sites of injury or inflammation; and mediate immune response, fibrosis, and tissue remodeling.37,38 Cultured fibrocytes are functionally and phenotypically distinct from monocytes, dendritic cells, and other antigen-presenting cells.39 Both circulating and cultured fibrocytes express fibroblast products such as collagen 1 (Col1) but also hematopoietic antigens, including CD45 and the stem cell antigen CD34. It is unclear whether peripheral blood fibrocytes share analogous functional features to those derived in culture. The investigation of TSH-mediated cytokine expression by circulating fibrocytes as described herein may be critical for therapeutic development.
We have identified CD34+ fibrocytes infiltrating orbital tissues in TAO, and they comprise the majority of orbital fibroblasts in these patients.37 Moreover, TAO orbital fibroblasts and cultured fibrocytes share phenotypic and functional features.37 Unlike control fibroblasts, TAO orbital fibroblasts and fibrocytes both express CD34 and CD40 and readily differentiate into myofibroblasts or adipocytes.22 We hypothesize that TAO orbital fibroblasts arise from CD34+ fibrocytes in the peripheral blood, accounting for their unique phenotype.
Increased numbers of circulating fibrocytes have been shown in human autoimmune fibrotic diseases, including idiopathic pulmonary fibrosis.38 The fibrocyte level, defined as the relative number of fibrocytes in the peripheral circulation, reflects disease severity in these patients.38,40 In addition, treatments that alter fibrocyte migration in postburn patients diminish inflammatory infiltration and fibrosis of the target tissues.41 We have reported that levels of fibrocytes are elevated in the peripheral blood from TAO patients compared to controls,37 and fibrocytes infiltrate the thyroid and orbital tissues of TAO patients but not of controls.
Given the potential importance of TSHR and CD40 expression to the pathogenesis of TAO, we investigated TSH- and CD40-mediated cytokine production by peripheral blood fibrocytes. In our study we examined the expression of IL-8 given the previous implication of this cytokine in the pathogenesis of TAO.
METHODS
CHEMICALS
Histopaque-1077 and sodium azide were purchased from Sigma-Aldrich (St Louis, Missouri), and Dulbecco’s modified Eagle medium, Dulbecco’s phosphate-buffered saline, fetal bovine serum (Gibco FBS), and penicillin-streptomycin mixture (Gibco PenStrep) were purchased from Life Technologies (Grand Island, New York). Bovine TSH and Akt inhibitor IV were purchased from Calbiochem EMD Biosciences (La Jolla, California). Soluble CD40 ligand (MegaCD40L) was provided by Enzo Life Sciences (Farmingdale, New York).
FIBROCYTE ISOLATION
The leukocyte reduction filters from American Red Cross blood donations were the source of all fibrocyte preparation. Peripheral blood mononuclear cells were isolated by layering leukocytes over Histopaque-1077 and centrifuging according to Bucala and associates.39 Peripheral blood mononuclear cells were removed and washed with phosphate-buffered saline solution twice and resuspended in medium with 10% or 1% bovine serum for culture.
CELL CULTURE AND TREATMENTS
Fibrocytes were cultivated as described by Douglas and associates.37 Each well in a 6-well plate was inoculated with approximately 107 peripheral blood mononuclear cells in 3 mL medium supplemented with 10% serum. Cultures were incubated at 37°C and 5% of CO2. Nonadherent cells were removed after 7 days of culture. After the initial weeklong incubation, the medium was changed every 3 to 4 days. After 10 to 14 days in culture, fibrocytes reached approximately 90% purity.
Next, 24 hours prior to experimental conditions, the medium was replaced with medium containing 1% serum. CD40 ligand and TSH were added to cultures at final concentrations of 100 ng/mL or 5 mU/mL, respectively, based on previous dose-response experiments. In some experiments, fibrocytes were pretreated with 100 nM Akt inhibitor IV for 1 hour prior to stimulation.
RNA ISOLATION AND QUANTITATIVE RT-PCR
Total RNA was isolated using Aurum total RNA mini kit from Bio-Rad (Hercules, California). Quantitect reverse transcription kit (Qiagen, Valencia, California) was used for the reverse transcriptase reaction. Relative IL-8 mRNA levels in cultivated fibrocytes were measured by quantitative real-time polymerase chain reaction (PCR) using SYBR Green technique (Bio-Rad) with a Bio-Rad CFX96 thermocycler. For PCR studies of IL-8, the following primers were used: forward primer 5′-GGCAGCCTTCCTGATTTCTG-3′ and reverse primer 5′-GGGTGGAAAGGTTTGGAGTATG-3′. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the housekeeping gene control, using a forward primer (5′-TTGCCATCAATGACCCCTT-3′) and a reverse primer (5′-CGCCCCACTTGATTTTGGA-3′).
EXTRACELLULAR INTERLEUKIN-8 PROTEIN DETERMINATION BY LUMINEX ANALYSIS
Extracellular IL-8 produced by cultivated fibrocytes was determined from the culture media collected after 24-hour treatment with TSH or CD40 ligand. The supernatants of untreated and treated cultures were subjected to cytokine analysis using Luminex technology with IL-8 Human Singleplex Bead Kit (Life Technologies, catalog No. LHC0081).
INTRACELLULAR INTERLEUKIN-8 PROTEIN DETERMINATION BY FLOW CYTOMETRY
Peripheral blood mononuclear cells or fibrocytes cultured for 10 to 14 days were isolated as described and resuspended in medium supplemented with 1% serum and treated with the stimulating agents for 24 hours. After 12 hours of stimulation, golgi stop was added to prevent the excretion of cytokines. After the 24-hour treatment, cells were centrifuged (500g for 5 minutes) and washed with staining buffer containing 2% serum and 0.1% sodium azide. The following anti-human, fluorochrome-conjugated antibodies were added: CD40-PE (catalog No. 555589), CD34 PE-Cy7, mouse IgG1, κ isotype control-FITC (catalog No. 555748), mouse IgG1, κ isotype control-PE (catalog No. 554680), CD34 PE-Cy7 (catalog No. 560710) from BD Biosciences (San Jose, California); CD45-PerCP (catalog No. MHCD4531) from Life Technologies; and TSHR-PE (catalog No. 53542) from Santa Cruz Biotechnology (Santa Cruz, California).
Cells were incubated with the antibodies for 30 minutes at 4°C in the dark, followed by two washes. Cells were then permeabilized with Cytofix/Cytoperm (BD Biosciences, catalog No. 554722) for 20 minutes at 4°C, washed twice, and resuspended in 100 µL Perm/Wash buffer (BD Biosciences, catalog No. 554723). Anti-human IL-8-PE (catalog No. 340510) from BD Biosciences and collagen type-I-FITC (catalog No. FCMAB412) from Millipore (Temecula, California) antibodies were added to the cells and incubated for 30 minutes in the dark on ice. Cells were washed again twice and fixed with 1% paraformaldehyde. Analysis was performed with LSR II flow cytometer (BD Biosciences). At least 1×106 events were collected. Mean fluorescence intensity (MFI) was calculated as a ratio of mean fluorescence sample over mean fluorescence isotype control. MFI of one indicates same fluorescent intensity as background or isotype.
AKT PHOSPHORYLATION
Akt phosphorylation in circulating fibrocytes was studied by flow cytometry analysis. Peripheral blood mononuclear cells were isolated as described earlier and cultured. Cells were stimulated with CD40 ligand or TSH and incubated at 37°C. After treatment, cells were placed on ice and immediately resuspended in fixation buffer (BD Biosciences, catalog No. 554655) for 30 minutes. Antibodies for surface markers (CD45 and CD34) and isotype controls were added and incubated for 30 minutes on ice in dark. Washed cells were permeabilized with ice cold Perm Buffer III (BD Biosciences, catalog No. 558050) for 30 minutes. After two washes, cells were subjected to anti-Akt (pS473) (catalog No. 560378) or PE Mouse anti-Akt1 (catalog No. 560049) antibodies from BD Biosciences and incubated for 30 minutes in dark. Cells were resuspended in 1% paraformaldehyde after the final washes.
STATISTICAL ANALYSIS
Unless otherwise stated, data values are reported as the mean ± standard deviation. Statistical analysis was performed using analysis of variance (ANOVA) with a confidence level greater than 95%.
RESULTS
CULTURED AND CIRCULATING FIBROCYTES EXPRESS CD40 AND THYROTROPIN RECEPTOR
Fibrocytes appear to infiltrate the thyroid and orbit of patients with TAO and express proinflammatory interleukins and chemokines that may be critical for the development of TAO.22,37 Given the substantial role of CD40 and TSHR in this disease, we examined IL-8 expression after stimulation of fibrocytes derived from culture (10 to 14 days) and those directly isolated from peripheral blood. A homogenous fibrocyte population can be derived by culturing peripheral blood for approximately 2 weeks. Thus “cultured” fibrocytes are mature, homogenous, and well differentiated, providing insight into cellular response mechanisms. On the other hand, “circulating” fibrocytes assessed directly from the peripheral blood are investigated without cell separation and within 24 hours of blood donation. Assessment of circulating fibrocytes requires multiparameter flow cytometry to evaluate individual cells within a heterogeneous mixture and reflects an immature stage of fibrocyte development. Examination of both populations is necessary considering their functional and differentiative capacity. It is unclear whether peripheral blood fibrocytes share analogous functional features to those derived in culture. The identification and characterization of circulating fibrocytes and investigation of TSH- and CD40-mediated cytokine expression are examined here and may be critical for therapeutic development.
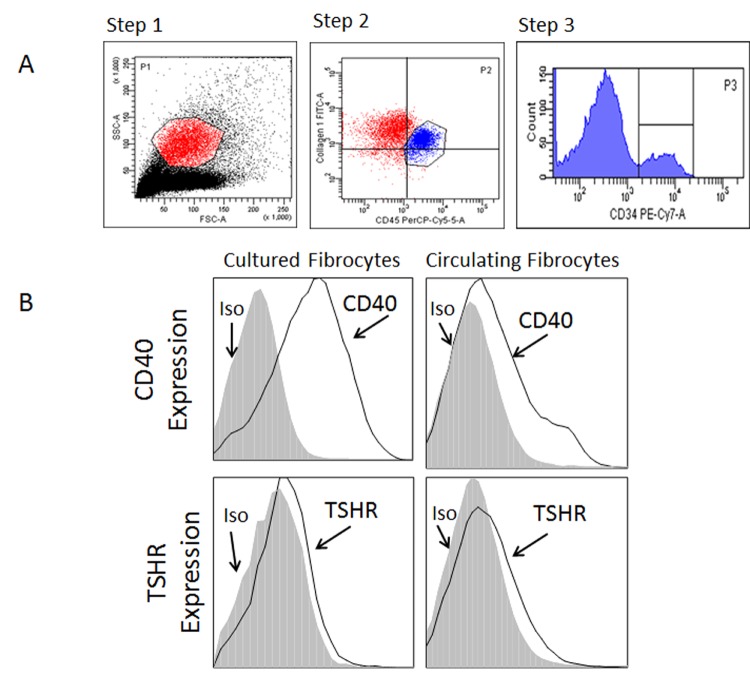
We initially examined whether CD40 and TSHR are expressed by circulating fibrocytes to levels analogous to fibrocytes differentiated in culture. In order to identify peripheral blood fibrocytes, flow cytometry and a three-step gating strategy were applied (Figure 1, panel A). Initially monocytes were identified by the size and granularity profile as demonstrated (step 1; FSC vs SSC). In step 2, CD45+ Col 1+ cells were identified within the monocyte population. Last, the CD34+ CD45+ Col1+ fibrocytes were selected (step 3). This strategy allowed identification of CD45+ Col1+ CD34+ fibrocytes. Fibrocytes identified in the peripheral blood constitutively express high levels of CD40 and TSHR (Figure 1, panel B). The expression of these molecules is analogous to expression by cultured fibrocytes in agreement with our previous findings.42,43
FIGURE 1.
CD40 and TSHR are constitutively expressed by fibrocytes as determined by flow cytometry. We have previously reported that levels of fibrocytes are elevated in the peripheral blood from patients with thyroid-associated orbitopathy compared to controls.37 Panel A demonstrates the three-step gating strategy for discriminating circulating fibrocytes among peripheral mononuclear cells. Fibrocytes are determined by forward and side scatter (step 1), then CD45 and collagen expression (step 2) and CD34 expression (step 3). Panel B demonstrates that fibrocytes constitutively express CD40 and TSHR (gray-filled histogram: isotype control; solid line: CD40 or TSHR). Mean fluorescent intensity of CD40 expression was 5.39 and 1.75, and of TSHR expression was 1.99 and 1.29, for cultured and circulating fibrocytes, respectively. The results are a representative example of three experiments.
CD40 LIGAND AND THYROID-STIMULATING HORMONE STIMULATE INTERLEUKIN-8 PRODUCTION IN FIBROCYTES
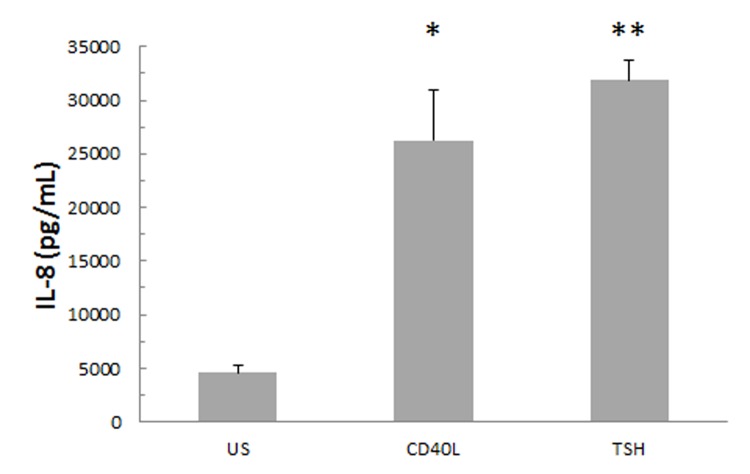
The addition of CD40 ligand and TSH for 24 hours to fibrocytes isolated after culture increases IL-8 protein expression (Figure 2). Fibrocytes isolated and differentiated in culture exhibited a low but detectable basal level of IL-8 expression. After 24-hour stimulation with CD40 ligand or TSH, a 6- to 7-fold increase in the IL-8 protein concentration was observed.
FIGURE 2.
IL-8 secretion is increased in cultures of 10- to 14-day-old fibrocytes that were stimulated for 24 hours with CD40 ligand or TSH. Increasing evidence shows that these signal pathways are involved in the manifestation of thyroid-associated orbitopathy by stimulating proinflammatory cytokines such as IL-8. Culture media were collected and subjected to IL-8 analysis using Luminex technology (n=3, *P <.05; **P <.01).
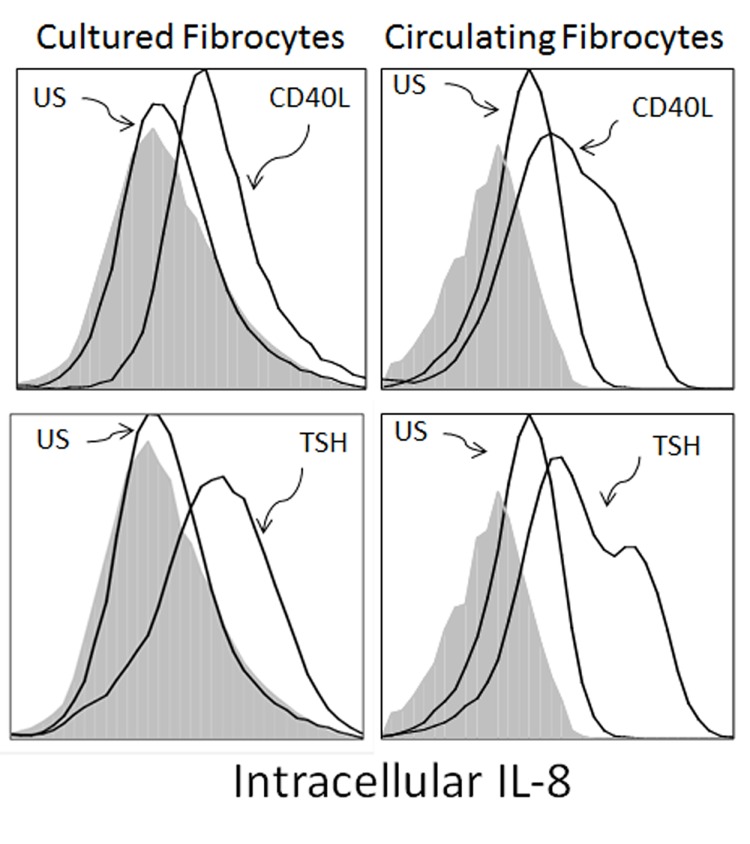
Since IL-8 is important during the initiation phase of an immune response, we next determined if CD40 ligand and TSH induce IL-8 production in peripheral blood fibrocytes. Freshly isolated peripheral blood mononuclear cells were stimulated with CD40 ligand, TSH, or nothing while intracellular IL-8 accumulation was determined by flow cytometry. Identification of fibrocytes within the mixed population of blood mononuclear cells was characterized by expression of CD45, CD34, and Col1 performed analogous to that shown in Figure 1. Peripheral blood fibrocytes express intracellular IL-8 without stimulation. The addition of TSH or CD40 ligand increased IL-8 expression resulting in a 2.1- and 1.6-fold increase in logarithmic mean fluorescent intensity when compared to that of untreated cells. Intracellular IL-8 was also assessed in fibrocytes differentiated in culture for 2 weeks as a comparison (Figure 3). Both TSH and CD40 ligand induced IL-8 expression in these cells, albeit to a lesser extent compared to peripheral blood fibrocytes (1.7- and 1.3-fold increase in logarithmic mean fluorescent intensity with TSH or with CD40 ligand vs untreated cells). Cultured fibrocytes expressed scant IL-8 without stimulation in contrast to fibrocytes from the peripheral blood (unstimulated mean fluorescent intensity = 1.48 of peripheral blood vs mean fluorescent intensity = 0.98 from cultured fibrocytes) (Figure 3). Expression of constitutive intracellular IL-8 in unstimulated fibrocytes derived from the circulation suggests ongoing IL-8 production in vivo. As a negative control, lymphocytes in the peripheral blood do not express IL-8 under any of these conditions (data not shown). Overall, our data indicate that CD45+ Col1+ CD34+ peripheral blood fibrocytes are unique in their ability to constitutively express IL-8 while both peripheral blood and cultured fibrocytes increase IL-8 expression in response to TSH and CD40 ligand.
FIGURE 3.
Intracellular IL-8 production is stimulated with CD40 ligand and TSH in fibrocytes. Fibrocytes infiltrate the orbit in thyroid-associated orbitopathy and participate in tissue remodelling. Cells were stimulated for 24 hours. CD40 induction results in 1.3- and 1.6-fold increase in logarithmic fluorescent intensity compared to those of unstimulated cells in cultured and circulating fibrocytes, respectively. TSH also induces IL-8 production resulting in 1.7- and 2.1-fold increase in fluorescent intensity in cultured and circulating fibrocytes, respectively. Isotype controls are shown as histograms filled with gray. Figure is representative of three experiments.
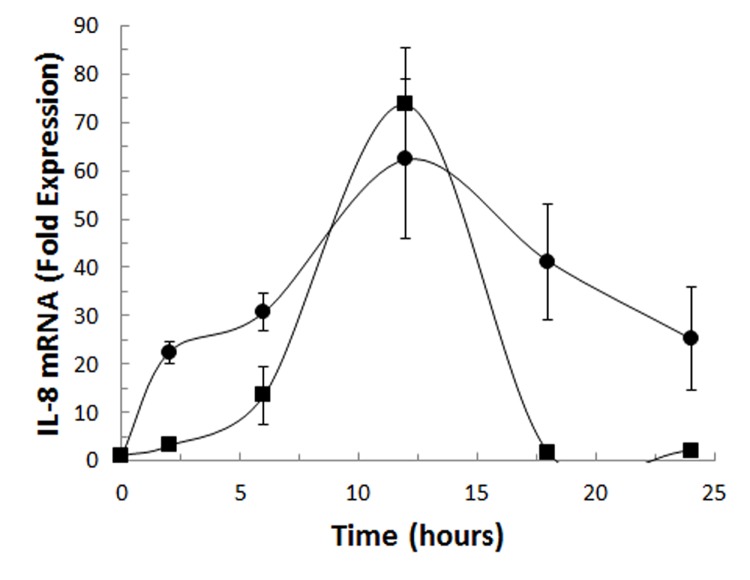
Since CD40 ligand and TSH induce IL-8, we evaluated the role of these mediators on IL-8 mRNA (Figure 4). CD40 ligand and TSH increased IL-8 mRNA in fibrocytes differentiated in culture, reaching peak expression after 12 hours. While both CD40 ligand and TSH induce IL-8 mRNA 60- to 70-fold compared to unstimulated cultures, the kinetics of induction were divergent. Fibrocytes stimulated with CD40 ligand demonstrated increased IL-8 mRNA expression as early as 2 hours, and this was sustained at 24 hours. In contrast, TSH stimulation increased IL-8 mRNA but was not sustained. Thus given the de novo expression of IL-8 mRNA, we have established that CD40 ligand- and TSH-mediated expression is at the pretranslational level.
FIGURE 4.
Time course of IL-8 mRNA expression stimulated with CD40 ligand (•) or TSH (▪) in cultured fibrocytes was determined by qRT-PCR. This experiment shows that these ligands, which participate in the development of thyroid-associated orbitopathy, induce IL-8 production at the pretranslational level. Fibrocytes were isolated for 10 to 14 days in culture and stimulated with CD40 ligand or TSH for times indicated. The cells were harvested and IL-8 RNA was assessed by PCR. Values are reported as mean “fold” induction.
CELLULAR SIGNALING MECHANISMS OF INTERLEUKIN-8 PRODUCTION IN FIBROCYTES: THE ROLE OF AKT
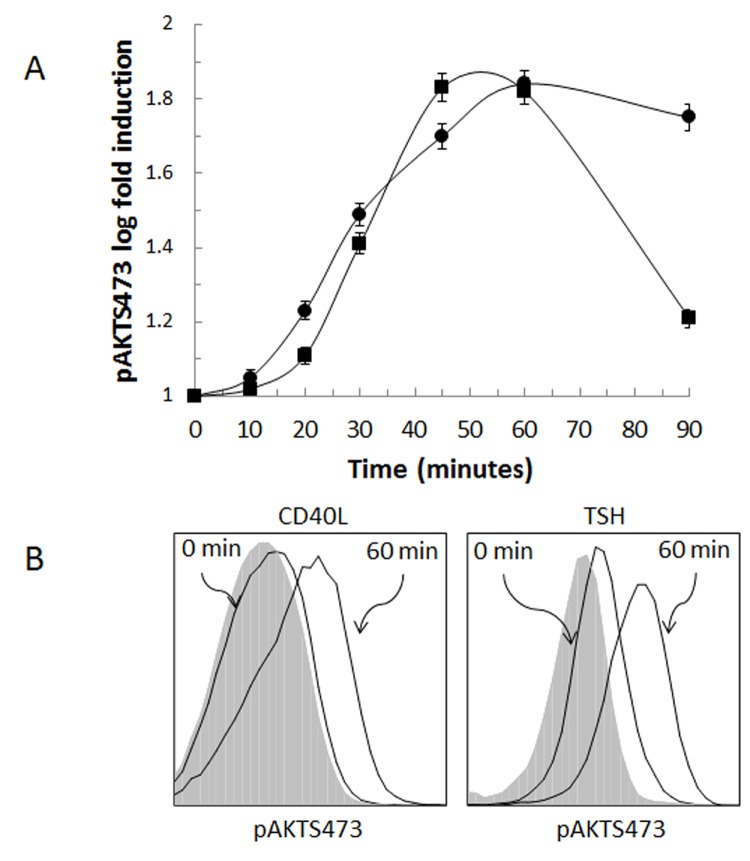
IL-8 and IL-6 are two of several cytokines and chemokines that coordinately regulate early cellular inflammatory processes. Phosphorylation of Akt has been previously implicated in the CD40-mediated up-regulation of IL-6 in fibrocytes43 and the TSHR-mediated up-regulation in thyroid fibroblasts.44 We now investigate the role of Akt phosphorylation regulating CD40- and TSHR-mediated IL-8 production in circulating fibrocytes. Peripheral blood was stimulated with CD40 ligand or TSH at intervals up to 90 minutes and immediately fixed as described in the “Methods” section. To determine the relative state of fibrocyte Akt phosphorylation in the peripheral blood, multiparameter flow cytometry was utilized. Quantification of phosphorylation was expressed as a ratio between the MFI at a sampling point and the fluorescent intensity at baseline (time 0). CD40 ligand- and TSH-induced phosphorylation of AktS473 reached an approximately 1.8-fold increase compared to baseline (Figure 5). The peak phosphorylation was achieved between 45 and 60 minutes after addition of CD40 ligand and 60 minutes after addition of TSH. CD40-mediated Akt phosphorylation declined rapidly compared to that of TSH-stimulated cells (solid square, Figure 5, panel B). The total Akt, irrespective of its phosphorylation state, did not change significantly during the course of the experiment, indicating that neither CD40 ligand nor TSH alters induction and/or degradation of Akt protein (data not shown).
FIGURE 5.
CD40 ligand and TSH stimulate the phosphorylation of AktS473 in circulating fibrocytes as identified by flow cytometry. Phosphorylation of Akt is a common signal transduction pathway for inflammatory mediators, and our research indicates that Akt activation is involved in the development of thyroid-associated orbitopathy. Panel A shows the time course of phosphorylation of Akt as a result of CD40 ligand (•) or TSH (▪) stimulation. In Panel B, phosphorylation of AktS473 is shown for fibrocytes stimulated with CD40 ligand or TSH at time 0 and 60 minutes. Gray-filled histograms are the isotype controls.
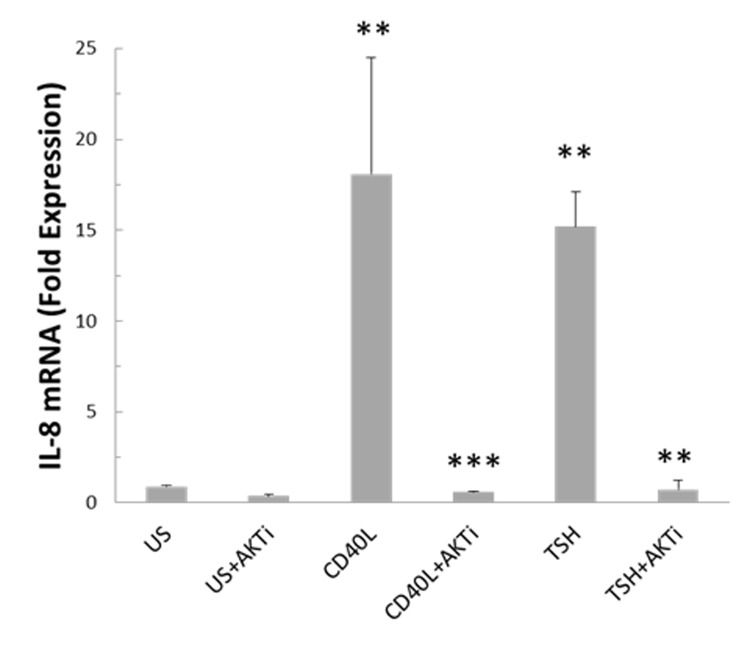
Since phosphorylation of Akt occurs after addition of CD40 ligand or TSH, we utilized a chemical inhibitor of Akt to determine specificity. The inhibitor was added 1 hour prior to stimulation of fibrocytes. IL-8 mRNA level was measured by PCR in the presence or absence of Akt inhibitor and stimulation for 6 hours with CD40 ligand or TSH. Inhibition of Akt signaling suppressed CD40- and TSHR-mediated IL-8 mRNA to basal levels in cultured fibrocytes (Figure 6). Therefore Akt phosphorylation appears to be instrumental in CD40- and TSHR-mediated IL-8 expression.
FIGURE 6.
CD40 ligand- and TSH-mediated IL-8 transcription is suppressed by Akt inhibitor IV in cultured fibrocytes. In this experiment the role of Akt phosphorylation in IL-8 production was demonstrated at the pretranslational level. Akt is a common member of the TSHR and CD40 signaling pathways, which partake in the onset of thyroid-associated orbitopathy. Cells were stimulated for 6 hours with CD40 ligand or TSH with or without Akt inhibitor (AKTi) added 1 hour prior. CD40 ligand- or TSH-treated cultures significantly increased IL-8 mRNA compared to unstimulated cultures (n=3, **P <.01). Pretreatment for 1 hour with AKTi abrogated CD40 ligand- and TSH-mediated IL-8 mRNA expression (n=3, **P <.01; ***P <.001).
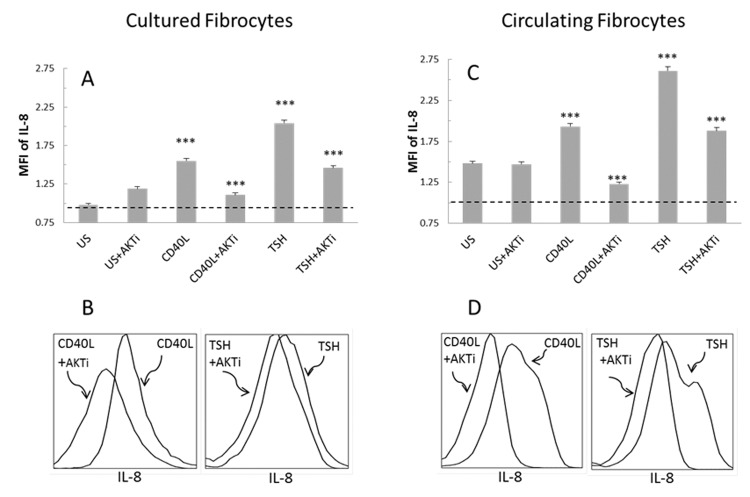
The role of Akt phosphorylation in CD40- and TSHR-mediated IL-8 protein production was also assessed in peripheral blood fibrocytes identified by multiparameter flow cytometry. CD40 ligand- and TSH-mediated intracellular IL-8 production was assessed in the presence and absence of an Akt inhibitor (Figure 7). As described in the “Methods” section, peripheral blood and cultured fibrocytes were stimulated for 24 hours with CD40 ligand or TSH with or without Akt inhibitor. Addition of Akt inhibitor significantly blunted intracellular IL-8 expression after CD40 ligand and TSH stimulation in peripheral blood and cultured fibrocytes. While the inhibitor did not significantly alter the basal level of IL-8, the inhibition of Akt significantly abrogated the CD40- and TSHR-mediated IL-8 production.
FIGURE 7.
CD40 ligand and TSH stimulate IL-8 production through Akt signaling in cultured (A, B) and circulating fibrocytes (C, D). This figure shows the effect of Akt inhibition on the protein level, indicating its potential role in thyroid-associated eye disease. The addition of Akt inhibitor (AKTi) abrogates the stimulatory effect of CD40 ligand and TSH. Fibrocytes were selected based on their CD34+ CD45+ Col1+ expression using the same gating strategy as in Figure 1. A and C, CD40 ligand or TSH induction of intracellular IL-8 (MFI of 1 equals no expression). AKTi addition inhibits intracellular IL-8 accumulation after CD40 ligand or TSH signaling (n=3, ***P <.001). B and D, Histograms demonstrate the inhibition of CD40 ligand- and TSH-mediated IL-8 expression with addition of AKTi. Figure is representative of three experiments.
DISCUSSION
PATHOGENESIS OF TAO: ROLE OF TSHR AND CD40
Graves disease is among the most common autoimmune diseases in the United States.37 Its ocular manifestation, TAO, remains a potentially sight-threatening process that can result in life-altering disfigurement. The molecular mechanisms underlying the pathogenesis of thyroid gland and orbital involvement remain an unsolved mystery. Thyroid overactivity is the result of autoantibodies generated against the thyrotropin receptor (TSHR); however, the cause of orbital inflammation and remodeling is less clear. Pathologically, TAO is associated with immune activation and orbital fibroblast expansion, leading to enlargement of the extraocular muscles, cellular infiltration of interstitial tissues, and an increase in orbital fat and connective tissue.
Understanding the distinct molecular and immunologic attributes of orbital tissues and how these influence the manifestations of TAO remains central to novel treatment development. Thus far several lines of investigation have indicated a critical role for fibrocytes. Fibrocytes display the hematopoietic marker CD34 and infiltrate tissues during fibrosis and tissue remodeling.42,45 Bone marrow–derived CD34+ fibrocytes infiltrate the orbit and thyroid in Graves disease and appear to participate in the pathogenesis of TAO.46 Following their tissue infiltration, they retain expression of these proteins as CD34+ fibroblasts. Fibrocyte immune activation and tissue remodeling may provide the missing link between manifestations of Graves disease that affect the thyroid and orbit. Supporting this rationale, recent observations indicate that circulating fibrocytes unexpectedly express high levels of functional TSHR and thyroglobulin.47 These proteins were originally thought to be expressed only in the thyroid gland, as both are autoantigens implicated in thyroid autoimmunity.48,49 Increased fibrocyte TSHR expression and the presence of circulating activating autoantibodies lead to the question of whether these antibodies may be contributing to disease process.
Immune recognition of “foreign” and “self” is predicated on molecular recognition of target epitopes and costimulation of host immune cells providing T- and B-cell “help.”50,51 CD40 activation functions as the fulcrum between immune activation and anergy or unresponsiveness. In several autoimmune model systems, CD40 activation overcomes peripheral tolerance.52,53 In contrast, antigen expression in the absence of CD40 activation leads to peripheral anergy.22 The importance of CD40 in Graves disease and TAO has been established from genetic and immunologic perspectives.22,27,30 In summary, we have identified an increased frequency of CD40+ fibroblasts in the tissues of TAO patients.54 Moreover, they are particularly responsive to CD40 activation and express IL-6, IL-8, and MCP-1, and produce hyaluronan, all of which are hallmarks of TAO.55
Expression of major histocompatibility complex and costimulatory molecules by fibrocytes suggests that they can present antigen to T lymphocytes and initiate immune responses.56–58 We have previously demonstrated that CD40 is overexpressed by CD34+ TAO fibroblasts and CD34+ fibrocytes compared to those of control donors.22
PROGRESS TOWARD IN VIVO IDENTIFICATION AND FUNCTIONAL ANALYSIS
The preponderance of previous data detailing fibrocyte biology has been derived from cells placed in culture and differentiated for 10 to 14 days. This technique has been necessitated by practical demands to yield substantial numbers of cells for analysis. Our work herein advances our understanding of fibrocyte biology in TAO by identifying and functionally characterizing these cells in the peripheral blood. Identification of these cells by multiparameter flow cytometry is essential, since they constitute less than 1% of the peripheral blood cells in controls. 39 Furthermore, potentially important markers of disease and/or disease activity may be present in cells obtained directly from the peripheral blood. For example, previous studies in rheumatoid arthritis have demonstrated an increased activation of intracellular signaling molecules in patients with active disease compared to quiescent disease.59 Currently there are not biomarkers available in TAO, and delineation of cells believed to be important in the disease process is critical.
THE ROLE OF IL-8
IL-8 is an early response, proinflammatory chemokine discovered in 1987.60 IL-8 is produced by multiple cell types, including monocytes and fibroblasts,61 and is involved in the directional migration of neutrophils and T lymphocytes.62 IL-8 also demonstrates proangiogenic properties via stimulation of endothelial cell proliferation and capillary vessel formation; recruitment of neutrophils, basophils, and T lymphocytes; inhibition of apoptosis of endothelial cells; and protease activation by inducing increased endothelial cell mRNA expression of matrix metalloproteinases and gelatinase activity.63 IL-8 is an early response proinflammatory chemokine, which is produced as early as 4 hours after exposure to a foreign agent or injury of the body.22,27,64–66 The major sources of IL-8 in the body are monocytes and macrophages, but it is produced by many other types of cells, such as epithelial cells and fibroblasts.67 It also has been detected in tissues relevant to Graves disease, such as thyroid tissue, orbital fibroblasts, and the tears and blood of Graves patients.68,69 Our result shows that circulating fibrocytes may significantly contribute to the elevated IL-8 levels in Graves patients in tissues mentioned previously.
We have demonstrated that increased numbers of TSHR+ CD40+ circulating fibrocytes infiltrate into the orbital tissue and may be the major source of IL-8 production. TSHR+ CD40+ fibrocytes may be activated by TSH (and/or TSHR-stimulating immunoglobulin) or cells expressing CD40 ligand, thus contributing to the inflammatory process via IL-8 expression (Figure 3). Constitutive production of IL-8 is exceptionally rare outside of neoplastic transformation.70 However, fibrocytes derived from the peripheral blood consistently demonstrate measurable intracellular levels of IL-8 (Figures 2 and 3). This suggests that circulating fibrocytes are poised to facilitate immune responses as occurs in the orbital tissue. The stimuli for constitutive intracellular IL-8 production will require additional investigation, but gene expression of IL-8 is particularly sensitive to reactive oxygen species (ROS).70,71 Increased ROS are detected in the blood of Graves patients and may be attributed to the imbalance of intracellular oxidants and antioxidants.69,72 Constitutive IL-8 production may also yield insight into the unfavorable impact of certain environmental factors and consequent oxidative stress in these tissues.44 Apart from ROS, IL-8 production is stimulated by many agents, including lipopolysaccharide, bacteria, viruses, TNF-α, and IL-1.44 Nonetheless, our findings demonstrate a dramatic increase in IL-8 production in response to TSHR and CD40 ligation. This finding implies that increased IL-8 found in these patients may be secondary to fibrocyte stimulation, possibly by stimulatory antibodies.
IL-8 expression by orbital or thyroid fibroblasts is stimulated by CD40 ligand42,43 and TSH.68 Our study confirms that TSHR and CD40 stimulation plays a role in IL-8 production in fibrocytes as well. These may be significant pathways for IL-8 production in Graves disease and TAO, since the number of fibrocytes and, perhaps more important, the number of CD40+ THSR+ circulating fibrocytes are augmented in patients with TAO.63 In this way, fibrocytes may also provide the link between the thyroid and orbital manifestations of disease. This finding is especially relevant, since we demonstrate for the first time that fibrocytes identified from the circulation also express IL-8 constitutively and in response to TSHR and CD40 stimulation.
While IL-8 can be produced rapidly, the protein and downstream physiologic consequences are evident for days or weeks.63,73,74 This may be due to the complex regulation of expression involving multiple pathways of transcriptional signaling and posttranscriptional stabilization of mRNA.75 One of the dominant pathways in IL-8 induction is mediated by the PI3K-Akt-NF-κB pathway.43 This pathway also appears to be important in circulating fibrocytes, since both TSH and CD40 ligand activate Akt by promoting the phosphorylation of serine 473 (Figure 5). Inhibition of Akt phosphorylation by a chemical inhibitor also blocked the induction of IL-8 production with CD40 ligand and TSH (Figure 7). Phosphorylation of Akt is a common signal transduction pathway for inflammatory mediators.76 We have previously reported the role of Akt and NF-κB in CD40-mediated signaling for IL-6 expression by cultured fibrocytes75. Here we have shown that Akt is involved in the TSHR- and CD40-mediated regulation of IL-8 transcription in cultured and circulating fibrocytes. Our current result demonstrates that these mediators both utilize Akt phosphorylation to generate IL-8. Targeted interruption of the fibrocyte signaling pathways of TSHR- and CD40-mediated cytokine production could provide novel therapies.76
One of the most exciting aspects of the study is the identification and functional evaluation of fibrocytes isolated directly from peripheral blood. In our studies we identified immature fibrocytes using multiparameter flow cytometry and found that the CD40- and TSHR-mediated IL-8 production is analogous to those fibrocytes differentiated in culture for 10 to 14 days. This finding now allows further evaluation of these cells and their responses as biomarkers of disease activity and severity. These studies hold exceptional promise given the functional assessment of Akt phosphorylation and IL-8 production we demonstrate.
This study further supports our hypothesis that circulating fibrocytes contribute to the site-specific immune function in TAO. TSHR- and CD40-mediated production of IL-8 may play a role in Graves disease and TAO-related thyroid and orbital tissues. Both pathways involve Akt in their signaling, which may be a therapeutic target for TAO and Graves disease.
Acknowledgments
Funding/Support: This work was supported in part by National Institutes of Health grants EY008976, EY011708, DK063121, EY016339, EY021197, and EY007003 Eye Core Grant; an unrestricted grant from Research to Prevent Blindness; a Research to Prevent Blindness Career Development Award; Research to Prevent Blindness Lew Wasserman Merit Award; and the Bell Charitable Foundation.
Financial Disclosures: None.
Author Contributions: Design and conduct of the study (R.S.D, T.M, A.G.); Collection, management, analysis, and interpretation of data (R.S.D, T.M.); Preparation, review, and approval of the manuscript (R.S.D, T.M., A.G., D.S.K.).
REFERENCES
- 1.Khoo TK, Bahn RS. Pathogenesis of Graves’ ophthalmopathy: the role of autoantibodies. Thyroid. 2007;17:1013–1018. doi: 10.1089/thy.2007.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacobson DL, Gange SJ, Rose NR, Graham NM. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin Immunol Immunopathol. 1997;84:223–243. doi: 10.1006/clin.1997.4412. [DOI] [PubMed] [Google Scholar]
- 3.Gould DJ, Roth FS, Soparkar CNS. The diagnosis and treatment of thyroid-associated ophthalmopathy. Aesthetic Plast Surg. 2012;36:638–648. doi: 10.1007/s00266-011-9843-4. [DOI] [PubMed] [Google Scholar]
- 4.Kazim M, Goldberg RA, Smith TJ. Insights into the pathogenesis of thyroid-associated orbitopathy: evolving rationale for therapy. Arch Ophthalmol. 2002;120:380–386. doi: 10.1001/archopht.120.3.380. [DOI] [PubMed] [Google Scholar]
- 5.Bartley GB, Fatourechi V, Kadrmas EF, et al. Clinical features of Graves’ ophthalmopathy in an incidence cohort. Am J Ophthalmol. 1996;121:284–290. doi: 10.1016/s0002-9394(14)70276-4. [DOI] [PubMed] [Google Scholar]
- 6.Perros P, Crombie AL, Matthews JN, Kendall-Taylor P. Age and gender influence the severity of thyroid-associated ophthalmopathy: a study of 101 patients attending a combined thyroid-eye clinic. Clin Endocrinol (Oxf) 1993;38:367–372. doi: 10.1111/j.1365-2265.1993.tb00516.x. [DOI] [PubMed] [Google Scholar]
- 7.Ing E. Thyroid-associated orbitopathy. Medscape. 2012. Available at: http://emedicine.medscape.com/article/1218444-overview [Accessed September 24, 2013].
- 8.Prummel MF, Wiersinga WM. Smoking a risk factor for hypothyroidism [correction of hyperthyroidism] J Endocrinol Invest. 1993;16:827. doi: 10.1007/BF03348935. [DOI] [PubMed] [Google Scholar]
- 9.Bartalena L, Pinchera A, Marcocci C. Management of Graves’ ophthalmopathy: reality and perspectives. Endocr Rev. 2000;21:168–199. doi: 10.1210/edrv.21.2.0393. [DOI] [PubMed] [Google Scholar]
- 10.Dickinson AJ. Clinical manifestations. In: Wiersinga WM, Kahaly GJ, editors. Graves’ Orbitopathy. Vol. 0. Basel: KARGER; 2010. pp. 1–25. Available at: http://www.karger.com/doi/10.1159/000320423 [Accessed September 24, 2013]. [Google Scholar]
- 11.Isbister J, Rundle FF. Antithyroid therapy: clinical trials with mercazole. Med J Aust. 1954;41:78–81. [PubMed] [Google Scholar]
- 12.Garrity JA, Bahn RS. Pathogenesis of graves ophthalmopathy: implications for prediction, prevention, and treatment. Am J Ophthalmol. 2006;142:147–153. doi: 10.1016/j.ajo.2006.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bartalena L, Baldeschi L, Dickinson A, et al. Consensus statement of the European Group on Graves’ orbitopathy (EUGOGO) on management of GO. Eur J Endocrinol Eur Fed Endocr Soc. 2008;158:273–285. doi: 10.1530/EJE-07-0666. [DOI] [PubMed] [Google Scholar]
- 14.Le Moli R, Baldeschi L, Saeed P, et al. Determinants of liver damage associated with intravenous methylprednisolone pulse therapy in Graves’ ophthalmopathy. Thyroid. 2007;17:357–362. doi: 10.1089/thy.2006.0267. [DOI] [PubMed] [Google Scholar]
- 15.Stamato FJ, Maciel RM, Manso PG, et al. Colchicine in the treatment of the inflammatory phase of Graves’ ophthalmopathy: a prospective and randomized trial with prednisone. Arq Bras Oftalmol. 2006;69:811–816. doi: 10.1590/s0004-27492006000600006. [DOI] [PubMed] [Google Scholar]
- 16.Mallika P, Tan A, Aziz S, et al. Thyroid associated ophthalmopathy—a review. Malays Fam Physician. 2009;4:8–14. Available at: http://www.e-mfp.org/2009v4n1/pdf/Thyroid_assoc_ophthalmopathy.pdf [Accessed September 24, 2013]. [PMC free article] [PubMed] [Google Scholar]
- 17.Terwee CB, Gerding MN, Dekker FW, et al. Development of a disease specific quality of life questionnaire for patients with Graves’ ophthalmopathy: the GO-QOL. Br J Ophthalmol. 1998;82:773–779. doi: 10.1136/bjo.82.7.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tomer Y. Genetic susceptibility to autoimmune thyroid disease: past, present, and future. Thyroid. 2010;20:715–725. doi: 10.1089/thy.2010.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bahn RS, Heufelder AE. Role of connective tissue autoimmunity in Graves’ ophthalmopathy. Autoimmunity. 1992;13:75–79. doi: 10.3109/08916939209014638. [DOI] [PubMed] [Google Scholar]
- 20.Feldon SE, Park DJJ, O’Loughlin CW, et al. Autologous T-lymphocytes stimulate proliferation of orbital fibroblasts derived from patients with Graves’ ophthalmopathy. Invest Ophthalmol Vis Sci. 2005;46:3913–3921. doi: 10.1167/iovs.05-0605. [DOI] [PubMed] [Google Scholar]
- 21.Naik VM, Naik MN, Goldberg RA, et al. Immunopathogenesis of thyroid eye disease: emerging paradigms. Surv Ophthalmol. 2010;55:215–226. doi: 10.1016/j.survophthal.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hwang CJ, Afifiyan N, Sand D, et al. Orbital fibroblasts from patients with thyroid-associated ophthalmopathy overexpress CD40: CD154 hyperinduces IL-6, IL-8, and MCP-1. Invest Ophthalmol Vis Sci. 2009;50:2262–2268. doi: 10.1167/iovs.08-2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ujhelyi B, Gogolak P, Erdei A, et al. Graves’ orbitopathy results in profound changes in tear composition: a study of plasminogen activator inhibitor-1 and seven cytokines. Thyroid. 2012;22:407–414. doi: 10.1089/thy.2011.0248. [DOI] [PubMed] [Google Scholar]
- 24.Huang D, Xu N, Song Y, Wang P, Yang H. Inflammatory cytokine profiles in the tears of thyroid-associated ophthalmopathy. Graefes Arch Clin Exp Ophthalmol. 2012;250:619–625. doi: 10.1007/s00417-011-1863-x. [DOI] [PubMed] [Google Scholar]
- 25.Kumar S, Bahn RS. Relative overexpression of macrophage-derived cytokines in orbital adipose tissue from patients with graves’ ophthalmopathy. J Clin Endocrinol Metab. 2003;88:4246–4250. doi: 10.1210/jc.2003-030380. [DOI] [PubMed] [Google Scholar]
- 26.Smith TJ. Orbital fibroblasts exhibit a novel pattern of responses to proinflammatory cytokines: potential basis for the pathogenesis of thyroid-associated ophthalmopathy. Thyroid. 2002;12:197–203. doi: 10.1089/105072502753600133. [DOI] [PubMed] [Google Scholar]
- 27.Sempowski GD, Rozenblit J, Smith TJ, Phipps RP. Human orbital fibroblasts are activated through CD40 to induce proinflammatory cytokine production. Am J Physiol. 1998;274:C707–714. doi: 10.1152/ajpcell.1998.274.3.C707. [DOI] [PubMed] [Google Scholar]
- 28.Elner VM, Burnstine MA, Kunkel SL, et al. Interleukin-8 and monocyte chemotactic protein-1 gene expression and protein production by human orbital fibroblasts. Ophthal Plast Reconstr Surg. 1998;14:119–125. doi: 10.1097/00002341-199803000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Han R, Smith TJ. Induction by IL-1 beta of tissue inhibitor of metalloproteinase-1 in human orbital fibroblasts: modulation of gene promoter activity by IL-4 and IFN-gamma. J Immunol 1950. 2005;174:3072–3079. doi: 10.4049/jimmunol.174.5.3072. [DOI] [PubMed] [Google Scholar]
- 30.Cao HJ, Wang HS, Zhang Y, et al. Activation of human orbital fibroblasts through CD40 engagement results in a dramatic induction of hyaluronan synthesis and prostaglandin endoperoxide H synthase-2 expression. Insights into potential pathogenic mechanisms of thyroid-associated ophthalmopathy. J Biol Chem. 1998;273:29615–29625. doi: 10.1074/jbc.273.45.29615. [DOI] [PubMed] [Google Scholar]
- 31.Han R, Chen B, Smith TJ. Jak2 dampens the induction by IL-1beta of prostaglandin endoperoxide H synthase 2 expression in human orbital fibroblasts: evidence for divergent influence on the prostaglandin E2 biosynthetic pathway. J Immunol 1950. 2007;179:7147–7156. doi: 10.4049/jimmunol.179.10.7147. [DOI] [PubMed] [Google Scholar]
- 32.Han R, Smith TJ. T helper type 1 and type 2 cytokines exert divergent influence on the induction of prostaglandin E2 and hyaluronan synthesis by interleukin-1beta in orbital fibroblasts: implications for the pathogenesis of thyroid-associated ophthalmopathy. Endocrinology. 2006;147:13–19. doi: 10.1210/en.2005-1018. [DOI] [PubMed] [Google Scholar]
- 33.Tsui S, Fernando R, Chen B, Smith TJ. Divergent Sp1 protein levels may underlie differential expression of UDP-glucose dehydrogenase by fibroblasts: role in susceptibility to orbital Graves disease. J Biol Chem. 2011;286:24487–24499. doi: 10.1074/jbc.M111.241166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang Y, Yap S, Ge X, et al. DNA vaccination with an insulin construct and a chimeric protein binding to both CTLA4 and CD40 ameliorates type 1 diabetes in NOD mice. Gene Ther. 2005;12:1679–1685. doi: 10.1038/sj.gt.3302578. [DOI] [PubMed] [Google Scholar]
- 35.Ogawa N, List JF, Habener JF, Maki T. Cure of overt diabetes in NOD mice by transient treatment with anti-lymphocyte serum and exendin-4. Diabetes. 2004;53:1700–1705. doi: 10.2337/diabetes.53.7.1700. [DOI] [PubMed] [Google Scholar]
- 36.Tooley JE, Waldron-Lynch F, Herold KC. New and future immunomodulatory therapy in type 1 diabetes. Trends Mol Med. 2012;18:173–181. doi: 10.1016/j.molmed.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Douglas RS, Afifiyan NF, Hwang CJ, et al. Increased generation of fibrocytes in thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 2010;95:430–438. doi: 10.1210/jc.2009-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moeller A, Gilpin SE, Ask K, et al. Circulating fibrocytes are an indicator of poor prognosis in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;179:588–594. doi: 10.1164/rccm.200810-1534OC. [DOI] [PubMed] [Google Scholar]
- 39.Bucala R, Spiegel LA, Chesney J, et al. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- 40.Wang J, Jiao H, Stewart TL, et al. Increased severity of bleomycin-induced skin fibrosis in mice with leukocyte-specific protein 1 deficiency. J Invest Dermatol. 2008;128:2767–2776. doi: 10.1038/jid.2008.164. [DOI] [PubMed] [Google Scholar]
- 41.Wang J, Chen H, Shankowsky HA, Scott PG, Tredget EE. Improved scar in postburn patients following interferon-alpha2b treatment is associated with decreased angiogenesis mediated by vascular endothelial cell growth factor. J Interferon Cytokine Res. 2008;28:423–434. doi: 10.1089/jir.2007.0104. [DOI] [PubMed] [Google Scholar]
- 42.Gillespie EF, Papageorgiou KI, Fernando R, et al. Increased expression of TSH receptor by fibrocytes in thyroid-associated ophthalmopathy leads to chemokine production. J Clin Endocrinol Metab. 2012;97:E740–746. doi: 10.1210/jc.2011-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gillespie EF, Raychaudhuri N, Papageorgiou KI, et al. Interleukin-6 production in CD40-engaged fibrocytes in thyroid-associated ophthalmopathy: involvement of Akt and NF-κB. Invest Ophthalmol Vis Sci. 2012;53:7746–7753. doi: 10.1167/iovs.12-9861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith TJ, Padovani-Claudio DA, Lu Y, et al. Fibroblasts expressing the thyrotropin receptor overarch thyroid and orbit in Graves’ disease. J Clin Endocrinol Metab. 2011;96:3827–3837. doi: 10.1210/jc.2011-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fernando R, Atkins S, Raychaudhuri N, et al. Human fibrocytes coexpress thyroglobulin and thyrotropin receptor. Proc Natl Acad Sci U S A. 2012;109:7427–7432. doi: 10.1073/pnas.1202064109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chesney J, Bacher M, Bender A, Bucala R. The peripheral blood fibrocyte is a potent antigen-presenting cell capable of priming naive T cells in situ. Proc Natl Acad Sci U S A. 1997;94:6307–6312. doi: 10.1073/pnas.94.12.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weaver CT, Hatton RD. Interplay between the TH17 and TReg cell lineages: a (co-)evolutionary perspective. Nat Rev Immunol. 2009;9:883–889. doi: 10.1038/nri2660. [DOI] [PubMed] [Google Scholar]
- 48.Arvan P, Di Jeso B. Thyroglobulin structure, function, and biosynthesis. In: Werner SC, Ingbar SH, Braverman LE, Utiger RD, editors. Werner & Ingbar’s The Thyroid: A Fundamental and Clinical Text. 9th ed. Philadelphia: Lippincott Williams & Wilkins; 2005. pp. 77–95. [Google Scholar]
- 49.Spaulding SW. Biological actions of thyrotropin. In: Werner SC, Ingbar SH, Braverman LE, Utiger RD, editors. Werner & Ingbar’s The Thyroid: A Fundamental and Clinical Text. 9th ed. Philadelphia: Lippincott Williams & Wilkins; 2005. pp. 183–197. [Google Scholar]
- 50.Aoki-Ota M, Kinoshita M, Ota T, et al. Tolerance induction by the blockade of CD40/CD154 interaction in pemphigus vulgaris mouse model. J Invest Dermatol. 2006;126:105–113. doi: 10.1038/sj.jid.5700016. [DOI] [PubMed] [Google Scholar]
- 51.Bagenstose LM, Agarwal RK, Silver PB, et al. Disruption of CD40/CD40-ligand interactions in a retinal autoimmunity model results in protection without tolerance. J Immunol 1950. 2005;175:124–130. doi: 10.4049/jimmunol.175.1.124. [DOI] [PubMed] [Google Scholar]
- 52.Jacobson EM, Tomer Y. The CD40, CTLA-4, thyroglobulin, TSH receptor, and PTPN22 gene quintet and its contribution to thyroid autoimmunity: back to the future. J Autoimmun. 2007;28:85–98. doi: 10.1016/j.jaut.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Houston FA, Wilson V, Jennings CE, et al. Role of the CD40 locus in Graves’ disease. Thyroid. 2004;14:506–509. doi: 10.1089/1050725041517039. [DOI] [PubMed] [Google Scholar]
- 54.Yoshimura T, Matsushima K, Oppenheim JJ, Leonard EJ. Neutrophil chemotactic factor produced by lipopolysaccharide (LPS)-stimulated human blood mononuclear leukocytes: partial characterization and separation from interleukin 1 (IL 1) J Immunol. 1987;139:788–793. [PubMed] [Google Scholar]
- 55.Baggiolini M, Walz A, Kunkel SL. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest. 1989;84:1045–1049. doi: 10.1172/JCI114265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haase C, Markholst H. CD40 is required for development of islet inflammation in the RIP-CD154 transgenic mouse model of type 1 diabetes. Ann N Y Acad Sci. 2007;1107:373–379. doi: 10.1196/annals.1381.039. [DOI] [PubMed] [Google Scholar]
- 57.Toubi E, Shoenfeld Y. The role of CD40-CD154 interactions in autoimmunity and the benefit of disrupting this pathway. Autoimmunity. 2004;37:457–464. doi: 10.1080/08916930400002386. [DOI] [PubMed] [Google Scholar]
- 58.Zhang-Hoover J, Sutton A, Stein-Streilein J. CD40/CD40 ligand interactions are critical for elicitation of autoimmune-mediated fibrosis in the lung. J Immunol. 2001;166:3556–3563. doi: 10.4049/jimmunol.166.5.3556. [DOI] [PubMed] [Google Scholar]
- 59.Criswell LA. Gene discovery in rheumatoid arthritis highlights the CD40/NF-κB signaling pathway in disease pathogenesis. 2010;233:55–61. doi: 10.1111/j.0105-2896.2009.00862.x. [DOI] [PubMed] [Google Scholar]
- 60.Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217–242. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 61.Ghasemi H, Ghazanfari T, Yaraee R, et al. Roles of IL-8 in ocular inflammations: a review. Ocul Immunol Inflamm. 2011;19:401–412. doi: 10.3109/09273948.2011.618902. [DOI] [PubMed] [Google Scholar]
- 62.DeForge LE, Remick DG. Kinetics of TNF, IL-6, and IL-8 gene expression in LPS-stimulated human whole blood. Biochem Biophys Res Commun. 1991;174:18–24. doi: 10.1016/0006-291x(91)90478-p. [DOI] [PubMed] [Google Scholar]
- 63.Hoffmann E, Dittrich-Breiholz O, Holtmann H, Kracht M. Multiple control of interleukin-8 gene expression. J Leukoc Biol. 2002;72:847–855. [PubMed] [Google Scholar]
- 64.Bossowski A, Urban M. Serum levels of cytokines in children and adolescents with Graves’ disease and non-toxic nodular goiter. J Pediatr Endocrinol Metab. 2001;14:741–747. doi: 10.1515/jpem.2001.14.6.741. [DOI] [PubMed] [Google Scholar]
- 65.Watson PF, Pickerill AP, Davies R, Weetman AP. Semi-quantitative analysis of interleukin-1 alpha, interleukin-6 and interleukin-8 mRNA expression by human thyrocytes. J Mol Endocrinol. 1995;15:11–21. doi: 10.1677/jme.0.0150011. [DOI] [PubMed] [Google Scholar]
- 66.Weetman AP, Bennett GL, Wong WL. Thyroid follicular cells produce interleukin-8. J Clin Endocrinol Metab. 1992;75:328–330. doi: 10.1210/jcem.75.1.1619027. [DOI] [PubMed] [Google Scholar]
- 67.Zhu YM, Woll PJ. Mitogenic effects of interleukin-8/CXCL8 on cancer cells. Future Oncol. 2005;1:699–704. doi: 10.2217/14796694.1.5.699. [DOI] [PubMed] [Google Scholar]
- 68.DeForge LE, Fantone JC, Kenney JS, Remick DG. Oxygen radical scavengers selectively inhibit interleukin 8 production in human whole blood. J Clin Invest. 1992;90:2123–2129. doi: 10.1172/JCI116097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.DeForge LE, Preston AM, Takeuchi E, et al. Regulation of interleukin 8 gene expression by oxidant stress. J Biol Chem. 1993;268:25568–25576. [PubMed] [Google Scholar]
- 70.Tsai C-C, Wu S-B, Cheng C-Y, et al. Increased response to oxidative stress challenge in Graves’ ophthalmopathy orbital fibroblasts. Mol Vis. 2011;17:2782–2788. [PMC free article] [PubMed] [Google Scholar]
- 71.Prabhakar BS, Bahn RS, Smith TJ. Current perspective on the pathogenesis of Graves’ disease and ophthalmopathy. Endocr Rev. 2003;24:802–835. doi: 10.1210/er.2002-0020. [DOI] [PubMed] [Google Scholar]
- 72.Standiford TJ, Kunkel SL, Basha MA, et al. Interleukin-8 gene expression by a pulmonary epithelial cell line. A model for cytokine networks in the lung. J Clin Invest. 1990;86:1945–1953. doi: 10.1172/JCI114928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tong K-M, Shieh D-C, Chen C-P, et al. Leptin induces IL-8 expression via leptin receptor, IRS-1, PI3K, Akt cascade and promotion of NF-kappaB/p300 binding in human synovial fibroblasts. Cell Signal. 2008;20:1478–1488. doi: 10.1016/j.cellsig.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 74.Unger BL, McGee DW. Hepatocyte growth factor and keratinocyte growth factor enhance IL-1-induced IL-8 secretion through different mechanisms in Caco-2 epithelial cells. In Vitro Cell Dev Biol Anim. 2011;47:173–181. doi: 10.1007/s11626-010-9365-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hirsch E, Katanaev VL, Garlanda C, et al. Central role for G protein-coupled phosphoinositide 3-kinase gamma in inflammation. Science. 2000;287:1049–1053. doi: 10.1126/science.287.5455.1049. [DOI] [PubMed] [Google Scholar]
- 76.Pasparakis M. Regulation of tissue homeostasis by NF-kappaB signalling: implications for inflammatory diseases. Nat Rev Immunol. 2009;9:778–788. doi: 10.1038/nri2655. [DOI] [PubMed] [Google Scholar]