Significance
Rapid evolution on an ecological time scale has been increasingly recognized. Ecological and evolutionary dynamics can be tightly linked and important to predict future dynamics, but there is a significant gap between theoretical predictions and empirical tests, especially on the effects of the nature of genetic variation such as the form of a fitness tradeoff. Using a predator–prey experimental system, we show for the first time to our knowledge that different forms of a fitness tradeoff produce remarkably divergent eco-evolutionary dynamics. A mathematical model supports the observed dynamics. Our results suggest that without knowing the details of genetic variation that is usually variable among wild populations, it is difficult to understand how evolution and ecology interact and what form of eco-evolutionary dynamics results.
Keywords: allele-specific quantitative PCR, Chlorella vulgaris, clonal models, grazing resistance, rapid evolution
Abstract
Evolution on a time scale similar to ecological dynamics has been increasingly recognized for the last three decades. Selection mediated by ecological interactions can change heritable phenotypic variation (i.e., evolution), and evolution of traits, in turn, can affect ecological interactions. Hence, ecological and evolutionary dynamics can be tightly linked and important to predict future dynamics, but our understanding of eco-evolutionary dynamics is still in its infancy and there is a significant gap between theoretical predictions and empirical tests. Empirical studies have demonstrated that the presence of genetic variation can dramatically change ecological dynamics, whereas theoretical studies predict that eco-evolutionary dynamics depend on the details of the genetic variation, such as the form of a tradeoff among genotypes, which can be more important than the presence or absence of the genetic variation. Using a predator–prey (rotifer–algal) experimental system in laboratory microcosms, we studied how different forms of a tradeoff between prey defense and growth affect eco-evolutionary dynamics. Our experimental results show for the first time to our knowledge that different forms of the tradeoff produce remarkably divergent eco-evolutionary dynamics, including near fixation, near extinction, and coexistence of algal genotypes, with quantitatively different population dynamics. A mathematical model, parameterized from completely independent experiments, explains the observed dynamics. The results suggest that knowing the details of heritable trait variation and covariation within a population is essential for understanding how evolution and ecology will interact and what form of eco-evolutionary dynamics will result.
Evolutionary dynamics, changes in intraspecific genotype frequency over generations, can have a time scale similar to that of ecological dynamics (1–3). Selection mediated by ecological interactions causes evolutionary dynamics, and evolution of traits, in turn, changes ecological interactions. Thus, understanding population dynamics needs to take account of the feedbacks between trait evolution and ecological interactions (i.e., eco-evolutionary feedbacks). These feedbacks have increasingly attracted ecologists’ attention since Pimentel (4) proposed genetic feedback as a mechanism regulating animal populations (e.g., refs. 5–11). This integration of evolutionary biology and ecology has important implications in both basic and applied problems in biology (12–17).
Empirical studies have shown that rapid evolution can affect many ecological interactions, including predator–prey (18–20), host–parasite (21), herbivore–plant (22), competitive interactions (23), and interactions with abiotic environments (24–27). Previous empirical studies on eco-evolutionary feedbacks have usually compared the dynamics of populations with and without genetic variation, but recent theoretical models predicted that not only the presence or absence of genetic variation (28–30) but also the form of the evolutionary tradeoff among genotypes is important in generating qualitatively different dynamics (31–35). Indeed, the forms of evolutionary tradeoffs within populations are known to be remarkably variable in plants and microbes (36–38). Thus, there should be various eco-evolutionary dynamics depending on the form of evolutionary tradeoffs existing in wild populations. Nevertheless, to our knowledge, no empirical study has directly demonstrated the theoretically predicted effects of the evolutionary tradeoff on eco-evolutionary dynamics, and it is still unclear how different forms of an evolutionary tradeoff in real organisms can result in different eco-evolutionary dynamics.
Here, using a predator–prey (rotifer–algal) system cultured in continuous flow-through microcosms (chemostats), we examined how different forms of an evolutionary tradeoff between defense and growth in algal prey (Chlorella vulgaris) affect the population dynamics of the predator–prey system and the evolutionary changes in the clonal frequency of the algal prey. Experimental studies using laboratory microcosms have been a powerful approach in exploring eco-evolutionary dynamics and testing theoretical predictions because of the constant environment and simple community structure (39–41). We used two different pairs of algal clones originally obtained from the University of Texas (UTEX) algal collection that showed different forms of a fitness tradeoff between antipredator defense and competitive ability to obtain the resource limiting population growth in the experimental system (inorganic nitrogen). Each pair of algal clones was cultured with an obligately asexual lineage of rotifer predators (Brachionus calyciflorus). Population dynamics of the predators and prey and clonal frequency changes in the algal pair were observed in long-term chemostat runs. We recorded evolutionary dynamics (genotype frequency change) by using an allele-specific quantitative PCR (AsQ-PCR) technique based on microsatellite DNA that allowed us to measure the relative abundance of algal clones (42). We also developed a mathematical model for the experimental system, based on a model of Jones and Ellner (43), parameterized the model using data from separate experiments, and compared the model’s predictions to the observed population and genotype dynamics.
Results
Both pairs of algal clones that we used showed an evolutionary tradeoff between defense against rotifer predation and reproductive ability (Fig. 1). The pair of UTEX1809 and UTEX1811 clones had a relatively “costly defense” tradeoff: Defense is not very effective despite a huge reduction in growth rate. UTEX1809 is a fast-growing but undefended alga, and UTEX1811 is a more defended but slowly growing alga (maximum growth rate, t test, t = 4.992, P < 0.01; defense, t test, t = 3.683, P < 0.05; Fig. 1). The pair of UTEX396 and UTEX265 clones, which was already known to have a tradeoff (42), had a relatively “cheap defense” tradeoff compared with the UTEX1809–1811 pair: Defense is effective, even though the difference in growth rate is small. UTEX396 has higher population growth rate, whereas UTEX265 is more defended against rotifer predation (maximum growth rate, t test, t = 2.138, P < 0.05; palatability, t test, t = 3.338, P < 0.05; Fig. 1). Meyer et al. (42) showed that the rotifers fed on the algal clones unselectively, but the defended clone was defecated in a viable state by rotifers much more frequently than the undefended clone in the UTEX396–265 pair.
Fig. 1.
Different forms of an evolutionary tradeoff between two pairs of algal clones. The pair of UTEX396 and UTEX265 had a cheap defense tradeoff (better defended clone has only slightly lower maximum growth rate) compared with the pair of UTEX1809 and UTEX1811 showing a costly defense tradeoff. Error bars represent SD (n = 3 for palatability, n = 9 for maximum growth rate).
In the costly defense tradeoff pair, population and evolutionary dynamics were similar among the three replicate experiments (Fig. 2). Before rotifers increased in abundance at the beginning of the experiment, the competitive clone (UTEX1809) was dominant in the algal population. As rotifers increased, the defended clone (UTEX1811) became advantageous and increased in frequency, whereas the total abundance of algae declined dramatically. However, the dominance of the defended clone was temporary. The competitive clone eventually increased again and went to near fixation (remarkably dominant in the population), probably because of the high cost of defense in this pair of algal clones (Fig. 1). Meanwhile, rotifer abundance gradually decreased after 30 d, whereas the competitive, undefended clone increased slightly more, which might suggest that the undefended algal clone evolved to be less palatable. Then, rotifer and algal densities stayed almost constant. Note that one of the replicates had to be terminated owing to bacterial contamination of the inflowing fresh medium before reaching equilibrium of rotifers and algae (Fig. 2 E and F).
Fig. 2.
Population dynamics and evolutionary dynamics for the pair showing a costly defense tradeoff (panels in the same row are data from the same run of chemostat). A, C, and E show rotifer and algal population dynamics, corresponding to B, D, and F, respectively, which show the changes in algal clonal frequencies in the same chemostat. The mean and range of dilution rates during the experiments were 0.55 (0.49–0.58) d−1 (A and B), 0.52 (0.49–0.55) d−1 (C and D), and 0.52 (0.50–0.54) d−1 (E and F).
In the cheap defense tradeoff pair, we observed two different types of population and evolutionary dynamics (Fig. 3), both of which were quite different from those with the costly defense tradeoff pair. One type of dynamics was characterized by coexistence of the two algal clones at similar frequency (Fig. 3 B and D) and relatively low abundance of rotifers (Fig. 3 A and C). At the beginning of the experiment, when algal abundance quickly declined as rotifers increased, the algal clonal frequencies fluctuated greatly. This was followed by dampening of the fluctuations to some extent and resulted in the coexistence of the algal clones. Fluctuation of rotifer abundance followed the fluctuations in algal genotype frequency rather than the fluctuations in total algal abundance in Fig. 3 C and D, suggesting the influence of algal clonal frequency on rotifer population growth (Fig. S1). The second type of dynamics with the cheap defense pair was characterized by dominance or near fixation of the defended algal clone (Fig. 3 F and H), probably because of the cheap defense. Rotifer density tended to be higher when the defended clone was selected for, followed by decline of rotifer density as the defended clone continued to be dominant in the algal population (Fig. 3 E–H). This type of dynamics with this pair of algal clones was consistent with previous results for the same pair (42), whereas the first type of dynamics (Fig. 3 A–D) was not observed in the previous study.
Fig. 3.
Population dynamics and evolutionary dynamics for the pair showing a cheap defense tradeoff (panels in the same row are data from the same run of chemostat). A, C, E, and G show rotifer and algal population dynamics, corresponding to B, D, F, and H, respectively, which show the changes in algal clonal frequencies in the same chemostat. The mean and range of dilution rates during the experiments were 0.44 (0.40–0.50) d−1 (A and B), 0.49 (0.46–0.52) d−1 (C and D), 0.48 (0.42–0.56) d−1 (E and F), and 0.49 (0.48–0.51) d−1 (G and H).
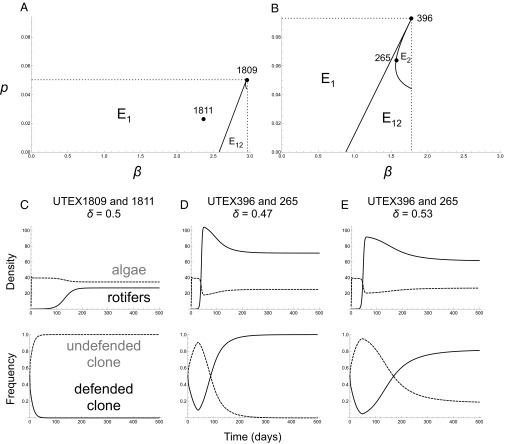
To understand the experimental results, we analyzed a mathematical model based on a model of Jones and Ellner (43). Here we briefly explain the model and results (see SI Text for details). The model describes the population and evolutionary dynamics of the rotifer–algal system cultured in a chemostat as in our experiment. The algal population consists of two clones that have a tradeoff between defense against predation and reproductive ability. The model was parameterized completely from previous and present experimental results separate from the chemostat runs (Table S1), except for the parameter representing algal “palatability” (vulnerability to rotifer predation). Our measured palatability cannot be used directly as the palatability parameter in the model; however, the ratio of measured palatabilities provides an estimate for the relative values of the palatability parameter (see SI Text for details). We therefore assumed that the palatability parameters of the algal strains were proportional to the experimentally measured values (Fig. 1). We calculated the palatability parameters of the four clones so that the relative palatability values were the same in the model as in the observed data. We found a set of palatability parameters (Fig. S2), subject to this constraint, such that the model reproduced the observed population and evolutionary dynamics of both clone pairs (Fig. 4).
Fig. 4.
(A and B) Phase diagram for each pair of algal clones showing different eco-evolutionary dynamics. Parameters p and β are palatability and maximum recruitment rate, respectively, in the mathematical model (SI Text). Black circles represent the estimated parameters based on the experimental results. E1, stable equilibrium with undefended prey; E2, stable equilibrium with defended prey; E12, stable equilibrium with coexisting undefended and defended prey. (C–E) Population and evolutionary dynamics when p11 = 0.055 (SI Text) for the UTEX1809–1811 pair when δ = 0.5 (C) and the UTEX396–265 pair when δ = 0.47 (D) and δ = 0.53 (E). Eco-evolutionary dynamics shown in C, D, and E correspond to E1 in A and E2 and E12 in B, respectively. Solid lines in upper panels, rotifers (individuals per milliliter); dashed lines in upper panels, total algae (105 cells per milliliter); dashed lines in lower panels, frequency of undefended clone; solid lines in lower panels, frequency of defended clone.
With the costly defense tradeoff between UTEX1809 and UTEX1811, the model predicted the fixation of the undefended, competitive clone (UTEX1809) (i.e., competitive exclusion of the defended clone UTEX1811) and equilibrium of rotifer and algal densities (Fig. 4). The fixation of the undefended clone allows the rotifer population to persist, which would go extinct only if the defended clone is present (Fig. S3). With the cheap defense tradeoff between UTEX396 and UTEX265, the tradeoff parameters were very near the border of two different types of dynamics (Fig. 4). One type is the fixation of defended clone (competitive exclusion of undefended clone) and the equilibrium of predators and prey, and the other type is the coexistence of two clones and the equilibrium of predators and prey (Fig. 4). This suggests that an experimental system could display either type of dynamics (as we observed in our experiments with this clone pair), depending on slight changes in conditions such as the chemostat dilution rate (i.e., the rate at which nutrient is continuously added to the chemostat and all components are removed). Thus, the model analysis suggests that the form of the tradeoff is important in determining the resulting eco-evolutionary dynamics. This is supported by the additional analysis of the model assuming the scaled tradeoffs with the same mean trait values and the different forms, showing the consistent results with the model having the original, unscaled tradeoffs (Fig. S4). Also, the model predicts that the system will reach equilibrium irrespective of whether the algal population can evolve or not (Fig. S3). Overall, the model predictions are qualitatively consistent with the experimental data, capturing some quantitative aspects as well (Discussion).
Discussion
Our experimental and theoretical results showed that different forms of an evolutionary tradeoff result in qualitatively different eco-evolutionary dynamics. Although theoretical models have often suggested that the details of evolutionary tradeoffs are important in determining eco-evolutionary dynamics, as was predicted in our predator–prey system (43, 44), empirical studies using real organisms have not tested this prediction so far. Here we show, for the first time to our knowledge, that intraspecific genetic variation within an algal species can be large enough to produce different consequences in eco-evolutionary dynamics as a result of differences in the slope of a tradeoff curve. This confirms that not only the presence or absence of genetic variation but also the actual components of the genetic diversity are important to understand eco-evolutionary feedbacks (45–47). Intraspecific trait variation has been often measured quantitatively, such as the frequency distribution of trait values (14, 24, 48). However, even when the variation of trait values is the same, the form of a tradeoff between different traits can be variable (i.e., the same trait means and variances can be associated with different genetic covariances), and this can result in distinct eco-evolutionary outcomes, as in our study. Thus, measurements of intraspecific trait variation need to include not only the variation of each trait but also the relationships between different traits.
Evolutionary dynamics (clonal frequency changes) were especially different between the two pairs of algal clones showing different forms of tradeoff. For the costly defense tradeoff pair that had relatively large difference in reproductive ability, the palatable, undefended clone became dominant toward the end of the experiment (Fig. 2). In contrast, the defended clone became dominant, or the two clones coexisted with comparable frequencies, for the cheap defense tradeoff pair (Fig. 3). These results make sense because a high cost of defense favors the undefended clone, whereas cheap defense favors the defended clone. This intuitive understanding was supported by the mathematical model that showed the influence of the tradeoff form on eco-evolutionary dynamics (Fig. 4).
Two qualitatively different dynamics were observed for the cheap defense tradeoff pair. The defended clone was dominant when rotifer density was relatively high, whereas the two clones coexisted with comparable frequencies when rotifer density was relatively low. This can be explained by the mathematical model if the cheap defense tradeoff lies at the boundary of the two different dynamics in the phase diagram shown in Fig. 4. Then, which dynamics the predator–prey system takes can depend on the slight change in the dilution rate of chemostat, which influences the pattern of the phase diagram as well (43, 49, 50). Indeed, the dilution rate was slightly different among the replicated runs of chemostats, as a result of small but unavoidable fluctuations in dilution rate over time. The higher rotifer density when the defended clone was dominant than when the two clones coexisted (Fig. 3) would not be intuitively understandable because rotifer density was lower when palatable, undefended clone was more abundant. Our model predicts that the rotifer density is higher when the defended clone is dominant than when the two clones coexist (Fig. 4), suggesting that the higher rotifer density should have selected the defended clone.
An alternative explanation of the different dynamics with the cheap defense tradeoff pair would be a dependence on the initial densities of the algal clones and rotifers. Initial clonal frequencies were slightly different among the replicated chemostats, even though the two clones were inoculated into the chemostats with almost identical densities. If the difference in the initial condition affects the following dynamics, it means that the predator–prey system has a bistability (i.e., there are two locally stable states or attractors). However, our mathematical model did not show the bistability corresponding to the observed dynamics. Stage- or age-structured models often show complex multistability, and our results of the UTEX 396–265 pair may be explained by alternative stable states driven by structured interactions. For example, McCauley et al. (51, 52) demonstrated that small- and large-amplitude cycles coexisted in Daphnia–algal microcosm systems owing to resource-dependent mortality and a dynamic development delay in consumers (Daphnia). However, in our case, consumers are rotifers that do not have as distinct an age structure as daphnids have. Also, previous theoretical studies found that age structure of rotifers (senescence) did not change the dynamics substantially (43, 44). Therefore, the different dynamics with the cheap defense tradeoff pair were likely due to the slight change in the dilution rate, although it remains a challenge for future research to investigate bistability in the predator–prey system (35). It should be noted that the observed different eco-evolutionary dynamics between the two pairs with the different tradeoff forms cannot be explained by the slight change in the dilution rate we had (Fig. S5), although the dilution rate has the significant influence on dynamics.
With respect to eco-evolutionary dynamics, the equilibria of rotifer and algal densities can be seen as qualitatively different depending on the form of the tradeoff. For the costly defense tradeoff, rotifer persistence depends on the evolution of algal prey, in which the palatable clone is selected for and the defended one is selected against (Fig. 4). The defended clone itself cannot support the rotifer population (Fig. S3). However, for the cheap defense tradeoff, the persistence of rotifer population is independent from the algal evolution (Fig. S3).
Our results were in accord with previous studies showing that rapid evolutionary changes can affect the ecological interaction and population dynamics in a predator–prey system (39, 42, 44, 53). Our study provides a previously unidentified insight into the importance of the details of genetic diversity. The details are likely to be very variable owing to intraspecific variation in evolutionary tradeoffs (36–38, 54). Theory predicts that numerous details can greatly affect eco-evolutionary dynamics (55): tradeoffs between defense cost and resource availability (32, 34), interactions between phenotypic plasticity and evolution (33, 56–58), and spatial heterogeneity and gene flow (59, 60). However, empirical studies were lacking. Our experiments demonstrate that the details of genetic diversity can be more important in understanding ecological and evolutionary dynamics in nature than we assumed before. The form of fitness tradeoffs matters.
Materials and Methods
The predator–prey system we used in this study consisted of B. calyciflorus (asexually reproducing rotifer predator) and C. vulgaris (asexually reproducing algal prey), which was the same system used in previous studies (42, 49, 53). Because the original algal strains can be composed of multiple clones (50), we isolated a single clone from each strain for the tradeoff and chemostat experiments described below. The all-algal cultures were kept axenic. To examine the clonal frequency changes in the algal population (i.e., natural selection in the population), we used the AsQ-PCR technique developed by Meyer et al. (42), in which the frequencies of a pair of clones can be quantified by using a microsatellite-DNA marker. Because a pair of algal clones had different microsatellite-DNA sequences, the amount of PCR products amplified from each allele can be used to quantify their frequencies. Note that this method cannot be applied to any arbitrarily chosen pair of clones, but it works for some specific pairs of clones. We used two pairs of clones, UTEX396 and UTEX265, and UTEX1809 and UTEX1811, for each of which the clonal frequency can be accurately quantified by AsQ-PCR, because the correlation between known and estimated frequencies was highly significant (r2 > 0.97 for the UTEX396–265 pair and r2 > 0.98 for the UTEX1809–1811 pair).
Measuring a Tradeoff Between Palatability and Reproductive Ability.
We examined the evolutionary tradeoff for each pair of algal clones. First, we measured the reproductive ability of each clone in the culture medium that was used for the chemostat experiments. The medium was the same as in previous studies (42, 49, 53) and had the limiting nutrient (nitrate) at 80 μmol⋅L−1. We inoculated algal cells of each clone (1 × 104 cells per milliliter) into 50 mL of fresh medium with nine replicates per clone and maintained at 24 °C in continuous light (120 μE⋅m−2⋅s−1). Algal density was monitored daily until population growth saturated. Algal densities exponentially increased from low but observable density to nearly saturation, and we estimated the maximum growth rate as the slope of a linear function fitted to log (algal density) versus time using the data during the exponential growth.
To measure the vulnerability to predation (“palatability”) of algal clones, we inoculated algal cells of each pair of clones (3.5 × 106 cells per milliliter for each clone) into 50 mL of fresh medium with 100 rotifers. To prevent algal growth, the medium lacked nitrate and the culture was kept in darkness. Three replicates for each clone pair were continuously mixed at 1 rpm on a rotary shaker at 24 °C. Algal density was monitored daily, and we used the data during the period of exponential decline. Clonal frequencies for each pair were determined by using AsQ-PCR at the beginning and end of the exponential decline. Three additional replicates without rotifers were used for the control. Mortality rate d was calculated by
| [1] |
where Cend and Cstart were densities of each clone at the end and beginning of the exponential decline (calculated from total algal density and the clone frequencies), respectively, and t is time period of the experiment (days). The palatability of each clone was estimated as the difference between the d with rotifers present and the d with rotifers absent.
Ecological and Evolutionary Dynamics: Chemostat Experiment.
We ran rotifer–algal chemostat experiments following the methods of previous studies (44, 49, 53). Our rotifer population consisted of a strain that reproduced only asexually in the chemostat (40). For the algal population, we used two pairs of algal clones (UTEX396 and UTEX265; UTEX1809 and UTEX1811) that showed the different forms of tradeoff (Results). The culture medium was the same as used for the tradeoff experiment, and the dilution rate of chemostat was 0.5 ± 0.1 per day. Chemostats were held at 24 °C in continuous light (120 μE⋅m−2⋅s−1). The rotifer and algal densities were measured at 1- to 2-d intervals using a microscope and a cell counter (CASY Model TTC; Roche), respectively. We checked bacterial contamination by monitoring the particle size distribution in fresh samples using the cell counter, but no significant sign of bacterial contamination was detected during the experiments. The frequencies of algal clones were determined by AsQ-PCR as described above.
Supplementary Material
Acknowledgments
We thank R. Iwadate, O. Mashita, K. Miura, T. Muranaka, M. Ohue, M. Saido, M. Seki, M. Shinya, K. Suzuki, K. Yokoi, and K. Yoshimura for their assistance with laboratory experiments and S. P. Ellner, N. G. Hairston Jr., B. E. Miner, and members of the Ellner laboratory for their helpful comments on the manuscript. We also thank the editor and the anonymous reviewers for their comments that improved the manuscript. This study is supported by a research fellowship from the Japan Society for the Promotion of Science (JSPS) for young scientists (23-8508) (to M.K.), a JSPS postdoctoral fellowship for research abroad (24-869) (to M.Y.), and the Nakajima Foundation, Japan Science and Technology Agency Precursory Research for Embryonic Science and Technology, JSPS Grants-in-Aid for Scientific Research (Grants 19687002, 20370009, and 26291088) (to T.Y.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. R.M.N. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1406357111/-/DCSupplemental.
References
- 1.Thompson JN. Rapid evolution as an ecological process. Trends Ecol Evol. 1998;13(8):329–332. doi: 10.1016/s0169-5347(98)01378-0. [DOI] [PubMed] [Google Scholar]
- 2.Hendry AP, Kinnison MT. The pace of modern life: Measuring rates of contemporary microevolution. Evolution. 1999;53(6):1637–1653. doi: 10.1111/j.1558-5646.1999.tb04550.x. [DOI] [PubMed] [Google Scholar]
- 3.Hairston NG, Jr, Ellner SP, Geber MA, Yoshida T, Fox JA. Rapid evolution and the convergence of ecological and evolutionary time. Ecol Lett. 2005;8(10):1114–1127. [Google Scholar]
- 4.Pimentel D. Animal population regulation by the genetic feed-back mechanism. Am Nat. 1961;95(881):65–79. [Google Scholar]
- 5.Sinervo B, Svensson E, Comendant T. Density cycles and an offspring quantity and quality game driven by natural selection. Nature. 2000;406(6799):985–988. doi: 10.1038/35023149. [DOI] [PubMed] [Google Scholar]
- 6.Stockwell CA, Hendry AP, Kinnison MT. Contemporary evolution meets conservation biology. Trends Ecol Evol. 2003;18(2):94–101. [Google Scholar]
- 7.Saccheri I, Hanski I. Natural selection and population dynamics. Trends Ecol Evol. 2006;21(6):341–347. doi: 10.1016/j.tree.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 8.Kokko H, López-Sepulcre A. The ecogenetic link between demography and evolution: Can we bridge the gap between theory and data? Ecol Lett. 2007;10(9):773–782. doi: 10.1111/j.1461-0248.2007.01086.x. [DOI] [PubMed] [Google Scholar]
- 9.Lennon JT, Martiny JBH. Rapid evolution buffers ecosystem impacts of viruses in a microbial food web. Ecol Lett. 2008;11(11):1178–1188. doi: 10.1111/j.1461-0248.2008.01225.x. [DOI] [PubMed] [Google Scholar]
- 10.Pelletier F, Garant D, Hendry AP. Eco-evolutionary dynamics. Phil Trans R Soc B. 2009;364(1523):1483–1489. doi: 10.1098/rstb.2009.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Post DM, Palkovacs EP. Eco-evolutionary feedbacks in community and ecosystem ecology: Interactions between the ecological theatre and the evolutionary play. Phil Trans R Soc B. 2009;364(1523):1629–1640. doi: 10.1098/rstb.2009.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson MTJ, Agrawal AA. The ecological play of predator-prey dynamics in an evolutionary theatre. Trends Ecol Evol. 2003;18(11):549–551. [Google Scholar]
- 13.Carroll SP, Hendry AP, Reznick DN, Fox CW. Evolution on ecological time-scales. Funct Ecol. 2007;21(3):387–393. [Google Scholar]
- 14.Hughes AR, Inouye BD, Johnson MTJ, Underwood N, Vellend M. Ecological consequences of genetic diversity. Ecol Lett. 2008;11(6):609–623. doi: 10.1111/j.1461-0248.2008.01179.x. [DOI] [PubMed] [Google Scholar]
- 15.Matthews B, et al. Toward an integration of evolutionary biology and ecosystem science. Ecol Lett. 2011;14(7):690–701. doi: 10.1111/j.1461-0248.2011.01627.x. [DOI] [PubMed] [Google Scholar]
- 16.Schoener TW. The newest synthesis: Understanding the interplay of evolutionary and ecological dynamics. Science. 2011;331(6016):426–429. doi: 10.1126/science.1193954. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez A, Ronce O, Ferriere R, Hochberg ME. Evolutionary rescue: An emerging focus at the intersection between ecology and evolution. Phil Trans R Soc B. 2013;368(1610):20120404. doi: 10.1098/rstb.2012.0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Post DM, Palkovacs EP, Schielke EG, Dodson SI. Intraspecific variation in a predator affects community structure and cascading trophic interactions. Ecology. 2008;89(7):2019–2032. doi: 10.1890/07-1216.1. [DOI] [PubMed] [Google Scholar]
- 19.Palkovacs EP, Post DM. Experimental evidence that phenotypic divergence in predators drives community divergence in prey. Ecology. 2009;90(2):300–305. doi: 10.1890/08-1673.1. [DOI] [PubMed] [Google Scholar]
- 20.terHorst CP, Miller TE, Levitan DR. Evolution of prey in ecological time reduces the effect size of predators in experimental microcosms. Ecology. 2010;91(3):629–636. doi: 10.1890/09-1481.1. [DOI] [PubMed] [Google Scholar]
- 21.Duffy MA, Sivars-Becker L. Rapid evolution and ecological host-parasite dynamics. Ecol Lett. 2007;10(1):44–53. doi: 10.1111/j.1461-0248.2006.00995.x. [DOI] [PubMed] [Google Scholar]
- 22.Turcotte MM, Reznick DN, Hare JD. The impact of rapid evolution on population dynamics in the wild: experimental test of eco-evolutionary dynamics. Ecol Lett. 2011;14(11):1084–1092. doi: 10.1111/j.1461-0248.2011.01676.x. [DOI] [PubMed] [Google Scholar]
- 23.Grant PR, Grant BR. Evolution of character displacement in Darwin’s finches. Science. 2006;313(5784):224–226. doi: 10.1126/science.1128374. [DOI] [PubMed] [Google Scholar]
- 24.Whitham TG, et al. A framework for community and ecosystem genetics: From genes to ecosystems. Nat Rev Genet. 2006;7(7):510–523. doi: 10.1038/nrg1877. [DOI] [PubMed] [Google Scholar]
- 25.Harmon LJ, et al. Evolutionary diversification in stickleback affects ecosystem functioning. Nature. 2009;458(7242):1167–1170. doi: 10.1038/nature07974. [DOI] [PubMed] [Google Scholar]
- 26.Palkovacs EP, et al. Experimental evaluation of evolution and coevolution as agents of ecosystem change in Trinidadian streams. Phil Trans R Soc B. 2009;364(1523):1617–1628. doi: 10.1098/rstb.2009.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bassar RD, et al. Local adaptation in Trinidadian guppies alters ecosystem processes. Proc Natl Acad Sci USA. 2010;107(8):3616–3621. doi: 10.1073/pnas.0908023107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abrams PA, Matsuda H. Prey adaptation as a cause of predator-prey cycles. Evolution. 1997;51(6):1742–1750. doi: 10.1111/j.1558-5646.1997.tb05098.x. [DOI] [PubMed] [Google Scholar]
- 29.Abrams PA. The evolution of predator-prey interactions: Theory and evidence. Annu Rev Ecol Syst. 2000;31:79–105. [Google Scholar]
- 30.Cortez MH, Ellner SP. Understanding rapid evolution in predator–prey interactions using the theory of fast–slow dynamical systems. Am Nat. 2010;176(5):E109–E127. doi: 10.1086/656485. [DOI] [PubMed] [Google Scholar]
- 31.Jones LE, et al. Rapid contemporary evolution and clonal food web dynamics. Phil Trans R Soc B. 2009;364(1523):1579–1591. doi: 10.1098/rstb.2009.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mougi A, Iwasa Y. Evolution towards oscillation or stability in a predator-prey system. Proc R Soc B. 2010;277(1697):3163–3171. doi: 10.1098/rspb.2010.0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamamichi M, Yoshida T, Sasaki A. Comparing the effects of rapid evolution and phenotypic plasticity on predator-prey dynamics. Am Nat. 2011;178(3):287–304. doi: 10.1086/661241. [DOI] [PubMed] [Google Scholar]
- 34.Tien RJ, Ellner SP. Variable cost of prey defense and coevolution in predator-prey systems. Ecol Monogr. 2012;82(4):491–504. [Google Scholar]
- 35.Yamamichi M, Yoshida T, Sasaki A. Timing and propagule size of invasion determine its success by a time-varying threshold of demographic regime shift. Ecology. 2014;95(8):2303–2315. doi: 10.1890/13-1527.1. [DOI] [PubMed] [Google Scholar]
- 36.Koricheva J. Meta-analysis of sources of variation in fitness costs of plant antiherbivore defenses. Ecology. 2002;83(1):176–190. [Google Scholar]
- 37.Gagneux S, et al. The competitive cost of antibiotic resistance in Mycobacterium tuberculosis. Science. 2006;312(5782):1944–1946. doi: 10.1126/science.1124410. [DOI] [PubMed] [Google Scholar]
- 38.Andersson DI, Hughes D. Antibiotic resistance and its cost: Is it possible to reverse resistance? Nat Rev Microbiol. 2010;8(4):260–271. doi: 10.1038/nrmicro2319. [DOI] [PubMed] [Google Scholar]
- 39.Fussmann GF, Ellner SP, Hairston NG., Jr Evolution as a critical component of plankton dynamics. Proc R Soc B. 2003;270(1519):1015–1022. doi: 10.1098/rspb.2003.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Becks L, Ellner SP, Jones LE, Hairston NG., Jr Reduction of adaptive genetic diversity radically alters eco-evolutionary community dynamics. Ecol Lett. 2010;13(8):989–997. doi: 10.1111/j.1461-0248.2010.01490.x. [DOI] [PubMed] [Google Scholar]
- 41.Becks L, Ellner SP, Jones LE, Hairston NG., Jr The functional genomics of an eco-evolutionary feedback loop: Linking gene expression, trait evolution, and community dynamics. Ecol Lett. 2012;15(5):492–501. doi: 10.1111/j.1461-0248.2012.01763.x. [DOI] [PubMed] [Google Scholar]
- 42.Meyer JR, Ellner SP, Hairston NG, Jr, Jones LE, Yoshida T. Prey evolution on the time scale of predator-prey dynamics revealed by allele-specific quantitative PCR. Proc Natl Acad Sci USA. 2006;103(28):10690–10695. doi: 10.1073/pnas.0600434103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jones LE, Ellner SP. Effects of rapid prey evolution on predator-prey cycles. J Math Biol. 2007;55(4):541–573. doi: 10.1007/s00285-007-0094-6. [DOI] [PubMed] [Google Scholar]
- 44.Yoshida T, et al. Cryptic population dynamics: Rapid evolution masks trophic interactions. PLoS Biol. 2007;5(9):e235. doi: 10.1371/journal.pbio.0050235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fussmann GF, Loreau M, Abrams PA. Eco-evolutionary dynamics of communities and ecosystems. Funct Ecol. 2007;21(3):465–477. [Google Scholar]
- 46.Bolnick DI, et al. Why intraspecific trait variation matters in community ecology. Trends Ecol Evol. 2011;26(4):183–192. doi: 10.1016/j.tree.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hersch-Green EI, Turley NE, Johnson MTJ. Community genetics: What have we accomplished and where should we be going? Phil Trans R Soc B. 2011;366(1569):1453–1460. doi: 10.1098/rstb.2010.0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Violle C, et al. The return of the variance: Intraspecific variability in community ecology. Trends Ecol Evol. 2012;27(4):244–252. doi: 10.1016/j.tree.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 49.Fussmann GF, Ellner SP, Shertzer KW, Hairston NG., Jr Crossing the Hopf bifurcation in a live predator-prey system. Science. 2000;290(5495):1358–1360. doi: 10.1126/science.290.5495.1358. [DOI] [PubMed] [Google Scholar]
- 50.Jones LE, Ellner SP. Evolutionary tradeoff and equilibrium in an aquatic predator-prey system. Bull Math Biol. 2004;66(6):1547–1573. doi: 10.1016/j.bulm.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 51.McCauley E, Nisbet RM, Murdoch WW, de Roos AM, Gurney WSC. Large-amplitude cycles of Daphnia and its algal prey in enriched environments. Nature. 1999;402(6762):653–656. [Google Scholar]
- 52.McCauley E, Nelson WA, Nisbet RM. Small-amplitude cycles emerge from stage-structured interactions in Daphnia-algal systems. Nature. 2008;455(7217):1240–1243. doi: 10.1038/nature07220. [DOI] [PubMed] [Google Scholar]
- 53.Yoshida T, Jones LE, Ellner SP, Fussmann GF, Hairston NG., Jr Rapid evolution drives ecological dynamics in a predator-prey system. Nature. 2003;424(6946):303–306. doi: 10.1038/nature01767. [DOI] [PubMed] [Google Scholar]
- 54.Yoshida T, Hairston NG, Jr, Ellner SP. Evolutionary trade-off between defence against grazing and competitive ability in a simple unicellular alga, Chlorella vulgaris. Proc R Soc B. 2004;271(1551):1947–1953. doi: 10.1098/rspb.2004.2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ellner SP. Rapid evolution: From genes to communities, and back again? Funct Ecol. 2013;27(5):1087–1099. [Google Scholar]
- 56.Chevin LM, Lande R, Mace GM. Adaptation, plasticity, and extinction in a changing environment: Towards a predictive theory. PLoS Biol. 2010;8(4):e1000357. doi: 10.1371/journal.pbio.1000357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cortez MH. Comparing the qualitatively different effects rapidly evolving and rapidly induced defences have on predator-prey interactions. Ecol Lett. 2011;14(2):202–209. doi: 10.1111/j.1461-0248.2010.01572.x. [DOI] [PubMed] [Google Scholar]
- 58.Kovach-Orr C, Fussmann GF. Evolutionary and plastic rescue in multitrophic model communities. Phil Trans R Soc B. 2013;368(1610):20120084. doi: 10.1098/rstb.2012.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leibold MA, et al. The metacommunity concept: A framework for multi-scale community ecology. Ecol Lett. 2004;7(7):601–613. [Google Scholar]
- 60.Urban MC, Skelly DK. Evolving metacommunities: Toward an evolutionary perspective on metacommunities. Ecology. 2006;87(7):1616–1626. doi: 10.1890/0012-9658(2006)87[1616:emtaep]2.0.co;2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.