Abstract
Fibronectin is a globular protein that circulates in the blood and undergoes fibrillogenesis if stretched or under other partially denaturing conditions, even in the absence of cells. Stretch assays made by pulling fibers from droplets of solutions containing high concentrations of fibronectin have previously been introduced in mechanobiology, particularly to ask how bacteria and cells exploit the stretching of fibronectin fibers within extracellular matrix to mechano-regulate its chemical display. Our electron microscopy analysis of their ultrastructure now reveals that the manually pulled fibronectin fibers are composed of densely packed lamellar spirals, whose interlamellar distances are dictated by ion-tunable electrostatic interactions. Our findings suggest that fibrillogenesis proceeds via an irreversible sheet-to-fiber transition as the fibronectin sheet formed at the air-liquid interface of the droplet is pulled off by a sharp tip. This far from equilibrium process is driven by the externally applied force, interfacial surface tension, shear-induced fibronectin self-association, and capillary force-induced buffer drainage. The ultrastructural characterization is then contrasted with previous FRET studies that characterized the molecular strain within these manually pulled fibers. Particularly relevant for stretch-dependent binding studies is the finding that the interior fiber surfaces are accessible to nanoparticles smaller than 10 nm. In summary, our study discovers the underpinning mechanism by which highly hierarchically structured fibers can be generated with unique mechanical and mechano-chemical properties, a concept that might be extended to other bio- or biomimetic polymers.
Keywords: Fibronectin, Fibrillogenesis, Sheet-to-fiber transition, Monolayer-to-lamella transition, Electron microscopy, Kinetically trapped supramolecular system
Abbreviations: FRET, Förster resonance energy transfer; MEMS, micro-electro-mechanical system; PBS, phosphate buffer solution; PDMS, polydimethylsiloxane; SEM, scanning electron microscopy; TEM, transmission electron microscopy
1. Introduction
Fibronectin is a large (∼500 kDa) glycoprotein found in a fibrillar state within the extracellular matrix of a great variety of tissues and guides multiple cell physiological processes, from cell adhesion and proliferation to differentiation [1], [2], [3]. A prominent feature of fibronectin is the ability to undergo fibrillogenesis through a variety of different induction methods, all of which have in common the ability to promote fibronectin self-interactions. Cells assemble fibronectin into a fibrillar meshwork [4], via interactions with integrins and the application of mechanical forces through the acto-myosin cytoskeleton [5], [6]. However, fibronectin fibers form also in cell-free systems. Chemical agents, such as polyamines [7] and guanidinium hydrochloride [8], or mechanical perturbations [9], [10], [11], [12] can initiate fibronectin fibrillogenesis. One of these techniques of artificial fibrillogenesis, whereby fibers are generated by pulling at the air-liquid interface of fibronectin solutions [9], has been adopted and used to study the mechano-regulated properties of fibronectin fibers in various stretch assays at the molecular and cellular level [13], [14], [15]. The stretch-dependent molecular conformation of fibronectin within these fibers has been investigated previously using FRET-based probes [14]. We have shown that the tunable conformational range within such fibers is similar to the conformations fibronectin adopts within cell-derived extracellular matrix [14], and sensitive to the application of mechanical force [16]. Since we still lack information about the ultrastructure of these fibers and their 3D architecture, as well as the physical mechanisms that lead to their assembly, the present study aims at investigating the ultrastructure of the manually pulled fibronectin fibers with electron microscopy, thus completing the structural characterization of this biomaterial at different length scales, from the molecular to the macroscopic.
The need for experimental model systems that can serve as stretch assays to explore fibronectin's mechano-regulated functions motivates the urgency of understanding the structure and properties of the manually pulled fibronectin fibers presented here. As an example, morphogenetic processes that require complex and highly coordinated cell behavior, such as embryonic development, wound healing, angiogenesis and carcinogenesis, depend on the presence of fibronectin [17], [18], however, we only begin to understand which aspects of cell behavior are directly affected by fibronectin's mechano-regulated biochemical and/or biophysical factors [15]. One of the confounding factors in the study of such problems is the structural flexibility of fibronectin. Depending on the chemical and physical properties of its microenvironment, fibronectin (also in a surface-adsorbed state) assumes different tertiary/quaternary structures [19], [20], which could lead to distinct functionalities [21]. The structural flexibility of fibronectin results from its molecular architecture: it consists of two almost identical monomeric chains linked by disulfide bridges on their C-termini [22] and each chain consists in turn of a series of structural repeats connected via linkers [23], which can participate in various interactions with one another, depending on the physicochemical properties of the environment, leading to different final structures of the protein [19]. A consequence of this structural flexibility is the differential exposure of the numerous binding sites on fibronectin, including sites for growth factors [24], cytokines [25], integrins [26], proteoglycans [27], heparin [28], collagens [29], fibrin [30] and bacterial adhesins [13], [31]. Any factor, either chemical or mechanical, which can affect fibronectin conformation, has thus the potential to alter the availability of binding sites for a number of ligands [13], [32]. The large number of fibronectin interactions that can be regulated in such a way leads to the hypothesis that fibronectin could be the extracellular equivalent of a mechano-tunable signaling node, which integrates various stimuli to guide an appropriate cell response [3]. One of the mechanisms employed by cells to regulate the availability of binding sites on fibronectin is unfolding of the protein by mechanical forces [3], [5]. Experimental [33] and computational [34] studies have shown the ability of fibronectin molecules to unfold under mechanical strain, first by losing the tertiary structure and subsequently, by sequential unfolding of the individual domains. The unfolding is reversible and upon release of the mechanical strain the protein assumes its original configuration. However, in the extracellular matrix, fibronectin experiences the mechanical stress not as a single molecule but as part of fibrillar structures. Due to the complex interlaced architecture of fibrillar extracellular matrix, a large range of conformations is seen in native extracellular matrix and often, major conformational alterations are seen at distances along a extracellular matrix fibril of one micron or shorter [35]. To study correlations between mechanical unfolding of fibronectin, availability of binding sites and subsequent biological responses within fibrillar structures similar to the in vivo substrates, manually pulled fibronectin fibers, as characterized in this study, present a suitable model system with a much more narrow conformational heterogeneity, at least as concluded from FRET studies [14]. They can be produced at any desired orientation, deposited on flat (stretchable) substrates or microfabricated structures and the molecular conformation of fibronectin fine-tuned by the application of biomechanical forces. By manipulating these parameters, the mechanosensitive binding of various ligands to fibronectin and the subsequent cellular response has been studied in a controlled manner [13], [15], [36]. In addition to the applications in basic research on fibronectin biology, the manually pulled fibers can be used for tissue engineering applications [37].
Here, we thus characterized the internal structure of manually pulled fibronectin fibers by electron microscopy. Our findings revealed a lamellar structure, stabilized mainly by electrostatic interactions, and which originates from the insoluble monolayer that fibronectin is known to form at the air–liquid interface [38], [39]. Knowledge of the ultrastructure of manually pulled fibers and how it is altered by mechanical stress would benefit not only the numerous applications for which these fibers are used, but it can also shed light on the mechanism for their assembly, as well as provide a basis for comparisons between this model system of fibronectin fibrillogenesis and the cell-derived fibronectin fibrils within the extracellular matrix.
2. Materials and methods
2.1. Fibronectin isolation from human plasma
Fibronectin was isolated from human plasma with two-step affinity chromatography as previously described [35]. Briefly, the plasma was passed through a sepharose 4B size exclusion chromatography column. The flow through was subsequently applied to a gelatin-sepharose column. The column was washed with PBS and 1 m NaCl, until no protein was detected (monitored by absorbance at 280 nm). Gelatin bound fibronectin was eluted from the column either under denaturing conditions with 6 m urea or under non-denaturing conditions with 1 m arginine. In the case of arginine elution, the gelatin column was washed additionally with 0.2 m arginine prior to elution. Typical yields ranged from 1 to 4 mg/ml. Fibronectin was stored at −80 °C as eluted from the column and was dialyzed against PBS prior to use. There was no difference in the ultrastructure of fibers produced from fibronectin purified under denaturing and non-denaturing conditions (data not shown).
2.2. Production of manually pulled fibers
Following previously published protocols [14], fibronectin was diluted in the appropriate buffer to a final concentration of 0.4 mg/ml. A droplet of this solution was deposited on a silicone sheet. A sharp tip was immersed in the droplet and, as it was withdrawn, it pulled a fiber from the surface of the droplet. The fiber could be pulled to 0.5–1 cm final length before it was deposited to the substrate. Following deposition onto the substrate, pressing the fiber down with the pulling tip severed the connection of the fiber to the droplet. From a typical droplet of 2.5 μl, an average of 35 fibers could be produced. Any remaining of the droplet was aspirated, the fibers were washed three times with the buffer and they were kept in buffer prior to any further processing. For the purpose of the present study, two types of pulling tips were used: plastic pipet tips (1–200 μl volume capacity), which were cut at the very front to produce a concave, sharp tip and microfabricated metal tips with a defined radius of 2 μm, produced from a solid tungsten wire with an electrochemically etched taper (American Probes & Technologies, model 72T-J3). As described in the results, the morphology and size of the tip did not affect the fiber ultrastructure, as seen by TEM.
2.3. Stretching of manually pulled fibers
Manually pulled fibronectin fibers were deposited onto a silicone sheet, mounted on a uniaxial stretching device and were either left as deposited or subjected to different amounts of strain. According to previous studies in our group [14], by relaxing or further stretching manually pulled fibronectin fibers, samples with strains ranging from 0% to 500% could be prepared. Briefly, the fibers just pulled out of the solution are mechanically strained due to the pulling process itself, and relaxing them to a third of their initial length is required to produce fully relaxed fibers (0% strain). The maximum strain that can be applied on fibers deposited on silicone sheets [14] before fibers start breaking amounts to 500% (stretch fully relaxed fibers six times their length).
2.4. Force-extension curves
Measurement of force–extension curves of fibronectin fibers in solutions of different ionic strength were conducted with a MEMS actuator as previously described [16]. Briefly, fibers were pulled on microfabricated PDMS trenches, out of a fibronectin solution in PBS. Through glutaraldehyde functionalization of the surface, the fibers were covalently linked to the top of the trenches, whereas they were freely suspended over the wells. Before the measurements, the fibers were fully relaxed, until the point where they started sagging over the wells. Using the MEMS device, the fibers were pushed laterally and force–extension curves were recorded in PBS (up to 3× extension). Curves for 12 individual fibers were recorded. Following this first set of measurements, the PBS was exchanged with distilled H2O, the fibers were incubated for 10 min at room temperature and a new set of force–extension curves was recorded, for the same set of fibers, but on an adjacent well from the one used for the PBS measurements. The H2O was exchanged once more with PBS and after 10 min of incubation, the last set of force–extension curves was acquired in PBS. Again, the same set of fibers was used and the measurements were done in a different well.
2.5. Sample preparation for electron microscopy
2.5.1. Chemical fixation
The fibers, while kept hydrated in the pulling buffer, were enclosed by a PDMS chamber, which was glued on the underlying PDMS substrate with silicone glue able to withstand all the subsequent fixative/solvent treatments. After 1 h fixation with 2% glutaraldehyde in the pulling buffer, the fibers were stained for 1 h with 1% uranyl acetate in H2O and then, gradually dehydrated with ethanol in five steps, 15 min each: 70%, 90% and 96% ethanol in H2O, followed by two incubations in anhydrous ethanol. The dehydrated fibers were embedded in epoxy resin (Fluka): the first two incubations (1 h and overnight, respectively) were performed with 33% and 66% epoxy resin in anhydrous ethanol, and the final step with 100% epoxy resin for 1 h at room temperature, followed by polymerization at 60 °C for at least 48 h. Ultrathin sections and serial sections (50–60 nm) were cut from the blocks with an ultramicrotome (Reichert Ultracut S, Leica), perpendicular to the plane of the fibers, post-stained with 2% uranyl acetate for 5 min and observed in a 100 kV FEI Morgagni 268 transmission electron microscope.
2.5.2. High pressure freezing and freeze substitution
Manually pulled fibronectin fibers were deposited on 6 mm sapphire discs in PBS and were high pressure frozen (BAL-TEC, HPM100). The samples were transferred to a freeze-substitution device pre-cooled to −90 °C, and were dehydrated in three successive steps: at −90 °C, −60 °C and −35 °C, for 8 h each, with the substitution medium being 2% uranyl acetate in a 10% methanol: 90% acetone mixture. The samples were washed with anhydrous acetone for 30 min at −35 °C, followed by two washes with anhydrous ethanol also at −35 °C, 30 min each. For TEM, the samples were embedded in HM20 at −35 °C (twice 30% HM20 in ethanol, 30 min each; twice 70% HM20 in ethanol, 1 h each; twice 100% HM20, 1 h and 5 h respectively). Polymerization of the resin was carried at −35 °C under UV for 24 h, followed by room temperature UV-polymerization for at least 48 h. The blocks were sectioned and processed as described above for the chemical fixation procedure. For SEM, instead of resin embedding, the dehydrated samples in acetone were brought from −35 °C to 0 °C, then plunge frozen at liquid N2 and freeze dried in a pre-cooled freeze-fracturing system BAF 060 (Bal-Tec/Leica, Vienna) to −85 °C at a rate of 30 °C/h). At −85 °C, the samples were coated with 2 nm tungsten at 45°, followed by 2 nm under continuous angle changes from 45° to 90° to 45° and were transferred onto the cryo-stage of a Zeiss Gemini 1530 FEG scanning electron microscope. Imaging was done at −120 °C.
2.6. Image processing
To extract quantitative information from the images of fiber cross-sections, the contrast of the 8-bit electron microscopic images was enhanced by using an unsharp mask filter (ImageJ; radius = 10 pixels, mask weight = 0.7) and the noise reduced with a median filter (ImageJ; radius = 5 pixels). The processed images were then subjected to a trainable segmentation algorithm (Fiji, [40]), to produce binary images. From these images, the values for domain size, layer thickness and interlamellar distances were calculated with ImageJ by a line profile drawn across the domain. For 3D reconstructions of serial sections, the stack of such segmented images was aligned with the SIFT linear elastic alignment algorithm (Fiji, [40]) and was reconstructed in Imaris 6.3.1 (Bitplane), using smoothing surface area detail level equal to 2 nm.
2.7. Permeability to nanoparticles
Fibers were pulled from a 0.4 mg/ml fibronectin solution in PBS as previously described, and were incubated at room temperature with a nanoparticle colloidal solution for 1 h prior to fixation. Alternatively, fibronectin was diluted in the colloidal nanoparticle solution in PBS to a final concentration of 0.4 mg/ml and fibers were pulled from these solutions in the presence of the nanoparticles. The samples were chemically fixed and further processed for TEM, as described above. Nanoparticles used for these studies were: 10 nm gold colloid nanoparticles from BBI Life Sciences, UK, 10 nm silver colloidal nanoparticles (Sigma) and 2.5 nm nanogold particles, with a gold core of 1.4 nm in diameter and an organic shell that is either neutral, positively or negatively charged (Nanonprobes, USA; Cat. No. 2010, 2022 and 2023 respectively).
3. Results
3.1. Pulling of fibronectin fibers from the air-liquid interface
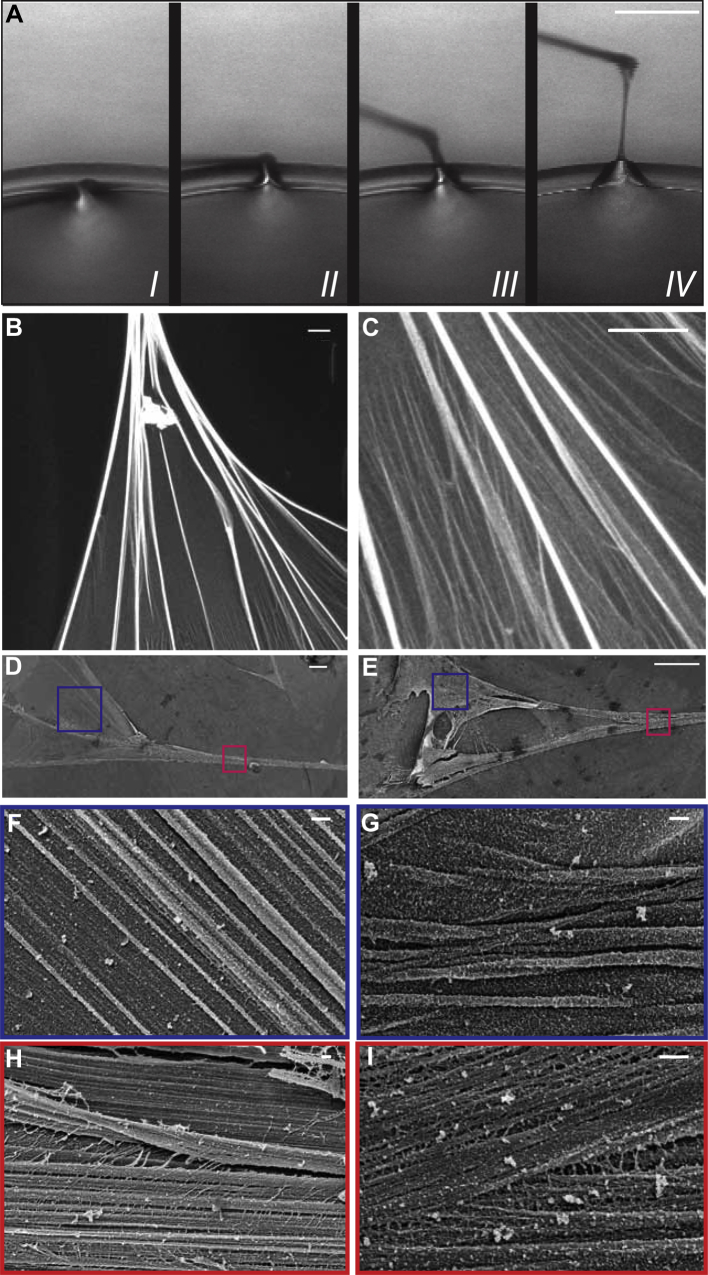
Fibronectin is known to form stable, insoluble monolayers at air-liquid interfaces [38], [39]. Based on previous work ([14], [16]), a fiber can be drawn by pulling a sharp tip away from a fibronectin monolayer that had formed at the surface of a droplet of concentrated plasma fibronectin (0.4 mg/ml) in PBS solution (Fig. 1A). At first, the droplet surface deforms due to capillary forces that tend to minimize the total liquid surface area. Once the surface tension is overcome by the externally applied force, the fibronectin monolayer adsorbed at the interface undergoes a monolayer–fiber transition and the length of the newly formed fiber increases by the continuous transfer of material from the droplet surface. The material loss from the droplet surface is refurbished if fibronectin adsorption from the droplet solution to the air–water interface can happen sufficiently fast, and such fibers can then reach lengths up to 1 cm. The forces necessary to induce a monolayer-to-fiber transition have previously been measured by pulling the fibers with a force sensor, while simultaneously monitoring the length of the forming fiber [16], and forces are in the μN range. The pulled fibers are deposited on the underlying substrate and the connection of each fiber to the droplet is then severed by pressing the fiber down with the pulling tip. Immediately following pulling and deposition, the fibers are immersed in a droplet of the appropriate buffer, and subsequently fixed with glutaraldehyde in the same buffer.
Fig. 1.
Fibronetin fibers can be generated by pulling their monolayers away from the air-liquid interface. Fibers were pulled out of a 0.4 mg/ml fibronectin solution in PBS with a constant speed of 8 μm/s, using the tip of a MEMS force sensor (A). Similar fibers, pulled with a pipet tip out of a 0.4 mg/ml fibronectin (labeled with Alexa488 (B, C)) or unlabeled (D–J)) solution in PBS, deposited on the substrate and the fan-like region that connects the fiber to the droplet of the fibronectin solution was imaged by confocal microscopy (B, C; 63× oil objective) or by cryo-SEM, following high pressure freezing, freeze substitution and freeze drying of the specimen (D–I). The regions highlighted by the blue and red boxes in D and E are shown at higher magnification at F, G and H, I respectively. Scale bar: 200 μm for A, 10 μm for B, C, 20 μm for D, E and 200 nm for F–I.
A similar behavior has been observed when lipid tubules are drawn from phospholipid vesicles [41]. However, whereas the lipid tubules retract immediately if they are not connected to a vesicle at both ends [42], the pulled fibronectin fibers remain stable even after they are cut from the fibronectin droplet, indicating that the transition is irreversible and that the resulting fiber forms a stable structure. It is likely that additionally to the sheet-to-fiber transition, transformations within the fibronectin monolayer possibly through self-association of fibronectin molecules, further stabilize the resulting fiber. Indeed, hints for such transformations are given from direct SEM observations of the region that connects the fiber to the droplet. As the drawn fiber is deposited onto the substrate, this region assumes a fan-like shape, consisting at the light microscopic level (Fig. 1B, C) of strands originating from the droplet surface and progressively merging towards the main fiber. Cryo-SEM images of this region reveal more insights into this nanoscale topography (Fig. 1D–I). The resulting surface morphology, with nanoscale strands and groves parallel to the long fiber axis, is preserved along the whole length of fully assembled fibers (Fig. 2A, B).
Fig. 2.
Manually pulled fibronectin fibers show a lamellar organization. Manually pulled fibers were fixed by high pressure freezing, freeze dried and imaged in the SEM at −120 °C (A, B) or were chemically fixed with glutaraldehyde, ethanol dehydrated, embedded in Epon, ultrathin sectioned (50 nm) and observed by TEM (C, D). Scale bar: 1 μm for A, 100 nm for B and 200 nm for C and D.
3.2. Fiber ultrastructure
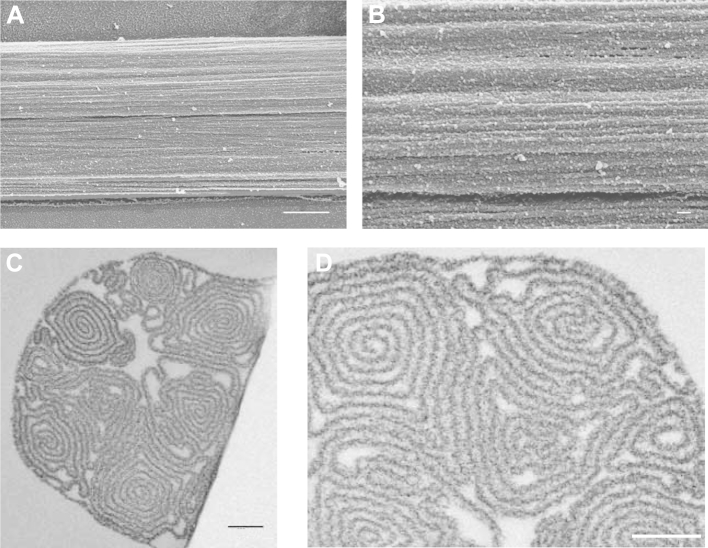
The internal structure of the fibers at a distance at least several mm away from the droplet location was then imaged by TEM using cross sections of chemically fixed fibers and revealed a densely packed, lamellar organization (Fig. 2C, D). The fiber cross-section consists of an outer protein layer connected internally to several topologically segregated spiral segments (highlighted in Fig. 2C with different colors). The total length of the protein layer that forms a spiral segment can be several times the fiber diameter, significantly increasing the internal fiber surface (Supplementary Fig. 1). Comparison of chemically fixed fibers with freeze-substituted fibers after high pressure freezing (Supplementary Fig. 2) shows a very similar morphology, suggesting that chemical fixation preserves the structure well and does not introduce any obvious artifacts. Based on this observation, the fibers were chemically fixed with glutaraldehyde for the rest of our study. The observed fiber ultrastructure seems to be an inherent property of the fibrillogenesis process during fiber pulling since it is unaffected by the specific morphology of the tip used for their production (Supplementary Fig. 3). Moreover, the ultrastructure was retained even after air-drying for 1 h at RT (Supplementary Fig. 4), suggesting that the solvent molecules in the fiber interior are tightly associated with the adjacent protein layers.
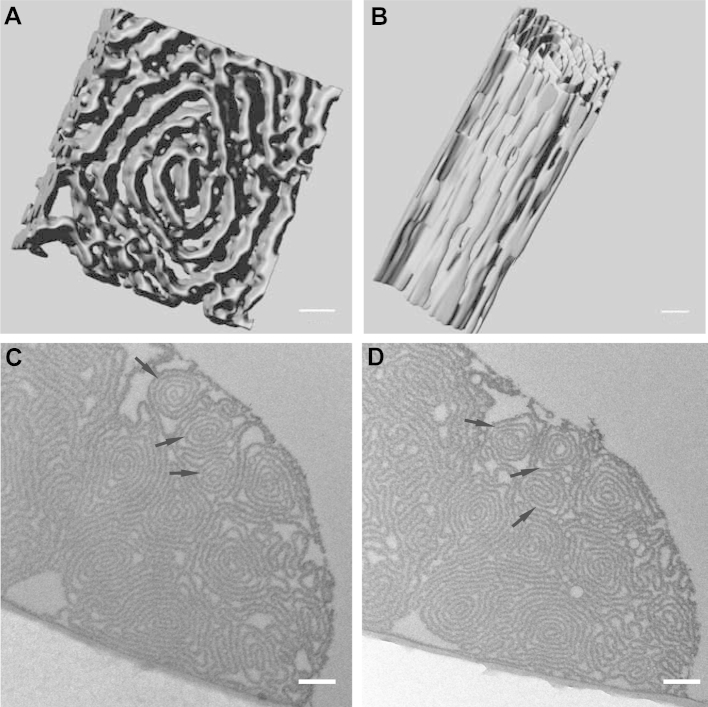
3.3. Self-similarity of fiber ultrastructure in 3D
To gain insight in the 3D architecture of manually pulled fibers, a 550 nm deep volume was reconstructed from serial TEM sections. In Fig. 3A and B two perspectives of one spiral segment are shown (see Supplementary Movie 1 for a 3D reconstruction of the whole volume), from which one can see that the segment retains its overall morphology and organization along the z-axis in the μm range. No helical twisting was observed along the fiber axis, while groves and undulations on both facets of the protein layer were evident, similar to the outer fiber surface as observed by SEM (Fig. 2A, B). Moreover, there are several points where adjacent layers come into direct contact with one another. Comparison of sections from the same fiber 10 μm apart (Fig. 3C and D) reveal that the spiral segment organization is retained along the fiber for much larger distances, compared to the dimensions of both segment and fiber. However, one can also observe plasticity, as for example in the case of the three segments pointed at by arrows in Fig. 3C and D, which are differently aligned with respect to each other in the two sections.
Fig. 3.
Three dimensional ultrastructure of manually pulled fibronectin fibers. Fibers were pulled out of a 0.4 mg/ml fibronectin solution in PBS, chemically fixed with glutaraldehyde, ethanol dehydrated and embedded in Epon. 50 nm thick serial sections were cut from the blocks and were observed under the TEM. The images were processed as described for Fig. 2, and the segmented images were used for a 3D reconstruction. The reconstruction of one spiral domain from 11 serial sections is shown (A, B). In C and D, sequential sections 10 μm apart from a single fiber are shown. Scale bars: 20 nm for A, B and 500 nm for C, D.
Supplementary video related to this article can be found at http://dx.doi.org/10.1016/j.biomaterials.2014.08.012.
The following is the supplementary video related to this article:
Suppl. Video: 3D reconstruction of a 550 nm volume of a manually pulled fiber. Fibers pulled out of a 0.4 mg/ml fibronectin solution in PBS were chemically fixed with glutaraldehyde, ethanol dehydrated and embedded in epoxy resin. Serial ultrathin sections (11 sections, 50 nm each) were imaged by TEM. The images were registered and aligned in Fiji and the fiber volume was reconstructed in Imaris.
3.4. Effects of ionic strength on fiber ultrastructure
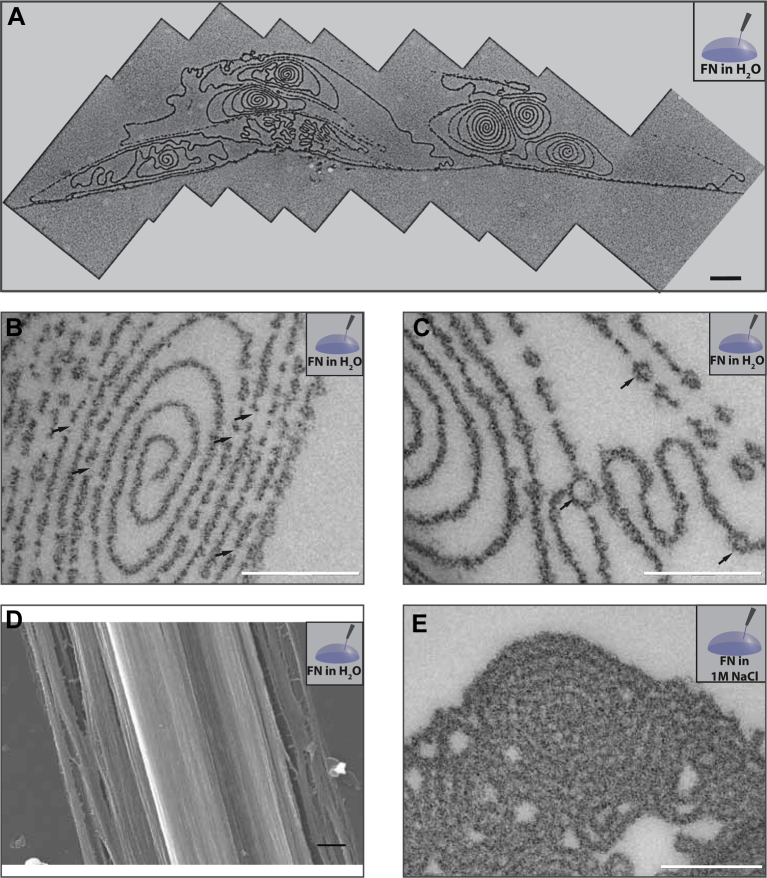
To investigate the factors that regulate fiber architecture, we first looked at electrostatic interactions, since it is known from the literature that they play an important role in stabilizing various surfactant, lipid and clay lamellar systems [43], [44]. For this purpose, we analyzed the structure of fibers pulled out of solutions of different ionic strengths. Fibronectin was dissolved in H2O, PBS (containing 0.15 m NaCl) or 1 m NaCl and fibers were pulled from these solutions as described above. Fibers could be produced similarly from all three solutions. Importantly, the ionic strength affected the internal organization of the fibers (Fig. 4). In H2O, the fiber cross-sections appeared much more elongated, assuming a more elliptical shape (Fig. 4A). Spiral segments were still present but a large part of the fiber interior was free of lamellae. The protein layer appeared often discontinuous (Fig. 4B) and ring-like structures were observed (Fig. 4C). Several of these rings were found along the length of the protein lamella, but often they appeared to connect different lamellae. Cryo-SEM imaging of the fibers pulled out of the fibronectin solution in H2O showed a similar surface topography as for the fibers in PBS (Fig. 4D). The fibers pulled out of the highest ionic strength solution (1 m NaCl) had an overall architecture similar to the ones pulled out of PBS, although the lamellar structures appeared denser (Fig. 4E).
Fig. 4.
The internal structure of fibers is stabilized by repulsive electrostatic interactions. Fibers pulled out of a 0.4 mg/ml fibronectin solution in distilled H2O (A,B,C,D) or 1 m NaCl (E) were chemically fixed with glutaraldehyde, ethanol dehydrated and embedded in Epon, ultrathin sectioned (50 nm) and observed by TEM (A, B, C, E) or high pressure frozen, freeze substituted and freeze dried and imaged in the SEM at −120 °C (D). For A, several images were required to capture the entire fiber cross section, and are shown stitched together. Scale bar: 500 nm for A, D and 200 nm for B, C, E.
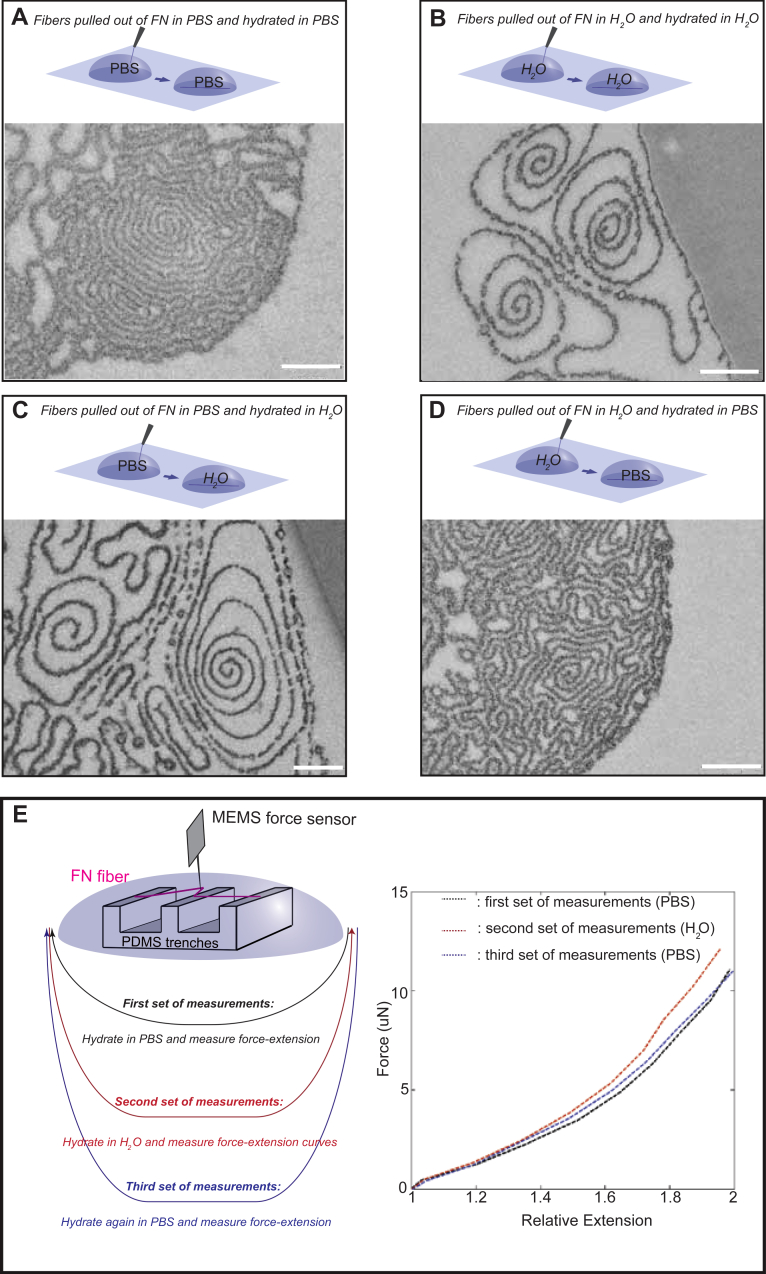
The distinct features of the fibers pulled out of fibronectin solutions in H2O, as compared to solutions in PBS, suggest that electrostatic interactions during fiber assembly regulate primarily the interlamellar spacing. But once the structure has been formed, can it still respond to alterations of the ionic strength of the environment? Indeed, fibers that were pulled out of solutions of fibronectin in H2O had a very similar organization to fibers initially pulled out of PBS solutions and then incubated with H2O for as short times as 1 min (Fig. 5A, B). Swelled structures, with few spiral segments that show large free spaces, abundant ring-like structures and discontinuities in the protein lamellae were observed in both types of fibers. Similarly, fibers pulled out of H2O solutions and subsequently incubated with PBS were indistinguishable from fibers pulled directly out of PBS (Fig. 5C, D). Force-extension curves (Fig. 5E) were measured for fibers pulled out of PBS (black lines) and deposited onto microfabricated trenches, by using a MEMS device as described previously [16]. The measurements were repeated on the same set of fibers after they were incubated with H2O (red lines) and once again after they were re-incubated with PBS (blue lines). All three sets of force–extension curves, as presented for an average of 12 fibers, were identical (Fig. 5E), suggesting that the tunable morphological differences induced by hydrating the fibers in buffers of different ionic strength are not associated with any material loss.
Fig. 5.
The swelling effect induced by ionic strength on the fiber structure is reversible. Fibers were pulled out of a 0.4 mg/ml fibronectin solution in PBS and remained in PBS (A) or incubated with H2O (C) prior to fixation. Alternatively, fibers were pulled out of the H2O solution and remained in H2O (B) or incubated with PBS (D) prior to fixation. All samples were chemically fixed with glutaraldehyde, dehydrated in ethanol and embedded in Epon. 50 nm sections were observed by TEM. (E) Fibers were pulled out of a 0.4 mg/ml fibronectin solution in PBS onto microfabricated PDMS trenches. Using a MEMS sensor, force–extension curves were determined for the segments of the fibers freely suspended over the wells. Measurements were performed in PBS (black curves), after treating the fibers with H2O (red curves) and after rehydrating them again with PBS (blue curves). Curves for an average of 12 fibers are shown. Scale bar: 200 nm.
3.5. Effects of stretching on fiber ultrastructure
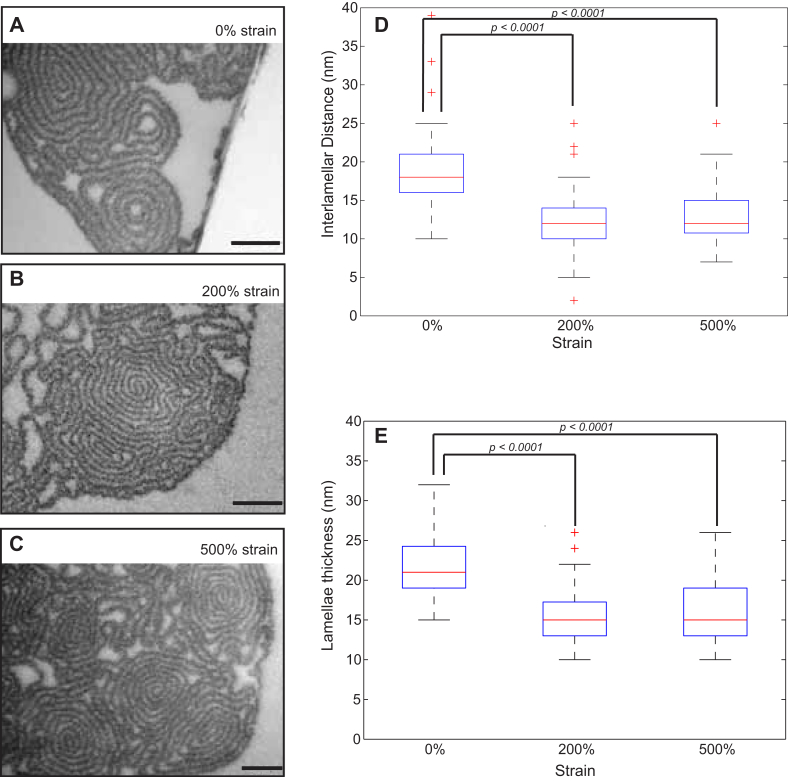
For reasons mentioned in the Introduction, the manually pulled fibronectin fibers serve as a model system to study the mechanosensitive binding of various ligands to fibronectin. In such stretch assays, fibers are typically deposited onto a silicone sheet and subsequently subjected to various strains, within a range of 0%–500% strain [13], [14]. To understand how the fiber structure is affected by mechanical strain, previous measurements were conducted using FRET-based probes [16], which indicated that cryptic residues on the fibronectin type III modules started becoming exposed above 100% strain, suggesting that loss of tertiary/secondary structure of individual fibronectin molecules within the fibers start taking place at these strain levels. In the present study, cross sections of fibers at 0%, 200% and 500% absolute strains (Fig. 6A, B and C) revealed a very similar lamellar organization. To extract quantitative information, the raw images were processed as described in Methods to generate binary images, which could be used to measure the interlamellar distances within a spiral segment (Fig. 6E), as well as the thickness of the lamellae (Fig. 6F). Fibers at 0% strain, showed thicker lamellae (22 ± 4 nm) than fibers at 200% and 500% strain (15 ± 3 nm and 16 ± 4 nm, respectively). Similarly, the interlamellar distance increased for the relaxed fibers (0% strain) to 20 ± 6 nm, compared to 12 ± 4 nm and 13 ± 4 nm for fibers at 200% and 500% strain. These results suggest that before protein unfolding occurs at strains above 100% [16], decrease of the fiber interlamellar distances and lamellar thickness also contributes to the overall extension of the fibers. Additionally, since a fibronectin type III repeat has a diameter of roughly 3 nm [45], the observed lamellar thicknesses suggest fibronectin multimerization during pulling and thus, the transition from a monolayer into a sheet.
Fig. 6.
Mechanical relaxation increases lamellar thickness and the interlamellar distance. Fibers were pulled out of a 0.4 mg/ml fibronectin solution in PBS and were subjected to three different strain levels using a uniaxial stretching device: 0% (A), 200% (B) and 500% (C) absolute strain. Values for the interlamellar distance (D) and lamellar thickness (E) were calculated for spiral segments for each of the strain values. For 0% absolute strain, 24 segments were analyzed, for 200% strain 67 segments and for 500% strain 55 segments. The results are presented as boxplots, where the central mark is the median, the edges of the box are the 25th and 75th percentiles, the whiskers extend to the most extreme data points not considered outliers, and outliers are plotted individually as crosses. Scale bar: 200 nm.
3.6. Fiber permeability to nanoparticles
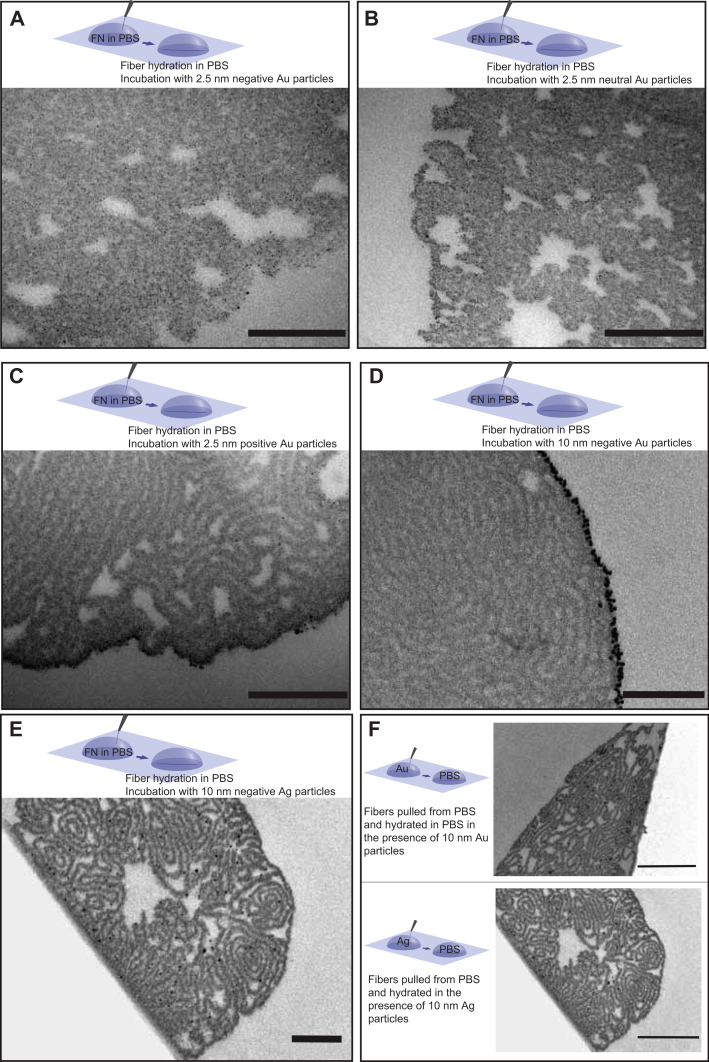
The existence of a densely packed but hydrated lamellar interior suggests that the initial sheet is folded up to form large intrinsic surface areas. This prompts another question related to the usage of the manually pulled fibers in binding assays, particularly how to best take their large internal surface into consideration. To ask whether this internal surface is available for the ligand, or if the interaction takes place only at the outer fiber surface, the fibers were incubated with nanoparticles of different sizes and surface chemistries. Nanogold particles were used which consist of a 0.82 nm gold core surrounded by an organic shell that can either be neutral, negatively or positively charged, giving the final particle a total diameter of 2.5 nm (from Nanoprobes, see Methods). Here we find that the neutral and negative nanoparticles were uniformly distributed throughout the fiber interior irrespectively of surface charge (Fig. 7A, B). Positive nanoparticles were also found in the fiber interior but they tended to accumulate on the fiber surface, reflecting either a negative charge on the surface of the fibers or the propensity of these nanoparticles to aggregate compared to their negative and neutral counterparts (Fig. 7C). On the contrary, 10 nm negatively charged gold colloid nanoparticles, which did not contain any organic shell (from BBI, see Methods) were not able to enter the fiber interior (Fig. 7D). Similarly, 10 nm negatively charged silver colloid nanoparticles were accumulated on the fiber surface and did not enter the fiber interior (Fig. 7E). When fibers were pulled from fibronectin solutions in the presence of 10 nm gold or silver nanoparticles, fibrillogenesis was not affected and the nanoparticles became associated with the internal fiber lamellae (Fig. 7F).
Fig. 7.
The fibers are permeable to 2.5 nm nanoparticles irrespective of their surface charge. Fibers were pulled out of a 0.4 mg/ml fibronectin solution in PBS and were treated with neutral (A), negatively (B), and positively (C) charged nanogold particles (2.5 nm) or with 10 nm negatively charged colloidal gold (D) or silver (E) nanoparticles for 1 h at room temperature prior to fixation. Alternatively, fibronectin was diluted in the colloidal solution of 10 nm negatively charged colloidal gold or silver nanoparticles to a final concentration of 0.4 mg/ml and fibers were pulled from these solutions in the presence of the nanoparticles (F). All samples were chemically fixed, ethanol dehydrated and embedded in Epon. 50 nm thick sections were observed by TEM. Scale bar: 200 nm for A-E and 500 nm for F.
3.7. Molecular requirements for fiber assembly
The ability to adsorb at the air-liquid interface among proteins is not unique for fibronectin. We tested BSA, laminin and collagen type I, which can all form insoluble monolayers at the air-liquid interface [46], [47], [48], [49]. However, we were not able to generate manually pulled fibers from any of these solutions (Table 1). The same was true for fibronectin fragments, suggesting that additional features in the intact fibronectin molecule are required for the air-liquid fibrillogenesis process (Table 1). Loss of fibronectin secondary structure by adding in the solution urea or guanidinium hydrochloride (GdnHCl) at a concentration higher than 1 m [50] was detrimental for fiber assembly (Table 1). Similarly, treatment of fibers with more that 1 m urea or GdnHCl led to their complete dissolving (Table 2). Denaturation of fibronectin with 1% SDS also led to complete fiber dissolving, whereas the same concentration of deoxycholate or Tween20 had no macroscopic effect on the fibers (Table 2).
Table 1.
Requirements for fiber assembly. The table summarizes the results of attempting to pull fibers from solutions of full length fibronectin (in the absence or presence of denaturants), fibronectin fragments, BSA, collagen I and laminin.
| Fiber assembly | |
|---|---|
| Full length fibronectin | Yes |
| 70 kDa N-terminal fibronectin fragment | No |
| 40 kDa C-terminal fibronectin fragment | No |
| 120 kDa central fibronectin fragment | No |
| BSA | No |
| Collagen I | No |
| Laminin | No |
| Full length fibronectin in the presence of urea | Yes (only when urea < 1 m) |
| Full length fibronectin in the presence of GdnHCl | Yes (only when GdnHCl < 1 m) |
Table 2.
Fiber stability on denaturants and detergents. The table summarizes results of experiments testing fiber stability in various denaturants and detergents. Fiber dissolution was observed microscopically using fibers pulled in the presence of fluorescently labeled fibronectin.
| Fiber dissolution | |
|---|---|
| Urea | Yes (only >1 m) |
| GdnHCl | Yes (only >1 m) |
| 1% SDS | Yes |
| 1% Deoxycholate | No |
| 1% Tween20 | No |
4. Discussion
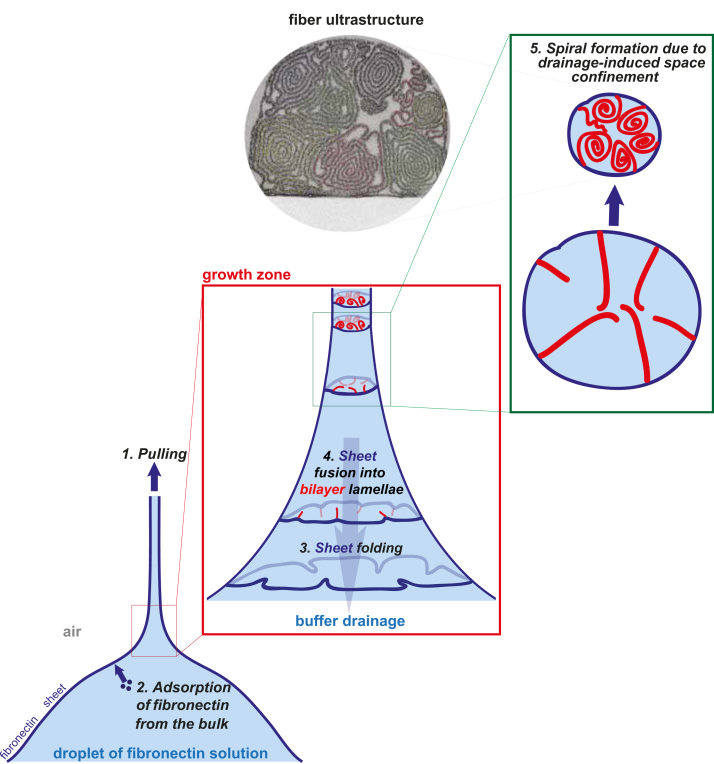
One of the prominent properties of fibronectin is its ability to form fibers either when mechanically stretched or under partially denaturing conditions [4], [8], [9], [10], [11]. A simple technique to produce stable, macroscopic fibronectin fibers in the absence of cells is to pull at the air-liquid interface of a droplet of a concentrated fibronectin solution [9]. Electron microscopy was used here to characterize the ultrastructure of such fibronectin fibers. Internally, these fibers are largely organized into a number of spiral lamellar segments, stabilized primarily through electrostatic interactions (Fig. 2). These manually pulled fibronectin fibers present a large internal surface to volume area, which is accessible to nanoparticles smaller than 10 nm (Fig. 7). Our findings suggest that fibrillogenesis under these conditions proceeds through an irreversible sheet-to-fiber phase transition. Taken together all of our observations, we propose a kinetic model of how the pulling process dictates the resulting molecular conformation and ultrastructure, as sketched in Fig. 8. First, during the adsorption process to the air-liquid interface, the fibronectin modules will structurally rearrange such as to minimize the overall energy. Fibronectin will thus expose hydrophobic patches or residues towards the air while the hydrophilic and charged residues are immersed in water. Second, as the tip is pulled away from the droplet surface, capillary forces cause the droplet surface to deform and vertical tension is applied to the fibronectin sheet. Third and to keep the surface tension constant, the increase in droplet surface area would spontaneously be compensated by fibronectin adsorption from the bulk, a process that would be accelerated as monolayer material is transferred into the fiber. Due to the high viscosity of fibronectin monolayers [38], [39], shear forces are generated during pulling, which is expected to cause partial alignment of fibronectin molecules along the direction of flow [11]. Such shear forces may already initiate partial fibronectin self-association within these rapidly drifting, highly sheared monolayers, and indeed we have observed by SEM strands in the monolayer connecting the droplet with the fiber (Fig. 1G–J). We thus, refer to these sheared monolayers as sheets.
Fig. 8.
Proposed kinetic model for the sequential assembly of manually pulled fibronectin fibers. The formation of manually pulled fibers is initiated by pulling at a fibronectin monolayer adsorbed at the air-liquid interface, depicted here as dark blue. As the tip is pulled away from the droplet surface (step 1), capillary forces cause the droplet surface to deform and vertical tension is applied to the fibronectin monolayer. As fibronectin is transferred towards the fiber, the material loss needs to be compensated. Fibronectin must thus adsorb fast enough to the droplet surface to keep the surface tension constant (step 2). As pulling continues, capillary force-induced buffer drainage leads to a rapid loss of fiber volume. In what we call the fiber growth zone, driven by the drainage-associated reduction in volume, the sheet is forced to buckle (step 3) and finally collapse into bilayer lamellar structures that point towards the interior of the fiber (step 4), while the rest of the sheet remains under tension along the fiber axis. Since the sheet collapses towards the interior of the fibers, its hydrophobic side, initially in contact with air, will form intralamellar contacts (bilayers, red), while the charged and hydrophilic residues on the other side remain strongly hydrated and eventually define the interlamellar distances through electrostatic repulsion. As the fibers loose most of their water content, the lamella tips start to get pushed against each other in the center of the fibers, leading to their curving away from the centripetal direction and ultimately inducing their spiraling we have observed in the TEM cross-sections (step 5).
As pulling continues, capillary force-induced buffer drainage leads to a rapid loss of fiber volume. Driven by the accompanying drainage-associated reduction in surface area, the sheet is forced to buckle, as seen in draping films or upon overcompression of other monolayer systems [51], [52], but here it is happening exclusively in the plane perpendicular to the tensile pulling force. Finally, the sheet, remaining under tension along the fiber axis, collapses into lamellar structures that point towards the interior of the fiber. Since the sheet undergoes a transition into lamellar structures towards the interior of the fiber, the surfaces that were in contact with air will form intralamellar contacts, while the charged residues remain strongly hydrated and define the interlamellar distances through electrostatic repulsion (Fig. 4, Fig. 5). As the fibers loose most of their water content, the lamellae tips start to get pushed against each other in the center of the fibers, leading to their curving away from the centripetal direction and ultimately inducing their spiraling. This model is further supported by the experimental findings that the self-similar lamella structures extend throughout the length of the fibers (Fig. 3), and that the interlamellar distance increases when lowering the ionic strength of the aqueous medium due to increased repulsive interactions (Fig. 4). Increasing the tension along the fiber axis furthermore reduces the lamellar thickness (Fig. 6) and increases the molecular strain of fibronectin molecules as previously shown by FRET [16].
In summary, we propose a transformation of the initial fibronectin monolayer at the droplet surface, which then collapses into lamellar structures that curve up into spirals as the water drains out. The resulting supramolecular architecture thus, originates from a kinetically trapped and irreversible process by which the sheet-to-fiber transition is forced to progress. At the end of this process, a sheet surface with a circumference in the order of ∼200 μm is being packed within a fiber of ∼2 μm in diameter (Supplementary Fig. 1). This assembly process occurs continuously due to surface tension-driven sheet transfer from the droplet surface into the fiber in what we call the fiber growth zone (Fig. 8). Replenishment of the monolayer at the air-liquid interface is ensured by protein adsorption from the bulk due to the high protein concentration. Such a mechanism for fiber formation suggests that during pulling, the growth zone always produces self-similar spiral patterns, which will therefore be retained throughout the full fiber length as confirmed by serial sectioning (Fig. 3). Finally, reversible swelling and de-swelling when altering the ionic buffer concentration (Fig. 5) shows complete solvent access to the interior of the fiber. Additionally, the ability of particles to penetrate into the interior of the fibers if they have a diameter of less than 10 nm (Fig. 7), suggest that under appropriate conditions, the large internal fiber surface remains accessible. This knowledge is important when utilizing these fibers for binding assays, whereby these fibers are used as a model system to study the interactions between fibronectin and various ligands that can vary in size, from small peptides to full-length proteins [13], [36]. More specifically, in the quantitative analysis of binding events using fiber stretch assays, the signal would have to be normalized with respect to the externally available fiber surface area when the peptides or proteins would only bind to the outer surface of the fibers. In contrast and if all of the interior surfaces are accessible as well, the signal has to be normalized with respect to the total internal and external fiber surfaces area. If the area per fibronectin is the same throughout the fiber, it is thus a reasonable approximation to calibrate the signal of the binding peptide per fibronectin molecule.
Finally, previous work from our group using FRET based fibronectin probes [14] has shown that the molecular conformations that fibronectin molecules assume within manually pulled fibers, which have been tuned by stretching/relaxing to experience a range of strain values from 0% to 500% as described above, is similar to the conformation range of fibronectin within cell-derived fibrils in the extracellular matrix. The similarity in the molecular configuration of fibronectin on cell-derived and manually pulled fibers is intriguing if one takes into consideration the different processes involved during fibrillogenesis. A common element in both processes is the presence of mechanical forces acting along the fiber axis, which are necessary in both cases to expose self-association sites in individual fibronectin molecules and possibly align the molecules as they assemble into fibrils. During assembly of cell-derived fibronectin fibers in the extracellular matrix, such forces originate from the actomyosin cytoskeleton and are transmitted to fibronectin through interactions with integrins at the cell surface [5]. In both cases, mechanical forces partially open up self-association sites, which can facilitate fibrillogenesis [53] and one would expect similarities in the way individual fibronectin molecules interact with each other in both types of fibers. Supporting such similarities, we observe that, as is the case for cell-derived fibronectin fibers [54], manually pulled fibers cannot form when fibronectin fragments are used, which lack either self-association sites or the disulfide bridges between the monomeric chains, and that they are deoxycholate insoluble (Table 1). Additional hints for the organization of fibronectin molecules within the fiber lamellae are provided from our denaturation experiments (Table 2), which showed that loss of secondary structure either prior or after fiber formation leads to inability for fiber assembly or fiber dissolving, respectively. It has been shown that fibronectin molecules can form supramolecular β-sheet structures [55], which may be involved during fibronectin multimerization within the drifting monolayer, and in the subsequent fiber assembly. Our observations that fibronectin fragments and other proteins such as BSA, collagen and laminin, although form monolayers at the air-liquid interface, cannot undergo fibrillogenesis by pulling at the interface, suggest that unique elements in the complete structure of fibronectin are necessary for the production of manually pulled fibers.
In addition to basic research in extracellular matrix and mechanobiology, manually pulled fibronectin fibers have the potential to be used as substrates to guide cell migration and growth in tissue engineering applications [37]. Insights into the dynamics of the fiber assembly process, and the molecular requirements to generated highly organized microscopic lamellar structures also offers the potential for the production of novel fibers from other biopolymers alone or in combination with fibronectin.
5. Conclusions
In this study, we have used electron microscopy to characterize the ultrastructure of macroscopic fibers generated by pulling at the air-liquid interface of a fibronectin solution. Such manually pulled fibronectin fibers have been used as an experimental model system to study various aspects of fibronectin mechanobiology, as well as in tissue engineering applications, highlighting the importance of a detailed characterization of their ultrastructure. Our results here revealed a dense interior, largely organized into spiral, lamellar segments, whose interlamellar distances are regulated primarily via repulsive electrostatic interactions. We have shown that this large internal surface of the fibers is accessible to nanoparticles smaller than 10 nm. Furthermore, based on our observations, we have proposed a mechanism for fiber assembly whereby the fibronectin monolayer adsorbed at the air-liquid interface undergoes an irreversible sheet-to-fiber transition through the combined action of the externally applied pulling force, interfacial surface tension, capillary force-induced buffer drainage and shear-induced fibronectin self-association within the monolayer. In addition to underlying the generation of manually pulled fibronectin fibers, our findings exemplify a mechanism for the assembly of hierarchically structured fibers, which may be extended to other bio- or biomimetic polymers.
Acknowledgments
We gratefully acknowledge financial support from an ERC Advanced Grant (233157, VV), the CCMX Competence Centre for Materials Science and Technology (CCMX grant title: ‘Fibroblast growth factor 2 delivery for tissue repair: From natural concepts to engineered systems’) and funding from the Swiss National Science Foundation (SNF Nr. 310030B_133122). E.K. was in part supported by a FEBS Long-term Fellowship.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.biomaterials.2014.08.012.
Appendix A. Supplementary data
Suppl. Fig. 1: The length of the spiral segments in the fiber interior can reach up to several times the fiber dimensions. Fibers were pulled out of a 0.4 mg/ml fibronectin solution, chemically fixed with glutaraldehyde, ethanol dehydrated, embedded in epoxy resin, ultrathin sectioned (50 nm) and observed by TEM. Spiral segments were traced and highlighted in the image with different colors (A). The length of each segment was measured, along with the overall fiber height and diameter as designated in (A) by the yellow dashed lines (B).
Suppl. Fig. 2: Chemical fixation does not introduce artifacts compared to high pressure freezing. Fibers were pulled out of a 0.4 mg/ml fibronectin solution and were either chemically fixed with glutaraldehyde and ethanol dehydrated (A) or high pressure frozen and freeze substituted in acetone (B). Both samples were embedded in epoxy resin, ultrathin sectioned (50 nm) and observed under the TEM. Scale bar: 500 nm.
Suppl. Fig. 3: The pulling tip morphology does not affect the ultrastructure of the resulting fibronectin fibers. Fibers were pulled out of a 0.4 mg/ml fibronectin solution in PBS with a plastic pipet tip cut on the front (A, B, C) or with a metal microfabricated tip with a defined radius of 2 μm (E, F, G). Both types of tips were visualized with SEM after coating with platinum (A, B, E, F) and the fibers produced were chemically fixed, dehydrated with ethanol and embedded in Epon. 50 nm thick sections were visualized by TEM (C, G). Scale bar: 100 µm for (A), 20 µm for (E) and 1000 nm for (B), (C), (F) and (G).
Suppl. Fig. 4: Air-drying does not alter the internal organization of the fibers. Fibers were pulled out of a 0.4 mg/ml fibronectin solution in PBS and were either chemically fixed with glutaraldehyde in PBS (A), dried in air for 1 h prior to fixation (B) or rehydrated with PBS for another 1 h after air drying (C, D). All samples were chemically fixed, dehydrated in ethanol and embedded in epoxy resin. 60 nm thick sections were observed by TEM. Scale bar: 1 μm.
References
- 1.Ruoslahti E. Fibronectin in cell adhesion and invasion. Cancer Metastasis Rev. 1984;3:43–51. doi: 10.1007/BF00047692. [DOI] [PubMed] [Google Scholar]
- 2.Miyamoto S., Katz B.Z., Lafrenie R.M., Yamada K.M. Fibronectin and integrins in cell adhesion, signaling, and morphogenesis. Ann N Y Acad Sci. 1998;857:119–129. doi: 10.1111/j.1749-6632.1998.tb10112.x. [DOI] [PubMed] [Google Scholar]
- 3.Vogel V. Mechanotransduction involving multimodular proteins: converting force into biochemical signals. Annu Rev Biophys Biomol Struct. 2006;35:459–488. doi: 10.1146/annurev.biophys.35.040405.102013. [DOI] [PubMed] [Google Scholar]
- 4.Singh P., Carraher C., Schwarzbauer J.E. Assembly of fibronectin extracellular matrix. Annu Rev Cell Dev Biol. 2010;26:397–419. doi: 10.1146/annurev-cellbio-100109-104020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhong C., Chrzanowska-Wodnicka M., Brown J., Shaub A., Belkin A.M., Burridge K. Rho-mediated contractility exposes a cryptic site in fibronectin and induces fibronectin matrix assembly. J Cell Biol. 1998;141:539–551. doi: 10.1083/jcb.141.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pankov R., Cukierman E., Katz B.Z., Matsumoto K., Lin D.C., Lin S. Integrin dynamics and matrix assembly: tensin-dependent translocation of alpha(5)beta(1) integrins promotes early fibronectin fibrillogenesis. J Cell Biol. 2000;148:1075–1090. doi: 10.1083/jcb.148.5.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vuento M., Vartio T., Saraste M., von Bonsdorff C.H., Vaheri A. Spontaneous and polyamine-induced formation of filamentous polymers from soluble fibronectin. Eur J Biochem. 1980;105:33–42. doi: 10.1111/j.1432-1033.1980.tb04471.x. [DOI] [PubMed] [Google Scholar]
- 8.Peters D.M.P., Chen Y., Zardi L., Brummel S. Conformation of fibronectin fibrils varies: discrete globular domains of type III repeats detected. Microsc Microanal. 1998;4:385–396. doi: 10.1017/s1431927698980369. [DOI] [PubMed] [Google Scholar]
- 9.Ejim O.S., Blunn G.W., Brown R.A. Production of artificial-orientated mats and strands from plasma fibronectin: a morphological study. Biomaterials. 1993;14:743–748. doi: 10.1016/0142-9612(93)90038-4. [DOI] [PubMed] [Google Scholar]
- 10.Ulmer J., Geiger B., Spatz J.P. Force-induced fibronectin fibrillogenesis in vitro. Soft Matter. 2008;4:1998–2007. [Google Scholar]
- 11.Phillips J.B., King V.R., Ward Z., Porter R.A., Priestley J.V., Brown R.A. Fluid shear in viscous fibronectin gels allows aggregation of fibrous materials for CNS tissue engineering. Biomaterials. 2004;25:2769–2779. doi: 10.1016/j.biomaterials.2003.09.052. [DOI] [PubMed] [Google Scholar]
- 12.Baneyx G., Vogel V. Self-assembly of fibronectin into fibrillar networks underneath dipalmitoyl phosphatidylcholine monolayers: role of lipid matrix and tensile forces. Proc Natl Acad Sci U S A. 1999;96:12518–12523. doi: 10.1073/pnas.96.22.12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chabria M., Hertig S., Smith M.L., Vogel V. Stretching fibronectin fibres disrupts binding of bacterial adhesins by physically destroying an epitope. Nat Commun. 2010;1:135. doi: 10.1038/ncomms1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Little W.C., Smith M.L., Ebneter U., Vogel V. Assay to mechanically tune and optically probe fibrillar fibronectin conformations from fully relaxed to breakage. Matrix Biol. 2008;27:451–461. doi: 10.1016/j.matbio.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li B., Moshfegh C., Lin Z., Albuschies J., Vogel V. Mesenchymal stem cells exploit extracellular matrix as mechanotransducer. Sci Rep. 2013;3:2425. doi: 10.1038/srep02425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klotzsch E., Smith M.L., Kubow K.E., Muntwyler S., Little W.C., Beyeler F. Fibronectin forms the most extensible biological fibers displaying switchable force-exposed cryptic binding sites. Proc Natl Acad Sci U S A. 2009;106:18267–18272. doi: 10.1073/pnas.0907518106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Astrof S., Hynes R.O. Fibronectins in vascular morphogenesis. Angiogenesis. 2009;12:165–175. doi: 10.1007/s10456-009-9136-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruoslahti E. Fibronectin and its integrin receptors in cancer. Adv Cancer Res. 1999;76:1–20. doi: 10.1016/s0065-230x(08)60772-1. [DOI] [PubMed] [Google Scholar]
- 19.Erickson H.P., Carrel N.A. Fibronectin in extended and compact conformations. Electron microscopy and sedimentation analysis. J Biol Chem. 1983;258:14539–14544. [PubMed] [Google Scholar]
- 20.Klotzsch E., Schoen I., Ries J., Renn a., Sandoghdar V., Vogel V. Conformational distribution of surface-adsorbed fibronectin molecules explored by single molecule localization microscopy. Biomater Sci. 2014;2:883. doi: 10.1039/c3bm60262a. [DOI] [PubMed] [Google Scholar]
- 21.Garcia A.J., Vega M.D., Boettiger D. Modulation of cell proliferation and differentiation through substrate-dependent changes in fibronectin conformation. Mol Biol Cell. 1999;10:785–798. doi: 10.1091/mbc.10.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohashi T., Kiehart D.P., Erickson H.P. Dual labeling of the fibronectin matrix and actin cytoskeleton with green fluorescent protein variants. J Cell Sci. 2002;115:1221–1229. doi: 10.1242/jcs.115.6.1221. [DOI] [PubMed] [Google Scholar]
- 23.Potts J.R., Campbell I.D. Structure and function of fibronectin modules. Matrix Biol. 1996;15:313–320. doi: 10.1016/s0945-053x(96)90133-x. Discussion 321. [DOI] [PubMed] [Google Scholar]
- 24.Martino M.M., Hubbell J.A. The 12th-14th type III repeats of fibronectin function as a highly promiscuous growth factor-binding domain. Faseb J. 2010;24:4711–4721. doi: 10.1096/fj.09-151282. [DOI] [PubMed] [Google Scholar]
- 25.Ariel a, Hershkoviz R., Cahalon L., Williams D.E., Akiyama S.K., Yamada K.M. Induction of T cell adhesion to extracellular matrix or endothelial cell ligands by soluble or matrix-bound interleukin-7. Eur J Immunol. 1997;27:2562–2570. doi: 10.1002/eji.1830271015. [DOI] [PubMed] [Google Scholar]
- 26.Johansson S., Svineng G., Wennerberg K., Armulik A., Lohikangas L. Fibronectin-integrin interactions. Front Biosci. 1997;2:d126–d146. doi: 10.2741/a178. [DOI] [PubMed] [Google Scholar]
- 27.Woods A., Longley R.L., Tumova S., Couchman J.R. Syndecan-4 binding to the high affinity heparin-binding domain of fibronectin drives focal adhesion formation in fibroblasts. Arch Biochem Biophys. 2000;374:66–72. doi: 10.1006/abbi.1999.1607. [DOI] [PubMed] [Google Scholar]
- 28.Ingham K.C., Brew S.A., Migliorini M.M., Busby T.F. Binding of heparin by type III domains and peptides from the carboxy terminal hep-2 region of fibronectin. Biochemistry. 1993;32:12548–12553. doi: 10.1021/bi00097a035. [DOI] [PubMed] [Google Scholar]
- 29.Skorstengaard K., Thogersen H.C., Petersen T.E. Complete primary structure of the collagen-binding domain of bovine fibronectin. Eur J Biochem. 1984;140:235–243. doi: 10.1111/j.1432-1033.1984.tb08092.x. [DOI] [PubMed] [Google Scholar]
- 30.Makogonenko E., Ingham K.C., Medved L. Interaction of the fibronectin COOH-terminal Fib-2 regions with fibrin: further characterization and localization of the Fib-2-binding sites. Biochemistry. 2007;46:5418–5426. doi: 10.1021/bi7001373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henderson B., Nair S., Pallas J., Williams M.A. Fibronectin: a multidomain host adhesin targeted by bacterial fibronectin-binding proteins. FEMS Microbiol Rev. 2011;35:147–200. doi: 10.1111/j.1574-6976.2010.00243.x. [DOI] [PubMed] [Google Scholar]
- 32.Mitsi M., Hong Z., Costello C.E., Nugent M.A. Heparin-mediated conformational changes in fibronectin expose vascular endothelial growth factor binding sites. Biochemistry. 2006;45:10319–10328. doi: 10.1021/bi060974p. [DOI] [PubMed] [Google Scholar]
- 33.Oberhauser A.F., Badilla-Fernandez C., Carrion-Vazquez M., Fernandez J.M. The mechanical hierarchies of fibronectin observed with single-molecule AFM. J Mol Biol. 2002;319:433–447. doi: 10.1016/S0022-2836(02)00306-6. [DOI] [PubMed] [Google Scholar]
- 34.Gao M., Craig D., Lequin O., Campbell I.D., Vogel V., Schulten K. Structure and functional significance of mechanically unfolded fibronectin type III1 intermediates. Proc Natl Acad Sci U S A. 2003;100:14784–14789. doi: 10.1073/pnas.2334390100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith M.L., Gourdon D., Little W.C., Kubow K.E., Eguiluz R.A., Luna-Morris S. Force-induced unfolding of fibronectin in the extracellular matrix of living cells. PLoS Biol. 2007;5:e268. doi: 10.1371/journal.pbio.0050268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Little W.C., Schwartlander R., Smith M.L., Gourdon D., Vogel V. Stretched extracellular matrix proteins turn fouling and are functionally rescued by the chaperones albumin and casein. Nano Lett. 2009;9:4158–4167. doi: 10.1021/nl902365z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahmed Z., Underwood S., Brown R.A. Nerve guide material made from fibronectin: assessment of in vitro properties. Tissue Eng. 2003;9:219–231. doi: 10.1089/107632703764664693. [DOI] [PubMed] [Google Scholar]
- 38.Zhou N.F., Pethica B.A. Monolayers of human plasma fibronectin at the air/water interface. Langmuir. 1986;2:47–50. [Google Scholar]
- 39.Vogel V. Fibronectin in a surface-adsorbed state – insolubilization and self-assembly. Proteins Interfaces II. 1995;602:505–518. [Google Scholar]
- 40.Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rossier O., Cuvelier D., Borghi N., Puech P.H., Derenyi I., Buguin A. Giant vesicles under flows: extrusion and retraction of tubes. Langmuir. 2003;19:575–584. [Google Scholar]
- 42.Evans E., Yeung A. Hidden dynamics in rapid changes of bilayer shape. Chem Phys Lipids. 1994;73:39–56. [Google Scholar]
- 43.Kegel W.K., Lekkerkerker H.N.W. Evidence for enhanced electrostatic interactions in lamellar monolayer systems. Europhys Lett. 1994;26:425–430. [Google Scholar]
- 44.Rowan D.G., Hansen J.P., Trizac E. Screened electrostatic interactions between clay platelets. Mol Phys. 2000;98:1369–1378. [Google Scholar]
- 45.Leahy D.J., Aukhil I., Erickson H.P. 2.0 A crystal structure of a four-domain segment of human fibronectin encompassing the RGD loop and synergy region. Cell. 1996;84:155–164. doi: 10.1016/s0092-8674(00)81002-8. [DOI] [PubMed] [Google Scholar]
- 46.Noskov B.A., Mikhailovskaya A.A., Lin S.Y., Loglio G., Miller R. Bovine serum albumin unfolding at the air/water interface as studied by dilational surface rheology. Langmuir. 2010;26:17225–17231. doi: 10.1021/la103360h. [DOI] [PubMed] [Google Scholar]
- 47.Fadeev A.S., Levachev S.M., Yampol’skaya G.P., Rudoy V.M., Izmailova V.N. Properties of collagen monolayers formed at the water-air interface: the effect of pH and the ionic strength of a subphase. Colloid J. 1999;61:520–528. [Google Scholar]
- 48.Lakshmanan M., Dhathathreyan A. Amphiphilic laminin peptides at air/water interface – effect of single amino acid mutations on surface properties. J Colloid Interface Sci. 2006;302:95–102. doi: 10.1016/j.jcis.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 49.Bhattacharya M., Jain N., Mukhopadhyay S. Insights into the mechanism of aggregation and fibril formation from bovine serum albumin. J Phys Chem B. 2011;115:4195–4205. doi: 10.1021/jp111528c. [DOI] [PubMed] [Google Scholar]
- 50.Khan M.Y., Medow M.S., Newman S.A. Unfolding transitions of fibronectin and its domains. Stabilization and structural alteration of the N-terminal domain by heparin. Biochem J. 1990;270:33–38. doi: 10.1042/bj2700033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Holmes D.P., Crosby A.J. Draping films: a wrinkle to fold transition. Phys Rev Lett. 2010:105. doi: 10.1103/PhysRevLett.105.038303. [DOI] [PubMed] [Google Scholar]
- 52.Schief W.R., Antia M., Discher B.M., Hall S.B., Vogel V. Liquid-crystalline collapse of pulmonary surfactant monolayers. Biophys J. 2003;84:3792–3806. doi: 10.1016/S0006-3495(03)75107-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vakonakis I., Staunton D., Rooney L.M., Campbell I.D. Interdomain association in fibronectin: insight into cryptic sites and fibrillogenesis. Embo J. 2007;26:2575–2583. doi: 10.1038/sj.emboj.7601694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schwarzbauer J.E. Identification of the fibronectin sequences required for assembly of a fibrillar matrix. J Cell Biol. 1991;113:1463–1473. doi: 10.1083/jcb.113.6.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Litvinovich S.V., Brew S.A., Aota S., Akiyama S.K., Haudenschild C., Ingham K.C. Formation of amyloid-like fibrils by self-association of a partially unfolded fibronectin type III module. J Mol Biol. 1998;280:245–258. doi: 10.1006/jmbi.1998.1863. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Suppl. Video: 3D reconstruction of a 550 nm volume of a manually pulled fiber. Fibers pulled out of a 0.4 mg/ml fibronectin solution in PBS were chemically fixed with glutaraldehyde, ethanol dehydrated and embedded in epoxy resin. Serial ultrathin sections (11 sections, 50 nm each) were imaged by TEM. The images were registered and aligned in Fiji and the fiber volume was reconstructed in Imaris.