Abstract
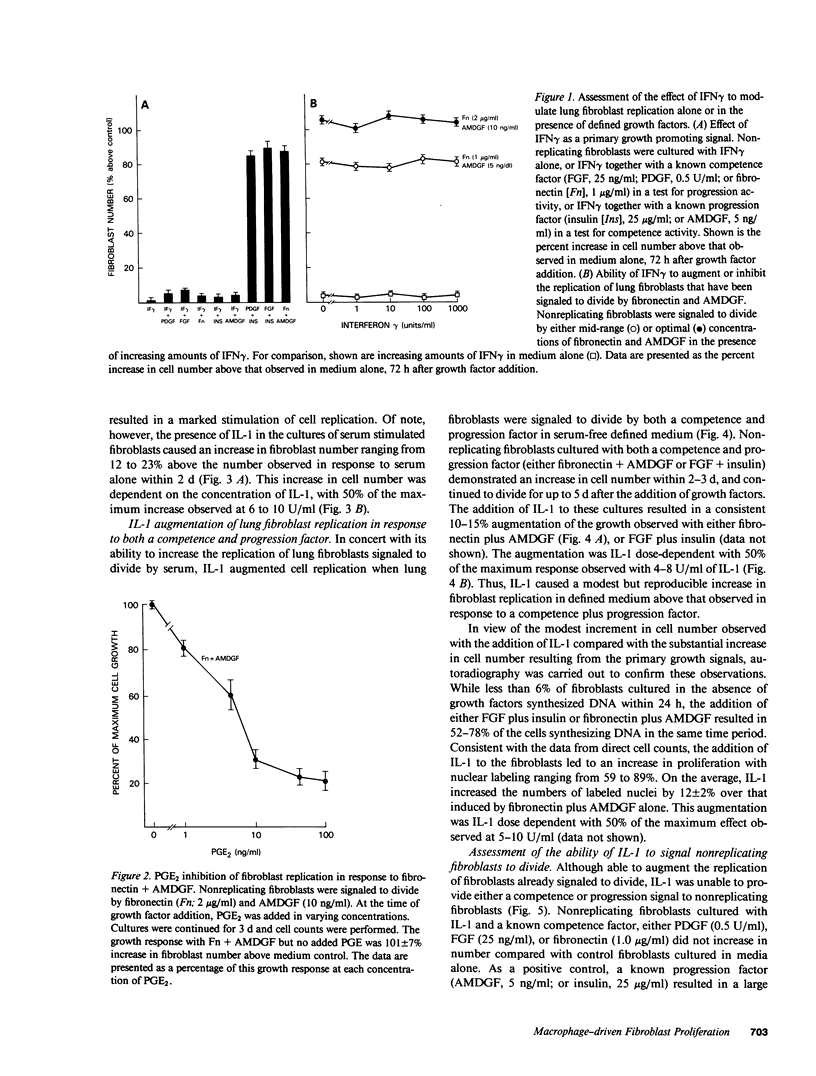
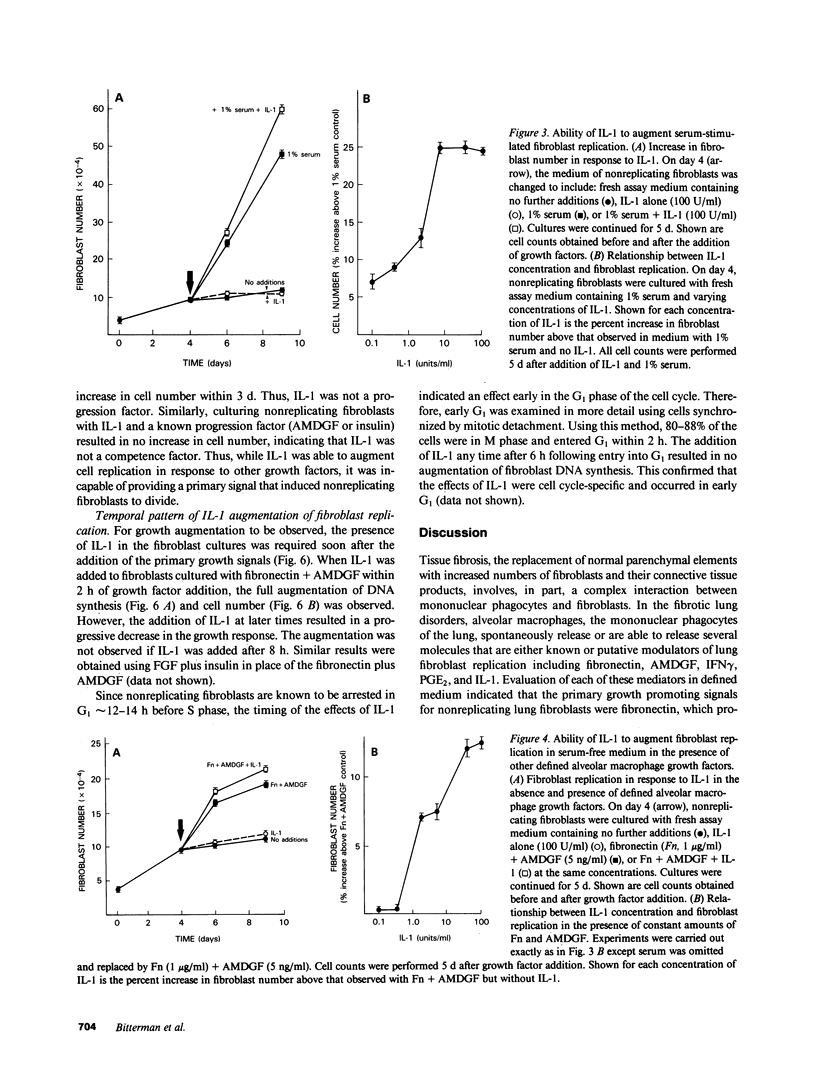
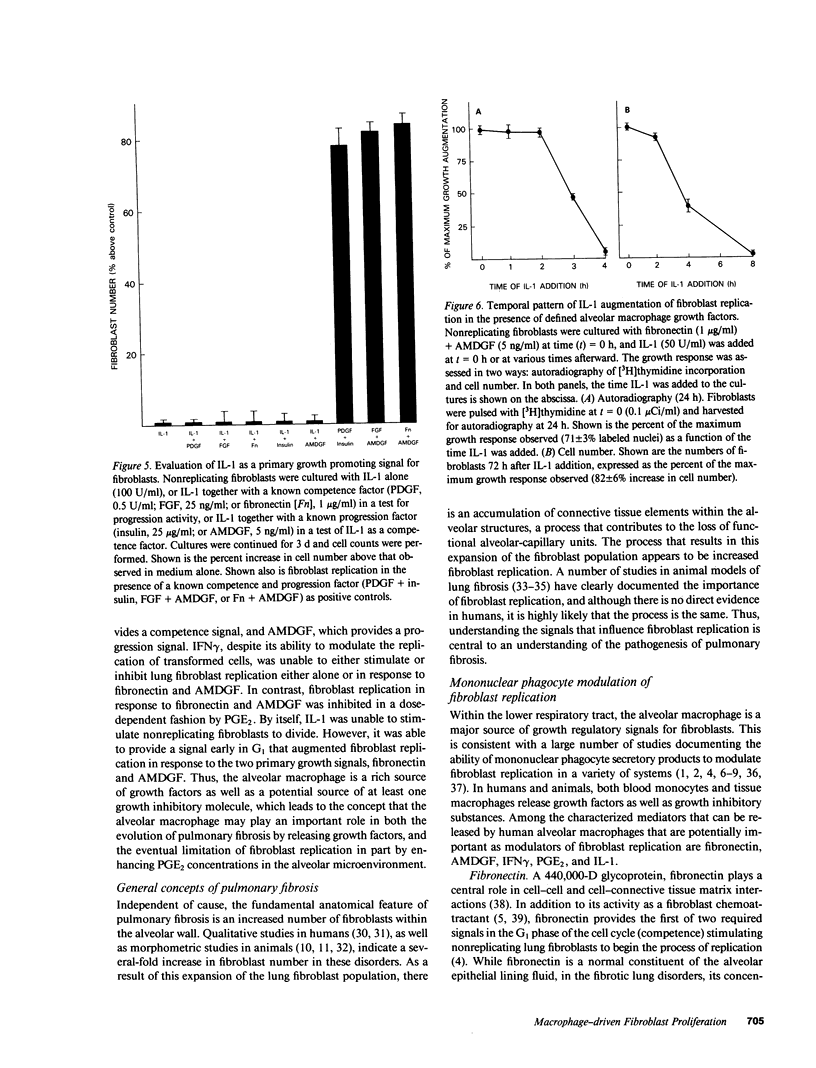
Tissue fibrosis results, in part, from an interaction between growth regulatory molecules released by mononuclear phagocytes and fibroblasts. In the chronic interstitial lung disorders, alveolar macrophages, the mononuclear phagocytes of the lung, are known to spontaneously release two growth factors for fibroblasts, fibronectin and alveolar macrophage-derived growth factor (AMDGF) that together stimulate nonreplicating lung fibroblasts to divide. In addition to these two primary growth promoting signals, alveolar macrophages are able to release other mediators that may have a potential role in modulating lung fibroblast replication in response to these primary signals, including interferon gamma (IFN gamma), prostaglandin E2 (PGE2), and interleukin 1 (IL-1). To evaluate this possibility, we examined the effect of each of these other mediators on lung fibroblast replication in response to fibronectin and AMDGF in serum-free, defined medium. IFN gamma had no effect on fibroblast replication. In contrast, PGE2 resulted in a dose-dependent inhibition of fibroblast replication in response to fibronectin and AMDGF with 50% of the maximum inhibition observed at a PGE2 concentration of less than 10 ng/ml. IL-1, while not active as a primary growth promoting signal, at concentrations of 4-10 U/ml, augmented fibroblast replication in response to fibronectin and AMDGF by 10 to 15%. Temporally, the growth augmenting effect of IL-1 occurred early in the G1 phase of the cell cycle. These data indicate that lung fibroblast replication in response to two of the primary growth promoting signals spontaneously released by alveolar macrophages in the interstitial lung disorders, while uninfluenced by IFN gamma, can be inhibited by PGE2 and modestly augmented by IL-1. Understanding the relevant fibroblast growth modulatory signals within the alveolar microenvironment in the chronic interstitial disorders may lead to rational therapeutic strategies designed to interrupt the fibrotic process.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson I. Y., Bowden D. H., Cote M. G., Witschi H. Lung injury induced by butylated hydroxytoluene: cytodynamic and biochemical studies in mice. Lab Invest. 1977 Jan;36(1):26–32. [PubMed] [Google Scholar]
- Adamson Y. I., Bowden D. H. Pulmonary injury and repair. Organ culture studies of murine lung after oxygen. Arch Pathol Lab Med. 1976 Dec;100(12):640–643. [PubMed] [Google Scholar]
- Bitterman P. B., Adelberg S., Crystal R. G. Mechanisms of pulmonary fibrosis. Spontaneous release of the alveolar macrophage-derived growth factor in the interstitial lung disorders. J Clin Invest. 1983 Nov;72(5):1801–1813. doi: 10.1172/JCI111140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitterman P. B., Rennard S. I., Adelberg S., Crystal R. G. Role of fibronectin as a growth factor for fibroblasts. J Cell Biol. 1983 Dec;97(6):1925–1932. doi: 10.1083/jcb.97.6.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitterman P. B., Rennard S. I., Hunninghake G. W., Crystal R. G. Human alveolar macrophage growth factor for fibroblasts. Regulation and partial characterization. J Clin Invest. 1982 Oct;70(4):806–822. doi: 10.1172/JCI110677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitterman P. B., Saltzman L. E., Adelberg S., Ferrans V. J., Crystal R. G. Alveolar macrophage replication. One mechanism for the expansion of the mononuclear phagocyte population in the chronically inflamed lung. J Clin Invest. 1984 Aug;74(2):460–469. doi: 10.1172/JCI111443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blalock J. E., Georgiades J. A., Langford M. P., Johnson H. M. Purified human immune interferon has more potent anticellular activity than fibroblast or leukocyte interferon. Cell Immunol. 1980 Feb;49(2):390–394. doi: 10.1016/0008-8749(80)90041-6. [DOI] [PubMed] [Google Scholar]
- Brinckerhoff C. E., Guyre P. M. Increased proliferation of human synovial fibroblasts treated with recombinant immune interferon. J Immunol. 1985 May;134(5):3142–3146. [PubMed] [Google Scholar]
- Brody A. R., Craighead J. E. Interstitial associations of cells lining air spaces in human pulmonary fibrosis. Virchows Arch A Pathol Anat Histol. 1976 Nov 22;372(1):39–49. doi: 10.1007/BF00429715. [DOI] [PubMed] [Google Scholar]
- Carrington C. B. Structure and function in sarcoidosis. Ann N Y Acad Sci. 1976;278:265–283. doi: 10.1111/j.1749-6632.1976.tb47038.x. [DOI] [PubMed] [Google Scholar]
- Clark J. G., Kostal K. M., Marino B. A. Modulation of collagen production following bleomycin-induced pulmonary fibrosis in hamsters. Presence of a factor in lung that increases fibroblast prostaglandin E2 and cAMP and suppresses fibroblast proliferation and collagen production. J Biol Chem. 1982 Jul 25;257(14):8098–8105. [PubMed] [Google Scholar]
- Crapo J. D., Peters-Golden M., Marsh-Salin J., Shelburne J. S. Pathologic changes in the lungs of oxygen-adapted rats: a morphometric analysis. Lab Invest. 1978 Dec;39(6):640–653. [PubMed] [Google Scholar]
- Crystal R. G., Bitterman P. B., Rennard S. I., Hance A. J., Keogh B. A. Interstitial lung diseases of unknown cause. Disorders characterized by chronic inflammation of the lower respiratory tract (first of two parts). N Engl J Med. 1984 Jan 19;310(3):154–166. doi: 10.1056/NEJM198401193100304. [DOI] [PubMed] [Google Scholar]
- DeLustro F., Sherer G. K., LeRoy E. C. Human monocyte stimulation of fibroblast growth by a soluble mediator(s). J Reticuloendothel Soc. 1980 Dec;28(6):519–532. [PubMed] [Google Scholar]
- Elias J. A., Zurier R. B., Rossman M. D., Berube M. L., Daniele R. P. Inhibition of lung fibroblast growth by human lung mononuclear cells. Am Rev Respir Dis. 1984 Nov;130(5):810–816. doi: 10.1164/arrd.1984.130.5.810. [DOI] [PubMed] [Google Scholar]
- Engineer D. M., Niederhauser U., Piper P. J., Sirois P. Release of mediators of anaphylaxis: inhibition of prostaglandin synthesis and the modification of release of slow reacting substance of anaphylaxis and histamine. Br J Pharmacol. 1978 Jan;62(1):61–66. doi: 10.1111/j.1476-5381.1978.tb07006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977 Jul 15;20(1):1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- Estes J. E., Pledger W. J., Gillespie G. Y. Macrophage-derived growth factor for fibroblasts and Interleukin-1 are distinct entities. J Leukoc Biol. 1984 Jan;35(1):115–129. doi: 10.1002/jlb.35.1.115. [DOI] [PubMed] [Google Scholar]
- Evans M. J., Bils R. F. Identification of cells labeled with tritiated thymidine in the pulmonary alveolar walls of the mouse. Am Rev Respir Dis. 1969 Sep;100(3):372–378. doi: 10.1164/arrd.1969.100.3.372. [DOI] [PubMed] [Google Scholar]
- Gauss-Müller V., Kleinman H. K., Martin G. R., Schiffmann E. Role of attachment factors and attractants in fibroblast chemotaxis. J Lab Clin Med. 1980 Dec;96(6):1071–1080. [PubMed] [Google Scholar]
- Glenn K. C., Ross R. Human monocyte-derived growth factor(s) for mesenchymal cells: activation of secretion by endotoxin and concanavalin A. Cell. 1981 Sep;25(3):603–615. doi: 10.1016/0092-8674(81)90168-9. [DOI] [PubMed] [Google Scholar]
- Hance A. J., Douches S., Winchester R. J., Ferrans V. J., Crystal R. G. Characterization of mononuclear phagocyte subpopulations in the human lung by using monoclonal antibodies: changes in alveolar macrophage phenotype associated with pulmonary sarcoidosis. J Immunol. 1985 Jan;134(1):284–292. [PubMed] [Google Scholar]
- Haschek W. M., Reiser K. M., Klein-Szanto A. J., Kehrer J. P., Smith L. H., Last J. A., Witschi H. P. Potentiation of butylated hydroxytoluene-induced acute lung damage by oxygen. Cell kinetics and collagen metabolism. Am Rev Respir Dis. 1983 Jan;127(1):28–34. doi: 10.1164/arrd.1983.127.1.28. [DOI] [PubMed] [Google Scholar]
- Hayashi M., Yamada K. M. Differences in domain structures between plasma and cellular fibronectins. J Biol Chem. 1981 Nov 10;256(21):11292–11300. [PubMed] [Google Scholar]
- Hayatdavoudi G., O'Neil J. J., Barry B. E., Freeman B. A., Crapo J. D. Pulmonary injury in rats following continuous exposure to 60% O2 for 7 days. J Appl Physiol Respir Environ Exerc Physiol. 1981 Nov;51(5):1220–1231. doi: 10.1152/jappl.1981.51.5.1220. [DOI] [PubMed] [Google Scholar]
- Humes J. L., Bonney R. J., Pelus L., Dahlgren M. E., Sadowski S. J., Kuehl F. A., Jr, Davies P. Macrophages synthesis and release prostaglandins in response to inflammatory stimuli. Nature. 1977 Sep 8;269(5624):149–151. doi: 10.1038/269149a0. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W. Release of interleukin-1 by alveolar macrophages of patients with active pulmonary sarcoidosis. Am Rev Respir Dis. 1984 Apr;129(4):569–572. [PubMed] [Google Scholar]
- Johnson H. M., Farrar W. L. The role of a gamma interferon-like lymphokine in the activation of T cells for expression of interleukin 2 receptors. Cell Immunol. 1983 Jan;75(1):154–159. doi: 10.1016/0008-8749(83)90314-3. [DOI] [PubMed] [Google Scholar]
- Ko S. D., Page R. C., Narayanan A. S. Fibroblast heterogeneity and prostaglandin regulation of subpopulations. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3429–3432. doi: 10.1073/pnas.74.8.3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn J. H., Halushka P. V., LeRoy E. C. Mononuclear cell modulation of connective tissue function: suppression of fibroblast growth by stimulation of endogenous prostaglandin production. J Clin Invest. 1980 Feb;65(2):543–554. doi: 10.1172/JCI109698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel S. L., Duque R. E. The macrophage adherence phenomenon: its relationship to prostaglandin E2 and superoxide anion production and changes in transmembrane potential. Prostaglandins. 1983 Dec;26(6):893–904. doi: 10.1016/0090-6980(83)90152-1. [DOI] [PubMed] [Google Scholar]
- Lacronique J. G., Rennard S. I., Bitterman P. B., Ozaki T., Crystal R. G. Alveolar macrophages in idiopathic pulmonary fibrosis have glucocorticoid receptors, but glucocorticoid therapy does not suppress alveolar macrophage release of fibronectin and alveolar macrophage derived growth factor. Am Rev Respir Dis. 1984 Sep;130(3):450–456. doi: 10.1164/arrd.1984.130.3.450. [DOI] [PubMed] [Google Scholar]
- Leibovich S. J., Ross R. A macrophage-dependent factor that stimulates the proliferation of fibroblasts in vitro. Am J Pathol. 1976 Sep;84(3):501–514. [PMC free article] [PubMed] [Google Scholar]
- Morley J., Bray M. A., Jones R. W., Nugteren D. H., van Dorp D. A. Prostaglandin and thromboxane production by human and guinea-pig macrophages and leucocytes. Prostaglandins. 1979 May;17(5):729–746. doi: 10.1016/s0090-6980(79)80044-1. [DOI] [PubMed] [Google Scholar]
- Pledger W. J., Stiles C. D., Antoniades H. N., Scher C. D. An ordered sequence of events is required before BALB/c-3T3 cells become committed to DNA synthesis. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2839–2843. doi: 10.1073/pnas.75.6.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennard S. I., Berg R., Martin G. R., Foidart J. M., Robey P. G. Enzyme-linked immunoassay (ELISA) for connective tissue components. Anal Biochem. 1980 May 1;104(1):205–214. doi: 10.1016/0003-2697(80)90300-0. [DOI] [PubMed] [Google Scholar]
- Rennard S. I., Crystal R. G. Fibronectin in human bronchopulmonary lavage fluid. Elevation in patients with interstitial lung disease. J Clin Invest. 1982 Jan;69(1):113–122. doi: 10.1172/JCI110421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennard S. I., Hunninghake G. W., Bitterman P. B., Crystal R. G. Production of fibronectin by the human alveolar macrophage: mechanism for the recruitment of fibroblasts to sites of tissue injury in interstitial lung diseases. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7147–7151. doi: 10.1073/pnas.78.11.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinderknecht E., O'Connor B. H., Rodriguez H. Natural human interferon-gamma. Complete amino acid sequence and determination of sites of glycosylation. J Biol Chem. 1984 Jun 10;259(11):6790–6797. [PubMed] [Google Scholar]
- Robinson B. W., McLemore T. L., Crystal R. G. Gamma interferon is spontaneously released by alveolar macrophages and lung T lymphocytes in patients with pulmonary sarcoidosis. J Clin Invest. 1985 May;75(5):1488–1495. doi: 10.1172/JCI111852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenwasser L. J., Dinarello C. A. Ability of human leukocytic pyrogen to enhance phytohemagglutinin induced murine thymocyte proliferation. Cell Immunol. 1981 Sep 1;63(1):134–142. doi: 10.1016/0008-8749(81)90034-4. [DOI] [PubMed] [Google Scholar]
- Scadding J. G., Hinson K. F. Diffuse fibrosing alveolitis (diffuse interstitial fibrosis of the lungs). Correlation of histology at biopsy with prognosis. Thorax. 1967 Jul;22(4):291–304. doi: 10.1136/thx.22.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J. A., Mizel S. B., Cohen D., Green I. Interleukin 1, a potential regulator of fibroblast proliferation. J Immunol. 1982 May;128(5):2177–2182. [PubMed] [Google Scholar]
- Schmidt J. A., Oliver C. N., Lepe-Zuniga J. L., Green I., Gery I. Silica-stimulated monocytes release fibroblast proliferation factors identical to interleukin 1. A potential role for interleukin 1 in the pathogenesis of silicosis. J Clin Invest. 1984 May;73(5):1462–1472. doi: 10.1172/JCI111350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J. A. Purification and partial biochemical characterization of normal human interleukin 1. J Exp Med. 1984 Sep 1;160(3):772–787. doi: 10.1084/jem.160.3.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer H. Interstitial pneumonia. Annu Rev Med. 1967;18:423–442. doi: 10.1146/annurev.me.18.020167.002231. [DOI] [PubMed] [Google Scholar]
- Taylor L., Polgar P. Self regulation of growth by human diploid fibroblasts via prostaglandin production. FEBS Lett. 1977 Jul 1;79(1):69–72. doi: 10.1016/0014-5793(77)80352-9. [DOI] [PubMed] [Google Scholar]
- Vogel A., Raines E., Kariya B., Rivest M. J., Ross R. Coordinate control of 3T3 cell proliferation by platelet-derived growth factor and plasma components. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2810–2814. doi: 10.1073/pnas.75.6.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wewers M. D., Rennard S. I., Hance A. J., Bitterman P. B., Crystal R. G. Normal human alveolar macrophages obtained by bronchoalveolar lavage have a limited capacity to release interleukin-1. J Clin Invest. 1984 Dec;74(6):2208–2218. doi: 10.1172/JCI111647. [DOI] [PMC free article] [PubMed] [Google Scholar]



