Abstract
Evolution of insect resistance to transgenic crops containing Bacillus thuringiensis (Bt) genes is a serious threat to the sustainability of this technology. However, field resistance related to the reduced efficacy of Bt maize has not been documented in any lepidopteran pest in the mainland U.S. after 18 years of intensive Bt maize planting. Here we report compelling evidence of field resistance in the fall armyworm, Spodoptera frugiperda (J.E. Smith), to Cry1F maize (TC 3507) in the southeastern region of the U.S. An F2 screen showed a surprisingly high (0.293) Cry1F resistance allele frequency in a population collected in 2011 from non-Bt maize in south Florida. Field populations from non-Bt maize in 2012–2013 exhibited 18.8-fold to >85.4-fold resistance to purified Cry1F protein and those collected from unexpectedly damaged Bt maize plants at several locations in Florida and North Carolina had >85.4-fold resistance. In addition, reduced efficacy and control failure of Cry1F maize against natural populations of S. frugiperda were documented in field trials using Cry1F-based and pyramided Bt maize products in south Florida. The Cry1F-resistant S. frugiperda also showed a low level of cross-resistance to Cry1A.105 and related maize products, but not to Cry2Ab2 or Vip3A. The occurrence of Cry1F resistance in the U.S. mainland populations of S. frugiperda likely represents migration of insects from Puerto Rico, indicating the great challenges faced in achieving effective resistance management for long-distance migratory pests like S. frugiperda.
Introduction
Fall armyworm, Spodoptera frugiperda (J.E. Smith), is a well-known long-distance migratory insect that is distributed from Argentina to Canada [1]. In the U.S., populations from overwintering areas in south Texas (TX) and south Florida (FL) migrate annually into various regions across the country [2]. S. frugiperda is a major target of both Bt maize and Bt cotton in North and South America [3], [4]. In 2013 alone, approximately 50 MHa of Bt crops were planted in the Americas for insect pest management [5], [6]. Effective insect resistance management (IRM) is crucial to ensure the long-term durability of these Bt crops [7]–[9]. Resistance monitoring must be addressed in IRM plans for Bt crops [10]. Although disagreements over the definition of ‘field resistance’ still exist [9], [11]–[13], the possibility of field resistance should be considered when there is a field control failure or significantly reduced efficacy [11]. Based on this criterion, field resistance to Bt crops has been clearly documented in at least four cases around the world [9], [11], including resistance of S. frugiperda to Cry1F maize in Puerto Rico [14], [15].
In recent years, unexpected survival of S. frugiperda on Cry1F maize has been reported on several occasions in the southeastern U.S. and in Brazil (F.H., R.L.M., J.A.Q., and D.D.R., unpublished data). However, scientific documentation of field resistance to Bt maize in S. frugiperda has not been reported anywhere except Puerto Rico [16]. During 2011–2013, an F2 screen, diet-incorporated bioassays, greenhouse tests, and field studies with various maize products (Table S1) were conducted in four southeastern U.S. states and the results documented that the unexpected survival of S. frugiperda on Cry1F maize in the region was due to resistance. The occurrence of field resistance of S. frugiperda in the U.S. mainland indicates a great challenge in resistance management for migratory targets of Bt crops.
Results and Discussion
In 2011, a total of 142 F2 two-parent families of S. frugiperda were established using single-pair mating of field-collected individuals, which included 70 families from Rapides and Franklin parishes in central and northeast Louisiana (LA) and 72 families from Collier County in south FL. F2 neonates of these families were screened on leaf tissue of Herculex I (HX1) maize expressing the Cry1F protein. The F2 screen showed that Cry1F resistance alleles were not rare in the LA and FL populations. The parents of 47.2% families in the two populations were found to carry ≥1 resistance allele (Table 1). For the LA population, parents of 49 families were negative for the presence of resistance alleles (genotype SSSS), 14 families carried 1 resistance allele (RSSS), and 7 families carried 2 resistance alleles (RSRS or RRSS). Among the 72 FL families, only 26 were negative, while 15, 25, 5, and 1 families were identified to carry 1, 2, 3 (RRRS), and 4 (RRRR) resistance alleles, respectively. The Cry1F resistance allele frequency estimated using a multinomial Bayesian statistical model (Methods S1) was 0.103 with a 95% credibility interval (CI) of 0.070 to 0.141 for the LA population and 0.293 with a 95% CI of 0.242 to 0.347 for the FL population. The resistant families initiated with five neonates per plant in the greenhouse grew well and survived on 40–80% of the Cry1F maize plants after 13 d (Table S2). A significant level of resistance (>270-fold) was also observed when these families were tested against purified Cry1F protein in diet-incorporated bioassays (Table 2).
Table 1. Expected Cry1F resistance allele frequency (RAF) and corresponding 95% credibility interval (CI) in Louisiana (LA) and Florida (FL) populations of Spodoptera frugiperda.
| Population | No. F2 families screened | No. RAs (genotype) in the two parents of each family | Total no. RAs | RAF(95% CI) | ||||
| 0 (SSSS) | 1 (RSSS) | 2 (RSRS or RRSS) | 3 (RRRS) | 4 (RRRR) | ||||
| LA | 70 | 49 | 14 | 7 | 0 | 0 | 28 | 0.103 (0.070, 0.141) |
| FL | 72 | 26 | 15 | 25 | 5 | 1 | 84 | 0.293 (0.242, 0.347) |
Table 2. Susceptibility of Spodoptera frugiperda collected from multiple locations to purified Cry1F protein using diet-incorporated bioassays.
| Insect population | State/county/parish | Year | Host source | LC50 (95% CL) (µg/g) | Resistance ratio | % growth inhibition at 10 µg/g (Mean ± SEM) |
| SS | LA, FL, TX | 2008–2013 | NBt | 0.37 (0.27, 0.49) | - | 100±0.0 e |
| LA-RD-34 | Rapides, LA | 2011 | NBt | >100 | >270 | 28.8±6.1bc |
| FL-39 | Collier, FL | 2011 | NBt | >100 | >270 | 41.8±4.0 cd |
| LA-RD-nBt-12 | Rapides, LA | 2012 | NBt | 23.1 (17.3, 34.2) | 62.4 | 85.6±1.3 e |
| LA-FK-nBt-12 | Franklin, LA | 2012 | NBt | 10.9 (8.2, 15.3) | 29.5 | 97.2±0.3 e |
| GA-GB-nBt-12 | Tift, GA | 2012 | NBt | 1.33 (1.00, 1.76) | 3.6 | n/a |
| GA-VT-nBt-12 | Tift, GA | 2012 | NBt | 4.94 (1.50, 75.6) | 13.4 | 60.4±7.9 d |
| FL-CL-nBt-12 | Collier, FL | 2012 | NBt | 6.97 (4.12, 14.4) | 18.8 | 87.1±3.0 e |
| FL-HD-nBt-12 | Hendry, FL | 2012 | NBt | >31.6 (0.0±0.0) | >85.4 | 31.4±4.9 c |
| FL-MI-nBt-13 | Miami-Dade, FL | 2013 | NBt | 7.35 (5.35, 10.8) | 19.9 | 86.9±4.1e |
| FL-HD-nBt-13 | Hendry, FL | 2013 | NBt | 29.5 (18.9, 55.8) | 79.7 | 33.3±4.3c |
| FL-CL-nBt-13 | Collier, FL | 2013 | NBt | 20.7 (13.4, 41.0) | 55.9 | 18.3±4.7abc |
| FL-AC-12 | Alachua. FL | 2012 | Bt | >31.6 (0.0±0.0) | >85.4 | −1.5±7.4a |
| FL-CL-Bt-12 | Collier, FL | 2012 | Bt | >31.6 (0.0±0.0) | >85.4 | 27.9±4.3bc |
| FL-CL-Bt-13 | Collier, FL | 2013 | Bt | >31.6 (0.0±1.9) | >85.4 | 0.5±5.5a |
| NC-Bt-13 | Hyde, NC | 2013 | Bt | >31.6 (7.1±5.1) | >85.4 | 5.9±6.0ab |
Larval mortality was calculated as the number of dead larvae divided by the total number of larvae in the test. During this study, three Cry1F-susceptibile (SS) strains (SS-FL, SS-LA, and SS-TX) were used as references. SS-FL was initiated from larvae collected from Hendry Co., FL in 2011; SS-LA was established from insects collected from Franklin Parish, LA in 2008; and SS-TX was developed from insects collected from Hidalgo Co., TX in 2013. All three SS strains were highly susceptible to both Cry1F maize plants and Cry1F protein in diet. Because the overall performance on maize plants and diet were similar among the three strains, SS was used to denote all three strains unless mentioned specifically. FL-HD-nBt-12 was collected from a heavily infested non-Bt sweet corn field that was close to an early-planted Bt maize field. The Bt maize field was heavily damaged by S. frugiperda and the population infesting the non-Bt sweet corn was believed to be the progeny of moths that came out of the Bt maize field. LA-RD-24 and FL-39 were two resistant families isolated from populations from Rapides Parish, LA and Collier Co., FL, respectively, using the F2 screen. FL-CL-nBt-12, FL-CL-nBt-13, FL-CL-Bt-12, and FL-CL-Bt-13 were collected from non-Bt and Bt plants in two field trials in Collier Co., FL in 2012 and 2013. LA: Louisiana, FL: Florida, GA: Georgia, NC: North Carolina. NBt: non-Bt maize, Bt: Bt maize. The LC50 value of a population was considered to be greater than the highest Cry1F concentration tested if its larval mortality was <50% at the highest concentration in the bioassays. Limited by the cost of Cry1F protein, the highest concentrations used in the bioassays varied depending on the sources of the populations. The highest concentration assayed for LA-RD-24 and FL-39 was 100 µg/g, while it was 31.6 µg/g for other populations. Mortality at 100 µg/g was 20.6±3.9% for LA-RD-24 and 0.0±0.0% for FL-39. Mortality at 31.6 µg/g was 7.1±5.1% for FL-SC-Bt-13 and zero for FL-HD-nBt-12 and all other populations collected from Bt maize plants. Resistance ratio was calculated as the LC50 of the field populations divided by that of the SS strain. Analysis of variance for growth inhibition: F14,46 = 59.75, P<0.0001. Mean values for growth inhibition followed by a common letter were not significantly different at α = 0.05 (Tukey’s HSD test). n/a: Data are not available.
We interpreted the high Cry1F resistance allele frequency estimated by the F2 screen in the FL population as an indication of field resistance as defined above. To confirm this hypothesis, 13 additional populations of S. frugiperda were collected from LA, Georgia (GA), FL, and North Carolina (NC) during 2012–2013 (Table 2), which included 9 populations (2 LA, 2 GA, 5 FL) from non-Bt maize and 4 populations from Cry1F maize. Two of the four populations from Cry1F maize were collected from fields that showed unexpected damage by feral populations of S. frugiperda, which included one from FL and one from NC. The other two of the four populations from Cry1F maize were collected from two field trials in FL in 2012 and 2013. Diet-incorporated bioassays showed that, relative to the Cry1F-susceptible (SS) strains, larvae of S. frugiperda collected from non-Bt maize were 3.6- to >85.4-fold less susceptible to purified Cry1F protein (Table 2). All four populations collected from Cry1F plants in FL and NC were highly resistant (>85.4-fold) to Cry1F protein. No significant mortality was observed at the Cry1F concentration of 31.6 µg/g, the highest concentration tested, for any of the four populations. The results confirmed that the unexpected damage by S. frugiperda observed in the fields in FL and NC was due to resistance to the Cry1F protein in the plants.
There also was clear evidence of Cry1F resistance in the field when trials were conducted in 2012 and 2013 at the location in FL where S. frugiperda were collected for the F2 screen. In 2012, an average leaf injury rating of 4.3 (Davis 1–9 scale) [17] due to the damage by S. frugiperda was recorded on Cry1F maize plants during the V2–V10 plant stages [18] (Table S3). Additional greenhouse tests showed that five out of 20 Cry1F plants each infested with 10 F1 neonates of S. frugiperda collected from non-Bt plants in the FL field trial were heavily injured, with a leaf injury rating of 6–9, and five live 4th–5th instars were recovered from the five plants (1 larva/plant) after 12 d (Table S4). In contrast, the Cry1F plants killed all of the SS larvae placed on them and had virtually no leaf injury. More importantly, the field trial in 2013 showed that Cry1F plants were essentially ineffective against the feral populations of S. frugiperda (Fig. 1). There were no significant differences in the leaf injury ratings and the percentage of plants containing live larvae of S. frugiperda between the non-Bt and Cry1F maize (HX1) plants. Both non-Bt and Cry1F plants were heavily injured by S. frugiperda, with a leaf injury rating of 8.24 on the non-Bt maize and 8.09 on HX1 at V9–V12 and >80% plants at the R1 plant stage contained large, live larvae (most of which were 5th instars) (Fig. 1). Diet-incorporated bioassays showed that the larvae collected from the non-Bt maize plants had 18.8-fold (for FL-CL-NBt-2012) and 55.9-fold (FL-CL-NBt-2013) resistance to Cry1F protein (Table 2). As described above, for the two populations (FL-CL-Bt-2012 and FL-CL-Bt-2013) collected from the Cry1F plants, no mortality was observed at 31.6 µg/g of diet. Thus, the performance of the Cry1F maize in the 2012 trial showed reduced efficacy of Cry1F because the non-Bt maize plants had significantly greater leaf injury, while the 2013 trial demonstrated failure of Cry1F against S. frugiperda. The results of the field trials confirmed that field resistance to Cry1F maize in S. frugiperda had occurred in FL and NC.
Figure 1. Leaf injury ratings (A) and occurrence of feral populations of Spodoptera frugiperda (B) on non-Bt and Bt maize containing single and pyramided genes in a field trial in 2013 in Collier Co., Florida.
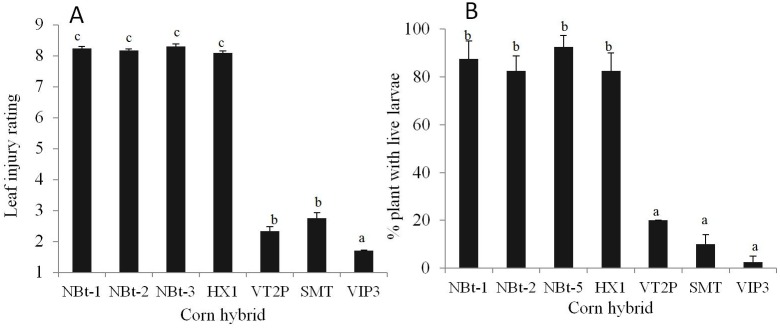
Leaf injury ratings were measured at V9–V12 plant stages using Davis’s 1–9 scale17, while larval occurrence was recorded at R1 plant stage. NBt-1, Pioneer 31P40; NBt-2, DKC 61-22; NBt-5, N78N-GT; HX1, Pioneer 31D59; VT2P, DKC 61-49; SMT, DKC 62-08; VIP3, N78N-3111. Bars represent means (±SEM) and those with a common letter were not significantly different at α = 0.05 (Tukey’s HSD test).
The geographical range and distribution of Cry1F resistance in S. frugiperda in the mainland U.S. remains unknown. A recent independent study found an resistance allele frequency of 0.132 to Cry1F in three samples of S. frugiperda collected from Palm Beach and Hendry counties in FL in 2011 and 2012 [16]. They found no unexpected field survival, but one population collected from Palm Beach in 2012 showed a resistance allele frequency of 0.247. Although unexpected field survival of S. frugiperda has not been reported in LA, the resistance allele frequency (0.103) of the LA populations detected in this study was also relatively high. The results of our study, together with other published data, indicate that the range of Cry1F resistance in S. frugiperda may be geographically extensive in the southeast coastal region of the U.S.
The factors that led to the field resistance of S. frugiperda to Cry1F maize in FL and NC are unknown. Local selection pressure due to the planting of Bt maize appears not to be a major factor driving the development of field resistance. In most years, S. frugiperda in the U.S. mainland overwinters only in south FL and south TX [2], [19]. Maize is not a major crop in FL, which had a total planting area of <40,000 ha/year [5]. A high proportion of maize in the state is sweet corn, and most sweet corn does not contain Bt genes. In addition, S. frugiperda is a polyphagous insect with a wide host range [19]. For these reasons, local selection pressure by Bt maize in FL should be limited. Although it is unclear if local selection caused by the use of Bt microbial insecticides is a contributing factor, the more plausible reason for the field resistance appears to be the migration of resistant populations from Puerto Rico through other Caribbean islands to FL. Northerly movement of FL populations along the U.S. East Coast has been documented for years [20]. This hypothesis is supported by a recent comparative study of mitochondrial haplotype ratios in S. frugiperda [21]. The study showed that the Puerto Rico populations of S. frugiperda had only very limited interactions with TX populations, but could have substantial exchanges with FL populations. In addition, the areas with unexpected damage by S. frugiperda on Bt maize also match the expected migration patterns of S. frugiperda from the Caribbean islands to the mainland U.S. that were generated based on weather patterns [20].
While further studies are warranted to reveal the geographical ranges and factors leading to field resistance in the U.S. mainland, effective management of Cry1F- resistant populations of S. frugiperda is needed to ensure the continued success of Bt crop technologies. To generate essential information for IRM, additional F2 screen, and laboratory, greenhouse, and field studies were performed to analyze the cross-resistance to other commonly used Bt proteins and Bt maize products containing single and pyramided traits.
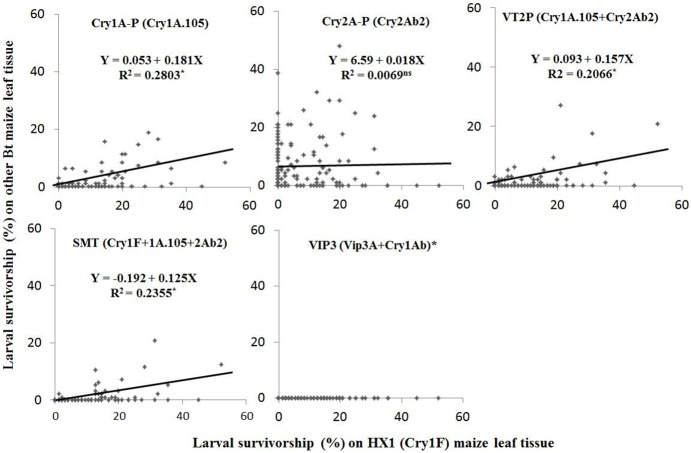
We analyzed the cross-resistance of S. frugiperda between HX1 and five other Bt maize products based on the survival of the 142 families in an F2 screen that was performed simultaneously with the F2 screen against HX1 mentioned above. The five Bt maize products included two experimental Bt maize lines, Cry1A.105Ln (Cry1A-P) and Cry2Ab2Ln (Cry2A-P), as well as three commercial products: Genuity VT Double (VT2P), Genuity SmartStax (SMT), and Agrisure Viptera 3111 (VIP3). Cry1A-P and Cry2A-P produce a single Bt protein, Cry1A.105 and Cry2Ab2, respectively, whereas VT2P expresses both Cry1A.105 and Cry2Ab2 (Table S1) [22]. SMT produces six Bt proteins including the two in VT2P and Cry1F for controlling Lepidoptera plus Cry3Bb1 and Cry34/35Ab1 for Coleoptera. VIP3 produces three Bt proteins including Vip3A and Cry1Ab for Lepidoptera and mCry3A for Coleoptera. Correlation analysis showed that there was a significantly (P<0.05) positive relationship in larval survival of the 142 families between Cry1F maize and three other maize products, namely, Cry1A-P, VT2P, and SMT, but not with Cry2A-P or VIP3 (Fig. 2). The correlation coefficients calculated based on larval survivorship between HX1 and other products were 0.534 (P<0.05) for Cry1A-P, 0.461 (P<0.05) for VT2P, and 0.491 (P<0.05) for SMT, but only 0.021 (not significant) for Cry2A-P (Table S5). No correlation coefficients could be calculated with VIP3 which killed all of the F2 larvae in the 142 families and has previously been reported to be extremely toxic towards S. frugiperda [23]–[25]. The results suggest that some level of cross-resistance exists between HX1 and Cry1A-P, VT2P, and SMT, but not VIP3 and Cry2A-P.
Figure 2. Correlation analysis on larval survivorship (%) of 142 F2 two-parent families between HX1 vs. five other Bt maize products.
Analysis was performed by treating survival on HX1 as the independent variable (X) and survival on the other five Bt products as the dependent variable (Y) [38]. HX1, Pioneer 31D59; Cry1A-P, an experimental line expressing the Cry1A.105 protein; Cry2A-P, an experimental line expressing the Cry2Ab2 protein; VT2P, DKC 61-49; SMT, DKC 62-08; VIP3, N78N-3111. “*” indicates statistical significance (P<0.05), while “ns” indicates not significant (P≥0.05).
To understand the cross-resistance patterns observed in the F2 screen, diet-incorporated bioassays were conducted to determine the susceptibility of a known Bt-susceptible strain collected from TX in 2013 (SS-TX) and a resistant strain (FL-39) to five individual purified Cry proteins: Cry1Aa, Cry1Ab, Cry1Ac, Cry1A.105, and Cry2Ab2. FL-39 was isolated from a two-parent family of the FL population using the F2 screen mentioned above. Relative to SS-TX, FL-39 exhibited 4.8-fold less susceptibility to Cry1A.105, while susceptibility to Cry2Ab2 was similar between SS-TX and FL-39 (Table 3). Individual Cry1Aa, Cry1Ab, and Cry1Ac proteins were not very effective against either strain. LC50s of the three proteins were ≥23.8 µg/g against the two strains, and larvae of both strains showed a considerable weight gain at 31.6 µg/g. Survival of SS-TX and FL-39 was also evaluated in the greenhouse on whole plants of YieldGard corn borer (YG), VIP3, and three Bt maize lines: Cry1A-P, Cry2A-P, and Cry2Ab2Hn (Cry2A-HP, an experimental line expressing a ‘high level’ of Cry2Ab2 protein). In these tests, YG was virtually ineffective against S. frugiperda, with an average of 58.3% plants containing live larvae at 12 d after infestation with five neonates of SS-TX or FL-39 per plant (Table 4). In contrast, no larvae of either strain survived on VIP3. Cry1A-P also killed all of the SS-TX larvae, while 37.5% of the Cry1A-P plants infested with FL-39 contained live larvae. SS-TX and FL-39 survived on 18.8% and 31.3% of the Cry2A-P plants, respectively, but no survivors of either strain were observed on Cry2A-HP. The results of the greenhouse tests further confirmed that some level of cross-resistance exists in S. frugiperda between Cry1F and Cry1A.105, but not between Cry1F and Cry2Ab2 or Vip3A.
Table 3. Median lethal concentrations (LC50) and 95% confidence limits (CL) based on larval mortality of Cry1F-susceptible (SS-TX) and -resistant (FL-39) strains of Spodoptera frugiperda to five individual Cry proteins.
| Cry protein | Insectstrain | N | Slope ± SE | LC50 (95% CL)(µg/g) | Resistanceratio | % growth inhibition at31.6 µg/g (mean ± SEM) |
| Cry1Aa | SS-TX | 495 | - | >31.6 | 72.6±4.4 bc | |
| FL-39 | 512 | - | >31.6 | - | 42.0±10.8 a | |
| Cry1Ab | SS-TX | 938 | 0.83±0.17 | 28.3 (12.3, 141.8) | 62.3±9.3 ab | |
| FL-39 | 507 | 3.20±1.0 | 23.8 (14.5, 56.7) | −1.2 | 66.7±4.3 ab | |
| Cry1Ac | SS-TX | 608 | n/a | >31.6 | 74.6±7.4 bcd | |
| FL-39 | 577 | 0.83±0.24 | 29.2 (12.7, 347.8) | −1.1 | 71.6±2.5 ab | |
| Cry1A.105 | SS-TX | 576 | 1.94±0.31 | 9.0 (6.3, 13.8) | 100±0.0 e | |
| FL-39 | 640 | 1.30±0.24 | 43.5 (27.6, 84.1) | 4.8 | 81.4±4.7 bcd | |
| Cry2Ab2 | SS-TX | 543 | 0.87±0.28 | 17.7 (7.4, 260.4) | 94.2±1.9 cde | |
| FL-39 | 576 | 1.28±0.33 | 12.5 (5.8, 88.6) | −1.4 | 95.0±1.4 de |
SS-TX was developed from insects collected from Hidalgo Co., TX in 2013 and documented to be susceptible to Cry1F maize and Cry1F protein. FL-39 was a resistant family isolated from an FL population collected in 2011 using an F2 screen. n = total number of neonates assayed. Limited by the amount of Cry proteins available, the highest concentrations used in some bioassays didn’t cause a 50% or greater larval mortality. LC50 value of an insect strain was considered to be greater than the highest Cry concentration assayed if its larval mortality was <50% at the highest concentration. Mortality at 31.6 µg/g was 6.7±1.1% for SS-TX and 26.1±9.5% for FL-39 for Cry1Aa, and 24.1±10.2% for SS-TX for Cry1Ac. Resistance ratios for a Cry protein were calculated by dividing the greater LC50 value by the smaller one. A negative sign was given if the LC50 of FL-39 was smaller than that of SS-TX. Analysis of variance for growth inhibition: F4,29 = 22.19, P<0.0001 for protein; F1,29 = 12.18, P = 0.0016 for insect strain; and F4,29 = 4.79, P = 0.0043 for the interaction. Mean values followed by a common letter in a column were not significantly different at α = 0.05 (Tukey’s HSD test).
Table 4. Leaf injury rating and % plants containing live larvae (mean ± SEM) of Cry1F-susceptible (SS-TX) and -resistant (FL-39) strains of Spodoptera frugiperda on whole plants of non-Bt and Bt maize containing single or pyramided genes.
| Maize hybrid/line | Maize trait | Leaf injury rating after 7 d | % plants containing live larvae after 12 d | ||
| SS-TX | FL-39 | SS-TX | FL-39 | ||
| Non-Bt | NBt | 3.75±0.16 c | 4.1±0.15 c | 87.5±5.59 e | 84.4±5.98 de |
| Pioneer 31D50 | HX1 | 1.13±0.07 a | 4.31±0.33 c | 0.00±0.00 a | 75.00±10.21 cde |
| DKC 69–70 | YG | 3.21±0.36 c | 3.50±0.40 c | 60.4±6.25 bcde | 56.25±6.25 bcd |
| Cry1A.105Ln | Cry1A-P | 1.06±0.06 a | 3.31±0.19 c | 0.00±0.00 a | 37.50±7.22 bc |
| Cry2Ab2Ln | Cry2A-P | 2.06±0.12 b | 1.88±0.07 b | 18.75±6.25 ab | 31.25±6.25 abc |
| Cry2Ab2Hn | Cry2A-HP | 1.00±0.00 a | 1.00±0.00 a | 0.00±0.00 a | 0.00±0.00 a |
| DKC 64-04 | VT2P | 1.06±0.06 a | 1.19±0.12 a | 0.00±0.00 a | 0.00±0.00 a |
| DKC 62-08 | SMT | 1.00±0.00 a | 1.00±0.00 a | 0.00±0.00 a | 0.00±0.00 a |
| N78N-3111 | VIP3 | 1.00±0.00 a | 1.00±0.00 a | 0.00±0.00 a | 0.00±0.00 a |
| Analysis of variance | Insect | F 1,75 = 89.73, P<0.0001 | F 1,75 = 14.78, P = 0.0003 | ||
| Maize | F 8,75 = 196.09, P<0.0001 | F 8,75 = 66.72, P<0.0001 | |||
| Interaction | F 8,75 = 31.6, P<0.0001 | F 8,75 = 6.39, P<0.0001 | |||
Data were pooled for three non-Bt maize hybrids/lines which included DKC 61-22 (NBt-2), N78N-GT (NBt-5), and ExpH (NBt-6). n/a: Information is not available. Mean values followed by a common letter within a parameter measured were not significantly different at α = 0.05 (Tukey’s HSD test).
The observed cross-resistance in the F2 screen between HX1 and the two pyramided products VT2P and SMT is likely due to the similar (Cry1A.105) and/or shared (Cry1F in SMT) protein domains in the products. VT2P and SMT contain the same Cry1A.105 gene [22]. Cry1A.105 is a chimeric protein incorporating domains I and II from Cry1Ab or Cry1Ac, domain III from Cry1F, and the C-terminal domain from Cry1Ac [26]. Limited by the technology available, the expression levels of Cry1F or Cry1A.105 in the pyramided Bt maize plants were not determined. Based on the gene structures, the overall amino acid sequence identity of Cry1A.105 to Cry1Ac, Cry1Ab, and Cry1F is 93.6%, 90.0%, and 76.7%, respectively [26]. As shown in both the diet-incorporated bioassays and the whole-plant tests, both Cry1Ab and Cry1Ac were ineffective against S. frugiperda. Thus, if S. frugiperda develops resistance to Cry1F protein, Cry2Ab2 is the only protein in VT2P and SMT still fully active against S. frugiperda (with incomplete resistance to Cry1A.105). Because Cry2Ab2 has a mode of action distinct from that of Cry1F or Cry1A [27], cross-resistance between Cry2Ab2 and Cry1F or Cry1A is unlikely [16], [28]–[31]. The results of our study are consistent with those of a recent protein binding study [32] which showed that S. frugiperda shares binding sites for Cry1A.105 and Cry1F. The high effectiveness of VIP3 against S. frugiperda is most likely due to the Vip3A protein. As mentioned above, neither YG plants nor purified Cry1Ab protein are very effective against S. frugiperda, indicating a limited activity of Cry1Ab protein in VIP3 for the insect. Cross-resistance between Cry1F and Vip3A is unlikely because the two proteins do not share binding sites [33] and was not seen in the case of the Puerto Rico Cry1F-resistant population of S. frugiperda [16].
In North and South America, pyramided Bt maize products are becoming prevalent and thus it is necessary to know the performance of these products in order to effectively manage Cry1F-resistant populations of S. frugiperda. In a greenhouse trial, we observed no larval survival of either SS-TX or FL-39 on three pyramided Bt products (VT2P, SMT, and VIP3) (Table 4). Niu et al. [25] also showed that these pyramided Bt maize products were effective in controlling a Puerto Rico Cry1F-resistant population of S. frugiperda in the greenhouse. To validate the performance of these products in the field, the HX1 field trial in Collier Co., FL in 2013 was extended to include VT2P, SMT, and VIP3 along with closely related non-Bt maize hybrids. As described above, the feral population of S. frugiperda at the trial site (FL-CL-nBt-13 and FL-CL-Bt-13) was highly resistant to both HX1 maize (Fig. 1) and purified Cry1F protein (Table 2). The field trial showed that the natural population of S. frugiperda caused very limited leaf injury on the pyramided (VT2P, SMT, and VIP3) Bt-plants (1.7–2.8 on the Davis scale), with 2.5–20.0% of the plants containing live larvae (Fig. 1). Some larvae could have moved between plots, but sampling was avoided at the plot ends where this risk was high. A positive correlation was observed between the survival in the F2 screen and the open field trial for the three pyramided products, suggesting that the low level of cross-resistance to Cry1A.105 could allow limited survival of Cry1F-resistant S. frugiperda on maize plants with pyramided traits related to Cry1A.105.
Our documentation of field resistance of S. frugiperda to Cry1F maize in the continental U.S. indicates that the Cry1F-based crop technologies may face a great challenge due to the migration of the Cry1F-resistant populations of S. frugiperda. It appears that geographic isolation and withdrawal of Cry1F maize (TC1507) from Puerto Rico [14] were not enough to stop the spread of resistance. Cry1F maize was first registered in 2001 in the U.S. and later commercially planted in Puerto Rico in 2003 for controlling lepidopteran pests including S. frugiperda, which is the most important maize pest in the territory [14], [34]. With the extensive use of TC1507 maize products along with several other factors [11], [25], field resistance to Cry1F maize occurred in Puerto Rico in 2006 [14], [15]. Upon an initial confirmation of field resistance in 2006 and as a part of IRM, the commercial sale of Cry1F maize seeds was stopped in Puerto Rico [14], [35]. However, resistance is still persistent after several years of not planting TC1507 products [15], [25], [36]. In addition, unlike Bt resistance in most other insects, the Cry1F resistance in S. frugiperda is likely complete resistance [25] and not associated with any fitness costs [16], [37]. Thus, the Cry1F based Bt maize and Bt cotton products currently planted in North and South America could be at risk. For example, Cry1F-resistant S. frugiperda could migrate north and damage Bt maize fields. The resistance observed in NC in this study may be a good example of such a situation. In the southern US, resistant populations of S. frugiperda could impact WideStrike cotton that contains the Cry1F protein. In Brazil and Argentina, >18 MHa of Bt crops were planted in 2013, much of it targeted against S. frugiperda [6]. Therefore, effective IRM for S. frugiperda and other similar migratory polyphagous pests will require careful consideration of their movement patterns and of possible Bt crop deployment strategies.
Materials and Methods
Insect collections
Third to fifth instars of S. frugiperda were collected during 2011–2013 from multiple locations in four southeastern U.S. states: LA, GA, FL, and NC. Insects collected in 2011 were used to establish two-parent families for an F2 screen [39]. A total of >1,200 larvae of S. frugiperda were collected from sorghum fields in Franklin and Rapides parishes in LA and from non-Bt sweet corn fields in Collier Co. in south FL. Field-collected larvae were reared individually on a meridic diet as described in Yang et al [40]. Newly emerged virgin male and female adults derived from the field collections were paired. Progeny (F1) produced by each pair were separately reared on diet and the F1 adults were sib-mated within each two-parent family to produce F2 offspring. The number of viable F1 pupae in each family ranged from 55 to 80 with an average of 76.5±1.0 (mean ± SE) for the LA populations and 50 to 80 with an average of 67.9±1.7 (mean ± SE) for the FL population. The F2 neonates were used in an F2 screen on Bt maize leaf tissue as described below.
In addition, 13 field populations of S. frugiperda were collected during 2012–2013 from Bt and non-Bt maize fields in 10 locations in LA, GA, FL, and NC (Table 2). Sample size was 35 larvae for one population (FL-CL-Bt-13) and 92–300 for other populations. Field-collected larvae were reared on a meridic diet [36] and F1 progeny of the field-collected populations, except NC-13, were used to determine the susceptibility to purified Cry1F protein. For NC-13, F3 progeny were used in the bioassay. Purified (99.9%) Cry 1F protein was obtained from Case Western Reserve University, Cleveland, Ohio, USA [41].
F2 screen
A total of 142 two-parent families of S. frugiperda were established from the field collections in 2011, which included 70 families from LA and 72 from FL (Table 1). Among the 70 LA families, 47 families were collected from Rapides Parish and 23 were from Franklin Parish. F2 neonates of the families were screened on leaf tissues of HX1, Cry1A-P, Cry2A-P, VT2P, SMT, and VIP3 maize as described in Yang et al [40]. Limited by the technology available, expression levels of Bt proteins in plants were not measured, but Cry protein expression for a maize hybrid/line was confirmed using the ELISA-based assays (EnviroLogix, Quantiplate kits, Portland, ME. In each family, 96 neonates were placed in 24 wells (4 neonates/well) (Bio-Smart-32, C–D International, Pitman, NJ) containing leaf tissue excised from greenhouse-grown maize plants at V4–V9. The decision to use four neonates per well was based on a previous study to minimize larval cannibalism [40]. All bioassay trays containing maize leaf tissue and larvae of S. frugiperda were incubated in environmental chambers maintained at 28°C, ∼50% RH and a 16-h: 8-h (L:D) photoperiod. Fresh leaf tissue was added every 2–3 d. Larval survival and development were recorded after 7 d. Live larvae were separated into two groups based on their growth: small (1st or 2nd instars) and large (≥3rd instars).
Definition of potential positive families possessing resistance alleles to Cry1F maize
During the study, three SS strains (SS-FL, SS-LA, and SS-TX) were used as references for laboratory bioassays and greenhouse tests. SS-FL was initiated from larvae collected from non-Bt maize fields in Hendry Co., FL in 2011 [36]; SS-LA was established from cotton and maize fields in 2008 in LA [42]; and SS-TX was developed from insects collected from non-Bt maize in TX in 2013. All three SS strains were highly susceptible to both Cry1F maize plants and Cry1F protein in diet. Because the overall performance on maize plants and diet were similar among the three strains, SS was used to denote all three strains unless mentioned specifically. Baseline survival assays showed that all three Cry1F-susceptible strains (SS) of S. frugiperda survived well on non-Bt maize leaf tissue after 7 d with an average survivorship of 63.4% and a larval mass of 44.2 mg/larva (Table S6). In contrast, on HX1 leaf tissue, only a small percentage (2.3%) of larvae survived and all survivors were 1st or 2nd instars. The results suggested that survivorship of large larvae (≥3rd instars) in the F2 screen on HX1 leaf tissue could be used to identify potential positive families carrying resistance alleles to Cry1F. Correspondingly, ≤2nd instars that survived the F2 screen were treated as dead larvae in determining resistance alleles.
Theoretically, if one of the two parents of a family contains a recessive resistance allele, 6.25% of the F2 larvae are expected to be homozygous (RR) for Bt resistance and should survive in the F2 screen [39]. Based on the baseline survival data of SS, an average of 3.59 [ = 96 (neonates screened)×6.25% (RR frequency)×59.9% (baseline survivorship on HX1)] live larvae were expected in a family in the F2 screen if one parent of the family possessed a resistance allele. A χ2-test showed that a survival of one larva in a family was not significantly (P>0.05) different from the expected survivorship (3.59 larvae/family), and thus a family with one or more survivors was considered as a potential positive family for resistance alleles to Cry1F maize.
Cry1F resistance confirmation
Based on the larval survival in the F2 screen, 21 of the 70 LA families of S. frugiperda and 46 of the 72 FL families were identified to be potential positive families (Table S7). To confirm if a potential positive family actually possessed resistance alleles, six strains were established from the survivors of six potential positive families including three families (LA-RD-24, LA-RD-34, and LA-RD-37) from Rapides Parish, LA and three families (FL-13, FL-37, and FL-39) from FL (Table S8). To increase the chance of success in the strain establishments, all F2 survivors (both large and small larvae) of a family were transferred to the diet [36] and reared in varied temperatures to synchronize their development. Progeny of the strains established were then selected on Cry1F maize leaf tissue for 1–2 times using the similar methods as described in the F2 screen. Initial confirmation for the six potential positive families was performed by measuring larval survival of the potential positive families and SS on HX1 leaf tissue using the same method as described in the F2 screen. Then, resistance of three potential positive families (LA-RD-24, LA-RD-34, and FL-39) was reconfirmed on whole plants of greenhouse-grown HX1 plants (Table S2). In the reconfirmation tests, five neonates of a potential positive family were placed into the whorl of a plant at the V6–VT stages. Leaf injury ratings, larval survival, and larval mass were recorded 12–14 d after the initial insect infestation. In addition, non-Bt maize and SS-FL were also included in the tests as the controls of the experiment. A potential positive family was considered to actually possess resistance alleles if it showed a significant survivorship with live ≥3rd instars on the leaf tissue and on whole plants in the confirmation tests.
In addition, susceptibility to purified Cry1F protein of two families (LA-RD-34 and FL-39) that were already confirmed to be resistant to Cry1F maize was examined, along with SS, using a diet-incorporated bioassay [36] (Table 2). In the bioassay, larval survival (both small and large larvae) and masses of live larvae were recorded 7 d after neonate infestations. Corrected dose/mortality data [43] of SS were subjected to probit analysis [38], [44] to determine LC50 and 95% CL. For the two resistant families, the LC50 value was considered to be greater than the highest Cry concentration (100 µg/g) tested because the larval mortalities were <50% at 100 µg/g. Resistance ratios were calculated using the LC50 value of a HX1-resistant strain divided by the LC50 of SS. In addition, the percentage of larval growth inhibition at 10 µg/g was calculated as described in Huang et al [45]. Growth inhibition data were analyzed using a one-way analysis of variance (ANOVA) [38]. Comparison among insect strains was made using the Tukey’s HSD test at α = 0.05.
Estimate of Cry1F resistance allele frequency
Results of the resistance confirmation studies showed that all six potential positive families examined possessed resistance alleles against HX1 maize plants. Diet-incorporated bioassays further confirmed that the survival of S. frugiperda on HX1 maize plants was due to resistance to the Cry1F protein in the plants. Therefore, all of the 21 LA and 46 FL potential positive families identified in the F2 screen were considered to carry resistance alleles. Revisiting the F2 screen data (Table S7), we found that the survivorship of F2 progeny of some families in the F2 screen was much greater than the expected survival of 3.59 larvae/family, suggesting that there was >1 resistance allele in the two parents of some families. To accurately estimate the resistance allele frequency, a χ2-test with the assumption of single-gene Mendelian inheritance was used to determine the number of resistance alleles in the two parents of each family (Tables S7, S9, S10, S11). We then framed a Bayesian statistical model [39], [46], [47] as a multinomial problem to calculate the expected resistance allele frequency and the corresponding 95% CI (Methods S1).
Susceptibility of field populations of S. frugiperda to Cry1F protein
The surprisingly high Cry1F resistance allele frequency in the populations of S. frugiperda detected in the F2 screen, especially for the FL population, suggests that it should be possible to detect resistance using a convenient dose-response bioassay method [48]. During 2012–2013, a total of 13 field populations of S. frugiperda were collected from LA, GA, FL, and NC (Table 2). Susceptibility of these field populations, along with SS, to purified Cry1F protein was determined using the diet-incorporated bioassay method as described above. Limited by the cost of Cry1F protein, these populations were assayed with Cry1F concentrations up to only 31.6 µg/g. LC50s and larval growth inhibition (%) were analyzed using the methods mentioned above.
Survival and leaf injury of natural populations of S. frugiperda on non-Bt and HX1 under field and greenhouse conditions
Larval survival and plant injury of natural populations of S. frugiperda were evaluated in 2012 and 2013 in the same field (26° 28′N, 81° 26′W) at the Southwest Florida Research and Education Center, University of Florida in Collier Co., FL where insects were collected for the F2 screen. The field trials were permitted by the Southwest Florida Research and Education Center, University of Florida. The field work did not involve any endangered or protected species. No human participants, specimens or tissue samples, or vertebrate animals, embryos or tissues were involved in the study. A randomized completely block (RCB) design with four replications was used in both years. There were 200 plants/replication in 2012 and 504 plants/replication in 2013. Only an HX1 hybrid and a closely related non-Bt maize hybrid were included in the trial in 2012, while the test in 2013 also contained three pyramided Bt maize traits (VT2P, SMT, and VIP3) along with closely related non-Bt maize hybrids (Fig. 1). Leaf injury by S. frugiperda was rated using Davis’ 1–9 scale [17] in V2–V10 plant stage for the trial in 2012 and V9–V12 plant stages for the trial in 2013. In addition, in the 2013 trial, larval occurrence of S. frugiperda was recorded at the R1 plant stage, when the plants showed maximum leaf injury. Occurrence of S. frugiperda was not recorded for the trial in 2012. Transformed data [25] on leaf injury ratings and percentage plants containing live larvae were analyzed using a one-way ANOVA [38]. Treatment means for each trial were separated using Tukey’s HSD test at α = 0.05.
In addition, susceptibility to Cry1F protein of field-collected populations (F1) from non-Bt and Bt maize plants of the two trials was determined using the diet-incorporated bioassay as described above. Larval survival and plant injury of the field population (F1 of FL-CL-nBt-12) collected from non-Bt maize plants in the trial in 2012 along with SS were also tested on whole plants of greenhouse-grown HX1 and non-Bt maize plants to demonstrate the biological activity of HX1 against susceptible S. frugiperda and resistance in the field-collected population. In the greenhouse tests, 10 neonates of FL-CL-nBt-12 and SS were placed into the whorl of a plant at V5–V7. Larval survival and leaf injury ratings were recorded at 12 d after insect infestation. A RCB was used in the test with four replications and 5 plants/replication. Transformed data [25] were analyzed using a two-way ANOVA (38). Treatment means were separated using Tukey’s HSD test at α = 0.05.
Determination of cross-resistance
Cross-resistance of Cry1F-resistant S. frugiperda was examined using two methods. First, correlation and regression analyses [38] were performed to examine if there were significant relationships in the survivorship of the 142 families of S. frugiperda in the F2 screen on leaf tissue of HX1 and the five other Bt maize products. A significant positive correlation would suggest the existence of cross-resistance among Bt maize products. Second, susceptibility of FL-39 and SS-TX to five common individual Cry proteins (Cry1Aa, Cry1Ab, Cry1Ac, Cry1A.105, and Cry2Ab2) was determined using the diet-incorporated bioassay method as described above. All five proteins were provided by Monsanto Company (St. Louis, MO, USA).
Larval survival and plant injury of SS and Cry1F-resistant S. frugiperda on Bt maize plants containing single and pyramided traits
Both greenhouse and field studies were utilized to evaluate if pyramided Bt maize and other related traits were effective against the Cry1F- resistant S. frugiperda. In the greenhouse study, larval survival and plant injury of FL-39 and SS-TX were investigated on four non-Bt and eight Bt maize products using a method similar to that described in Niu et al [25]. The eight Bt maize products included five commercial products (HX1, YG, VT2P, SMT, and VIP3) and three experimental lines (Cry1A-P, Cry2A-P, and Cry2A-HP). Expression/non-expression of Bt proteins for a maize hybrid/line was also confirmed using the ELISA-based assays mentioned above. The four non-Bt maize products were genetically closely related to 1–2 of the Bt products. At the V5–V7 plant stages, five neonates of a S. frugiperda strain were placed into the whorl of a plant. Leaf injury was rated with Davis’ 1–9 scale at 7 d after larval release, and larval survival was recorded after 12 d. A RCB was used in the tests containing four replications with four plants/replication. Transformed data [25] were analyzed using a two-way ANOVA [38]. Treatment means were separated using Tukey’s HSD test at α = 0.05.
Supporting Information
Non-Bt and Bt maize products evaluated in this study.
(DOCX)
Plant injury and larval survival of a Cry1F-susceptible (SS-FL) strain and three Cry1F-resistant families (LA-RD-24, LA-RD-34, and FL-39) of Spodoptera frugiperda on whole plants of non-Bt and Cry1F maize hybrids in the greenhouse.
(DOCX)
Leaf injury ratings (mean ± SEM) of non-Bt and HX1 plants caused by feral populations of Spodoptera frugiperda in a field trial in Collier Co., FL in 2012.
(DOCX)
Leaf injury rating and survival (mean ± SEM) of Cry1F-susceptible strain (SS-FL) and a field population (FL-CL-NBt-2012) of Spodoptera frugiperda collected from non-Bt maize from a field in Collier Co., FL in 2012 and tested in the greenhouse.
(DOCX)
Correlation coefficients of larval survivorship of 142 two-parent families of Spodoptera frugiperda in an F2 screen with maize leaf tissue containing single or pyramided Bt genes.
(DOCX)
Baseline survivorship (mean ± SEM) of a susceptible strain (SS-FL) of Spodoptera frugiperda on leaf tissue of Bt and non-Bt maize plants.
(DOCX)
Potential positive families (PPF) possessing resistance alleles (RAs) to Cry1F maize that were identified in the F2 screen in three populations of Spodoptera frugiperda collected from Louisiana (LA) and Florida (FL).
(DOCX)
Larval survivorship (%) of potential positive families of Spodoptera frugiperda on leaf tissue of Cry1F maize plants.
(DOCX)
Baseline survival (mean ± SEM) of Cry1F-susceptible (SS-FL), -resistant (RR), and -heterozygous (RS) genotypes of Spodoptera frugiperda on leaf tissue of HX1 and non-Bt (NBt) maize plants.
(DOCX)
Expected frequency of genotypes of in the F2 progeny of a two-parent family of Spodoptera frugiperda.
(DOCX)
Six different parental (P1) crosses of two-parent families and the related frequencies and genetic models.
(DOCX)
Supplementary methods.
(DOCX)
Acknowledgments
This article is published with the approval of the Director of the Louisiana Agricultural Experiment Station as manuscript No. 2014-234-15414.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
This project represents work supported by the Louisiana Soybean and Feed Grain Promotion Board, Monsanto Company, and Hatch funds from the USDA National Institute of Food and Agriculture. The funder Monsanto provided support in the form of salaries for the coauthor (G.P.H.), but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. All other funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the “author contributions” section. The authors also confirm that this does not alter their adherence to PLOS ONE policies on sharing data and materials. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by Louisiana State University Agricultural Center, University of Florida or the U.S. Department of Agriculture.
References
- 1. Sparks AN (1979) A review of the biology of the fall armyworm. Fla Entomol 62: 82–87. [Google Scholar]
- 2. Pashley DP (1988) The current status of fall armyworm host strains. Fla Entomol 71: 227–234. [Google Scholar]
- 3.Buntin D, Flanders K (2012) Bt corn products for the southeastern United States. http://www.caes.uga.edu/commodities/fieldcrops/gagrains/documents/2012BtcornSEBtcorntraitstableNov21.pdf. Accessed 17 July 2014.
- 4. Frizzas MR, Neto SS, de Oliveira CM, Omoto C (2014) Genetically modified corn on fall armyworm and earwig populations under field conditions. Ciência Rural 44: 203–209. [Google Scholar]
- 5.NASS (National Agricultural Statistics Service) (2013). Acreage. USDA, Washington DC. USA. http://usda01.library.cornell.edu/usda/current/Acre/Acre-06-28-2013.pdf. Accessed 17 July 2014.
- 6.James C (2013) Global status of commercialized biotech/GM crops: 2013. ISAAA Brief No. 45. ISAAA: Ithaca, NY, USA.
- 7. Gould F (1998) Sustainability of transgenic insecticidal cultivars: integrating pest genetics and ecology. Ann Rev Entomol 43: 701–726. [DOI] [PubMed] [Google Scholar]
- 8.Matten SR, Frederick RJ, Reynolds AH (2012) United States Environmental Protection Agency insect resistance management programs for plant-incorporated protectants and use of simulation modeling. In: Wozniak CA, McHughen A, editors. Regulation of agricultural biotechnology: The United States and Canada. Springer. 175−267.
- 9. Tabashnik BE, Brévault T, Carrière Y (2013) Insect resistance to Bt crops: lessons from the first billion acres. Nat Biotechnol 3: 510–521. [DOI] [PubMed] [Google Scholar]
- 10.US-EPA (U.S. Environmental Protection Agency) (2001) Biopesticide registration action document: Bacillus thuringiensis plant-incorporated protectants. http://www.epa.gov/oppbppd1/biopesticides/pips/bt_brad2/1-overview.pdf. Accessed 17 July 2014.
- 11. Huang F, Andow DA, Buschman LL (2011) Success of the high dose/refuge resistance management strategy after 15 years of Bt crop use in North America. Entomol Exp App 140: 1–16. [Google Scholar]
- 12. Tabashnik BE, van Rensburg JBJ, Carriere Y (2009) Field-evolved insect resistance to Bt crops: definition, theory, and data. J Econ Entomol 102: 2011–2025. [DOI] [PubMed] [Google Scholar]
- 13. Sumerford DV, Head GP, Shelton A, Greenplate J, Moar W (2013) Field-evolved resistance: assessing the problem and ways to move forward. J Econ Entomol 106: 1525–1534. [DOI] [PubMed] [Google Scholar]
- 14. Storer NP, Babcock JM, Schlenz M, Meade T, Thompson GD, et al. (2010) Discovery and characterization of field resistance to Bt maize: Spodoptera frugiperda (Lepidoptera: Noctuidae) in Puerto Rico. J Econ Entomol 103: 1031–1038. [DOI] [PubMed] [Google Scholar]
- 15. Storer NP, Thompson GD, Head GP (2012) Application of pyramided traits against lepidoptera in insect resistance management for Bt crops. GM Crop 3: 154–162. [DOI] [PubMed] [Google Scholar]
- 16. Vélez AM, Spencer TA, Alves AP, Moellenbeck D, Meagher RL, et al. (2013) Inheritance of Cry1F resistance, cross-resistance and frequency of resistant alleles in Spodoptera frugiperda (Lepidoptera: Noctuidae). Bull Entomol Res 103: 700–713. [DOI] [PubMed] [Google Scholar]
- 17.Davis FM, Ng SS, Williams WP (1992) Visual rating scales for screening whorl-stage corn for resistance to fall armyworm. Miss Agric Forestry Exp Stn Tech Bull 186.
- 18.Ritchie S W, Hanway JJ, Benson GO, Herman JC (1993) How a corn plant develops. Iowa State Uni Coop Ext Ser Sp Rep No. 48, Ames, Iowa, USA.
- 19.Capinera JL (1999) Fall armyworm. Uni. Fla. Publi. no. EENY-98. http://entnemdept.ufl.edu/creatures/field/fall_armyworm.htm. Accessed 17 July 2014.
- 20. Mitchell ER, McNeil JN, Westbrook JK, Silvant JF, Lalanne-Cassou B, et al. (1991) Seasonal periodicity of fall armyworm (Lepidoptera: Noctuidae) in the Caribbean basin and northward to Canada. J Entomol Sci 26: 39–51. [Google Scholar]
- 21. Nagoshi RN, Meagher RL, Jenkins DJ (2010) Puerto Rico fall armyworm has only limited interactions with those from Brazil or Texas but could have substantial exchanges with Florida populations. J Econ Entomol 103: 360–367. [DOI] [PubMed] [Google Scholar]
- 22.DiFonzo C, Cullen E (2012) Handy Bt trait table. http://www3.ag.purdue.edu/agry/PCPP/Documents/Entry%20forms/Handy_Bt_Trait_Table.pdf. Accessed 17 July 2014.
- 23. Burkness EC, Dively G, Patton T, Morey AC, Hutchison WD (2010) Novel Vip3A Bacillus thuringiensis (Bt) maize approaches high-dose efficacy against Helicoverpa zea (Lepidoptera: Noctuidae) under field conditions. GM Crop 1: 1–7. [DOI] [PubMed] [Google Scholar]
- 24. Yang F, Huang F, Qureshi JA, Leonard BR, Niu Y, et al. (2013) Susceptibility of Louisiana and Florida populations of Spodoptera frugiperda (Lepidoptera: Noctuidae) to transgenic Agrisure Viptera 3111 maize. Crop Protect 50: 37–39. [Google Scholar]
- 25. Niu Y, Yang F, Dangal V, Huang F (2014) Larval survival and plant injury of Cry1F-susceptible, -resistant, and -heterozygous fall armyworm (Lepidoptera: Noctuidae) on non-Bt and Bt corn containing single or pyramided genes. Crop Protect 59: 22–28. [Google Scholar]
- 26.Biosafety Cleaning-House. Gene and DNA sequence, Cry1A.105. https://bch.cbd.int/database/record.shtml?documentid=43771. Accessed 10 October 2014.
- 27. Storer NP, Kubiszak ME, King JE, Thompson GD, Santos AC (2012) Status of resistance to Bt maize in Spodoptera frugiperda: Lessons from Puerto Rico. J Invert Pathol 110: 294–300. [DOI] [PubMed] [Google Scholar]
- 28. Sivasupramaniam S, Moar WJ, Ruschke LG, Osborn JA, Jiang C, et al. (2008) Toxicity and characterization of cotton expressing Bacillus thuringiensis Cry1Ac and Cry2Ab2 proteins for control of lepidopteran pests. J Econ Entomol 101: 546–554. [DOI] [PubMed] [Google Scholar]
- 29. Brévault T, Prudent P, Vaissayre M, Carrière Y (2009) Susceptibility of Helicoverpa armigera (Lepidoptera: Noctuidae) to Cry1Ac and Cry2Ab2 insecticidal proteins in four countries of the West African cotton belt. J Econ Entomol 102: 2301–2309. [DOI] [PubMed] [Google Scholar]
- 30. Wu X, Leonard BR, Zhu Y-C, Abel CA, Head GP, et al. (2009) Susceptibility of Cry1Ab-resistant and -susceptible sugarcane borer (Lepidoptera: Crambidae) to four Bacillus thuringiensis toxins. J Invert Pathol 100: 29–34. [DOI] [PubMed] [Google Scholar]
- 31.Sumerford DV, Head GP (2014) Patterns of cross resistance among insecticidal Bacillus thuringiensis proteins and their implications for the durability of pyramided Bt crops. Crop Prot. In press.
- 32. Hernández-Rodríguez CS, Hernández-Martínez P, Van Rie J, Escriche B, Ferré J (2013) Shared midgut binding sites for Cry1A.105, Cry1Aa, Cry1Ab, Cry1Ac and Cry1Fa proteins from Bacillus thuringiensis in two important corn pests, Ostrinia nubilalis and Spodoptera frugiperda . PLoS ONE 8(7), e68164 doi:10.1371/journal.pone.0068164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sena JDA, Hernandez-Rodriguez CS, Ferré J (2009) Interaction of Bacillus thuringiensis Cry1 and Vip3A proteins with Spodoptera frugiperda midgut binding sites. App Environ Microbiol 75: 2236–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.US-EPA (U.S. Environmental Protection Agency) (2007) TC1507 maize and fall armyworm in Puerto Rico, MRID 47176001. USEPA, Washington, DC, USA.
- 35.Matten SR, Head GP, Quemada HD (2008) How governmental regulation can help or hinder the integration of Bt crops within IPM programs. In: Romeis J, Shelton AM, Kennedy GG editors. Integration of insect resistant genetically modified crops with IPM programs. Springer USA. 27−39.
- 36. Niu Y, Meagher Jr RL, Yang F, Huang F (2013) Susceptibility of field populations of the fall armyworm (Lepidopteran: Noctuidae) from Florida and Puerto Rico to purified Cry1F protein and corn leaf tissue containing single and pyramided Bt genes. Fla Entomol 96: 701–713. [Google Scholar]
- 37. Jakka SRK, Knight VR, Jurat-Fuentes JL (2014) Fitness costs associated with field-evolved resistance to Bt maize in Spodoptera frugiperda (Lepidoptera: Noctuidae). J Econ Entomol 107: 342–351. [DOI] [PubMed] [Google Scholar]
- 38.SAS Institute Inc (2010) SAS/STAT: 9.3 User’s Third Edition. Cary, NC, USA.
- 39. Andow DA, Alstad DN (1998) F2 screen for rare resistance alleles. J Econ Entomol 91: 572–578. [Google Scholar]
- 40. Yang F, Qureshi JA, Leonard BR, Head GP, Niu Y, et al. (2013) Susceptibility of Louisiana and Florida populations of Spodoptera frugiperda (Lepidoptera: Noctuidae) to pyramided Bt corn containing Genuity VT Double Pro and SmartStax traits. Fla Entomol 96: 714–723. [Google Scholar]
- 41. Zhang L, Huang F, Leonard BR, Chen M, Clark T, et al. (2013) Susceptibility of Cry1Ab maize.-resistant and –susceptible strains of sugarcane borer (Lepidoptera: Crambidae) to four individual Cry proteins. J Invertebr Pathol 112: 267–272. [DOI] [PubMed] [Google Scholar]
- 42. Hardke JT, Leonard BR, Huang F, Jackson RE (2011) Damage and survivorship of fall armyworm (Lepidoptera: Noctuidae) on transgenic field corn expressing Bacillus thuringiensis Cry proteins. Crop Protect 30: 168–172. [Google Scholar]
- 43. Abbott WS (1925) A method of computing the effectiveness of an insecticide. J Econ Entomol 18: 265–267. [Google Scholar]
- 44.Finney DJ (1971) Probit analysis (Cambridge University Press, England).
- 45. Huang F, Ghimire MN, Leonard BR, Zhu Y-C, Head G (2012) Susceptibility of field populations of sugarcane borer from non-Bt and Bt maize plants to five individual Cry toxins. Ins Sci 19: 570–578. [Google Scholar]
- 46. Andow DA, Alstad DN (1999) Credibility interval for rare resistance allele frequencies. J Econ Entomol 92: 755–758. [Google Scholar]
- 47. Stodola TJ, Andow DA (2004) F2 screen variations and associated statistics. J Econ Entomol 97: 1756–1764. [DOI] [PubMed] [Google Scholar]
- 48. Huang F Detection, monitoring of insect resistance to transgenic Bt crops (2006) Ins Sci. 13: 73–84. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Non-Bt and Bt maize products evaluated in this study.
(DOCX)
Plant injury and larval survival of a Cry1F-susceptible (SS-FL) strain and three Cry1F-resistant families (LA-RD-24, LA-RD-34, and FL-39) of Spodoptera frugiperda on whole plants of non-Bt and Cry1F maize hybrids in the greenhouse.
(DOCX)
Leaf injury ratings (mean ± SEM) of non-Bt and HX1 plants caused by feral populations of Spodoptera frugiperda in a field trial in Collier Co., FL in 2012.
(DOCX)
Leaf injury rating and survival (mean ± SEM) of Cry1F-susceptible strain (SS-FL) and a field population (FL-CL-NBt-2012) of Spodoptera frugiperda collected from non-Bt maize from a field in Collier Co., FL in 2012 and tested in the greenhouse.
(DOCX)
Correlation coefficients of larval survivorship of 142 two-parent families of Spodoptera frugiperda in an F2 screen with maize leaf tissue containing single or pyramided Bt genes.
(DOCX)
Baseline survivorship (mean ± SEM) of a susceptible strain (SS-FL) of Spodoptera frugiperda on leaf tissue of Bt and non-Bt maize plants.
(DOCX)
Potential positive families (PPF) possessing resistance alleles (RAs) to Cry1F maize that were identified in the F2 screen in three populations of Spodoptera frugiperda collected from Louisiana (LA) and Florida (FL).
(DOCX)
Larval survivorship (%) of potential positive families of Spodoptera frugiperda on leaf tissue of Cry1F maize plants.
(DOCX)
Baseline survival (mean ± SEM) of Cry1F-susceptible (SS-FL), -resistant (RR), and -heterozygous (RS) genotypes of Spodoptera frugiperda on leaf tissue of HX1 and non-Bt (NBt) maize plants.
(DOCX)
Expected frequency of genotypes of in the F2 progeny of a two-parent family of Spodoptera frugiperda.
(DOCX)
Six different parental (P1) crosses of two-parent families and the related frequencies and genetic models.
(DOCX)
Supplementary methods.
(DOCX)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.