Significance
With no lysine (K) (WNK)1, which is mutated in pseudohypoaldosteronism type II (PHAII) autosomal dominant hypertension, is a large, complex enzyme essential for development, blood pressure control, and many cellular functions. WNK1 signaling is largely mediated by two downstream protein kinases, OSR1 (oxidative stress responsive 1) and SPAK (STE20/SPS1-related proline-, alanine-rich kinase), sometimes considered redundant in terms of WNK1 function. This study characterizes an essential contribution of WNK1 in angiogenesis and presents a mechanism of clear bifurcation in WNK1-dependent functions between OSR1 and SPAK, with SPAK regulating WNK1 effects on proliferation and OSR1 mediating effects on invasion. Our work also identifies a previously unidentified link between WNK1 and the zinc-finger transcription factor Slug, with implications in cancer biology. This study also suggests potential mechanisms for cardiovascular defects associated with PHAII.
Keywords: endothelium, angiogenesis, migration, EMT, Slug
Abstract
The with no lysine (K) (WNK) family of enzymes is best known for control of blood pressure through regulation of the function and membrane localization of ion cotransporters. In mice, global as well as endothelial-specific WNK1 gene disruption results in embryonic lethality due to angiogenic and cardiovascular defects. WNK1−/− embryos can be rescued by endothelial-specific expression of a constitutively active form of the WNK1 substrate protein kinase OSR1 (oxidative stress responsive 1). Using human umbilical vein endothelial cells (HUVECs), we explored mechanisms underlying the requirement of WNK1–OSR1 signaling for vascular development. WNK1 is required for cord formation in HUVECs, but the actions of the two major WNK1 effectors, OSR1 and its close relative SPAK (STE20/SPS1-related proline-, alanine-rich kinase), are distinct. SPAK is important for endothelial cell proliferation, whereas OSR1 is required for HUVEC chemotaxis and invasion. We also identified the zinc-finger transcription factor Slug in WNK1-mediated control of endothelial functions. Our study identifies a separation of functions for the WNK1-activated protein kinases OSR1 and SPAK in mediating proliferation, invasion, and gene expression in endothelial cells and an unanticipated link between WNK1 and Slug that is important for angiogenesis.
With no lysine (K) (WNK) enzymes constitute a family of large (up to 300-kDa) serine/threonine protein kinases with a unique N-terminal kinase domain characterized by the presence of the catalytic lysine replacing a conserved glycine in the phosphate anchor ribbon (1–3). This unique organization of the active site is essential for WNK regulation by chloride ion concentration (4), and may also contribute to the phosphorylation of otherwise inaccessible substrates (5). Mutations in WNK1 and WNK4 can cause an autosomal dominant hypertension, PHAII (pseudohypoaldosteronism type II) (6). This has led to a focus on WNKs in hypertension and characterization of their roles in ion balance, mediated by regulation of the membrane localization and activity of various ion transporters via WNK downstream targets (5, 7–10). WNK1 is activated by osmotic stress, resulting in phosphorylation and activation of two of its substrates, the closely related protein kinases oxidative stress responsive 1 (OSR1) and STE20/SPS1-related proline/alanine-rich kinase (SPAK), which phosphorylate and regulate the activities of the SLC12 family of ion cotransporters, including the Na+, Cl− cotransporter (NCC), Na+, K+, 2Cl− cotransporters 1 and 2 (NKCC1 and NKCC2), and K+, Cl− cotransporters (KCCs) (7–9, 11–17). Although WNK1+/− heterozygous mice are viable (18, 19), a study using whole-embryo and endothelial-specific WNK1 knockout mice showed embryonic lethality due to angiogenic and cardiovascular defects (20, 21). Similar findings were made in zebrafish embryos following WNK1 knockout (22). The defects observed in mice were not due to problems with primary vasculogenesis, which is the differentiation of progenitor cells into endothelial cells to form the primary vascular structures of the embryo (20, 23). Rather, the defect occurs at the remodeling/angiogenesis step, which requires growth, sprouting, and branching of vessels to remodel the primordial vascular plexus into a mature vascular system (20, 23). The WNK1 angiogenic and cardiac defects are phenocopied by global or endothelial-specific OSR1 deletion, and can be rescued by expressing an endothelial-specific constitutively active OSR1 (21). PHAII patients regularly manifest cardiovascular symptoms (24, 25); however, effects of WNK mutations on these symptoms have not been analyzed.
Angiogenesis is the process by which endothelial cells organize to form new, functional vasculature (26). This process is essential during embryonic development, with the cardiovascular system being the first system to develop and reach a functional state in embryos (27, 28). Whereas angiogenesis is necessary for wound healing, reproduction, and other physiological processes, angiogenesis is detrimental in diseases such as diabetic retinopathy and cancer; for example, angiogenesis can be a mark of tumor progression and is a target for therapeutics (26, 29–34). Angiogenesis requires multiple steps, including endothelial cell proliferation, disruption of the surrounding extracellular matrix, secretion of angiogenic factors, migration toward stimuli, and organization to form the 3D vessel architecture. Many angiogenic growth factors are secreted by a variety of cells, including endothelial cells themselves, and these include vascular endothelial growth factor (VEGF), endothelin 1, angiopoietin 1, stromal-derived factor 1 (SDF-1/CXCL12), and transforming growth factor beta (TGF-β) (34, 35). Cells also produce angiostatic molecules that block angiogenesis, including thrombospondin 1, endostatin, angiostatin, and angiopoietin 2 (34, 35). The ability of endothelial cells to undergo angiogenesis depends on pathways that regulate the expression and secretion of angiogenic and angiostatic molecules to control invasion, migration, and organization of endothelial cells. Transcription factors that regulate these molecules include nuclear factor kappa B, hypoxia-inducible factor 1, and zinc-finger transcription factors such as the Kruppel-like factor KLF4 and Slug, among others (36–40).
The impact of WNK1 loss on endothelial cells and angiogenesis has been described (20, 21); however, the underlying mechanisms are less understood. In this study, we extend the previous mouse studies using two primary endothelial cell lines, human umbilical vein endothelial cells (HUVECs) and human dermal microvascular endothelial cells (HDMECs), and show a central role for WNK1 in angiogenesis. We further investigate the mechanism by which WNK1 exerts its angiogenic actions, identifying distinct contributions of OSR1 and SPAK, as well as an unexpected mechanism involving the zinc-finger transcription factor Slug, in conveying WNK1 functions.
Results
WNK1 Is Required for in Vitro Angiogenesis.
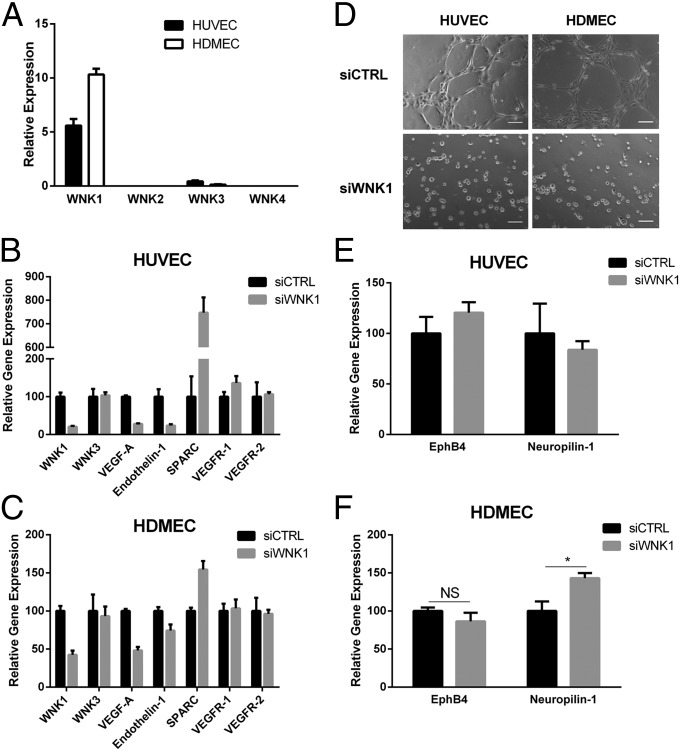
Previous studies in mice had shown that WNK1 is required for embryonic cardiovascular development and angiogenesis (20, 21). To begin to define the underlying mechanisms, we confirmed that WNK1 is the major WNK mRNA expressed in HUVECs and HDMECs. Much less WNK3 mRNA is present, and WNK2 and WNK4 mRNAs are undetectable (Fig. 1A). WNK3−/− mice are viable and do not show any angiogenic or cardiovascular defects (41), consistent with its low expression in endothelial cells. Therefore, we focused solely on WNK1. We tested the effect of WNK1 knockdown on angiogenic regulatory genes and found decreased VEGF-A and endothelin 1 mRNAs in both HUVECs and HDMECs, but no changes in transcripts encoding VEGF receptors 1 and 2 [Fig. 1 B and C, compare small interfering RNA directed against WNK1 (siWNK1) with siCTRL]. WNK1 depletion also increased mRNA encoding SPARC (secreted protein, acidic and rich in cysteine/osteonectin) (Fig. 1 B and C). SPARC has context-dependent pro- and antiangiogenic effects (42); in HUVECs, SPARC appears to repress invasion and angiogenesis (43). Knockdown of WNK1 in both HUVECs and HDMECs did not affect amounts of the WNK1 downstream kinases OSR1 and SPAK (Fig. S1 A and B). Because of the significant changes in angiogenesis-regulating genes following WNK1 knockdown, we tested the ability of WNK1-depleted HUVECs and HDMECs to form cords, an in vitro angiogenesis assay. Consistent with the angiogenic defect observed in WNK1−/− mice, HUVECs and HDMECs depleted of WNK1 failed to form cords within the same 5-h assay as knockdown control HUVECs and HDMECs that organized into an extensive network of cords in that time (Fig. 1D). Similar effects on cord formation were seen with two different WNK1 siRNAs (Fig. S1C). Huang and colleagues showed ectopic expression of arterial and venous markers, neuropilin 1 and EphB4, respectively, in the endothelium of WNK1−/− mice (20). To test whether HUVECs differentially regulated venous and arterial markers following WNK1 knockdown, we performed quantitative (q)RT-PCR for EphB4 and neuropilin 1 and detected no changes in expression (Fig. 1E). This may be because HUVECs pass oxygenated blood during embryonic development. Thus, we tested venous and arterial markers in HDMECs, a microvascular cell line with some venular characteristics; qRT-PCR showed an increase in the arterial marker neuropilin 1 in siWNK1 HDMECs compared with siCTRL, whereas the venous marker EphB4 did not change (Fig. 1F). These data support previous observations by Huang and colleagues (20), and suggest that WNK1 may regulate factors involved in arterial–venous differentiation.
Fig. 1.
WNK1 is required for cord formation in HUVECs and HDMECs. (A) Amounts of mRNAs encoding WNKs 1–4 in HUVECs and HDMECs relative to β-actin measured by qRT-PCR. (B and C) Changes in mRNAs encoding angiogenesis-related genes in (B) HUVECs and (C) HDMECs following depletion of WNK1 measured by qRT-PCR. (D) Effect of WNK1 depletion on cord formation by HUVECs and HDMECs. (Scale bars, 100 μm.) (E and F) Effect of siWNK1 on expression of venous and arterial markers in (E) HUVECs and (F) HDMECs. Data are mean ± SEM; n = 3 (A–C, E, and F); representative images are from three experiments (C). *P < 0.05. NS, not significant.
We next tested the impact of WNK1 on angiogenic sprouting. First, we performed bead sprouting assays, in which endothelial cells coated on beads embedded in a matrix can sprout vessels with a lumen (36, 44–46). This assay mimics blood vessel sprouting and development. Although we were unable to coat beads using HUVECs, we successfully performed the assay using HDMECs. Control cells showed from two to six sprouts per bead after 3 wk, but siWNK1 HDMECs displayed at most one sprout per bead, with the majority of beads showing no sprouts (Fig. S2A). We also performed a spheroid sprouting assay in which HUVECs were grown in spheres and then embedded in a collagen I matrix. Sprouting toward medium containing proangiogenic factors was monitored (47, 48). We observed a lack of sprouting in the siWNK1 HUVEC spheroids, whereas control spheroids showed significant sprouts and invasion from spheroids into the surrounding matrix (Fig. S2B). These observations support a role of WNK1 in angiogenic sprouting and vasculogenesis (20, 22).
WNK1 Is Required for HUVEC Proliferation and Motility.
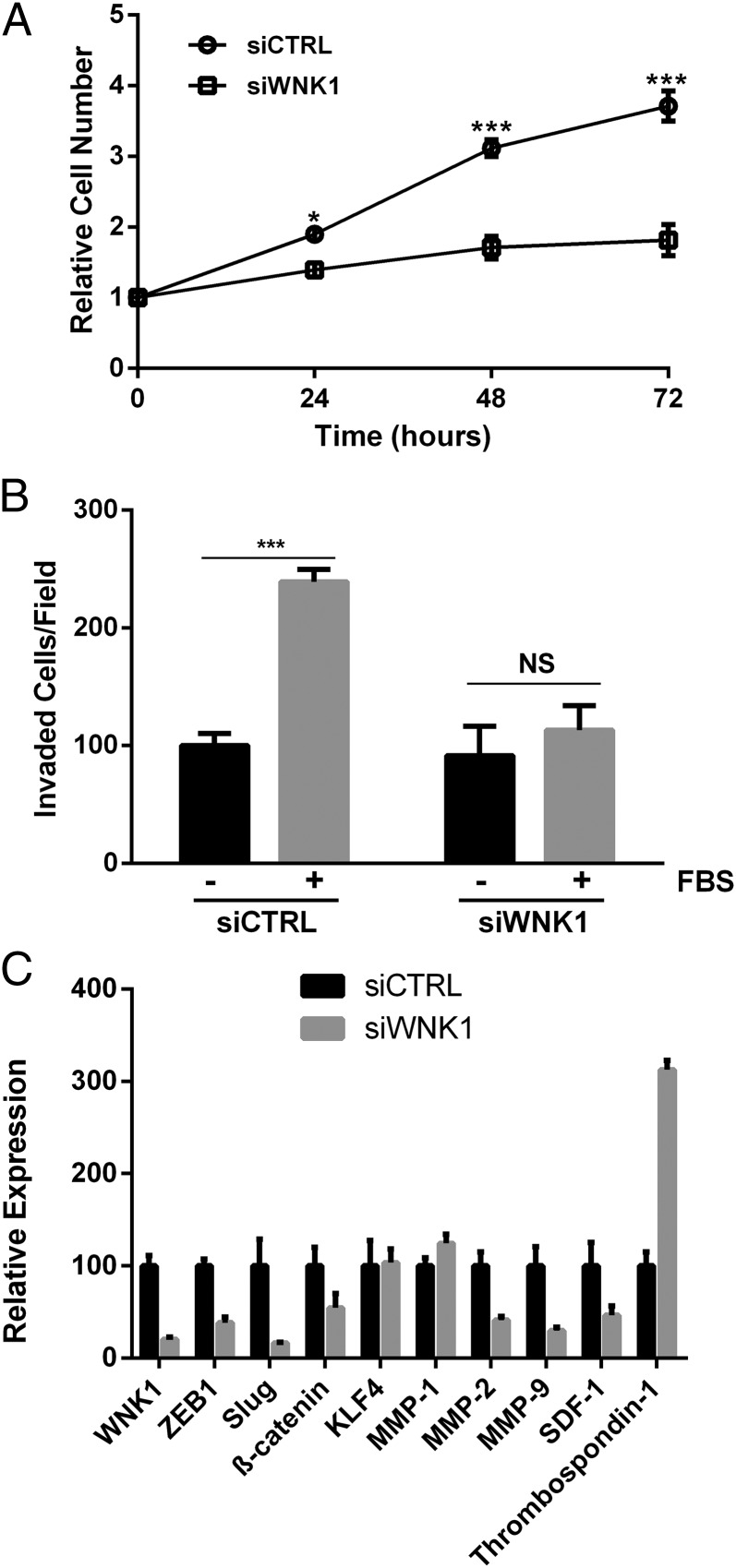
Angiogenesis involves endothelial cell proliferation and migration toward angiogenic stimuli. Because we see greater depletion of WNK1 in HUVECs compared with HDMECs (Fig. 1 B and C; 80–90% knockdown in HUVECs vs. 50–60% knockdown in HDMECs), we focused on HUVECs for the majority of the studies below. We tested the effect of WNK1 knockdown on HUVEC proliferation and growth, and noted a significantly slower proliferation rate at all times tested (Fig. 2A), consistent with earlier findings (49, 50). We tested the ability of WNK1-depleted HUVECs to migrate toward serum in a Boyden chamber assay. Serum increased migration of HUVECs, approximately doubling the number of cells crossing the membrane. In contrast, depletion of WNK1 nearly prevented the serum-induced increase in migration observed in the control group; instead, migration of the knockdown cells was similar in stimulated and unstimulated conditions (Fig. 2B). To outline a mechanism for the WNK1 effect on migration, we measured mRNAs encoding genes involved in invasion and migration. A decrease in the epithelial-to-mesenchymal (EMT)–associated transcription factors Slug and ZEB-1 was noted in WNK1-depleted HUVECs. In contrast, Snail mRNA was not detected in these cells. KLF4, a zinc-finger transcription factor with vascular roles (51), was not affected by WNK1 knockdown (Fig. 2C). We also measured matrix metalloproteinases (MMPs), regulators of the invasive ability of cells and drug targets for vascular diseases (52). MMP-2 and MMP-9 mRNAs were markedly reduced by WNK1 siRNA, whereas that encoding thrombospondin 1, an inhibitor of endothelial migration and angiogenesis (53), was significantly increased (Fig. 2C). Similar changes in Slug, MMP-2, MMP-9, and thrombospondin 1 transcripts were seen in HDMECs upon WNK1 knockdown (Fig. S3), again confirming the similarities in WNK1 knockdown phenotypes in the two endothelial cell lines.
Fig. 2.
WNK1 affects HUVEC proliferation and motility. (A) Proliferation of HUVECs over 72 h; cell numbers are relative to time 0. (B) Migration through a membrane toward medium without or with 10% (vol/vol) FBS. (C) Changes in mRNAs in HUVECs following depletion of WNK1 measured by qRT-PCR. Data are mean ± SEM; n = 3. *P < 0.05, ***P < 0.001.
Loss of WNK1 Substrate Kinases OSR1 or SPAK Blocks in Vitro Angiogenesis.
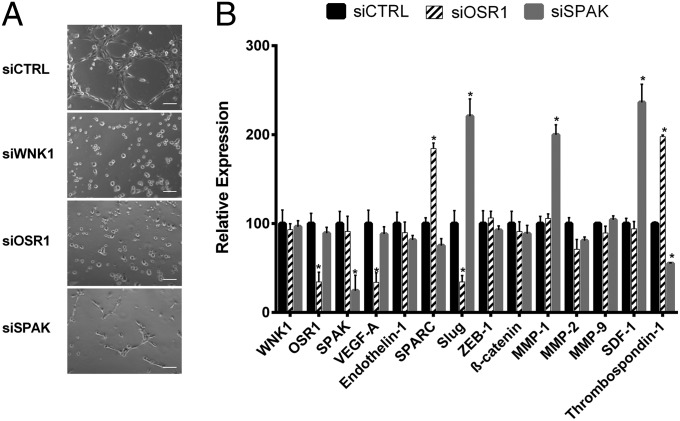
The best-defined WNK1 signaling is to OSR1 and SPAK, which mediate WNK1 effects on cotransporters and cell-volume control (3). A previous study showed that loss of OSR1 phenocopied the angiogenic defect due to loss of WNK1, and constitutively active OSR1 rescued WNK1−/− cardiovascular defects (21). In contrast to WNK1 or OSR1 null mice, SPAK−/− mice are viable but display vasodilation, which contributes to hypotension (54). To test the impact of OSR1 and SPAK on in vitro angiogenesis, we monitored cord formation, comparing HUVECs depleted of WNK1, OSR1, or SPAK. siOSR1 reduced the protein by half, whereas siSPAK resulted in an ∼70–80% decrease (Fig. S4A). Knockdown of OSR1 recapitulated the siWNK1 phenotype, with no cords formed (Fig. 3A). Reducing SPAK yielded only small cords, a phenotype that was intermediate between the control and knockdown of WNK1 or OSR1 (Fig. 3A). These results were confirmed in HUVECs using a second set of targeted siRNAs (Fig. S4B) and were also replicated in HDMECs (Fig. S5A). It is possible that SPAK is required for the elongation, branching, or fusion of vessels once the initial small vessels have formed. To obtain more insight into differences in these knockdown phenotypes, we measured mRNA expression in OSR1- and SPAK-depleted HUVECs. Depletion of OSR1 replicated some of the changes detected in WNK knockdown cells (Figs. 1B and 2C); amounts of VEGF-A and Slug mRNAs decreased, and SPARC and thrombospondin 1 mRNAs increased (Fig. 3B). In contrast, depletion of SPAK did not change amounts of mRNAs encoding the angiogenesis-related genes VEGF-A and endothelin 1 but did increase amounts of Slug, MMP-1, and SDF-1 mRNAs and decrease thrombospondin 1 mRNA (Fig. 3B). These data suggest significant differences in the pathways controlled by OSR1 and SPAK downstream of WNK1.
Fig. 3.
OSR1 and SPAK impact HUVEC cord formation. (A) Effect of depletion of WNK1, OSR1, or SPAK on cord formation by HUVECs. (Scale bars, 100 μm.) (B) Changes in angiogenesis- and EMT-related genes in HUVECs following depletion of the indicated proteins. Data are mean ± SEM; n = 3 (B); representative images are from three experiments (A). *P < 0.05.
OSR1 and SPAK Regulate Different Aspects of WNK1 Functions in HUVECs.
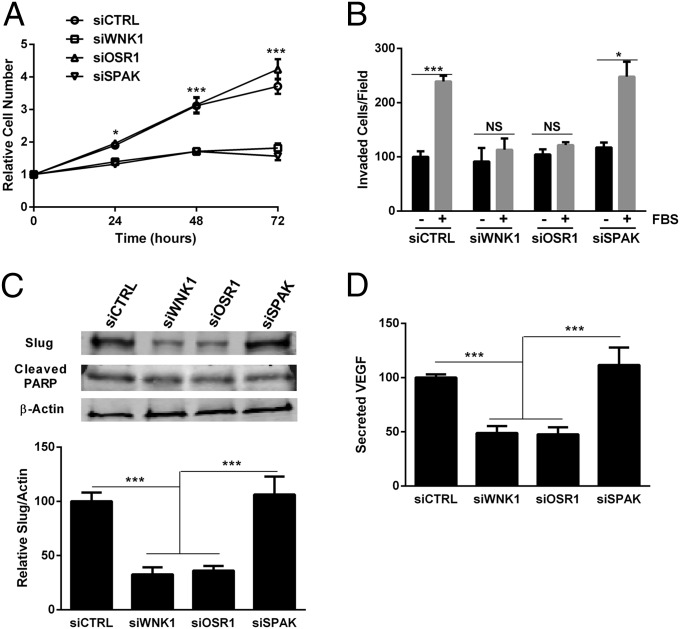
We tested the impact of OSR1 and SPAK on other WNK1 loss-of-function phenotypes in endothelial cells. Knockdown of WNK1 decreased proliferation of HUVECs (Fig. 2A), as did knockdown of SPAK (Fig. 4A). Knockdown of OSR1 in HUVECs had no effect on proliferation (Fig. 4A), as suggested by studies in HeLa cells (50). In contrast, migration of HUVECs in a serum gradient was reduced by depletion of WNK1 or OSR1, yet SPAK knockdown had no effect on migration of HUVECs (Fig. 4B). Similar proliferation and migration phenotypes were observed in kinase-depleted HDMECs (Fig. S5 B and C).
Fig. 4.
OSR1 and SPAK regulate different WNK1 functions in HUVECs. (A) Proliferation of HUVECs over 72 h. Cell numbers are relative to time 0. (B) Migration through a membrane toward medium without or with 10% FBS. (C) Lysates from HUVECs treated as indicated were immunoblotted with anti-Slug, anti-cleaved PARP, and anti–β-actin. Blots were quantified via LI-COR imaging. (D) Secreted VEGF-A was measured by ELISA following a 2-h starvation. Data are mean ± SEM; n = 3 (A–C), n = 5 (D). *P < 0.05, ***P < 0.001.
Effects on migration were consistent with changes in expression of EMT-related genes; mRNA encoding Slug, one of the master transcriptional regulators of EMT, was decreased by siWNK1 and siOSR1, whereas knockdown of SPAK increased Slug mRNA (Figs. 2C and 3B). The changes in Slug and VEGF-A mRNAs were mirrored by changes in protein expression. Less immunoreactive Slug and VEGF-A were apparent in cells depleted of either WNK1 or OSR1, but no change in either was detected following depletion of SPAK (Fig. 4 C and D). No differences in cleaved PARP (Fig. 4C) were seen in knockdown cells, suggesting that differences in cell proliferation were not due to increased apoptosis.
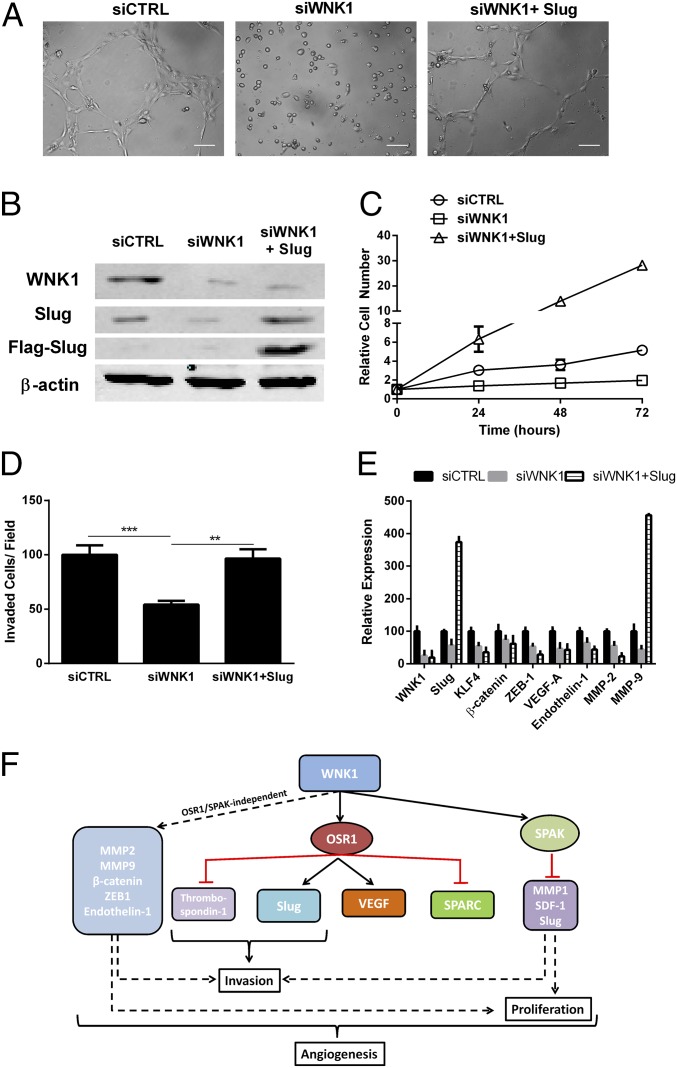
We attempted to rescue the WNK1 phenotypes by overexpressing OSR1 or SPAK. Expression of either in siWNK1 HUVECs led to a partial rescue of cord formation (Fig. S6A). As might be predicted from the observed phenotype of SPAK knockdown on proliferation, overexpression of SPAK partially rescued the proliferation defect in siWNK1 HUVECs (Fig. S6B); overexpression of either OSR1 or SPAK partially rescued the decreased invasion observed in siWNK1 HUVECs (Fig. S6C). To test the significance of Slug (36) as a downstream mediator of WNK1 in angiogenesis, we examined formation of cords by control and WNK1-depleted HUVECs as well as siWNK1 cells expressing Flag–Slug (Fig. 5 A and B). Heterologous expression of Slug rescued the cord formation defect observed in siWNK1 HUVECs (Fig. 5A). Slug dramatically increased proliferation in WNK1-depleted cells (Fig. 5C) and restored invasion toward chemoattractant to that observed in the controls (Fig. 5D). Slug also increased MMP-9 transcript (Fig. 5E). These data suggest that the WNK1 effects on angiogenesis are differentially mediated by OSR1 and SPAK, with some actions independent of both (Fig. 5F). Furthermore, these data provide evidence linking WNK1 and Slug, although the mechanism connecting them has not yet been identified.
Fig. 5.
Slug rescues cord formation by WNK1-depleted HUVECs. (A) Effect of Flag–Slug on cord formation by cells depleted of WNK1; representative images are from two experiments. (Scale bars, 100 μm.) (B) Lysates from HUVECs treated as indicated were immunoblotted with anti-Flag, anti-Slug, anti-WNK1, and anti–β-actin; n = 2. (C) Proliferation of cells treated as indicated over 72 h. Cell numbers are relative to time 0. (D) Migration of cells toward medium without or with 10% FBS. (E) Changes in expression of angiogenesis- and EMT-related genes in HUVECs treated as indicated. (F) Schematic diagram of the mechanisms of WNK1 actions on endothelial cell functions. Data are mean ± SD; n = 2 (C–E). **P < 0.01, ***P < 0.001.
Discussion
Canonical WNK1 signaling is stimulated by osmotic stress and results in activation of OSR1 and SPAK, both of which regulate ion cotransporter activity and localization (7, 8, 13–17). WNK1 knockout mice die before birth, with gross defects in the development of the cardiovascular system and angiogenesis, rescued by endothelial-restricted expression of constitutively active OSR1 (20, 21). We identified distinct functions for OSR1 and SPAK in mediating WNK1 activity in endothelial cells and angiogenesis. OSR1 knockdown was most similar to the WNK1 loss-of-function phenotype with a complete absence of cords, whereas SPAK knockdown yielded a partial phenotype with small, undeveloped cords. Overexpression of neither OSR1 nor SPAK fully rescued WNK1 depletion, suggesting that WNK1 requires both OSR1 and SPAK, as well as one or more other downstream targets and/or interacting partners that are yet to be characterized.
Transcripts encoding three factors are decreased upon WNK1 depletion, independent of OSR1 and SPAK: the angiogenic factor endothelin 1; ZEB-1, an EMT-associated transcription factor involved in HUVEC proliferation and viability (55); and β-catenin, which can regulate cell adhesion as well as Wnt signaling. A recent study suggested a positive effect of WNK1 on Wnt/β-catenin signaling in Drosophila (56), and our data support the notion that WNK1 up-regulates β-catenin, which in turn can enhance Wnt/β-catenin–mediated signaling and transcription. Such a mechanism could enable WNK1 functions in development.
WNK1 has been shown to be required for proliferation in a mouse neural progenitor cell line (57), HeLa cells (38), and Akt-stimulated proliferation in 3T3-L1 cells (50, 58–60). The WNK1 requirement for endothelial cell proliferation appears to be controlled by SPAK, whose knockdown also decreases both HUVEC and HDMEC proliferation. In contrast to reports for WNK3 (61), WNK1 loss apparently does not induce apoptosis. Preliminary analysis suggests that loss of WNK1, OSR1, or SPAK also had no effect on the cell-cycle distribution of HUVECs. We had previously shown that WNK1 localizes to the mitotic spindle and is required for completion of mitosis and abscission, also independent of OSR1 (50). It is possible that SPAK may be involved in these actions in cell division that result in the slowed cell proliferation observed in WNK1 and SPAK knockdown HUVECs. This may also be related to the earlier finding that SPAK induces apoptosis in a caspase-dependent manner in response to genotoxic stress (62). Modulating SPAK activity directly or indirectly via WNK1 knockdown could lead to proliferation defects mediated by deregulation of cellular responses to DNA damage or other stress-induced apoptotic mechanisms. SPAK may act as a switch, promoting cell proliferation under normal conditions but inducing apoptosis under stress.
WNKs 1, 2, and 3 have also been shown to regulate migration (63–65). We find that WNK1 is required for endothelial cell migration toward a chemoattractant that is dependent on OSR1 but not SPAK. Our data provide some insight into the mechanism behind WNK1 effects on migration. Notably, the EMT-enhancing transcription factor Slug accumulates in the presence of WNK1 and OSR1 but is oppositely affected by SPAK. Slug is important in tumor invasion and in development (66, 67). It regulates angiogenesis and is connected to vessel sprouting and lumen formation (36). Increased Slug accumulation in tumor-associated endothelial cells may provide a mechanism by which WNK1 could impact metastasis (68). It will be important to determine whether WNK1 regulates Slug by stimulating transcription, translation, or mRNA stability, whether this effect of WNK1 on Slug is universal across different cell types, and whether it extends to other WNK family members.
If transcription is involved, the mechanism is not obvious. WNK1 has not been found in the nucleus. Previous studies have shown that OSR1 can localize to the nucleus (50, 69) whereas a caspase-cleaved fragment of SPAK also translocates to the nucleus (70), although nuclear functions have not been determined. The significance of gene transcription during the short duration of the angiogenesis assay has been debated, with some studies suggesting that transcription is not required in assays performed on Matrigel (71) whereas others point to transcriptional changes occurring during the onset of HUVEC cord formation (72, 73).
In summary, our data shed light on vascular defects in WNK1−/− animals, with at least three different arms of WNK1 downstream pathways required for the angiogenic process (Fig. 5F). WNK1 controls endothelial cells by mechanisms including the regulation of several transcription factors, such as Slug, ZEB-1, and β-catenin, which have far-reaching roles in disease. WNK1 is mutated in PHAII, and although research focus has been on the impact on kidney function, many patients exhibit cardiovascular disorders (24, 25). The detailed mechanisms by which WNK1 regulates the cardiovascular system need to be further elucidated, as they may provide a basis for targeting WNK1 functions in disease.
Materials and Methods
Materials.
Silencer select siRNAs were from Ambion. Flag–Slug plasmid (Addgene; plasmid 25696) was from Eric Fearon (University of Michigan, Ann Arbor, MI). Growth Factor Reduced Matrigel was from BD Biosciences. Antibodies against WNK1, OSR1, SPAK, cleaved PARP, and Slug were from Cell Signaling Technologies, and β-actin and Flag–M2 antibodies were from Sigma.
Cell Culture and Transfection.
HUVECs (CRL-1730) and HDMECs (PCS-110-010) were from the American Type Culture Collection (ATCC) and were cultured in F12K medium supplemented with 0.1 mg/mL heparin (Sigma), 0.03 mg/mL endothelial cell growth supplement (Sigma), and 10% (vol/vol) FBS and in Vascular Cell Basal Medium supplemented with the Microvascular Endothelial Cell Growth Kit-BBE (ATCC; PCS-110-040), respectively. Cells were transfected with 20 nM siRNA using Lipofectamine RNAiMax reagent (Life Technologies) or 2.5 μg Flag–Slug using Lipofectamine LTX (Life Technologies) per the manufacturer’s instructions.
Cell Proliferation.
HUVECs were treated with siRNA, and 2 × 103 cells were plated in 96-well dishes. At 0, 24, 48, and 72 h postplating, 20 μL CellTiter-Blue reagent (Promega) was added to each well for 1.5 h at 37 °C. Emission at 590 nm was measured after excitation at 560 nm using the Synergy 2 multimode microplate reader (BioTek) with Gen5 software. Data were plotted relative to emission at day 0.
Boyden Chamber Migration.
HUVECs were transfected with siRNAs. After 48 h, serum was removed for 2 h. Cells (5 × 104) were plated in serum-free medium in the upper chamber and medium, either without or with 10% (vol/vol) FBS, in the lower chamber. After 5 h, the cells were fixed in 4% (vol/vol) paraformaldehyde (Electron Microscopy Sciences) and insert membranes were removed, stained, and mounted on coverslips with DAPI Fluoromount (Southern Biotech). Images were collected using a DeltaVision RT deconvolution microscope at 40× magnification, and cells were counted using ImageJ software (National Institutes of Health).
Cord Formation.
HUVECs (2 × 105 in a six-well dish) were treated with 20 nM siRNAs for 2 d and then starved for 2 h before 3 × 104 cells were plated in serum-free medium on polymerized Matrigel in 24-well dishes and then imaged after 4–5 h. For experiments with Flag–Slug rescue, cells were first transfected with siRNA as above for 24 h and then transfected for 24 h with Flag–Slug (2.5 μg) before starvation and plating. All images were acquired in ImageJ (version 1.45) using a microscope (Axiovert 200M; Carl Zeiss) with a 10× objective lens and equipped with a digital camera (SensiCam; Cooke).
Statistical Analysis.
Student t test and one-way analysis of variance were used to determine statistical significance.
Supplementary Material
Acknowledgments
The authors thank Drs. Michael Kalwat, Andrès Lorente-Rodríguez, Aileen Klein, and other members of the M.H.C. laboratory for valuable discussions and Dionne Ware for administrative assistance. This work was supported by National Institutes of Health Grants R01 GM53032 (to M.H.C.), R01 CA118240 (to R.A.B.), and R01 CA115241 (to G.P.), start-up funds from the Department of Surgery, University of Texas Southwestern Medical Center (to M.T.D.), and The Effie Marie Cain Scholarship for Angiogenesis Research (to R.A.B.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1419057111/-/DCSupplemental.
References
- 1.Xu B, et al. WNK1, a novel mammalian serine/threonine protein kinase lacking the catalytic lysine in subdomain II. J Biol Chem. 2000;275(22):16795–16801. doi: 10.1074/jbc.275.22.16795. [DOI] [PubMed] [Google Scholar]
- 2.Min X, Lee BH, Cobb MH, Goldsmith EJ. Crystal structure of the kinase domain of WNK1, a kinase that causes a hereditary form of hypertension. Structure. 2004;12(7):1303–1311. doi: 10.1016/j.str.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 3.McCormick JA, Ellison DH. The WNKs: Atypical protein kinases with pleiotropic actions. Physiol Rev. 2011;91(1):177–219. doi: 10.1152/physrev.00017.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piala AT, et al. Chloride sensing by WNK1 involves inhibition of autophosphorylation. Sci Signal. 2014;7(324):ra41. doi: 10.1126/scisignal.2005050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He G, Wang HR, Huang SK, Huang CL. Intersectin links WNK kinases to endocytosis of ROMK1. J Clin Invest. 2007;117(4):1078–1087. doi: 10.1172/JCI30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson FH, et al. Human hypertension caused by mutations in WNK kinases. Science. 2001;293(5532):1107–1112. doi: 10.1126/science.1062844. [DOI] [PubMed] [Google Scholar]
- 7.Anselmo AN, et al. WNK1 and OSR1 regulate the Na+, K+, 2Cl− cotransporter in HeLa cells. Proc Natl Acad Sci USA. 2006;103(29):10883–10888. doi: 10.1073/pnas.0604607103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moriguchi T, et al. WNK1 regulates phosphorylation of cation-chloride-coupled cotransporters via the STE20-related kinases, SPAK and OSR1. J Biol Chem. 2005;280(52):42685–42693. doi: 10.1074/jbc.M510042200. [DOI] [PubMed] [Google Scholar]
- 9.Vitari AC, Deak M, Morrice NA, Alessi DR. The WNK1 and WNK4 protein kinases that are mutated in Gordon’s hypertension syndrome phosphorylate and activate SPAK and OSR1 protein kinases. Biochem J. 2005;391(Pt 1):17–24. doi: 10.1042/BJ20051180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heise CJ, et al. Serum and glucocorticoid-induced kinase (SGK) 1 and the epithelial sodium channel are regulated by multiple with no lysine (WNK) family members. J Biol Chem. 2010;285(33):25161–25167. doi: 10.1074/jbc.M110.103432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deaton SL, Sengupta S, Cobb MH. WNK kinases and blood pressure control. Curr Hypertens Rep. 2009;11(6):421–426. doi: 10.1007/s11906-009-0072-z. [DOI] [PubMed] [Google Scholar]
- 12.Sengupta S, et al. Regulation of OSR1 and the sodium, potassium, two chloride cotransporter by convergent signals. Proc Natl Acad Sci USA. 2013;110(47):18826–18831. doi: 10.1073/pnas.1318676110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sengupta S, et al. Interactions with WNK (with no lysine) family members regulate oxidative stress response 1 and ion co-transporter activity. J Biol Chem. 2012;287(45):37868–37879. doi: 10.1074/jbc.M112.398750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vitari AC, et al. Functional interactions of the SPAK/OSR1 kinases with their upstream activator WNK1 and downstream substrate NKCC1. Biochem J. 2006;397(1):223–231. doi: 10.1042/BJ20060220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Los Heros P, et al. The WNK-regulated SPAK/OSR1 kinases directly phosphorylate and inhibit the K+-Cl− co-transporters. Biochem J. 2014;458(3):559–573. doi: 10.1042/BJ20131478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gagnon KB, England R, Delpire E. Volume sensitivity of cation-Cl− cotransporters is modulated by the interaction of two kinases: Ste20-related proline-alanine-rich kinase and WNK4. Am J Physiol Cell Physiol. 2006;290(1):C134–C142. doi: 10.1152/ajpcell.00037.2005. [DOI] [PubMed] [Google Scholar]
- 17.Piechotta K, Lu J, Delpire E. Cation chloride cotransporters interact with the stress-related kinases Ste20-related proline-alanine-rich kinase (SPAK) and oxidative stress response 1 (OSR1) J Biol Chem. 2002;277(52):50812–50819. doi: 10.1074/jbc.M208108200. [DOI] [PubMed] [Google Scholar]
- 18.Zambrowicz BP, et al. Wnk1 kinase deficiency lowers blood pressure in mice: A gene-trap screen to identify potential targets for therapeutic intervention. Proc Natl Acad Sci USA. 2003;100(24):14109–14114. doi: 10.1073/pnas.2336103100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Susa K, et al. Effect of heterozygous deletion of WNK1 on the WNK-OSR1/SPAK-NCC/NKCC1/NKCC2 signal cascade in the kidney and blood vessels. Clin Exp Nephrol. 2012;16(4):530–538. doi: 10.1007/s10157-012-0590-x. [DOI] [PubMed] [Google Scholar]
- 20.Xie J, et al. Endothelial-specific expression of WNK1 kinase is essential for angiogenesis and heart development in mice. Am J Pathol. 2009;175(3):1315–1327. doi: 10.2353/ajpath.2009.090094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie J, Yoon J, Yang SS, Lin SH, Huang CL. WNK1 protein kinase regulates embryonic cardiovascular development through the OSR1 signaling cascade. J Biol Chem. 2013;288(12):8566–8574. doi: 10.1074/jbc.M113.451575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lai JG, et al. Zebrafish WNK lysine deficient protein kinase 1 (wnk1) affects angiogenesis associated with VEGF signaling. PLoS ONE. 2014;9(8):e106129. doi: 10.1371/journal.pone.0106129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Risau W, Flamme I. Vasculogenesis. Annu Rev Cell Dev Biol. 1995;11:73–91. doi: 10.1146/annurev.cb.11.110195.000445. [DOI] [PubMed] [Google Scholar]
- 24.Mayan H, et al. Pseudohypoaldosteronism type II: Marked sensitivity to thiazides, hypercalciuria, normomagnesemia, and low bone mineral density. J Clin Endocrinol Metab. 2002;87(7):3248–3254. doi: 10.1210/jcem.87.7.8449. [DOI] [PubMed] [Google Scholar]
- 25.Siew K, O’Shaughnessy KM. Extrarenal roles of the with-no-lysine[K] kinases (WNKs) Clin Exp Pharmacol Physiol. 2013;40(12):885–894. doi: 10.1111/1440-1681.12108. [DOI] [PubMed] [Google Scholar]
- 26.Folkman J, Shing Y. Angiogenesis. J Biol Chem. 1992;267(16):10931–10934. [PubMed] [Google Scholar]
- 27.Ferrara N. Vascular endothelial growth factor: Basic science and clinical progress. Endocr Rev. 2004;25(4):581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 28.Flamme I, Frölich T, Risau W. Molecular mechanisms of vasculogenesis and embryonic angiogenesis. J Cell Physiol. 1997;173(2):206–210. doi: 10.1002/(SICI)1097-4652(199711)173:2<206::AID-JCP22>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 29.Antonetti DA, Lieth E, Barber AJ, Gardner TW. Molecular mechanisms of vascular permeability in diabetic retinopathy. Semin Ophthalmol. 1999;14(4):240–248. doi: 10.3109/08820539909069543. [DOI] [PubMed] [Google Scholar]
- 30.Folkman J. The role of angiogenesis in tumor growth. Semin Cancer Biol. 1992;3(2):65–71. [PubMed] [Google Scholar]
- 31.Harris SR, Thorgeirsson UP. Tumor angiogenesis: Biology and therapeutic prospects. In Vivo. 1998;12(6):563–570. [PubMed] [Google Scholar]
- 32.Jones A, Harris AL. New developments in angiogenesis: A major mechanism for tumor growth and target for therapy. Cancer J Sci Am. 1998;4(4):209–217. [PubMed] [Google Scholar]
- 33.Folkman J. Angiogenesis. Annu Rev Med. 2006;57:1–18. doi: 10.1146/annurev.med.57.121304.131306. [DOI] [PubMed] [Google Scholar]
- 34.Yoo SY, Kwon SM. Angiogenesis and its therapeutic opportunities. Mediators Inflamm. 2013;2013:127170. doi: 10.1155/2013/127170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liakouli V, et al. Angiogenic cytokines and growth factors in systemic sclerosis. Autoimmun Rev. 2011;10(10):590–594. doi: 10.1016/j.autrev.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 36.Welch-Reardon KM, et al. Angiogenic sprouting is regulated by endothelial cell expression of Slug. J Cell Sci. 2014;127(Pt 9):2017–2028. doi: 10.1242/jcs.143420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999;18(49):6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- 38.Hale AT, et al. Endothelial Kruppel-like factor 4 regulates angiogenesis and the Notch signaling pathway. J Biol Chem. 2014;289(17):12016–12028. doi: 10.1074/jbc.M113.530956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Semenza GL. HIF-1: Using two hands to flip the angiogenic switch. Cancer Metastasis Rev. 2000;19(1-2):59–65. doi: 10.1023/a:1026544214667. [DOI] [PubMed] [Google Scholar]
- 40.Lim CS, Kiriakidis S, Sandison A, Paleolog EM, Davies AH. Hypoxia-inducible factor pathway and diseases of the vascular wall. J Vasc Surg. 2013;58(1):219–230. doi: 10.1016/j.jvs.2013.02.240. [DOI] [PubMed] [Google Scholar]
- 41.Oi K, et al. A minor role of WNK3 in regulating phosphorylation of renal NKCC2 and NCC co-transporters in vivo. Biol Open. 2012;1(2):120–127. doi: 10.1242/bio.2011048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rivera LB, Bradshaw AD, Brekken RA. The regulatory function of SPARC in vascular biology. Cell Mol Life Sci. 2011;68(19):3165–3173. doi: 10.1007/s00018-011-0781-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chlenski A, et al. Anti-angiogenic SPARC peptides inhibit progression of neuroblastoma tumors. Mol Cancer. 2010;9:138. doi: 10.1186/1476-4598-9-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakatsu MN, Davis J, Hughes CC. Optimized fibrin gel bead assay for the study of angiogenesis. J Vis Exp. 2007;(3):e186. doi: 10.3791/186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakatsu MN, et al. Angiogenic sprouting and capillary lumen formation modeled by human umbilical vein endothelial cells (HUVEC) in fibrin gels: The role of fibroblasts and Angiopoietin-1. Microvasc Res. 2003;66(2):102–112. doi: 10.1016/s0026-2862(03)00045-1. [DOI] [PubMed] [Google Scholar]
- 46.Newman AC, Nakatsu MN, Chou W, Gershon PD, Hughes CC. The requirement for fibroblasts in angiogenesis: Fibroblast-derived matrix proteins are essential for endothelial cell lumen formation. Mol Biol Cell. 2011;22(20):3791–3800. doi: 10.1091/mbc.E11-05-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Korff T, Augustin HG. Integration of endothelial cells in multicellular spheroids prevents apoptosis and induces differentiation. J Cell Biol. 1998;143(5):1341–1352. doi: 10.1083/jcb.143.5.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vailhé B, Vittet D, Feige JJ. In vitro models of vasculogenesis and angiogenesis. Lab Invest. 2001;81(4):439–452. doi: 10.1038/labinvest.3780252. [DOI] [PubMed] [Google Scholar]
- 49.Moniz S, Jordan P. Emerging roles for WNK kinases in cancer. Cell Mol Life Sci. 2010;67(8):1265–1276. doi: 10.1007/s00018-010-0261-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tu SW, Bugde A, Luby-Phelps K, Cobb MH. WNK1 is required for mitosis and abscission. Proc Natl Acad Sci USA. 2011;108(4):1385–1390. doi: 10.1073/pnas.1018567108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suzuki T, Aizawa K, Matsumura T, Nagai R. Vascular implications of the Krüppel-like family of transcription factors. Arterioscler Thromb Vasc Biol. 2005;25(6):1135–1141. doi: 10.1161/01.ATV.0000165656.65359.23. [DOI] [PubMed] [Google Scholar]
- 52.Newby AC. Matrix metalloproteinase inhibition therapy for vascular diseases. Vascul Pharmacol. 2012;56(5-6):232–244. doi: 10.1016/j.vph.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 53.Iruela-Arispe ML, Luque A, Lee N. Thrombospondin modules and angiogenesis. Int J Biochem Cell Biol. 2004;36(6):1070–1078. doi: 10.1016/j.biocel.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 54.Yang SS, et al. SPAK-knockout mice manifest Gitelman syndrome and impaired vasoconstriction. J Am Soc Nephrol. 2010;21(11):1868–1877. doi: 10.1681/ASN.2009121295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Magenta A, et al. miR-200c is upregulated by oxidative stress and induces endothelial cell apoptosis and senescence via ZEB1 inhibition. Cell Death Differ. 2011;18(10):1628–1639. doi: 10.1038/cdd.2011.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Serysheva E, et al. Wnk kinases are positive regulators of canonical Wnt/β-catenin signalling. EMBO Rep. 2013;14(8):718–725. doi: 10.1038/embor.2013.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun X, Gao L, Yu RK, Zeng G. Down-regulation of WNK1 protein kinase in neural progenitor cells suppresses cell proliferation and migration. J Neurochem. 2006;99(4):1114–1121. doi: 10.1111/j.1471-4159.2006.04159.x. [DOI] [PubMed] [Google Scholar]
- 58.Jiang ZY, et al. Identification of WNK1 as a substrate of Akt/protein kinase B and a negative regulator of insulin-stimulated mitogenesis in 3T3-L1 cells. J Biol Chem. 2005;280(22):21622–21628. doi: 10.1074/jbc.M414464200. [DOI] [PubMed] [Google Scholar]
- 59.Björklund M, et al. Identification of pathways regulating cell size and cell-cycle progression by RNAi. Nature. 2006;439(7079):1009–1013. doi: 10.1038/nature04469. [DOI] [PubMed] [Google Scholar]
- 60.Conery AR, Harlow E. High-throughput screens in diploid cells identify factors that contribute to the acquisition of chromosomal instability. Proc Natl Acad Sci USA. 2010;107(35):15455–15460. doi: 10.1073/pnas.1010627107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Veríssimo F, Silva E, Morris JD, Pepperkok R, Jordan P. Protein kinase WNK3 increases cell survival in a caspase-3-dependent pathway. Oncogene. 2006;25(30):4172–4182. doi: 10.1038/sj.onc.1209449. [DOI] [PubMed] [Google Scholar]
- 62.Balatoni CE, et al. Epigenetic silencing of Stk39 in B-cell lymphoma inhibits apoptosis from genotoxic stress. Am J Pathol. 2009;175(4):1653–1661. doi: 10.2353/ajpath.2009.090091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Haas BR, et al. With-No-Lysine Kinase 3 (WNK3) stimulates glioma invasion by regulating cell volume. Am J Physiol Cell Physiol. 2011;301(5):C1150–C1160. doi: 10.1152/ajpcell.00203.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mäusbacher N, Schreiber TB, Daub H. Glycoprotein capture and quantitative phosphoproteomics indicate coordinated regulation of cell migration upon lysophosphatidic acid stimulation. Mol Cell Proteomics. 2010;9(11):2337–2353. doi: 10.1074/mcp.M110.000737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moniz S, et al. Loss of WNK2 expression by promoter gene methylation occurs in adult gliomas and triggers Rac1-mediated tumour cell invasiveness. Hum Mol Genet. 2013;22(1):84–95. doi: 10.1093/hmg/dds405. [DOI] [PubMed] [Google Scholar]
- 66.Alves CC, Carneiro F, Hoefler H, Becker KF. Role of the epithelial-mesenchymal transition regulator Slug in primary human cancers. Front Biosci (Landmark Ed) 2009;14:3035–3050. doi: 10.2741/3433. [DOI] [PubMed] [Google Scholar]
- 67.Cobaleda C, Pérez-Caro M, Vicente-Dueñas C, Sánchez-García I. Function of the zinc-finger transcription factor SNAI2 in cancer and development. Annu Rev Genet. 2007;41:41–61. doi: 10.1146/annurev.genet.41.110306.130146. [DOI] [PubMed] [Google Scholar]
- 68.Lu C, et al. Gene alterations identified by expression profiling in tumor-associated endothelial cells from invasive ovarian carcinoma. Cancer Res. 2007;67(4):1757–1768. doi: 10.1158/0008-5472.CAN-06-3700. [DOI] [PubMed] [Google Scholar]
- 69.Chen W, Yazicioglu M, Cobb MH. Characterization of OSR1, a member of the mammalian Ste20p/germinal center kinase subfamily. J Biol Chem. 2004;279(12):11129–11136. doi: 10.1074/jbc.M313562200. [DOI] [PubMed] [Google Scholar]
- 70.Johnston AM, et al. SPAK, a STE20/SPS1-related kinase that activates the p38 pathway. Oncogene. 2000;19(37):4290–4297. doi: 10.1038/sj.onc.1203784. [DOI] [PubMed] [Google Scholar]
- 71.Zimrin AB, Villeponteau B, Maciag T. Models of in vitro angiogenesis: Endothelial cell differentiation on fibrin but not Matrigel is transcriptionally dependent. Biochem Biophys Res Commun. 1995;213(2):630–638. doi: 10.1006/bbrc.1995.2178. [DOI] [PubMed] [Google Scholar]
- 72.Grove AD, et al. Both protein activation and gene expression are involved in early vascular tube formation in vitro. Clin Cancer Res. 2002;8(9):3019–3026. [PubMed] [Google Scholar]
- 73.Hahn CN, et al. Expression profiling reveals functionally important genes and coordinately regulated signaling pathway genes during in vitro angiogenesis. Physiol Genomics. 2005;22(1):57–69. doi: 10.1152/physiolgenomics.00278.2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.