Significance
Biochemical interactions between cells are crucial for regulating cell behavior, and many of these processes are mediated by integral membrane proteins. Fat4 and Dachsous1 cadherins are such proteins, and their interactions at intercellular contacts generate signals that play pivotal roles in the formation of planar cell polarity and proliferation. However, their molecular sizes are extremely large, raising questions of how these molecules fit in narrow intercellular spaces. Our present study reveals a unique compaction mechanism for these molecules. The repetitive Ca2+-binding domains of cadherins are normally important for stabilizing their linear conformation. Strikingly, some of these domains are mutated in Fat4 and Dachsous1, and these changes lead them to bend, thereby enabling them to fit in confined spaces.
Keywords: cadherin superfamily, Fat, Dachsous, cell junctions, electron microscope tomography
Abstract
The cadherins Fat and Dachsous regulate cell polarity and proliferation via their heterophilic interactions at intercellular junctions. Their ectodomains are unusually large because of repetitive extracellular cadherin (EC) domains, which raises the question of how they fit in regular intercellular spaces. Cadherins typically exhibit a linear topology through the binding of Ca2+ to the linker between the EC domains. Our electron-microscopic observations of mammalian Fat4 and Dachsous1 ectodomains, however, revealed that, although their N-terminal regions exhibit a linear configuration, the C-terminal regions are kinked with multiple hairpin-like bends. Notably, certain EC–EC linkers in Fat4 and Dachsous1 lost Ca2+-binding amino acids. When such non–Ca2+-binding linkers were substituted for a normal linker in E-cadherin, the mutant E-cadherins deformed more extensively than the wild-type molecule. To simulate cadherin structures with non–Ca2+-binding linkers, we used an elastic network model and confirmed that bent configurations can be generated by deformation of non–Ca2+-binding linkers. These findings suggest that Fat and Dachsous self-bend due to the loss of Ca2+-binding amino acids from specific EC–EC linkers, and can therefore adapt to confined spaces.
Cell–cell adhesion is achieved through interactions between adhesion receptors, by either homophilic or heterophilic binding with partner molecules. Cadherins are a group of cell–cell adhesion receptors, and the so-called “classical” cadherins are important for general cell–cell adhesion (1). The extracellular domain (ectodomain) of the classical cadherins is divided into five repetitive domains called extracellular cadherin (EC) domains, which are linked by a region containing a unique amino acid sequence called the Ca2+-binding motif (CBM) (2, 3). Binding of Ca2+ to this linker region confers a strand-like morphology on the cadherin molecules, which represents their active form, and this morphology breaks down when Ca2+ is depleted (4–6). The homophilic interactions via the N-terminal EC domains between classic cadherins result in the formation of adherens junctions (AJs), which span intercellular spaces that are ∼20 nm across (2, 3, 7).
The EC domains are conserved in many other molecules grouped under the cadherin superfamily (8). These include desmosomal cadherins, protocadherins, Flamingo/Celsr, Fat, and Dachsous. Curiously, the number of EC domains among these molecules is varied, ranging from 5 to 34. This variation might be related to the functional diversity of cadherin superfamily members. For example, cadherin-23 and protocadherin-15 have 27 and 11 EC domains, respectively, resulting in their large size. Their heterophilic complex organizes the “tip link” to connect a pair of stereocilia (9). The linearly extended configuration of this complex matches the 150- to 200-nm dimensions of the tip links. The size of a cadherin molecule, however, does not always correlate with the size of the intercellular space that holds it. “Fat” is ranked as the largest cadherin molecule among the cadherin superfamily members, with 34 EC domains. It regulates planar cell polarity and cell proliferation via its heterophilic interaction with “Dachsous,” another giant cadherin with 27 EC domains (10–12). Despite their large sizes, the mammalian Fat4 and Dachsous1 have been detected in intercellular spaces that are structurally contiguous to AJs (13). Likewise, mammalian Fat1 localizes at intercellular junctions with a 30- to 45-nm gap (only twice as wide as the AJs) to form glomerular slit diaphragms (14, 15). We questioned which mechanisms allow these large molecules to fit in relatively narrow spaces. Our present studies show that, in contrast with many other cadherins, Fat4 and Dachsous1 cadherins bend at multiple sites of the molecule, so as to adapt to narrow intercellular spaces, and this bending is induced by a loss of Ca2+-binding amino acids in certain EC–EC linkers.
Results
Structures of Purified Fat4 and Dachsous1 Ectodomains.
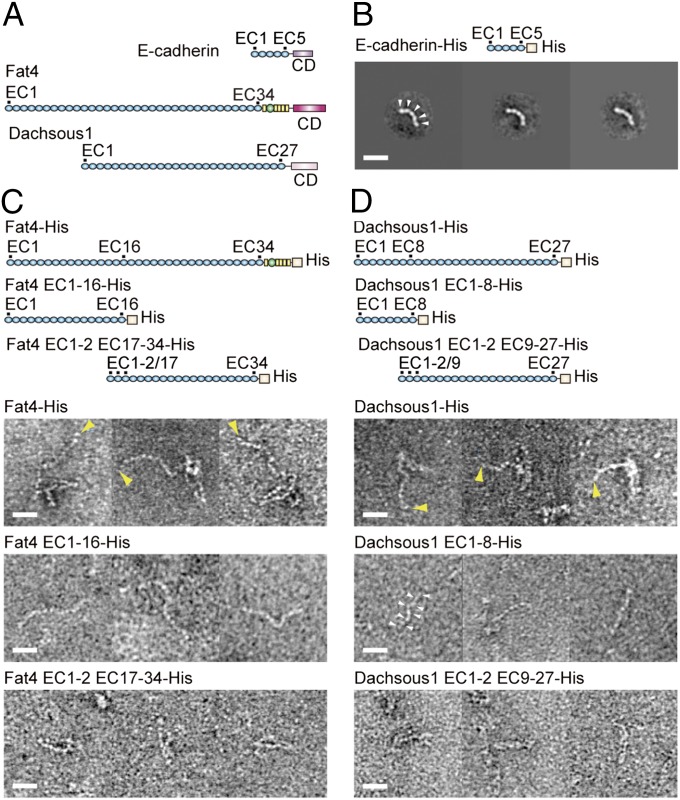
We expressed the entire ectodomain of mammalian Fat4 or Dachsous1 fused with a histidine (His) tag in HEK293 cells, and purified them (Fig. 1 and Fig. S1 A and B). For comparison, we also purified the E-cadherin ectodomain. Transmission electron microscopy (TEM) showed that the E-cadherin ectodomain exhibits a slightly bent, strand-like structure with five EC domains (Fig. 1B), as observed previously (2–4). Similar strand-like structures were also found for Fat4 and Dachsous1. However, the Fat4 and Dachsous1 ectodomains did not simply elongate; instead, portions of them appeared to kink (Fig. 1C, Fat4-His, and Fig. 1D, Dachsous1-His). The Fat4 ectodomain has non-EC domains at the juxtamembrane region. To confirm the possibility that this region might be involved in such a tangled configuration, we prepared a Fat4 ectodomain lacking this region. This protein still kinked, however (Fig. S1C).
Fig. 1.
Fat4 and Dachsous1 ectodomains in comparison with E-cadherin. (A) Schematic drawings of E-cadherin, Fat4, and Dachsous1. Blue spheres indicate EC domains. Yellow boxes and green spheres represent EGF-like and laminin A-G domains, respectively. CDs indicate cytoplasmic domains. (B) Representative class averages of His-tagged E-cadherin ectodomain. (C and D) Images of His-tagged, full-length Fat4 (Fat4-His) and Dachsous1 (Dachsous1-His) ectodomains, and their truncated mutants. Fat4 EC1-16-His and Dacsous1 EC1-8-His represent a N-terminal fragment of the respective molecule, comprising the EC domains indicated. Fat4 EC1-2 EC17-34-His and Dacsous1 EC1-2 EC9-27-His represent a fusion of EC1 and EC2 with the C-terminal fragment of the respective molecule that comprises EC17 to 34 for Fat4, and EC9 to 27 for Dachsous1. Three representative images are shown for each construct. White arrowheads point to examples of individual EC domains. Yellow arrowheads indicate the putative terminus of the extended portion of the molecule. (Scale bars, 20 nm.)
Despite the overall nonstraight appearance of Fat4 and Dachsous1 ectodomains, one end of each molecule always linearly extended from the compacted portion. To determine which side of the protein is linear, we split each of the Fat4 and Dachsous1 ectodomains into N- and C-terminal fragments (Fig. 1 C and D). In obtaining the C-terminal fragments, we attached the EC1 and 2, plus the signal peptides, to the N terminus of its C-terminal fragment to ensure protein secretion. In TEM images of these truncated proteins, the N-terminal fragments exhibited a linear appearance in both Fat4 and Dachsous1. Individual EC domains were identifiable as bead-like units in most specimens. The C-terminal fragments, on the other hand, displayed a complex configuration. In the case of Fat4, they exhibited a structure comprising straight sections with hairpin-like bend points. The Dachsous1 C-terminal fragments displayed a similar but more tightly packed appearance, suggesting a sharper bending of the strand at a single or multiple point(s). These observations suggest that, in both Fat4 and Dachsous1, the N-terminal side extends linearly, whereas the C-terminal side contains hairpin-like bending points, causing its kinked appearance.
Modifications of the CBM in Fat4 and Dachsous1.
Crystallographic analysis of Drosophila neural cadherin showed that its EC1–EC4 region (abbreviated as EC1-4 hereafter) adopts a V-shaped structure, due to loss of the conserved CBM from the EC2-3 linker (6). Bioinformatics analysis performed in this study also predicted the presence of the linkers that are unable to bind Ca2+ (non–Ca2+-binding linkers) in the Fat and Dachsous proteins. Because such mutated linkers could be responsible for the bent configuration of Fat4 and Dachsous1, we reanalyzed their amino acid sequences using a different method: We compared them with the E-cadherin EC1-2 linker sequences that contain 10 Ca2+-binding amino acid residues to coordinate with three Ca2+ ions (2, 3) (Fig. S2 A–C). We identified the 10 residues in Fat4 and Dachsous1 as those involved in Ca2+ binding, of which D, E, and N are most highly conserved. At the level of individual CBMs, however, the sequences were modified particularly at the linkers between EC15-16 and EC24-25 of Fat4, and between EC7-8, EC9-10, EC12-13, and EC19-20 of Dachsous1 (Fig. S2 D and E), consistent with previous findings (6). Such loss of conservation was not observed in E-cadherin. For comparison, we also analyzed cadherin-23 and protocadherin-15, both of which exhibit a straight configuration (9). All of their linker regions had a conserved CBM (Fig. S2 F and G).
Mutational Analysis of Deformed Cadherin Structures.
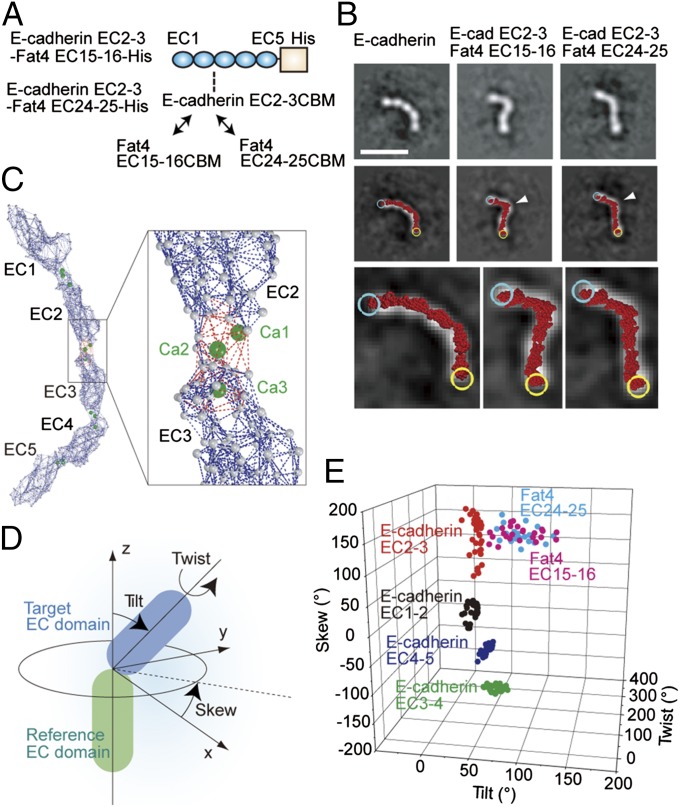
To test whether the modified CBMs are responsible for the unique appearance of Fat4 and Dachsous1, we prepared two mutants of the E-cadherin ectodomain in which the CBM of E-cadherin EC2-3 was replaced with that of Fat4 EC15-16 or EC24-25 (Fig. 2A and Fig. S3A). In TEM images, the mutated E-cadherins appeared to bend more sharply than wild-type E-cadherin (Fig. 2B). To confirm the shape changes, we observed their behavior in gel filtration, finding a clear peak shift of the mutant E-cadherins toward slowed elution (Fig. S3B). For comparison, we also eluted the wild-type E-cadherin in Ca2+-free buffer and found that its elution was markedly slower. These results verify that Ca2+ binding to the cadherin ectodomain is critical for its shape determination.
Fig. 2.
Fat4-derived CBMs cause deformation of E-cadherin ectodomain. (A) Schematic drawing of the mutated E-cadherin ectodomain in which the CBM of EC2-3 is replaced with that of Fat4 EC15-16 or Fat4 EC24-25. (B) Representative views of the wild-type and CBM-substituted E-cadherin (Top) in class averages, and atomic model fitting (Middle). The molecular images were overlaid with fitted atomic models (red), which are enlarged to show individual amino acid residues at the bottom. Cyan and yellow circles represent the N and C termini of the atomic models, respectively. White arrows point to the position where CBMs were replaced. (Scale bar, 20 nm.) (C) Elastic network model of E-cadherin ectodomain. Gray balls represent amino acid residues. Dark blue and red dotted lines indicate the springs connecting amino acid residues. The red dotted lines were eliminated in the non–Ca2+-binding EC–EC linkers. (D) Geometric definition of a target EC domain against the reference EC domain. (E) Scattergram of EC–EC linkage geometries in wild-type and mutated E-cadherins.
To quantitatively evaluate the shape changes in CBM-substituted E-cadherin, we constructed an elastic network model (16, 17) from the X-ray crystal structure of E-cadherin (see Materials and Methods for details), as done previously (18). In the model, the interactions between two neighboring amino acid residues are represented by a “spring” (Fig. 2C). At the Ca2+-bound EC–EC linkers, the existence of Ca2+ is expected to induce additional interactions between residues, and these potential interactions were included in the model, as shown with red broken lines in Fig. 2C. In modeling CBM-substituted E-cadherin ectodomains, the Ca2+-binding–dependent springs were eliminated from the mutated EC–EC linkers. To generate cadherin atomic models with varying degrees of deformation, the elastic network model was deformed along the lowest-frequency normal modes. These atomic models were fitted over each TEM image of E-cadherins, and the one with the highest fitting score was chosen as the atomic model of best fit (Fig. 2B, Middle and Bottom, and Fig. S3 C–E). We then quantified the geometry of the EC–EC linkages in the atomic models (Fig. 2 D and E). The EC–EC linkers with a Fat4 EC15-16 or 24–25 CBMs were more diversely deformed than those found in wild-type E-cadherin. These findings are in accord with the hypothesis that CBM modifications control the EC–EC linkage morphology.
Building Molecular Models of Fat4 and Dachsous1 Ectodomain.
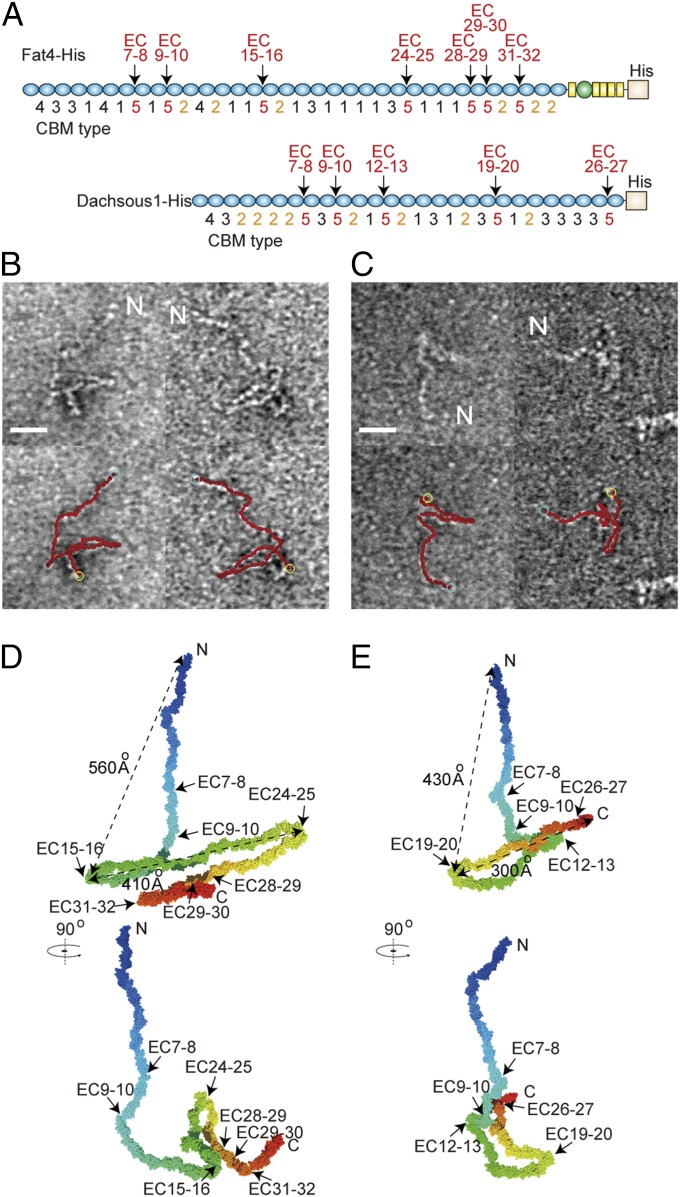
Then, we investigated whether Fat4 and Dachsous1 have any defined structures. We performed classification and averaging analysis using TEM images of each molecule, as done for E-cadherin mutants. However, we failed to identify defined structures from the TEM images due to their heterogeneity (Fig. S4 A–D). Therefore, we decided to construct their atomic models based on the dynamics of the elastic network model, as we could successfully predict the configuration of E-cadherin mutants (Fig. 2). The atomic models were built by linking the X-ray crystal structures of the E-cadherin EC2 domain, because the X-ray crystal structures of Fat4 and Dachsous1 have not yet been resolved. To cover the variations in CBMs across the entire length of the molecule, we classified the CBMs into five types: Those with three or more amino acid substitutions in the consensus sequences or with other amino acid substitutions that could suppress Ca2+ binding were defined as type 5, and other CBMs were defined as types 1–4, based on their amino acid variations at the EC–EC linkages of multiple classical cadherins (Table S1). All of the CBMs of Fat4 and Dachsous1 were thus classified (Fig. 3A and Tables S2 and S3). In the atomic models, the EC–EC linkages with CBM type 1, 3, or 4 were restrained to a single fixed geometry, and those with CBM type 2 were given several geometries, as shown in Table S4. The linkages with CBM type 5 were allowed to take a wide range of geometries (see Materials and Methods for details). These atomic models were fitted over each of 25 TEM images of a Fat4 or Dachsous1 ectodomain without defining the C and N terminus, and the models of best fit were chosen for each image (Fig. 3 B and C). The best fit was found when the N terminus was assigned to the end of the linear portion of the molecule, whereas when the N to C terminus polarity was reversed, fitting efficiency decreased (Fig. S1 D and E). This result is consistent with the experimental finding that the linear portion of the molecules corresponds to the N-terminal region.
Fig. 3.
Reconstitution of atomic models of Fat4 and Dachsous1. (A) Typing of CBMs in Fat4 and Dachsous1. CBM types 1–4 were defined according to the classification in Tables S1–S3. CBM type 5 corresponds to the EC–EC linkers with modified Ca2+-binding abilities. (B and C) Fitting of atomic models to TEM image of Fat4 (B) and Dachsous1 (C). TEM images of the molecule were overlaid with the best-fit atomic models shown in red. Out of 25 images analyzed for each molecule, two representative images were chosen, which are identical to those used in Fig. 1 C and D. Cyan and yellow circles indicate N and C termini of the models, respectively. “N” represents the putative N terminus of the molecule, inferred from its distal position of the linear strand. (Scale bar, 20 nm.) (D and E) Representative atomic models of Fat4 (D) and Dachsous1 (E). The models were drawn as representatives of the structures obtained through the analysis of 25 TEM images for each molecule.
Analysis of the geometry of EC–EC linkages in the atomic models indicated that the CBM-modified EC–EC linkers in Fat4 and Dachsous1 always display certain levels of deformation (Fig. S4 E–J). They were more diversely disordered than the native EC1-2 of E-cadherin, and Fat EC15-16 and EC24-25 in these models were more deformable than those introduced into E-cadherin (compare Fig. 2E and Fig. S4E), probably due to differences in the neighboring structures between the models. The CBM modifications clearly affected the tilt angles; the angles were particularly high at EC24-25 of Fat4 and EC19-20 of Dachsous1. However, the tilting occurred only within limited degrees. Especially, the tilt angles at these two sites were less variable compared with those in other modified EC–EC linkers (Fig. S4 H and I, Tilt). In addition, the CBM modifications did not significantly alter the twist and skew angles at any modified linkers. These analyses confirmed that CBM-modified EC–EC linkers do deform, but they also suggested that their shapes would fluctuate in a nonrandom fashion. Thus, we conclude that the overall shapes of Fat4 and Dachsous1 may vary but within a restricted range. From the best-fit models thus analyzed, we reconstituted a representative structure of Fat4 and Dachsous1 (Fig. 3 D and E).
Heterophilic Interactions Between Fat4 and Dachsous1 via Their N-Terminal EC Domains.
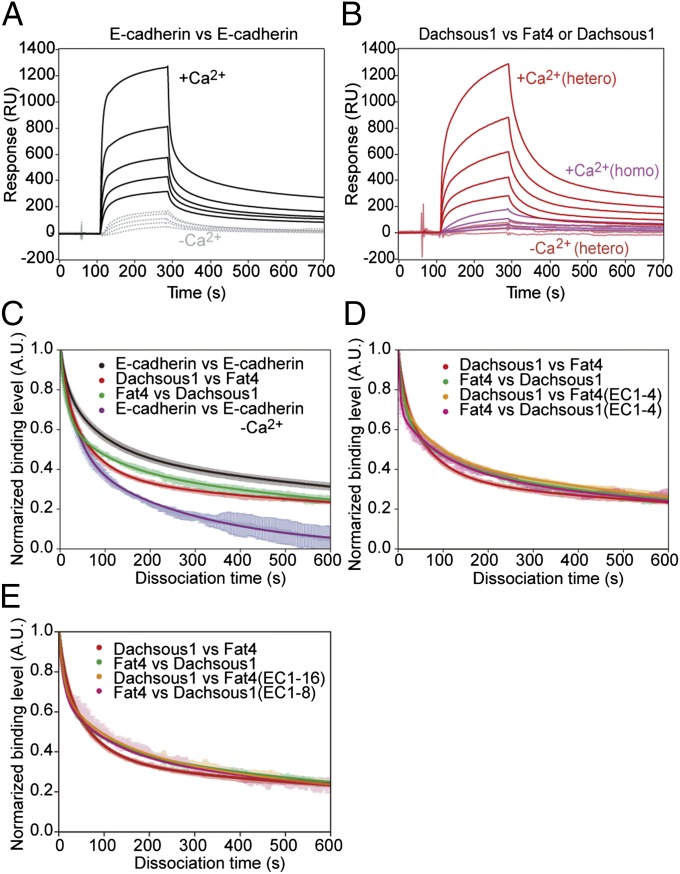
To determine whether the interaction between Fat4 and Dachsous1 occurs via their extended N-terminal regions, we used surface plasmon resonance (SPR) analysis. As a technical control, we first measured the homophilic interactions between E-cadherin ectodomains, confirming their Ca2+ dependence (Fig. 4A). We also confirmed that full-length Fat4 ectodomains interacted with full-length Dachsous1 ectodomains in a Ca2+-dependent manner and that their heterophilic binding effectively exceeded the homophilic binding between Dachsous molecules (Fig. 4B). Next, we prepared N-terminal fragments of Fat4 and Dachsous1, Fat4 EC1-4, Fat4 EC1-16, Dachsous1 EC1-4, and Dachsous1 EC1-8 (Fig. S1 A and B). To evaluate the binding properties of these fragments, we measured the dissociation processes, because the association processes are difficult to analyze due to the self-association of the molecules as well as the presence of multiple bound states of cadherins (19, 20). The dissociation process of E-cadherin matched well with the multiple exponential fits as previously observed (20), and this was also the case for Dachsous1 and Fat4 (Fig. 4C and Fig. S5 A–C). The interactions between full-length Dachsous1 ectodomains and Fat4 EC1-4, and between full-length Fat4 ectodomains and Dachsous1 EC1-4, exhibited similar dissociation profiles and lifetimes to those between the full-length Fat4 and Dachsous1 ectodomains (Fig. 4D and Table S5). Longer fragments, Fat4 EC1-16 and Dachsous1 EC1-8, also showed similar dissociation profiles (Fig. 4E and Table S5). These observations suggest that the four N-terminal EC domains are sufficient for the heterophilic binding of Fat4 and Dachsous1.
Fig. 4.
Heterophilic interactions between Fat4 and Dachsous1 via their N-terminal domains. (A and B) Surface plasmon resonance (SPR) analysis of the binding between E-cadherin ectodomains (A) or between Dachsous1 and Fat4 ectodomains (B, hetero) in the presence or absence of Ca2+. In B, the binding between Dachsous1 and Dachsous1 (homo) was also assayed. (C) Dissociation processes of E-cadherin from E-cadherin, Fat4 from Dachsous1, and Dachsous1 from Fat4. E-cadherin was analyzed also in the absence of Ca2+. (D and E) Comparisons between the dissociation processes of Fat4 from Dachsous1 (and vice versa), Dachsous1 fragments from Fat4, and Fat4 fragments from Dachsous1. Two fragments of Fat4, EC1-4 and EC1-16, and those of Dachsous1, EC1-4 and EC1-8, were used. In all of the molecular pairs shown by “vs.,” the one indicated at the left was adsorbed to the assay substrate, and that at the right was dissolved in the reaction solution.
Electron Tomographic Analysis of Cell–Cell Contacts Formed by Fat4 and Dachsous1.
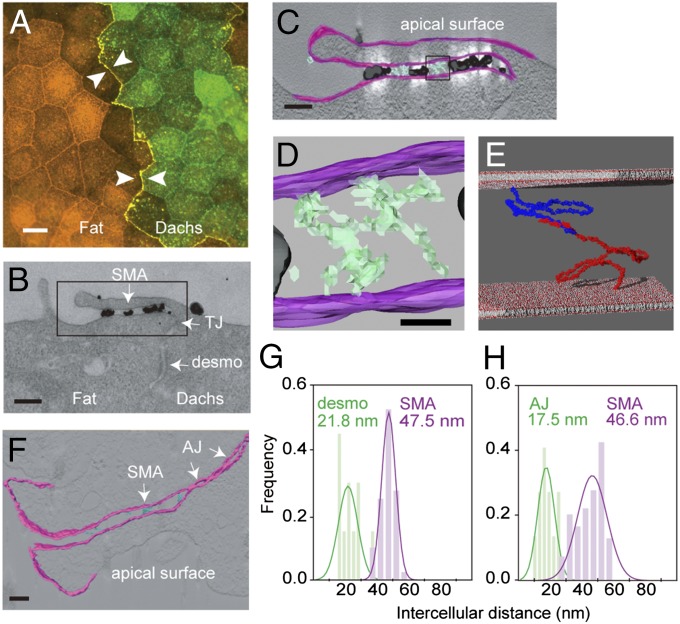
To visualize Fat4 and Dachsous1 in vivo, we prepared MDCK cells transfected with Fat4 or Dachsous1 full-length cDNAs, and cocultured them. As reported previously (13), the two molecules accumulated most intensely at the heterotypic boundaries between the transfectants (Fig. 5A). Then, we treated the mixed cultures with antibodies specific to the Dachsous1 ectodomain and visualized them with secondary antibodies conjugated with colloidal gold. TEM examination of heterotypic cell boundaries detected gold particles from cell–cell contact structures formed directly above the tight junction (Fig. 5B). These Dachsous1-positive contacts, which we named “subapical membrane apposition” (SMA), measured 47.5 nm across on average, a little wider than a desmosome with a 21- to 22-nm intercellular gap (Fig. 5G). Tomographic images of these TEM sections detected strand-like structures at the intercellular spaces, which were likely Fat4–Dachsous1 complexes that did not react with the antibodies (cyan in Fig. 5 C and D). Intriguingly, atomic models of Fat4 and Dachsous1, which were arbitrarily arranged to form a heterophilic pair via their N-terminal regions, could fit in a 40- to 50-nm intercellular space (Fig. 5E). Untransfected MDCK cells did not show any junctional structures at the corresponding regions. In addition, we observed neuroepithelial SMA in the mouse embryonic brain cortex, in which Fat4 and Dachsous1 localize (13), as well as the AJs located below the SMA. Tomographic analysis showed that the SMA, which again contained strand-like materials, measured 46.6 nm across on average (Fig. 5 F and H).
Fig. 5.
Electron microscopy of cell–cell contacts formed by Fat4 and Dachsous1. (A) Double immunostaining for Fat4 (red) and Dachsous1 (green) in a mixed culture of MDCK cells stably transfected with respective cDNAs. Arrows indicate a boundary between the two transfectants. (B) TEM image of a heterotypic boundary between MDCK cells expressing Dachsous1 (Right) and Fat4 (Left). Black dots represent colloidal gold particles conjugated to anti-Dachsous1 antibodies. The Dachsous1-expressing cell is positioned at the Right, which is identified by the presence of immunogolds on the free cell surface. (Scale bar, 200 nm.) (C) Tomographic visualization of the area boxed in B. Intercellular structures are colored light cyan. (Scale bar, 100 nm.) (D) A close-up view of the area boxed in C. Strand-like structures are detected in the intercellular space. (Scale bar, 20 nm.) (E) Atomic models of Fat4 (in red) and Dachsous1 (in dark blue), chosen from their best-fit models, are arranged to touch one another via their N-terminal four EC domains, and placed in an intercellular space that is 47.5 nm across. (F) Electron-tomographic visualization of the apical cell–cell contacts in neuroepithelial cells of the embryonic cerebral cortex. Strand-like materials visible in the intercellular space are colored cyan. (Scale bar, 100 nm.) (G and H) Quantification of the distances between the cell membranes at the subapical membrane apposition (SAM) formed by Fat4 and Dachsous1 transfectants (G), and those in neuroepithelial cells of the mouse embryonic brain cortex (H). In the latter, the intermembrane distances were measured at the sites where strand-like structures were detectable, and also at neighboring regions. The desmosomes and adherens junctions were also analyzed. AJ, adherens junctions; Dachs, Dachsous1; desmo, desmosome; Fat, Fat4; TJ, tight junction. Cell membranes are colored magenta in tomographic images.
Discussion
All of our findings support the idea that Fat4 and Dachsous1 can fit into a given intercellular space due to their bending at EC–EC linkers that are unable to bind Ca2+. Atomic models built from the EM images gave theoretical evidence that the non–Ca2+-binding linkers are deformable, enabling these molecules to bend. The intermembrane distances in the Fat4/Dachsous1-bearing junctions are probably autonomously determined based on the dimensions of the Fat4/Dachsous1 heterophilic complexes, because the 47.5-nm junctions emerged de novo as a result of exogenous expression of Fat4 and Dachsous1 in MDCK cells. Likewise, in neuroepithelial cells, the 46.6-nm SMA developed depending on the expression of Fat4 (13). Formation of junctions with such fixed widths suggests that Fat4 and Dachsous1 may have a confined conformation in vivo, despite their deformable non–Ca2+-binding EC–EC linkers. Whether such restricted conformation is regulated solely by a limited range of deformation of non–Ca2+-binding linkers, or by other mechanisms such as long-range interactions between different domains of the molecule, remains to be elucidated.
Mammals have four Fat cadherins, Fat1 to 4, and Drosophila have two, Fat and Fat-like. Among these, Fat4 is thought to be the ortholog of Drosophila Fat (21). Intriguingly, the positions of non–Ca2+-binding linkers are well conserved between the interspecies orthologs of Fat4, and this is also the case for Dachsous1 (6). This implies that the molecular shape revealed here has some relevance to the evolutionarily preserved functions of Fat and Dachsous cadherins. In addition to Fat and Dachsous, even the molecules categorized as classical cadherins, which are responsible for AJ formation, generally exhibit large sizes in invertebrate species (22). Our results provide a clue to the structural and evolutionary significance of such unusually sized cadherin superfamily members in cell–cell interactions.
Materials and Methods
Expression and Purification of His-Tagged Proteins.
HEK293 cells stably expressing ectodomains were cultured, and the culture medium containing secreted ectodomains was collected and subjected to gel chromatography with anti-6× Histidine tag monoclonal antibody, 2D8 (MBL). His-tagged proteins were eluted with excess 6× Histidine peptides (Wako) and subjected to gel filtration for exchanging the buffer to HBSS using TSKgel G4000SW columns (TOSOH). Purified proteins were separated on 2–15% (wt/vol) gradient SDS/PAGE gels (Cosmo Bio) and analyzed by CBB staining and Western blotting with anti-6× Histidine tag monoclonal antibody, 9F2 (Wako).
Animals.
Animal experiments were approved by the Institutional Animal Care and Use Committee of RIKEN Center for Developmental Biology, and mice were maintained and handled in accordance with the protocols approved by this committee.
Construction of Classical Cadherin Structural Model and Its Fitting to TEM Images.
The elastic network model was built from the X-ray crystal structure of E-cadherin [Protein Data Bank (PDB) ID code 3Q2V] (3). To incorporate the influence of calcium ion, the amino acid residues near the calcium ion (within 4 Å) were interconnected to each other by springs (Fig. 2C). In the modified CBMs that have lost the Ca2+-binding ability, on the other hand, the same residues were connected only to close residues, in terms of residue numbers, to represent the interactions in the backbone structure.
In the previous study (23), the deformed atomic models have been built by the following:
where is the integer, and () is a series of deformed atomic models built along the th lowest-frequency normal mode of the X-ray crystal structure by the iterative calculations. The advantage of this method is that a deformed atomic model is obtained easily by giving a set of integers . However, the local structure of the model was sometimes destroyed partly due to the summation in the atomic () coordinate system. To avoid this, the atomic models (and ) were described temporarily by the internal coordinates (and ), such as bond lengths, bond angles, and dihedral angles. Then, the summation was performed in the internal coordinate system []. Finally, the internal coordinates were translated into the atomic coordinates (). In this study, the rmsd of from was 0.1 Å.
We assumed that the TEM image was on the plane, and the electron beam was projected along the axis. Similarly, the deformed atomic models were projected onto the plane along the axis. Considering that the TEM images of E-cadherin were obtained by class averaging, it would be reasonable to assume that the molecules were in stable orientations, in another word, they would lie on the plane. To find such orientations, we considered a function defined by the following:
where and are the mass and the coordinate of the th atom of the atomic model, respectively, and the minimum value of . The orientations at the global minimum or local minima, whose function values were not so high compared with the global minimum, were used to make projections. Each projection was then translated and rotated in the plane to reach the maximum value of the fitting score, which was defined as follows:
where was the intensity of the TEM image at position . and were the integers that satisfied and , respectively, where and were the and coordinates of the th atom of the atomic model, and was the pixel size of the image. This process was repeated for many deformed atomic models, and the best-deformed atomic model in terms of the fitting score was selected.
Construction of Atomic Models for Fat4 and Dachsous1.
We constructed atomic models of Fat4 and Dachsous1 by linking the X-ray crystal structures of an EC domain of classical cadherins in tandem. We assumed that the geometry of an EC–EC linkage was determined by the CBM type: thus, the linkage geometries in Fat4 or Dachsous1 were deduced from those in the classical cadherins. The CBM types in Fat4 and Dachsous1 were defined according to the classification in Tables S1–S3 (Fig. 3A): The CBMs with three or more amino acid substitutions in the consensus sequences or with other amino acid substitutions that could suppress Ca2+ binding were defined as type 5, and the other CBMs were defined as types 1–4, based on the amino acid variations at the position corresponding to N12 of E-cadherin. The linkage geometries were determined from the best-fitting atomic models of E-cadherin obtained in this study (CBM types 1–3) and the previously resolved crystal structures of classical cadherins (CBM type 4) (Table S4). Because the conformational variations of CBM types 1, 3, and 4 in E-cadherin were small, we assumed that the linkages had a fixed conformation. On the other hand, we assumed that CBM type 2 could have several different conformations because of the relatively large variations. Concerning the CBM type 5, we assumed that the linkages could have diverse (∼500) conformations, which were determined as follows; we built an elastic network model from EC2 and 3 of the X-ray crystal structure of E-cadherin, in which the CBM was modeled to have lost the Ca2+-binding ability, and deformed it iteratively (23, 24) along a variety of directions defined by the combination of the three lowest-frequency normal modes, of which two modes were bending modes and one, a twisting mode. The resultant (>2 million) conformations were then classified with respect to the bending angle (tilt), bending direction (skew), and twisting angle (twist) between the two EC domains, and a representative conformation was selected for each class. Because of the presence of deformable EC–EC linkages (CBM types 2 and 5) in Fat4 and Dachsous1, the atomic models could have a huge number of different conformations. They were projected onto the plane (TEM image plane) along the axis. More than 100 different orientations, which were chosen so that the projections covered the angular space almost evenly, were used for each atomic model. Similar to the case of E-cadherin, we assumed that the molecule would lie on the plane. Thus, only the projections in the orientations where all atoms are within a small range (∼100 Å) along the axis were used for the fitting calculations. Different from the case of E-cadherin, the positional information on the terminal points of the molecule, which were identified by visual inspections of the TEM image, was used to help fitting to the low-quality image. The projections were fitted into each TEM image without defining the C and N terminus, and the one with the highest fitting score was selected as the model of best fit. To build representative atomic models (Fig. 3 D and E), 25 structures of the best-fit models were then analyzed, and the representative conformation of each EC–EC linkage was identified. For CBM type 2, we selected the most frequently occurring one as the representative, and for CBM type 5, the one most similar to the average was selected. Because the conformational variations of the latter were generally large, only the conformations in the most densely distributed conformational space were averaged.
Supplementary Material
Acknowledgments
We thank C. Yoshii, M. Nomura-Harada, K. Misaki, K. Matoba, and M. Hirosaki for technical support; T. Ishiuchi, N. Tanaka, S. Hayashi, K. Kubota, and K. Tanabe for technical suggestions and discussion; and B. Honig for critical reading of the manuscript. This work was supported by the Targeted Proteins Research Program of the Ministry of Education, Culture, Sports, Science and Technology of the Japanese Government (MEXT) (M.T. and K.I.). Part of this work was supported by Core Research for Evolutional Science and Technology of the Japan Science and Technology Agency (K.I.), and by the Platform for Drug Discovery, Informatics, and Structural Life Science from the MEXT (A.M. and K.I.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1418990111/-/DCSupplemental.
References
- 1.Hirano S, Takeichi M. Cadherins in brain morphogenesis and wiring. Physiol Rev. 2012;92(2):597–634. doi: 10.1152/physrev.00014.2011. [DOI] [PubMed] [Google Scholar]
- 2.Boggon TJ, et al. C-cadherin ectodomain structure and implications for cell adhesion mechanisms. Science. 2002;296(5571):1308–1313. doi: 10.1126/science.1071559. [DOI] [PubMed] [Google Scholar]
- 3.Harrison OJ, et al. The extracellular architecture of adherens junctions revealed by crystal structures of type I cadherins. Structure. 2011;19(2):244–256. doi: 10.1016/j.str.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pokutta S, Herrenknecht K, Kemler R, Engel J. Conformational changes of the recombinant extracellular domain of E-cadherin upon calcium binding. Eur J Biochem. 1994;223(3):1019–1026. doi: 10.1111/j.1432-1033.1994.tb19080.x. [DOI] [PubMed] [Google Scholar]
- 5.Sotomayor M, Schulten K. The allosteric role of the Ca2+ switch in adhesion and elasticity of C-cadherin. Biophys J. 2008;94(12):4621–4633. doi: 10.1529/biophysj.107.125591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jin X, et al. Crystal structures of Drosophila N-cadherin ectodomain regions reveal a widely used class of Ca2+-free interdomain linkers. Proc Natl Acad Sci USA. 2012;109(3):E127–E134. doi: 10.1073/pnas.1117538108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farquhar MG, Palade GE. Junctional complexes in various epithelia. J Cell Biol. 1963;17:375–412. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hulpiau P, van Roy F. Molecular evolution of the cadherin superfamily. Int J Biochem Cell Biol. 2009;41(2):349–369. doi: 10.1016/j.biocel.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 9.Kazmierczak P, et al. Cadherin 23 and protocadherin 15 interact to form tip-link filaments in sensory hair cells. Nature. 2007;449(7158):87–91. doi: 10.1038/nature06091. [DOI] [PubMed] [Google Scholar]
- 10.Saburi S, et al. Loss of Fat4 disrupts PCP signaling and oriented cell division and leads to cystic kidney disease. Nat Genet. 2008;40(8):1010–1015. doi: 10.1038/ng.179. [DOI] [PubMed] [Google Scholar]
- 11.Mao Y, et al. Characterization of a Dchs1 mutant mouse reveals requirements for Dchs1-Fat4 signaling during mammalian development. Development. 2011;138(5):947–957. doi: 10.1242/dev.057166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matis M, Axelrod JD. Regulation of PCP by the Fat signaling pathway. Genes Dev. 2013;27(20):2207–2220. doi: 10.1101/gad.228098.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishiuchi T, Misaki K, Yonemura S, Takeichi M, Tanoue T. Mammalian Fat and Dachsous cadherins regulate apical membrane organization in the embryonic cerebral cortex. J Cell Biol. 2009;185(6):959–967. doi: 10.1083/jcb.200811030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodewald R, Karnovsky MJ. Porous substructure of the glomerular slit diaphragm in the rat and mouse. J Cell Biol. 1974;60(2):423–433. doi: 10.1083/jcb.60.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inoue T, et al. FAT is a component of glomerular slit diaphragms. Kidney Int. 2001;59(3):1003–1012. doi: 10.1046/j.1523-1755.2001.0590031003.x. [DOI] [PubMed] [Google Scholar]
- 16.Tirion MM. Large amplitude elastic motions in proteins from a single-parameter, atomic analysis. Phys Rev Lett. 1996;77(9):1905–1908. doi: 10.1103/PhysRevLett.77.1905. [DOI] [PubMed] [Google Scholar]
- 17.Bahar I, Atilgan AR, Erman B. Direct evaluation of thermal fluctuations in proteins using a single-parameter harmonic potential. Fold Des. 1997;2(3):173–181. doi: 10.1016/S1359-0278(97)00024-2. [DOI] [PubMed] [Google Scholar]
- 18.Wu Y, Vendome J, Shapiro L, Ben-Shaul A, Honig B. Transforming binding affinities from three dimensions to two with application to cadherin clustering. Nature. 2011;475(7357):510–513. doi: 10.1038/nature10183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chappuis-Flament S, Wong E, Hicks LD, Kay CM, Gumbiner BM. Multiple cadherin extracellular repeats mediate homophilic binding and adhesion. J Cell Biol. 2001;154(1):231–243. doi: 10.1083/jcb.200103143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsukasaki Y, et al. Role of multiple bonds between the single cell adhesion molecules, nectin and cadherin, revealed by high sensitive force measurements. J Mol Biol. 2007;367(4):996–1006. doi: 10.1016/j.jmb.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 21.Tanoue T, Takeichi M. New insights into Fat cadherins. J Cell Sci. 2005;118(Pt 11):2347–2353. doi: 10.1242/jcs.02398. [DOI] [PubMed] [Google Scholar]
- 22.Oda H, Takeichi M. Evolution: Structural and functional diversity of cadherin at the adherens junction. J Cell Biol. 2011;193(7):1137–1146. doi: 10.1083/jcb.201008173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsumoto A, Ishida H. Global conformational changes of ribosome observed by normal mode fitting for 3D Cryo-EM structures. Structure. 2009;17(12):1605–1613. doi: 10.1016/j.str.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 24.Matsumoto A, Kamata T, Takagi J, Iwasaki K, Yura K. Key interactions in integrin ectodomain responsible for global conformational change detected by elastic network normal-mode analysis. Biophys J. 2008;95(6):2895–2908. doi: 10.1529/biophysj.108.131045. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.