Abstract
Background
To investigate the association between the single nucleotide polymorphism (SNP) of hypoxia-inducible factor1 α (HIF-1α) and the susceptibility to cervical spondylotic myelopathy (CSM) and its outcome after surgical treatment.
Method
A total of 230 CSM patients and 284 healthy controls were recruited. All patients received anterior cervical corpectomy and fusion (ACF) and were followed for 12 months. The genotypes for two HIF-1α variants (1772C>T and 1790G>A) were determined.
Results
In the present study, we found that the HIF-1α polymorphism at 1790G>A significantly affects the susceptibility to CSM and its clinical features, including severity and onset age. In addition, the 1790A>G polymorphism also determines the prognosis of CSM patients after ACF treatment. The GG genotype of 1790G>A polymorphism is associated with a higher risk to develop CSM, higher severity and earlier onset age. More importantly, we found that the 1790G>A polymorphism determines the clinical outcome in CSM patients who underwent ACF treatment.
Conclusion
Our findings suggest that the HIF-1α 1790G>A polymorphism is associated with the susceptibility to CSM and can be used as predictor for the clinical outcome in CSM patients receiving ACF treatment.
Introduction
Degenerative changes in the cervical spine are an inevitable response to the aging process. Impairment of cervical nerve roots may result from instability, disc degeneration, herniation or spinal stenosis. Cervical spondylotic myelopathy (CSM) is one of the most common degenerative spinal cord disorders affecting the elderly [1], [2], [3]. The mechanism of CSM development remains unclear. Some environmental factors, such as age, gender, smoking and trauma are reported to be associated with CSM risk [4], [5]. Previous studies show that the genetic factors also play an important role in the CSM development [6], [7]. Some candidate genes predicting the occurrence and development of CSM have been reported [8], [9]. Anterior cervical corpectomy and fusion (ACF) is a widely used surgical treatment for CSM patients. A recent study shows that the patient's genetic background affects the clinical outcome of CSM patients receiving ACF treatment [10].
The effect of hypoxia on the development of chronic spine disease has aroused interest. Hypoxia differentially regulates human nucleus pulposus and annulus fibrosus cell extracellular matrix production in 3D scaffolds [11]. As the largest avascular structure in the body, intervertebral disc is characterized by low oxygen tension in vivo [12]. Hypoxia-inducible factor α (HIF-1α) is a master transcription factor that regulates the cellular responses to hypoxic environments. HIF-1α is expressed in nucleus pulposus cells and plays an important role in regulating energy metabolism and matrix synthesis [13], [14], [15]. A recent study revealed that HIF-1α plays a crucial role in the survival of disc cells and resorption of the herniated disc in human [16]. HIF-1α is involved in the homeostasis of intervertebral disc cells. HIF-1α regulates apoptosis of intervertebral disc cells [16] [26].
Two HIF1α polymorphisms, namely, 1772C>T (P582S) and 1790G>A (A588T) have been reported to significantly increase HIF1α gene transcriptional activity [17], [18]. A recent study suggests that HIF-1α polymorphism affects lumbar disc degeneration and confers the susceptibility to lumbar disc disease (LDD) in Chinese cohort [19]. To date, the role of HIF-1α polymorphism in CSM remains unknown. In this study, we enrolled the Chinese CSM patients to investigate the association of HIF-1α polymorphism with the susceptibility, clinical feature and prognosis of CSM patients after ACF treatment.
Methods
Ethics statement
The ethical committee of Shanghai Jiaotong University approved the study. All participants provided their written informed consent to participate in this study.
Enrolment
In our study, the sample size required to achieve statistically significant associations were calculated using the power calculator for case control genetic association studies (PGA). According to the estimated sample size, we enrolled 230 patients with CSM. The diagnose was established on the basis of findings from the history, physical examination and confirmed by magnetic resonance imaging (MRI). Patients with one of the following conditions were excluded from this study: cervical trauma, autoimmune disease, chronic inflammatory disease, severe osteoporosis, and chronic renal or liver insufficiency. The control group consisted of 288 sex and age matched healthy Chinese individuals. All controls underwent the MRI and show no evidence of spondylosis, cord or nerve root compression and osteophyte formation in spine. The clinical characteristics including sex, age, weight, height, body mass index (BMI), daily desk work time, smoking status and family history of intervertebral degenerative disc disease were collected. The severity of CSM was scored according to the modified Japanese Orthopedic Association (modified JOA) score for CSM [20].
Follow-up
All 230 patients received anterior cervical corpectomy and fusion (ACF) and were followed for 2 years. The patients were dichotomized into two groups according to the mJOA scores: improvement group (at least 50% or higher improvement in mJOA score at the last follow-up compared with pre-operative score) and a non-improvement group (the improvement of mJOA score at last follow-up was less than 50%, equal, or less than pre-operative mJOA score) [10].
HIF-1α genotyping
Genomic DNA was isolated from the peripheral blood leukocytes by using standard protocols. Polymerase chain reaction (PCR) was performed to amplify the 178-bp fragment of the exon 12 of the HIF-1α human gene, using the 5′-CAT GTA TTT GCT GTT TTA AAG-3′ forward primer and 5′-GAG TCT GCT GGA ATA CTG TAA CTG-3′ reverse primer. The mixture for PCR was in 30 µL, containing 200 ng template DNA, 0.2 mM of each dNTP, 0.5 µM of each forward and reverse primer, 1.5 mM MgCl2, 0.5 U of Taq polymerase and 3 µL of 10× PCR buffer. The conditions for the PCR reaction were: denaturation at 95°C for 5 min, followed by 35 cycles of denaturation at 95°C for 30 sec, annealing at 61°C for 30 sec, extension at 70°C for 1 min, and a final extension at 72°C for 10 min. PCR products were purified and sequenced using Big Dye Terminator kit on an ABI Prism 3100 Automated DNA sequencer according to the manufacturer's protocol (Applied Biosystems, Foster City, CA).
Western blot assay
The intervertebral discs were collected during surgery from patients during ACF treatment. Samples were homogenized and lysed. Extracts were resolved on SDS-polyacrylamide gels followed by transfer to nitrocellulose membranes. Proteins were resolved by electrophoresis on 8–12% sodium dodecyl sulfate–polyacrylamide gels and transferred by electroblotting to polyvinylidene difluoride membranes. The membranes were blocked with 5% nonfat dry milk and incubated overnight at 4°C with the anti-HIF-1α (Novus Biological, 1;1000), anti-vascular endothelial growth factor (anti-VEGF) (Santa Cruz, 1∶1000), anti-VEGF receptor (anti-VEGFR) (Santa Cruz, 1∶1000), anti-NF-kB (Santa Cruz, 1∶1000), anti-interleukin 1 (anti-IL1) (Santa Cruz, 1∶1000), anti-interleukin6 (anti-IL6) (Santa Cruz, 1∶1000), anti-Osteopontin (OPN) (Santa Cruz, 1∶1000), anti-Osteoprotegerin (OPG) and anti-GAPDH (Santa Cruz, 1∶2000), antibodies. Immunolabeling was detected using the enhanced chemiluminescence Reagent (Amersham Biosciences).
Statistical analysis
Data on quantitative characteristics are expressed as means ± SD. Data on qualitative characteristics are expressed as percent values or absolute numbers, as indicated. Differences in demographic characteristics and vascular risk factors between patients and controls were compared by using Student's t test or ANOVA for continuous variables and the χ2 test for all categorical variables. To estimate the deviation of frequency of gene alleles in tested population, we performed the Hardy-Weinberg equilibrium using χ2 tests. Genotypes and allele frequencies were compared by χ2 analysis or Fisher's exact test. Multivariate logistic regression analysis was used to determine the influence of HIF-1α polymorphism on CSM, controlling potential confounding conventional risk factors. A forward stepwise (Likelihood Ratio) procedure was used for multivariable analysis. Data were analyzed with the SPSS 16.0 package (Statistical Package for the Social Sciences, version 16.0, SPSS Inc, Chicago, IL, USA). The results were considered statistically significant at P<0.05 using a 2-tailed test.
Results
Table 1 shows the clinical characteristics of CSM patients and controls. There was no significant difference in age, sex and BMI between two groups. However, CSM patients had a significantly higher rate of smoker, family history for spine disorders, Diabetes mellitus (DM) and daily desk work time than controls (all P<0.001).
Table 1. Characteristics of subjects.
| Variables | CSM patients | Controls | P value |
| Age(mean ± SD) | 45.3±4.4 | 45.2±2.5 | 0.853 |
| Gender (Male,%) | 57.4 | 58.1 | 0.654 |
| BMI(mean ± SD) | 23.2±2.3 | 23.1±2.5 | 0.753 |
| Smoker (%) | 35.3 | 20.5 | <0.001 |
| DM | 21.3 | 9.5 | <0.001 |
| Spine disorder family history (%) | 20.5 | 7.8 | <0.001 |
| Desk worktime (hour/d) | 5.5±1.2 | 3.6±0.9 | <0.001 |
| Operation cervical segment number | |||
| 1 | 156 | ||
| 2 | 54 | ||
| 3 | 20 |
Table 2 describes the genotype distributions and allele frequencies of HIF-1α polymorphisms in CSM and control subjects. The genotype frequencies for both polymorphisms were not significantly different from those expected under Hardy–Weinberg equilibrium (all P>0.05). There were no significant difference in the 1772C>T genotypes between CSM patients and controls. For the 1790G>A polymorphism, the CSM patients had a significant higher prevalence of GG genotype than controls (29.13% % vs. 17.96%, P<0.001). To determine the independent risk factor for CSM, we preformed the multivariate logistic regression analysis with the adjustment of age, sex, BMI, smoking status, family history status and daily desk work time. With the 1790AA genotype as reference, our data showed that the 1790GG genotype carriers had a higher risk for CSM development (adjust OR = 2.37, 95%CI: 1.47–3.83, adjusted P<0.001). The 1790G allele also represented a higher risk for CSM (adjusted OR = 1.62, adjusted P<0.001). In contrast, the 1772 C>T polymorphism did not affect the risk for CSM in our study.
Table 2. The genotype and allele frequencies of HIF-1α polymorphism in CSM and control subjects.
| Genotype | CSM (n) | % | Control(n) | % | adjusted OR | 95%CI | adjusted P | |
| 1790AA | 62 | 26.96% | 112 | 39.44% | 1.00 | |||
| 1790GA | 101 | 43.91% | 121 | 42.61% | 1.51 | 1.00 | 2.27 | 0.07 |
| 1790GG | 67 | 29.13% | 51 | 17.96% | 2.37 | 1.47 | 3.83 | <0.001 |
| A | 225 | 48.91% | 345 | 60.74% | 1.00 | |||
| G | 235 | 51.09% | 223 | 39.26% | 1.62 | 1.26 | 2.07 | <0.001 |
| 1772CC | 89 | 20.41% | 84 | 15.79% | 1.00 | |||
| 1772CT | 104 | 23.85% | 146 | 27.44% | 0.67 | 0.46 | 0.99 | 0.18 |
| 1772TT | 37 | 8.49% | 54 | 8.27% | 0.79 | 0.47 | 1.35 | 0.21 |
| C | 442 | 50.69% | 526 | 49.44% | 1.00 | |||
| T | 430 | 49.31% | 538 | 50.56% | 0.95 | 0.80 | 1.14 | 0.76 |
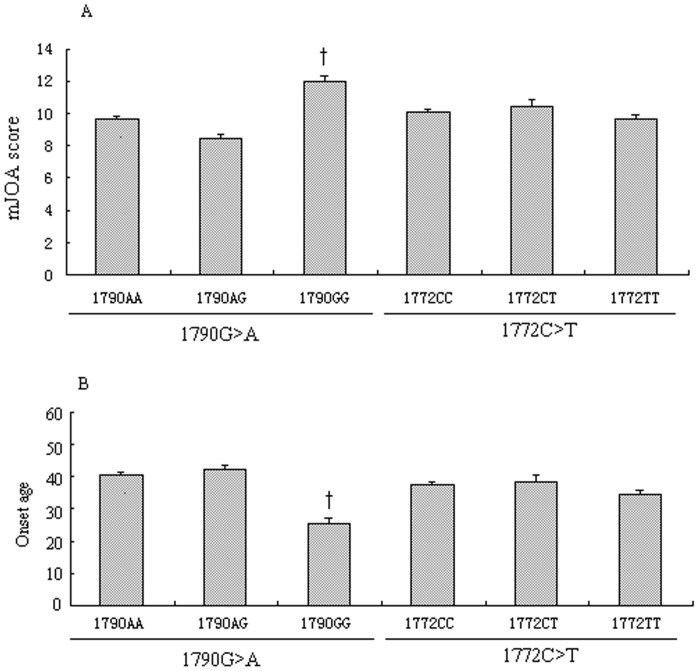
Among all CSM patients, we evaluated the association between the HIF-1α polymorphisms and the clinical features of CSM patients before their surgical treatment. The 1790G>A and 1772C>T did not affect the smoking status, daily desk work time and family history status. However, we found the 1790G>A polymorphism dramatically affects the severity and onset age of CSM patients. The 1790GG patients had higher mJOA score (Figure 1A) and earlier on set age (Figure 1B) than 1790GA and 1790AA carriers (†, P<0.001).
Figure 1. HIF-1α polymorphisms with the clinical features of CSM patients.
Figure 1 shows that the 1790G>A dramatically affects the severity (Figure 1A) and onset age (Figure 1B) of CSM patients. Patients with the 1790GG had a higher mJOA score (Figure 1A) and earlier on set age (Figure 1B) than those with 1790GA and 1790AA genotypes (†, P<0.001).
We next compared the protein expressions of HIF-1α, VEGF, VEGFR and a series of inflammatory factors in disc samples from CSM patients (Figure 2). We found that only the 1790A>G polymorphism significantly affected the above mentioned factor expression levels (Figure 2). The 1790GG genotype carriers had higher levels of HIF-1α, VEGF, VEGFR, IL1, IL6 and NF-kB compared to the 1970AA and 1970AG carriers, but did not affect the OPG and OPN levels (Figure 2). In contrast, the 1772C>T genotype did not influence any of the above mentioned factors expression levels.
Figure 2. The protein expressions of HIF-1α, VEGF, VEGFR and a series of inflammatory factors based on HIF-1α polymorphisms.
Figure 2 shows that only the 1790A>G polymorphism significantly affects the expression level of HIF-1α, VEGF, VEGFR, IL1, IL6 and NF-kB protein expressions compared to 1970AA and 1970AG. The OPG and OPN levels were not changed when stratified by 1790A>G polymorphism (Figure 2). The 1772C>T genotype did not influence the above mentioned factors expression levels.
All CSM subjects receiving ACF treatment were alive and completed the 12 months follow-up. All patients According to the modified JOA scores, 147 patients were attributed into improvement group and 83 into non-improvement groups. Again, we found that the 1790A>G polymorphism distribution were significantly different between the improvement and non-improvement groups. The 1790GG genotype was more prevalent in CSM patients with poor outcome than those with good outcome (Table 3). Multiple logistic regression analysis showed the 1790GG polymorphism was associated with higher risk for a poor outcome (non-improvement) after ACF treatment (adjusted OR = 2.66, adjusted P = 0.019, compared to 1790AA genotype).
Table 3. The effect of genotype distributions and allele frequencies of HIF-1α polymorphisms on the clinical outcome after ACF treatment.
| Genotype | Non-Improvement | Improvement | Adjusted OR | 95%CI | Adjusted P | |||
| 1790AA | 15 | 18.07% | 34 | 23.13% | 1.00 | |||
| 1790AG | 41 | 49.40% | 90 | 61.22% | 1.03 | 0.51 | 2.10 | 0.930 |
| 1790GG | 27 | 32.53% | 23 | 15.65% | 2.66 | 1.17 | 6.06 | 0.019 |
| A | 71 | 42.77% | 158 | 53.74% | 1.00 | |||
| G | 95 | 57.23% | 136 | 46.26% | 1.55 | 1.06 | 2.28 | 0.024 |
| HIF-1α | ||||||||
| Low | 25 | 30.12% | 77 | 52.38% | 1.00 | |||
| High | 58 | 69.88% | 70 | 47.62% | 2.55 | 1.44 | 4.51 | 0.001 |
| VEGF | ||||||||
| Low | 28 | 33.73% | 81 | 55.10% | 1.00 | |||
| High | 55 | 66.27% | 66 | 44.90% | 2.41 | 1.38 | 4.22 | 0.002 |
| VEGFR | ||||||||
| Low | 37 | 44.58% | 95 | 64.63% | 1.00 | |||
| High | 46 | 55.42% | 52 | 35.37% | 2.27 | 1.31 | 3.93 | 0.003 |
Discussion
In the present study, we found that the HIF-1α polymorphism at 1790G>A significantly affects the susceptibility to CSM and is associated with its clinical features in CSM patients, including the severity and the onset age. In addition, the 1790A>G polymorphism also determines the prognosis of CSM patients after ACF treatment. The GG genotype of 1790G>A polymorphism is associated with higher risk to develop CSM, higher severity and earlier onset age. This genotype also presents a higher possibility for a poorer clinical outcome after CAF treatment. Our findings suggest that the HIF-1α polymorphism at 1790G>A may be used as a molecular marker for the CSM.
Hypoxia is a main characteristic of bone diseases like osteonecrosis and osteoarthritis [21], [22] [23]. HIF-1α is the major transcriptional regulator triggered in hypoxia to promote adaptation to the new environment. Under normal oxygen conditions, HIF-1α is continuously produced and destroyed. However, under hypoxic conditions, the expression of HIF-1α is stabilized and translocates to the nucleus where it dimerizes with HIF-1β, thus promots the transcription of its target genes, including VEGF [24], [25], [26] [10].
Several studies have shown that HIF1α plays an important role in growth plate morphogenesis, fracture healing, and distraction osteogenesis [25], [27], [28], [29]. To date, little is know about the association of HIF-1α polymorphism and bone disorders. In a previous study, the HIF1α polymorphism at +45319C>T (the 1772C>T in our study) and several other loci are associated with idiopathic osteonecrosis of the femoral head (ONFH) in Korean men [22], [30], [31], suggesting that HIF1α variations play a role in the pathogenesis of ONFH. However, in our current study, the HIF1α polymorphism at +45319C>T was not associated with the CSM susceptibility in Chinese patients. In contrast, another SNP at locus, 1790A>G was shown to be closely related to the risk, severity, onset age of CSM patients. Also it should be noted that the HIF1α polymorphisms distribution was quite different from Koreans and Chinese based on the genotype distribution data from their study and ours. Our results are consistent with another study in Chinese patients, in which the authors found that the 1790A>G polymorphism affects the risk and severity of lumbar disc degeneration (LDD) [19].
To date, only one study reported that the association of the gene polymorphism of a candidate gene with the clinical outcome of surgical treatment of ACF [10]. Bone morphogenic proteins-4 (BMP-4) polymorphism is associated with the functional improvement from ACF surgery [10]. In our study, we found that the 1790A>G polymorphism determines clinical improvement of CSM patients after ACF treatment. Our findings suggest that the HIF-1α polymorphism at 1790G>A be used as a prognostic marker for the CSM underwent ACF treatment.
In hypoxic condition, the up-regulation of VEGF is consistent with increasing HIF-1α in acute periods. HIF-1α/VEGF signaling pathway is thought to play a dual role following acute spinal cord injury [32], [33]. In the present study, we found that the HIF-1α 1790G>A influences local expression of VEGF and VEGFR in cervical disc tissues. The 1790GG genotype carriers tend to have higher HIF-1α and VEGF expressions, which is consistent with a previous study [19]. In addition, we observed higher expressions of VEGFR, NF-KB, IL1 and IL6. However, the OPN and OPG levels were not affected by 1790A>G polymorphism. We postulate that the 1790A>G polymorphism may affect the local inflammation level in the intervertebral discs among patients with different genotype carriers, thus confers the susceptibility to CSM in these patients.
Some limitations in this study should be addressed. First, this was a single-center based study and only Chinese patients were enrolled. Thus the findings of this study need validation by another duplicate study. Secondly, we did not illustrate the mechanism under which the HIF-1α gene polymorphism affects CSM development.
Data Availability
The authors confirm that, for approved reasons, some access restrictions apply to the data underlying the findings. Data contain patient-identifying information and are unsuitable for public deposition for ethical reasons. Readers may contact Dr. Hua Lu (drhualu@163.com) to request the data.
Funding Statement
The authors have no support or funding to report.
References
- 1. Green C, Butler J, Eustace S, Poynton A, O'Byrne JM (2012) Imaging modalities for cervical spondylotic stenosis and myelopathy. Adv Orthop 2012: 908324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tamburrelli F, Di Lazzaro V, Pola E, Genitiempo M, Pilato F, et al. (2008) Cervical spondylotic myelopathy: proposal of a surveillance algorithm. Eur Rev Med Pharmacol Sci 12: 161–165. [PubMed] [Google Scholar]
- 3. Tracy JA, Bartleson JD (2010) Cervical spondylotic myelopathy. Neurologist 16: 176–187. [DOI] [PubMed] [Google Scholar]
- 4. Oga M, Yuge I, Terada K, Shimizu A, Sugioka Y (1996) Tortuosity of the vertebral artery in patients with cervical spondylotic myelopathy. Risk factor for the vertebral artery injury during anterior cervical decompression. Spine (Phila Pa 1976) 21: 1085–1089. [DOI] [PubMed] [Google Scholar]
- 5. Emery SE (2001) Cervical spondylotic myelopathy: diagnosis and treatment. J Am Acad Orthop Surg 9: 376–388. [DOI] [PubMed] [Google Scholar]
- 6. Sakai Y, Matsuyama Y, Hasegawa Y, Yoshihara H, Nakamura H, et al. (2007) Association of gene polymorphisms with intervertebral disc degeneration and vertebral osteophyte formation. Spine (Phila Pa 1976) 32: 1279–1286. [DOI] [PubMed] [Google Scholar]
- 7. Noponen-Hietala N, Kyllonen E, Mannikko M, Ilkko E, Karppinen J, et al. (2003) Sequence variations in the collagen IX and XI genes are associated with degenerative lumbar spinal stenosis. Ann Rheum Dis 62: 1208–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lu YJ, Wu CS, Li HP, Liu HP, Lu CY, et al. (2010) Aberrant methylation impairs low density lipoprotein receptor-related protein 1B tumor suppressor function in gastric cancer. Genes Chromosomes Cancer 49: 412–424. [DOI] [PubMed] [Google Scholar]
- 9. Setzer M, Vrionis FD, Hermann EJ, Seifert V, Marquardt G (2009) Effect of apolipoprotein E genotype on the outcome after anterior cervical decompression and fusion in patients with cervical spondylotic myelopathy. J Neurosurg Spine 11: 659–666. [DOI] [PubMed] [Google Scholar]
- 10. Wang D, Liu W, Cao Y, Yang L, Liu B, et al. (2013) BMP-4 polymorphisms in the susceptibility of cervical spondylotic myelopathy and its outcome after anterior cervical corpectomy and fusion. Cell Physiol Biochem 32: 210–217. [DOI] [PubMed] [Google Scholar]
- 11. Feng G, Li L, Liu H, Song Y, Huang F, et al. (2013) Hypoxia differentially regulates human nucleus pulposus and annulus fibrosus cell extracellular matrix production in 3D scaffolds. Osteoarthritis Cartilage 21: 582–588. [DOI] [PubMed] [Google Scholar]
- 12. Li H, Liang CZ, Chen QX (2013) Regulatory role of hypoxia inducible factor in the biological behavior of nucleus pulposus cells. Yonsei Med J 54: 807–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fujita N, Chiba K, Shapiro IM, Risbud MV (2012) HIF-1alpha and HIF-2alpha degradation is differentially regulated in nucleus pulposus cells of the intervertebral disc. J Bone Miner Res 27: 401–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Agrawal A, Guttapalli A, Narayan S, Albert TJ, Shapiro IM, et al. (2007) Normoxic stabilization of HIF-1alpha drives glycolytic metabolism and regulates aggrecan gene expression in nucleus pulposus cells of the rat intervertebral disk. Am J Physiol Cell Physiol 293: C621–631. [DOI] [PubMed] [Google Scholar]
- 15. Risbud MV, Guttapalli A, Stokes DG, Hawkins D, Danielson KG, et al. (2006) Nucleus pulposus cells express HIF-1 alpha under normoxic culture conditions: a metabolic adaptation to the intervertebral disc microenvironment. J Cell Biochem 98: 152–159. [DOI] [PubMed] [Google Scholar]
- 16. Fu XS, Choi E, Bubley GJ, Balk SP (2005) Identification of hypoxia-inducible factor-1alpha (HIF-1alpha) polymorphism as a mutation in prostate cancer that prevents normoxia-induced degradation. Prostate 63: 215–221. [DOI] [PubMed] [Google Scholar]
- 17. Vainrib M, Golan M, Amir S, Dang DT, Dang LH, et al. (2012) HIF1A C1772T polymorphism leads to HIF-1alpha mRNA overexpression in prostate cancer patients. Cancer Biol Ther 13: 720–726. [DOI] [PubMed] [Google Scholar]
- 18. Kim TH, Park YJ, Lim JA, Ahn HY, Lee EK, et al. (2012) The association of the BRAF (V600E) mutation with prognostic factors and poor clinical outcome in papillary thyroid cancer: a meta-analysis. Cancer 118: 1764–1773. [DOI] [PubMed] [Google Scholar]
- 19. Lin WP, Wang XJ, Wang CR, Zhang LQ, Li N, et al. (2013) Polymorphism in the hypoxia-inducible factor 1alpha gene may confer susceptibility to LDD in Chinese cohort. PLoS One 8: e73158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Koc RK, Menku A, Akdemir H, Tucer B, Kurtsoy A, et al. (2004) Cervical spondylotic myelopathy and radiculopathy treated by oblique corpectomies without fusion. Neurosurg Rev 27: 252–258. [DOI] [PubMed] [Google Scholar]
- 21. Zhang C, Yang F, Cornelia R, Tang W, Swisher S, et al. (2011) Hypoxia-inducible factor-1 is a positive regulator of Sox9 activity in femoral head osteonecrosis. Bone 48: 507–513. [DOI] [PubMed] [Google Scholar]
- 22. Hong JM, Kim TH, Chae SC, Koo KH, Lee YJ, et al. (2007) Association study of hypoxia inducible factor 1alpha (HIF1alpha) with osteonecrosis of femoral head in a Korean population. Osteoarthritis Cartilage 15: 688–694. [DOI] [PubMed] [Google Scholar]
- 23. Hou X, Hu Z, Huang X, Chen Y, He X, et al. (2014) Serum osteopontin, but not OPN gene polymorphism, is associated with LVH in essential hypertensive patients. J Mol Med (Berl) 92: 487–495. [DOI] [PubMed] [Google Scholar]
- 24.Yi L, Hou X, Zhou J, Xu L, Ouyang Q, et al.. (2014) HIF-1alpha Genetic Variants and Protein Expression Confer the Susceptibility and Prognosis of Gliomas. Neuromolecular Med. [DOI] [PubMed]
- 25. Li L, Zeng H, Hou X, He X, Chen JX (2013) Myocardial injection of apelin-overexpressing bone marrow cells improves cardiac repair via upregulation of Sirt3 after myocardial infarction. PLoS One 8: e71041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hou X, Hu Z, Xu H, Xu J, Zhang S, et al. (2014) Advanced glycation endproducts trigger autophagy in cadiomyocyte via RAGE/PI3K/AKT/mTOR pathway. Cardiovasc Diabetol 13: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schipani E, Ryan HE, Didrickson S, Kobayashi T, Knight M, et al. (2001) Hypoxia in cartilage: HIF-1alpha is essential for chondrocyte growth arrest and survival. Genes Dev 15: 2865–2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Komatsu DE, Hadjiargyrou M (2004) Activation of the transcription factor HIF-1 and its target genes, VEGF, HO-1, iNOS, during fracture repair. Bone 34: 680–688. [DOI] [PubMed] [Google Scholar]
- 29. Mori S, Akagi M, Kikuyama A, Yasuda Y, Hamanishi C (2006) Axial shortening during distraction osteogenesis leads to enhanced bone formation in a rabbit model through the HIF-1alpha/vascular endothelial growth factor system. J Orthop Res 24: 653–663. [DOI] [PubMed] [Google Scholar]
- 30. Jing M, Li B, Hou X, Shoba J, Li C, et al. (2013) OPN gene polymorphism and the serum OPN levels confer the susceptibility and prognosis of ischemic stroke in Chinese patients. Cell Physiol Biochem 32: 1798–1807. [DOI] [PubMed] [Google Scholar]
- 31. Xu HY, Hou XW, Wang LF, Wang NF, Xu J (2010) Association between transforming growth factor beta1 polymorphisms and left ventricle hypertrophy in essential hypertensive subjects. Mol Cell Biochem 335: 13–17. [DOI] [PubMed] [Google Scholar]
- 32.Hou X, Zeng H, He X, Chen JX (2014) Sirt3 is essential for apelin-induced angiogenesis in post-myocardial infarction of diabetes. J Cell Mol Med. [DOI] [PMC free article] [PubMed]
- 33. Long HQ, Li GS, Hu Y, Wen CY, Xie WH (2012) HIF-1alpha/VEGF signaling pathway may play a dual role in secondary pathogenesis of cervical myelopathy. Med Hypotheses 79: 82–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that, for approved reasons, some access restrictions apply to the data underlying the findings. Data contain patient-identifying information and are unsuitable for public deposition for ethical reasons. Readers may contact Dr. Hua Lu (drhualu@163.com) to request the data.