Abstract
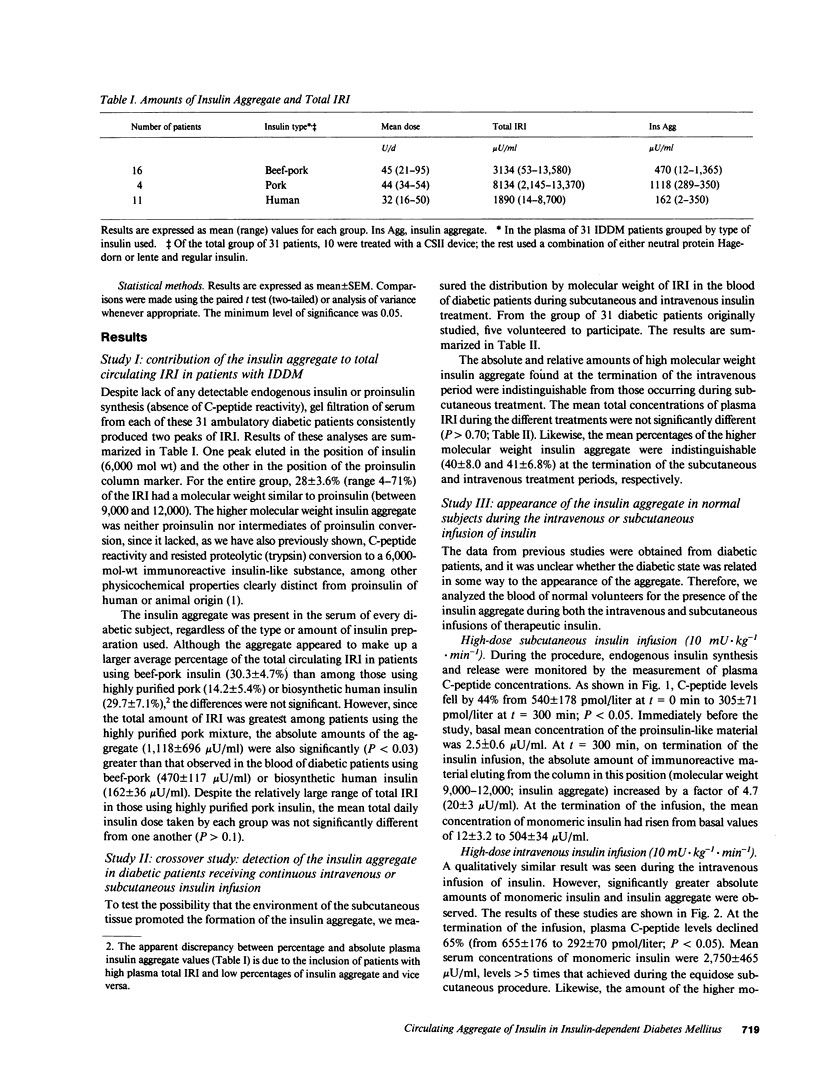
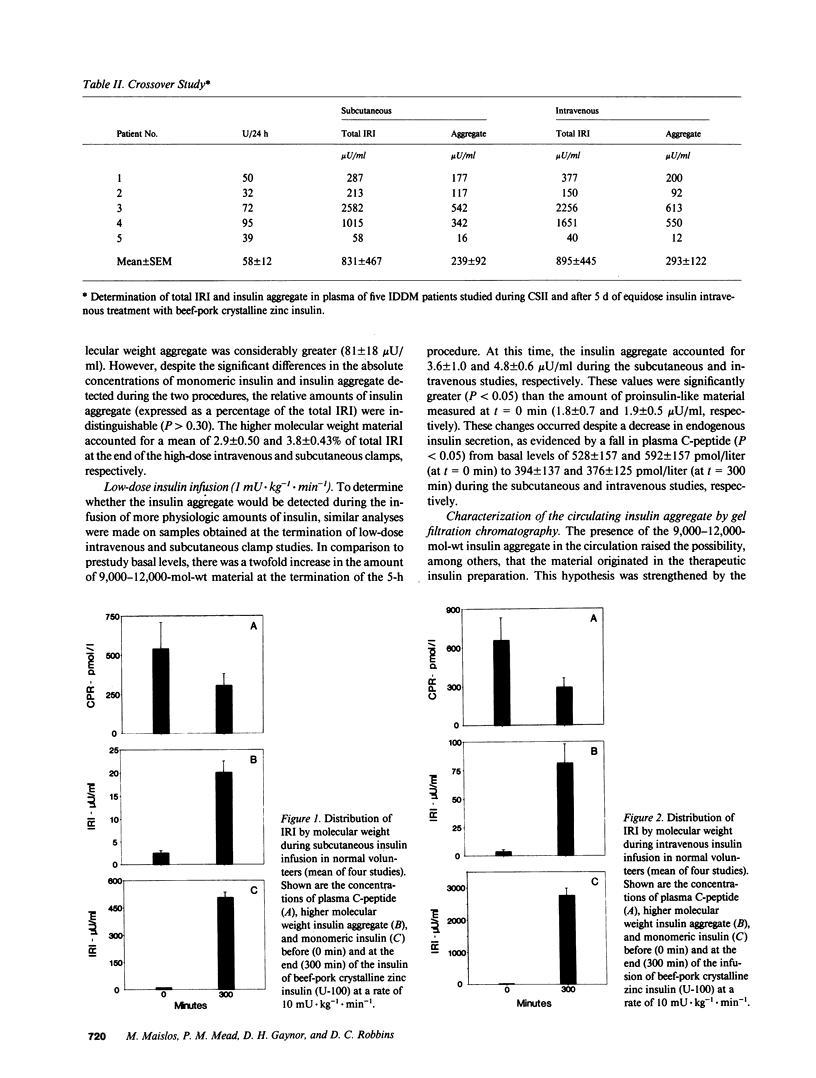
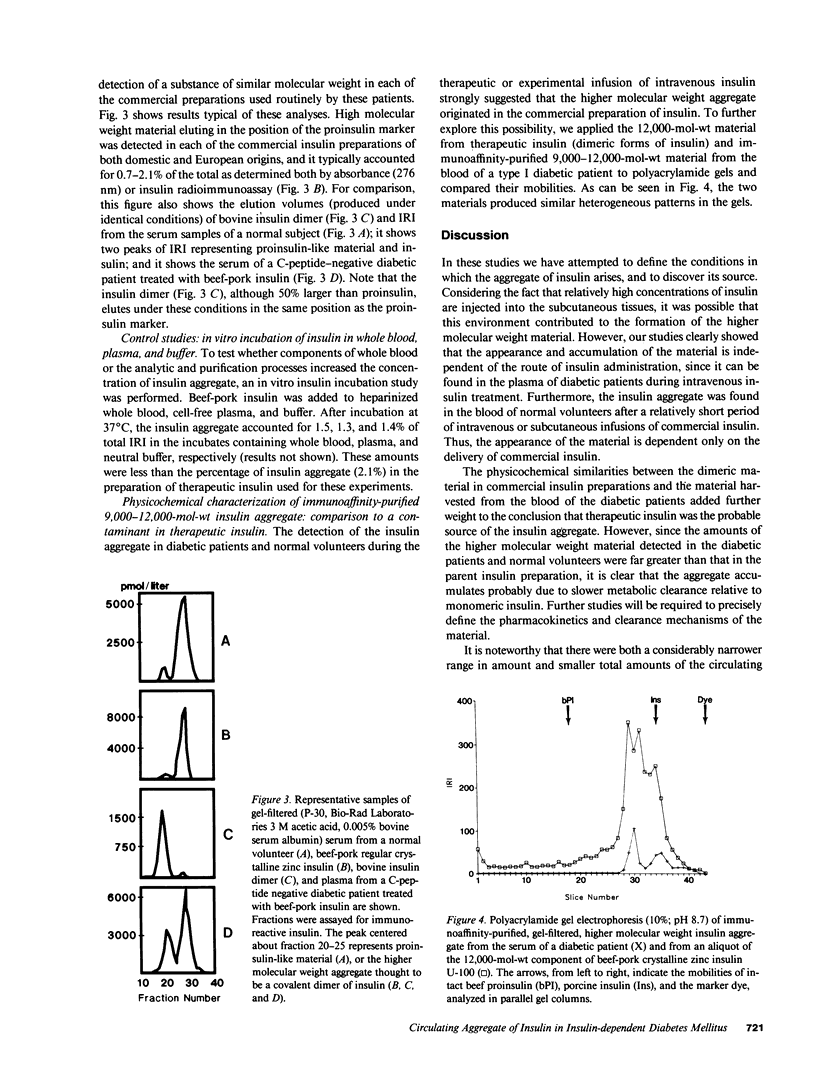
Circulating insulin immunoreactivity (IRI) in type I diabetic patients (insulin-dependent diabetes mellitus [IDDM]) includes a covalent aggregate about twice the size of insulin. These studies were designed to determine the source and conditions promoting the accumulation of this material. Among 31 IDDMs, the aggregate made up 28 +/- 3.6% of the mean fasting plasma IRI. Five of these patients were restudied after 5 d of treatment with equidose intravenous insulin. The relative amount of the aggregate during subcutaneous treatment (40 +/- 8.0%) was indistinguishable (P greater than 0.7) from that at the termination of intravenous treatment (41 +/- 6.8%). To determine whether previous exposure to therapeutic insulin influenced the appearance and accumulation of the aggregate, we intravenously or subcutaneously infused insulin for 5 h in nine healthy volunteers (euglycemic clamp). At the termination of the high-dose intravenous infusion (10 mU X kg-1 X min-1), the concentration of the aggregate was 81 +/- 18 microU/ml, and it accounted for 2.9% of total IRI. At the conclusion of the other infusion protocols, the absolute amounts of aggregate were somewhat less, but they accounted for similar percentages. On polyacrylamide gel electrophoresis, the circulating aggregate was indistinguishable from a material of similar molecular weight contaminating commercial insulin. We conclude that the insulin aggregate found in the blood of IDDMs originates in commercial insulin. Its appearance is independent of the route of insulin administration. Prolonged and continuous use of insulin may increase its concentration but is not necessary for its appearance. The potential biologic and immunologic consequences of the aggregate are important matters that need to be addressed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albisser A. M., Lougheed W., Perlman K., Bahoric A. Nonaggregating insulin solutions for long-term glucose control in experimental and human diabetes. Diabetes. 1980 Mar;29(3):241–243. doi: 10.2337/diab.29.3.241. [DOI] [PubMed] [Google Scholar]
- Burke M. J., Rougvie M. A. Cross- protein structures. I. Insulin fibrils. Biochemistry. 1972 Jun 20;11(13):2435–2439. doi: 10.1021/bi00763a008. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Tobin J. D., Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979 Sep;237(3):E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- Faber O. K., Binder C. C-peptide response to glucagon. A test for the residual beta-cell function in diabetes mellitus. Diabetes. 1977 Jul;26(7):605–610. doi: 10.2337/diab.26.7.605. [DOI] [PubMed] [Google Scholar]
- Fisher B. V., Porter P. B. Stability of bovine insulin. J Pharm Pharmacol. 1981 Apr;33(4):203–206. doi: 10.1111/j.2042-7158.1981.tb13758.x. [DOI] [PubMed] [Google Scholar]
- Fujikawa K., Legaz M. E., Davie E. W. Bovine factors X 1 and X 2 (Stuart factor). Isolation and characterization. Biochemistry. 1972 Dec 19;11(26):4882–4891. doi: 10.1021/bi00776a002. [DOI] [PubMed] [Google Scholar]
- Hayford J. T., Thompson R. G. Free and total insulin integrated concentrations in insulin dependent diabetes. Metabolism. 1982 Apr;31(4):387–397. doi: 10.1016/0026-0495(82)90116-0. [DOI] [PubMed] [Google Scholar]
- Heding L. G. Radioimmunological determination of human C-peptide in serum. Diabetologia. 1975 Dec;11(6):541–548. doi: 10.1007/BF01222104. [DOI] [PubMed] [Google Scholar]
- Martindale H., Marsh J., Hallett F. R., Albisser A. M. Examination of insulin formulations using quasi-elastic light scattering. Diabetes. 1982 Apr;31(4 Pt 1):364–366. doi: 10.2337/diab.31.4.364. [DOI] [PubMed] [Google Scholar]
- Pongor S., Brownlee M., Cerami A. Preparation of high-potency, non-aggregating insulins using a novel sulfation procedure. Diabetes. 1983 Dec;32(12):1087–1091. doi: 10.2337/diab.32.12.1087. [DOI] [PubMed] [Google Scholar]
- Robbins D. C., Shoelson S. E., Rubenstein A. H., Tager H. S. Familial hyperproinsulinemia. Two cohorts secreting indistinguishable type II intermediates of proinsulin conversion. J Clin Invest. 1984 Mar;73(3):714–719. doi: 10.1172/JCI111264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins D. C., Shoelson S. E., Tager H. S., Mead P. M., Gaynor D. H. Products of therapeutic insulins in the blood of insulin-dependent (type I) diabetic patients. Diabetes. 1985 May;34(5):510–519. doi: 10.2337/diab.34.5.510. [DOI] [PubMed] [Google Scholar]
- Sato S., Ebert C. D., Kim S. W. Prevention of insulin self-association and surface adsorption. J Pharm Sci. 1983 Mar;72(3):228–232. doi: 10.1002/jps.2600720307. [DOI] [PubMed] [Google Scholar]
- Schade D. S., Eaton R. P., DeLongo J., Saland L. C., Ladman A. J., Carlson G. A. Electron microscopy of insulin precipitates. Diabetes Care. 1982 Jan-Feb;5(1):25–30. doi: 10.2337/diacare.5.1.25. [DOI] [PubMed] [Google Scholar]
- Schernthaner G., Borkenstein M., Fink M., Mayr W. R., Menzel J., Schober E. Immunogenicity of human insulin (Novo) or pork monocomponent insulin in HLA-DR-typed insulin-dependent diabetic individuals. Diabetes Care. 1983 Mar-Apr;6 (Suppl 1):43–48. [PubMed] [Google Scholar]
- Smith D. J., Venable R. M., Collins J. Separation and quantitation of insulins and related substances in bulk insulin crystals and in injectables by reversed-phase high performance liquid chromatography and the effect of temperature on the separation. J Chromatogr Sci. 1985 Feb;23(2):81–88. doi: 10.1093/chromsci/23.2.81. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Hallund O., Rubenstein A., Cho S., Bayliss C. Isolation and properties of proinsulin, intermediate forms, and other minor components from crystalline bovine insulin. Diabetes. 1968 Dec;17(12):725–736. doi: 10.2337/diab.17.12.725. [DOI] [PubMed] [Google Scholar]
- Tatnell M. A., Jones R. H., Sönksen P. H. Covalently-linked insulin dimers: their metabolism and biological effects in vivo as partial competitive antagonists of insulin clearance. Diabetologia. 1984 Jul;27(1):27–31. doi: 10.1007/BF00253497. [DOI] [PubMed] [Google Scholar]
- de Haën C., Little S. A., May J. M., Williams R. H. Characterization of proinsulin-insulin intermediates in human plasma. J Clin Invest. 1978 Oct;62(4):727–737. doi: 10.1172/JCI109183. [DOI] [PMC free article] [PubMed] [Google Scholar]



